Introduction
Idiopathic pulmonary fibrosis (IPF) is a chronic and
progressive interstitial lung disease that is primarily
characterized by marked infiltration of macrophages and abnormal
deposition of the extracellular matrix (ECM) (1,2).
This disease severely impacts the quality of life of patients and
has a high mortality rate, with a 5-year survival rate usually
between 20-30% (3,4). Although pirfenidone and nintedanib
have been approved for the treatment of IPF, their efficacy in
improving patient prognosis is limited (5,6).
In order to improve the survival rate and quality of life for
patients with IPF, it is necessary to conduct further research into
the pathogenesis of the disease and explore more effective
treatment options and therapeutic targets.
Macrophages, immune cells with both phagocytic and
bactericidal capabilities, can transition from an M1 to an M2 state
in response to stimulation or alterations in their microenvironment
(7,8). M1 macrophages are closely
associated with inflammation and tissue damage, whereas M2
macrophages contribute to tissue repair and fibrosis by releasing
pro-fibrotic factors (9-11). Given these insights, developing
therapeutic strategies targeting M2 macrophages holds promise for
achieving significant breakthroughs in the treatment of IPF
(12,13).
Secreted phosphoprotein 1 (SPP1), also known as
osteopontin, is a multifunctional secreted phosphoprotein (14,15). It was previously revealed that
high expression of SPP1 in lung cancer is commonly associated with
the severity of the tumor and poor patient prognosis (16). In terms of its function, SPP1 is
primarily synthesized by both activated macrophages and epithelial
cells. It plays a key role in regulating cell chemotaxis and
adhesion, as well as impacting cell proliferation and migration.
Ultimately, it contributes significantly to the development of
fibrosis in the liver and kidneys (17-20). Studies reported that SPP1 can
promote epithelial-mesenchymal transition (EMT) in epithelial
cells, thereby promoting lung fibrosis (21). Furthermore, it was recently
suggested that inhibiting the expression of the SPP1 gene in club
cells can alleviate the progression of IPF and it also points out
that macrophages may be a key source of SPP1 in IPF (22); however, the aforementioned
studies did not continue to delve into the specific functions of
SPP1 in macrophages and its mechanisms of action. On this basis, in
the present study it was aimed to further investigate the function
of SPP1 in macrophages and its mechanism of action in IPF.
The current study comprehensively employed RNA
sequencing (RNA-seq) data and clinical information from lung
tissues of patients with IPF to meticulously analyze the expression
of SPP1 in these patients and its association with disease
prognosis. Utilizing single-cell sequencing technology, the present
study further elucidated the specific cellular localization and
expression patterns of SPP1 within lung tissue. In vitro
experiments investigated the effects of SPP1 on macrophage M2
polarization and its role in the EMT process of epithelial and
fibroblastic cells. Concurrently, through in vivo animal
experiments, the present study explored the potential intervention
mechanism of SPP1 inhibitors on the pathological process of IPF.
The present results enhanced the comprehension of the mechanisms by
which SPP1 operates in the pathological process of IPF,
establishing a scientific basis for the investigation of potential
future treatment pathways.
Materials and methods
Download and analysis of data sets
The present study utilized the Gene Expression
Omnibus (GEO) database, specifically the GSE70866 dataset
(https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE70866),
which is based on the GPL14550 platform and includes RNA-seq data
for 20 healthy control samples and 112 patients with IPF (23). The Limma package within R
software version 4.0.2 (https://www.r-project.org/) was employed for
comparative analysis to elucidate differences in SPP1 expression
between healthy individuals and patients with IPF. Moreover,
Kaplan-Meier survival curve analysis was conducted to explore the
relationship between SPP1 expression levels and survival rates in
patients with IPF. To gain a deeper understanding of SPP1's
expression pattern at the cellular level, the GSE132771 single-cell
RNA-seq dataset (https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE132771)
was retrieved from the GEO database and SPP1 cell expression
localization analysis was conducted on three IPF samples (24). Concurrently, single-sample gene
set enrichment analysis was applied to assess immune cell gene sets
associated with SPP1 expression, aiming to reveal the connection
between SPP1 and immune cell infiltration.
Cell isolation and cultivation
Initially, 10 ml of venous blood was drawn from
healthy volunteers (Table SI)
and transferred into a centrifuge tube. An equal volume of PBS
solution (Biosharp Life Sciences; cat. no. BL601A) was added for
dilution and after gentle mixing, 5 ml of Ficoll Pague PLUS
solution (Cytiva; cat. no. 17144002) was drawn into a new
centrifuge tube. Then, the diluted blood was gently added to the
top layer of Ficoll. After 15 min of centrifugation at 4°C and 300
× g, the middle white layer represents peripheral blood mononuclear
cells (PBMCs). Subsequently, the PBMCs were transferred to another
15 ml centrifuge tube with 10 ml of PBS solution and centrifuged at
4°C and 1,000 × g for 10 min and the process was repeated twice.
After discarding the supernatant, the cells were resuspended in
complete RPMI-1640 medium (Thermo Fisher Scientific, Inc.; cat. no.
11875093) supplemented with 10% fetal bovine serum (Beyotime
Institute of Biotechnology; cat. no. C0235) and 1%
penicillin/streptomycin (Beyotime Institute of Biotechnology; cat.
no. C0222) and cultured for 7 days under 100 ng/ml macrophage
colony-stimulating factor (M-CSF) (MedChemExpress; cat. no.
HY-P7050A) to induce differentiation of PBMCs into macrophage-like
cells (PBMC-m). THP-1 cells (cat. no. SCSP-567) were purchased from
the Cell Bank of the Chinese Academy of Sciences and cultured in
complete RPMI-1640 medium (90% RPMI-1640 + 10% fetal bovine serum +
1% penicillin/streptomycin). THP-1 cells are treated with 100 nM
PMA (MedChemExpress; cat. no. HY-18739) for 48 h to induce
macrophage differentiation. BEAS-2B and WI-38 cells (cat. nos.
GNHu27 and SCSP-521, respectively) were likewise obtained from the
Cell Bank of the Chinese Academy of Sciences, with BEAS-2B being
cultured in complete DMEM medium (Thermo Fisher Scientific, Inc.;
cat. no. 11965092) (90% DMEM + 10% fetal bovine serum + 1%
penicillin/streptomycin). WI-38 cells were grown in complete MEM
medium (Thermo Fisher Scientific, Inc.; cat. no. 11095080) (90% MEM
+ 10% fetal bovine serum + 1% penicillin/streptomycin) and all
cells were maintained in an incubator at 37°C with 5%
CO2. Venous blood was collected from all volunteers
after obtaining their consent, and an informed consent document was
signed by all participants. The human tissue samples were sourced
from the Oncology Hospital of the Huainan Dongfang Hospital Group
(Huainan, China) in May 2023.
Small interfering (si)RNA
transfection
PBMC-m and THP-1 cells were initially plated in
separate culture dishes. Once the cell growth density reached
60-80%, the transfection was performed. In total, 50 pmol of siRNA
was diluted in 200 μl of serum-free RPMI-1640 medium.
Additionally, 4 μl of Lipofectamine™ 2000 (Thermo Fisher
Scientific, Inc.; cat. no. 11668500) was diluted in 200 μl
of RPMI-1640 medium. The reagents were mixed well and then allowed
to stand for 15 min. The mixture was then added to the culture
dishes that had already been seeded with cells and the dishes were
gently rocked to ensure even distribution of the complex. After 4-6
h of incubation in an incubator at 37°C and 5% CO2, the
medium was changed to complete RPMI-1640 medium (90% RPMI 1640 +
10% fetal bovine serum + 1% penicillin/streptomycin) for continued
culture. The relevant siRNA sequences used in the experiment were
as follows: Negative control siRNA (si_NC; sense:
5′-UUCUCCGAACGUGUCACGUTT-3); and SPP1 specific siRNA (si_SPP1;
sense: 5′-CUGUGCCAUACCAGUUAAATT-3).
Cell coculture and wound scratch
assay
Bleomycin (BLM; MedChemExpress; cat. no. HY-17565A)
was initially dissolved in DMSO (MedChemExpress; cat. no. HY-Y0320)
to create a 10 mM stock solution, while Stattic (MedChemExpress;
cat. no. HY-13818) was dissolved in DMSO to form a 5 mM stock
solution. Initially, the supernatant was transferred from PBMC-m
and THP-1 cells that were treated with 5 μM BLM, 5 μM
STAT3 inhibitor (Stattic) and si_SPP1 into centrifuge tubes. Then,
the supernatant was collected and transferred to a centrifuge tube.
After centrifugal filtration, the supernatant was mixed with
serum-free medium in a 1:1 ratio to prepare the conditioned medium
(CM), which was stored at 4°C. BEAS-2B and WI-38 cells with a
density of 80-90% were counted after trypsin (Thermo Fisher
Scientific, Inc.; cat. no. 25200072) digestion, and then added to
DMEM/MEM complete medium. The concentration of BEAS-2B and WI-38
cells was ~5×105 cells/ml. The culture-dish was placed
into the incubator at 37°C and 5% CO2 for cultivation;
once the cells were confluent, 10-μl pipette tip was used to
draw three straight lines along a ruler at the bottom of the dish.
Washing was performed twice with PBS solution (Bioshare Life
Sciences; cat. no. BL601A), then the pre-prepared CM was aspirated
and added to the culture dish for further cultivation. The scratch
area of the cells was recorded by capturing images at 0, 24 and 48
h. Finally, the migration and healing of the cells was assessed
using ImageJ 1.52a software (National Institutes of Health).
RNA extraction and reverse
transcription-quantitative PCR (RT-qPCR)
Based on a previous protocol, total RNA was
extracted from the collected mouse lung tissues and cells (25) and the RNA concentration was
determined using a spectrophotometer. The RNA purity was considered
acceptable when the optical density ratio at 260 and 280 nm
(OD260/OD280) was between 1.8 and 2.0. The RevertAid First Strand
cDNA Synthesis Kit (Thermo Fisher Scientific, Inc.; cat. no. K1622)
was used to perform reverse transcription of RNA according to the
manufacturer's protocol, followed by gene amplification using the
Genious 2X SYBR Green Fast qPCR Mix (ABclonal Biotech Co., Ltd.;
cat. no. RK21204). The thermocycling protocol was as follows: 95°C
for a duration of 3 min, then 95°C for 5 sec, 60°C for 32 sec, and
72°C for 40 sec; this cycling process was repeated 43 times. To
standardize the results, the 2−∆∆Cq method was applied,
with GAPDH serving as the internal control gene (23). The primers used in the experiment
were designed and synthesized by Sangon Biotech (Shanghai) Co.,
Ltd. The specific primer sequences are detailed in Table SII.
Western blot (WB) analysis
Based on a previous protocol (25), proteins were extracted from the
collected cells, and their concentrations were determined using the
BCA protein assay kit (Thermo Fisher Scientific, Inc.; cat. no.
A55864) as per the manufacturer's protocol. Subsequently, the
protein samples (50 μg per lane) were subjected to
electrophoresis using a 10% SDS-PAGE gel and transferred to a
0.2-μm PVDF membrane (MilliporeSigma; cat. no. ISEQ00010).
After blocking the membrane with 5% skim milk at room temperature
for 2 h, it was incubated with the corresponding primary antibodies
at 4°C overnight. On the following day, the membrane was incubated
with a diluted secondary antibody solution for 2 h. Subsequently,
the protein bands were visualized using a chemiluminescent agent
(MilliporeSigma; cat. no. WBKLS100) and an imaging system (Cytiva;
Amersham™ ImageQuant™ 800; cat. no. 29399481), and analysis was
performed with ImageJ 1.52a software. The antibodies used in the
experiment included: SPP1 (Proteintech Group, Inc.; cat. no.
22952-1-AP; 1:2,000), COL3A1 (Proteintech Group, Inc.; cat. no.
68320-1-Ig; 1:5,000), E-cadherin (Cell Signaling Technology, Inc.;
cat. no. 3195T; 1:1,000), N-cadherin (Cell Signaling Technology,
Inc.; cat. no. 4061T; 1:1,000), Vimentin (Cell Signaling
Technology, Inc.; cat. no. 5741T; 1:1,000), phosphorylated (p)-JAK2
(ABclonal Biotech Co., Ltd.; cat. no. AP0531; 1:1,000), p-STAT3
(ABclonal Biotech Co., Ltd.; cat. no. AP0705; 1:1,000), JAK2
(ABclonal Biotech Co., Ltd.; cat. no. A11497; 1:1,000), STAT3
(ABclonal Biotech Co., Ltd.; cat. no. A1192; 1:1,000), GAPDH
(ABclonal Biotech Co., Ltd.; cat. no. AC002; 1:5,000) and
HRP-conjugated secondary antibody (ABclonal Biotech Co., Ltd.; cat.
no. AS063; 1:10,000). Densitometric analysis was performed using
ImageJ 1.52a software (National Institutes of Health).
Immunofluorescence staining analysis
Based on a previous protocol (25), the cells that were fixed in
formalin solution and the paraffin-embedded tissues from both
healthy and patients with IPF, were incubated with 5% BSA (Wuhan
Servicebio Technology Co., Ltd.; cat. no. GC305010-25g) at room
temperature for 1 h. Then, they were incubated with the
corresponding antibody overnight at 4°C. After adding the
corresponding fluorescent secondary antibody working solution, the
DAPI working solution (5 μg/ml) (Thermo Fisher Scientific,
Inc.; cat. no. 62248) was dropwise added. Finally, the specimens
were observed and images were collected under a fluorescence
microscope (Leica Microsystems, Inc.; cat. no. DMI3000 B). The
antibodies employed in the experiment included: SPP1 (Proteintech
Group, Inc.; cat. no. 22952-1-AP; 1:200), FAM13A (Proteintech
Group, Inc.; cat. no. 55401-1-AP; 1:100), p-JAK2 (ABclonal Biotech
Co., Ltd.; cat. no. AP0531; 1:100), p-STAT3 (ABclonal Biotech Co.,
Ltd.; cat. no. AP0705; 1:200), F4/80 (Proteintech Group, Inc.; cat.
no. 29414-1-AP; 1:500), CD68 (ABclonal Biotech Co., Ltd.; cat. no.
A23205; 1:200), CD206 (Proteintech Group, Inc.; cat. no.
60143-1-Ig; 1:500) and CD3 (Proteintech Group, Inc.; cat. no.
60181-1-Ig; 1:500).
Construction of animal models
All mice utilized in the present study were procured
from Henan Skobes Biotechnology Co., Ltd., consisting of 15
SPF-grade 8-10-week-old male C57BL/6J mice, with body weights
ranging from 18-22 g. These mice were housed in the animal facility
of the School of Medicine at Anhui University of Science and
Technology. All animal experimental protocols were granted approval
by the Animal Ethics Committee at Anhui University of Science and
Technology (approval no. NO. HX-001; Huainan, China). The mice were
maintained under controlled conditions (temperature 21±1°C,
humidity 50±16% and 12-h light/dark cycle), with a daily supply of
food and water, and body weight was measured once the mice were
acclimated to the environment. The mice were randomly divided into
three groups to construct animal models: PBS control group (n=5);
BLM-induced IPF disease group (n=5); and BLM + SPP1 inhibitor
(MedChemExpress; cat. no. HY-146064) treatment group (n=5). For the
PBS control group, mice were given 25 μl of 0.5% sodium
carboxymethyl cellulose (CMC-Na; Beijing Solarbio Science &
Technology Co., Ltd.; cat. no. IS9000) solution dissolved in
sterile PBS via intraperitoneal injection every 3 days. For the
establishment of the IPF disease group mouse model, isoflurane
(RWD; cat. no. R510-22-10) was first introduced into the induction
chamber of the anesthetic machine, with a concentration set at
3-4%. After waiting for 1 min until the induction chamber was
filled with isoflurane, the mice were placed into the box for
anesthesia. After ~2 min, the maintenance concentration was
adjusted to 1-1.5% and the mice were then removed and administered
5 mg/kg of BLM via nasal drops to induce a pulmonary fibrosis
model, once every 3 days. After three rounds of BLM stimulation,
this was discontinued and the mice were administered every three
days via intraperitoneal injection with 25 μl of 0.5% CMC-Na
solution. In the treatment group, mice were subjected to BLM
stimulation and simultaneously received 25 μl of SPP1
inhibitor solution (5 mg/kg) through intraperitoneal injection at
3-day intervals. The SPP1 inhibitor was dissolved by incorporating
it with 0.5% CMC-Na solution. Prior to the end of the animal model
experiments, all mice participating did not fulfill the conditions
necessitating humane euthanasia. Specific criteria for the humane
euthanasia of animals were as follows: i) Animals are unable to
walk on their limbs, completely losing their mobility; ii) dyspnea
is observed, characterized by the discharge of saliva or foam from
the mouth and nose; iii) urine and feces output is reduced, with
diarrhea or incontinence; iv) respiratory and pulse rates are
abnormal (either excessively high or low). These criteria should be
comprehensively assessed by professional veterinarians or
researchers according to specific circumstances to ensure the
welfare of the animals and the ethics of the experiment. Upon
completion of the 28-day animal model period, the mice were
subjected to an induction concentration of 3-4% isoflurane, within
an environment maintained at 1-1.5% concentration, after which they
were rendered unconscious to perform euthanasia through cervical
dislocation. Once it was determined that the animal was motionless,
without breathing or heartbeat, and the pupils were dilated, the
death of the animal was confirmed after an additional 2-3 min of
observation, and lung tissues were collected for subsequent
pathological examination.
H&E, Masson and Sirius red staining
analysis
Based on a previous study (25), mouse lung tissue samples were
initially fixed in a 10% formalin solution at room temperature for
48 h, followed by paraffin embedding and sectioning. The 5
μm-thick sections were stained with H&E and then
observed under a light microscope. Subsequently, the collagen
deposits were examined using Sirius Red and Masson's trichrome
staining solutions. Finally, all pathological images were analyzed
using ImageJ 1.52a software.
Statistical analysis
The present study utilized GraphPad Prism 9
(Dotmatics) software for data processing and analysis, with data
represented as the mean ± standard deviation. When the sample size
was ≤50, the Shapiro-Wilk test was applied to evaluate the
distribution of the data; when the sample size was >50, the
Kolmogorov-Smirnov test was applied, and a P>0.05 indicated a
normal distribution. After normality testing, the two-tailed
unpaired Student's t-test was used to compare between two groups,
whereas one-way ANOVA was used for comparisons among multiple
groups under a single variable and multi-way ANOVA was used for
comparisons among multiple groups under multiple variables,
followed by Tukey's post hoc test to analyze significant
differences between groups. P<0.05 was considered to indicate a
statistically significant difference.
Results
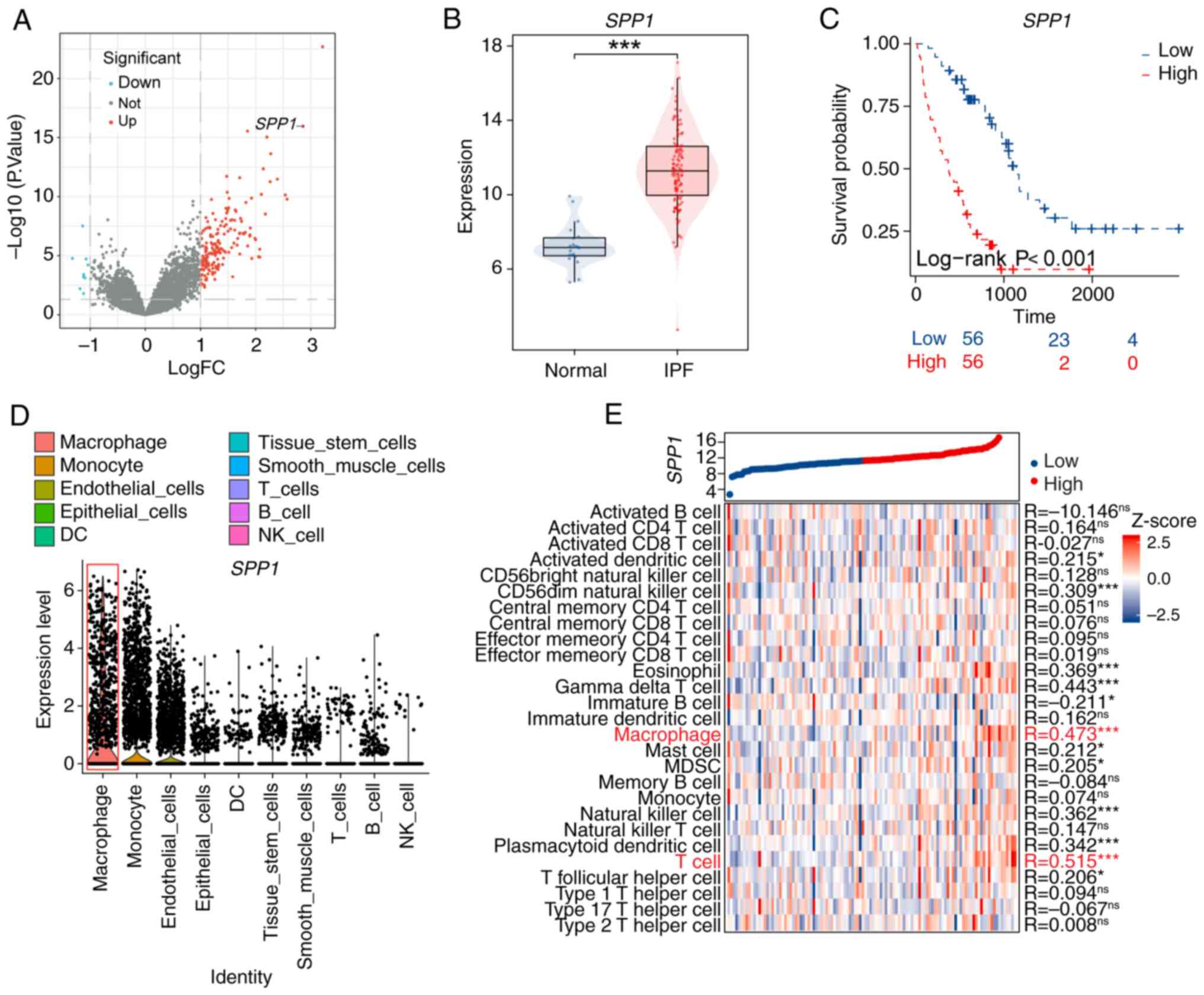
High expression of SPP1 is negatively
associated with the prognosis of patients with IPF
To explore further the expression pattern SPP1 in
patients with IPF and its relationship with prognosis, the present
study examined the RNA-seq data from the GEO database. The results
revealed that SPP1 gene expression is increased in the lung tissue
of patients with IPF (Fig. 1A and
B), and this upregulation correlates significantly with poor
prognosis (Fig. 1C). Single-cell
RNA-seq analysis demonstrated that SPP1 is primarily overexpressed
in macrophages and monocytes within the lung tissue of patients
with IPF (Fig. 1D). Additional
analysis revealed a significant positive association between SPP1
expression and immune cell expression, particularly in macrophages
and T cells, in the lungs of patients with IPF (Fig. 1E). These findings suggested a
potential role for SPP1 in macrophage function within IPF.
SPP1 promotes macrophage polarization
towards the M2 phenotype
Given the significant positive correlation between
SPP1 expression and macrophages and T cells, the present study
utilized immunofluorescence to assess SPP1 expression in
macrophages and T cells of lung tissues from normal controls and
patient with IPF. The results showed increased SPP1 expression in
macrophages (Fig. 2A and B). To
investigate the impact of SPP1 on macrophage function, PBMC-m and
THP-1 cells were stimulated with 5 μM BLM for 12 h and then
transfected with si_SPP1 and si_NC. After 24 h, the expression
levels of SPP1 and inflammation/fibrosis-related factors were
measured through RT-qPCR. The results revealed that BLM stimulation
increased the expression of inflammation and fibrosis-related
factors in macrophages, and knockdown of SPP1 significantly reduced
the expression levels of IL-10 and TGF-β, while the expression of
IL-1α, IL-1β and TNF-α remained unchanged (Fig. 2C and D). To verify transfection
efficiency, under conditions without BLM stimulation, PBMC and
THP-1 cells were transfected with si-NC and si-SPP1, respectively.
Subsequently, RT-qPCR technology was used to measure the expression
levels of SPP1 mRNA. The experimental results identified that, in
the absence of BLM stimulation, there was no significant difference
in the expression levels of SPP1 in PBMC cells and THP-1 cells
transfected with si-NC and si-SPP1 (Fig. S1A). Previous studies have
reported that M1 macrophages primarily secrete pro-inflammatory
factors such as IL-1α, IL-1β and TNF-α, while M2 macrophages
primarily secrete factors promoting tissue repair, including IL-4,
IL-10 and TGF-β (26-28); the present immunofluorescence
analysis revealed a significant increase in the expression of M2
macrophage marker CD206 in PBMC-m and THP-1 cells following BLM
stimulation. Moreover, the knockdown of SPP1 decreased CD206
expression (Fig. 2E and F).
These findings suggested that SPP1 knockdown inhibits BLM-induced
M2 macrophage polarization.
 | Figure 2SPP1 promotes macrophage polarization
towards the M2 phenotype. (A and B) Immunofluorescence analysis of
SPP1 (red) expression in (A) macrophages (CD68, green) and (B) T
cells (CD3, green) of normal and idiopathic pulmonary fibrosis
patient lung tissues, with nuclear staining (DAPI, blue) (scale
bar, 5 μm). (C and D) Expression and analysis of
inflammation and fibrosis-related genes in (C) PBMC-M and (D) THP-1
cells treated with PBS, BLM, BLM + si_NC, and BLM + si_SPP1
(experiments repeated three times). (E and F) Immunofluorescence
analysis of CD68 and CD206 expression in (E) PBMC-M and (F) THP-1
cells treated with PBS, BLM, and BLM + si_SPP1, with macrophage
marker (CD68, green), M2 macrophage marker (CD206, red) and nuclear
staining (DAPI, blue) (scale bar, 20 μm). Data are presented
as the mean ± standard deviation. *P<0.05,
**P<0.01 and ***P<0.001. SPP1, secreted
phosphoprotein 1; BLM, bleomycin; si-, small interfering; PBMC,
peripheral blood mononuclear cells; ns, not significant. |
SPP1 promotes the induction of EMT in
epithelial and fibroblastic cells by macrophages
Previous studies by the authors revealed that high
expression of SPP1 can promote macrophage polarization towards the
M2 phenotype, which is known to secrete substantial levels of TGF-β
and induce EMT in epithelial and fibroblastic cells, thereby
promoting the development of pulmonary fibrosis (29,30). To investigate the role of SPP1 in
macrophage-induced EMT, a cellular model where macrophages (PBMC-m
and THP-1) induce EMT in epithelial (BEAS-2B) and fibroblastic
(WI-38) cells was established in the present study. The supernatant
was collected from macrophages treated with PBS, BLM and BLM +
si_SPP1 for 24 h and formulated into conditioned media (PBS_CM,
BLM_CM and BLM + si_SPP1_CM), which were used to stimulate
epithelial (BEAS-2B) and fibroblastic (WI-38) cells, respectively.
Scratch assay results showed that the supernatant from macrophages
stimulated with BLM (BLM_CM) significantly enhanced the migration
of both epithelial (BEAS-2B) and fibroblastic (WI-38) cells.
However, when SPP1 expression was knocked down in macrophages after
BLM stimulation, their supernatant (BLM + si_SPP1_CM) significantly
inhibited the migration of these cells (Fig. 3). WB analysis further confirmed
that BLM_CM promoted the expression of COL3A1, N-cadherin and
Vimentin proteins in epithelial (BEAS-2B) and fibroblastic (WI-38)
cells while reducing the level of E-cadherin. By contrast, BLM +
si_SPP1_CM suppressed these changes, suggesting that inhibition of
SPP1 may help reverse the EMT process and may have a positive role
in slowing down the fibrotic process.
 | Figure 3SPP1 promotes the induction of EMT in
epithelial and fibroblastic cells by macrophages. (A and B)
Schematic diagrams illustrating the indirect co-culture method of
PBMC-m and THP-1 with BEAS-2B cells. After treating (A) PBMC-m and
(B) THP-1 cells under various conditions, their supernatants were
used to culture BEAS-2B cells. Cell scratch assays (magnification,
×100) and assessments of EMT-related protein expression levels
(E-cadherin, N-cadherin, Vimentin and COL3A1) were performed (with
the experiment repeated three times). (C and D) Schematic diagrams
respectively depict the indirect co-culture methods of PBMC-M and
THP-1 with WI-38 cells. After treating (C) PBMC-m and (D) THP-1
cells under different conditions, their supernatants were utilized
to culture WI-38 cells. Subsequent cellular scratch assays
(magnification, ×100) and assessments of EMT-related protein
expression levels (E-cadherin, N-cadherin, Vimentin and COL3A1)
were conducted. (with the experiment repeated three times). Data
are presented as the mean ± standard deviation.
*P<0.05, **P<0.01 and
***P<0.001. SPP1, secreted phosphoprotein 1; EMT,
epithelial-to-mesenchymal transition; PBMC, peripheral blood
mononuclear cells; ns, not significant. |
SPP1 induces macrophage M2 polarization
through the JAK2/STAT3 pathway
To uncover the molecular mechanisms by which SPP1
regulates the polarization of M2 macrophages in IPF, the present
study integrated previous research findings suggesting that SPP1
may function by influencing the activation of STAT3 (31,32). After transfecting si_SPP1 into
PBMC-m and THP-1 cells stimulated by BLM, the protein expression
levels of SPP1, STAT3 and p-STAT3 were determined. The results
indicated that after downregulating the expression of SPP1, the
expression levels of p-STAT3 and the ratio of p-STAT3 to STAT3 in
macrophages were significantly reduced (Figs. 4A, B and S1B). JAK2 is a key kinase involved in
STAT3 activation (33,34), and our detection showed that
after downregulating SPP1 expression, the protein expression levels
of p-JAK2 and the ratio of p-JAK2 to JAK2 in macrophages were also
reduced (Figs. 4A, B and
S1B), suggesting that SPP1 may
influence the polarization of M2 macrophages through the JAK2/STAT3
signaling pathway. Cell scratch assays revealed that BEAS-2B cells
cultured with BLM-stimulated macrophage supernatant (BLM_CM)
exhibited enhanced cell migration, which was significantly
inhibited after treatment with 5 μM STAT3 inhibitor
(stattic; Fig. 4C and D).
Similarly, in the co-cultivation model with macrophages and
fibroblast line WI-38, the STAT3 inhibitor significantly suppressed
the migration of WI-38 cells (Fig.
4E and F). These findings collectively support the hypothesis
that SPP1 promotes M2 macrophage polarization through the
JAK2/STAT3 signaling pathway, thereby facilitating the progression
of pulmonary fibrosis.
 | Figure 4SPP1 induces macrophage M2
polarization through the JAK2/STAT3 pathway. (A and B) After
treating PBMC-m and THP-1 cells with PBS, BLM, and BLM + si_SPP1
for 48 h, the protein expression of SPP1, JAK2, STAT3, p-JAK2 and
p-STAT3 were detected (with the experiment repeated three times).
(C-F) PBS, BLM and BLM + Stattic were used to treat PBMC-m and
THP-1 cells, and the supernatants were collected and co-cultured
with BEAS-2B and WI-38 cells, respectively, for cell scratch assays
(magnification, ×100) (with the experiment repeated three times).
Data are presented as the mean ± standard deviation.
**P<0.01 and ***P<0.001. SPP1, secreted
phosphoprotein 1; BLM, bleomycin. PBMC, peripheral blood
mononuclear cells; p-, phosphorylated; ns, not significant. |
In vivo inhibition of SPP1 expression can
effectively treat IPF in mice
To elucidate the role of SPP1 in IPF, three groups
of mouse animal models were established: Control: IPF disease and
an SPP1 inhibitor treatment group (Fig. 5A). Through monitoring the body
weight of the mice, it was found that the mice treated with SPP1
inhibitors gradually regained and stabilized their body weight
starting from the fifth day of treatment (Fig. 5B). At the end of the experiment,
the mice were sacrificed and their lung tissues were examined
histologically (Fig. 5C).
H&E staining, Masson's trichrome staining and Sirius Red
staining revealed a significant reduction in fibrotic areas and
inflammatory cell infiltration in the lung tissues of the treated
mice, along with a decrease in collagen fiber deposition (Figs. 5D and S1C). In addition, three lung tissue
specimens were randomly selected from each group of mice models to
carry out RT-qPCR to examine the expression levels of genes
associated with inflammation and fibrosis. The results indicated
that compared with the disease group, the levels of inflammatory
genes (IL-α, IL-β and IL-6) and fibrotic genes (TGF-β, α-SMA and
COL3A1) in the lung tissues of the treated mice were significantly
reduced (Fig. S1D). In
addition, immunofluorescence analysis revealed a significant
increase in the expression level of FAM13A (a marker of pulmonary
function) (35) in the treated
group compared with the disease group (Fig. 5E). Further immunofluorescence
detection revealed high expression of SPP1 in alveolar macrophages
of IPF mice and that SPP1 inhibitor treatment significantly reduced
SPP1 expression in macrophages (Fig.
5F). These results suggested that SPP1 expression is closely
associated with the development of IPF. Moreover, assessing the
expression levels of JAK2 and STAT3 in lung tissue macrophages of
the three groups, it was found that the expression levels of JAK2
and STAT3 in the SPP1 inhibitor group were significantly decreased
(Fig. 5G), further suggesting
that SPP1 may promote the development of IPF by activating the
JAK2/STAT3 signaling pathway.
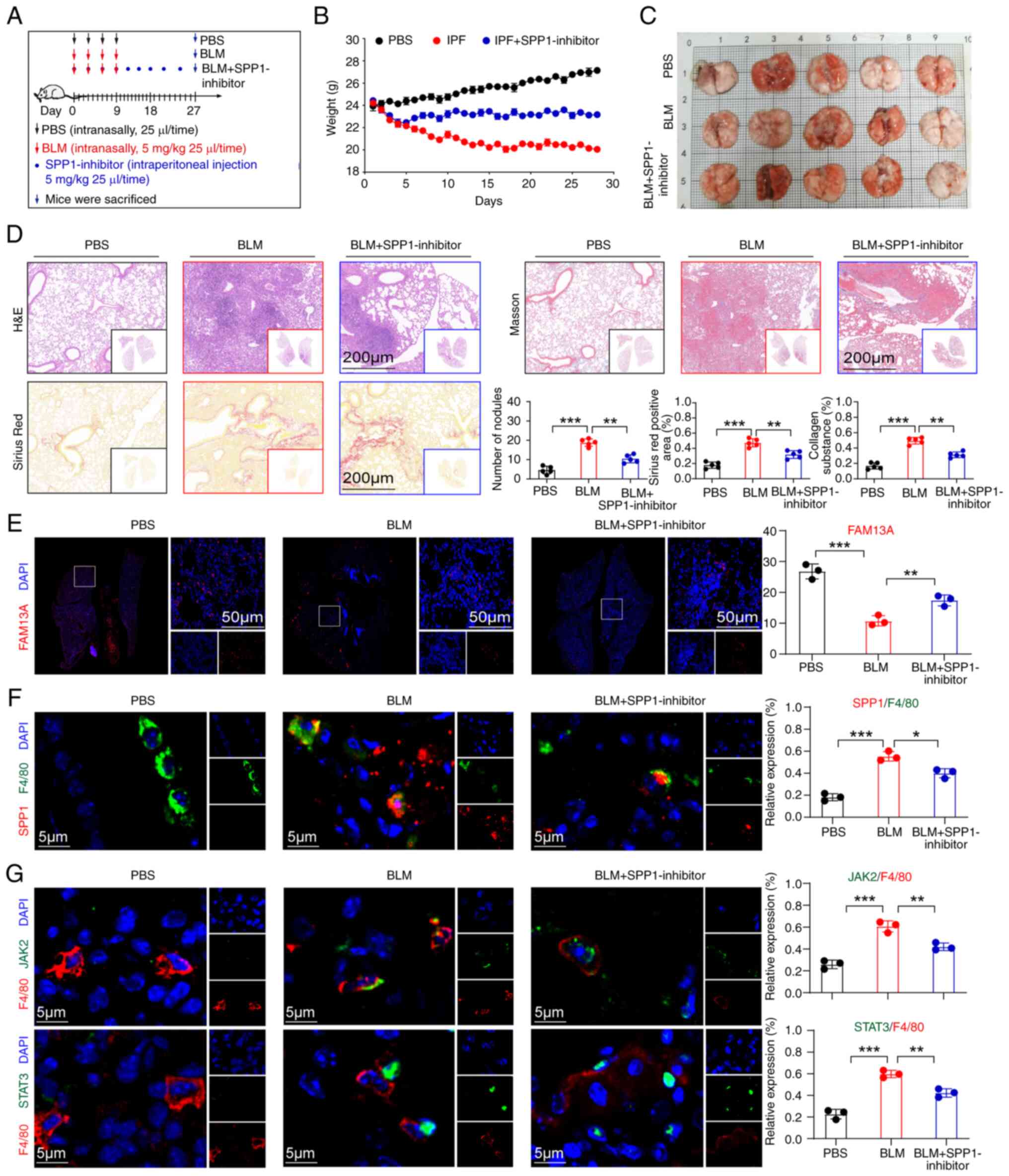 | Figure 5In vivo inhibition of SPP1
expression can effectively treat IPF in mice. (A) Schematic
illustration of the SPP1 inhibitor treatment in an IPF mouse model.
(B) Graphical representation of weight changes in three groups of
mice. (C) Images of lung tissues from three groups of mice at the
end of the experiment (scale bar, 1 cm). (D) Analysis of H&E,
Masson and Sirius Red staining of lung tissues from three groups of
mice (each dot represents one mouse; scale bar, 200 μm). (E)
Immunofluorescence detection of FAM13A expression in mouse lung
tissues (red) (scale bar, 50 μm). (F) Immunofluorescence
analysis of SPP1 (red) expression and localization in lung tissues
of normal, IPF and SPP1-inhibitor treated IPF mice, with
macrophages (F4/80, green) and cell nuclei (DAPI, blue) (scale bar,
5 μm). (G) Detection of p-JAK2 (green) and p-STAT3 (green)
expression in lung tissues from normal, IPF and SPP1
inhibitor-treated IPF mice, with macrophages (F4/80, red), cell
nuclei (DAPI, blue) (scale bar, 5 μm). Data are presented as
the mean ± standard deviation. *P<0.05,
**P<0.01 and ***P<0.001. SPP1, secreted
phosphoprotein 1; IPF, idiopathic pulmonary fibrosis; p-,
phosphorylated; BLM, bleomycin; ns, not significant. |
Discussion
The present study comprehensively analyzed the
crucial role of SPP1 in the development of IPF. The current
findings revealed that SPP1 expression is increased in lung
macrophages of patients with IPF, correlating negatively with their
prognosis. Experiments confirmed that SPP1 predominantly promotes
M2 macrophage polarization and accelerates fibrosis by activating
the JAK2/STAT3 signaling pathway. The findings demonstrated that
SPP1 has a crucial role in the progression of IPF, offering
beneficial insights for the future study and therapy of IPF.
Although the precise pathogenic mechanisms of IPF
have not been fully explained, research has indicated a strong
connection between IPF and the repeated injury to alveolar
epithelial cells. This includes abnormalities in epithelial repair
and the disturbances in the interactions between epithelial and
fibroblast cells. These factors collectively lead to the persistent
activation of mesenchymal cells and excessive deposition of the ECM
(36,37). In recent years, macrophages have
become a research focus due to their dual roles in fibrotic
remodeling, promoting fibrosis and anti-fibrosis (9). Recent studies observed infiltration
of M2-type macrophages in patients with IPF and those with lung
fibrosis caused by SARS-CoV-2, and have confirmed that inhibiting
M2-type macrophage polarization can mitigate BLM-induced lung
injury and fibrosis in model mice (34,38). Given the heterogeneity and
plasticity of macrophages under healthy and disease states,
elucidating their complex roles and interactions with other lung
cells presents a significant challenge. In the current study, it
was found through database analysis that high expression of SPP1 in
patients with IPF is associated with poor prognosis and positively
correlated with macrophage expression. Immunofluorescence
localization demonstrated that SPP1 is predominantly located in
macrophages in lung tissue of patients with IPF. After BLM
stimulation, the expression of CD206 (a marker of M2-type
macrophages) colocalizing with CD68 significantly increased, while
the expression of cytokines such as IL-10 and TGF-β secreted by
M2-type macrophages decreased following SPP1 knockdown, suggesting
that SPP1 inhibition significantly suppresses M2-type macrophage
polarization, hinting at a potential key role of SPP1 in the
progression of IPF. To further study the role of SPP1 in IPF,
BLM-stimulated macrophage supernatant was used to co-culture with
lung epithelial cells and it was found that BLM-stimulated
macrophages promote EMT and collagen expression in lung epithelial
cells, which can be reversed by inhibiting SPP1 in macrophages.
As the JAK2/STAT3 signaling pathway is a key pathway
for macrophage polarization, its role in M2-type macrophage
polarization and the progression of pulmonary fibrosis warrants
further investigation (39-41). Previous studies showed that
dihydroartemisinin can alleviate lung inflammation and fibrosis in
IPF mice by inhibiting the JAK2/STAT3 signaling pathway (42). Consistent with this, the present
study observed that BLM-stimulated macrophages not only enhanced
SPP1 expression but also activated the JAK2/STAT3 signaling
pathway. Treatment with inhibitors of this signaling pathway was
able to suppress cell healing. It was hypothesized that SPP1 may
promote M2-type polarization of macrophages induced by BLM through
activation of the JAK2/STAT3 signaling pathway, thereby driving IPF
progression. In the BLM-induced IPF model, the application of
SPP1-specific inhibitors significantly reduced lung fibrosis and
collagen deposition, further supporting the importance of SPP1 in
the IPF process. Although SPP1 inhibitors have shown significant
anti-fibrotic effects in animal models, future research is needed
to explore therapeutic dosing strategies or consider their
combination with existing treatments such as Stephania
tetrandra (43).
In summary, given the critical role of macrophages
in the pathological process of IPF, the current study has uncovered
that SPP1 regulates M2-type polarization of macrophages via the
JAK2/STAT3 signaling pathway, thereby promoting IPF progression.
The molecular mechanisms underlying this process offer a scientific
foundation for the clinical treatment of IPF in the future.
Supplementary Data
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
XLY designed cell and animal experiments, curated
data, developed methodology, conducted formal analysis and wrote
the original draft. ZQL curated data, conducted formal and software
analysis, and developed methodology. JWZ, JQG, TH, YFL, YYL, YB and
YRX collected the information and revised and finalized the
manuscript. JW and DH proposed the preliminary idea and design for
the present study and reviewed and revised the manuscript. XLY and
DH confirm the authenticity of all the raw data. All authors read
and approved the final manuscript.
Ethics approval and consent to
participate
All animal experimental protocols were granted
approval by the Animal Ethics Committee at Anhui University of
Science and Technology (approval no. HX-001; Huainan, China). Human
studies were approved by the Medical Research Ethics Committee of
Anhui University of Science and Technology (approval no. HX-001;
Huainan, China). Venous blood was collected from all volunteers
after obtaining their consent, and an informed consent document was
signed by all participants. The human tissue samples were sourced
from the Oncology Hospital of the Huainan Dongfang Hospital Group
(Huainan, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Anhui University of
Science and Technology Medical Special Cultivation Project (grant
no. YZ2023H2A001), the Anhui University of Science and Technology
Introduced Talent Fund (grant no. 2022yjrc14), the Open fund for
the Joint Research Center of Occupational Medicine and Health at
the Grand Health Institute (grant no. OMH-2023-09), the
Collaborative Innovation Project of Colleges and Universities of
Anhui (grant no. GXXT-2020-058), the Anhui Engineering Laboratory
of Occupational Health and Safety (grant nos. AYZJSGCLK202201001,
AYZJSGCLK202201002 and AYZJSGCLK202202001), the Key Laboratory of
Industrial Dust Deep Reduction and Occupational Health and Safety
of Anhui Higher Education Institutes (grant no.
AYZJSGXLK202202002), the Innovation and Entrepreneurship Project of
Anhui University of Science and Technology (grant nos. 2021CX2125,
2021CX2126 and 2021CX2124) and the Scientific Research Foundation
for High-level Talents of Anhui University of Science and
Technology (grant no. 2022yjrc14).
References
|
1
|
Chilosi M, Caliò A, Rossi A, Gilioli E,
Pedica F, Montagna L, Pedron S, Confalonieri M, Doglioni C, Ziesche
R, et al: Epithelial to mesenchymal transition-related proteins
ZEB1, β-catenin, and β-tubulin-III in idiopathic pulmonary
fibrosis. Mod Pathol. 30:26–38. 2017. View Article : Google Scholar
|
|
2
|
Hambly N, Shimbori C and Kolb M: Molecular
classification of idiopathic pulmonary fibrosis: Personalized
medicine, genetics and biomarkers. Respirology. 20:1010–1022. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Yan L, Su Y, Hsia I, Xu Y, Vincent-Chong
VK, Mojica W, Seshadri M, Zhao R and Wu Y: Delivery of
anti-microRNA-21 by lung-targeted liposomes for pulmonary fibrosis
treatment. Mol Ther Nucleic Acids. 32:36–47. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Liu YM, Nepali K and Liou JP: Idiopathic
pulmonary fibrosis: Current status, recent progress, and emerging
targets. J Med Chem. 60:527–553. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Sgalla G, Franciosa C, Simonetti J and
Richeldi L: Pamrevlumab for the treatment of idiopathic pulmonary
fibrosis. Expert Opin Investig Drugs. 29:771–777. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Tseng YH, Chen IC, Li WC and Hsu JH:
Regulatory cues in pulmonary fibrosis-with emphasis on the AIM2
inflammasome. Int J Mol Sci. 24:108762023. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Rao LZ, Wang Y, Zhang L, Wu G, Zhang L,
Wang FX, Chen LM, Sun F, Jia S, Zhang S, et al: IL-24 deficiency
protects mice against bleomycin-induced pulmonary fibrosis by
repressing IL-4-induced M2 program in macrophages. Cell Death
Differ. 28:1270–1283. 2021. View Article : Google Scholar :
|
|
8
|
Richeldi L, Collard HR and Jones MG:
Idiopathic pulmonary fibrosis. Lancet. 389:1941–1952. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Rui Y, Han X, Jiang A, Hu J, Li M, Liu B,
Qian F and Huang L: Eucalyptol prevents bleomycin-induced pulmonary
fibrosis and M2 macrophage polarization. Eur J Pharmacol.
931:1751842022. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Mehla K and Singh PK: Metabolic regulation
of macrophage polarization in cancer. Trends Cancer. 5:822–834.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Saradna A, Do DC, Kumar S, Fu QL and Gao
P: Macrophage polarization and allergic asthma. Transl Res.
191:1–14. 2018. View Article : Google Scholar :
|
|
12
|
Yan L, Hou C, Liu J, Wang Y, Zeng C, Yu J,
Zhou T, Zhou Q, Duan S and Xiong W: Local administration of
liposomal-based Plekhf1 gene therapy attenuates pulmonary fibrosis
by modulating macrophage polarization. Sci China Life Sci.
66:2571–2586. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yao Y, Wang Y, Zhang Z, He L, Zhu J, Zhang
M, He X, Cheng Z, Ao Q, Cao Y, et al: Chop deficiency protects mice
against bleomycin-induced pulmonary fibrosis by attenuating M2
macrophage production. Mol Ther. 24:915–925. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zeng B, Zhou M, Wu H and Xiong Z: SPP1
promotes ovarian cancer progression via Integrin β1/FAK/AKT
signaling pathway. Onco Targets Ther. 11:1333–1343. 2018.
View Article : Google Scholar :
|
|
15
|
Zeng P, Zhang X, Xiang T, Ling Z, Lin C
and Diao H: Secreted phosphoprotein 1 as a potential prognostic and
immunotherapy biomarker in multiple human cancers. Bioengineered.
13:3221–3239. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hu Z, Lin D, Yuan J, Xiao T, Zhang H, Sun
W, Han N, Ma Y, Di X, Gao M, et al: Overexpression of osteopontin
is associated with more aggressive phenotypes in human non-small
cell lung cancer. Clin Cancer Res. 11:4646–4652. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yi X, Luo L, Zhu Y, Deng H, Liao H, Shen Y
and Zheng Y: SPP1 facilitates cell migration and invasion by
targeting COL11A1 in lung adenocarcinoma. Cancer Cell Int.
22:3242022. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Richardson MM, Jonsson JR, Powell EE,
Brunt EM, Neuschwander-Tetri BA, Bhathal PS, Dixon JB, Weltman MD,
Tilg H, Moschen AR, et al: Progressive fibrosis in nonalcoholic
steatohepatitis: Association with altered regeneration and a
ductular reaction. Gastroenterology. 133:80–90. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhu C, Kim K, Wang X, Bartolome A, Salomao
M, Dongiovanni P, Meroni M, Graham MJ, Yates KP, Diehl AM, et al:
Hepatocyte Notch activation induces liver fibrosis in nonalcoholic
steatohepatitis. Sci Transl Med. 10:eaat03442018. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ophascharoensuk V, Giachelli CM, Gordon K,
Hughes J, Pichler R, Brown P, Liaw L, Schmidt R, Shankland SJ,
Alpers CE, et al: Obstructive uropathy in the mouse: Role of
osteopontin in interstitial fibrosis and apoptosis. Kidney Int.
56:571–580. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Xu CJ, Sun LC, Jiang CH, Zhou H, Gu L, Liu
Y and Xu Q: SPP1, analyzed by bioinformatics methods, promotes the
metastasis in colorectal cancer by activating EMT pathway. Biomed
Pharmacother. 91:1167–1177. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kumar A, Elko E, Bruno SR, Mark ZF,
Chamberlain N, Mihavics BK, Chandrasekaran R, Walzer J, Ruban M,
Gold C, et al: Inhibition of PDIA3 in club cells attenuates
osteopontin production and lung fibrosis. Thorax. 77:669–678. 2022.
View Article : Google Scholar
|
|
23
|
Luo Q, Deng D, Li Y, Shi H, Zhao J, Qian
Q, Wang W, Cai J, Yu W and Liu J: TREM2 insufficiency protects
against pulmonary fibrosis by inhibiting M2 macrophage
polarization. Int Immunopharmacol. 118:1100702023. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Charoentong P, Finotello F, Angelova M,
Mayer C, Efremova M, Rieder D, Hackl H and Trajanoski Z: Pan-cancer
immunogenomic analyses reveal genotype-immunophenotype
relationships and predictors of response to checkpoint blockade.
Cell Rep. 18:248–262. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zhou J, Yang X, Liu Y, Guo J, Liu Z, Li Y,
Bai Y, Xing Y, Wu J and Hu D: Mefloquine improves pulmonary
fibrosis by inhibiting the KCNH2/Jak2/Stat3 signaling pathway in
macrophages. Biomed Pharmacother. 171:1161382024. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Carrara SC, Davila-Lezama A, Cabriel C,
Berenschot EJW, Krol S, Gardeniers JGE, Izeddin I, Kolmar H and
Susarrey-Arce A: 3D topographies promote macrophage M2d-Subset
differentiation. Mater Today Bio. 24:1008972023. View Article : Google Scholar
|
|
27
|
Li K, Wu L and Jiang J: Apigenin
accelerates wound healing in diabetic mice by promoting macrophage
M2-type polarization via increasing miR-21 expression. Mol Cell
Biochem. Jan 23–2024.Epub ahead of print.
|
|
28
|
Kim YE, Sung DK, Bang Y, Sung SI, Yang M,
Ahn SY and Chang YS: SOCS3 protein mediates the therapeutic
efficacy of mesenchymal stem cells against acute lung injury. Int J
Mol Sci. 24:82562023. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Li S, Gao S, Jiang Q, Liang Q, Luan J,
Zhang R, Zhang F, Ruan H, Li X, Li X, et al: Clevudine attenuates
bleomycin-induced early pulmonary fibrosis via regulating M2
macrophage polarization. Int Immunopharmacol. 101:1082712021.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Wang J, Xu L, Xiang Z, Ren Y, Zheng X,
Zhao Q, Zhou Q, Zhou Y, Xu L and Wang Y: Microcystin-LR ameliorates
pulmonary fibrosis via modulating CD206+ M2-like
macrophage polarization. Cell Death Dis. 11:1362020. View Article : Google Scholar
|
|
31
|
Wu Q, Li L, Miao C, Hasnat M, Sun L, Jiang
Z and Zhang L: Osteopontin promotes hepatocellular carcinoma
progression through inducing JAK2/STAT3/NOX1-mediated ROS
production. Cell Death Dis. 13:3412022. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Wen Y, Feng D, Wu H, Liu W, Li H, Wang F,
Xia Q, Gao WQ and Kong X: Defective initiation of liver
regeneration in osteopontin-deficient mice after partial
hepatectomy due to insufficient activation of IL-6/Stat3 pathway.
Int J Biol Sci. 11:1236–1247. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Banerjee S, Biehl A, Gadina M, Hasni S and
Schwartz DM: Erratum to: JAK-STAT signaling as a target for
inflammatory and autoimmune diseases: Current and future prospects.
Drugs. 77:12612017. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Liu M, Li H, Zhang H, Zhou H, Jiao T, Feng
M, Na F, Sun M, Zhao M, Xue L and Xu L: RBMS1 promotes gastric
cancer metastasis through autocrine IL-6/JAK2/STAT3 signaling. Cell
Death Dis. 13:2872022. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Hobbs BD, de Jong K, Lamontagne M, Bossé
Y, Shrine N, Artigas MS, Wain LV, Hall IP, Jackson VE, Wyss AB, et
al: Genetic loci associated with chronic obstructive pulmonary
disease overlap with loci for lung function and pulmonary fibrosis.
Nat Genet. 49:426–432. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Hewlett JC, Kropski JA and Blackwell TS:
Idiopathic pulmonary fibrosis: Epithelial-mesenchymal interactions
and emerging therapeutic targets. Matrix Biol. 71-72:112–127. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Harari S and Caminati A: IPF: New insight
on pathogenesis and treatment. Allergy. 65:537–553. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Wendisch D, Dietrich O, Mari T, von
Stillfried S, Ibarra IL, Mittermaier M, Mache C, Chua RL, Knoll R,
Timm S, et al: SARS-CoV-2 infection triggers profibrotic macrophage
responses and lung fibrosis. Cell. 184:6243–6261.e27. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Xia T, Gu Y, Shen J, Zheng L and Xu C:
Limonin ameliorates acute pancreatitis by suppressing JAK2/STAT3
signaling pathway. Environ Toxicol. 36:2392–2403. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Li Q, Cheng Y, Zhang Z, Bi Z, Ma X, Wei Y
and Wei X: Inhibition of ROCK ameliorates pulmonary fibrosis by
suppressing M2 macrophage polarisation through phosphorylation of
STAT3. Clin Transl Med. 12:e10362022. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Zhao C, Zeng N, Zhou X, Tan Y, Wang Y,
Zhang J, Wu Y and Zhang Q: CAA-derived IL-6 induced M2 macrophage
polarization by activating STAT3. BMC Cancer. 23:3922023.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
You X, Jiang X, Zhang C, Jiang K, Zhao X,
Guo T, Zhu X, Bao J and Dou H: Dihydroartemisinin attenuates
pulmonary inflammation and fibrosis in rats by suppressing
JAK2/STAT3 signaling. Aging (Albany NY). 14:1110–1127. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Li J, Wang Y, Wang R, Wu MY, Shan J, Zhang
YC and Xu HM: Study on the molecular mechanisms of tetrandrine
against pulmonary fibrosis based on network pharmacology, molecular
docking and experimental verification. Heliyon. 8:e102012022.
View Article : Google Scholar : PubMed/NCBI
|