Introduction
Anaemia is a condition in which the body does not
have enough healthy red blood cells (RBCs) to carry adequate oxygen
to the body tissues. RBCs provide oxygen to body tissues, and
insufficient oxygen levels can lead to sensations of fatigue or
weakness. According to the World Health Organization (WHO), anaemia
is a serious global public health problem that particularly affects
young children, menstruating adolescent girls, and women, pregnant
and postpartum women. It is estimated that 500 million women
between the ages of 15 and 49 years and 269 million children
between the ages of 6 and 59 months are affected worldwide.
Globally, (1 May 2023) it is estimated that 40% of all children
aged 6-59 months, 37% of pregnant women, and 30% of women 15-49
years of age are affected by anaemia (sourced from https://www.who.int/news-room/fact-sheets/detail/anaemia#).
Similarly, it also states that ~37% (32 million) of pregnant women
were also affected by anaemia in 2019. In the world, African and
South-East Asian individuals are the most affected with an
estimated 106 million women and 103 million in African and 244
million women and 83 million children in South-East Asian regions.
The contributing reasons can be malnutrition, poverty, infections,
inflammations, and gynaecological and obstetric conditions.
According to a survey in India by National Family Health Survey
(NFHS-5) in 2019-2021, 25.0% of men (15-49 years), and 57.0% of
women (15-49 years), 31.1% in adolescent boys (15-19 years), 59.1%
in adolescent girls, 52.2% in pregnant women (15-49 years) and
67.1% in children (6-59 months) were found anaemic, which was a
drastic change from the NFHS-4, where the prevalence of anaemia was
~50.3% totally (1,2). Anaemia is a syndrome of an
underlying illness of various conditions such as cancer, rheumatoid
arthritis, chronic renal disease, etc., rather than a disease in
and of itself (3,4). Anaemia can be caused by a
combination of several factors, such as chronic diseases,
nutritional deficiencies, bone marrow disease, and blood disorders.
In some cases, it can be due to a combination of factors, and thus
identification and classification may help in further
treatment.
Anaemia and its types
The term anaemia is generalised and is used to refer
to all diverse types/subtypes of anaemia. According to the WHO,
anaemia is identified by haemoglobin (Hb) levels and is further
classified into three types based on severity: mild anaemia (9 to
10.9 g/dl), moderate anaemia (7-8.9 g/dl) and severe anaemia (less
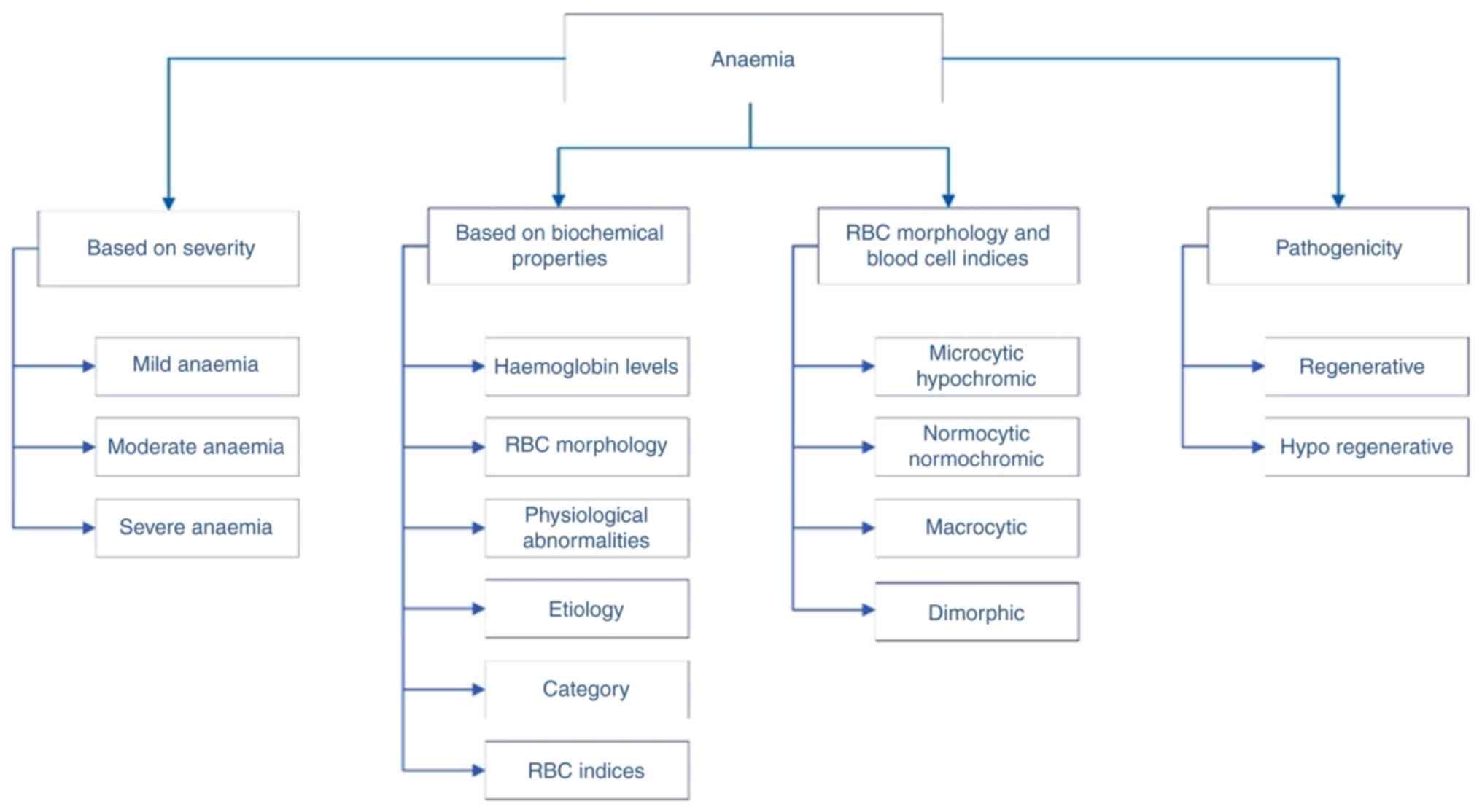
than 7 g/dl). It has been stated that it is classified into six
types, roughly based on Hb levels, RBC morphology, physiological
abnormality, aetiology, category and RBC indices; anaemia can be
classified based on its pathogenic behaviour and morphology
(5,6). In its morphology of RBCs and blood
cell indices, it is further classified into microcytic hypochromic,
normocytic normochromic, macrocytic, and dimorphic anaemia.
Meanwhile, the pathogenic classification is regenerative and
hypo-regenerative. There are >400 types of anaemia, and it is
further classified into three types, namely: Anaemia caused by
blood loss, anaemia caused by decreased or faulty RBCs' production,
and anaemia caused by the destruction of RBCs (7). Thus, based on the aforementioned
surveys, anaemia can be also classified broadly into three types
based on: i) nutritional deficiencies; ii) genetic changes such as
mutations and autoimmune conditions; and iii) chronic disorders,
(Fig. 1). In the present review,
focus was addressed on anaemia which is caused by genetic
conditions such as mutations, autoimmune conditions, and
inheritance. Apart from genetic conditions, anaemia can also be
caused by other factors such as overuse of drugs, viral infections
and overexposure to chemicals. The several types of anaemia caused
by genetic factors are haemolytic anaemia, sickle cell anaemia,
thalassemia, aplastic anaemia (AA), Diamond-Blackfan anaemia (DBA),
Fanconi anaemia (FA) and sideroblastic anaemia.
Haemolytic anaemia
Haemolytic anaemia is a condition characterised by
the early destruction of premature RBCs before their life span. It
can be caused by a range of factors, such as autoimmune disorders,
genetic defects, infections and toxins. Haemolytic anaemia has two
distinct types, namely, inherited and acquired. Inheritance is
caused by genetic conditions like a mutation in the PKLR, SPTA1,
ANK1, SPTB, SLC4A1 and AK1 genes (8,9).
Apart from the mentioned genes, numerous genes are involved
depending on the type and underlying conditions. Inherited
haemolytic anaemia is classified into numerous forms depending on
the abnormalities in the structure or function of RBCs. Acquired
haemolytic anaemia is caused by specific conditions and their types
(10).
Sickle cell anaemia
It is an inherited blood disorder where the RBCs are
crescent in shape rather than round, which makes them rigid and
sticky. This was caused by mutation in the Hb beta gene (HBB), a
point mutation at chromosome 11p15.5. This point mutation leads to
the production of abnormal RBCs. Thus, this type of anaemia is
known as sickle cell anaemia. The sickle-shaped cells will last
only for 10-20 days, leading to a shortage of RBCs in the body.
Apart from this, the sickle shape can block or slow down the blood
flow. It is the most common genetic disorder in the United States,
affecting 1 in 500 African Americans (11).
Thalassemia
Thalassemia is a genetic disorder caused by mutation
or deletion of the Hb genes. Hb is the protein that carries oxygen
throughout the body. It is also an inherited disorder, as it
transfers from one of the parents or the parent acts as a carrier.
In normal human Hb, six globin chains (α, β, γ, δ, ε, ζ) are found
at various stages of development (12). Among the six globin chains, alpha
(HBA1 and HBA2) and beta (HBB) globin chains play a vital role in
the ability of Hb to bind and release oxygen as it travels
throughout the body. In the case of thalassemia, mutation or
deletion in either alpha globin or beta globin can induce an excess
of other types of globin chains. This provokes an instability that
inclines to affect the production of Hb which paves the way for
anaemia and other complications. There are two types of
thalassemia, namely alpha thalassemia and beta thalassemia, which
are further categorised into various subtypes such as α-thalassemia
silent carriers, α-thalassemia treat, α-thalassemia major with Hb,
β-thalassemia minor, β-thalassemia intermediate and β-thalassemia
major which is also known as Cooley's anaemia (12,13).
AA
AA, as the name suggests, refers to the lack of bone
marrow cells, which is the primary source of RBCs' production.
Depletion or insufficient bone marrow cells can lead to a lack of
erythrocytes as well as other components of the blood. It is an
autoimmune disorder that is caused by assorted reasons, such as
exposure to chemicals, over-intake of drugs, some viral infections,
and in some rare pregnancy cases. AA is classified into two types,
namely inherited and acquired. T-cells are found to play a leading
role in the onset of the disease in both inherited and acquired
cases. Apart from these types, idiopathic AA is one of them, where
the cause of the disease and the relationship are unknown (14).
DBA
DBA is a rare genetic disorder caused by mutation in
any one of numerous genes, including RPL5, RPL11, RPL35A, RPS10,
RPS17, RPS19, RPS24 and RPS26. These genes provide instructions for
making ribosomal proteins. Mutations in these genes lead to defects
in ribosome biogenesis and function, impairing the production of
erythrocytes, especially in their development and maturation. DBA
is characterised by red blood aplasia, which means that the bone
marrow does not produce enough RBCs (15). Apart from ribosomal protein, the
GATA1 gene has been involved in DBA, because it is a haematopoietic
transcription factor that is required for normal erythropoiesis.
Mutations in this particular gene can also favour this disease
(16).
FA
FA is a rare genetic disorder caused by mutations in
at least 23 genes, known as FA genes, comprising FANC genes (A, B,
C, D1, D2, E, F, G, I, J, L, M, N, O, P, Q, R, S, T, U, V, W, Y)
that result in an impaired response to DNA damage. Due to mutations
in these genes, there is a disruption in the normal function of FA
genes, which results in bone marrow failure, physical
abnormalities, and an increased risk of developing cancer. This
type of anaemia is also associated with birth defects and affects
almost all organs of the body (17).
Sideroblastic anaemia
Sideroblastic anaemia is a rare blood disorder
caused by a mutation of genes involved in heme biosynthesis,
iron-sulphur cluster biogenesis, and mitochondrial metabolism. In
this type of anaemia, the bone marrow produces ring sideroblasts
(immature RBCs with nuclei surrounded by rings of iron). It is
classified into two types, namely inherited and acquired, in which
inherited is classified into two forms, namely X-linked
sideroblastic anaemia (mutation in ALAS2) and autosomal recessive
congenital sideroblastic anaemia (mutation in various genes,
including TRNT1, PUS1, LARS2, YARS2, FECH, GLRX5 and HSPA9).
Similarly, acquired sideroblastic anaemia has two forms, namely
primary and secondary. The primary is associated with
myelodysplastic anaemia and the secondary is associated with
exposure to certain chemicals, medications, and toxicity (18,19). Several types of anaemia and the
features associated with them are consolidated in Table I.
 | Table IDifferent types of anaemia, its
features and involved genes. |
Table I
Different types of anaemia, its
features and involved genes.
| S. no. | types | causes | Features | Genes involved | (Refs.) |
|---|
| 1 | Haemolytic
anaemia |
Autoimmune/mutation | Abnormality in the
structure of RBC | PKLR, SPTA1, ANK1,
SPTB, SLC4A1, AK1 | (8,9) |
| 2 | Sickle cell
anaemia | Inherited blood
disorder/point mutation | RBC crescent
shape | HBB - 11p15.5 | (11) |
| 3 | Thalassaemia | genetic
disorder | Microcytic
RBCs | HBA1, HBA2 and
HBB | (12,13) |
| 4 | Aplastic
anaemia |
Autoimmune/acquired/genetic | Depletion of bone
marrow cells | TERT, PIGA, FANCA,
FANCC, DNMT3A, FANCGs | (14) |
| 5 | Diamond-Blackfan
anaemia | Genetic
disorder/mutation | Impairment in the
development of erythrocytes | RPL5, RPL11,
RPL35A, RPS10, RPS17, RPS19, RPS24 and RPS26 genes | (15,16) |
| 6 | Fanconi
anaemia | Genetic | Bone marrow
failure | FANC (A, B, C, D1,
D2, E, F, G, I, J, L, M, N, O, P, Q, R, S, T, U, V, W, Y) | (17) |
| 7 | Sideroblastic
anaemia | Mutation | Immature RBCs with
nuclei surrounded by rings of iron | TRNT1, PUS1, LARS2,
YARS2, FECH, GLRX5 and HSPA9 | (18,19) |
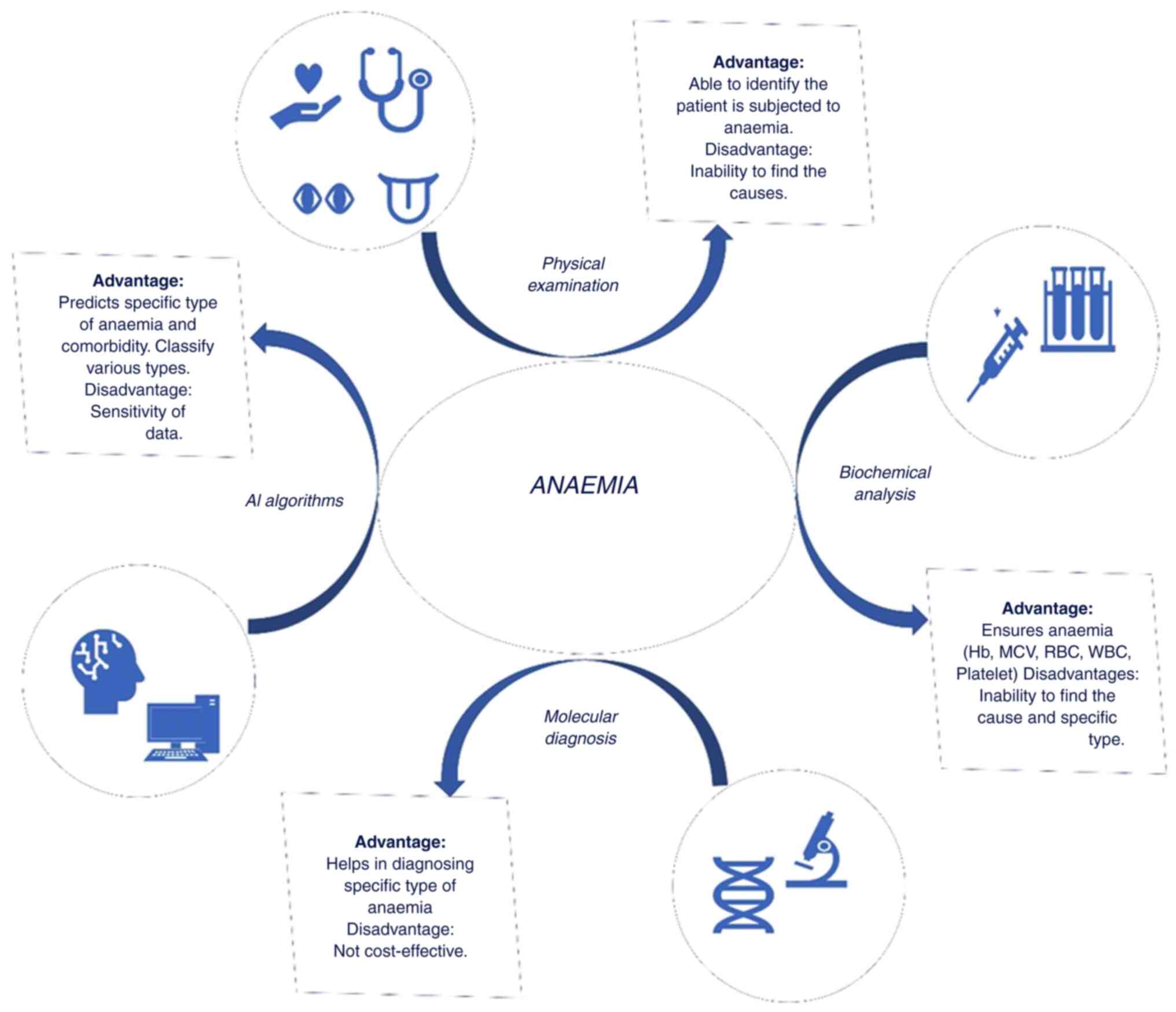
Diagnosis of anaemia
The process of diagnosing diverse types of anaemia
encompasses a multifaceted process that integrates clinical
evaluation, laboratory techniques, in some instances molecular
techniques, and in recent days prognosis and classification using
artificial intelligence (AI) methods, as illustrated in Fig. 2.
Physical examination
When we consult a physician, a comprehensive
physical examination is conducted, entailing the assessment of the
retina of the eye, nails, tongue, and skin. Additionally, the
patient's age and past medical history are gathered. The tips of
the nails are examined for signs of weakening Hb levels, such as
brittleness and spoon-shaped malformations. Pallor or glossitis are
checked for on the tongue, and any colour, texture, or nutritional
deficiency indicators are noted. The examination of the skin
involves noting any pallor in the conjunctiva, nail beds, or palms;
jaundice, which indicates potential haemolysis or liver failure, is
also noted. A detailed examination of the retina is also carried
out in order to find any vascular abnormalities or alterations in
blood vessels associated with anaemia, as well as any potential
indications of retinopathy. This information is crucial for a
proper diagnosis.
Typically, most anaemia share common symptoms
including dizziness, fatigue, paleness, loss of appetite, brittle
nails, an increase in heartbeat and frequent infections. Apart from
the above symptoms, they may vary depending on the type of anaemia.
In the case of genetic disorders, it may affect the nervous system,
muscle contraction, eye problems, neutropenia, heart problems,
hypospadias and physical abnormalities. Consequently, the range and
severity of symptoms are contingent on the underlying type of
anaemia.
Biochemical analysis
Biochemical parameters are essential in assessing
the body's physiological state since they provide important
information on a range of health and illness-related topics. These
characteristics, which are quantifiable indicators present in
biological samples like blood, urine, or tissues, offer crucial
details regarding the operation of organs, metabolic processes, and
the existence of particular compounds. A complete blood count (CBC)
test will be followed, which measures numerous different parts and
features of blood, such as RBCs, white blood cells (WBCs),
Platelets (PLT), Hb, haematocrit, Mean Corpuscles Volume (MCV) and
Mean Corpuscles Haemoglobin (MCH). Furthermore, it is recommended
assessing serum 25-hydroxyvitamin D levels in the diagnosis of
anaemia, as vitamin D deficiency can contribute to various anaemia
and serves as a potential risk factor for underlying conditions
(20).
Immunomodulatory role in anaemia
Vitamin D is essential for our bone strength and
supports our immune system and its function. Deficiency in vitamin
D is primarily associated with skeletal health and calcium
metabolism; however, vitamin D is indirectly intertwined with
anaemia (21). According to a
previous study, calcitriol (1,25-dihydroxy vitamin D) which is an
active form of vitamin D, increases erythropoietin-receptor
expression and synergistically stimulates proliferation along with
erythropoietin (22).
Erythropoietin is a hormone that is responsible for stimulating the
production of RBCs in the bone marrow. A deficiency in vitamin D
may impair this process and potentially contribute to anaemia.
Chronic kidney disease or malabsorption disorders are associated
with vitamin D deficiency and can lead to anaemia through various
unrelated mechanisms related to vitamin D deficiency itself. The
relationship between anaemia and vitamin D is as follows: i)
Several observational studies (23-25) have indicated the reverse
relationship between vitamin D levels and anaemia in adults; ii) it
was found that vitamin D was associated with the prevention of
chronic diseases, modulation of immunity, regulation of cellular
growth, and differentiation and induction of erythropoiesis in bone
marrow cells (22,23); iii) low levels of vitamin D may
affect the body's ability to produce new erythrocytes, which may
lead to anaemia (26,27); and iv) deficiency of vitamin D is
associated with a greater prevalence of anaemia, especially in
children and adolescents (25,26).
Vitamin D has been found to be associated with
anaemia in various healthy and diseased populations. It has been
revealed that vitamin D may have a positive impact on anaemia
through its down regulatory effects on inflammatory cytokines and
hepcidin (28). Therefore, its
deficiency may lead to opposite effects, whose sequelae consist of
depressed erythropoiesis and accentuation of the anaemia.
Similarly, vitamin D and other calciotropic hormones, such as PTH
and FGF33, have been found to establish their effect on iron
metabolism and erythropoiesis. Polymorphisms in vitamin D receptors
are associated with inducing anaemic conditions (29). Single nucleotide polymorphisms at
the vitamin D receptor gene (ApaI; rs7975232, TaqI; rs731236, BsmI;
rs1544410, FokI; rs10735810) might also contribute to anaemic
conditions. It has been also stated that Jessica Cusato has proved
the role of vitamin D receptor gene polymorphisms in
ribavirin-induced anaemia in patients with HCV during the second
and fourth weeks of medication. An increase in the intake of
vitamin D-rich foods has been shown to improve Hb levels in
patients with sickle cell anaemia as well as in anaemic individuals
(30). In the aforementioned
studies, it has been reported that calcitriol, which represents the
active form of vitamin D, is involved in haematopoiesis.
Apart from the aforementioned relationships between
anaemia and vitamin D, they were also found to play a significant
role in AA. It has been previously found that decreased expression
of vitamin D receptor (VDR) might contribute to the hyperimmune
status of AA (31). Appropriate
vitamin D supplements would partly rectify the immune dysfunction
by strengthening signal transduction through VDR in patients with
AA. A meta-analysis study, which includes 14 studies with 1,385
participants, showed a positive association between vitamin D
deficiency and the incidence of anaemia (23). It also indicated that there is a
possible reverse relationship, especially in adults. Vitamin D was
found to be associated with the prevention of chronic diseases,
modulation of immunity, regulation of cellular growth,
differentiation and induction of erythropoiesis in bone marrow
cells. As aforementioned, the importance of vitamin D in anaemic
conditions and molecular biomarkers can also be used to detect this
deficiency. Serum 25-hydroxyvitamin D can also be one of the best
molecular biomarkers to identify its deficiency in its marginal
levels as well as in its overt deficiency (21).
Even though vitamin D was considered a nutritional
deficiency, it was found to play a significant role in managing
certain genetic types of anaemia specific to impaired nutrient
absorption. It was previously stated that vitamin D supplements
were associated with an increase in Hb concentration in anaemic
conditions (23,32). Vitamin D supplementation has
demonstrated positive effects on transferrin saturation and iron
status, and thus it has a cohort role in anaemia. Similarly, it was
found that vitamin D has immunomodulatory properties, which may
influence the immune system response, and deficiency in vitamin D
might contribute to the dysregulation of anaemic conditions
(33,34).
Molecular techniques
Biomarkers are specific molecules or genetic
signatures that can be used to measure and analyse various
biological samples such as blood, urine, saliva and tissue.
Biomarkers can provide information about the presence, severity, or
progression of a disease. Anaemia is a common disease that is
generally detected with a blood test, which mentions the Hb content
in them. As aforementioned about its type, the detection of
specific types of anaemia is a tedious task. Hb concentration and
hepcidin are the essential molecular biomarkers that are used for
the detection of anaemia in general, which essentially do not
classify its types (35). The
use of molecular biomarkers varies for each type of anaemia
(Table II).
 | Table IIMolecular biomarkers associated with
different types of anaemia. |
Table II
Molecular biomarkers associated with
different types of anaemia.
| S. no. | Anaemia type | Biomarkers | Description | (Refs.) |
|---|
| 1 | Haemolytic
anaemia | LDH, Bilirubin,
Reticulocytes | Combined haemolytic
index assesses multiple markers of haemolysis. Elevated LDH and
bilirubin indicate RBC destruction; increased reticulocytes
indicate increased RBC turnover. | (32,36) |
| 2 | Sickle cell
anaemia | Haemoglobin
Electrophoresis, LDH, Bilirubin, Reticulocytes, Cytokines (IL-6,
TNF-α), Chemokines (MCP-1) | Identifies abnormal
haemoglobin (Hb S). Elevated LDH, bilirubin, reticulocytes, and
inflammatory markers indicate vaso-occlusive crisis. | (37-39) |
| 3 | Thalassaemia | LDH, Bilirubin,
Endocan-1, Lcn2, Genetic Testing (Gap-PCR), Plasma Proteome
Profiling, Cytokines (IL-2, TNF-α), Chemokines (MCP-1, MIP-1β) | Endocan-1 and Lcn2
correlate with disease complications. Genetic mutations (α, β
chains) via Gap-PCR. Plasma proteome profiling identifies
dysregulated proteins. | (40-43) |
| 4 | Aplastic
anaemia | Circulating
microRNAs, Apolipoprotein-A, Anti-COX-2, Leucocyte Telomere Length,
TERF2, GAS2L3, MK167, TMSB15 A, Flow Cytometry, Bone Marrow
Biopsy | MicroRNAs monitor
disease/treatment. Apolipoprotein-A/anti-COX-2 as novel biomarkers.
Telomere length and other molecular markers for diagnosis | (14,44-47) |
| 5 | Diamond-Blackfan
anaemia | Genetic Testing
(Ribosomal Protein Genes), Erythrocyte Adenosine Deaminase
Activity, GATA1, Apolipoprotein-A, Plasma microRNAs,
anti-COX-2 | Genetic mutations
and elevated erythrocyte adenosine deaminase activity. GATA1
mutations and other novel biomarkers for diagnosis. | (44,48-50) |
| 6 | Fanconi
anaemia | FANC Genes,
Chromosomal Breakage Test, Microarray analysis | FANC gene mutations
and chromosomal breakage test are primary diagnostics. Microarray
analysis identifies gene expression patterns. | (16,17,51,52) |
| 7 | Sideroblastic
anaemia | ALAS2, TRNT1, PUS1,
LARS2, YARS2, FECH, GLRX5, HSPA9, SLC25A38, ABCB7, SF3B1 | Genetic mutations
in several genes indicate different forms. SF3B1 mutations
differentiate clonal vs. non-clonal causes. | (48,53) |
As the authors of the present review came across
numerous types of biomarkers through a literature survey, an
analysis has been done with all types of biomarkers in the Marker
dB database (https://markerdb.ca/). It is an
electronic database that has consolidated information on molecular
biomarkers. The molecular biomarkers are categorised into four
types: Chemical, protein, genetic (DNA) and karyotypic; and further
four biomarkers are placed into categories such as diagnostic,
predictive, prognostic and exposure. Currently, the database
contains 142 protein biomarkers, 1,089 chemical biomarkers, 154
karyotype biomarkers, and 26,374 genetic markers. These are
categorised into 25,560 diagnostic biomarkers, 102 prognostic
biomarkers, 265 exposure biomarkers, and 6,746 predictive
biomarkers or biomarker panels. Collectively, these markers can be
used to detect, monitor, or predict 670 specific human conditions,
which are grouped into 27 broad condition categories (54). The biomarkers Coproporphyrin I
(MDB00000206) and Coproporphyrin III (MDB00000191), which emerge as
metabolites during heme synthesis, are used to identify
sideroblastic anaemia (55,56). Important roles are played by
pyridoxine (MDB00000117), pyridoxal (MDB00000335) and homocysteine
(MDB00013433) in the conditions of sickle cell anaemia detection.
Vitamin B6 in the form of pyridoxine helps produce RBCs and
controls the proportion of sodium to potassium. Pyridoxal converts
to pyridoxal phosphate, an essential coenzyme in the production of
amino acids, sphingolipids, neurotransmitters, and aminolevulinic
acid; low levels may indicate sickle cell anaemia. Methionine
metabolism produces homocysteine, which is linked to endothelial
dysfunction, a characteristic of sickle cell disease that
exacerbates vaso-occlusive crises and causes tissue damage
(57,58). A crucial part of energy synthesis
is played by coenzyme Q8, sometimes referred to as ubiquinol 8
(MDB00000298). Genetic testing and Hb analysis are the main
techniques used to determine the type and presence of
beta-thalassemia, with ubiquinol 8 acting as a crucial biomarker
for thalassemia diagnosis (59).
The bile pigment bilirubin (MDB00000027), which is generated during
heme breakdown, is important for diagnosing haemolytic anaemia.
Increased bilirubin levels are an essential marker of the illness,
emphasising their significance in the diagnostic procedure
(60).
AI methods for anaemia detection
Computational prediction can be implemented for fast
processes and time saving. Numerous machine learning and deep
learning algorithms are recently used for the detection or
prediction of anaemia in patients using their past health records
and other details that can be obtained from electronic medical or
health records, such as facial images, images of other parts of the
body, electrocardiograms (ECG), electrohepatography (EHG), X-ray
scans and magnetic resonance imaging (MRI) scans. The multitude of
data types that can be used to train a machine model for accurate
diagnosis makes it an efficient and cost-effective method to be
explored. The elaborate usage of ECG and patient images for the
detection of various types of anaemia has been previously shown
(61). Other features such as
age, CBC and other medical conditions of patients were also
considered for further detection and analysis. Most of the machine
learning and deep learning algorithms have used classification,
identification, or predictive algorithms for detection. Some
studies have shown the detection of iron-deficiency anaemia,
thalassemia, and AA, which have been discussed below.
A previous study has predicted β-thalassemic
carriers from CBC (62). The
datasets were obtained from the Punjab Thalassemic Prevention and
Program (PTPP) database (https://ptpp.punjab.gov.pk) and have 12 features, out
of which 9 features have the details of CBC. The data imbalance was
observed in the aforementioned dataset and was rectified with the
Synthetic Minority Oversampling Technique and Adaptive Synthetic.
The primary feature selection was done with principal component
analysis and singular vector decomposition. Binary classification
was their primary target, and supervised machine learning models
including decision tree (DT), gradient boosting machine (GBM),
support vector classifier, random forest (RF), extra tree
classifier (ETC) and logistic regression (LR) were implemented to
identify the best models. Among the aforementioned supervised
models, ETC had the best performance with 0.96 accuracy and GBM
with 0.89 accuracy. Similarly, in another study (63), the datasets were obtained from
PTPP, which has data on 5,066 patients. A federated method was
followed, and a global model was developed, which obtained an
accuracy of 97.89%. The developed model has classified 2015
thalassemic patients and 3051 non-thalassemic patients from the
dataset.
Extreme learning machine (ELM) models have been used
in a four-class anaemic dataset, which has been used in the
classification of iron-deficiency anaemia, β-thalassemia and HbE.
The datasets were obtained from the clinical pathology laboratory
in Indonesia, and the Department of Clinical Pathology and
Laboratory, in Gadjah Mada. The datasets have been split into 67%
for training and 33% for testing. A comparative study was conducted
with other machine learning models such as RF, K-neighbour nearest
(KNN), and support vector machine (SVM), in which the ELM model
outperformed with 99.21% accuracy (64). A unique IDEAL-IQ was designed to
distinguish AA and MDS. The IDEAL-IQ-based SVM classifier model was
used for the quantification of bone marrow fat. The dataset
collected has bone marrow biopsy and pelvic region of Magnetic
Resonance Imaging (MRI), where the acetabulum is irregular in
shape. The radiomic features of the biopsy samples were selected
and some incomplete data was removed for curation. The model
outperformed with 87.2% accuracy, and a comparative study was
performed with LR and SVM (65).
The aforementioned studies deal with the detection
of diverse types of anaemia. However, a previous study has
mentioned a model for the treatment of the disease and the
progression of recovery after the treatment with immunosuppressive
therapy (IST) (66). The
electronic medical records of 203 children affected with severe AA
were collected from the Chinese Academy of Medical Sciences and
Peking Union Medical College from 2000 to 2016 and classified based
on the disease using LR, RF, multi-layer perceptron and SVM. The
multi-layer perceptron has been used for the electroencephalogram
dataset and SVM for pattern recognition. The model was built based
on predictor sets of regular monitoring of the patient's remedy for
IST. The receiving operating characteristic area under the curve
(AUC) for binary classification has been achieved at around
0.962.
Researchers conducted a study focused on binary
classification was outlined to distinguish Hb of haemolytic and
sickle cell anaemia using various models such as the KNN the model,
Naïve-Bayes model, DT and deep learning model with 7-fold
cross-validation. Among the four models, the DT and deep learning
model performed well with 0.99 accuracies in both models (67). By contrast, another study, built
a deep neural network with sparse categorical cross entropy
function as loss function and Adam optimizer and attained an
accuracy of 0.897 with 8 features mapped to the model (68). The datasets were sourced from
Guangzhou Medical University, China, and have two parts. The first
part contains details such as RBC, RBC distribution width (RDW),
MCV and age; while the other part has details of gene deletions and
17 β-globin point mutations. Because of this additional
information, the model can be categorized into thalassemia and
iron-deficiency anaemia. RDW was larger in iron deficiency anaemia
than in thalassemia, a salient feature recognized in the study.
Bone marrow smears are employed in machine learning
for the detection of AA. Apart from AA, they are also utilised in
the detection of MDS and AML. The image datasets have existed in
some clinics (432) and the ASH Image Bank (115). Two- and three-way
classification methods were executed through the Resnet 50
algorithm (69). The two-way
classification was performed, which initially classifies MDS and
other samples but in three-way classification, they classified AA
and AML from the sample. The model outperformed in the
classification of bone marrow diseases with an accuracy of 0.926.
Likewise, the detection of iron-deficiency anaemia was done through
medical images such as images of the palm. The developed model has
utilised CIE L*a*b colour space operation to identify the region of
interest (ROI). A comparative study between various machine
learning models was performed, and it was found that the
naïve-Bayes algorithm outperformed and has an accuracy of 99.96%;
Convolutional Neural Network and KNN have an accuracy of 99.92%, DT
has an accuracy of 96.34%, and SVM has the least accuracy of
96.34%. A total of 1,520 anaemic patients were identified from the
datasets (70). A previous study
used facial images and deep learning technology to predict anaemia
in patients in the emergency department (71). The video images of the patients
are recorded live and then they are converted into readable images
by GRAD-CAM. The five models have been used, namely Mobilenet,
Resnet50, DenseNet121, Efficient NetB0, and Inception V3. In this
model, Inception V3 outperformed well with 84.02%. Simultaneously,
a comparative study with clinical assessment was performed, and the
deep learning model (Inception V3) has higher accuracy when
compared with clinical assessment. Digital image processing has
also been used in the counting of RBCs from the blood smear in the
detection of anaemia (72).
Case-based reasoning (CBR) and the KNN algorithm were developed to
predict anaemia severity using a machine learning algorithm. CBR is
a technique that uses past experiences to derive results for new
cases. It is a cyclic system and has four activities, namely
retrieve, reuse, revise and retain. The experimental results have
shown that the KNN models have 92% accuracy (73). Retinal fundus images also play an
efficient role in the prediction of anaemia using a deep learning
model. The datasets were obtained from UKBiobank, and the AUC for
anaemia was 0.89 (74).
An ECG is usually used to record the electric
signals from the heart to check for different heart diseases. A
retrospective, multicentre study used deep learning algorithms for
the detection of anaemia using ECGs (61). The real-time data was collected
from Sejong General Hospital and Mediplex Sejong Hospital in South
Korea, which has been classified into 12-lead, 6-lead and
single-lead raw data. Additionally, they have other features such
as the name, age and sex of the individuals. In total, they have
used 57,435 ECGs from 31,898 patients. The DeLong method with the
Sun & Su optimization method was used and obtained an AUROC
(ROC-AUC) of 0.923 internally and 0.901 externally. Big data
applications have been used in the classification of cancer by gene
expression analysis (75).
Similarly, it can also be applied to the classification of anaemia
which requires further studies and investigation.
Genetic data can also be incorporated into text
mining under the framework of classifying anaemia. Text mining can
help extract pertinent genetic information from research
publications, clinical notes and genetic databases. Genetic
variables are important in numerous forms of anaemia. Finding
certain genes linked to anaemia, comprehending genetic mutations or
changes that can exacerbate the illness, and learning more about
the inherited characteristics of anaemia subtypes are some examples
of this. Treatment can be more individualised when genetic
information is incorporated into the classification process, which
improves the accuracy of diagnostic models. Healthcare
practitioners can benefit from text mining techniques by using them
to stay up-to-date on the most recent genetic markers and
breakthroughs related to anaemia categorization, as they can help
close the knowledge gap between genetic research discoveries and
clinical applications. This full integration of textual data, which
includes insights from literature, genetics, and clinical settings,
advances our understanding of anaemia and facilitates its efficient
classification (76,77).
Bioinformatics approaches to anaemia
In recent years, bioinformatics has emerged as an
irreplaceable tool, providing a comprehensive study of the
intricate molecular mechanisms underlying various types of anaemia.
It also plays a pivotal role in advancing the understanding and
management of different types of anaemia, characterised by diverse
genetic and phenotypic features.
A variety of bioinformatics techniques are being
used for various anaemia forms, such as haemolytic, sickle cell,
thalassemia, AA, DBA, FA and sideroblastic anaemia. Through the
identification of genetic variants, sequence variations, and
microdeletions, next-generation sequencing (NGS) approaches such as
whole exome sequencing, targeted NGS and exome sequencing are
essential in the diagnosis of various anaemia (48,51,78-86). In particular, genome-wide
association studies provide valuable insights into the genetic
pathways and variations related to sickle cell anaemia (79,87-89). Understanding molecular
mechanisms, cellular composition, and heterogeneity can be gained
through molecular profiling using RNA-Seq, proteomics, and
single-cell genomics. Identification of microRNAs and meta-analyses
using microarray technology aid in the discovery of hub genes,
transcriptional regulation, and shared markers (90-92).
The development of possible treatment interventions
is another benefit of bioinformatics techniques. Especially in the
case of thalassemia (80), tiny
compounds are evaluated using molecular docking for potential
therapeutic uses. In numerous anaemias, including DBA and FA, the
molecular targets for therapies are identified (93). To determine the functional
properties of the proteins involved in haemolytic anaemia, machine
learning-based predictions are conducted. Finding possible drugs is
made easier with the use of bioinformatic analysis on proteomic and
microarray datasets (94,95).
Additionally, to close the gap between genetic discoveries and
clinical practice-particularly in the case of sickle cell
anaemia-efforts are being made to establish bioinformatics
infrastructure in areas with high occurrences (89).
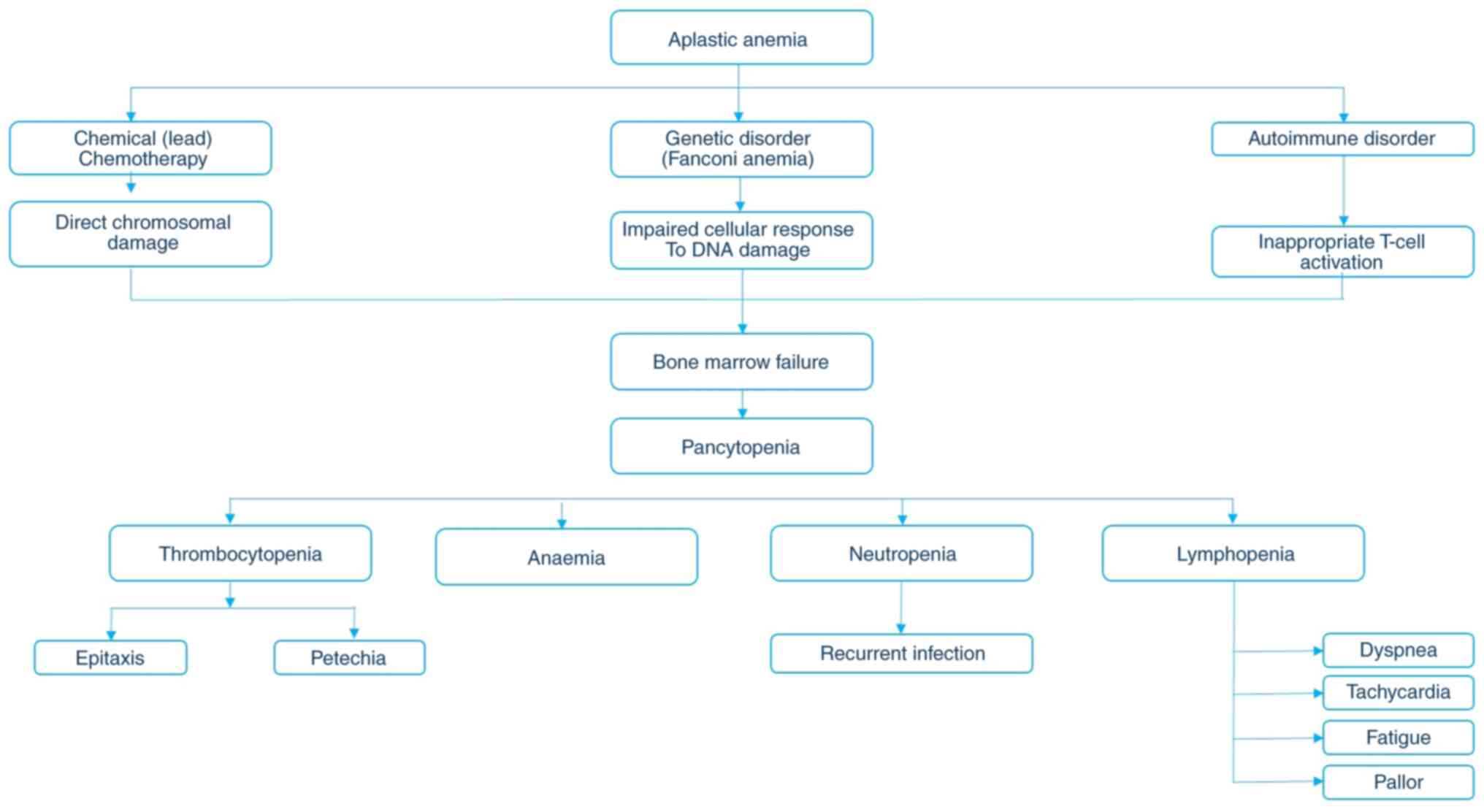
Aplastic pancytopenia
AA refers to an anaemic condition in an individual
in which he or she cannot produce sufficient erythrocytes, WBCs and
PLTs due to a lack of hematopoietic progenitor cells in them. The
word 'aplastic' refers to the inability of the marrow to form
blood, which might cause diverse pathogenic conditions, and anaemia
refers to an anaemic condition. AA is also known as 'idiopathic' or
'idiopathic anaemia'. The first case of AA was reported by Paul
Ehrlich in the year 1884 (14).
AA is not specific to any age group. It is sparsely found in all
age groups, from children to elderly individuals. AA is similar to
FA and dyskeratosis congenita, which are genetic disorders caused
by lack of DNA repair (14). AA
can lead to other health concerns, such as an irregular heartbeat,
an enlarged heart and heart failure. It can be caused by injury to
blood stem cells due to exposure to certain drugs, chemotherapy,
congenital disorders, drug therapy to suppress the immune system,
pregnancy, radiation therapy, or toxins such as benzene or arsenic.
The cause and symptoms of AA are described in Fig. 3.
AA can be classified into two types: These are
immune AA (IAA) and acquired AA (AAA). IAA is an auto-immune
disorder that can be transferred from one individual to another
individual (for example, from the parents to their children). It is
a very rare autoimmune disorder in which the T-cells act against
the healthy cells. Functionally and phenotypically activated
cytokine T-cells are skewed to produce type-1 cytokines. These
cytokines induce apoptosis and cause cell destruction (96,97). Apart from cytokines, somatic
mutations at the STAT3 signalling pathway and some
histocompatibility antigens, such as human leukocyte antigen (HLA),
are also responsible for the disease (98,99). It has been revealed that Treg
cells are decreased in AA conditions, which further leads to
hematologic processes (100).
AAA is a disorder resulting from damage to progenitor cells by
chemicals, exposure to ionising radiation, drugs, viral infections,
constitutional genetic defects, or autoimmune destruction (101). AAA is a condition in which AA
is attained due to some other factors apart from immunological
reasons. Numerous factors are responsible for AA, such as
chemoradiotherapy, viral hepatitis, overexposure to pesticides and
insecticides, certain drugs, exposure to chemicals, and in some
rare pregnancy cases. In all these conditions, the signalling
pathway is still a mystery. Apart from these two types, AA is said
to be idiopathic because there was no test to confirm a
cause-and-effect relationship in most of the cases (102).
According to a previous survey, it was found that AA
is higher in Asian regions when compared with Europe and North
American regions. It was also identified that the incidence of the
disease is higher in Asia when compared with the West (46,103). According to the National
Organization of Rare Diseases (NORD), AAA affects male and female
patients in about equal numbers in most cases, as well as older
children, teenagers, or young adults (104). The incidence rate is two or
three times greater in Asia. The exact incidence rates that exist
in the United States are unknown, although some sources report that
approximately 500-1,000 new cases of AA are diagnosed each year.
Similarly, NORD states that certain disorders share similar
symptoms as AA, such as MDS and PNH. Apart from this, AA may also
occur as part of an inherited disorder such as FA, telomere
diseases, Shwachman-Diamond syndrome, ataxia-pancytopenia syndrome,
and others.
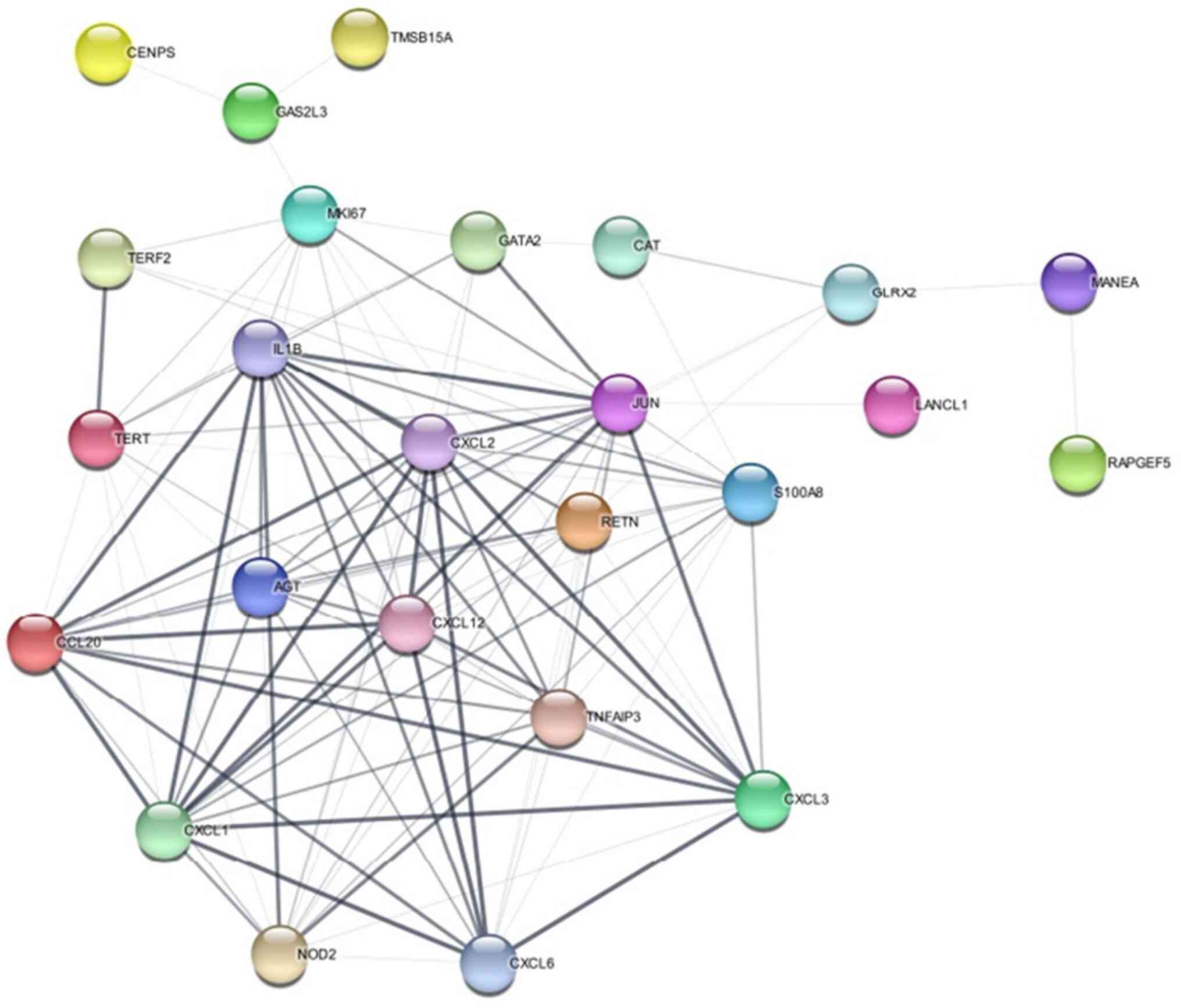
According to the literature review, the most
commonly expressed genes are discussed in Table III. Certain genes were also
found to be co-similar with other diseases, such as FA, and
telomerase diseases. Likewise, most of the genes are involved in
the inter and mitotic phases of cell division. Genes are subjected
to network analysis using the STRING database (https://string-db.org). STRING is an online
bioinformatics tool that provides information on protein-protein
interaction, functional annotation, enrichment analysis, and
network analysis. The specified genes are input into STRING with
low confidence, allowing no more than five interactions in their
configuration. The resulting network is visually represented in
Fig. 4.
 | Table IIIAssociation of the genes with AA and
its function. |
Table III
Association of the genes with AA and
its function.
| S. no. | Gene | Functions | Relation with
AA | (Refs.) |
|---|
| 1 | RAPGEF5 | Regulation of RAS
protein | Downregulated in
AA | (103,105,106) |
| 2 | MANEA | Sialic acid
production | Involved in
adipogenesis regulation | (107) |
| 3 | TERF2 | Telomere
maintenance | Genetic changes
connected to congenital dyskeratosis | (106,108,109) |
| 4 | TERT | Telomerase
enzyme | Mutations linked to
telomere shortening | (106,110) |
| 5 | AGT | Angiotensin
regulation | Downregulated in
bone marrow MSCs of AA patients | (107,111) |
| 6 | GAS2L3 | Cytoskeletal
dynamics | Role in controlling
MSCs characteristics | (112) |
| 7 | MKI67 | Proliferation
marker | Identified as a
proliferation marker in AA patients | (46,100) |
| 8 | TMSB15A | Thymosin beta
family | Expression linked
to better prognosis in paediatric AA | (108,109,113) |
| 9 | CAT | Catalase
enzyme | Essential for
reducing oxidative stress | (102,114,115) |
| 10 | GSH | Master
antioxidant | Homozygous deletion
linked to AA | (116) |
| 11 | SDF-1 | Chemokine
regulating BM | Altered expression
in AA patients | (117) |
| 12 | SOD-2 | Antioxidant
enzyme | Presence in bone
marrow of experimental AA | (118,119) |
| 13 | S100A8 | Calcium-binding
protein | Elevated in plasma
of AA patients | (120,121) |
| 14 | RETN | Resistin
hormone | Differentially
expressed in haematopoietic cells | (46,111) |
| 15 | TNFAIP3 | Negative regulator
of NF-κB | Upregulated in
T-cell differentiation of AA patients | (122,123) |
| 16 | JUN | AP-1 transcription
factor | Differentially
expressed in AA patients | (46,96,107,111) |
| 17 | IL1B | Proinflammatory
cytokine | Elevated in
patients with AA | (114) |
| 18 | FANC | Maintain genomic
integrity | Pancytopenia caused
by a hereditary disease resulting in defective DNA repair | (17,84) |
| 19 | GATA2 | Transcription
factor | Mutations linked to
paediatric neutropenia, AA | (124) |
| 20 | CD | Cluster of
differentiation | Immune cell markers
| CD8+, CD38+ implicated in AA | (125,126) |
The network analysis has revealed a number of
considerable enriched pathways and processes including IL-17
signalling pathway, TNF signalling pathway, Rheumatoid arthritis
pathway, Chemokine signalling, inflammatory response, and immune
system processes. A number of enriched terms are associated with
the regulation of cell migration, proliferation, and apoptosis,
indicating that the genes are involved in the regulation of these
biological processes. Databases such as Kyoto Encyclopedia of Genes
and Genomes (https://www.genome.jp/kegg/) pathways, Gene Ontology
(https://geneontology.org/) terms,
InterPro domains and UniProt keywords have shown enrichment,
suggesting that the input gene collection is functionally diverse
and engaged in a variety of biological processes. Ex aggravation of
the diseased state is not clear and our future study emphasises on
identification of specific pathways that can be targeted to
modulate the immune response and for further treatment.
Treatment of AA
Hematopoietic growth factors such as erythropoietin
and Neupogen are not effective in AA, but surprisingly Eltrombopag,
a stimulator of PLT production, was effective in improving blood
counts in refractory AA (RAA). In 2014, Promacta was approved to
treat patients with severe AA who have had an insufficient response
to IST and are not candidates for a hematopoietic stem cell
transplant. When eltrombopag was combined with standard
immunosuppression as first-line therapy, response and complete
response rates were higher than with immunosuppression alone
(104).
IST was given to the individuals affected with AA
for 6 months. If it lacks response after a course, then it is known
as RAA. Eltrombopag was found to be effective in refractory cases
(14). Antithymocyte globulin
(ATG) therapy and allogeneic stem transplantation were also found
to be efficient in AA conditions (103). Apart from Eltrombopag, bone
marrow transplantation was conducted in immune AA cases, but it was
not successful in certain cases due to graft rejection. The
survival of individuals is also reduced after transplantation.
Later, umbilical cord transplantation was performed and it was
found to be successful with 90% survival inpatients.
Transplantation was done in severe cases of IAA and AAA. All these
treatments depend upon the severity of the disease, age, and the
initial treatment (14).
A new therapeutic intervention for AA in paediatric
conditions was obtained through the telomere maintenance pathway
and gene expression profiling of mesenchymal stem cells. Leukocyte
telomere shortening was observed in individuals affected byAA.
TERF2 gene was said to be involved in telomerase shortening. So,
these genes can be focused on a therapeutic basis for AA-affected
individuals in terms of molecular characterization (106,110).
Role of mesenchymal stem cells (MSCs) in
AA treatment
(MSCs) are multipotent stem cells that are found in
bone marrow for making and repairing skeletal tissues. Apart from
this, they can also be found bounteous in the treatment of AA
individuals. Previous studies found that the MSCs provide a
specialised microenvironment for haematopoiesis (127,128). MSCs contribute to the
organisation and function of the haematopoietic niche through their
immunomodulatory properties. They were found to cause
morphological, functional, and genetic alterations in both AA and
MDS. They have also been shown to suppress T-cell proliferation and
activation, which can help to reduce the autoimmune response in AA.
However, some of the clinical manifestations of AA can be explained
by mesenchymal dysfunction (129). This remains a query in the case
of immune-related AA. Modulation of immune responses by secreting
various cytokines and growth factors can promote the survival and
proliferation of haematopoietic stem cells. These modulations are
performed by MSCs, and thus these modifications can help in the
further recovery of the disease (127). Previous studies have shown that
MSC-derived extracellular vesicles can significantly reverse
radiation damage to bone marrow, which could have implications for
the treatment of AA (130,131). A combination of umbilical cord
MSC and standard immunosuppressive drugs can effectively treat
severe AA in paediatric patients.
Pathway analysis
Currently, there is no approved medication for AA.
An in-depth analysis of the pathway could lead to a better
understanding of the illness and potentially facilitate the
development of target-specific medications. A total of 300
differentially expressed genes that were involved in apoptosis,
adipogenesis and the immunological response were found in AA
patients as compared with healthy individuals.
Adipogenesis-cytokine signalling, chemotaxis, cell division,
proliferation, the cell cycle, and hematopoietic cell lineage
differentiation are among the other processes with which these
genes are considered to be associated with. The cytokine genes are
important in AA and also suggests that the disease may be caused by
the deregulation of these genes. In addition to genes, chemical
substances also play an indirect role in the pathway disruption.
Lead has been shown to disrupt heme synthesis, which impacts
erythrocyte development and function (102).
Patients with AA exhibit a Treg/Th17 imbalance,
which is managed by MSCs via the production of exosomes.
Sphingosine-1-phosphate (S1P), which is present in these exosomes,
is enriched due to SphK1. MSCs use sphingosine 1-phosphate
(S1P)-containing exosomes to produce and regulate the Treg/Th17
imbalance in AA. SphK1 rather than SphK2 is the source of S1P
enrichment in MSCs-Exos. The exosomal S1P receptor S1PR1, which is
expressed on CD4+ T cells, mediates the exosomal
S1P-induced increase in Treg production. In vivo experiments
revealed that MSCs-Exos corrected the elevated Th17/Treg and
prevented the advancement of AA in AA mice (130).
Oxidative stress
Oxidative stress alters several important signalling
pathways and accelerates the ageing process of hematopoietic stem
cells, according to (103).
Oxidative stress reduces antioxidant activity, which results in the
production of free radicals (132). The onset of AA could be caused
by oncogenic Ras activating the mitogen-activated protein kinase
(MAPK) pathway after oxidative damage. It was found that that the
mechanism underlying the contraction of vascular smooth muscle may
also apply to AA (103). This
gene has been linked to the Treg phenotype associated with
autoimmune diseases (103).
Despite the fact that several genes exhibit differential expression
in AA, it is unclear how exactly these genes relate to one another
and the disease.
Apart from this, a lot of AA agents cause oxidative
stress. The overuse of alkylating medications such as
cyclophosphamide and busulfan resulted in a decrease in antioxidant
agents such as SDF-1 and SOD-2 (105). Chemokine SDF-1 is involved in
stem cell homing, angiogenesis and cell migration. One enzyme that
contributes to the antioxidant defence system is SOD-2.
Furthermore, the bone marrow's CD150+ and
C-Kit+ cell counts decreased. According to flow
cytometry investigations, there was a considerable decline in
RUNX-2 and ALPH-1, which had an impact on the CD45-ve population.
This decreased activity has shown that reactive oxygen species
(ROS), which preceded the decline in antibodies such as STRO-1 and
CFU-F, are involved.
Challenges in the classification of
AA
There were numerous conflicts arising from the
identification of AA in different illnesses. It was discovered that
MDS and PNH had symptoms that were comparable to those of AA. They
were also discovered to be associated with other illnesses, which
further complicates the illness's diagnosis and analysis. A
discussion of the diseases that were discovered to co-occur and be
similar follows.
Complexity with similar diseases
The complex interactions among MDS, clonal
haematopoiesis (CH), PNH, and AA are examined in detail. It draws
attention to PNH is potentially fatal character, its possible
emergence following coronavirus immunisation, and its diagnostic
importance in figuring out the immune-mediated pathophysiology of
AA's marrow aplasia. The higher risk of subsequent MDS or AML in
patients with AA is examined, as well as the morphological
parallels between AA and MDS. The following examines the recently
developed notion of CH and its correlation with late clonal
diseases following IST in AA, with a focus on the molecular aspects
and its treatment and diagnostic implications.
PNH and AA
PNH is a rare, life-threatening disease of the
blood. PNH is caused by somatic mutation in the
phosphatidylinositol glycan class A (PIG-A). It is characterised by
the destruction of erythrocytes, blood clots, and impaired bone
marrow function and it is also closely related to AA (133). According to a previous study
(134), individuals were
subjected to AA after completion of two doses of vaccination for
coronavirus which was observed in a 74-year-old man. A PNH clone
was found in the blood sample which was developed after the
vaccination and that specified clone might have caused AA.
Similarly, PNH clones were observed in 63 patients who were
affected by the disease in a case study (135). Patients with immune-mediated
bone marrow failure frequently have PNH clones. PNH clones form in
>50% of individuals with AAA, a T-cell-mediated autoimmune bone
marrow failure illness. In AAA, it is considered that PNH cells can
avoid the HSPC-directed autoimmune onslaught, which causes PNH
cells to overrun wild-type cells and generate PNH clones. Patients
with MDS and, less frequently, myeloproliferative neoplasms can
also have PNH clones. Although immune-mediated bone marrow failure
is the main risk factor for the emergence of PNH clones, patients
might be given a diagnosis of classical PNH without having any
history of cytopenia or a recognized diagnosis of bone marrow
failure. Prior subclinical marrow failure is generally assumed in
these situations, however subsequent proliferative mutations inside
a PNH HSPC are another possibility (133).
A PNH clone can offer a critical diagnostic clue
about the immune-mediated pathophysiology of marrow aplasia
(133). The clonal growth of
PNH cells is intimately linked to the HSPC-directed autoimmune
onslaught in AA. Pancytopenia and hypocellular bone marrow are the
presenting symptoms of AA; however, they are not always indicative
of the disease. The rigorous exclusion of all potential causes of
marrow failure, such as dietary deficiencies, infections, or
toxins, is therefore necessary for the diagnosis of AA. The
exclusion of inherited bone marrow failure disorders, a costly and
time-consuming technique that might leave persistent diagnostic
uncertainty, is especially crucial for children and young and
middle-aged adults. PNH clones were found in 46% of patients with
AA. This disease is found to have similar symptoms to AA as well as
found comorbid with AA (136).
MDS and AA
As they cope with a shortage of bone marrow
environment, AA and MDS exhibit substantial morphological
similarities (124,137). Although the pathophysiology of
AA and MDS differs, there are significant similarities. A clonal
condition called MDS is defined by the build-up of genetic
abnormalities in hematopoietic stem cells, which results in
defective blood cell formation and inefficient haematopoiesis
(138). On the other hand,
hematopoietic stem cells are destroyed by the immune system in AA,
a non-clonal illness that results in pancytopenia. Acute myeloid
leukaemia (AML) or MDS, however, may develop in certain AA
instances. Although the precise processes that lead from AA to MDS
or AML are not entirely understood, new genetic mutations or
epigenetic modifications may be acquired (138).
There is an association between MDS and AA (139). Patients with AA have an
increased risk of developing MDS and AML. In total, ~15-20% of
patients with AA and 2-6% of patients with PNH were found to to
develop secondary MDS/AML by 10 years of follow-up. The exact
mechanisms underlying the progression from AA to MDS or AML are not
fully understood, but they may involve the acquisition of
additional genetic mutations or epigenetic changes.
CH and AA
Recent research has shown that CH is closely linked
to the evolution of late clonal disorders, including PNH and
MDS/AML, which are common complications after successful IST in AAA
(140). The widespread
discovery of somatic mutations that promote clonal evolution has
shed light on the molecular component of CH in AA (134,135). Based on recent research,
autoimmune destruction of early hematopoietic stem cells may be the
cause of AAA, a rare condition that manifests as bone marrow
failure syndrome and might be regarded as a clonal disorder of
hematopoietic stem cells (136). Investigations on the prognostic
importance of somatic mutations driving clonal evolution in AA are
ongoing (134). Overall, new
knowledge on CH in AA has shed light on the disease's
pathophysiology and may have consequences for both diagnosis and
treatment.
Comorbid nature of AA
The discussion below explores the causes of AA, with
particular attention to telomere shortening, COVID-19 immunisation,
AA caused by cancer, FA, and the relationship to chronic gut
inflammation. It examines comorbidities, environmental factors, and
genetic components related to AA, providing a comprehensive picture
of the disease's pathogenesis. The genetic foundation of FA,
COVID-19 vaccine-related instances, telomerase deficiency, and the
relationship between severe AA and persistent gut inflammation are
also addressed in the discussion.
Telomere shortening and AA
Telomerase is the end-part of the chromosomes. It is
a hexanucleotide (TTAGGG) with tandem repeats of DNA. Telomerase is
also known as the 'mitotic cycle' which has the record of a cell's
proliferative history. Telomerase and its associated protein were
known as 'Sheltrin'. The Telomerase region consists of genes such
as TERT, TERC and Dyskerin. The diseases that are associated with
telomerase shortening and deficiency are dyskeratosis congenita,
Hoyerral Hreidarsson syndrome, and Revesz syndrome. Apart from the
aforementioned disease telomerase shortening can take place due to
ageing and ROS which were formed due to environmental toxins and
irradiation (141). AA can also
be caused due to somatic mutations at TERT and TERC genes which are
the main proteins that are present in the telomerase region. In
10-20% of patients affected with AA, it is known as a clonal
malignant disorder or premalignant disease (142).
Telomeropathy can cause damage to organs including
the bone marrow, lungs and liver, which indirectly indicates that
telomere shortening is observed in patients with AA. Adults with
acute AA have been observed with base change in the TERT A 1062T
variant which might be due to the environmental context. Likewise,
the telomerase disease TERT gene was also accompanied by some
clones of PNH (143).
Similarly, telomerase shortening was observed in individuals who
are affected with AA especially in children of the age group 3 to
15 (46).
COVID-19 and AA
A few case studies have been reported in recent days
regarding the diagnosis of AA after COVID-19 vaccination. In all
these case studies, the individuals do not have any past medical
history of pancytopenia namely, thrombocytopenia, anaemia,
neutropenia and lymphopenia. They were found to develop
pancytopenia after vaccination. The first case of AA was reported
in a 76-year-old man, who has been presented with severe AA with a
PNH clone one month after the second dose of the Pfizer coronavirus
vaccine. The individual could not survive because there were
multifactorial reasons for it. However, his medical history does
not show any pancytopenia symptoms (134). It was reported that the
COVID-19 vaccination has led to the development of AA in the
following cases (144). A
64-year-old woman has been found to develop AA after the first dose
of Ad 26. Cov.2. S (Janssen vaccine). In the initial stages, the
CBCs were found to be moderate neutropenia, thrombocytopenia, and
anaemia. But later it exaggerated into severe conditions. The
symptoms were petechiae and fever immediately after a week of
vaccination.
Similarly, numerous case studies show that
individuals were affected with AA after the completion of one or
two doses of corona vaccination. It can be considered a drop in Hb,
leading to haematological conditions in healthy individuals. Focus
should be addressed on the aftermath of vaccines and this should be
brought to light for the prevention of the disease (10,145-147). The correlation between these
two diseases is still unclear and a detailed study between them
should be performed for further medication and treatment.
Cancer and AA
Cancer is one of the deadliest diseases and it is
incurable. The incidence rate of cancer increases every year. The
primary cancer treatment is radiotherapy and chemotherapy. But
chemotherapy can also lead to AA. Temozolomide is also known as
'Temodal', which is a type of chemotherapy used in the treatment of
a type of brain tumour cells malignant gliomas. Malignant gliomas
include glioblastoma multiforme and anaplastic astrocytoma
(148). A 55-year-old female
patient was detected with glioblastoma multiforme through MRI. The
first cyclic dose of partial brain radiotherapy with Temozolomide
(75 mg/m2) for 6 weeks and 150 mg/m2
afterward. After 19 sessions, there was bleeding from the infection
sites on her thighs and bruises. The blood profile revealed that
the individual was affected with pancytopenia (AA) with a
reticulocyte count of 0.2 indicating a hypo-proliferative marrow.
Bone marrow biopsy shows the hypocellularity with increased plasma
cells. Immunosuppressive drugs did not apply to the patients as
they are affected with malignancy and pulmonary embolism. Despite
this condition, the affected female was given a trial of IV
immunoglobulin, which did not show any response (149,150). AA can be caused due to numerous
factors, in chemotherapy is one of the reasons for it. According to
the aforementioned studies, therapists should be aware that it
should not cause any hematologic suppression or damage to the
individuals under chemotherapy.
FA
FA is a rare genetic disorder in the bone marrow
which leads to a decrease in the production of all types of blood
cells. AA can be caused due to multiple factors and the mechanism
of the disease remains a query. FA is caused due to mutation in the
FANC gene and it can occur in any of 23 genes. It does not show any
symptoms in childhood but has a high risk of developing cancer
(17,104). AA and FA are two different
disorders with similar features of pancytopenia. FA is
characterised by chromosomal instability and congenital anomalies
while AA has a diverse aetiology. A chromosome breakage test in the
blood is the only diagnostic technique that can be used in the
differentiation of FA from AA (151).
Patients with FA have a higher risk of developing AA
than the general population. FA is one of the most prevalent causes
of AA concerning genetic causes. The core complex of FA is produced
by the FANCA gene, which is involved in the mutation and repair of
DNA damage. This damage leads to the accumulation of ICLs
(interstrand crosslinks) that can cause AA (17). Most of the patients with FA have
at least one of the physical anomalies but it is not well
characterised in patients with AA and not present in all cases
(152). A previous study showed
that the frequency of parental consanguinity among patients with AA
was 73% and the parental consanguinity among patients with FA was
also high (153). The
aforementioned study also suggested that there may be a higher
prevalence of comorbidity between AA and FA than in the general
population. In this scenario of comorbidity, bone marrow
transplants are the only treatment for patients with both AA and
FA, but they are more complex (154).
Chronic gut inflammation
Chronic gut inflammation refers to a group of
disorders that causes enduring inflammation of tissues in the
digestive tract. The most common conditions of chronic gut
inflammation are Crohn's disease and ulcerative colitis. AA and
chronic gut inflammation were found to be comorbid in certain
research studies. The individuals who are affected with severe AA
conditions are found to suffer from long-lasting gut inflammation
or vice-versa. Active chronic gut infections in the gut may sustain
aberrant immune responses in AAA. The development of AAA conditions
was due to the formation of polyps in the sigmoid of the colon and
rectal regions in the mucosal membrane. These polyps were enlarged
lymphoid follicles with infiltrated large numbers of lymphocytes
and plasma cells. An excellent response of SAA to the treatment of
gut inflammation was also reported (155). Patients with severe AA (SAA)
frequently present with inflammatory episodes; during flared
inflammatory episodes, haematopoietic cells in the bone marrow are
replaced by adipocytes (156).
Similarly, the plasma metabolomic and intestinal microbial profiles
of patients with SAA were analyzed and it was found that the
intestinal microbial composition of patients with SAA was
significantly different from that of healthy controls (157).
Scope of the review
Anaemia is found to be associated with other
diseases such as cancer, chronic kidney diseases, gastrointestinal
disorder, inflammatory disorders, autoimmune disorders, endocrine
disorders and so on (4,158-161). This network of links highlights
the intricate interactions that exist between anaemia and more
general health situations, indicating the need for a detailed
investigation of these interconnections. Researchers are turning
more to genetic analysis to identify the underlying genetic causes
causing anaemia and associated comorbidities to gain deeper
insights. This genomic research lays the groundwork for furthering
our comprehension of the pathophysiology of anaemia.
Simultaneously, the healthcare landscape is changing
and includes the idea of incorporating AI predictive modelling
tools. Future research aims to use AI to create advanced prediction
models for the early identification of anaemia and related
disorders. Through the integration of genetic studies into these
models, investigators hope to customise diagnostic methods more
precisely, opening the door to proactive and individualised
treatment approaches. In addition to enhancing early detection,
this comprehensive and progressive strategy presents AI as a
game-changing instrument that will revolutionise healthcare
practices for haematological illnesses and eventually improve
patient outcomes.
Another crucial thing we analysed was that the
genetic types of anaemia such as AA, FA and DBA have some features
which have certain concerns to aggravate MDS (117). This could require exigent and
targeted personalised medicine to cease its further growth.
Conclusion
The present analysis highlights the varied
classification of anaemia based on a range of characteristics,
including aetiology, Hb levels, RBC morphology, and genetic
factors, and the importance of identifying the type of anaemia. The
paper explores the intricacies of numerous forms of anaemia, with a
particular emphasis on those resulting from genetic diseases such
as heredity, autoimmune disorders, and mutations. The genetic
anaemias included in this study are haemolytic anaemia,
thalassemia, sideroblastic anaemia, DBA, FA and AA. Each kind is
distinguished by distinct physical properties of RBCs, pathogenic
behaviours, and genetic factors. The roles that each kind's
associated genes and mutations play have been clarified, offering a
comprehensive grasp of the underlying genetic processes. The
immunomodulatory role of vitamin D in anaemia is also examined in
this work, with special attention to its connection to
erythropoiesis and the potential impact of vitamin D deficiency on
various forms of anaemia. The importance of considering dietary
deficiencies, genetic changes, and chronic illnesses as potential
causes of anaemia is emphasised throughout the study.
The use of biomarkers for identifying distinct forms
of anaemia is highlighted, along with the diagnostic component of
anaemia. The study identifies distinct molecular biomarkers linked
to each kind, providing information on their possible use in the
detection and tracking of anaemia. Even though machine learning and
deep learning models have been used in the detection of various
types of anaemia, there are numerous challenges associated with
them. Medical data was the type of data that was used in the
majority of cases. These data are extremely sensitive and subjected
to strict privacy regulations. The data are imbalanced, and
restricted and have numerous ethical and legal concerns. Being
limited, the biological data obtained from different individuals
can vary significantly and thus it becomes a bigger challenge to
create a universally applicable model. Apart from the data, there
are numerous factors such as age, gender, locality, geographical
region, and past medical history of individuals that should also be
considered. Identification of relevant feature selection is the
secondary challenge in these types of data. Inadequate feature
selection may lead to suboptimal model performance and proper
dimensionality reduction was required in processing and analysing
the data. They deal with a lot of ethical and legal concerns in
handling the data. The usage of EHR would be complex and requires a
seamless integration of practical use as they require precise
statistical and genetic analysis. Lack of domain expertise in
understanding the intricacies of the diseases and further analysis
in machine learning aspects. Anaemia is a disease which is dynamic
and exhibits diverse and evolving characteristics. Machine learning
and deep learning models should be continuously updated and adapted
to handle new patterns and variations.
AA is a multifactorial and intricate illness
typified by the bone marrow's incapacity to generate a sufficient
quantity of RBCs. It can be caused by a number of things, including
immunological-related conditions, exposure to chemicals,
chemotherapy, genetic abnormalities, and in rare cases-such as the
COVID-19 vaccine cases that have been reported recently-by
particular immunisations. AA is difficult to classify because of
its similarities to other disorders such FA, MDS and PNH. These
diseases may require specialised testing, such as the chromosomal
breakage test, due to their overlapping symptoms and comorbidities,
which make diagnosis and distinction challenging. The kind and
severity of an illness determine which AA treatment plan is best.
Some immunomodulatory therapies have shown promise, such as ATG and
allogeneic stem cell transplantation. Furthermore, it has been
demonstrated that MSCs can regulate immune responses and offer a
particular environment for the process of hemopoiesis. The
association between AA and other conditions such as cancer,
telomere shortening, clonal haematopoiesis, and chronic
inflammatory bowel disease emphasises how complex the disease is
and how important it is to fully understand its aetiology. For
example, Alzheimer's disease has been associated with telomere
shortening, and new discoveries regarding the genes associated with
telomerase present new avenues for therapeutic methods. The sudden
appearance of AA following COVID-19 vaccination raises serious
questions about potential links between vaccinations and
haematological disorders. Even though these incidents are uncommon,
they serve as a reminder of the value of continuing observation and
investigation to learn more about the possible adverse effects of
immunisations.
Availability of data and materials
Not applicable.
Authors' contributions
DS and IRO conceived the present study. DS developed
methodology, conducted investigation, wrote the original draft. DS
and IRO wrote, reviewed and edited the manuscript. IRO supervised
the study. Both authors read and approved the final manuscript.
Data authentication is not applicable.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Abbreviations:
|
AA
|
aplastic anaemia
|
|
IAA
|
immune AA
|
|
AAA
|
acquired AA
|
|
RAA
|
refractory AA
|
|
MSCs
|
mesenchymal stem cells
|
|
ATG
|
anti-thymocyte globulin
|
|
MDS
|
myelodysplastic syndrome
|
|
AML
|
acute myeloid leukaemia
|
|
NORD
|
National Organization of Rare
Diseases
|
Acknowledgements
Not applicable.
Funding
No funding was received.
References
|
1
|
Kalaivani K and Ramachandran P: Time
trends in prevalence of anaemia in pregnancy. Indian J Med Res.
147:2682018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Belwal E, Pandey S and Sarkar S: Anemia
prevalence in india over two decades: Evidence from National Family
Health Survey (NFHS). Int J Sci Healthcare Res. 6:335–340. 2021.
View Article : Google Scholar
|
|
3
|
Baradhi KM and Badireddy M: Chronic
Anemia. StatPearls. StatPearls Publishing; Treasure Island, FL:
2024
|
|
4
|
Anemia of Inflammation or Chronic
Disease-NIDDK. (n.d.). https://rarediseases.org/.
|
|
5
|
Moreno Chulilla JA, Romero Colás MS and
Gutiérrez Martín M: Classification of anemia for
gastroenterologists. World J Gastroenterol. 15:4627–4637. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Abusharib AB: Morphological patterns of
anaemia among pregnant women from Sudan. Afr J Lab Med. 8:7432019.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Sheikh Z: Anemia. Available from:
https://www.webmd.com/a-to-z-guides/understanding-anemia-basics.
|
|
8
|
Dongerdiye R, Sampagar A, Devendra R,
Warang P and Kedar P: Rare hereditary nonspherocytic hemolytic
anemia caused by a novel homozygous mutation, c.301C>A, (Q101K),
in the AK1 gene in an Indian family. BMC Med Genomics. 14:1912021.
View Article : Google Scholar
|
|
9
|
Mohandas N: Inherited hemolytic anemia: A
possessive Beginner's guide. Hematol Am Soc Hematol Educ Program.
2018:377–381. 2018. View Article : Google Scholar
|
|
10
|
Baldwin C, Pandey J and Olarewaju O:
Hemolytic Anemia. StatPearls. StatPearls Publishing; Treasure
Island, FL: 2024
|
|
11
|
Tanabe P, Spratling R, Smith D, Grissom P
and Hulihan M: CE: Understanding the complications of sickle cell
disease. Am J Nurs. 119:26–35. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Bajwa H and Basit H: Thalassemia.
StatPearls. StatPearls Publishing; Treasure Island, FL: 2024
|
|
13
|
Huang TL, Zhang TY, Song CY, Lin YB, Sang
BH, Lei QL, Lv Y, Yang CH, Li N, Tian X, et al: Gene mutation
spectrum of thalassemia among children in yunnan province. Front
Pediatr. 8:1592020. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Young NS: Aplastic Anemia. N Engl J Med.
379:1643–1656. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Engidaye G, Melku M and Enawgaw B: Diamond
blackfan Anemia: Genetics, pathogenesis, diagnosis and treatment.
EJIFCC. 30:67–81. 2019.PubMed/NCBI
|
|
16
|
van Dooijeweert B, Kia SK, Dahl N,
Fenneteau O, Leguit R, Nieuwenhuis E, van Solinge W, van Wijk R, Da
Costa L and Bartels M: GATA-1 defects in diamond-blackfan Anemia:
Phenotypic characterization points to a specific subset of disease.
Genes (Basel). 13:4472022. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Bhandari J, Thada PK, Killeen RB and
Puckett Y: Fanconi Anemia. StatPearls. StatPearls Publishing;
Treasure Island, FL: 2024
|
|
18
|
Fouquet C, Le Rouzic MA, Leblanc T,
Fouyssac F, Leverger G, Hessissen L, Marlin S, Bourrat E, Fahd M,
Raffoux E, et al: Genotype/phenotype correlations of
childhood-onset congenital sideroblastic anaemia in a European
cohort. Br J Haematol. 187:530–542. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Nzelu D, Shangaris P, Story L, Smith F,
Piyasena C, Alamelu J, Elmakky A, Pelidis M, Mayhew R and Sankaran
S: X-linked sideroblastic anaemia in a female fetus: A case report
and a literature review. BMC Med Genomics. 14:2962021. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ovesen L and Boeing H; EFCOSUM Group: The
use of biomarkers in multicentric studies with particular
consideration of iodine, sodium, iron, folate and vitamin D. Eur J
Clin Nutr. 56(Suppl 2): S12–S17. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Liu T, Zhong S, Liu L, Liu S, Li X, Zhou T
and Zhang J: Vitamin D deficiency and the risk of anemia: A
meta-analysis of observational studies. Ren Fail. 37:929–934. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Patel NM, Gutiérrez OM, Andress DL, Coyne
DW, Levin A and Wolf M: Vitamin D deficiency and anemia in early
chronic kidney disease. Kidney Int. 77:715–720. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Arabi SM, Ranjbar G, Bahrami LS, Vafa M
and Norouzy A: The effect of vitamin D supplementation on
hemoglobin concentration: A systematic review and meta-analysis.
Nutr J. 19:112020. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Nur-Eke R and Özen M: The relationship
between vitamin D levels and iron deficiency and anemia in adults
applied for periodic medical examination. Clin Lab. Jun 1–2020.Epub
ahead of print. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Thomas CE, Guillet R, Queenan RA, Cooper
EM, Kent TR, Pressman EK, Vermeylen FM, Roberson MS and O'Brien KO:
Vitamin D status is inversely associated with anemia and serum
erythropoietin during pregnancy. Am J Clin Nutr. 102:1088–1095.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Lee JA, Hwang JS, Hwang IT, Kim DH, Seo JH
and Lim JS: Low Vitamin D levels are associated with both iron
deficiency and anemia in children and adolescents. Pediatr Hematol
Oncol. 32:99–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Chowdhury R, Taneja S, Bhandari N, Strand
TA and Bhan MK: Vitamin D deficiency and mild to moderate anemia in
young North Indian children: A secondary data analysis. Nutrition.
57:63–68. 2019. View Article : Google Scholar
|
|
28
|
Uwaezuoke S: Vitamin D deficiency and
anemia risk in children: A review of emerging evidence. Pediatric
Health Med Ther. 8:47–55. 2017. View Article : Google Scholar
|
|
29
|
Yassin FA, Said NM and Tarek D:
Association between Vitamin D receptor gene polymorphisms and
anemic patients. Biochemistry Lett. 13:1–10. 2017. View Article : Google Scholar
|
|
30
|
Ochogwu OL, Salawu L, Owojuyigbe TO and
Adedeji TA: Vitamin D deficiency and its association with anemia
and blood transfusion requirements in Nigerian adults with sickle
cell anemia. Plasmatology. 15:2021. View Article : Google Scholar
|
|
31
|
Yu W, Ge M, Lu S, Shi J, Feng S, Li X,
Zhang J, Wang M, Huang J, Shao Y, et al: Decreased expression of
vitamin D receptor may contribute to the hyperimmune status of
patients with acquired aplastic anemia. Eur J Haematol. 96:507–516.
2016. View Article : Google Scholar
|
|
32
|
Napolitano LM: Vitamin D supplementation
and hemoglobin: Dosing matters in prevention/treatment of Anemia.
Nutr J. 20:232021. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Athanassiou L, Mavragani CP and
Koutsilieris M: The immunomodulatory properties of Vitamin D.
Mediterr J Rheumatol. 33:7–13. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Rak K and Bronkowska M: Immunomodulatory
effect of Vitamin D and its potential role in the prevention and
treatment of type 1 diabetes Mellitus-A narrative review.
Molecules. 24:532018. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Fathi ZH, Mohammad JA, Younus ZM and
Mahmood SM: Hepcidin as a potential biomarker for the diagnosis of
Anemia. Turk J Pharm Sci. 19:603–609. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Michalak SS, Olewicz-Gawlik A,
Rupa-Matysek J, Wolny-Rokicka E, Nowakowska E and Gil L: Autoimmune
hemolytic anemia: Current knowledge and perspectives. Immun Ageing.
17:382020. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Saah E, Fadaei P, Gurkan UA and Sheehan V:
Sickle cell disease pathophysiology and related molecular and
biophysical biomarkers. Hematol Oncol Clin North Am. 36:1077–1095.
2022. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Kalpatthi R and Novelli EM: Measuring
success: Utility of biomarkers in sickle cell disease clinical
trials and care. Hematology Am Soc Hematol Educ Program.
2018:482–492. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Silva-Junior AL, Garcia NP, Cardoso EC,
Dias S, Tarragô AM, Fraiji NA, Gomes MS, Amaral LR,
Teixeira-Carvalho A, Martins-Filho OA, et al: Immunological
hallmarks of inflammatory status in Vaso-Occlusive crisis of sickle
cell Anemia patients. Front Immunol. 12:5599252021. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Vlachou M, Kamperidis V, Giannakoulas G,
Karamitsos T, Vlachaki E and Karvounis H: Biochemical and imaging
markers in patients with thalassaemia. Hellenic J Cardiol. 62:4–12.
2021. View Article : Google Scholar
|
|
41
|
Botta A, Forest A, Daneault C, Pantopoulos
K, Tantiworawit A, Phrommintikul A, Chattipakorn S, Chattipakorn N,
Des Rosiers C, Sweeney G, et al: Identification of circulating
Endocan-1 and ether phospholipids as biomarkers for complications
in thalassemia patients. Metabolites. 11:702021. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Li N, An P, Wang J, Zhang T, Qing X, Wu B,
Sun L, Ding X, Niu L, Xie Z, et al: Plasma proteome profiling
combined with clinical and genetic features reveals the
pathophysiological characteristics of β-thalassemia. iScience.
25:1040912022. View Article : Google Scholar
|
|
43
|
Caprari P, Profumo E, Massimi S, Buttari
B, Riganò R, Regine V, Gabbianelli M, Rossi S, Risoluti R,
Materazzi S, et al: Hemorheological profiles and chronic
inflammation markers in transfusion-dependent and
non-transfusion-dependent thalassemia. Front Mol Biosci.
9:11088962023. View Article : Google Scholar
|
|
44
|
Kelkka T, Tyster M, Lundgren S, Feng X,
Kerr C, Hosokawa K, Huuhtanen J, Keränen M, Patel B, Kawakami T, et
al: Anti-COX-2 autoantibody is a novel biomarker of immune aplastic
anemia. Leukemia. 36:2317–2327. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Liu Q, Dong H, Li Y, Shen Y, Hong Y, Chen
Y, Liu S, Wu X, Liu W, Hu H, et al: Apolipoprotein-a is a potential
prognosis biomarker for severe aplastic Anemia patients treated
with ATG-based immunosuppressive therapy: A Single-center
retrospective study. Blood. 140(Suppl 1): S11043–S11044. 2022.
View Article : Google Scholar
|
|
46
|
Adhikari S, Nayek K, Bandyopadhyay A and
Mandal P: Implication of therapeutic outcomes associated with
molecular characterization of paediatric aplastic anaemia. Biochem
Biophys Rep. 25:1008992021.PubMed/NCBI
|
|
47
|
Gurnari C, Pagliuca S, Prata PH, Galimard
JE, Catto LFB, Larcher L, Sebert M, Allain V, Patel BJ, Durmaz A,
et al: Clinical and molecular determinants of clonal evolution in
aplastic anemia and paroxysmal nocturnal hemoglobinuria. J Clin
Oncol. 41:132–142. 2023. View Article : Google Scholar :
|
|
48
|
Da Costa L, O'Donohue MF, van Dooijeweert
B, Albrecht K, Unal S, Ramenghi U, Leblanc T, Dianzani I, Tamary H,
Bartels M, et al: Molecular approaches to diagnose Diamond-Blackfan
Anemia: The EuroDBA experience. Eur J Med Genet. 61:664–673. 2018.
View Article : Google Scholar
|
|
49
|
Khan A, Ali A, Junaid M, Liu C, Kaushik
AC, Cho WCS and Wei DQ: Identification of novel drug targets for
Diamond-Blackfan Anemia based on RPS19 gene mutation using
protein-protein interaction network. BMC Syst Biol. 12(Suppl 4):
S392018. View Article : Google Scholar
|
|
50
|
Sieff C: Diamond-Blackfan Anemia.
GeneReviews®; Seattle, WA: 2023
|
|
51
|
Moreno OM, Paredes AC, Suarez-Obando F and
Rojas A: An update on Fanconi anemia: Clinical, cytogenetic and
molecular approaches (Review). Biomed Rep. 15:742021. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Miele E, Mastronuzzi A, Po A, Carai A,
Alfano V, Serra A, Colafati GS, Strocchio L, Antonelli M,
Buttarelli FR, et al: Characterization of medulloblastoma in
Fanconi Anemia: A novel mutation in the BRCA2 gene and SHH
molecular subgroup. Biomark Res. 3:132015. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Hoving V, Nijssen LE, Donker AE, Roelofs
R, Schols SEM and Swinkels DW: Erythropoiesis-Hepcidin-Iron axis in
patients with X-linked sideroblastic anaemia: An explorative
biomarker study. Br J Haematol. 202:1216–1219. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Wishart DS, Bartok B, Oler E, Liang KYH,
Budinski Z, Berjanskii M, Guo A, Cao X and Wilson M: MarkerDB: An
online database of molecular biomarkers. Nucleic Acids Res.
49:D1259–D1267. 2021. View Article : Google Scholar :
|
|
55
|
Hindmarsh JT, Oliveras L and Greenway DC:
Biochemical differentiation of the porphyrias. Clin Biochem.
32:609–619. 1999. View Article : Google Scholar
|
|
56
|
Zuijderhoudt FM, Koehorst SG, Kluitenberg
WE and Dorresteijn-de Bok J: On accuracy and precision of a HPLC
method for measurement of urine porphyrin concentrations. Clin Chem
Lab Med. 38:227–230. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
van der Dijs FP, Schnog JJ, Brouwer DA,
Velvis HJ, van den Berg GA, Bakker AJ, Duits AJ, Muskiet FD and
Muskiet FA: Elevated homocysteine levels indicate suboptimal folate
status in pediatric sickle cell patients. Am J Hematol. 59:192–198.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Nelson MC, Zemel BS, Kawchak DA, Barden
EM, Frongillo EA Jr, Coburn SP, Ohene-Frempong K and Stallings VA:
Vitamin B6 status of children with sickle cell disease. J Pediatr
Hematol Oncol. 24:463–469. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
De Luca C, Filosa A, Grandinetti M, Maggio
F, Lamba M and Passi S: Blood antioxidant status and urinary levels
of catecholamine metabolites in β-thalassemia. Free Radic Res.
30:453–462. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Iranpour R, Akbar MR and Haghshenas I:
Glucose-6-phosphate dehydrogenase deficiency in neonates. Indian J
Pediatr. 70:855–857. 2003. View Article : Google Scholar
|
|
61
|
Kwon JM, Cho Y, Jeon KH, Cho S, Kim KH,
Baek SD, Jeung S, Park J and Oh BH: A deep learning algorithm to
detect anaemia with ECGs: A retrospective, multicentre study.
Lancet Digit Health. 2:e358–e367. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Rustam F, Ashraf I, Jabbar S, Tutusaus K,
Mazas C, Barrera AEP and de la Torre Diez I: Prediction of
[Formula: See text]-Thalassemia carriers using complete blood count
features. Sci Rep. 12:199992022. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Farooq M and Ali Younas H: Beta
thalassemia carriers detection empowered federated Learning. 2023,
Available from: https://doi.org/10.48550/arXiv.2306.01818.
|
|
64
|
Saputra DCE, Sunat K and Ratnaningsih T: A
new artificial intelligence approach using extreme learning machine
as the potentially effective model to predict and analyze the
diagnosis of Anemia. Healthcare (Basel). 11:6972023. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Xiang P, Wu X, Zeng Z, Lin Z, Guo Y, Ma X,
Lin J and Wang W: Quantitative analysis of pelvic bone marrow fat
using an MRI-based machine learning method for distinguishing
aplastic anaemia from myelodysplastic syndromes. Clin Radiol.
78:e463–e468. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Chang L, Yan M, Zhang J, Binghang L, Guo
Y, Wan Y, Yi M, Lan Y, Cai Y, Feng J, et al: Long-term outcome
prediction after immunosuppressive therapy for severe aplastic
Anemia in childhood by machine learning methods. SSRN Electronic J.
2020, Available from: https://ssrn.com/abstract=3582734 or http://dx.doi.org/10.2139/ssrn.3582734.
|
|
67
|
Uçucu S, Karabıyık T and AzikF M: Machine
learning models can predict the presence of variants in hemoglobin:
Artificial neural network-based recognition of human hemoglobin
variants by HPLC. Turkish J Biochemistry. 485–411. 2023.
|
|
68
|
Mo D, Zheng Q, Xiao B and Li L: Predicting
thalassemia using deep neural network based on red blood cell
indices. Clin Chim Acta. 543:1173292023. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Wang M, Dong C, Gao Y, Li J, Han M and
Wang L: A deep learning model for the automatic recognition of
aplastic Anemia, myelodysplastic syndromes, and acute myeloid
leukemia based on bone marrow smear. Front Oncol. 12:8449782022.
View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Appiahene P, Asare JW, Donkoh ET, Dimauro
G and Maglietta R: Detection of iron deficiency Anemia by medical
images: A comparative study of machine learning algorithms. BioData
Min. 16:22023. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Zhang A, Lou J, Pan Z, Luo J, Zhang X,
Zhang H, Li J, Wang L, Cui X, Ji B and Chen L: Prediction of Anemia
using facial images and deep learning technology in the emergency
department. Front Public Health. 10:9643852022. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Aparna V, Sarath TV and Ramachandran KI:
Simulation model for anemia detection using RBC counting algorithms
and Watershed transform. In: 2017 International Conference on
Intelligent Computing Instrumentation and Control Technologies
(ICICICT); IEEE; pp. 284–291. 2017
|
|
73
|
De S and Chakraborty B: Case-based
reasoning (CBR)-based Anemia Severity Detection System (ASDS) using
machine learning algorithm. Advanced Machine Learning Technologies
and Applications. 621–632. 2021. View Article : Google Scholar
|
|
74
|
Mitani A, Huang A, Venugopalan S, Corrado
GS, Peng L, Webster DR, Hammel N, Liu Y and Varadarajan AV:
Detection of anaemia from retinal fundus images via deep learning.
Nat Biomed Eng. 4:18–27. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Amruth A, Ramanan R, Rhea P, Vishal S and
Saravanan S: Big Data Application in Cancer Classification by
Analysis of RNA-seq Gene Expression. In: 2023 3rd International
Conference on Intelligent Technologies (CONIT); IEEE; pp. 1–6.
2023
|
|
76
|
Manoharan S and Iyyappan OR: A hybrid
protocol for finding novel gene targets for various diseases using
microarray expression data analysis and text mining. Methods Mol
Biol. 2496:41–70. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Iyyappan OR and Manoharan S: Finding gene
associations by text mining and annotating it with gene ontology.
Methods Mol Biol. 2496:71–90. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Perveen G, Alturise F, Alkhalifah T and
Daanial Khan Y: Hemolytic-Pred: A machine learning-based predictor
for hemolytic proteins using position and composition-based
features. Digit Health. 9:205520762311807392023. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Mansour-Hendili L, Aissat A, Badaoui B,
Sakka M, Gameiro C, Ortonne V, Wagner-Ballon O, Pissard S, Picard
V, Ghazal K, et al: Exome sequencing for diagnosis of congenital
hemolytic anemia. Orphanet J Rare Dis. 15:1802020. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Fermo E, Vercellati C, Marcello AP, Keskin
EY, Perrotta S, Zaninoni A, Brancaleoni V, Zanella A, Giannotta JA,
Barcellini W and Bianchi P: Targeted next generation sequencing and
diagnosis of congenital hemolytic anemias: A three years experience
monocentric study. Front Physiol. 12:6845692021. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Jamwal M, Aggarwal A, Palodhi A, Sharma P,
Bansal D, Trehan A, Malhotra P, Maitra A and Das R: Next-generation
sequencing-based diagnosis of unexplained inherited hemolytic
anemias reveals wide genetic and phenotypic heterogeneity. J Mol
Diagn. 22:579–590. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Pagliuca S, Gurnari C, Hercus C, Hergalant
S, Nadarajah N, Wahida A, Terkawi L, Mori M, Zhou W, Visconte V, et
al: Molecular landscape of immune pressure and escape in aplastic
anemia. Leukemia. 37:202–211. 2023. View Article : Google Scholar :
|
|
83
|
Hou H, Li D, Yao YH, Lu J, Sun YN, Hu YX,
Wu SY, Chu XR, Xiao PF, Xu GQ and Hu SY: Proteomic analysis for
identifying the differences in molecular profiling between fanconi
anaemia and aplastic anaemia. Am J Transl Res. 11:6522–6533.
2019.PubMed/NCBI
|
|
84
|
Mehta S, Medicherla KM, Gulati S, Sharma
N, Gupta S, Parveen R, Mishra AK and Suravajhala P: Whole Exome
Sequencing of Aplastic Anemia Patients Specific to India Reveals
Unique Mutations. 2021, Available at SSRN: https://ssrn.com/abstract=4001799 or http://dx.doi.org/10.2139/ssrn.4001799.
|
|
85
|
Wang B, Wang C, Wan Y, Gao J, Ma Y, Zhang
Y, Tong J, Zhang Y, Liu J, Chang L, et al: Decoding the
pathogenesis of Diamond-Blackfan anemia using single-cell RNA-seq.
Cell Discov. 8:412022. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Bartels M and Bierings M: How I manage
children with Diamond-Blackfan anaemia. Br J Haematol. 184:123–133.
2019. View Article : Google Scholar
|
|
87
|
Judy J, Wang X, Seifuddin F, Tumburu L,
Pirooznia M and Thein SL: RNA Seq profiles and bioinformatics
validation in a large sample of sickle cell disease patients.
Blood. 136(Suppl 1): S13–S14. 2020. View Article : Google Scholar
|
|
88
|
Ben Hamda C, Sangeda R, Mwita L, Meintjes
A, Nkya S, Panji S, Mulder N, Guizani-Tabbane L, Benkahla A, Makani
J, et al: A common molecular signature of patients with sickle cell
disease revealed by microarray meta-analysis and a genome-wide
association study. PLoS One. 13:e01994612018. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Mwita LA, Mawalla WF, Mtiiye FR, Kandonga
D, Kent J, Makani J and Sangeda RZ: Infrastructure for
bioinformatics applications in Tanzania: Lessons from the sickle
cell programme. PLoS Comput Biol. 19:e10108482023. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Kalaigar SS, Rajashekar RB, Nataraj SM,
Vishwanath P and Prashant A: Bioinformatic tools for the
identification of MicroRNAs regulating the transcription factors in
patients with β-thalassemia. Bioinform Biol Insights.
16:117793222211155362022. View Article : Google Scholar
|
|
91
|
Chowdhury A and Sruthi VS: A
bioinformatics approach for the treatment of thalassemia using
molecular docking. Biological Forum An Int J. 13:332–338. 2021.
|
|
92
|
Hameed AR, Fakhri Ali S, N Almanaa T,
Aljasir MA, Alruwetei AM, Sanami S, Ayaz H, Ali I, Ahmad F and
Ahmad S: Exploring the hub genes and potential drugs involved in
Fanconi anemia using microarray datasets and bioinformatics
analysis. J Biomol Struct Dyn. Dec 27–2023.Epub ahead of print.
View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Reyes P, García-de Teresa B, Juárez U,
Pérez-Villatoro F, Fiesco-Roa MO, Rodríguez A, Molina B,
Villarreal-Molina MT, Meléndez-Zajgla J, Carnevale A, et al:
Fanconi Anemia patients from an indigenous community in Mexico
Carry a new founder pathogenic variant in FANCG. Int J Mol Sci.
23:23342022. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Fang R, Zhang J, Yang H, Shi J, Zeng H,
Zhu X, Wei D, Yuan P, Cheng T and Zhang Y: Highly efficient gene
editing and single cell analysis of hematopoietic stem/progenitor
cells from X-linked sideroblastic anemia patients. Signal Transduct
Target Ther. 6:2482021. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
An W, Zhang J, Chang L, Zhang Y, Wan Y,
Ren Y, Niu D, Wu J, Zhu X and Guo Y: Mutation analysis of Chinese
sporadic congenital sideroblastic anemia by targeted capture
sequencing. J Hematol Oncol. 8:552015. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Hosokawa K, Kajigaya S, Keyvanfar K, Qiao
W, Xie Y, Townsley DM, Feng X and Young NS: T cell transcriptomes
from paroxysmal nocturnal hemoglobinuria patients reveal novel
signaling pathways. J Immunol. 199:477–488. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Zoumbos NC, Gascón P, Djeu JY, Trost SR
and Young NS: Circulating activated suppressor T lymphocytes in
aplastic anemia. N Engl J Med. 312:257–265. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Deng XZ, Du M, Peng J, Long JX, Zheng CJ,
Tan Y, Li LJ, Chen HY, Qing C, Pang YY, et al: Associations between
the HLA-A/B/DRB1 polymorphisms and aplastic anemia: Evidence from
17 case-control studies. Hematology. 23:154–162. 2018. View Article : Google Scholar
|
|
99
|
Bo L, Mei-Ying L, Yang Z, Shan-Mi W and
Xiao-Hong Z: Aplastic anemia associated with pregnancy: Maternal
and fetal complications. J Matern Fetal Neonatal Med. 29:1120–1124.
2016. View Article : Google Scholar
|
|
100
|
Kordasti S, Costantini B, Seidl T, Perez
Abellan P, Martinez Llordella M, McLornan D, Diggins KE,
Kulasekararaj A, Benfatto C, Feng X, et al: Deep phenotyping of
Tregs identifies an immune signature for idiopathic aplastic anemia
and predicts response to treatment. Blood. 128:1193–1205. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Lee NCJ, Patel B, Etra A, Bat T, Ibrahim
IF, Vusirikala M, Chen M, Rosado F, Jaso JM, Young NS and Chen W:
SARS-CoV-2 infection associated with aplastic anemia and pure red
cell aplasia. Blood Adv. 6:3840–3843. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Ahamed M, Akhtar MJ, Verma S, Kumar A and
Siddiqui MK: Environmental lead exposure as a risk for childhood
aplastic anemia. Biosci Trends. 5:38–43. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Adhikari S and Mandal P: Integrated
analysis of global gene and microRNA expression profiling
associated with aplastic anaemia. Life Sci. 228:47–52. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Putkowski S: The national organization for
rare disorders (NORD). NASN School Nurse. 25:38–41. 2010.
View Article : Google Scholar
|
|
105
|
Sinha S, Chatterjee SS, Biswas M, Nag A,
Banerjee D, De R and Sengupta A: SWI/SNF subunit expression
heterogeneity in human aplastic anemia stem/progenitors. Exp
Hematol. 62:39–44.e2. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Khanna-Gupta A, Sarvepalli S, Majumder S,
Karunakaran C, Manoharan M, Prabhu S, Bafna V, Murugan S, Bose C,
Gupta R, et al: Mutations in the telomerase complex and expression
levels of the TERT gene determine severity and outcome of disease
in aplastic anemia patients. Blood. 128:1502. 2016. View Article : Google Scholar
|
|
107
|
Medinger M, Drexler B, Lengerke C and
Passweg J: Pathogenesis of acquired aplastic anemia and the role of
the bone marrow microenvironment. Front Oncol. 8:5872018.
View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Stelzer G, Rosen N, Plaschkes I, Zimmerman
S, Twik M, Fishilevich S, Stein TI, Nudel R, Lieder I, Mazor Y, et
al: The GeneCards Suite: From gene data mining to disease genome
sequence analyses. Curr Protoc Bioinformatics. 54:1.30.1–1.30.33.
2016.PubMed/NCBI
|
|
109
|
Johns Hopkins University and Baltimore M:
McKusick-Nathans Institute of Genetic Medicine OMIM®
-Online Mendelian Inheritance in Man. World Wide Web. URL:
https://omim.org/.
|
|
110
|
Yamaguchi H, Calado RT, Ly H, Kajigaya S,
Baerlocher GM, Chanock SJ, Lansdorp PM and Young NS: Mutations in
TERT, the gene for telomerase reverse transcriptase, in aplastic
anemia. N Engl J Med. 352:1413–1424. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Adhikari S and Mandal P: Novel role of AGT
gene in aplastic anaemia among paediatric patients based on gene
expression profiling. bioRxiv: May 29, 2020 (Epub ahead of print).
View Article : Google Scholar
|
|
112
|
Zhou Y, Zhang L, Song S, Xu L, Yan Y, Wu
H, Tong X and Yan H: Elevated GAS2L3 expression correlates with
poor prognosis in patients with Glioma: A study based on
bioinformatics and immunohistochemical analysis. Front Genet.
12:6492702021. View Article : Google Scholar : PubMed/NCBI
|
|
113
|
Darb-Esfahani S, Kronenwett R, von
Minckwitz G, Denkert C, Gehrmann M, Rody A, Budczies J, Brase JC,
Mehta MK, Bojar H, et al: Thymosin beta 15A (TMSB15A) is a
predictor of chemotherapy response in triple-negative breast
cancer. Br J Cancer. 107:1892–1900. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
114
|
Shukla S, Tripathi AK, Verma SP, Yadav DK,
Tripathi RK, Maurya S and Awasthi N: Association of
Interleukin-1β-31C/T, -511T/C and -3954C/T single nucleotide
polymorphism and their blood plasma level in acquired aplastic
anemia. Indian J Hematol Blood Transfus. 37:210–219. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
115
|
Cotoraci C, Ciceu A, Sasu A and Hermenean
A: Natural antioxidants in anemia treatment. Int J Mol Sci.
22:18832021. View Article : Google Scholar : PubMed/NCBI
|
|
116
|
Babushok DV, Li Y, Roth JJ, Perdigones N,
Cockroft JD, Biegel JA, Mason PJ and Bessler M: Common polymorphic
deletion of glutathione S-transferase theta predisposes to acquired
aplastic anemia: Independent cohort and meta-analysis of 609
patients. Am J Hematol. 88:862–867. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
117
|
Li J and Bledsoe JR: Inherited bone marrow
failure syndromes and germline predisposition to myeloid neoplasia:
A practical approach for the pathologist. Semin Diagn Pathol.
40:429–442. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
118
|
Chatterjee R and Law S: Epigenetic and
microenvironmental alterations in bone marrow associated with ROS
in experimental aplastic anemia. Eur J Cell Biol. 97:32–43. 2018.
View Article : Google Scholar
|
|
119
|
Ighodaro OM and Akinloye OA: First line
defence antioxidants-superoxide dismutase (SOD), catalase (CAT) and
glutathione peroxidase (GPX): Their fundamental role in the entire
antioxidant defence grid. Alexandria J Med. 54:287–293. 2018.
View Article : Google Scholar
|
|
120
|
Wang S, Song R, Wang Z, Jing Z, Wang S and
Ma J: S100A8/A9 in inflammation. Front Immunol. 9:12982018.
View Article : Google Scholar : PubMed/NCBI
|
|
121
|
Giudice V, Wu Z, Kajigaya S, Fernandez
Ibanez MDP, Rios O, Cheung F, Ito S and Young NS: Circulating
S100A8 and S100A9 protein levels in plasma of patients with
acquired aplastic anemia and myelodysplastic syndromes. Cytokine.
113:462–465. 2019. View Article : Google Scholar :
|
|
122
|
Lundgren S, Keränen MAI, Kankainen M,
Huuhtanen J, Walldin G, Kerr CM, Clemente M, Ebeling F, Rajala H,
Brück O, et al: Somatic mutations in lymphocytes in patients with
immune-mediated aplastic anemia. Leukemia. 35:1365–1379. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
123
|
Yu Z, Chen C, Xiao Y, Chen X, Guo L, Tan
G, Huang G, Luo W, Zhou M, Li Y, et al: Abnormal miR-214/A20
expression might play a role in T cell activation in patients with
aplastic anemia. Blood Sci. 2:100–105. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
124
|
Ganapathi KA, Townsley DM, Hsu AP, Arthur
DC, Zerbe CS, Cuellar-Rodriguez J, Hickstein DD, Rosenzweig SD,
Braylan RC, Young NS, et al: GATA2 deficiency-associated bone
marrow disorder differs from idiopathic aplastic anemia. Blood.
125:56–70. 2015. View Article : Google Scholar :
|
|
125
|
You X, Yang Q, Yan K, Wang SR, Huang RR,
Wang SQ, Gao CY, Li L and Lian ZX: Multi-Omics profiling identifies
pathways associated with CD8+ T-Cell activation in severe aplastic
anemia. Front Genet. 12:7909902022. View Article : Google Scholar :
|
|
126
|
Zaimoku Y, Patel BA, Kajigaya S, Feng X,
Alemu L, Quinones Raffo D, Groarke EM and Young NS: Deficit of
circulating CD19+ CD24hi CD38hi regulatory B cells in severe
aplastic anaemia. Br J Haematol. 190:610–617. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
127
|
Fattizzo B, Giannotta JA and Barcellini W:
Mesenchymal stem cells in aplastic anemia and myelodysplastic
syndromes: The 'Seed and Soil' Crosstalk. Int J Mol Sci.
21:54382020. View Article : Google Scholar
|
|
128
|
Wang XA, Li JP, Wu KH, Yang SF and Chao
YH: Mesenchymal stem cells in acquired aplastic anemia: The
spectrum from basic to clinical utility. Int J Mol Sci.
24:44642023. View Article : Google Scholar : PubMed/NCBI
|
|
129
|
Broglie L, Margolis D and Medin JA: Yin
and Yang of mesenchymal stem cells and aplastic anemia. World J
Stem Cells. 9:219–226. 2017. View Article : Google Scholar
|
|
130
|
Wu D, Wen X, Liu W, Hu H, Ye B and Zhou Y:
Comparison of the effects of deferasirox, deferoxamine, and
combination of deferasirox and deferoxamine on an aplastic anemia
mouse model complicated with iron overload. Drug Des Devel Ther.
12:1081–1091. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
131
|
Lan Y, Liu F, Chang L, Liu L, Zhang Y, Yi
M, Cai Y, Feng J, Han Z, Han Z and Zhu X: Combination of umbilical
cord mesenchymal stem cells and standard immunosuppressive regimen
for pediatric patients with severe aplastic anemia. BMC Pediatr.
21:1022021. View Article : Google Scholar : PubMed/NCBI
|
|
132
|
Atere AD, Oseni BSA, Agbona TO, Idomeh FA,
Akinbo DB and Osadolor HB: Free radicals inhibit the haematopoietic
elements and antioxidant agents of rats exposed to pyrethroids
insecticides. J Exp Res. 7:66–74. 2019.
|
|
133
|
Babushok DV: When does a PNH clone have
clinical significance? Hematology Am Soc Hematol Educ Program.
2021:143–152. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
134
|
Cecchi N, Giannotta JA, Barcellini W and
Fattizzo B: A case of severe aplastic anaemia after SARS-CoV-2
vaccination. Br J Haematol. 196:1334–1336. 2022. View Article : Google Scholar
|
|
135
|
Ahmed P, Chaudhry QUN, Satti TM, Mahmood
SK, Ghafoor T, Shahbaz N, Khan MA, Satti HS, Akram Z, Iftikhar R,
et al: Epidemiology of aplastic anemia: A study of 1324 cases.
Hematology. 25:48–54. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
136
|
Contejean A, Resche-Rigon M, Tamburini J,
Alcantara M, Jardin F, Lengliné E, Adès L, Bouscary D, Marçais A,
Lebon D, et al: Aplastic anemia in the elderly: A nationwide survey
on behalf of the french reference center for aplastic anemia.
Haematologica. 104:256–262. 2019. View Article : Google Scholar :
|
|
137
|
Durrani J and Maciejewski JP: Idiopathic
aplastic anemia vs hypocellular myelodysplastic syndrome.
Hematology. 2019:97–104. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
138
|
Barrett J, Saunthararajah Y and Molldrem
J: Myelodysplastic syndrome and aplastic anemia: Distinct entities
or diseases linked by a common pathophysiology? Semin Hematol.
37:15–29. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
139
|
Sun L and Babushok DV: Secondary
myelodysplastic syndrome and leukemia in acquired aplastic anemia
and paroxysmal nocturnal hemoglobinuria. Blood. 136:36–49. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
140
|
Ogawa S: Clonal hematopoiesis in acquired
aplastic anemia. Blood. 128:337–347. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
141
|
Revy P and Touzot F: The immunologic
complications and genetic origins of telomere disorders.
Encyclopedia of Immunobiology. Elsevier; pp. 451–457. 2016,
View Article : Google Scholar
|
|
142
|
Barcellini W, Fattizzo B and Cortelezzi A:
Autoimmune hemolytic anemia, autoimmune neutropenia and aplastic
anemia in the elderly. Eur J Intern Med. 58:77–83. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
143
|
Townsley DM, Dumitriu B and Young NS: Bone
marrow failure and the telomeropathies. Blood. 124:2775–2783. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
144
|
Chen CY, Chen TT, Hsieh CY, Lien MY, Yeh
SP and Chen CC: Case reports of management of aplastic anemia after
COVID-19 vaccination: A single institute experience in Taiwan. Int
J Hematol. 117:149–152. 2023. View Article : Google Scholar
|
|
145
|
Kmira Z, Sabrine K, Monia G, Imen A, Dorra
C, Rania B, Neila F, Walid B, Monia Z, Yosra BY, et al: A case of
acquired aplastic Anemia after severe Hepatitis-probably induced by
the Pfizer/BioNTech vaccine: A case report and review of
literature. Vaccines (Basel). 11:12282023. View Article : Google Scholar
|
|
146
|
Sridhara S, Nair R and Stanek M: Severe
aplastic Anemia after receiving SARS-CoV-2 moderna mRNA
vaccination. J Hematol. 11:34–39. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
147
|
Tabata S, Hosoi H, Murata S, Takeda S,
Mushino T and Sonoki T: Severe aplastic anemia after COVID-19 mRNA
vaccination: Causality or coincidence? J Autoimmun. 126:1027822022.
View Article : Google Scholar
|
|
148
|
Park AK, Waheed A, Forst DA and Al-Samkari
H: Characterization and prognosis of temozolomide-induced aplastic
anemia in patients with central nervous system malignancies. Neuro
Oncol. 24:964–973. 2022. View Article : Google Scholar :
|
|
149
|
Ata F, Akkam Veettil SF, Gaber M, Omar NE,
Madani A, Mah Afifi H, Aldardouri MM, Amer A, Kohla S and Zar Gul
AR: Fatal temozolomide induced aplastic anemia in a female with
Glioblastoma multiforme : A case report and literature review. Clin
Case Rep. 9:1641–1646. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
150
|
Younan RG, Raad RA, Sawan BY and Said R:
Aplastic anemia secondary to dual cancer immunotherapies a
physician nightmare: Case report and literature review. Allergy
Asthma Clin Immunol. 17:1122021. View Article : Google Scholar : PubMed/NCBI
|
|
151
|
Hou JW and Wang TR: Differentiation of
Fanconi anemia from aplastic anemia by chromosomal breakage test.
Zhonghua Min Guo Xiao Er Ke Yi Xue Hui Za Zhi. 38:121–126.
1997.PubMed/NCBI
|
|
152
|
Green AM and Kupfer GM: Fanconi Anemia.
Hematol Oncol Clin North Am. 23:193–214. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
153
|
Ayas M, Siddiqui K, Al-Jefri A, Al-Ahmari
A, Ghemlas I, Al-Saedi H, Al-Anazi A, Khan S, El-Solh H and
Al-Seraihi A: Does mixed chimerism after allogeneic hematopoietic
cell transplantation in pediatric patients with fanconi anemia
impact on outcome? Transplant Cell Ther. 27:257.e1–257.e6. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
154
|
Cincinnati Children's: Fanconi Anemia.
https://www.cincinnatichildrens.org/health/f/fanconi-anemia.
Accessed December 22, 2022
|
|
155
|
Zhao XC, Zhao L, Sun XY, Xu ZS, Ju B, Meng
FJ and Zhao HG: Excellent response of severe aplastic anemia to
treatment of gut inflammation: A case report and review of the
literature. World J Clin Cases. 8:425–435. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
156
|
Zhao XC, Xue CJ, Song H, Gao BH, Han FS
and Xiao SX: Bowel inflammatory presentations on computed
tomography in adult patients with severe aplastic anemia during
flared inflammatory episodes. World J Clin Cases. 11:576–597. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
157
|
Shao Y, Qi W, Zhang X, Ran N, Liu C, Fu R
and Shao Z: Plasma metabolomic and intestinal microbial analyses of
patients with severe aplastic anemia. Front Cell Dev Biol.
9:6698872021. View Article : Google Scholar : PubMed/NCBI
|
|
158
|
Taderegew MM, Gebremariam T, Tareke AA and
Woldeamanuel GG: Anemia and its associated factors among type 2
diabetes mellitus patients attending debre berhan referral
hospital, North-East Ethiopia: A Cross-Sectional study. J Blood
Med. 11:47–58. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
159
|
Bohlius J, Bohlke K, Castelli R,
Djulbegovic B, Lustberg MB, Martino M, Mountzios G, Peswani N,
Porter L, Tanaka TN, et al: Management of Cancer-associated anemia
with erythropoiesis-stimulating agents: ASCO/ASH clinical practice
guideline update. J Clin Oncol. 37:1336–1351. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
160
|
Boennelykke A, Jensen H, Falborg AZ,
Granfeldt Østgård LS, Hansen AT, Christensen KS and Vedsted P:
Diagnostic workup of cancer in patients with new-onset anaemia: A
Danish cohort study in general practice. Scand J Prim Health Care.
39:391–402. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
161
|
van Vliet NA, Kamphuis AEP, den Elzen WPJ,
Blauw GJ, Gussekloo J, Noordam R and van Heemst D: Thyroid function
and risk of anemia: A Multivariable-adjusted and mendelian
randomization analysis in the UK Biobank. J Clin Endocrinol Metab.
107:e643–e652. 2022. View Article : Google Scholar :
|