Introduction
Organ damage and loss are generally caused by
congenital abnormalities or acquired disorders. The clinical
application of regenerative medicine includes the use of biological
products, stem cell therapy, tissue engineering, cellular
reprogramming and gene therapy (1). Recently, a regenerative approach,
in which the local milieu of the diseased tissue or organ is
modulated into a regenerative environment to aid in the healing
process, has attracted attention and provided new insights into
'translational medicine'. It is speculated that this approach will
replace traditional transplantology in the near future. Thus, this
new regenerative approach needs to be explored.
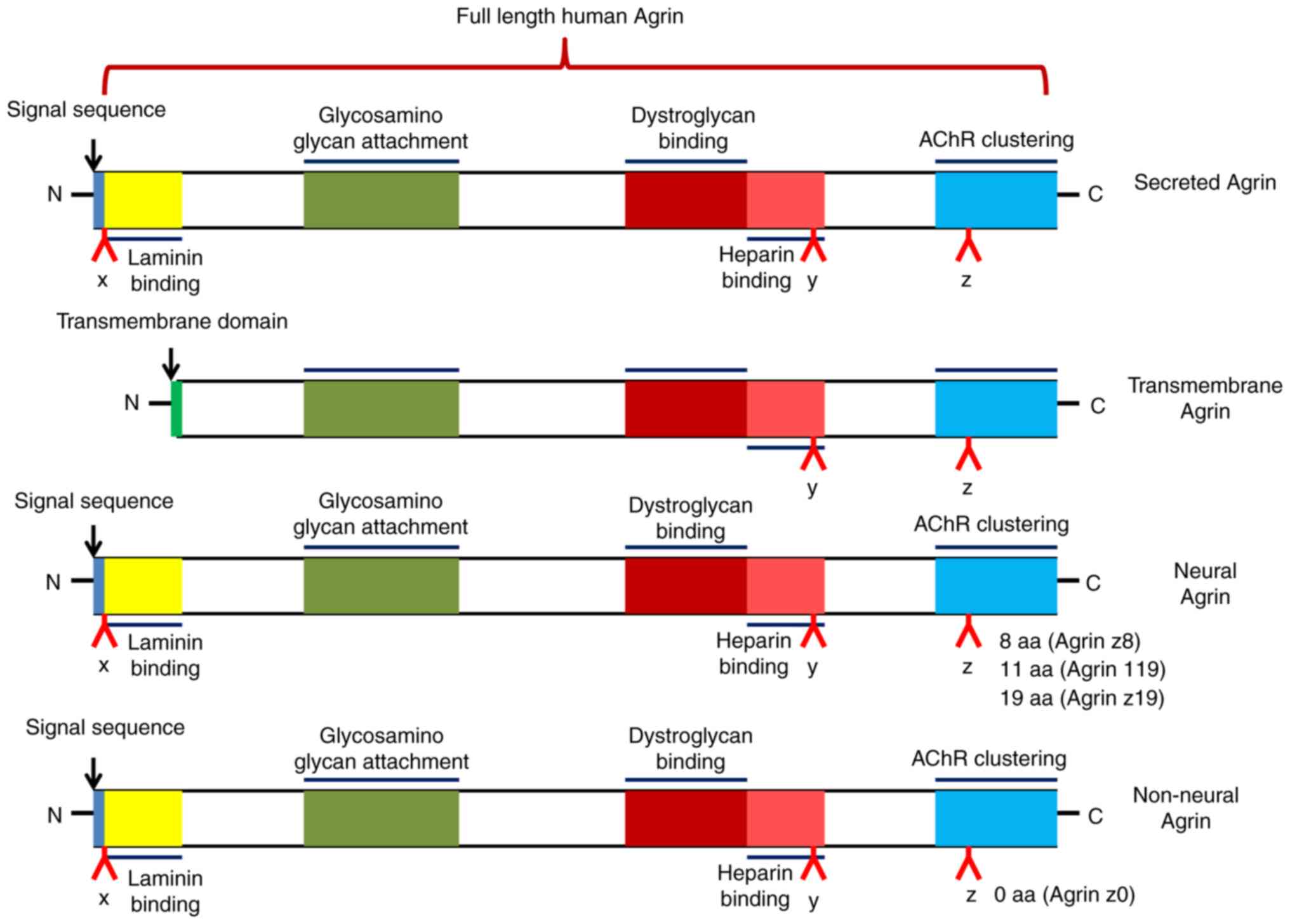
Agrin is an extracellular matrix protein that has
heparan sulfate proteoglycan as the core protein. It is encoded by
the AGRN gene and has a relative molecular weight of ~220
kDa, including nine protein kinase inhibitor domains, four
epidermal growth factor-like domains and one adhesion molecule G
homologous domain (2). The
AGRN transcript can be spliced to produce different
isoforms: Either a type II transmembrane protein or a secreted
protein (3) (Fig. 1). This splicing determines both
the localization and function of Agrin.
Agrin was originally isolated from tissue with
electrical activity. It binds to low-density lipoprotein
receptor-related protein 4 (LRP4) and activates muscle-specific
kinase (MuSK), thus forming a multiprotein complex at the
neuromuscular junction (NMJ) (4). The function of Agrin in the
formation and maintenance of the NMJ is relatively well known;
however, it also has roles in proliferation, motility, cell
adhesion and even the regulation of progression in certain types of
cancer (5-7). In a previous study, the role of
Agrin in regulating tissue proliferation and regeneration was
investigated, revealing that Agrin exerts a pleiotropic therapeutic
response, including intrinsic and extrinsic arms, which contributes
to the repair process in injured animals (8). Agrin therapy has also been reported
to promote both cardiac regeneration after myocardial infarction
and the proliferation of human induced pluripotent stem
cell-derived cardiomyocytes (9).
However, the role of Agrin during tissue repair and regeneration
remains largely unknown and investigations are needed to reveal
whether Agrin employs a similar strategy to stimulate the
proliferation of tissue and organs. In the subsequent sections of
the present review, the roles and mechanisms of Agrin in regulating
the repair of different tissues and organs were discussed, which
may offer important insights into novel strategies for regenerative
therapies. The search sequence (PRISMA flow diagram) is provided in
Fig. S1.
Regulation of Agrin in neurogenesis
Possible mechanisms of Agrin in
neurogenesis
Over the last several decades, Agrin has been
identified to play a central role in the formation of skeletal
neuromuscular synapses between presynaptic motor neurons and
postsynaptic muscle fibers (10). During this process, Agrin is
released by motor neurons and interacts with the transmembrane
LRP4, which leads to acetylcholine receptor (AChR) aggregation via
the activation of MuSK; this is important for the formation of a
functional NMJ (4).
A previous study demonstrated that local Agrin
stimulation induces the clustering of mitochondria and synaptic
vesicles, two well-known presynaptic markers, and regulates
vesicular trafficking (11).
Furthermore, Gautam et al (12) reported that postsynaptic AChR
aggregates are significantly reduced in number, size and density in
the muscles of Agrin-deficient mutant mice, suggesting that mice
lacking Agrin are unable to make synapses. This defect may be
caused by the absence of a neuron-specific isoform, with an exon
encoding only eight amino acids, which is a critical nerve-derived
inducer of postsynaptic differentiation (13). However, two reports have
indicated that synapses can form in the absence of Agrin (14,15). Cyclin-dependent kinase 5 is
activated by ACh agonists, and is required for the ACh
agonist-induced dispersion of AChR clusters that have not been
stabilized by Agrin (14).
Similarly, a study by Misgeld et al (15) demonstrated that the action of
Agrin in vivo is critically dependent on cholinergic
neurotransmission. Using double-mutant mice, these authors
demonstrated that synapses can form in the absence of Agrin,
provided that ACh is also absent. These results suggest that ACh
causes instability in newly generated postsynaptic sites and
indicate that a major physiological role of Agrin is to counteract
this 'anti-synaptogenic' influence (15).
Of note, ectopic MuSK expression promotes ectopic
synapse formation, and ectopic MuSK expression stimulates synapse
formation in the absence of Agrin and rescues the lethality of
AGRN-mutant mice. Furthermore, Agrin ablation is accompanied
by a second gene transformation, leading to increased postsynaptic
MuSK concentrations. These results further indicate that the
postsynaptic cell, and particularly MuSK, has a crucial role in the
regulation of synapse formation (16). Furthermore, studies suggest that
Agrin may stabilize existing AChR aggregates in the presence of
neurally derived dispersants (17,18). Although MuSK and the
synapse-specific cytoplasmic protein rapsyn are required in the
initial steps of postsynaptic differentiation and the formation of
an end-plate band, Agrin is not essential. However, nerve-derived
Agrin is required in the subsequent stages of synaptic growth and
maintenance (17).
Neurogenesis occurs at every stage of life,
including in adults. It has been reported that neurogenesis is
associated with learning, memory and mood regulation, and its
attenuation contributes to emotional and cognitive deficits during
aging and neurological diseases such as Alzheimer's disease
(19). In the adult hippocampus,
neural stem/progenitor cells of the subgranular zone reorganize and
proliferate to generate newborn neurons, which then integrate into
the granule cell layer of the dentate gyrus (20). During this dynamic process, the
activation and proliferation of quiescent stem cells, neuronal fate
specification, cell migration and synaptic integration are all
involved (21). In addition,
Agrin mRNA levels are reportedly increased in the mouse hippocampus
following exposure to an enriched environment, suggesting that
Agrin expression is dependent on activity (22). Rather than as a key synaptic
organizer, Agrin has been designated as a stabilizer that can
induce postsynaptic differentiation in the absence of nerves
(17,23). Another study reported that all
three members of the Agrin-MuSK-LRP4 complex are involved in the
induction of presynaptic differentiation (24), and particularly strong evidence
supports the role of LRP4 (25).
During Agrin-induced neurogenesis, multiple types of
cells and signaling pathways are involved (26). Transforming growth factor-β1 has
been reported to enhance synaptogenesis via the upregulation of
neuronal Agrin expression in Schwann cells, which is both
sufficient and necessary for mediating synapse-promoting effects in
the developing NMJ (27). In
addition, Zhang et al (28) revealed that the combined
treatment of Agrin and laminin in a co-culture system is able to
enhance functional NMJ formation through a primarily neural
mechanism, which has potential clinical importance for treating
denervation injuries and creating functional neuromuscular
constructs for muscle tissue. Zhang et al (22) have also reported that Agrin
activates the receptor tyrosine kinase orphan receptor 2 (ROR2)
through LRP4 in a mouse model, identifying a role for
Agrin-LRP4-ROR2 signaling in adult neurogenesis. Similarly, Ma
et al (29) determined
the role of Agrin in botulinum neurotoxin type A-induced nerve
sprouting in a rat model by regulating downstream MuSK and upstream
microRNA (miR)-144, and confirmed that Agrin can regulate nerve
sprouting via the miR-144-Agrin-MuSK signaling pathway. Yang et
al (23) reported that the
expression of the active Agrin isoforms B11 and B19 is upregulated
in Schwann cells during nerve regeneration in adults. As well as
reporting that neurons express active Agrin, they also noted that
glial cells express active Agrin and play a role in inducing AChR
clusters beneath perisynaptic Schwann cell sprouts (23), suggesting that Agrin may play an
indispensable role in the repair of central nervous system
injury.
Possible role of Agrin in nerve
disease
Myasthenia gravis (MG) is an autoimmune disease in
which antibodies against AChR, MuSK or other AChR-related proteins
in the NMJ cause localized or general muscle weakness (30). In patients with MG,
autoantibodies bind to the components of postsynaptic muscle
endplates and destroy the structure and function of NMJ, thus
leading to impaired neuromuscular transmission (31). Recent studies have revealed that
antibodies against Agrin and its receptor LRP4 are both critical
for NMJ formation and maintenance in patients with MG (32). Furthermore, Yu et al
(33) revealed that
anti-LRP4/Agrin antibodies in patients with MG are pathogenic; they
impair the NMJ by interrupting Agrin-dependent LRP4-MuSK
interactions. A multicenter study revealed that LRP4/Agrin
antibody-positive patients with double-seronegative MG have more
severe clinical disease than antibody-negative patients (34). These results suggest that
Agrin-LRP4-MuSK signaling may be a potential therapeutic target for
MG and other neuromuscular disorders (35). NT-1654 is a C-terminal fragment
of mouse neural Agrin; it possesses the same mechanism of action as
Agrin, by binding to LRP4 to activate MuSK protein Docking protein
7 signaling at the NMJ. It has also been demonstrated to induce
AChR aggregation and alleviate a sarcopenia-like phenotype
(36). Li et al (37) similarly reported that NT-1654
attenuates the clinical severity of this phenotype, effectively
promotes AChR aggregation at the NMJ and attenuates the repair of
NMJ transmission and the reduction of MuSK in rats with
experimental autoimmune MG.
Agrin may also play an important role in other
nerve-related diseases. Adult neurogenesis in the hippocampus may
represent the plasticity of brain functions, including emotions,
learning and memory. A decline in hippocampal neurogenesis is
thought to cause emotional and cognitive deficits in aging and
Alzheimer's disease. A recent study reported that neurogenesis in
the brains of healthy individuals may be more conservative
(38,39). Furthermore, Zhang et al
(22) revealed that Agrin is
upregulated in the hippocampus of mice stimulated by an enriched
environment. The genetic deletion of AGRN in excitatory
neurons decreases the proliferation of neural stem/progenitor cells
and increases depressive-like behavior (22). A further analysis led to a
working model in which Agrin activates ROR2 via LRP4 to promote
adult neurogenesis.
Sepsis is another infection-induced neuromuscular
dysfunction; it can induce denervation-like alterations in the NMJ,
which may cause muscle weakness (40). Lv et al (41) reported that decreased Agrin
expression may induce skeletal muscle dysfunction in sepsis,
whereas exogenous Agrin alleviates neuromuscular dysfunction and
downregulates γ- and α7-nicotinic AChR expression.
Role of Agrin in the tumor
microenvironment
In the process of tumor progression, tumors make new
capillaries by angiogenesis, and the targeting of angiogenesis
contributes to tumor treatment (42). Localized benign tumors are
surrounded by well-developed basement membranes, which limit tumors
from migrating from the surrounding tissue into blood vessels.
However, in malignant tumors, the tumor triggers an 'angiogenesis
switch', thus tipping the balance between pro- and anti-angiogenic
factors toward vascularization (43). Angiogenesis is a hallmark of
cancer, and although multiple naturally occurring compounds have
anti-angiogenic effects, these effects are not absolute (44,45). A recent study demonstrated that
different protein modules within proteoglycans can enhance tumor
cell plasticity and metastasis (46). However, the molecular mechanisms
underlying their recruitment of blood vessels within the tumor
niche remain largely elusive.
As a surface proteoglycan, the role of Agrin in
promoting cancer angiogenesis has been demonstrated recently
(7,47). He et al (47) reported a high expression of Agrin
in cholangiocarcinoma tissue compared with that in adjacent
non-tumor tissues; further analysis revealed that Agrin expression
is associated with poorer prognosis, such as portal vein tumor
thrombus and intrahepatic metastasis. Furthermore, forced Agrin
expression in cholangiocarcinoma cells appears to promote tumor
growth-related processes such as proliferation, colony formation,
migration and invasion. In rectal cancer, Agrin expression has also
been revealed to be markedly increased, and its upregulation is
associated with poor prognosis. However, Agrin inhibition
suppresses cell growth in rectal cancer, whereas Agrin
overexpression prompts these behaviors in vitro (7). Mechanistically, multiple signaling
pathways may participate in Agrin-regulated cancer progression.
Agrin reportedly activates the Hippo signaling pathway and induces
the translocation of yes-associated protein (YAP) to the nucleus in
cholangiocarcinoma (47).
However, Agrin also elevates Wnt pathway activity by increasing
cyclin D1, c-Myc, phosphorylated glycogen synthase kinase-3β and
phosphorylated β-catenin levels (7). These results indicate that Agrin
may function as an oncogenic indicator of cancer progression
through its activation of various pathways, which may be helpful
for developing optimized therapies for cancer.
In the healthy liver, Agrin expression in
hepatocytes is minimal and its expression is limited to certain
regions surrounding blood vessels. However, during the
transformation of liver cirrhosis and hepatocellular carcinoma,
Agrin levels in hepatocytes are significantly increased (22). The role of Agrin in extracellular
matrix sensing and mechanical conduction has been reported to
integrate integrin and focal adhesion, as well as the activation of
YAP/tafazzin (TAZ) to promote liver tumor growth (48,49). Furthermore, the depletion of
Agrin in cancer cells reduces blood vessel infiltration and
suppresses tumor growth, as well as the metastasis of
hepatocellular carcinoma cells to mouse lungs. Strikingly,
metastatic lesions that can colonize the lungs by Agrin deficiency
lack the capacity to attract peripheral pulmonary vessels within
these lesions (50).
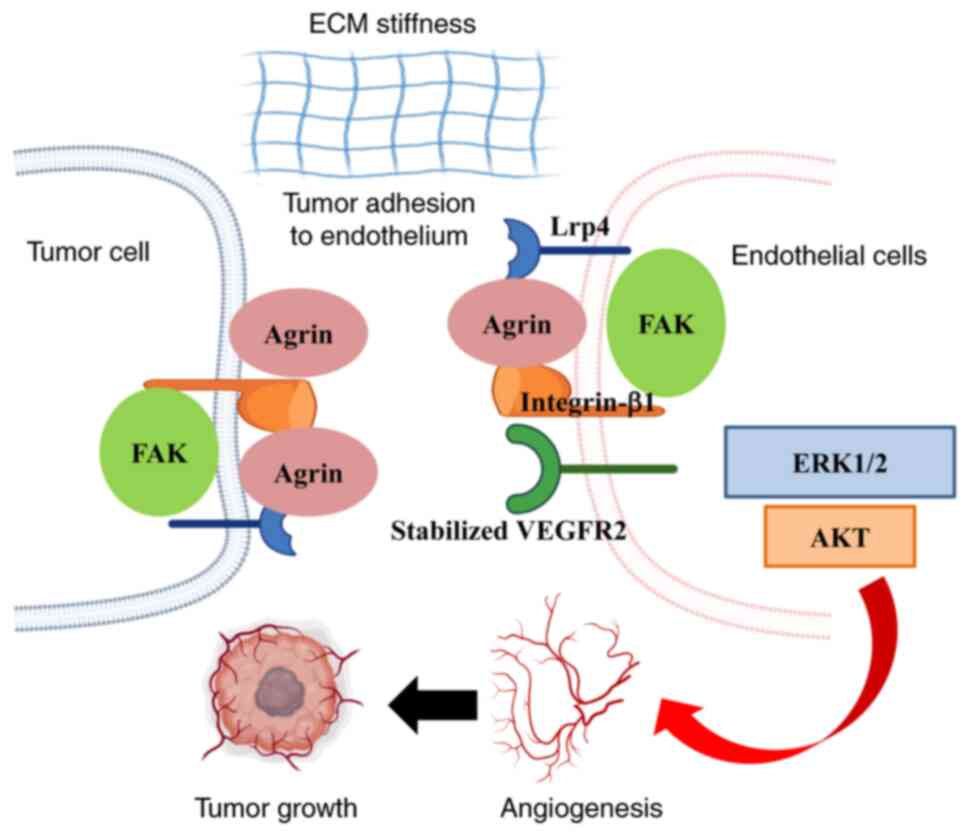
Endothelial cell recruitment is critical for tumor
vascularization. Njah et al (50) demonstrated that Agrin promotes
the adherence of endothelial cells to tumor cells by recruiting
blood vessels, and then facilitates tumor angiogenesis. Further
analysis revealed that Agrin stabilizes vascular endothelial growth
factor receptor 2 (VEGFR2) by enhancing its interactions with
LRP4-integrin-b1-focal adhesion kinase (FAK) (Fig. 2). This suggests that tumor
angiogenesis may be inhibited by targeting Agrin-induced VEGFR2
reductions. Furthermore, by inactivating the Hippo pathway, Agrin
can promote extracellular matrix remodeling and stiffness by
enhancing YAP/TAZ/transcriptional enhancer associated
domain-dependent transcription, which then promotes tumorigenesis
(49,51,52). Notably, consistent with other
proteoglycans that affect endothelial cell migration, it has been
reported that Agrin-either secreted by cancer cells or exogenously
supplied - is essential for angiogenesis (53).
In the context of oral squamous cell carcinoma
progression, Agrin is upregulated in oral squamous cell carcinoma
and promotes cell migration and adhesion, suggesting that Agrin
also plays an oncogenic role in oral cancer (54). Agrin can be cleaved through
protein hydrolysis to produce bioactive fragments (55). One of the cleavage products is
the C-terminal fragment, which reportedly has a role in multiple
pathological processes, including sarcopenia (56), renal dysfunction (57) and cancer (58). Rivera et al (59) reported that invasive oral
carcinomas have higher Agrin expression than benign tissue. These
results suggest that the presence of Agrin may promote cancer
progression in the tumor microenvironment. In oral squamous cell
carcinoma cells, Agrin can activate FAK by binding to integrins or
dystroglycan complexes, and can then induce cell invasion and
metastasis through growth factor receptor-bound protein 2,
proto-oncogene tyrosine-protein kinase Src, extracellular regulated
protein kinases and cyclin D (60,61). Nonetheless, the role of
C-terminal fragment Agrin in angiogenesis needs to be further
investigated in oral cancer, with a focus on tumor progression.
Other studies have reported that high Agrin
expression is also associated with tumor progression and poor
prognosis in hepatocellular carcinoma and lung adenocarcinoma
(62,63). Agrin-positive staining can be
used to identify patients with an increased risk of metastasis
after surgery for lung adenocarcinoma, and may therefore be a
valuable prognostic marker (62). Furthermore, the recurrence-free
survival rate of Agrin-positive patients with hepatocellular
carcinoma was reported to be significantly lower than that of
Agrin-negative patients (63).
The potential mechanism may be the result of Agrin-mediated
tumor-related angiogenesis in the tumor microenvironment (53). Furthermore, Agrin knockdown in
multiple human endothelial cell lines, including human umbilical
vascular, human retinal, human dermal microvascular and human
aortic endothelial cells, was observed to be associated with
significantly reduced in vitro angiogenesis after treatment
with soluble Agrin (50).
However, in endothelial cell-specific Agrin knockout mice, normal
endothelial and hematopoietic cell development was observed during
embryogenesis (64). Of note,
the growth of localized or metastatic cancer cells is not affected
after their implantation into mice with Agrin-depleted endothelial
cells. This finding suggests that Agrin may not play an important
role in endothelial development during physiological and
tumor-related angiogenesis; targeting endothelial-derived Agrin may
therefore not be effective in inhibiting tumor angiogenesis.
Any discrepancies among these studies may be
attributed to the availability of Agrin in vitro vs. in
vivo, which may rescue the loss of Agrin in endothelial cells
in AGRN-knockout mice during tumor angiogenesis. This
suggests that in the early stages of tumor growth, endothelial cell
recruitment does not require endothelial Agrin, as tumor-derived
Agrin can compensate for the endothelial loss of Agrin. Thus, the
profiles and mechanisms of Agrin in tumorigenesis need to be
further studied.
Agrin-mediated cardiac regeneration
At present, heart disease is the leading cause of
death world-wide, and repairing the damaged heart remains one of
the most critical challenges in cardiovascular disease. In mammals,
post-mitotic adult cardiomyocytes lose their proliferative capacity
for replenishing damaged tissue (65). Upon injury, cardiomyocytes are
replaced by fibrotic tissue, which usually has deleterious
consequences. Hypertrophy therefore becomes responsible for most
remaining heart growth. Notably, cardiomyocyte proliferation is
sufficient for the repair of cardiac injury in neonatal mice;
however, this ability is greatly diminished by 1 week after birth
(66). In the past decade,
however, researchers have questioned whether mature hearts truly
lack the ability to produce new myocardium after injury; indeed,
their findings suggest the possibility of a substantial endogenous
regenerative capacity.
Studies have demonstrated that the proteoglycan
Agrin can promote cardiomyocyte proliferation as an extracellular
matrix component and is involved in neonatal heart repair (9,67). Of note, Agrin is reportedly
enriched in the matrix on postnatal day 1 but decreased by
postnatal day 7. Crucially, the administration of recombinant Agrin
promotes cardiac regeneration in adult mice after myocardial
infarction in vivo through the reactivation of cardiomyocyte
proliferation. A porcine model of acute myocardial infarction,
which is closely related to human cardiac physiology, has been used
to demonstrate that recombinant human Agrin delivered to the
infarcted heart can target the affected regions in an efficient and
clinically relevant manner. These results indicate that recombinant
Agrin may be a novel therapy for acute myocardial infarction and
may prevent the onset of chronic heart failure (68). The data obtained from these mouse
and porcine models highlight that Agrin treatment may strengthen
pleiotropic effects and the broad cardiac repair process.
Agrin has been reported to bind and signal through
α-dystroglycan (DAG1) (69) with
C-terminus laminin G-like domains (LG1 and LG2) (60,70). In the heart, DAG1 serves as an
Agrin receptor that is expressed by cardiomyocytes and mediates
mild dedifferentiation and proliferation (9). The aforementioned reports emphasize
the potential therapeutic role of Agrin in cardiac repair; this
therapy is simple, safe and clinically relevant, and may be used in
patients. However, further research is needed regarding the
molecular mechanisms, effective dose ranges, toxicity and
pharmacokinetics of Agrin.
The results from mouse (9) and porcine (68) myocardial infarction models that
received local injections of recombinant Agrin into the damaged
heart indicate that Agrin may have an overall cardioprotective
effect. This was also accompanied by anti-inflammatory effects and
blood vessel production, which adds to cardiomyocyte protection and
induces proliferation. These findings suggest that Agrin has the
potential to regulate cardiomyocyte cell proliferation. However,
epicardial cells with multiple differentiation potential are also
of great importance for cardiac regeneration (71). Normal development of the
embryonic heart is reportedly regulated by epicardial cells through
differentiation and proliferation (72,73). However, it remains unclear
whether Agrin can promote the proliferation of embryonic epicardial
cells. Jing et al (74)
revealed that epicardial cell proliferation is promoted by Agrin
through its regulation of YAP activity. Furthermore, YAP is key for
the regulation of Hippo signaling (75), and Agrin has been reported to
inhibit the Hippo signaling pathway and promote YAP activity
(76). Although cardiac
dysplasia can be induced by specific exfoliation of the embryonic
epicardium (77), the abnormal
epithelial-mesenchymal transition, migration and differentiation of
epicardial cells can also be induced by proliferation disorders
(78,79). The migration and
epithelial-mesenchymal transition of epicardial cells are
controlled by interactions between epicardial cells and the
extracellular matrix (80,81). Thus, Agrin may play an important
role in cardiac regeneration through its regulation of epicardial
cells.
Considering the various risks and technical issues
of current cardiac regenerative strategies, Agrin therapy has
potential as a relatively safe and effective drug for repairing
damaged hearts. However, caution is needed when performing this
cardiac regenerative strategy because of the role of Agrin in
tumorigenesis and angiogenesis. Furthermore, to evaluate the
potential benefits of therapeutic Agrin, it should be considered
whether Agrin can stimulate human cardiac fibroblast
hyperproliferation. It would also be worthwhile to investigate
whether Agrin has a similar mechanism of action for stimulating
cardiomyocyte and cancer cell proliferation.
Agrin mediates cartilage formation
Cartilage loss leads to osteoarthritis; cartilage
has a low turnover and often fails to repair after injury because
it is devoid of blood vessels (82). Cartilage regeneration is
therefore a priority in medicine. A study by Erickson et al
(83) indicated that AGRN
was initially upregulated throughout fracture healing, suggesting
that Agrin may have a novel function in non-neural tissue,
including cartilage. Consistent with a role for Agrin in skeletal
development, a study by Hausser et al (84) demonstrated that Agrin is involved
in postnatal skeletal development and endochondral bone formation
in transgenic mice. Furthermore, chondrocytes have high Agrin
expression in the growth plate, suggesting that the expression of
Agrin in cartilage may have a critical role in normal skeletal
growth (84).
Agrin is composed of a large N-terminal portion,
which binds to components of the basal membrane, and a biologically
active C-terminal portion, which contains three globular domains
separated by epidermal growth factor-like repeats (85). Eldridge et al (86) reported that Agrin is expressed in
a splice isoform without y and z motifs; they also identified Agrin
as having strong therapeutic potential in cartilage regeneration.
Agrin is expressed in normal cartilage but is progressively lost in
osteoarthritis, and Agrin knockdown induces downregulation of the
cartilage transcription factor SOX9, as well as other
cartilage-specific extracellular matrix molecules. Conversely,
cartilage differentiation in vitro and ectopic cartilage
formation in vivo are supported by exogenous Agrin. These
results suggest that Agrin plays an important role in
chondrogenesis and the repair of osteochondral defects (87).
Reduced growth and impaired skeletal development
have been observed in Agrin-null mice, thus indicating an important
role for Agrin in chondrocyte biology (84). A study by Eldridge et al
(87) also revealed that Agrin
is upregulated in injured cartilage and induces chondrogenic
differentiation in synovial membrane mesenchymal stem cells. A
single intra-articular administration of Agrin induces the
long-lasting regeneration of critical-size osteochondral defects by
attracting joint-resident progenitor cells to the injury site.
Furthermore, the simultaneous activation of cAMP responsive
element-binding protein and the suppression of canonical Wnt
signaling downstream of b-catenin are critical for inducing stable
articular chondrocyte differentiation and the formation of stable
articular cartilage. In addition, considering the close
relationship between cartilage and bone, Agrin may be involved in
the replacement of cartilage by bone; this process involves
multiple steps and several components of the extracellular matrix
(84,86,88,89).
Agrin plays an important role in chondrocyte biology
and participates in multiple signal transduction pathways. The Wnt
pathway has been well characterized, and is thus an attractive
therapeutic target for bone repair and skeletal homeostasis
(90). As described in the
previous paragraph, β-catenin has various roles at different stages
of bone repair and regulates the ratio of osteoblasts and
chondrocytes in the callus, which arises from pluripotent
mesenchymal stem cells in the early phases after injury (91). Later in the bone healing process,
β-catenin induces osteoblast differentiation and osteoblastic
matrix production (92). As the
main receptor of Agrin, LRP4 is also involved in the modulation of
Wnt signal transduction, which is an important pathway in
osteogenic differentiation and bone formation (93-95). Souza et al (96) reported that Agrin and its
receptors (LRP4 and DAG1) are expressed during the differentiation
of osteoblasts from three different sources. Furthermore, Agrin
disruption impairs the expression of its receptors, as well as
osteoblast differentiation, and treatment with recombinant Agrin
slightly improves this process. Agrin knockdown also downregulates
the expression of genes related to Wnt and bone morphogenetic
protein (BMP) signaling pathways. These results highlight the
contribution of the Agrin-Wnt-BMP pathway to osteoblast
differentiation and suggest that Agrin is a candidate target in the
development of new therapeutic strategies for bone-related diseases
and injuries (96).
Together, the aforementioned results indicate that
Agrin may be an orchestrator of repair morphogenesis at the joint
surface through its modulation of multiple signaling pathways. We
therefore anticipate that it represents a unique therapeutic
opportunity in the field of osteoarthritis, which opens new avenues
of investigation for cartilage-regenerative medicine. However, in
the clinic, cartilage defects are often associated with
meniscal/ligament injury and are at times accompanied by
osteoarthritis. Given that joint instability can be compromised in
the presence of inflammation, it remains to be explored whether
Agrin can induce cartilage regeneration under these conditions.
Role of Agrin in aging and disease
During aging, AChR clusters at the NMJ become
fragmented and denervated. As the site of information exchange and
storage between motor neurons and muscles, deleterious
morphological, functional and molecular features are acquired in
the NMJ with advancing age, and the NMJ ultimately degenerates
(97). NMJ activity requires
communication (through molecular mechanisms) among all three
cellular components: The presynapse, postsynapse and postsynaptic
currents. These three cellular components collaborate through
synapse-associated molecules to regenerate adult NMJs following
injuries that cause severed motor axons, postsynaptic current loss
and muscle fiber atrophy (98).
A study suggested that age-related NMJ decline is induced by
compromised Agrin-LRP4-MuSK signaling (99). This highlights the
interdependence between all three cellular components of the NMJ,
as well as the roles of synapse-associated molecules, such as
Agrin, LRP4 and MuSK receptors, in the maintenance and repair of
adult NMJs. In this regard, a study has shown that Agrin deletion
in a subset of adult motor neurons causes postsynaptic
disintegration and motor axon degeneration, suggesting that
synaptic molecules-including Agrin-remain essential in adult NMJs
(100). This indicates that
synaptic molecules that are essential for NMJ formation continue to
play important functions in adulthood. Together, these findings
suggest that age-related changes in NMJs may be mitigated by
targeting these molecules. Studies that have assessed therapeutic
potential following injury and in disease have demonstrated that
Agrin and other integral components of the NMJ can repair
age-related damage. Furthermore, following sciatic nerve crush
surgery in young animals, administration of biologically active
Agrin fragments into skeletal muscles can accelerate the rate of
NMJ remodeling (36).
Sarcopenia is characterized by the loss of skeletal
muscle mass, strength and function, and is a strong predictor of
multiple adverse health outcomes, such as physical disability,
hospitalization and mortality (101,102). During the neuromuscular
remodeling process, the neuronal protease neurotrypsin
proteolytically cleaves and inactivates AGRN, thus
dissociating a 22-kDa C-terminal Agrin fragment (103). Recently, the C-terminal Agrin
fragment concentrations in the circulation have emerged as a
potential biomarker of skeletal muscle deterioration (104), which may signal the onset of
sarcopenia.
Intriguingly, a study has demonstrated that
appendicular lean mass, age and sex are significant explanatory
factors for C-terminal Agrin fragment concentrations. In male
individuals especially, there is a strong correlation between serum
C-terminal Agrin concentrations and appendicular lean mass.
Furthermore, vitamin D supplementation and physical exercise are
significantly associated with lower C-terminal Agrin concentrations
(105). This finding indicates
that C-terminal Agrin fragments may be a potential marker for
identifying sarcopenia in a subgroup of affected individuals in the
future. Furthermore, the viability of the C-terminal Agrin fragment
as a biomarker for sarcopenia has been confirmed in a variety of
subpopulations (106). These
results have also revealed that z-Agrin degradation during aging
may contribute to NMJ pathology. Pratt et al (107) reported that plasma C-terminal
Agrin fragment concentrations are significantly higher in
sarcopenic individuals than in nonsarcopenic individuals,
suggesting the potential relevance of C-terminal Agrin fragments as
an accessible biomarker for skeletal muscle health. Together, these
findings indicate that C-terminal Agrin fragments may be used for
the diagnosis of sarcopenia, and may also have potential as an
early indicator of denervation.
Functions of Agrin in other tissue and
organs
In tissues such as the kidneys and lungs, Agrin
plays a role in mechanotransduction by linking the cell
cytoskeleton to other basement membrane components, including DAG1
and laminin-γ1 (108,109), through either direct binding or
indirectly via integrins (60).
Raats et al (110)
concluded that the presence of Agrin in the glomerular capillary
wall and its ultrastructural localization are involved in linking
the podocyte cytoskeleton to the glomerular basement membrane.
However, other studies have directly demonstrated the contribution
of Agrin to glomerular basement membrane functionality in podocytes
and have reported that the glomerular basement membrane shows
normal renal function with no changes in glomerular architecture,
suggesting that Agrin is not required for the establishment or
maintenance of glomerular basement membrane architecture (111,112). Vestentoft et al
(113) reported that the levels
of extracellular matrix constituents, including Agrin, are
significantly upregulated after rat liver injury, mainly in the
second tier of defense. These findings suggest that Agrin may play
an important role in the hepatic progenitor cell response process
for tissue repair and indicate a potentially important biological
role for Agrin in other tissues and organs; however, the detailed
roles and mechanisms remain unclear.
Limbal stem cell deficiency is an ocular surface
disorder that is caused by the decreased population of corneal
epithelial stem or progenitor cells and their dysfunction, which
leads to vision loss and corneal blindness (114-116). Corneal epithelium regeneration
depends on limbal stem cells, which may contribute to maintaining
corneal epithelium homeostasis (117). Hou et al (76) reported that Agrin promotes limbal
stem cell proliferation in vitro and noted that Agrin
accelerates the wound-healing rate of corneal epithelium by
activating limbal stem-cell proliferation in vivo. Further
analysis of the underlying mechanism revealed that Agrin can
facilitate the nuclear translocation of YAP1 and the expression of
cyclin D1 induced by YAP1 dephosphorylation, which subsequently
promotes limbal stem cell proliferation (76).
Hematopoiesis is a dynamic process that refers to
the production of all hematopoietic-lineage cells generated by
multipotent hematopoietic stem cells (118). Among the extracellular matrix
components, heparan sulfate proteoglycans reportedly play a crucial
role in controlling the structural and functional organization of
the bone marrow hematopoietic stem cell niche (119). Agrin is expressed by
multipotent nonhematopoietic mesenchymal stem cells as well as by
differentiated osteoblasts from the surface of the bone
endometrium. Furthermore, Mazzon et al (69) reported that Agrin is a critical
niche-derived signal that controls the survival and proliferation
of hematopoietic stem cells, and demonstrated that, although
Agrin-deficient mice display impaired hematopoiesis, this can be
reverted by Agrin-sufficient stroma. These findings suggest that
Agrin may play a crucial role in the hematopoietic niche and in the
cross-talk between stromal and hematopoietic stem cells.
Wound healing represents a complex biological
program for restoring damaged tissue architecture to a normal state
(120). During this process, a
major factor for effective wound healing following injury is the
rate of deposition of new extracellular matrix and its components
that favor the healing process, which may subsequently support
keratinocyte proliferation, migration and angiogenesis (121). A clinical trial study by
Chakraborty et al (122)
revealed that the recombinant Agrin fragment has great potential to
accelerate wound healing as a bio additive material, and enrichment
of the proteoglycan Agrin occurs early in the wound
microenvironment, which indicates that it is essential for healing.
Importantly, Agrin enhances the actomyosin cables by sensing
geometric stress and force after injury, and then completely alters
the cytoskeletal structure. Furthermore, matrix
metalloproteinase-12 (MMP12) has been identified as a downstream
effector of Agrin (123), and
the Agrin-MMP12 pathway integrates a broad range of mechanical
stimuli to promote optimal mechanical biology for wound healing and
the generation of pro-angiogenic parameters. Therefore,
injury-triggered Agrin enrichment has been proposed to integrate a
broad range of mechanical stimulation and enhanced mechanical
sensation through MMP12 activity in keratinocytes, which ultimately
favors wound healing.
Conclusions and perspectives
Although Agrin was initially identified as a factor
that is critical for NMJ function, its function in other
tissues-including cardiac regeneration, tumor growth and cartilage
formation-implies a widespread role for this protein. Existing
evidence suggests that Agrin function is associated with the
regulation of tissue proliferation and regeneration. During this
process, multiple fundamental signaling pathways that are modulated
by Agrin are involved (3,9,22,74,76,86,87,122,124,125)
(Table I), and different
molecular mechanisms appear to play functional roles in different
cell types. For instance, Agrin promotes epithelial-mesenchymal
transition by decreasing β-catenin and promoting phosphorylated FAK
localization at focal adhesions in human embryonic stem
cell-derived epicardial-like cells, and enhances dystroglycan
aggregation in the Golgi apparatus. The absence of Agrin leads to
the dispersal of dystroglycan in vivo, thus disrupting
basement membrane integrity and impairing epithelial-mesenchymal
transition. Injury-triggered Agrin enrichment induces a broad range
of signaling pathways in different tissues, which ultimately favors
the development of an injury microenvironment. In summary, Agrin
represents a unique therapeutic opportunity in regenerative
medicine, and a new and exciting research avenue involves
understanding how to achieve a high specificity of biological
effects by regulating otherwise-pleiotropic signaling pathways.
However, despite initial findings, the precise function of Agrin in
repair and regeneration-at both the molecular and whole-organism
levels-remains to be elucidated.
 | Table ISignaling network during
Agrin-induced regeneration. |
Table I
Signaling network during
Agrin-induced regeneration.
| Author(s),
year | Cell type | Function | Targets | Mechanism | (Refs.) |
|---|
| Bassat et
al, 2017 | Cardiomyocyte | Promotes
division | DGC/YAP | Disassembly of the
DGC, and Yap- and ERK-mediated signalling | (9) |
| Zhang et al,
2019 | NSPCs | Neurogenesis | LRP4/Ror2 | Promotes adult
neurogenesis through proliferation and maturation of NSPCs | (22) |
| Jing et al,
2021 | Epicardial
Cells | Promotes
proliferation | YAP | Increases the
expression of Ki67, pH3 and Aurora B through YAP | (74) |
| Hou et al,
2020 | Limbal stem
cells | Promotes
proliferation | YAP/Cyclin D1 | Facilitates the
dephosphorylation of Yap1 and activates transcription of Cyclin
D1 | (76) |
| Eldridge et
al, 2016 | Chondrocyte |
Differentiation | LRP4/DAG/DKK1 | Inhibition of WNT
pathway and promotion of SOX9 expression | (86) |
| Eldridge et
al, 2020 | Chondrocyte |
Differentiation |
LRP4/DAG/CaMKII | Inhibition of WNT
signaling in a CREB-dependent manner | (87) |
| Chakraborty et
al, 2021 | Keratinocytes | Enhances
mechanically competent | MMP-12 | Overhauls
cytoskeletal architecture via enhancing actomyosin cables | (122) |
| Chakraborty et
al, 2018; Calvo et al, 2013 | Cancer cell | Carcinogenesis | MuSK | Inhibition of the
Hippo pathway by promoting the FAK-ILK-PAK1 axis | (3,124) |
| Sun et al,
2021 | Epicardial
cells | Regulator of
EMT | β-catenin/pFAK | Enhances EMT by
decreasing β-catenin and promoting pFAK localization and the
aggregation of dystroglycan | (125) |
Supplementary Data
Availability of data and materials
Not applicable.
Authors' contributions
YYM conceived the study. XL reviewed the literature
and wrote the draft. YX interpreted the information and checked the
manuscript. JXS and FG collected part of the information and
produced the figures. YYM supervised the preparation of the study
and revised the manuscript. All authors read and approved the final
manuscript. Data authentication is not applicable.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Abbreviations:
|
LRP4
|
low density lipoprotein
receptor-related protein 4
|
|
MuSK
|
muscle-specific kinase
|
|
NMJ
|
neuromuscular junction
|
|
AChR
|
acetylcholine receptor
|
|
NSPCs
|
neural stem/progenitor cells
|
|
MG
|
myasthenia gravis
|
|
DNMG
|
double-seronegative myasthenia
gravis
|
|
YAP
|
yes-associated protein
|
|
VEGFR2
|
vascular endothelial growth factor
receptor 2
|
|
FAK
|
focal adhesion kinase
|
|
ERK
|
extracellular regulated protein
kinases
|
|
CREB
|
cAMP responsive element binding
protein
|
|
BMP
|
bone morphogenetic protein
|
|
MMP12
|
matrix metalloproteinase-12
|
Acknowledgements
Not applicable.
Funding
This work was supported by the Natural Science Foundation of
Zhejiang Province (grant nos. LBY22H180006 and LY21H160048) and the
Medical Science and Technology Project of Zhejiang Province (grant
nos. 2021KY529 and 2022RC102).
References
|
1
|
Mao AS and Mooney DJ: Regenerative
medicine: Current therapies and future directions. Proc Natl Acad
Sci USA. 112:14452–14459. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Tsim KW, Ruegg MA, Escher G, Kroger S and
McMahan UJ: cDNA that encodes active agrin. Neuron. 8:677–689.
1992. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Chakraborty S and Hong W: Linking
extracellular matrix agrin to the hippo pathway in liver cancer and
beyond. Cancers (Basel). 10:452018. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Xie T, Xu G, Liu Y, Quade B, Lin W and Bai
XC: Structural insights into the assembly of the agrin/LRP4/MuSK
signaling complex. Proc Natl Acad Sci USA. 120:e23004531202023.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Adamiok-Ostrowska A, Grzanka M and
Czarnocka B: Agrin is a novel oncogenic protein in thyroid cancer.
Oncol Lett. 26:4832023. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Han L, Shi H, Ma S, Luo Y, Sun W, Li S,
Zhang N, Jiang X, Gao Y, Huang Z, et al: Agrin promotes non-small
cell lung cancer progression and stimulates regulatory T cells via
increasing IL-6 secretion through PI3K/AKT pathway. Front Oncol.
11:8044182022. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wang ZQ, Sun XL, Wang YL and Miao YL:
Agrin promotes the proliferation, invasion and migration of rectal
cancer cells via the WNT signaling pathway to contribute to rectal
cancer progression. J Recept Signal Transduct Res. 41:363–370.
2021. View Article : Google Scholar
|
|
8
|
Sarig R, Rimmer R, Bassat E, Zhang L,
Umansky KB, Lendengolts D, Perlmoter G, Yaniv K and Tzahor E:
Transient p53-mediated regenerative senescence in the injured
heart. Circulation. 139:2491–2494. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Bassat E, Mutlak YE, Genzelinakh A,
Shadrin IY, Baruch Umansky K, Yifa O, Kain D, Rajchman D, Leach J,
Riabov Bassat D, et al: The extracellular matrix protein agrin
promotes heart regeneration in mice. Nature. 547:179–184. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Li L, Xiong WC and Mei L: Neuromuscular
junction formation, aging, and disorders. Annu Rev Physiol.
80:159–188. 2018. View Article : Google Scholar
|
|
11
|
Oentaryo MJ, Tse AC and Lee CW: Neuronal
MT1-MMP mediates ECM clearance and Lrp4 cleavage for agrin
deposition and signaling in presynaptic development. J Cell Sci.
133:jcs2467102020. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Gautam M, Noakes PG, Moscoso L, Rupp F,
Scheller RH, Merlie JP and Sanes JR: Defective neuromuscular
synaptogenesis in agrin-deficient mutant mice. Cell. 85:525–535.
1996. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Burgess RW, Nguyen QT, Son YJ, Lichtman JW
and Sanes JR: Alternatively spliced isoforms of nerve- and
muscle-derived agrin: Their roles at the neuromuscular junction.
Neuron. 23:33–44. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lin W, Dominguez B, Yang J, Aryal P,
Brandon EP, Gage FH and Lee KF: Neurotransmitter acetylcholine
negatively regulates neuromuscular synapse formation by a
Cdk5-dependent mechanism. Neuron. 46:569–579. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Misgeld T, Kummer TT, Lichtman JW and
Sanes JR: Agrin promotes synaptic differentiation by counteracting
an inhibitory effect of neurotransmitter. Proc Natl Acad Sci USA.
102:11088–11093. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kim N and Burden SJ: MuSK controls where
motor axons grow and form synapses. Nat Neurosci. 11:19–27. 2008.
View Article : Google Scholar
|
|
17
|
Lin W, Burgess RW, Dominguez B, Pfaff SL,
Sanes JR and Lee KF: Distinct roles of nerve and muscle in
postsynaptic differentiation of the neuromuscular synapse. Nature.
410:1057–1064. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yang X, Arber S, William C, Li L, Tanabe
Y, Jessell TM, Birchmeier C and Burden SJ: Patterning of muscle
acetylcholine receptor gene expression in the absence of motor
innervation. Neuron. 30:399–410. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Grabrucker S, Marizzoni M, Silajdzic E,
Lopizzo N, Mombelli E, Nicolas S, Dohm-Hansen S, Scassellati C,
Moretti DV, Rosa M, et al: Microbiota from Alzheimer's patients
induce deficits in cognition and hippocampal neurogenesis. Brain.
146:4916–4934. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Goncalves JT, Schafer ST and Gage FH:
Adult neurogenesis in the hippocampus: From stem cells to behavior.
Cell. 167:897–914. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ming GL and Song H: Adult neurogenesis in
the mammalian brain: Significant answers and significant questions.
Neuron. 70:687–702. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhang H, Sathyamurthy A, Liu F, Li L,
Zhang L, Dong Z, Cui W, Sun X, Zhao K, Wang H, et al:
Agrin-Lrp4-Ror2 signaling regulates adult hippocampal neurogenesis
in mice. Elife. 8:e453032019. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Yang JF, Cao G, Koirala S, Reddy LV and Ko
CP: Schwann cells express active agrin and enhance aggregation of
acetylcholine receptors on muscle fibers. J Neurosci. 21:9572–9584.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Yu J, Oentaryo MJ and Lee CW: Local
protein synthesis of neuronal MT1-MMP for agrin-induced presynaptic
development. Development. 148:dev1990002021. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Uyen Dao TM, Barbeau S, Messeant J,
Della-Gaspera B, Bouceba T, Semprez F, Legay C and Dobbertin A: The
collagen ColQ binds to LRP4 and regulates the activation of the
Muscle-Specific Kinase-LRP4 receptor complex by agrin at the
neuromuscular junction. J Biol Chem. 299:1049622023. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Gao H, Zhao Z, Li J, Guo Z, Zhang F, Wang
K, Bai X, Wang Q, Guan Y, Wang Y, et al: Platelet-rich plasma
promotes skeletal muscle regeneration and neuromuscular functional
reconstitution in a concentration-dependent manner in a rat
laceration model. Biochem Biophys Res Commun. 672:185–192. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Feng Z and Ko CP: Schwann cells promote
synaptogenesis at the neuromuscular junction via transforming
growth factor-beta1. J Neurosci. 28:9599–9609. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zhang BG, Quigley AF, Bourke JL, Nowell
CJ, Myers DE, Choong PF and Kapsa RM: Combination of agrin and
laminin increase acetylcholine receptor clustering and enhance
functional neuromuscular junction formation In vitro. Dev
Neurobiol. 76:551–565. 2016. View Article : Google Scholar
|
|
29
|
Ma L, Pan L, Liu W, Liu Y, Xiang X, Pan Y,
Zhang X and Jin L: Agrin influences botulinum neurotoxin a-induced
nerve sprouting via miR-144-agrin-MuSK signaling. Front Cell Dev
Biol. 8:152020. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Gilhus NE, Tzartos S, Evoli A, Palace J,
Burns TM and Verschuuren JJGM: Myasthenia gravis. Nat Rev Dis
Primers. 5:302019. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Lazaridis K and Tzartos SJ: Autoantibody
specificities in myasthenia gravis; implications for improved
diagnostics and therapeutics. Front Immunol. 11:2122020. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Yan M, Xing GL, Xiong WC and Mei L: Agrin
and LRP4 antibodies as new biomarkers of myasthenia gravis. Ann N Y
Acad Sci. 1413:126–135. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Yu Z, Zhang M, Jing H, Chen P, Cao R, Pan
J, Luo B, Yu Y, Quarles BM, Xiong W, et al: Characterization of
LRP4/agrin antibodies from a patient with myasthenia gravis.
Neurology. 97:e975–e987. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Rivner MH, Quarles BM, Pan JX, Yu Z,
Howard JF Jr, Corse A, Dimachkie MM, Jackson C, Vu T, Small G, et
al: Clinical features of LRP4/agrin-antibody-positive myasthenia
gravis: A multicenter study. Muscle Nerve. 62:333–343. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Ohno K, Ohkawara B and Ito M:
Agrin-LRP4-MuSK signaling as a therapeutic target for myasthenia
gravis and other neuromuscular disorders. Expert Opin Ther Targets.
21:949–958. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Hettwer S, Lin S, Kucsera S, Haubitz M,
Oliveri F, Fariello RG, Ruegg MA and Vrijbloed JW: Injection of a
soluble fragment of neural agrin (NT-1654) considerably improves
the muscle pathology caused by the disassembly of the neuromuscular
junction. PLoS One. 9:e887392014. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Li Z, Li M, Wood K, Hettwer S, Muley SA,
Shi FD, Liu Q and Ladha SS: Engineered agrin attenuates the
severity of experimental autoimmune myasthenia gravis. Muscle
Nerve. 57:814–820. 2018. View Article : Google Scholar :
|
|
38
|
Kempermann G, Gage FH, Aigner L, Song H,
Curtis MA, Thuret S, Kuhn HG, Jessberger S, Frankland PW, Cameron
HA, et al: Human adult neurogenesis: Evidence and remaining
questions. Cell Stem Cell. 23:25–30. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Sorrells SF, Paredes MF, Cebrian-Silla A,
Sandoval K, Qi D, Kelley KW, James D, Mayer S, Chang J, Auguste KI,
et al: Human hippocampal neurogenesis drops sharply in children to
undetectable levels in adults. Nature. 555:377–381. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Li X, Sun B, Li J, Ye W, Li M, Guan F, Wu
S, Luo X, Feng J, Jia J, et al: Sepsis leads to impaired
mitochondrial calcium uptake and skeletal muscle weakness by
reducing the micu1: Mcu protein ratio. Shock. 60:698–706. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Lv B, Min S, Xie F, Yang J and Chen J:
Alleviating sepsis-induced neuromuscular dysfunction linked with
acetylcholine receptors by agrin. J Surg Res. 241:308–316. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Abdalla A, Murali C and Amin A: Safranal
inhibits angiogenesis via targeting HIF-1α/VEGF machinery: In vitro
and Ex vivo insights. Front Oncol. 11:7891722022. View Article : Google Scholar
|
|
43
|
Hanahan D and Folkman J: Patterns and
emerging mechanisms of the angiogenic switch during tumorigenesis.
Cell. 86:353–364. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Abdalla Y, Abdalla A, Hamza AA and Amin A:
Safranal prevents liver cancer through inhibiting oxidative stress
and alleviating inflammation. Front Pharmacol. 12:7775002022.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Bouabdallah S, Al-Maktoum A and Amin A:
Steroidal saponins: Naturally occurring compounds as inhibitors of
the hallmarks of cancer. Cancers (Basel). 15:39002023. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Wiedmann L, De Angelis Rigotti F,
Vaquero-Siguero N, Donato E, Espinet E, Moll I, Alsina-Sanchis E,
Bohnenberger H, Fernandez-Florido E, Mulfarth R, et al: HAPLN1
potentiates peritoneal metastasis in pancreatic cancer. Nat Commun.
14:23532023. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
He M, Cheng C, Tu J, Ji SS, Lou D and Bai
B: Agrin expression is correlated with tumor development and poor
prognosis in cholangiocarcinoma. J Int Med Res.
49:30006052110097222021. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Chakraborty S, Lakshmanan M, Swa HL, Chen
J, Zhang X, Ong YS, Loo LS, Akincilar SC, Gunaratne J, Tergaonkar
V, et al: An oncogenic role of agrin in regulating focal adhesion
integrity in hepatocellular carcinoma. Nat Commun. 6:61842015.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Chakraborty S, Njah K, Pobbati AV, Lim YB,
Raju A, Lakshmanan M, Tergaonkar V, Lim CT and Hong W: Agrin as a
mechanotransduction signal regulating YAP through the hippo
pathway. Cell Rep. 18:2464–2479. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Njah K, Chakraborty S, Qiu B, Arumugam S,
Raju A, Pobbati AV, Lakshmanan M, Tergaonkar V, Thibault G, Wang X
and Hong W: A role of agrin in maintaining the stability of
vascular endothelial growth factor receptor-2 during tumor
angiogenesis. Cell Rep. 28:949–965.e7. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Bordeleau F, Mason BN, Lollis EM, Mazzola
M, Zanotelli MR, Somasegar S, Califano JP, Montague C, LaValley DJ,
Huynh J, et al: Matrix stiffening promotes a tumor vasculature
phenotype. Proc Natl Acad Sci USA. 114:492–497. 2017. View Article : Google Scholar :
|
|
52
|
Frye M, Taddei A, Dierkes C,
Martinez-Corral I, Fielden M, Ortsater H, Kazenwadel J, Calado DP,
Ostergaard P, Salminen M, et al: Matrix stiffness controls
lymphatic vessel formation through regulation of a GATA2-dependent
transcriptional program. Nat Commun. 9:15112018. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Chakraborty S, Njah K and Hong W: Agrin
mediates angiogenesis in the tumor microenvironment. Trends Cancer.
6:81–85. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Kawahara R, Granato DC, Carnielli CM,
Cervigne NK, Oliveria CE, Rivera C, Yokoo S, Fonseca FP, Lopes M,
Santos-Silva AR, et al: Agrin and perlecan mediate tumorigenic
processes in oral squamous cell carcinoma. PLoS One. 9:e1150042014.
View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Neill T, Schaefer L and Iozzo RV: Decoding
the matrix: Instructive roles of proteoglycan receptors.
Biochemistry. 54:4583–4598. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Scherbakov N, Knops M, Ebner N, Valentova
M, Sandek A, Grittner U, Dahinden P, Hettwer S, Schefold JC, von
Haehling S, et al: Evaluation of C-terminal agrin fragment as a
marker of muscle wasting in patients after acute stroke during
early rehabilitation. J Cachexia Sarcopenia Muscle. 7:60–67. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Yu D, Li HX, Liu Y, Ying ZW, Guo JJ, Cao
CY, Wang J, Li YF and Yang HR: The reference intervals for serum
C-terminal agrin fragment in healthy individuals and as a biomarker
for renal function in kidney transplant recipients. J Clin Lab
Anal. 31:e220592017. View Article : Google Scholar
|
|
58
|
Sartori R, Hagg A, Zampieri S, Armani A,
Winbanks CE, Viana LR, Haidar M, Watt KI, Qian H, Pezzini C, et al:
Perturbed BMP signaling and denervation promote muscle wasting in
cancer cachexia. Sci Transl Med. 13:eaay95922021. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Rivera C, Zandonadi FS, Sanchez-Romero C,
Soares CD, Granato DC, Gonzalez-Arriagada WA and Paes Leme AF:
Agrin has a pathological role in the progression of oral cancer. Br
J Cancer. 118:1628–1638. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Bezakova G and Ruegg MA: New insights into
the roles of agrin. Nat Rev Mol Cell Biol. 4:295–308. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Sulzmaier FJ, Jean C and Schlaepfer DD:
FAK in cancer: Mechanistic findings and clinical applications. Nat
Rev Cancer. 14:598–610. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Li D, Gu Q, Xie Z, Shen Q and Li H:
Clinical significance of nuclear localisation of agrin in lung
adenocarcinoma. Pol J Pathol. 70:198–204. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Zhang QJ, Wan L and Xu HF: High expression
of agrin is associated with tumor progression and poor prognosis in
hepatocellular carcinoma. Math Biosci Eng. 16:7375–7383. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Ye P, Fu Z, Chung JY, Cao X, Ko H, Tian
XY, Tang PM and Lui KO: Endothelial agrin is dispensable for normal
and tumor angiogenesis. Front Cardiovasc Med. 8:8104772022.
View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Zhu Y, Do VD, Richards AM and Foo R: What
we know about cardiomyocyte dedifferentiation. J Mol Cell Cardiol.
152:80–91. 2021. View Article : Google Scholar
|
|
66
|
Porrello ER, Mahmoud AI, Simpson E, Hill
JA, Richardson JA, Olson EN and Sadek HA: Transient regenerative
potential of the neonatal mouse heart. Science. 331:1078–1080.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Zlatanova I, Sun F, Wu RS, Chen X, Lau BH,
Colombier P, Sinha T, Celona B, Xu SM, Materna SC, et al: An
injury-responsive mmp14b enhancer is required for heart
regeneration. Sci Adv. 9:eadh53132023. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Baehr A, Umansky KB, Bassat E, Jurisch V,
Klett K, Bozoglu T, Hornaschewitz N, Solyanik O, Kain D, Ferraro B,
et al: Agrin promotes coordinated therapeutic processes leading to
improved cardiac repair in pigs. Circulation. 142:868–881. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Mazzon C, Anselmo A, Cibella J, Soldani C,
Destro A, Kim N, Roncalli M, Burden SJ, Dustin ML, Sarukhan A and
Viola A: The critical role of agrin in the hematopoietic stem cell
niche. Blood. 118:2733–2742. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Burgess RW, Dickman DK, Nunez L, Glass DJ
and Sanes JR: Mapping sites responsible for interactions of agrin
with neurons. J Neurochem. 83:271–284. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Guadix JA, Orlova VV, Giacomelli E, Bellin
M, Ribeiro MC, Mummery CL, Perez-Pomares JM and Passier R: Human
pluripotent stem cell differentiation into functional epicardial
progenitor cells. Stem Cell Reports. 9:1754–1764. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Germani A, Foglio E, Capogrossi MC, Russo
MA and Limana F: Generation of cardiac progenitor cells through
epicardial to mesenchymal transition. J Mol Med (Berl). 93:735–748.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Smits AM, Dronkers E and Goumans MJ: The
epicardium as a source of multipotent adult cardiac progenitor
cells: Their origin, role and fate. Pharmacol Res. 127:129–140.
2018. View Article : Google Scholar
|
|
74
|
Jing X, Liu B, Deng S, Du J and She Q:
Agrin yes-associated protein promotes the proliferation of
epicardial cells. J Cardiovasc Pharmacol. 77:94–99. 2021.
View Article : Google Scholar
|
|
75
|
Sun K, Guo J, Guo Z, Hou L, Liu H, Hou Y,
He J, Guo F and Ye Y: The roles of the hippo-YAP signalling pathway
in cartilage and osteoarthritis. Ageing Res Rev. 90:1020152023.
View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Hou L, Fu W, Liu Y, Wang Q, Wang L and
Huang Y: Agrin promotes limbal stem cell proliferation and corneal
wound healing through hippo-yap signaling pathway. Invest
Ophthalmol Vis Sci. 61:72020. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Manner J, Schlueter J and Brand T:
Experimental analyses of the function of the proepicardium using a
new microsurgical procedure to induce
loss-of-proepicardial-function in chick embryos. Dev Dyn.
233:1454–1463. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Diman NY, Brooks G, Kruithof BP, Elemento
O, Seidman JG, Seidman CE, Basson CT and Hatcher CJ: Tbx5 is
required for avian and mammalian epicardial formation and coronary
vasculogenesis. Circ Res. 115:834–844. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
van Wijk B, Gunst QD, Moorman AF and van
den Hoff MJ: Cardiac regeneration from activated epicardium. PLoS
One. 7:e446922012. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Lan Y, Pan H, Li C, Banks KM, Sam J, Ding
B, Elemento O, Goll MG and Evans T: TETs regulate proepicardial
cell migration through extracellular matrix organization during
zebrafish cardiogenesis. Cell Rep. 26:720–732.e4. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Missinato MA, Tobita K, Romano N, Carroll
JA and Tsang M: Extracellular component hyaluronic acid and its
receptor Hmmr are required for epicardial EMT during heart
regeneration. Cardiovasc Res. 107:487–498. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Verzijl N, DeGroot J, Thorpe SR, Bank RA,
Shaw JN, Lyons TJ, Bijlsma JW, Lafeber FP, Baynes JW and TeKoppele
JM: Effect of collagen turnover on the accumulation of advanced
glycation end products. J Biol Chem. 275:39027–39031. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Erickson CB, Hill R, Pascablo D, Kazakia
G, Hansen K and Bahney C: A timeseries analysis of the fracture
callus extracellular matrix proteome during bone fracture healing.
J Life Sci (Westlake Village). 3:1–30. 2021.
|
|
84
|
Hausser HJ, Ruegg MA, Brenner RE and
Ksiazek I: Agrin is highly expressed by chondrocytes and is
required for normal growth. Histochem Cell Biol. 127:363–374. 2007.
View Article : Google Scholar
|
|
85
|
Campanelli JT, Ferns M, Hoch W, Rupp F,
von Zastrow M, Hall Z and Scheller RH: Agrin: A synaptic basal
lamina protein that regulates development of the neuromuscular
junction. Cold Spring Harb Symp Quant Biol. 57:461–472. 1992.
View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Eldridge S, Nalesso G, Ismail H,
Vicente-Greco K, Kabouridis P, Ramachandran M, Niemeier A, Herz J,
Pitzalis C, Perretti M and Dell'Accio F: Agrin mediates chondrocyte
homeostasis and requires both LRP4 and α-dystroglycan to enhance
cartilage formation in vitro and in vivo. Ann Rheum Dis.
75:1228–1235. 2016. View Article : Google Scholar
|
|
87
|
Eldridge SE, Barawi A, Wang H, Roelofs AJ,
Kaneva M, Guan Z, Lydon H, Thomas BL, Thorup AS, Fernandez BF, et
al: Agrin induces long-term osteochondral regeneration by
supporting repair morphogenesis. Sci Transl Med. 12:eaax90862020.
View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Gentili C and Cancedda R: Cartilage and
bone extracellular matrix. Curr Pharm Des. 15:1334–1348. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Grol MW and Lee BH: Gene therapy for
repair and regeneration of bone and cartilage. Curr Opin Pharmacol.
40:59–66. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Gomes KDN, Alves APNN, Dutra PGP and Viana
GSB: Doxycycline induces bone repair and changes in Wnt signalling.
Int J Oral Sci. 9:158–166. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Bao Q, Chen S, Qin H, Feng J, Liu H, Liu
D, Li A, Shen Y, Zhao Y, Li J and Zong Z: An appropriate
Wnt/β-catenin expression level during the remodeling phase is
required for improved bone fracture healing in mice. Sci Rep.
7:26952017. View Article : Google Scholar
|
|
92
|
Wang T, Zhang X and Bikle DD: Osteogenic
Differentiation of Periosteal Cells During Fracture Healing. J Cell
Physiol. 232:913–921. 2017. View Article : Google Scholar :
|
|
93
|
Ahn Y, Sims C, Murray MJ, Kuhlmann PK,
Fuentes-Antras J, Weatherbee SD and Krumlauf R: Multiple modes of
Lrp4 function in modulation of Wnt/β-catenin signaling during tooth
development. Development. 144:2824–2836. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Houschyar KS, Tapking C, Borrelli MR, Popp
D, Duscher D, Maan ZN, Chelliah MP, Li J, Harati K, Wallner C, et
al: Wnt pathway in bone repair and regeneration-what do we know so
far. Front Cell Dev Biol. 6:1702019. View Article : Google Scholar
|
|
95
|
Shen C, Xiong WC and Mei L: LRP4 in
neuromuscular junction and bone development and diseases. Bone.
80:101–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Souza ATP, Lopes HB, Oliveira FS, Weffort
D, Freitas GP, Adolpho LF, Fernandes RR, Rosa AL and Beloti MM: The
extracellular matrix protein Agrin is expressed by osteoblasts and
contributes to their differentiation. Cell Tissue Res. 386:335–347.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Willadt S, Nash M and Slater C:
Age-related changes in the structure and function of mammalian
neuromuscular junctions. Ann N Y Acad Sci. 1412:41–53. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Taetzsch T, Tenga MJ and Valdez G: Muscle
fibers secrete FGFBP1 to slow degeneration of neuromuscular
synapses during aging and progression of ALS. J Neurosci. 37:70–82.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Zhao K, Shen C, Li L, Wu H, Xing G, Dong
Z, Jing H, Chen W, Zhang H, Tan Z, et al: Sarcoglycan alpha
mitigates neuromuscular junction decline in aged mice by
stabilizing LRP4. J Neurosci. 38:8860–8873. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Samuel MA, Valdez G, Tapia JC, Lichtman JW
and Sanes JR: Agrin and synaptic laminin are required to maintain
adult neuromuscular junctions. PLoS One. 7:e466632012. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Benjumea AM, Curcio CL, Duque G and Gomez
F: Dynapenia and sarcopenia as a risk factor for disability in a
falls and fractures clinic in older persons. Open Access Maced J
Med Sci. 6:344–349. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Zhang X, Zhang W, Wang C, Tao W, Dou Q and
Yang Y: Sarcopenia as a predictor of hospitalization among older
people: A systematic review and meta-analysis. BMC Geriatr.
18:1882018. View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Stephan A, Mateos JM, Kozlov SV, Cinelli
P, Kistler AD, Hettwer S, Rulicke T, Streit P, Kunz B and
Sonderegger P: Neurotrypsin cleaves agrin locally at the synapse.
FASEB J. 22:1861–1873. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Kamiya K, Tachiki T, Sato Y, Kouda K,
Kajita E, Tamaki J, Kagamimori S and Iki M: Association between the
110-kDa C-terminal agrin fragment and skeletal muscle decline among
community-dwelling older women. J Cachexia Sarcopenia Muscle.
14:2253–2263. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Drey M, Sieber CC, Bauer JM, Uter W,
Dahinden P, Fariello RG and Vrijbloed JW; FiAT intervention group:
C-terminal Agrin Fragment as a potential marker for sarcopenia
caused by degeneration of the neuromuscular junction. Exp Gerontol.
48:76–80. 2013. View Article : Google Scholar
|
|
106
|
Racha P, Selvam S, Bose B, Bantwal G and
Sambashivaiah S: Circulating C-terminal agrin fragment: A potential
marker for sarcopenia among type 2 diabetes. Indian J Endocrinol
Metab. 26:334–340. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Pratt J, De Vito G, Narici M, Segurado R,
Pessanha L, Dolan J, Conroy J and Boreham C: Plasma C-terminal
agrin fragment as an early biomarker for sarcopenia: Results from
the GenoFit study. J Gerontol A Biol Sci Med Sci. 76:2090–2096.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Denzer AJ, Brandenberger R, Gesemann M,
Chiquet M and Ruegg MA: Agrin binds to the nerve-muscle basal
lamina via laminin. J Cell Biol. 137:671–683. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Denzer AJ, Schulthess T, Fauser C,
Schumacher B, Kammerer RA, Engel J and Ruegg MA: Electron
microscopic structure of agrin and mapping of its binding site in
laminin-1. EMBO J. 17:335–343. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Raats CJ, van den Born J, Bakker MA,
Oppers-Walgreen B, Pisa BJ, Dijkman HB, Assmann KJ and Berden JH:
Expression of agrin, dystroglycan, and utrophin in normal renal
tissue and in experimental glomerulopathies. Am J Pathol.
156:1749–1765. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Goldberg S, Harvey SJ, Cunningham J,
Tryggvason K and Miner JH: Glomerular filtration is normal in the
absence of both agrin and perlecan-heparan sulfate from the
glomerular basement membrane. Nephrol Dial Transplant.
24:2044–2051. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
112
|
Harvey SJ, Jarad G, Cunningham J, Rops AL,
van der Vlag J, Berden JH, Moeller MJ, Holzman LB, Burgess RW and
Miner JH: Disruption of glomerular basement membrane charge through
podocyte-specific mutation of agrin does not alter glomerular
permselectivity. Am J Pathol. 171:139–152. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
113
|
Vestentoft PS, Jelnes P, Andersen JB, Tran
TA, Jorgensen T, Rasmussen M, Bornholdt J, Grovdal LM, Jensen CH,
Vogel LK, et al: Molecular constituents of the extracellular matrix
in rat liver mounting a hepatic progenitor cell response for tissue
repair. Fibrogenesis Tissue Repair. 6:212013. View Article : Google Scholar : PubMed/NCBI
|
|
114
|
Deng SX, Borderie V, Chan CC, Dana R,
Figueiredo FC, Gomes JAP, Pellegrini G, Shimmura S and Kruse FE;
The International Limbal Stem Cell Deficiency Working Group: Global
consensus on definition, classification, diagnosis, and staging of
limbal stem cell deficiency. Cornea. 38:364–375. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
115
|
Kolli S, Ahmad S, Lako M and Figueiredo F:
Successful clinical implementation of corneal epithelial stem cell
therapy for treatment of unilateral limbal stem cell deficiency.
Stem Cells. 28:597–610. 2010. View Article : Google Scholar
|
|
116
|
Whitcher JP, Srinivasan M and Upadhyay MP:
Corneal blindness: A global perspective. Bull World Health Organ.
79:214–221. 2001.PubMed/NCBI
|
|
117
|
Sacchetti M, Rama P, Bruscolini A and
Lambiase A: Limbal stem cell transplantation: Clinical results,
limits, and perspectives. Stem Cells Int. 2018:80862692018.
View Article : Google Scholar : PubMed/NCBI
|
|
118
|
Ribatti D and d'Amati A: Hematopoiesis and
Mast Cell Development. Int J Mol Sci. 24:106792023. View Article : Google Scholar : PubMed/NCBI
|
|
119
|
Bruno E, Luikart SD, Long MW and Hoffman
R: Marrow-derived heparan sulfate proteoglycan mediates the
adhesion of hematopoietic progenitor cells to cytokines. Exp
Hematol. 23:1212–1217. 1995.PubMed/NCBI
|
|
120
|
Sorg H and Sorg CGG: Skin wound healing:
Of players, patterns, and processes. Eur Surg Res. 64:141–157.
2023. View Article : Google Scholar
|
|
121
|
Xue M and Jackson CJ: Extracellular matrix
reorganization during wound healing and its impact on abnormal
scarring. Adv Wound Care (New Rochelle). 4:119–136. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
122
|
Chakraborty S, Sampath D, Yu Lin MO,
Bilton M, Huang CK, Nai MH, Njah K, Goy PA, Wang CC, Guccione E, et
al: Agrin-matrix metalloproteinase-12 axis confers a mechanically
competent microenvironment in skin wound healing. Nat Commun.
12:63492021. View Article : Google Scholar : PubMed/NCBI
|
|
123
|
Yu Lin MO, Sampath D, Bosykh DA, Wang C,
Wang X, Subramaniam T, Han W, Hong W and Chakraborty S: YAP/TAZ
drive agrin-matrix metalloproteinase-12 mediated diabetic skin
wound healing. J Invest Dermatol. May 27–2024.Epub ahead of print.
View Article : Google Scholar
|
|
124
|
Calvo F, Ege N, Grande-Garcia A, Hooper S,
Jenkins RP, Chaudhry SI, Harrington K, Williamson P, Moeendarbary
E, Charras G and Sahai E: Mechanotransduction and YAP-dependent
matrix remodelling is required for the generation and maintenance
of cancer-associated fibroblasts. Nat Cell Biol. 637–646. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
125
|
Sun X, Malandraki-Miller S, Kennedy T,
Bassat E, Klaourakis K, Zhao J, Gamen E, Vieira JM, Tzahor E and
Riley PR: The extracellular matrix protein agrin is essential for
epicardial epithelial-to-mesenchymal transition during heart
development. Development. 148:dev1975252021. View Article : Google Scholar : PubMed/NCBI
|