Introduction
Uveal melanoma (UM) is the most prevalent primary intraocular type of cancer, accounting for up to 5% of all melanomas (1). Metastasis of UM develops in half the patients especially to the liver, with a historical median overall survival of approximately 1 year (2,3). Based on the genetic background of UM, in addition to classic chemotherapy, several adjuvant therapies have been developed to prevent UM metastasis and improve the survival of patients, such as bevacizumab, imatinib and mitogen-activated protein kinase kinase inhibitors such as tolametinib (4). Natural extracts, such as cucurbitacin B, salidroside and andrographis, exert anti-tumor activity against several types of cancer, including non-small cell lung (5), gastric (6) and colorectal cancer (7).
Piceatannol (PIC) is a phenolic compound extracted from grapes, passion fruit, white tea and Asian legume (8). As a homolog of resveratrol, PIC exhibits various biological activities, such as attenuation of atopic dermatitis (9), promotion of autophagy (10), induction of apoptosis (11) and vasorelaxant activity (12). In addition, PIC possesses potent anticancer properties. The molecular mechanisms involved in the anti-tumor effects of PIC include suppressed cell proliferation, increased reactive oxygen species (ROS) levels, enhanced inflammation and induction of autophagy. Previous in vitro and in vivo studies also suggested that PIC can promote tumor regression, cell apoptosis, invasion and migration and metastasis (13-15). For example, a previous study showed that the viability of breast cancer MCF-7 cells is inhibited following treatment with PIC for 48 h (13). Additionally, PIC decreases the activity of the antioxidant enzymes catalase (CAT) and superoxide dismutase (SOD) (13). Kwon et al (15) demonstrated that PIC decreases IL-6 signaling and inhibits the migration and invasion of prostate cancer cells. PIC also enhances beclin-1 activity and induces autophagy in gastric cancer cells, thus suppressing tumor progression (14). Although the anti-tumor effects of PIC have been well-documented (13-15), to the best of our knowledge, no studies have focused on the therapeutic effects and underlying mechanism of PIC on human UM.
The present study aimed to uncover the anti-cancer mechanisms underlying the effects of PIC on human UM cell lines C918 and Mum-2b and elucidate the promoting effects of PIC on oxidative stress, inflammation and pyroptosis both in vitro and in vivo.
Materials and methods
Cell culture and treatment
UM cell line C918 (cat. no. CL-0264), was purchased from Procell Life Science & Technology Co., Ltd. Mum-2b (cat. no. CTCC-003-0091) UM cells were obtained from Chinese Tissue Culture Collection. Cells were cultured in RPMI-1640 supplemented with 1% penicillin/streptomycin solution (cat. no. 15140122) and 10% FBS (cat. no. A3161001C; both Thermo Fisher Scientific, Inc.) in 5% CO2 at 37°C. PIC (cat. no. HY-13518) and COG1410 (COG; cat. no. HY-P2136) were obtained from MedChemExpress and dissolved in DMSO. AC-DEVD-CHO (cat. no. HY-P1001), an inhibitor of caspase 3, was obtained from MedChemExpress (MCE) and dissolved in H2O. C918 cells were treated with PIC (0, 40 or 80 μM) for 0, 24 h, 48 h or 72 h at 37°C. C918 cells were also treated with 25 μM COG or 10 μM AC-DEVD-CHO for 72 h at 37°C and collected for further experiments.
Establishment of UM mouse model and treatment
A total of 18 BALB/c-nu male mice (age, 4-6 weeks; weight, 15-20 g) were obtained from Chengdu Yaokang Biotechnology Co., Ltd. The animals were provided ad libitum access to water and housed at 20-25°C; humidity, 40-60%; 12-h light/dark cycle). A total of 3×106 C918 cells in 200 μl PBS were subcutaneously inoculated into the flanks of mice. Mice were allocated into three groups (NC, 30, 60; n=6/group) treated with 0, 30 and 60 mg/kg PIC, respectively. Tumor volume was calculated as follows: Tumor volume=a2 × b × 0.5, where a and b indicate the minimum diameter and height respectively, measured by caliper every 3 days. On day 10 after inoculation, the mice were intraperitoneally injected with 0, 30 or 60 mg/kg PIC once/day. Following treatment for 2 weeks, mice were euthanized, followed by xenograft tumor dissection and weighing. According to the AVMA Guidelines for the Euthanasia of Animals (16), mice were euthanized via CO2 asphyxiation with a flow rate of 60% chamber volume/min and animal death was verified by respiratory cessation and loss of muscular tension.
Cell viability assay
UM cells were seeded into 96-well plates (5,000 cells/well) and incubated at 37°C in a humidified incubator with 5% CO2. Cells were treated with 0, 40 or 80 μM PIC for 0, 24, 48 and 72 h at 37°C and cell viability was assessed using Cell Counting Kit 8 (CCK-8) assay. The medium was replaced with fresh medium supplemented with a CCK-8 working solution (cat. no. HY-K0301; MedChemExpress) and cells were incubated for 1 h. The optical density (OD) at a wavelength of 450 nm was measured using a microplate reader. The cell viability was calculated using the following formula: Cell viability (%)=[OD (sample)-OD (blank)]/[OD (control)-OD (blank)] ×100%.
Caspase 3/9 activity assay
Following treatment with PIC as aforementioned, cells were collected and lysed using a lysis buffer (cat. nos. BC3830 and BC3890; Beijing Solarbio Science & Technology Co., Ltd.). Subsequently, lysate was centrifuged at 15,000 × g at 4°C for 30 min and protein concentration was measured using a BCA kit and then diluted into 1 mg/ml. DEVD-pNA or LEHD-pNA were added into the reaction system, followed by incubation at 37°C for 1 h. OD at a wavelength of 405 nm was measured using a preheated microplate reader and the activity of caspase 3/9 was calculated using the formula: Activity (%)=[OD (sample)-OD (blank)]/[OD (control)-OD (blank)] ×100%.
Cell migration assay
Cells were plated onto 6-well plates at a density of 1×105 cells/ml and cultured at 37°C for 24 h. Artificial wound tracks on the monolayers were created with a 200 μl sterile micropipette tip in FBS-free medium when cell density reached at least 80%. Cells were treated with PIC as aforementioned. Images of the migrated cells were captured under the Olympus IX73 Light microscope at 24 h intervals in ×100. The width of wounds was measured and analyzed using Image J v1.8.0 (National Institutes of Health).
ROS detection
To determine the levels of ROS in C918 cells, ROS detection kit (cat. no. CA1410; Beijing Solarbio Science & Technology Co., Ltd.) was used. Cells were pre-treated with 0, 40 or 80 μM PIC or COG for 72 h in 6-well plates at 37°C. DCFH-DA was diluted in FBS-free RPMI-1640 at a final concentration of 10 μM and used to treat cells for 20 min at 37°C. Following washing with FBS-free medium three times, cells were collected and analyzed using a fluorescence microplate reader at excitation and emission wavelengths of 488 and 525 nm, respectively.
Antioxidative enzymatic activity assay
The activities of the antioxidant enzymes SOD, glutathione-S-transferase (GST), glutathione reductase (GR), glutathione peroxidase (GPx) and CAT were assessed using commercially available kits (cat. nos. BC0175, BC0355, BC1165, BC1195 and BC0205, respectively; Beijing Solarbio Science & Technology Co., Ltd.). All procedures were performed according to the manufacturer's instructions. Briefly, following treatment with PIC for 72 h as aforementioned, C918 cells were collected and washed with ice-cold PBS. Subsequently, cells were lysed using buffer contained in the kits. The protein concentration was measured using a BCA kit and isolated proteins were diluted to 1 mg/ml. To determine SOD levels, the reaction system was supplemented with xanthine oxidase, xanthine and nitroblue tetrazolium and mixed, followed by incubation at 37°C for 30 min. OD value at a wavelength of 560 nm was measured using a preheated microplate reader. For GST, the reaction system was incubated with glutathione (GSH) and 1-chloro-2,4,dinitrobenzene for 10 sec and 5 min at 37°C, respectively. OD values were obtained at 340 nm. For GR, the reaction system was supplemented with NADPH and oxidized glutathione and incubated for 10 sec and 3 min at 37°C, respectively. OD values were measured at 340 nm. For GPx, H2O2 and GSH were added into the reaction system and incubated for 5 min at 37°C. Subsequently, the mixture was centrifuged at 1,700 g for 5 min at room temperature and the deposit was then removed. The suspension was incubated with 5,5′-dithiobis-(2-nitrobenzoic acid) at room temperature for 15 min. OD value at a wavelength of 412 nm was obtained using a microplate reader. For CAT assessment, the reaction system was treated with H2O2 for 1 min at 37°C and OD value was measured at 240 nm.
Lactate dehydrogenase (LDH) activity assay
LDH detection kit (cat. no. BC0685; Beijing Solarbio Science & Technology Co., Ltd.) was used to assess LDH levels in C918 cells according to the manufacturer's instructions. Briefly, cells were collected and lysed. Following centrifugation in 15,000 g at 4°C for 30 min, NAD+ and L-lactic acid were added to the supernatant and incubated at 37°C for 15 min. Subsequently, the reaction system was supplemented with 2,4-nitrophenylhydrazine and sodium hydroxide at room temperature for 3 min. Finally, OD was measured at a wavelength of 450 nm using a preheated microplate reader.
Hoechst and PI staining
Hoechst (cat. no. H3570) and PI (cat. no. P3566) were obtained from Thermo Fisher Scientific, Inc. and used according to the manufacturer's instructions. Briefly, C918 cells were treated with PIC for 72 h as aforementioned and then collected. Following washing with PBS twice, cells were resuspended in cell staining buffer. Hoechst and PI working solution were added for 20 min at 4°C. Cells were washed with PBS and then visualized via Leica fluorescence microscope at ×200.
Reverse transcription-quantitative PCR (RT-qPCR)
For RT-qPCR, following C918 cell treatment with PIC as aforementioned, total RNA was extracted using Total RNA Isolation kit (cat. no. RC101; Vazyme Biotech Co., Ltd.). Subsequently, total RNA was quantified using a NanoDrop spectrophotometer and reverse-transcribed into cDNA using a commercial kit according to the manufacturer's protocol (cat. no. AH341-01; TransGen Biotech). The qPCR reaction system included cDNA, specific primers and SYBR Green Mix (cat. no. K0222; Thermo Scientific, Inc.), and relative expression levels of the target genes were assessed on the ABI PRISM® 7000 Sequence Detection system (Applied Biosystems; Thermo Fisher Scientific, Inc.). The relative mRNA expression was calculated using 2−∆∆Cq (17). The primer sequences are listed in Table I. The thermocycling conditions were as follows: Activation at 95°C for 10 min; 40 cycles of denaturation at 95°C for 30 sec, annealing at 60°C for 30 sec and extension at 72°C for 30 sec; and a melting curve between 55 and 95°C).
 |
Table I
Primer sequences.
|
Table I
Primer sequences.
| Gene |
Forward |
Reverse |
| IL-6 |
5′-ACTCACCTCTTCAGAACGAATTG-3′ |
5′-CCATCTTTGGAAGGTTCAGGTTG-3′ |
| TNF-α |
5′-CCTCTCTCTAATCAGCCCTCTG-3′ |
5′-GAGGACCTGGGAGTAGATGAG-3′ |
| IL-1β |
5′-ATGATGGCTTATTACAGTGGCAA-3′ |
5′-GTCGGAGATTCGTAGCTGGA-3′ |
| IL-18 |
5′-TCTTCATTGACCAAGGAAATCGG-3′ |
5′-TCCGGGGTGCATTATCTCTAC-3′ |
| NLRP3 |
5′-GATCTTCGCTGCGATCAACAG-3′ |
5′-CGTGCATTATCTGAACCCCAC-3′ |
| TREM2 |
5′-GGTCAGCACGCACAACTTG-3′ |
5′-CGCAGCGTAATGGTGAGAGT-3′ |
| GSDME |
5′-ACATGCAGGTCGAGGAGAAGT-3′ |
5′-TCAATGACACCGTAGGCAATG-3′ |
| Caspase-3 |
5′-CATGGAAGCGAATCAATGGACT-3′ |
CTGTACCAGACCGAGATGTCA-3′ |
| GAPDH |
5′-GGAGCGAGATCCCTCCAAAAT-3′ |
5′-GGCTGTTGTCATACTTCTCATGG-3′ |
RNA-seq
For RNA-seq, C918 cells were collected and total RNA was isolated as aforementioned. Total RNA was subjected to RNA-seq analysis using the DNBSEQ system (BGI Co., LTD.). For library construction, RNA was sheared and reverse-transcribed into cDNA using Optimal Dual-mode mRNA Library Prep kit (cat. no. LR00R96; BGI Co., LTD.) with random primers. PCR products were purified (AMPure XP system) and library quality was assessed on Agilent Bioanalyzer 2100 system. Sequencing was performed using the prepared library on the DNBSEQ-T7 platform and 100/50 bp single-end reads were generated. DNBSEQ-T7RS High-throughput Sequencing kit was provided by MGI Tech Co., Ltd. (cat. no. 1000028454). The concentration of loading concentration of the final library was 8 pM which was detected using Agilent 5300. The expression levels (fragments per kilobase per million fragments mapped) of the target genes was calculated by RSEM (v 1.3.1) (18) The read counts for each gene were determined using the SOAPnuke (v1.5.6) (19); normalization and differential expression analysis were performed using DeSeq2 package (1.4.5) (20). Gene Ontology (GO) as well as Kyoto Encyclopedia of Genes and Genomes (KEGG) analysis was performed using DAVID 2021 database (david.ncifcrf.gov/home.jsp) (21).
Western blot analysis
Total proteins were extracted from C918 cells using RIPA buffer (cat. no. R0010; Beijing Solarbio Science & Technology Co., Ltd.) and quantified by BCA kit (cat. no. PC0020; Beijing Solarbio Science & Technology Co., Ltd.). Then, 50 μg of protein were separated by SDS-PAGE in 4-20% Bis-Tris precast gel, followed by transfer onto PVDF membranes. Following blocking with 5% milk for 2 h at room temperature, membranes were incubated with primary antibodies diluted according to the manufacturer's instructions (Table II) at 4°C overnight. The membranes were washed with TBS-Tween-20 (0.5% Tween-20 in TBS) five times and incubated with HRP-conjugated secondary goat antibody (dilution, 1:2,000) for 2 h at room temperature. The protein bands were visualized using the ECL Western Blotting Detection Reagent (cat. no. 34096; Thermo Scientific, Inc.) and scanned in ChemiDoc MP (Bio-Rad Laboratories, Inc.). Blots were quantified using Image J v1.8.0 (National Institutes of Health).
 |
Table II
Antibodies for western blotting.
|
Table II
Antibodies for western blotting.
| Name |
Supplier |
Cat. no. |
| IL-18 |
Abcam |
ab243091 |
| IL-1β |
Abcam |
ab283818 |
| NLRP3 |
Abcam |
ab263899 |
| GSDMD |
Abcam |
ab210070 |
| N-GSDMD |
Abcam |
ab215203 |
| Caspase 1 |
Abcam |
ab207802 |
| p20 |
AdipoGen |
AG-20B-0048-C100 |
| Caspase 3 |
Abcam |
ab32351 |
| GSDME |
Abcam |
ab230482 |
| TREM2 |
Abcam |
ab318262 |
| GAPDH |
Abcam |
ab9484 |
Silencing using si-RNAs
The transfection of melanoma cells with si-TREM2 and the corresponding negative control (RiboBio Co. Ltd.) was performed according to the manufacturer's instructions of RNAiMAX (cat. no. 13778100; Thermo Fisher Scientific, Inc.). Briefly, C918 cells were seeded onto 6-well plates at a density of 1×106 cells/well and cultured at 37°C for 24 h. Lipofectamine RNAiMAX (cat. no. 13778030; Thermo Fisher Scientific, Inc.) and si-TREM2 were diluted in FBS-free RPMI-1640 to 10 μM (cat. no. 11875119; Thermo Fisher Scientific, Inc.) at room temperature. The diluted si-TREM2 and Lipofectamine RNAiMAX were mixed and incubated at room temperature for 5 min. C918 cells were treated with the si-TREM2/Lipofectamine complex and incubated at 37°C for 24 h. Following incubation, cells were treated with 80 μM PIC for 72 h and collected for further analysis. The sequence of si-TREM2 was 5′-GCCUCUUGGAAGGAGAAAUUU-3′ and that of the negative control was 5′-UUGUACUACACAAAAGUACUG-3′.
Immunohistochemical staining
Tumor tissue was collected, fixed in 4% paraformaldehyde at room temperature overnight, embedded in paraffin and cut into 5 μm. Immunohistochemical staining was performed according to a previous study (22). Briefly, sections were dewaxed in xylene for 10 min twice, then rehydrated in a descending ethanol series (hydrated in 100, 95, 90, 80 and 70% ethanol and water for 5 min each). Heat-induced epitope retrieval (cat. no. ZLI-9065; ZSGB-Bio) was used for antigen retrieval in a microwave (800 W) oven for 10 min. After that, sections were treated with 3% hydrogen peroxide for 10 min at room temperature and blocked with 2.5% goat serum (cat. no. R37624; Thermo Scientific, Inc.) for 30 min at room temperature. Tissue sections were incubated with primary antibodies [TREM2 (1:200; cat. no. ab318262; Abcam), GSDME (dilution, 1:200; cat. no. ab230482; Abcam)] at 4°C overnight and washed with PBS four times. Subsequently, the sections were incubated with the peroxidase-conjugated secondary antibody (dilution, 1:200; cat. no. ab214880; Abcam) at room temperature for 2 h. Following washing with PBS four times, tissue sections were stained with DAB (cat. no. ab64238; Abcam) and hematoxylin for 3 min at room temperature. All images were captured using the Olympus IX73 Light microscope (Olympus Corporation) at ×200. Data was quantified using Image J v1.8.0 (National Institutes of Health).
Statistical analysis
All data are expressed as the mean ± SEM. All experiments were repeated ≥3 times. The differences between two groups were compared by unpaired Student's t test, while those between three groups were compared with one-way ANOVA followed by Tukey's post hoc test. P<0.05 was considered to indicate a statistically significant difference. All data were analyzed using GraphPad v8.0 (GraphPad Software, Inc.; Dotmatics).
Results
Effects of PIC on proliferation and apoptosis of UM cells
Cell volume decreased following treatment with 40 μM PIC for 72 h (Fig. 1A). Following treatment with 80 μM PIC, cells were characterized by unclear boundary, fragmentation and floating. Viability of PIC-treated cells was significantly reduced in a dose-dependent manner (Fig. 1B). These findings indicated that PIC inhibited C918 cell viability. Compared with the control group, the viability of cells treated with 40 or 80 μM PIC was 70 and 47%, respectively, indicating decreased cell survival and enhanced cell death. PIC treatment for 72 h induced the activity of both caspase 3 and 9 (Fig. 1C and D). More specifically, the activities of caspase 3/9 were more potent in the 80 compared with the 40 μM PIC group. Similarly, the effects of PIC on Mum-2b cells were also investigated. PIC administration inhibited cell viability (Fig. S1A and B) and markedly enhanced levels of caspase 3 and 9 (Fig. S1C and D). PIC treatment induced cell death in a dose-dependent manner, as indicated by Hoechst-PI staining (Fig. S2). Overall, these findings suggested that PIC significantly promoted cell apoptosis and inhibited cell proliferation in human UM cell lines.
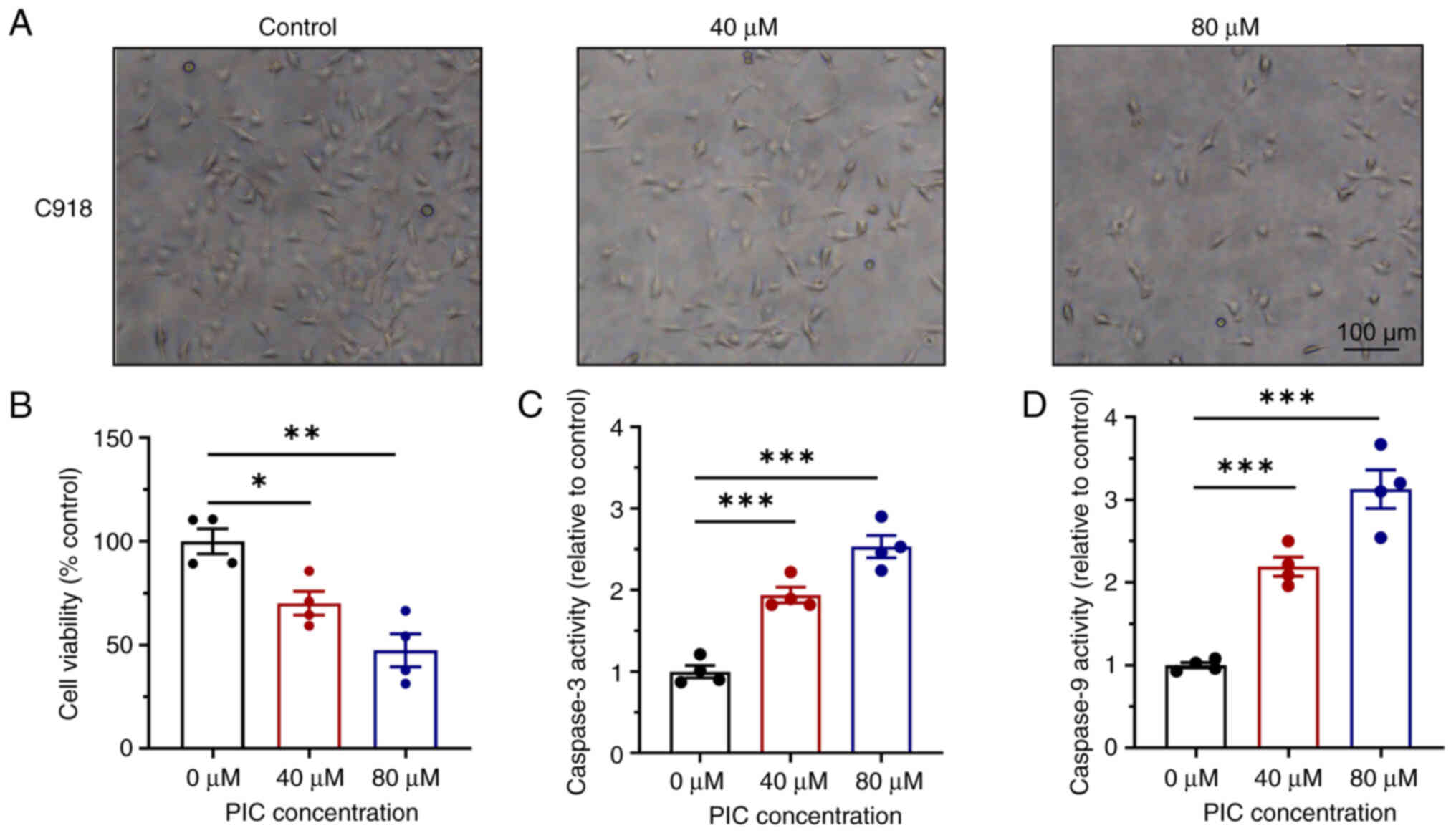 |
Figure 1
Effect of PIC on C918 cell proliferation and apoptosis. (A) Morphological changes of C918 cells after PIC treatment. Magnification, ×100. (B) Effects of PIC on cell viability. Activity of caspase (C) 3 and (D) 9 in C918 cells after PIC. n=4. *P<0.05, **P<0.01, ***P<0.001. PIC, piceatannol.
|
Effect of PIC on C918 cell migration
To investigate the effects of PIC on UM cell migration, wound-healing assay was performed. Untreated cells showed effectively heal the wounds after 72 h. However, treatment with both 40 and 80 μM PIC for 48 h markedly suppressed the wound healing ability of UM cells compared with the control (Fig. 2A and B). PIC also reduced the migration ability of Mum-2b cells in a dose-dependent manner (Fig. S3). The aforementioned findings indicated that PIC attenuated the migration of human UM cells.
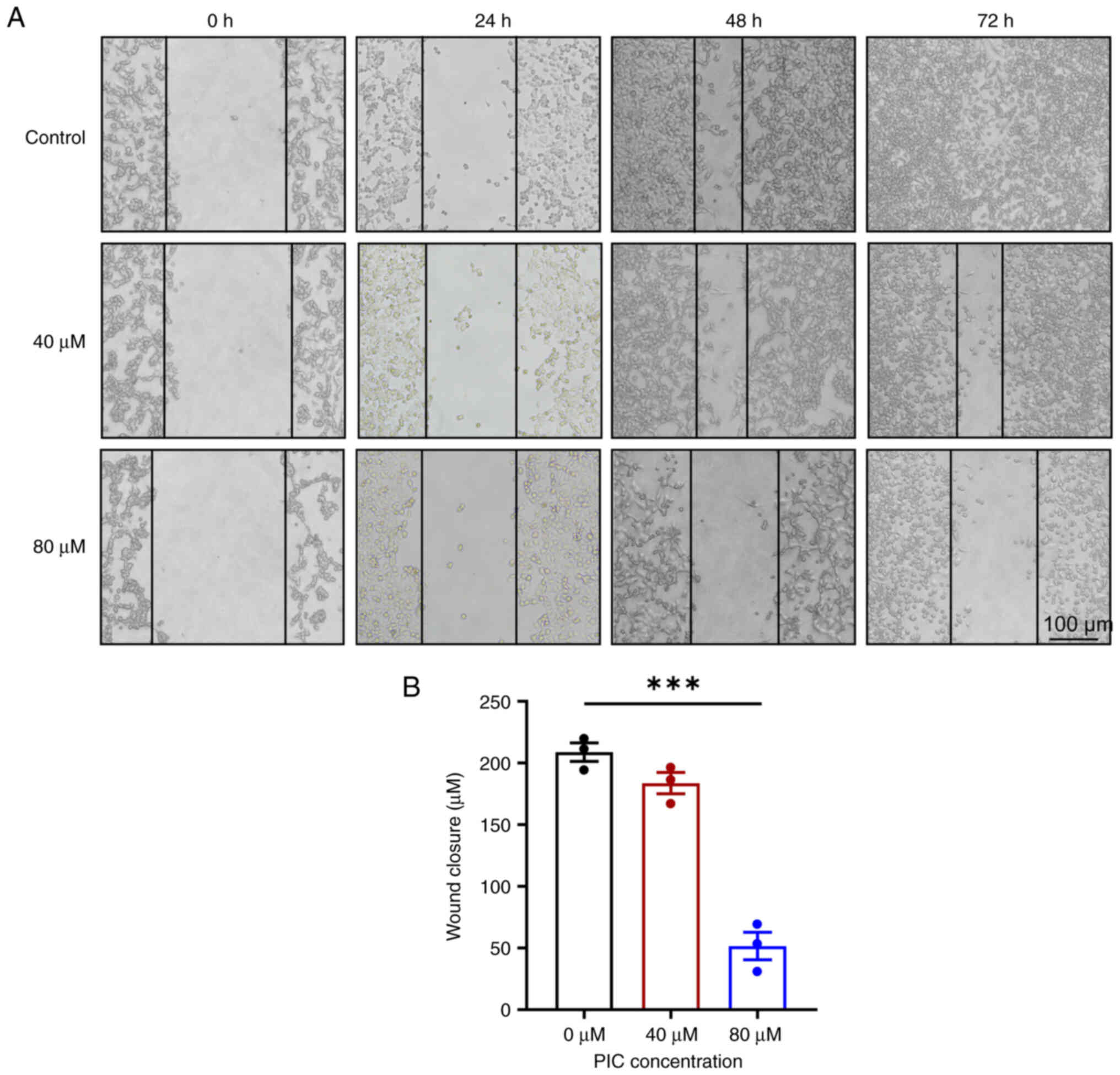 |
Figure 2
Effect of PIC on C918 cell migration. (A) Representative scratch assay on C918 cells. Magnification, ×100. (B) Wound closure of C918 cells after PIC administration for 48 h. n=3. ***P<0.001. PIC, piceatannol.
|
Effects of PIC on ROS regulation in C918 cells
Oxidative stress was assessed via measuring ROS and antioxidant enzyme levels. ROS content was significantly increased in PIC-treated cells compared with the control (Fig. 3A). In addition, following treatment with PIC for 72 h, the activities of the antioxidant enzymes SOD and GST were impaired in C918 cells (Fig. 3B and C). However, no significant differences were observed in the activity of GR (Fig. 3D). To verify the effect of PIC on antioxidant enzyme activity, the activity of GPx and CAT was also detected. Activities of GPx and CAT were significantly suppressed following PIC administration; these effects were greater in the 80 μM PIC group compared with the 40 μM PIC group (Fig. 3E and F). These results suggested the PIC suppressed superoxide anion scavenging and promoted ROS accumulation in C918 cells.
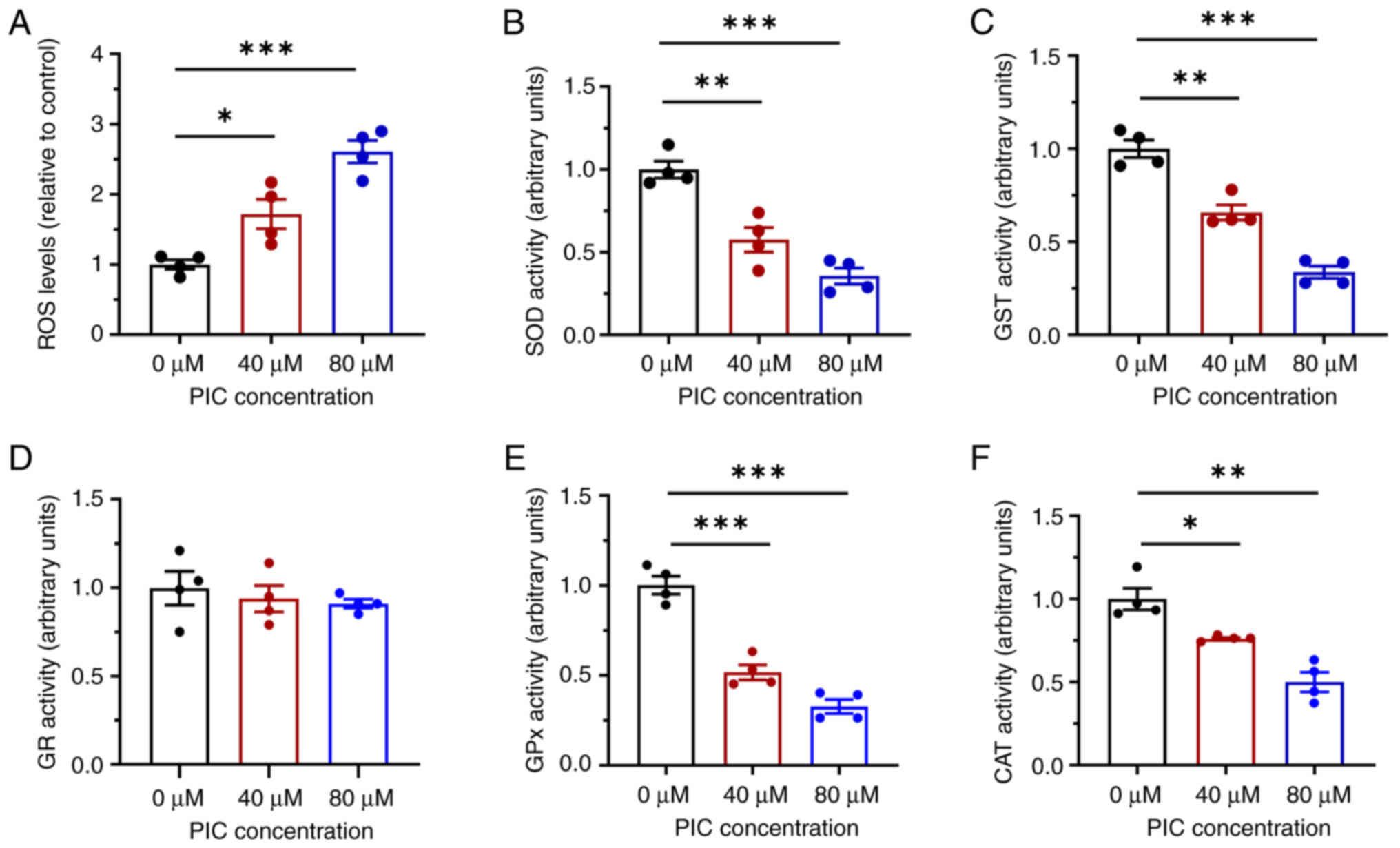 |
Figure 3
Effect of PIC on oxidative regulation in C918 cells. (A) ROS production in C918 cells after PIC administration. Effects of PIC on the activities of antioxidant enzymes (B) SOD, (C) GST, (D) GR, (E) GPx and (F) CAT. n=4. *P<0.05, **P<0.01, ***P<0.001. PIC, piceatannol; SOD, superoxide dismutase; GST, Glutathione S-transferase; GR, glutathione reductase; GPx, Glutathione Peroxidase; CAT, catalase; ROS, reactive oxygen species.
|
Effects of PIC on inflammatory responses in C918 cells
Previous studies have reported that PIC exerts anti-inflammatory activity in several biological processes, such as cell proliferation (23) and induction of regulatory T cells (24). Therefore, to investigate whether PIC promotes inflammatory responses in C918 cells, the levels of intracellular inflammatory mediators and cytokines, including IL-1β, IL-18, TNF-α, IL-6 and nod-like receptor protein 3 (NLRP3), were detected. RT-qPCR showed that mRNA expression levels of IL-6 and TNF-α were significantly reduced following treatment with PIC (Fig. 4A). By contrast, mRNA expression levels of IL-18 and IL-1β were significantly increased. mRNA expression levels of NLRP3 were enhanced in the 40 μM PIC group, while those in the 80 μM PIC group were markedly decreased (Fig. 4B). As NLRP3, IL-18 and IL-1β are biomarkers of pyroptosis (25), these results indicated that PIC promoted pyroptosis in C918 cells.
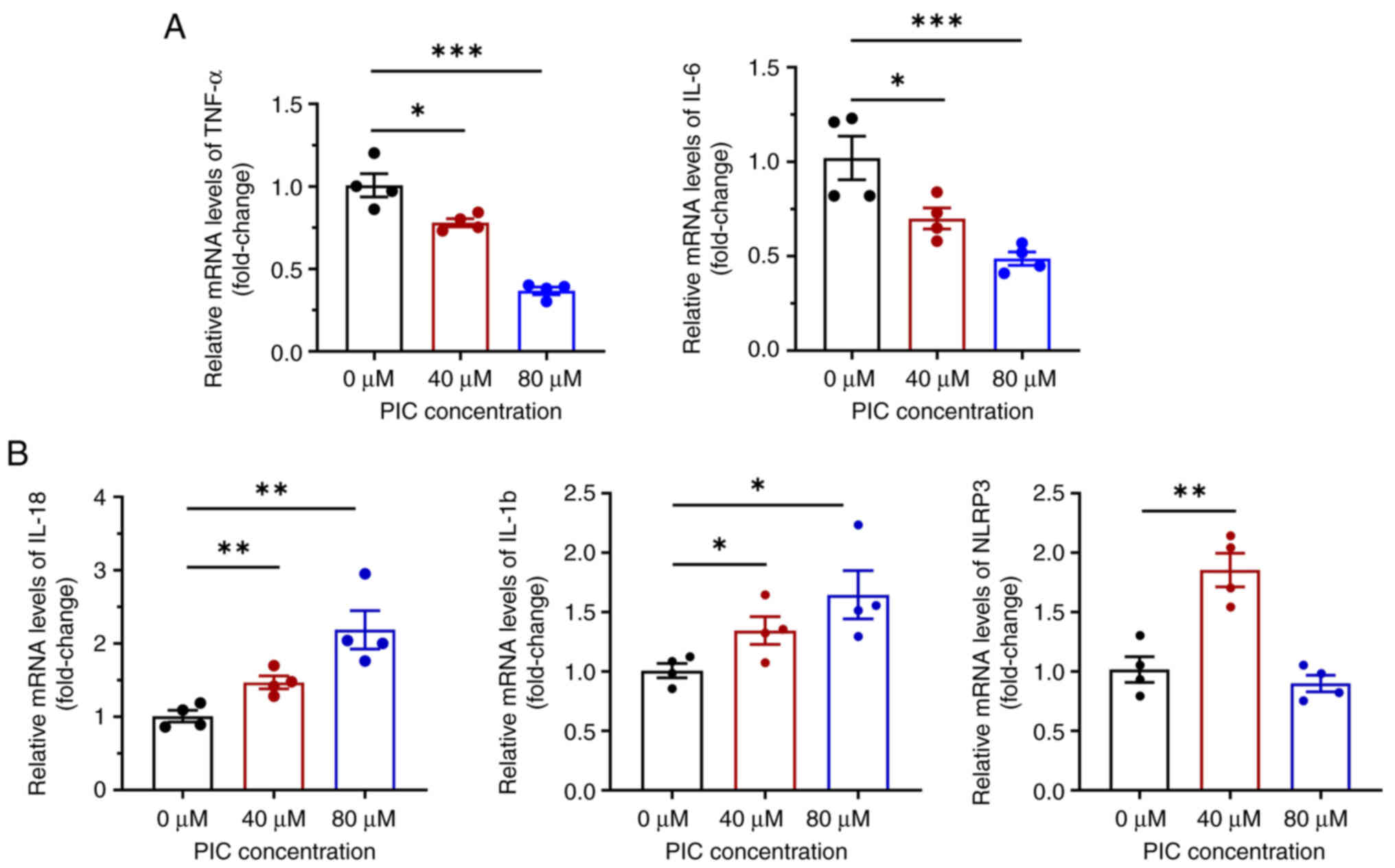 |
Figure 4
Effect of PIC on inflammation in C918 cells. mRNA levels of (A) TNF-α and IL-6 and (B) IL-18, IL-1β and NLRP3 in PIC-treated C918 cells. n=4. *P<0.05, **P<0.01, ***P<0.001. PIC, piceatannol.
|
Effect of PIC on pyroptosis in C918 cells
The aforementioned findings suggested that PIC affected pyroptosis in C918 cells. Therefore, western blot analysis was carried out to assess pyroptosis-associated protein levels in C918 cells. Consistent with the aforementioned results, the protein expression of both IL-18 and IL-1β was elevated in PIC-treated C918 cells (Fig. 5A). PIC treatment downregulated both gasdermin D (GSDMD) and N-GSDMD, while protein expressions levels of NLRP3, caspase 1 and p20 were only increased in 20 or 40 μM PIC groups (Fig. 5B), indicating that PIC did not enhance pyroptosis via conventional pathways. Levels of caspase 3 and GSDME were significantly increased (Fig. 5C). Overall, the aforementioned findings indicated that PIC enhanced pyroptosis in C918 cells via the caspase 3/GSDME pathway.
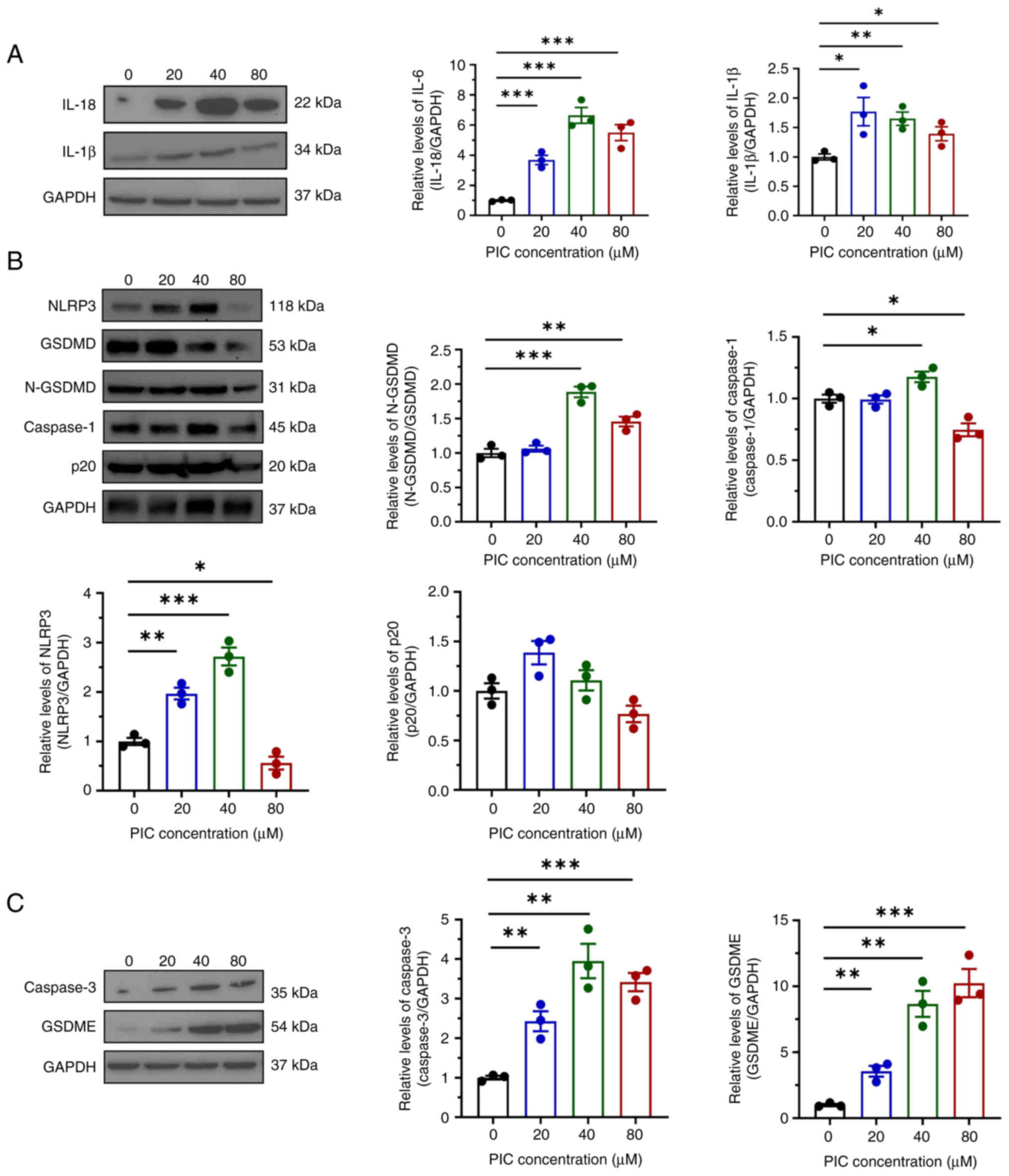 |
Figure 5
Effect of PIC on pyroptosis in C918 cells. (A) Western blot and quantification of IL-18 and IL-1β in C918 cells after PIC treatment. (B) Western blot and quantifications of NLRP3, GSDMD, N-GSDMD, caspase-1 and p20 in C918 cells after PIC treatment. (C) Western blot and quantifications of caspase-3 and GSDME in C918 cells after PIC treatment. *P<0.05, **P<0.01, ***P<0.001. N-GSDMD, N-Gasdermin D; PIC, piceatannol.
|
Role of TREM2 in regulating pyroptosis in C918 cells
To explore the mechanism underlying the effect of PIC on promoting pyroptosis in C918 cells, RNA-seq was performed. PIC notably increased the expression levels of genes associated with inflammation, such as STAT3, IL-18 and IL-1β, non-canonical pyroptosis-associated pathways, including caspase 3 and GSDME, and inflammasome, including caspase 1 and NLRP3. However, expression levels of GSDMD, a gene associated with conventional pyroptosis-associated pathways, were significantly decreased (Fig. 6A and B). Gene Ontology and Kyoto Encyclopedia of Genes and Genomes analysis showed that genes were enriched in 'metabolic pathways', 'ABC transporters', 'purine metabolism', 'spliceosome', 'RNA transport' and 'cell cycle' (Fig. 6C). In addition, pyroptosis-related genes were ranked by fold-change from high to low based on the results of differentially expressed genes (DEGs; Fig. 6D). TREM2 ranked first and was marked in red, suggesting a possible critical role in regulating pyroptosis. Furthermore, protein expression levels of TREM2 were detected via western blot analysis. TREM2 was upregulated in PIC-treated C918 cells (Fig. 7A and B). To investigate the role of TREM2 in regulating pyroptosis, TREM2 expression was silenced (Figs. 7C and S4). Following TREM2 knockdown, the inhibitory effect of PIC on viability of C918 cells was markedly reversed (Fig. 7D). In addition, TREM2 silencing decreased ROS production and LDH activity (Fig. 7E and F). Additionally, the expression levels of caspase 3 and GSDME were notably decreased following C918 cell transfection with si-TREM2 (Fig. 7G-J). AC-DEVD-CHO obviously reversed the suppressed viability induced by PIC in C918 cells (Fig. S5A) and PIC-induced upregulation of TREM2 was not affected by caspase-3 inhibition (Fig. S5B and C), indicating that TREM2 serve act as an upstream regulator of caspase-3. These results suggested that TREM2 silencing abrogated the effect of PIC on promoting pyroptosis in C918 cells.
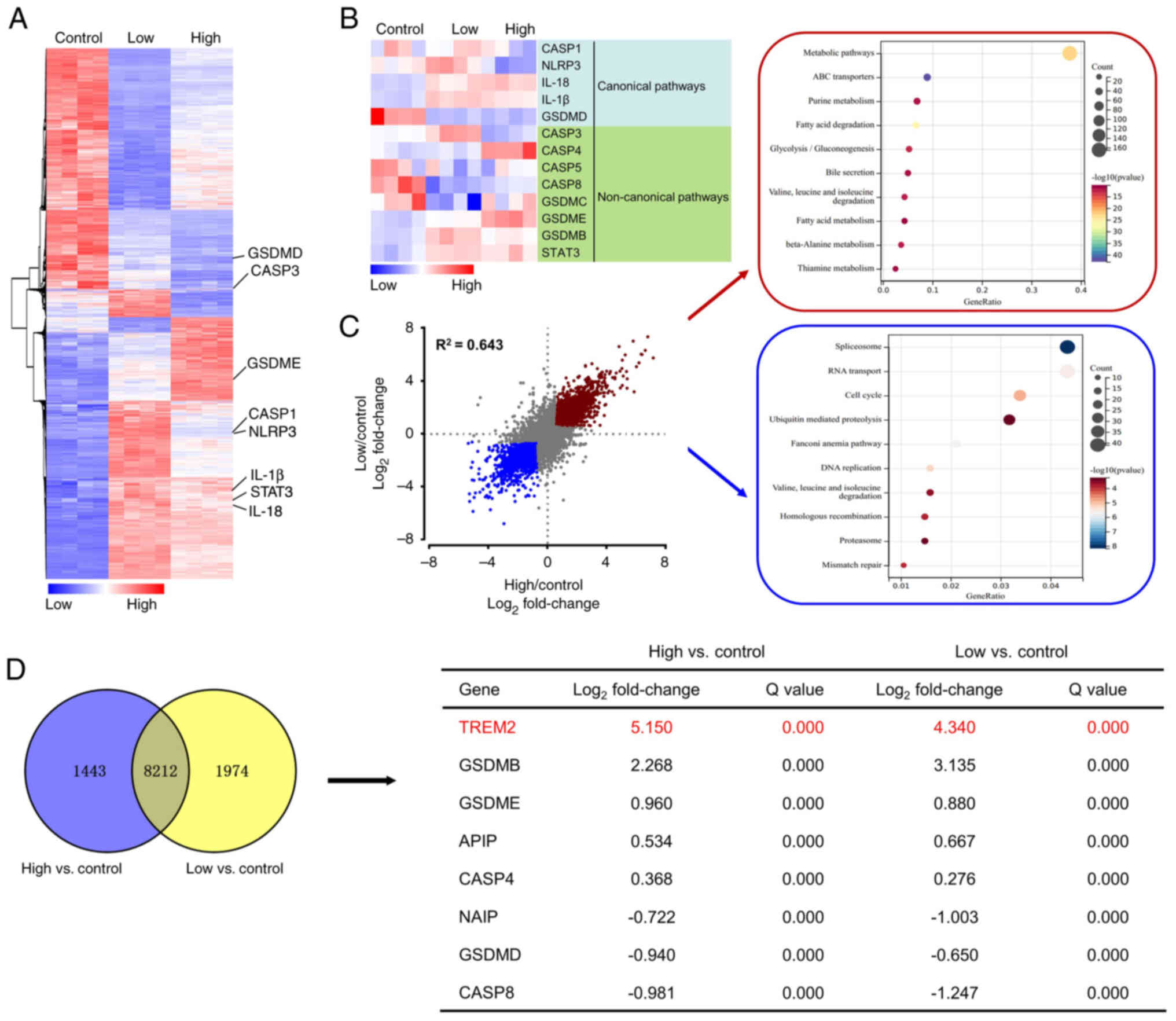 |
Figure 6
Gene expression analysis of C918 cells treated with PIC. (A) Hierarchical clustered heatmap of gene expression profiles. (B) Heatmap of DEGs. Red, upregulated; blue, downregulated genes. (C) Scatter plot of DEGs and Gene Ontology enrichment analysis (D) Venn diagram of top 8 overlapping DEGs (Q<0.05). PIC, piceatannol; DEG, differentially expressed genes; GSDM, gasdermin; TREM2, triggering receptor expressed on myeloid cells 2; APIP, apoptotic protease activator APAF1 action protein; NAIP, neuronal apoptosis inhibitory protein; CASP, caspase.
|
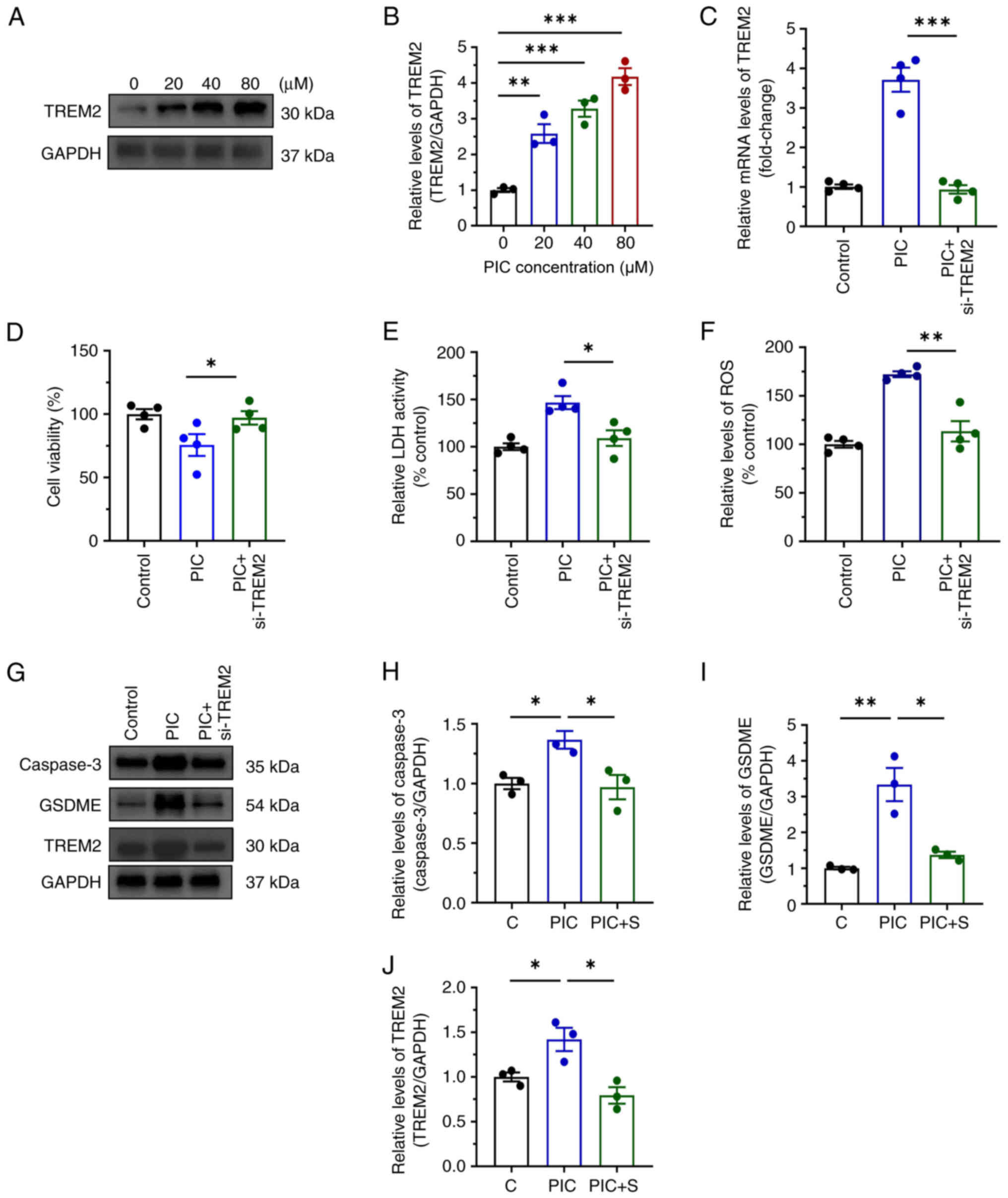 |
Figure 7
Role of TREM2 in regulation of pyroptosis in C918 cells. (A) Expression and (B) quantifications of TREM2 proteins in C918 cells following PIC treatment for 72 h. (C) mRNA expression, (D) viability, (E) LDH activity, (F) ROS production in C918 and (G) expression levels and quantifications of (H) caspase-3, GSDME (I) TREM2 (J) proteins in C918 cells following si-TREM2 transfection. n=3-4. *P<0.05, **P<0.01, ***P<0.001. PIC, piceatannol; LDH, lactate dehydrogenase; GSDME, Gasdermin E; C, control; S, si-TREM2; ROS, reactive oxygen species.
|
TREM2 activation suppresses C918 cell proliferation
The key role of TREM2 in the regulation of pyroptosis was previously verified (26). The present study aimed to investigate whether TREM2 overexpression suppressed UM cell proliferation. Cells were treated with COG, an agonist of TREM2. COG upregulated TREM2 at the protein level (Fig. 8A and B). Similar to the effects of PIC, the viability of C918 cells significantly decreased after COG treatment (Fig. 8C), accompanied by elevated LDH activity and ROS production (Fig. 8D and E). Furthermore, COG effectively enhanced pyroptosis in C918 cells, as evidenced by increased expression of caspase 3 and GSDME (Fig. 8F-H).
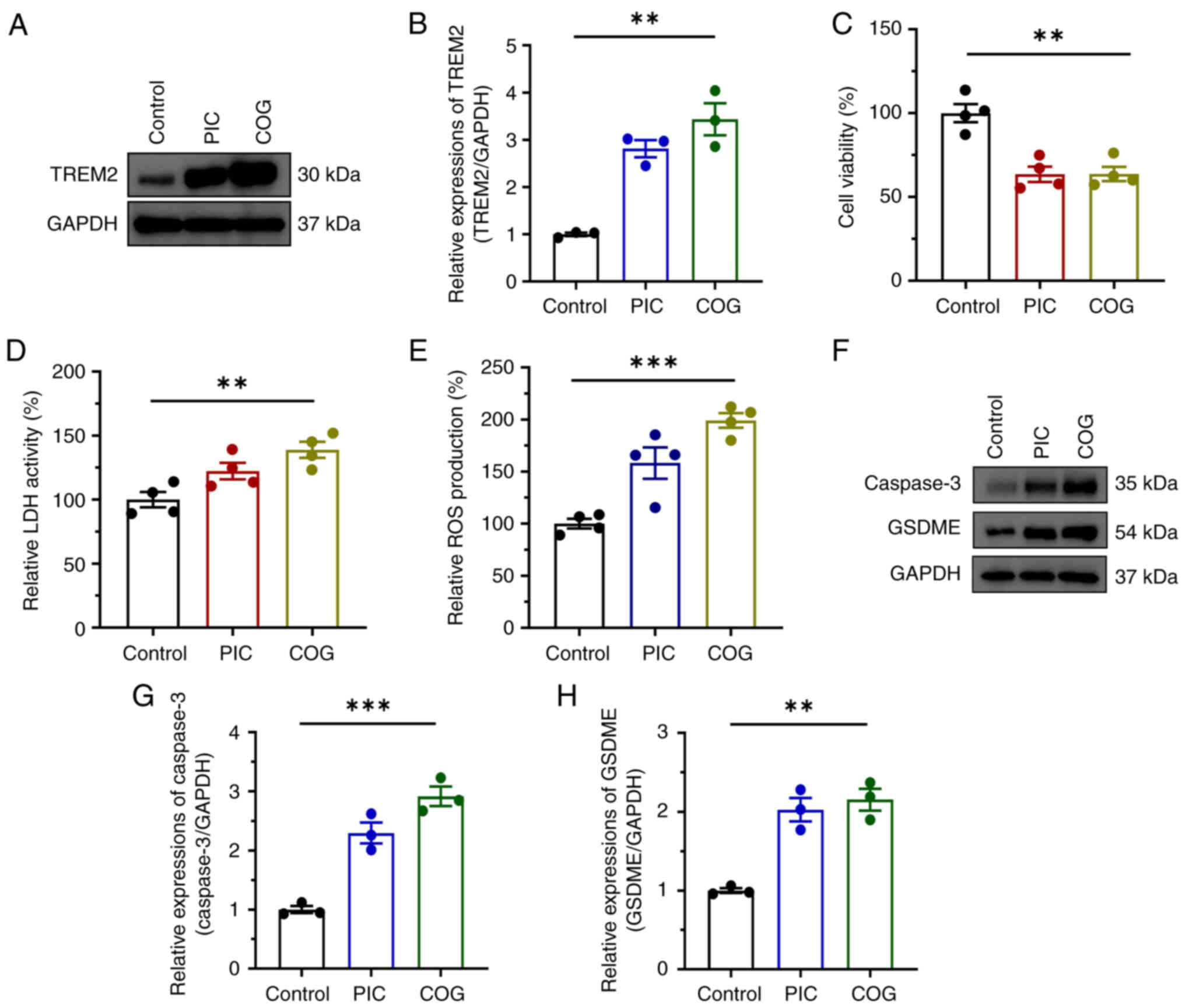 |
Figure 8
Effect of TREM2 activation in regulation of pyroptosis in C918 cells. (A) Expression as well as (B) quantifications of TREM2 protein, (C) viability, (D) LDH activity, (E) ROS production and (F) expression levels and quantification of (G) caspase-3 and GSDME (H) proteins in C918 cells following COG treatment. n=4. **P<0.01, ***P<0.001. TREM2, triggering receptor expressed on myeloid cells 2; LDH, lactate dehydrogenase; ROS, reactive oxygen species; GSDME, Gasdermin E; COG, COG1410; PIC, piceatannol.
|
PIC suppresses tumor growth in a UM mouse model
Based on the inhibitory effects of PIC on UM cells, the present study further investigated whether PIC alleviated tumor progress in a UM mouse model. Tumor size was notably reduced in the all PIC group compared with the control group in a dose-dependent manner (Fig. 9A). No significant differences were observed in body weight between the three groups (Fig. 9B). Increase in tumor volume and weight was slower in the PIC-treated group compared with control group, with a significant difference between the 30 and 60 mg/kg PIC groups (Fig. 9C and D). Consistent with the results obtained in C918 cells, RT-qPCR, western blot and immunohistochemical assay demonstrated that the expression of TREM2, caspase 3 and GSDME significantly increased following treatment with PIC (Fig. 9E-J). These results suggested that PIC effectively inhibited tumor cell proliferation in vivo via upregulating TREM2 and enhancing pyroptosis.
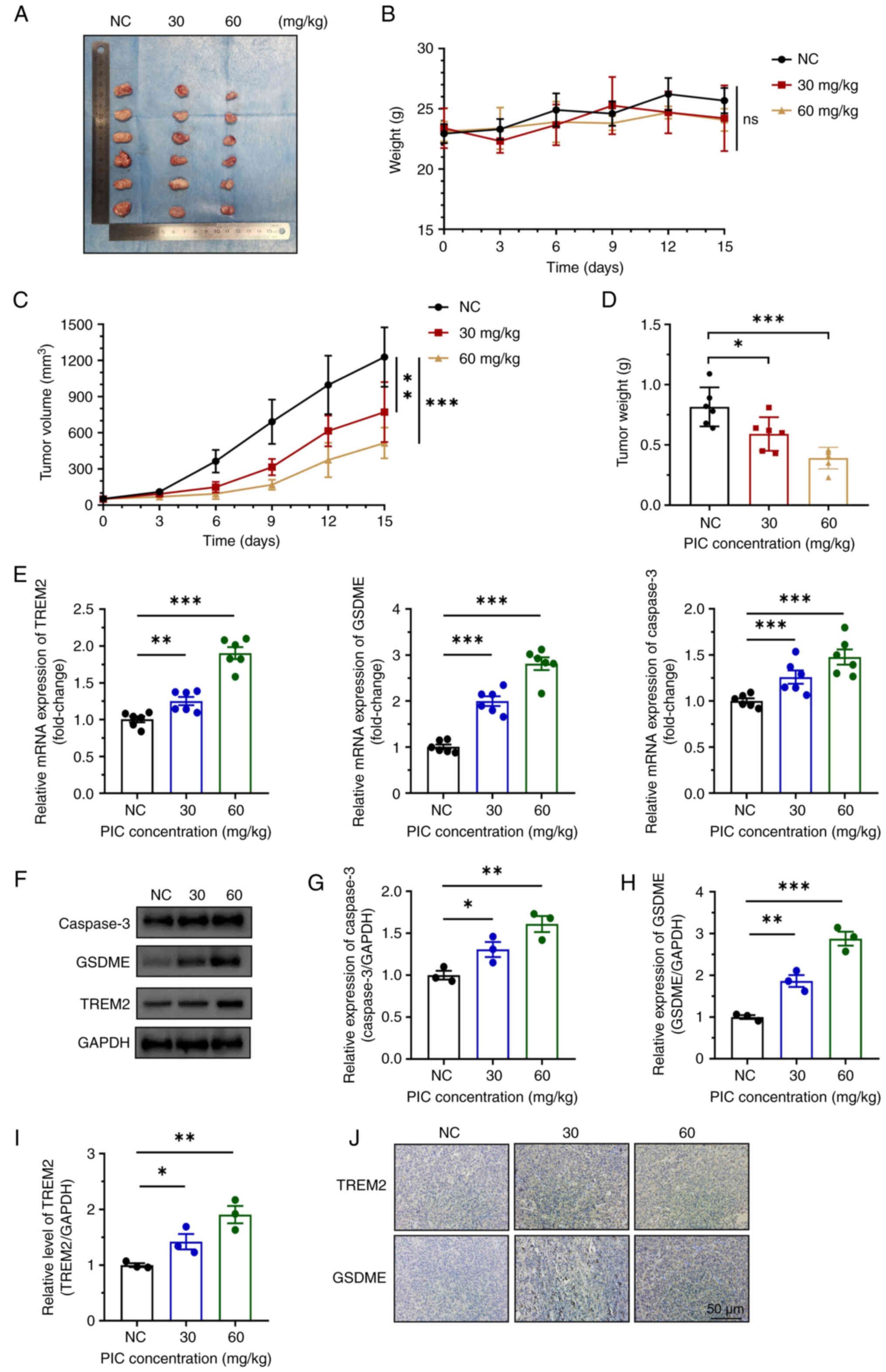 |
Figure 9
Effect of PIC on tumor growth in mice. (A) Representative tumors, (B) mouse weight and tumor (C) volume and (D) weight after PIC treatment. (E) Relative mRNA levels of TREM2, GSDME and caspase 3 in tumor tissue. (F) Western blot and quantification of (G) caspase 3, (H) GSDME and (I) TREM2 and (J) immunohistochemical staining of TREM2 and GSDME in tumor tissue. Magnification, ×200. n=6. *P<0.05, **P<0.01, ***P<0.001. PIC, piceatannol; GSDME, gasdermin E; ns, not significant; NC, negative control.
|
Discussion
UM is the most common type of primary malignant intraocular tumor in adults. In approximately half of UM cases (52%), clinical metastasis occurs (27). Although National Comprehensive Cancer Network guidelines agree that patients should be considered for surgical resection, ionizing radiation or radiosurgery (28), there is no consensus on how best to manage tumor masses. The present study verified that PIC exhibited anti-tumor activity in human UM cells via regulating apoptosis, inflammation and pyroptosis. Notably, the present study identified TREM2 as a key regulator of pyroptosis via inducing activation of caspase 3/GSDME signaling.
PIC is extracted from fruits and vegetables, such as grapes. Since the content of PIC in grapes is lower than that of resveratrol, less research has been conducted on the effects of PIC compared with those of resveratrol. The anti-tumor effects of resveratrol have been well-documented both in vitro and in vivo in previous studies (29-32). Therefore, it was hypothesized that PIC, a homolog of resveratrol, could exert similar activity. Recently, Siedlecka-Kroplewska et al (33) demonstrated that PIC induces caspase-dependent apoptosis characterized by caspase 3 activation. Furthermore, Çınar Ayan et al (34) showed that PIC activates caspase 3-dependent pathways and promotes ROS-mediated mitochondrial dysfunction, inducing apoptosis in pancreatic cancer cells. Therefore, the present study aimed to investigate whether PIC inhibits the proliferation of human UM cells. PIC inhibited C918 and Mum-2b cell viability in vitro in a dose-dependent manner. In addition, the migration ability of UM cells was also suppressed. These results indicated that PIC exhibited potent anti-tumor activity against human UM.
During tumor development, inflammation promotes tumor growth (35). Inflammation facilitates onset of cancer, either as a consequence of chronic inflammation or by inducing inflammatory responses. Recent studies have suggested that alleviation of inflammatory responses inhibits tumor cell proliferation (36,37). Guo et al (37) found that relieving Ganoderma lucidum-induced colitis inhibits tumorigenesis in the colon. Furthermore, clinical and epidemiological studies suggest that non-steroidal anti-inflammatory drugs, particularly aspirin, could be considered as broad-spectrum cancer-preventive agents (36,38). PIC exhibits potent anti-inflammatory effects similar to resveratrol (39). Therefore, in the present study the effects of PIC on inflammatory responses in C918 cells were investigated. PIC downregulated TNF-α and IL-6. Expression of IL-1β and NLRP3 was also enhanced in PIC-treated cells. NLRP3 and IL-1β are biomarkers of pyroptosis, a type of programmed cell death in mammals (40). The aforementioned results indicated that PIC inhibited the proliferation of C918 cells via promoting pyroptosis via canonical or non-canonical mechanisms. The increased levels of NLRP3 and IL-1β were also accompanied by the enhanced ROS production following UM cell treatment with PIC. This may be because ROS could promote the activation of NLRP3 and release of IL-1β (41).
Pyroptosis is a regulated cell death pathway involved in maintaining cell metabolism and regulating protein and organelle quality, thus eliminating damaged proteins and organelles that accumulate during stress to prevent tumor initiation (42). Emerging evidence has suggested that pyroptosis acts as a tumor suppressor (43,44). A previous study of clear cell renal cell carcinoma demonstrated that long intergenic non-protein coding RNA 0002 silencing-mediated pyroptosis inhibition significantly enhances ACHN cell proliferation via p53 downregulation (44). In addition, drug complex Ca@GOx delivery to mitochondria significantly enhances ROS generation and induces pyroptosis, thus promoting tumor infiltration of CD8+ T cells and improving anti-tumor effects in the 4T1 mouse mammary cancer cell line (45). It has been reported that TREM2 acts as a promotor to enhance pyroptosis (26). Zhang et al (26) showed that navitoclax upregulates TREM2 and decreases the Bcl2/Bax ratio, thus activating caspase 3 and enhancing GSDME cleavage. The present results showed PIC enhanced caspase 3/GSDME expression and triggered pyroptosis in C918 cells, potentially via TREM2 upregulation. Although studies have verified the inhibitory effect of TREM2 on caspase 3 activation, Bcl2 is downregulated following TREM2 knockdown (46,47), indicating enhanced caspase 3 activity. A previous study demonstrated that Bcl-2 inhibition induces pyroptosis in acute myeloid leukemia (AML) cells by activating GSDME (48). Additionally, treatment of AML cells with Bcl-2 inhibitor disrupts the interaction of Bcl-2 and Bax, promoting release of cytochrome C and activating the caspase 3/GSDME pathway (48). Therefore, it was hypothesized that enhanced pyroptosis is triggered by decreased Bcl2/Bax ratio, which in turn could promote caspase 3 activation and GSDME cleavage.
In summary, the present study suggested that PIC may serve as a potential anti-tumor drug against human UM. PIC promoted ROS production, alleviated inflammation and inhibited UM cell proliferation. PIC enhanced pyroptosis via the caspase 3/GSDME pathway by upregulating TREM2. However, the present study had limitations. Firstly, since PIC at high concentration showed cytotoxicity to macrophages, promoting immunosuppression, further studies should be performed to attenuate cytotoxicity, potentially via combination with other molecules. Secondly, further studies are necessary to clarify the regulatory effects of TREM2 on inhibiting PIC-treated human UM cells in vivo, such as elucidating cytotoxicity of PIC on other types of cells. Notably, a normal cell line should be used as a control. More experiments should be performed to explore the underlying interaction between TREM2 and pyroptosis, such as using specific inhibitors targeting pyroptosis to clarify the involvement of pyroptosis in the effects of PIC on UM tumors. To the best of our knowledge, only few studies have been conducted on the association between TREM2 and pyroptosis in cancers (49,50), including direct interaction and potential signaling pathways involved. Additionally, few studies have assessed the effects of PIC on human UM patients in clinic. Notably, the RNA-seq results should be further analyzed to assess the effects of PIC besides pyroptosis. Overall, the present study may provide insights into development of novel natural drugs for treating human UM.
Supplementary Data
Availability of data and materials
The data generated in the present study may be found in the Sequence Read Archive under accession number PRJNA1129681 or at the following URL: ncbi.nlm.nih.gov/bioproject/1129681.
Authors' contributions
XJ conceived and designed the study, performed experiments, analyzed data and wrote and reviewed the manuscript. WL, YL and LL performed the experiments. HL conceived and designed the study, edited the manuscript and supervised the study. XJ, WL, YL, LL and HL confirm the authenticity of all the raw data. All authors have read and approved the final manuscript.
Ethics approval and consent to participate
The present study was approved by the Experimental Animal Ethics Committee of The First Hospital of Lanzhou University (approval no. LDYYLL2021-249; Lanzhou; China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Acknowledgements
Not applicable.
Funding
The present study was supported by Natural Science Foundation of Gansu Province (grant no. 21JR1RA093) and Higher Education Innovation Fund Project of Gansu Province (grant no. 2021B-023).
References
|
1
|
Jager MJ, Shields CL, Cebulla CM, Abdel-Rahman MH, Grossniklaus HE, Stern MH, Carvajal RD, Belfort RN, Jia R, Shields JA and Damato BE: Uveal melanoma. Nat Rev Dis Primers. 6:242020. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Khoja L, Atenafu EG, Suciu S, Leyvraz S, Sato T, Marshall E, Keilholz U, Zimmer L, Patel SP, Piperno-Neumann S, et al: Meta-analysis in metastatic uveal melanoma to determine progression free and overall survival benchmarks: An international rare cancers initiative (IRCI) ocular melanoma study. Ann Oncol. 30:1370–1380. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Rantala ES, Hernberg M and Kivelä TT: Overall survival after treatment for metastatic uveal melanoma: A systematic review and meta-analysis. Melanoma Res. 29:561–568. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Spagnolo F, Caltabiano G and Queirolo P: Uveal melanoma. Cancer Treat Rev. 38:549–553. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Yuan R, Zhao W, Wang QQ, He J, Han S, Gao H, Feng Y and Yang S: Cucurbitacin B inhibits non-small cell lung cancer in vivo and in vitro by triggering TLR4/NLRP3/GSDMD-dependent pyroptosis. Pharmacol Res. 170:1057482021. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Rong L, Li Z, Leng X, Li H, Ma Y, Chen Y and Song F: Salidroside induces apoptosis and protective autophagy in human gastric cancer AGS cells through the PI3K/Akt/mTOR pathway. Biomed Pharmacother. 122:1097262020. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Sharma P, Shimura T, Banwait JK and Goel A: Andrographis-mediated chemosensitization through activation of ferroptosis and suppression of β-catenin/Wnt-signaling pathways in colorectal cancer. Carcinogenesis. 41:1385–1394. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wahdan SA, Azab SS, Elsherbiny DA and El-Demerdash E: Piceatannol protects against cisplatin nephrotoxicity via activation of Nrf2/HO-1 pathway and hindering NF-κB inflammatory cascade. Naunyn Schmiedebergs Arch Pharmacol. 392:1331–1345. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lee CH, Yang H, Park JHY, Kim JE and Lee KW: Piceatannol, a metabolite of resveratrol, attenuates atopic dermatitis by targeting Janus kinase 1. Phytomedicine. 99:1539812022. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kwon JY, Kershaw J, Chen CY, Komanetsky SM, Zhu Y, Guo X, Myer PR, Applegate B and Kim KH: Piceatannol antagonizes lipolysis by promoting autophagy-lysosome-dependent degradation of lipolytic protein clusters in adipocytes. J Nutr Biochem. 105:1089982022. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Du M, Zhang Z and Gao T: Piceatannol induced apoptosis through up-regulation of microRNA-181a in melanoma cells. Biol Res. 50:362017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Sano S, Sugiyama K, Ito T, Katano Y and Ishihata A: Identification of the strong vasorelaxing substance scirpusin B, a dimer of piceatannol, from passion fruit (Passiflora edulis) seeds. J Agric Food Chem. 59:6209–6213. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Komorowska D, Gajewska A, Hikisz P, Bartosz G and Rodacka A: Comparison of the effects of resveratrol and its derivatives on the radiation response of MCF-7 breast cancer cells. Int J Mol Sci. 22:95112021. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Huangfu L, Wang X, Tian S, Chen J, Wang X, Fan B, Yao Q, Wang G, Chen C, Han J, et al: Piceatannol enhances Beclin-1 activity to suppress tumor progression and its combination therapy strategy with everolimus in gastric cancer. Sci China Life Sci. 66:298–312. 2023. View Article : Google Scholar
|
|
15
|
Kwon GT, Jung JI, Song HR, Woo EY, Jun JG, Kim JK, Her S and Park JH: Piceatannol inhibits migration and invasion of prostate cancer cells: Possible mediation by decreased interleukin-6 signaling. J Nutr Biochem. 23:228–238. 2012. View Article : Google Scholar
|
|
16
|
Leary S, Underwood W, Anthony R, Cartner S, Grandin T, Greenacre C, Gwaltney-Brant S, McCrackin MA, Meyer R, Miller D, et al: AVMA guidelines for the euthanasia of animals: 2020 edition. AVMA. Accessed September 5, 2020
|
|
17
|
Jiu X, Liu Y and Wen J: Artesunate combined with verteporfin inhibits uveal melanoma by regulation of the MALAT1/yes-associated protein signaling pathway. Oncol Lett. 22:5972021. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Li B and Dewey CN: RSEM: Accurate transcript quantification from RNA-Seq data with or without a reference genome. BMC Bioinformatics. 12:3232011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Li R, Li Y, Kristiansen K and Wang J: SOAP: Short oligonucleotide alignment program. Bioinformatics. 24:713–714. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Love MI, Huber W and Anders S: Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 15:5502014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Sherman BT, Hao M, Qiu J, Jiao X, Baseler MW, Lane HC, Imamichi T and Chang W: DAVID: A web server for functional enrichment analysis and functional annotation of gene lists (2021 update). Nucleic Acids Res. 50(W1): W216–W221. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Yu C, Zhou G, Shi Z, Yu L and Zhou X: TREM1 facilitates the development of gastric cancer through regulating neutrophil extracellular traps-mediated macrophage polarization. Dig Liver Dis. 56:1237–1247. 2024. View Article : Google Scholar
|
|
23
|
Kuo PL and Hsu YL: The grape and wine constituent piceatannol inhibits proliferation of human bladder cancer cells via blocking cell cycle progression and inducing Fas/membrane bound Fas ligand-mediated apoptotic pathway. Mol Nutr Food Res. 52:408–418. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Rakib A, Mandal M, Showkat A, Kiran S, Mazumdar S, Singla B, Bajwa A, Kumar S, Park F and Singh UP: Piceatannol induces regulatory T cells and modulates the inflammatory response and adipogenesis. Biomed Pharmacother. 161:1145142023. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zheng X, Wan J and Tan G: The mechanisms of NLRP3 inflammasome/pyroptosis activation and their role in diabetic retinopathy. Front Immunol. 14:11511852023. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhang Y, Tang LH, Lu J, Xu LM, Cheng BL and Xiong JY: ABT-263 enhanced bacterial phagocytosis of macrophages in aged mouse through Beclin-1-dependent autophagy. BMC Geriatr. 21:2252021. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Rantala ES, Hernberg MM, Piperno-Neumann S, Grossniklaus HE and Kivelä TT: Metastatic uveal melanoma: The final frontier. Prog Retin Eye Res. 90:1010412022. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Rao PK, Barker C, Coit DG, Joseph RW, Materin M, Rengan R, Sosman J, Thompson JA, Albertini MR, Boland G, et al: NCCN guidelines insights: Uveal melanoma, version 1.2019. J Natl Compr Canc Netw. 18:120–131. 2020.PubMed/NCBI
|
|
29
|
Bian P, Hu W, Liu C and Li L: Resveratrol potentiates the anti-tumor effects of rapamycin in papillary thyroid cancer: PI3K/AKT/mTOR pathway involved. Arch Biochem Biophys. 689:1084612020. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ma J, Xue M, Zhang S, Cheng L, Qian W, Duan W and Shen X: Resveratrol inhibits the growth of tumor cells under chronic stress via the ADRB-2-HIF-1α axis. Oncol Rep. 41:1051–1058. 2019.
|
|
31
|
van Ginkel PR, Darjatmoko SR, Sareen D, Subramanian L, Bhattacharya S, Lindstrom MJ, Albert DM and Polans AS: Resveratrol inhibits uveal melanoma tumor growth via early mitochondrial dysfunction. Invest Ophthalmol Vis Sci. 49:1299–1306. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Xie C, Liang C, Wang R, Yi K, Zhou X, Li X, Chen Y, Miao D, Zhong C and Zhu J: Resveratrol suppresses lung cancer by targeting cancer stem-like cells and regulating tumor microenvironment. J Nutr Biochem. 112:1092112023. View Article : Google Scholar
|
|
33
|
Siedlecka-Kroplewska K, Wrońska A and Kmieć Z: Piceatannol, a structural analog of resveratrol, is an apoptosis inducer and a multidrug resistance modulator in HL-60 human acute myeloid leukemia cells. Int J Mol Sci. 22:105972021. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Çınar Ayan İ, Güçlü E, Vural H and Dursun HG: Piceatannol induces apoptotic cell death through activation of caspase-dependent pathway and upregulation of ROS-mediated mitochondrial dysfunction in pancreatic cancer cells. Mol Biol Rep. 49:11947–11957. 2022. View Article : Google Scholar
|
|
35
|
Denk D and Greten FR: Inflammation: The incubator of the tumor microenvironment. Trends Cancer. 8:901–914. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Chen H, Qi Q, Wu N, Wang Y, Feng Q, Jin R and Jiang L: Aspirin promotes RSL3-induced ferroptosis by suppressing mTOR/SREBP-1/SCD1-mediated lipogenesis in PIK3CA-mutant colorectal cancer. Redox Biol. 55:1024262022. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Guo C, Guo D, Fang L, Sang T, Wu J, Guo C, Wang Y, Wang Y, Chen C, Chen J, et al: Ganoderma lucidum polysaccharide modulates gut microbiota and immune cell function to inhibit inflammation and tumorigenesis in colon. Carbohydr Polym. 267:1182312021. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Yin J, Li N, Han Y, Xue J, Deng Y, Shi J, Guo W, Zhang H, Wang H, Cheng S and Cao G: Effect of antiviral treatment with nucleotide/nucleoside analogs on postoperative prognosis of hepatitis B virus-related hepatocellular carcinoma: A two-stage longitudinal clinical study. J Clin Oncol. 31:3647–3655. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Yang X, Wu Y, Zhang M, Zhang L, Zhao T, Qian W, Zhu M, Wang X, Zhang Q, Sun J and Dong L: Piceatannol protects against age-related hearing loss by inhibiting cellular pyroptosis and inflammation through regulated caspase11-GSDMD pathway. Biomed Pharmacother. 163:1147042023. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Faria SS, Costantini S, de Lima VCC, de Andrade VP, Rialland M, Cedric R, Budillon A and Magalhães KG: NLRP3 inflammasome-mediated cytokine production and pyroptosis cell death in breast cancer. J Biomed Sci. 28:262021. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Wang J, Tan L, Wang HF, Tan CC, Meng XF, Wang C, Tang SW and Yu JT: Anti-inflammatory drugs and risk of Alzheimer's disease: An updated systematic review and meta-analysis. J Alzheimers Dis. 44:385–396. 2015. View Article : Google Scholar
|
|
42
|
Rao Z, Zhu Y, Yang P, Chen Z, Xia Y, Qiao C, Liu W, Deng H, Li J, Ning P and Wang Z: Pyroptosis in inflammatory diseases and cancer. Theranostics. 12:4310–4329. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Wu Y, Pi D, Zhou S, Yi Z, Dong Y, Wang W, Ye H, Chen Y, Zuo Q and Ouyang M: Ginsenoside Rh3 induces pyroptosis and ferroptosis through the Stat3/p53/NRF2 axis in colorectal cancer cells. Acta Biochim Biophys Sin (Shanghai). 55:587–600. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Zhu A, Cheng C, Lin S, Hong Z, Shi Z, Deng H and Zhang G: Silence of linc00023 inhibits pyroptosis and promotes cell proliferation via regulating p53. Gene. 882:1476282023. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Deng Y, Jia F, Jiang P, Chen L, Xing L, Shen X, Li L and Huang Y: Biomimetic nanoparticle synchronizing pyroptosis induction and mitophagy inhibition for anti-tumor therapy. Biomaterials. 301:1222932023. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Zheng H, Jia L, Liu CC, Rong Z, Zhong L, Yang L, Chen XF, Fryer JD, Wang X, Zhang YW, et al: TREM2 promotes microglial survival by activating Wnt/β-catenin pathway. J Neurosci. 37:1772–1784. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Yan J, Zhang Y, Wang L, Li Z, Tang S, Wang Y, Gu N, Sun X and Li L: TREM2 activation alleviates neural damage via Akt/CREB/BDNF signalling after traumatic brain injury in mice. J Neuroinflammation. 19:2892022. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Ye F, Zhang W, Fan C, Dong J, Peng M, Deng W, Zhang H and Yang L: Antileukemic effect of venetoclax and hypomethylating agents via caspase-3/GSDME-mediated pyroptosis. J Transl Med. 21:6062023. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Qian F, Kong W, Wang S and Wei K: Predicting the prognosis of hepatocellular carcinoma based on the interaction between pyroptosis, apoptosis, and necroptosis. Clin Exp Med. 23:2087–2104. 2023. View Article : Google Scholar
|
|
50
|
Li G, Zhang D, Liang C, Liang C and Wu J: Construction and validation of a prognostic model of pyroptosis related genes in hepatocellular carcinoma. Front Oncol. 12:10217752022. View Article : Google Scholar : PubMed/NCBI
|























