Introduction
A survey carried out by the Global Cancer
Observatory, under the World Health Organization International
Agency for Research on Cancer, indicated that thyroid cancer (TC)
is the ninth most prevalent cancer worldwide (1,2).
In addition, TC is the most common cancer among adolescents and
young adults aged 16-33 years in the United States; however, this
trend may vary in other regions globally (3,4).
The American Cancer Society estimates that there will be ~44,020
new cases of TC in 2024, with ~2,170 deaths attributed to the
disease (5). Similarly, in 2022,
the National Cancer Center of China reported that the
age-standardized incidence rate for TC increased to 17.7%, along
with an upward trend in mortality rates (6). Notably, the majority of TC cases
respond well to conventional treatments, such as surgery; however,
refractory cases, such as poorly differentiated thyroid carcinoma
(PTC), anaplastic thyroid carcinoma (ATC) and some cases of
advanced differentiated thyroid carcinoma, often exhibit a poor
prognosis due to high levels of malignancy and a lack of effective
treatment options (7,8). Thus, identifying target genes in TC
is crucial for the development of effective treatment options.
In the present study, TC data were obtained from the
Gene Expression Omnibus (GEO) and The Cancer Genome Atlas (TCGA)
databases. Through a series of bioinformatics analyses, inhibin βA
(INHBA) was identified as a pivotal gene in TC, leading to further
investigation of its role. INHBA, a member of the transforming
growth factor-β superfamily, has been implicated in several vital
biological processes, including glucose metabolism (9), cell differentiation (10) and immune response (11). Furthermore, INHBA upregulation is
associated with aggressive phenotypes and poor outcomes in various
types of cancer, including gastric, colon and esophageal cancer
(12-15). Although one study proposed INHBA
as a potential biomarker for advanced PTC (16), its specific functions and
mechanisms in TC remain to be elucidated.
The present study, aimed to investigate the role of
INHBA in TC. Specifically, the study aimed to understand how
regulating INHBA expression in TC cells, such as CAL-62 and KTC-1,
affects the cytoskeleton and phenotypic changes in these cells. The
objective was to explore the potential impact of INHBA on the
morphological characteristics and behavior of TC cells. The role of
the cytoskeleton in cancer, particularly in association with cell
metastasis, is both complex and critical (17). Comprising actin filaments,
microtubules and intermediate filaments, the cytoskeleton is a
dynamic framework that maintains cell shape, structure and
motility. Notably, cytoskeletal reorganization is closely
associated with the invasiveness and metastatic capacity of cancer
cells (18,19). RhoA, a key member of the Rho
GTPase family, affects this reorganization, including the
configuration of actin filaments and cell migration (20-22). The present study aimed to explore
the mechanisms by which INHBA may influence the metastasis of TC
cells. Based on the role of cytoskeletal regulatory proteins, such
as RhoA, in cell migration and invasion, it was hypothesized that
INHBA could affect the metastatic potential of CAL-62 and KTC-1 TC
cells through the regulation of the RhoA signaling pathway.
Materials and methods
Public database analysis
Gene expression data for TC were sourced from the
GEO online repository (https://www.ncbi.nlm.nih.gov/geo), utilizing the GEO2R
online tool (https://www.ncbi.nlm.nih.gov/geo/geo2r/) to identify
differentially expressed genes (DEGs). The following GEO datasets
were used in the present study: GSE33630 (23), GSE605422 (24) and GSE29265, which compared PTC
with normal thyroid tissues, and DEGs were identified with a
significance cut-off level of FDR <0.05, adjusted P<0.01 and
log2 fold change >0. For the comparison between ATC and PTC, the
datasets GSE33630 and GSE27155 (25) were used, and DEGs were also
identified with a significance cut-off level of FDR <0.05,
adjusted P<0.01 and log2 fold change >0. In most current
studies (26,27), log2 fold change thresholds >1
or even 2 are commonly used, focusing on genes that exhibit large
expression changes. However, the present research design considers
the comparison of multiple datasets, with the goal of identifying
genes that are differentially expressed across these different
datasets. Even if some genes show only small changes in expression
in certain groups, they may exhibit consistent trends or larger
changes in other groups. Therefore, by setting the threshold at
log2 fold change >0, these potentially key genes can be
captured, providing a broader pool of candidate genes for
subsequent cross-group analyses. Although the log2 fold change
threshold is relaxed, a strict adjusted P-value standard
(P<0.01) was employed. This stringent statistical filtering
ensures that all selected genes were highly significant, reducing
the likelihood of false positives. Even genes with small expression
changes, after multiple testing corrections, still exhibited
differential expression across multiple groups, indicating that
they may have important biological roles. For the common DEGs
obtained through the intersection in the Venn diagram, the STRING
database (https://cn.string-db.org/) was used
to construct a protein-protein interaction network. Expression
datasets for TC, along with genes co-expressed with INHBA, were
retrieved from cBioPortal (https://cbioportal.org). The mRNA expression levels of
INHBA in TC tissues were analyzed. The top 25% of patients, based
on mRNA expression rank in the TC dataset, were classified as the
high INHBA expression group, and the remaining 75% of patients were
classified as the low INHBA expression group. In addition, the
Linked Omics database (https://www.linkedomics.org/login.php) was employed
for further analysis. Genes co-expressed with the INHBA gene in TC
were examined, and those with a Spearman's correlation value of
>0.5 and a q-value <0.05 were selected for further functional
Gene Ontology (GO) enrichment analysis (https://david.ncifcrf.gov/). For gene annotation, the
'org.Hs.eg.db' R package (https://bioconductor.org/packages/release/data/annotation/html/org.Hs.eg.db.html)
was utilized, and the 'clusterProfiler' R package (https://bioconductor.org/packages/release/bioc/html/clusterProfiler.html)
facilitated the enrichment analysis. The analysis was performed
using R software version 4.2.0 (https://cran.r-project.org/bin/windows/base/old/4.2.0/).
The GO analysis identified the top eight pathways across biological
processes, cellular components and molecular functions. P<0.05
was considered to indicate a statistically significant
difference.
Human samples
The patients were specifically recruited for the
present study between August and November 2022. The age range of
the participants was 27 to 67 years, with a mean age of 44 years,
and the male-to-female ratio was 1:3. A total of 20 pairs of normal
thyroid tissue and tumor tissue samples were collected from the
Department of Thyroid Surgery, Shandong ENT Hospital (Jinan, China)
and the study was approved by the Ethics Committee of Shandong ENT
Hospital (approval no. 20220713). For reverse
transcription-quantitative (RT-q)PCR, fresh tissues were directly
collected and RNA was extracted. For immunohistochemistry (IHC),
the tissues were stored in 4% paraformaldehyde for subsequent
processing. All samples were histologically confirmed. The study
objectives and procedures were explained to all participants, and
written informed consent was obtained from all participants.
TC cell culture
In total, four TC cell lines; namely, BCPAP, CAL-62,
KTC-1 and 8305C, were obtained from The Cell Bank of Type Culture
Collection of The Chinese Academy of Sciences and were all
certified as mycoplasma-free. BCPAP and KTC-1 cells were cultured
in RPMI-1640 medium (Gibco; Thermo Fisher Scientific, Inc.), CAL-62
cells were cultured in DMEM (Gibco; Thermo Fisher Scientific,
Inc.), and 8305C cells were cultured in MEM (Gibco; Thermo Fisher
Scientific, Inc.), all supplemented with 10% fetal bovine serum
(FBS) (cat. no. 04-001-1A; Biological Industries), streptomycin
(100 mg/ml) and penicillin (100 U/ml). The cells were passaged
every 2-3 days and maintained in a humidified incubator at 37°C
with 5% CO2. The BCPAP cell line was authenticated by
short tandem repeat profiling and tested for mycoplasma
contamination.
Cell transfection
In the present study, short hairpin (sh) RNA was
transfected into CAL-62 cells to downregulate INHBA expression, and
plasmids were transfected into KTC-1 cells to upregulate INHBA
expression. INHBA shRNA (shINHBA) and a scrambled non-targeting
negative control (NC) shRNA (shNC) were cloned into a lentiviral
vector, LV3-(H1/GFP&Puro). These lentiviral expression vectors
were synthesized and purchased from Shanghai GenePharma Co., Ltd.
The shRNA sequences were as follows: shINHBA, sense
5′-CCCTTTGCCAACCTCAAAT-3′, antisense 5′-ATTTGAGGTTGGCAAAGGG-3′; and
shNC, sense 5′-GTTCTCCGAACGTGTCACGT-3′, antisense
5′-ACGTGACACGTTCGGAGAAC-3′. A pEX-2 plasmid containing human INHBA
and an empty pEX-2 vector (NC) were obtained from Shanghai
GenePharma Co., Ltd., and were transfected into cells using
Lipofectamine® 2000 (Thermo Fisher Scientific, Inc.),
according to the manufacturer's instructions. When the density of
KTC-1 cells reached 70-80%, cell transfection was performed in
serum-free medium with a plasmid concentration of 1 μg/ml.
After incubating the cells at 37°C for 6 h, the medium was replaced
with complete medium containing 10% FBS. The cells were cultured
for a further 48 h before subsequent experiments were
conducted.
shINHBA and shNC were transduced into CAL-62 cells
using polybrene at a final concentration of 5 μg/ml
(Shanghai GenePharma Co., Ltd.) with a multiplicity of infection
(MOI) of 6 for lentiviral vectors. The duration of lentiviral
transduction into CAL-62 cells was 24 h at 37°C. Following
transduction, the cells were allowed to recover for 48 h before
proceeding with subsequent experiments. Stable cell lines were
established using puromycin selection at a concentration of 2
μg/ml, with maintenance at 1 μg/ml.
A pEX-2 plasmid containing human RhoA (RhoA-OE) and
an empty pEX-2 vector (NC) were also obtained from Shanghai
GenePharma Co., Ltd. Initially, the efficacy of the RhoA-OE plasmid
was validated in untreated CAL-62 cells. Subsequently, the RhoA-OE
plasmid was transfected into CAL-62 cells with INHBA knockdown
using Lipofectamine 2000, according to the manufacturer's protocol,
When the CAL-62 cells reached 70-80% confluence, transfection was
initiated in serum-free medium with a vector concentration of 1
μg/ml. After incubating at 37°C for 6 h, the medium was
replaced with complete medium containing 10% FBS. The cells were
then cultured for an additional 48 h before proceeding with
subsequent experiments. Transfection efficiency was determined
using western blotting and RT-qPCR.
RT-qPCR
Total RNA was extracted from cells or tissues using
TRIzol® reagent (cat. no. 15596026; Invitrogen; Thermo
Fisher Scientific, Inc.) and reverse transcribed into cDNA using
the RevertAid First Strand cDNA Synthesis kit (cat. no. K1622;
Thermo Fisher Scientific, Inc.) according to the conditions: 25°C
for 15 min, 42°C for 60 min and 70°C for 10 min The resulting cDNA
was analyzed using a TB Green® Premix Ex Taq™ II (Tli
RNaseH Plus) cat. no. RR820B; Takara Bio, Inc.). The primer
sequences for INHBA were as follows: Forward,
5′-CCTCCCAAAGGATGTACCCAA-3′ and reverse,
5′-CTCTATCTCCACATACCCGTTCT-3′ (Sangon Biotech Co., Ltd.). The
internal standard gene was β-actin, and its primer sequences were:
Forward 5′-AGAGCTACGAGCTGCCTGAC-3′ and reverse
5′-AGCACTGTGTTGGCGTACAG-3′. qPCR was conducted under the following
conditions: 95°C for 2 min (pre-denaturation), followed by 40
cycles at 95°C for 15 sec (denaturation), 52°C for 20 sec
(annealing) and 68°C for 20 sec (extension). Gene expression levels
were quantified using the 2−ΔΔCq method (28).
Cell Counting Kit-8 (CCK-8) assay
CAL-62 and KTC-1 cells were seeded in 96-well plates
(2×103 cells/well) and were cultured at 37°C for 24 h.
Subsequently, the cells were incubated with 10 μl CCK-8
solution (Beyotime Institute of Biotechnology) for 2 h at 37°C with
5% CO2 at the indicated time points (0, 24, 48 and 72
h). The absorbance of each well was detected at 450 nm using a
spectrophotometer.
Flow cytometric apoptosis detection
CAL-62 cells were digested with 0.25% trypsin to
create a single-cell suspension and were washed twice with cold
PBS. The experiment was performed using BD Pharmingen™ FITC Annexin
V Apoptosis Detection kit I (cat. no. 556547; BD Biosciences). The
cells (1×106 cells/ml) were then resuspended in 1X
Binding Buffer and 100 μl of this suspension was transferred
to a tube. FITC Annexin and PI were then added, vortexed gently,
and incubated for 15 min at room temperature in the dark. After
adding 400 μl 1X Binding Buffer, the cells were analyzed by
flow cytometry within 1 h to distinguish viable, apoptotic and
necrotic cells based on their staining patterns. Flow cytometric
analysis was performed using a flow cytometer (Accuri C6; BD
Biosciences) and analyzed using FlowJo v10 software (FlowJo,
LLC).
Cell invasion and migration
To evaluate cell migration, a wound healing assay
was performed. A total of 48 h post-transfection, CAL-62 and KTC-1
cells monolayers were cultured until they reached 80-90%
confluence. Subsequently, the monolayers were scratched using a
sterile 200-μl pipette tip, and the cells were incubated in
serum-free medium. Images were obtained after 0 and 24 h using a
light microscope. The extent of wound healing was quantified by
measuring the wound area using ImageJ software (version 1.53;
National Institutes of Health).
Cell invasion was assessed using a Transwell assay.
The assay utilized 24-well Falcon® Cell Culture Inserts
(pore size, 8.0 μm; cat. no. 353097; Corning, Inc.) and the
upper chamber was coated with Matrigel. The 24-well plate was
incubated at 37°C for 60 min to allow the Matrigel to solidify.
Subsequently, 5×104 cells/well were seeded into the
upper chamber in serum-free medium, while the lower chamber was
filled with 800 μl medium containing 20% FBS. After
incubating for 48 h at 37°C, the cells were fixed with 4%
paraformaldehyde at room temperature for 20 min and stained with
0.1% crystal violet (cat. no. G1063; Beijing Solarbio Science &
Technology Co., Ltd.) for 10 min at room temperature. Images of the
invaded cells were subsequently captured using a light microscope
(BX53; Olympus Corporation), and invasion was assessed.
Western blot analysis
CAL-62 and KTC-1 cells were lysed using freshly
prepared RIPA lysis buffer (cat. no. R0010; Beijing Solarbio
Science & Technology Co., Ltd.), which was supplemented with 10
μl PMSF solution (cat. no. ST506-2; Beyotime Institute of
Biotechnology), 10 μl protease inhibitor cocktail (cat. no.
CW2200S; CWBIO), and 10 μl phosphatase inhibitor cocktail
(cat. no. CW2383S; CWBIO) per 1 ml RIPA buffer. Protein
concentrations were determined using the BCA method following
protein extraction. Equal amounts of protein (30 μg) were
loaded per lane and subjected to sodium dodecyl-sulfate
polyacrylamide gel electrophoresis (SDS-PAGE) using 8, 10 or 12%
gels. Subsequently, proteins were separated by SDS-PAGE and were
transferred to PVDF membranes, which were blocked with 5% skimmed
milk powder at room temperature for 2 h, followed by overnight
incubation at 4°C with the following primary antibodies:
Anti-phosphorylated (p)-LIM kinase 1 (LIMK1; 1:1,000; cat. no.
3841T; CST Biological Reagents Co., Ltd.), anti-LIMK1 (1:1,000;
cat. no. ab108507; Abcam), anti-p-cofilin (1:1,000; cat. no. 3313T;
CST Biological Reagents Co., Ltd.), anti-cofilin (1:1,000; cat. no.
5175T; CST Biological Reagents Co., Ltd.), anti-INHBA (1:1,000;
cat. no. 10651-1-AP; Proteintech Group, Inc.), anti-RhoA (1:1,000;
cat. no. 2117T; CST Biological Reagents Co., Ltd.), anti-RhoB
(1:1,000; cat. no. 2098T; CST Biological Reagents Co., Ltd.),
anti-RhoC (1:1,000; cat. no. 3430T; CST Biological Reagents Co.,
Ltd.) and anti-GAPDH (1:1,000; cat. no. TA-08; OriGene
Technologies, Inc.). After primary antibody incubation for 24 h,
the membranes were incubated with HRP-labeled goat anti-rabbit IgG
(1:5,000; cat. no. ZB-2301; OriGene Technologies, Inc.) and goat
anti-mouse IgG (1:10,000; cat. no. ZB-2305; OriGene Technologies,
Inc.) secondary antibodies using 5% skimmed milk powder as the
dilution buffer at room temperature for 1.5 h. Immunoreactive
proteins were visualized using an ECL kit (cat. no. SQ202L; Epizyme
Biotech). The intensity of protein bands was determined using
ImageJ software (version 1.53).
Rho/Rho-kinase (ROCK) inhibitor Y27632
treatment
KTC-1 cells were incubated at 37°C. The Rho/ROCK
inhibitor Y27632 (cat. no. HY-10071; MedChemExpress) was added to
the culture medium at a final concentration of 10 μM and the
KTC-1 cells were treated for 24 h at 37°C with 5% CO2.
After the treatment, the KTC-1 cells were subjected to wound
healing and Transwell assays to evaluate the effects of Y27632.
Additionally, the downstream signaling proteins of the RhoA/ROCK
pathway were examined, including their phosphorylation status,
using western blot analysis to assess the impact of the treatment
on the signaling cascade.
Immunofluorescence assay
CAL-62 and KTC-1 cells were plated at
2×104 cells/well on coverslips in a 48-well plate. After
culturing for 24 h, the cells were fixed with 4% paraformaldehyde
for 30 min at room temperature, permeabilized with 0.1% Triton
X-100 in PBS for 10 min on ice, and blocked with 1% FBS at room
temperature for 30 min. Subsequently, 200 μl 100 nM
rhodamine phalloidin (cat. no. R415; Thermo Fisher Scientific,
Inc.) was added to each well, and the cells were incubated in the
dark at room temperature for 30 min. Nuclei were stained using 2
μg/ml DAPI in PBS for 10 min and the cells were examined
under a confocal inverted microscope (Leica Microsystems,
Inc.).
In vivo experiments
Animal experiments were performed according to the
Guide for the Care and Use of Laboratory Animals of the National
Research Council (29), and were
approved by the Ethics Committee of Shandong Provincial ENT
Hospital (approval no. 20220713).
Female BALB/c nude mice (age, 4-6 weeks; weight,
18-20 g) were purchased from Charles River Laboratories, Inc., and
were housed in a specific pathogen-free environment with controlled
temperature (22-24°C) and humidity (45-55%). Mice had ad
libitum access to sterilized rodent chow and autoclaved water
throughout the experiment, and they were maintained on a 12-h
light/dark cycle. A total of 12 mice were used in the experiment.
Subsequently, 4×105 CAL-62 cells were seeded into 6-well
plates. Lentivirus-encapsulated firefly luciferase reporter
plasmids (plasmid backbone, pLV-CMV-Luciferase-T2A-Hygro vector;
Shanghai GenePharma Co., Ltd.) were infected into both NC and
INHBA-KD CAL-62 cells at a MOI of 6 in the presence of 5
μg/ml polybrene. The infection was carried out at 37°C in a
humidified incubator with 5% CO2 for 24 h. After
infection, the medium was replaced with fresh complete medium, and
the cells were further incubated for an additional 48 h before
subsequent experiments. The cells infected with the firefly
luciferase reporter plasmids were diluted in PBS to a concentration
of 1×107 cells/ml. Mice were anesthetized using
isoflurane inhalation (3.0% induction; 1.5% maintenance), and 100
μl luciferase-expressing cell suspension (1×106
cells) was injected into mice via the tail vein. The overall
condition of the mice was monitored daily, including vocalization,
respiratory difficulty, weight loss, abnormal behavior and physical
appearance. Metastatic tumors were monitored weekly using an in
vivo imaging system (IVIS) to detect luciferase bioluminescence
at pre-determined time points. D-luciferin potassium salt solution
(15 mg/ml; cat. no. ST196-2g; Beyotime Institute of Biotechnology)
was injected intraperitoneally into the mice at a dose of 150 mg/kg
body weight. Within 10-15 min of the substrate injection, the mice
were placed in the IVIS Spectrum (PerkinElmer, Inc.), and the
imaging parameters (exposure time, filter settings, etc.) were
adjusted to ensure clear images covering the entire mouse body
surface. Humane endpoints included a loss in bodyweight of >20%
and a tumor volume of >1,500 mm3. No animals reached
the humane endpoints during the experiment and mice were active
during all observations. At the end of the experiments, mice were
euthanized via cervical dislocation following anesthesia with
isoflurane. Death was confirmed by respiratory and cardiac arrest,
and after pupil dilation had been observed for ≥10 min.
Two pairs of AB strain adult zebrafish were
generously provided by the Otolaryngology Research Laboratory,
Shandong Provincial ENT Research Institute (Jinan, China), and were
maintained at 28.5°C under a 14/10-h light/dark cycle. The embryos
were obtained from breeding pairs. CAL-62 TC cells were cultured,
harvested and suspended in PBS at 1×107 cells/ml. At 48
h post-fertilization, zebrafish embryos were randomly assigned to
the NC group (n=10) and the INHBA-KD group (n=10) and were
anesthetized by immersion in water containing 0.01% tricaine. Then
they were injected with 200-300 fluorescent-labeled CAL-62 cells
into the perivitelline space. Embryos were subsequently maintained
at 34°C, and metastatic spread was assessed using fluorescence
microscopy. All experiments complied with the AVMA Euthanasia
Guidelines 2020 (30), and
suffering and distress were minimized. At the end of the
experiments, the 5-day post-fertilization (dpf) zebrafish were
euthanized by immersion in 2-4°C water for ≥20 min following the
loss of operculum movement. Subsequently, a sodium hypochlorite
solution with a final concentration exceeding 1% was used. A stock
solution of 6.15% sodium hypochlorite was diluted at a ratio of 1
part bleach to 5 parts system water, achieving a final
concentration of ~1.025%. The zebrafish were immersed in this
solution for a minimum of 5 min to ensure death.
IHC
Human thyroid tissues and mouse lung tissues were
fixed in 10% neutral buffered formalin at room temperature
(22-25°C) for 24 h, then dehydrated and embedded in paraffin, and
subsequently cut into 4-μm sections. The paraffin-embedded
sections were deparaffinized using xylene and rehydrated through a
descending ethanol series (100, 95, 85 and 75%) for 5 min each.
Antigen retrieval was conducted at high heat (>100°C) for 10
min, followed by medium to low heat (50-60°C) for 20 min, and then
allowed to cool to room temperature for 1 h, using EDTA buffer (pH
9.0; cat. no. ZLI-9068; OriGene Technologies, Inc.). After dewaxing
and antigen retrieval, the sections were washed with PBS three
times (5 min each). A quenching step was performed using 3%
hydrogen peroxide for 10 min at room temperature to block
endogenous peroxidase activity. For blocking, human thyroid tissues
were incubated with 8% normal goat serum (cat. no. SL038; Beijing
Solarbio Science & Technology Co., Ltd.) in PBS at 37°C for 30
min to prevent nonspecific binding, with the excess blocking
solution gently shaken off without washing. The sections were then
incubated with INHBA primary antibody (1:200; cat. no. 10651-1-AP;
Proteintech Group, Inc.) overnight at 4°C. Following primary
antibody incubation, the tissues were incubated with a goat
anti-mouse IgG H&L (HRP) secondary antibody (1:500; cat. no.
ab6789; Abcam) at room temperature for 2 h. DAB staining was
performed for 5-10 min at room temperature.
H&E staining of the mouse lung tissues was
carried out using 0.5% hematoxylin and 0.5% eosin solutions at room
temperature (22-25°C) for 2 min each. Tissues were subsequently
dehydrated and sealed. Images were captured by light microscope for
both IHC and H&E staining. Integrated optical density was
determined from IHC images using ImageJ software (version
1.53).
GTPase activation assay
Detection of activated RhoA, RhoB and RhoC was
performed using a consistent protocol, with different antibodies
used for the final incubation. Cells were lysed using cold IP lysis
buffer (cat. no. P0013; Beyotime Institute of Biotechnology) and
were clarified by centrifugation at 10,000 × g for 10 min at 4°C.
Equal amounts of total proteins (700 μg) from cell lysates
were incubated with 25 μl Rhotekin-RBD agarose beads (cat.
no. P2061; Beyotime Institute of Biotechnology) on a rotator at 4°C
for 1 h. The Rhotekin-RBD agarose beads specifically capture
activated RhoA, RhoB and RhoC. The beads were then pelleted by
centrifugation at 6,000 × g for 30 sec at 4°C and washed three
times with 1X assay buffer (cat. no. P0013; Beyotime Institute of
Biotechnology). After the final wash, 25 μl 1X SDS-PAGE
sample buffer was added and the mixture was heated at 95°C for 5
min. The proteins were then separated by SDS-PAGE, transferred to
PVDF membranes, and probed with specific antibodies against RhoA
(1:1,000; cat. no. 2117T; CST Biological Reagents Co., Ltd.), RhoB
(1:1,000; cat. no. 2098T; CST Biological Reagents Co., Ltd.) and
RhoC (1:1,000; cat. no. 3430T; CST Biological Reagents Co., Ltd.).
This method specifically recognizes and binds to the active forms
of Rho proteins (RhoA, RhoB, RhoC) but not to their inactive
GDP-bound forms (Rho-GDP). Secondary antibodies and
chemiluminescence were used for protein detection, following the
same protocol described for western blotting. For the detection of
total RhoA, RhoB and RhoC, the lysates that remained after removing
the 700 μg portion were used, according to the same protocol
described for western blotting. The activation levels of RhoA, RhoB
and RhoC were normalized to the total levels of RhoA, RhoB and
RhoC, respectively.
Statistical analysis
Each experiment was repeated at least three times.
All statistical analyses were performed using SPSS software
(version 20.0; IBM Corporation) and GraphPad Prism 8 (Dotmatics).
Survival analysis was performed using Kaplan-Meier survival curves,
with differences between groups assessed using the log-rank test.
Hazard ratios and 95% confidence intervals were calculated to
evaluate risk. Differences between paired data (e.g., normal and
tumor tissues from the same patients) were compared using a paired
two-tailed Student's t-test. Differences between two unpaired
groups were compared using an unpaired two-tailed Student's t-test,
and differences between multiple groups were compared using one-way
ANOVA followed by Tukey's post hoc test. χ2 test or
Fisher's exact test was used to compare clinicopathological
features between the INHBA high and low expression groups.
P<0.05 was considered to indicate a statistically significant
difference.
Results
INHBA is associated with TC
RNA sequencing data were obtained from multiple GEO
datasets to identify DEGs between PTC samples and healthy thyroid
tissues, and ATC and PTC samples. Using these data, Venn diagrams
were constructed, identifying 26 intersecting genes (Fig. 1A-C). The STRING database was used
to determine genes that may be associated with TC (Fig. 1D). The results of the present
study highlighted that INHBA, a gene implicated in other types of
cancer, may have a role in TC. In addition, patient characteristics
and clinicopathological data were examined and the results
suggested that INHBA expression was significantly associated with
clinical stage (Table I). The
results of the quantitative analysis revealed significantly higher
mRNA expression levels of INHBA in stages T3 and T4 compared with
stages T1 and T2. (Fig. 1E), and
in patients with N1 stage cancer (Fig. 1F). Collectively, these results
demonstrated the potential role of INHBA in TC. Due to the overall
low mortality rate and high survival rate of TC, the statistical
analysis performed using TCGA data indicated no significant
association between INHBA expression and TC survival rates
(Fig. 1G). However, the overall
trend suggested that lower INHBA expression was associated with
better patient survival rates. The present study did not further
investigate the association between INHBA expression and TC
survival rates.
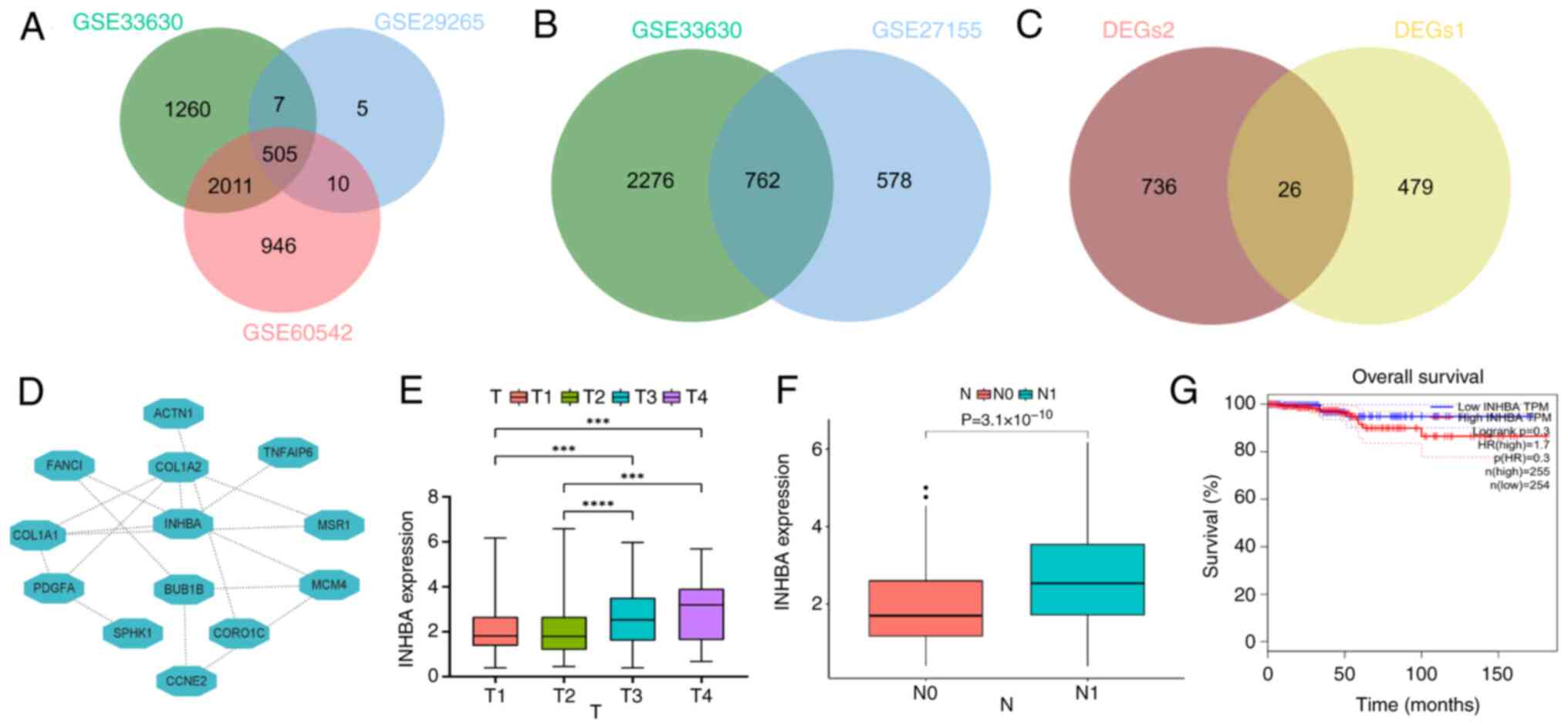 | Figure 1INHBA expression is positively
associated with TC tumor staging. (A) A Venn diagram depicting the
overlap of DEGs1 (PTC vs. normal) among three GEO datasets
(GSE33630, n=105; GSE29265, n=49; GSE60542, n=92). (B) A Venn
diagram depicting the overlap of DEGs2 (ATC vs. PTC) between
GSE33630 and GSE27155 datasets (GSE33630, n=105; GSE227155, n=99).
(C) A Venn diagram depicting the overlap of DEGs1 and DEGs2. (D)
Protein-protein interaction network was created using STRING. (E)
INHBA expression levels were increased in T3 and T4.
***P<0.001, ****P<0.0001. (F) INHBA
expression levels were increased in N1 compared with N0. (G)
Relationship between INHBA expression and the prognosis of patients
with TC. ATC, anaplastic thyroid carcinoma; DEGs, differentially
expressed genes; GEO, Gene Expression Omnibus; INHBA, inhibin βA;
PTC, poorly differentiated thyroid carcinoma; TC, thyroid
cancer. |
 | Table IAssociation between INHBA mRNA
expression and clinical characteristics of patients. |
Table I
Association between INHBA mRNA
expression and clinical characteristics of patients.
| Clinical
parameter | Low-INHBA
(n=377) | High-INHBA
(n=124) | P-value |
|---|
| Age (%) | | | 0.978 |
| <55 years | 265 (70.3) | 87 (70.2) | |
| ≥55 years | 112 (29.7) | 37 (29.8) | |
| Sex (%) | | | 0.135 |
| Female | 269 (71.4) | 97 (78.2) | |
| Male | 108 (28.6) | 27 (21.8) | |
| Clinical stage
(%) | | | 0.009 |
| I | 222 (58.9) | 61 (49.2) | |
| II | 44 (11.7) | 7 (5.6) | |
| III | 75 (19.9) | 35 (28.2) | |
| IV | 35 (9.3) | 20 (16.1) | |
| NA | 1 (0.2) | 1 (0.9) | |
| Metastasis (%) | | | 0.168 |
| M0 | 201 (53.3) | 78 (62.9) | |
| M1 | 7 (1.9) | 2 (1.6) | |
| NA | 169 (44.8) | 44 (35.5) | |
| N classification
(%) | | | <0.001 |
| N0 | 189 (50.2) | 37 (29.8) | |
| N1 | 149 (39.5) | 76 (61.3) | |
| NA | 39 (10.3) | 11 (8.9) | |
| T classification
(%) | | | 0.056 |
| T1 | 114 (30.2) | 28 (22.6) | |
| T2 | 112 (29.7) | 53 (42.7) | |
| T3 | 129 (34.2) | 40 (32.3) | |
| T4 | 20 (5.3) | 3 (2.4) | |
| NA | 2 (0.5) | 0 (0.0) | |
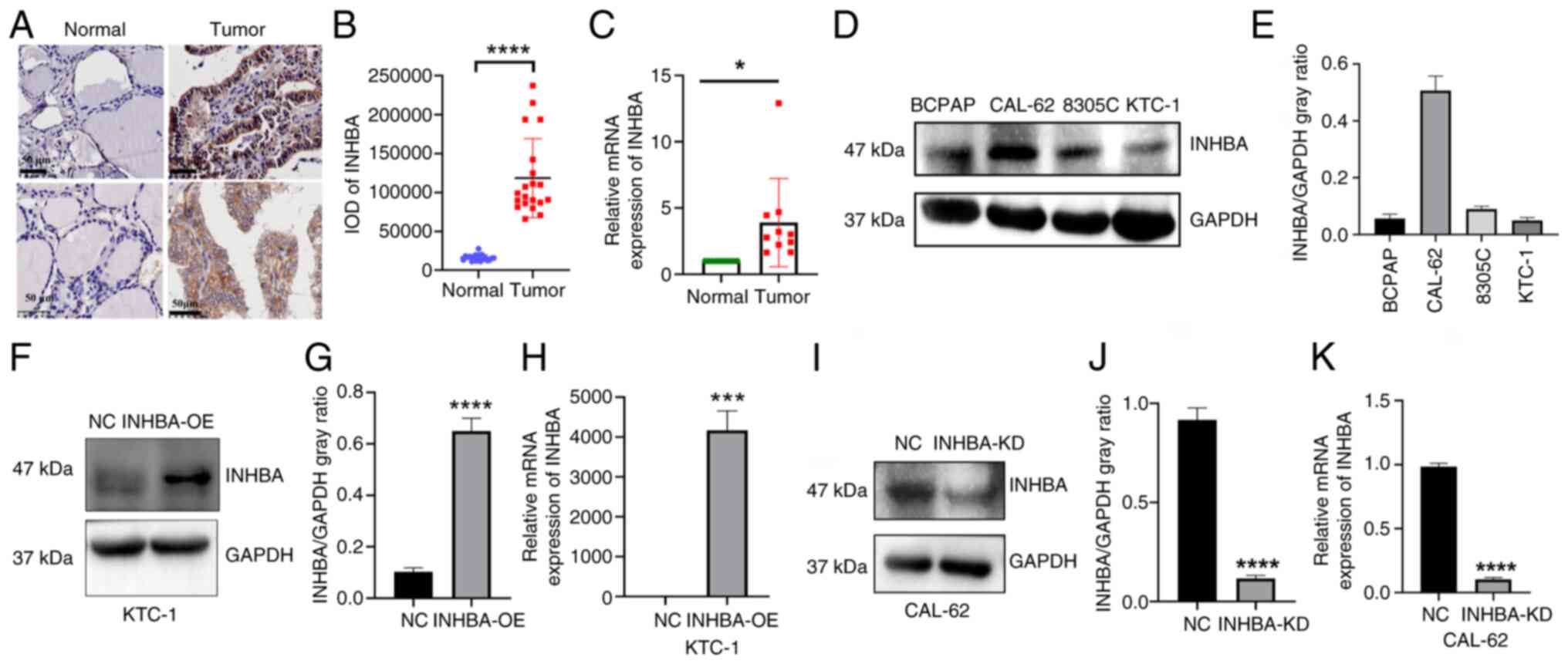
INHBA is upregulated in TC tissues
To further validate the aforementioned findings, 20
matched pairs of healthy thyroid and cancer tissues were analyzed.
INHBA expression was assessed using IHC, and brown coloration was
indicative of positive cells (Fig.
2A). The results of the present study indicated that INHBA was
predominantly localized in the cytoplasm, with significantly
increased expression levels in cancer tissues compared with those
in healthy tissues (Fig. 2B). In
addition, the results of RT-qPCR analysis demonstrated
significantly higher INHBA mRNA expression levels in tumor tissues
compared with those in healthy tissues (Fig. 2C).
To further explore the role of INHBA in TC,
corresponding expression levels were analyzed in four TC cell
lines; namely, BCPAP, CAL-62, KTC-1 and 8305C. INHBA expression was
high in CAL-62 cells and low in KTC-1 cells (Fig. 2D and E). Thus, INHBA knockdown
was carried out in CAL-62 cells and INHBA overexpression was
carried out in KTC-1 cells. INHBA mRNA and protein expression
levels were analyzed to confirm transfection efficiency (Fig. 2F-K).
INHBA promotes TC cell migration
Based on clinical observations that distant
metastasis significantly impacts the prognosis of patients with TC,
the effects of INHBA expression on TC progression were determined
in the present study. The results demonstrated that INHBA knockdown
or overexpression exerted no marked effect on TC cell proliferation
and apoptosis (Fig. 3A-C). The
results of the Transwell assay revealed that INHBA knockdown in
CAL-62 cells significantly inhibited invasion, whereas INHBA
overexpression in KTC-1 cells markedly enhanced invasion (Fig. 3D-G). The results of the migration
assay indicated that INHBA knockdown significantly reduced
migration in CAL-62 cells, compared with that in the NC group,
whereas KTC-1 cell migration was increased following INHBA
overexpression, compared with that in the NC group (Fig. 3H-K). Collectively, these results
demonstrated that INHBA overexpression exerted no effect on the
proliferation of TC cells; however, INHBA overexpression
significantly promoted the migration and invasion of TC cells,
providing a theoretical basis for the treatment of TC cell
metastasis.
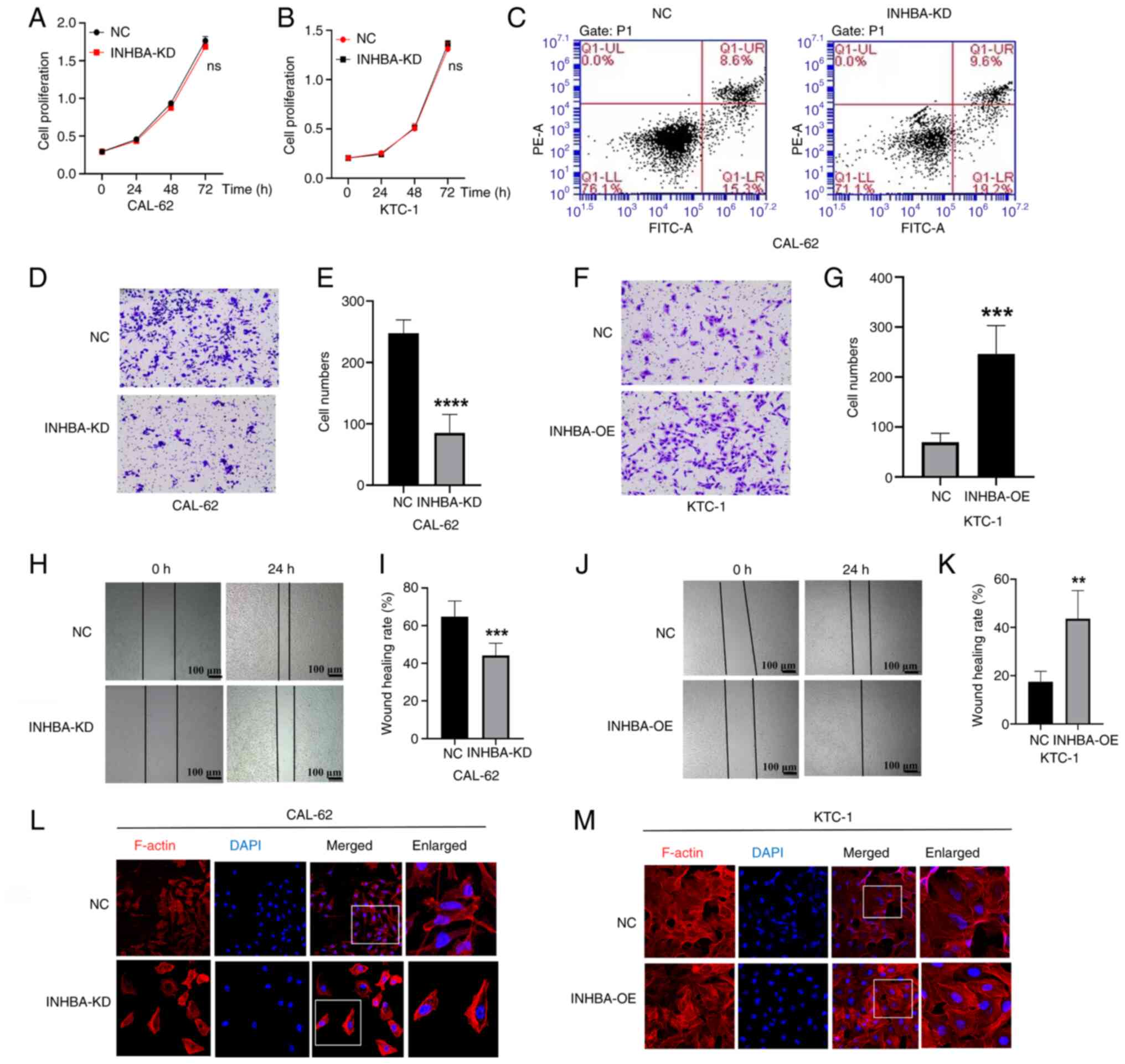 | Figure 3INHBA expression promotes the
migration and invasion of TC cells. In (A) CAL-62 cells and (B)
KTC-1 cells, the results of the Cell Counting Kit-8 assay
demonstrated that INHBA expression had no effect on the
proliferation of TC cells. (C) Effect of INHBA expression on
apoptosis of CAL-62 TC cells was detected using the Annexin V
assay. (D) INHBA-KD inhibited CAL-62 cell invasion; (E)
corresponding statistical analysis. (F) INHBA-OE promoted KTC cell
invasion; (G) corresponding statistical analysis. Magnification,
×200). (H) INHBA-KD inhibited the migration of CAL-62 cells; (I)
corresponding statistical analysis. (J) INHBA-OE promoted the
migration of TC cells; (K) corresponding statistical analysis.
**P<0.01, ***P<0.001,
****P<0.0001 vs. NC. (L) Cytoskeleton of CAL-62 cells
was visualized using confocal microscopy. (M) Cytoskeleton of KTC-1
cells was visualized using confocal microscopy. Magnification,
×400. Data are presented as the mean ± SD. INHBA, inhibin βA; KD,
knockdown; NC, negative control; OE, overexpression; TC, thyroid
cancer. |
The results of the immunofluorescence staining
analysis revealed alterations in the cytoskeleton of cells
following INHBA knockdown or overexpression. In the NC group of
CAL-62 cells, F-actin, which forms the main cytoskeletal structure,
was well-modeled and distributed, and the cell pseudopodia were
fully extended; however, in the INHBA knockdown group, the actin
filaments were disordered and the cell pseudopodia were reduced
(Fig. 3L). Following INHBA
overexpression, an increased number of actin filaments accumulated
at the leading edge of KTC-1 cells, compared with that in the
control group (Fig. 3M). These
results indicated that INHBA may impact the arrangement of the
cytoskeleton.
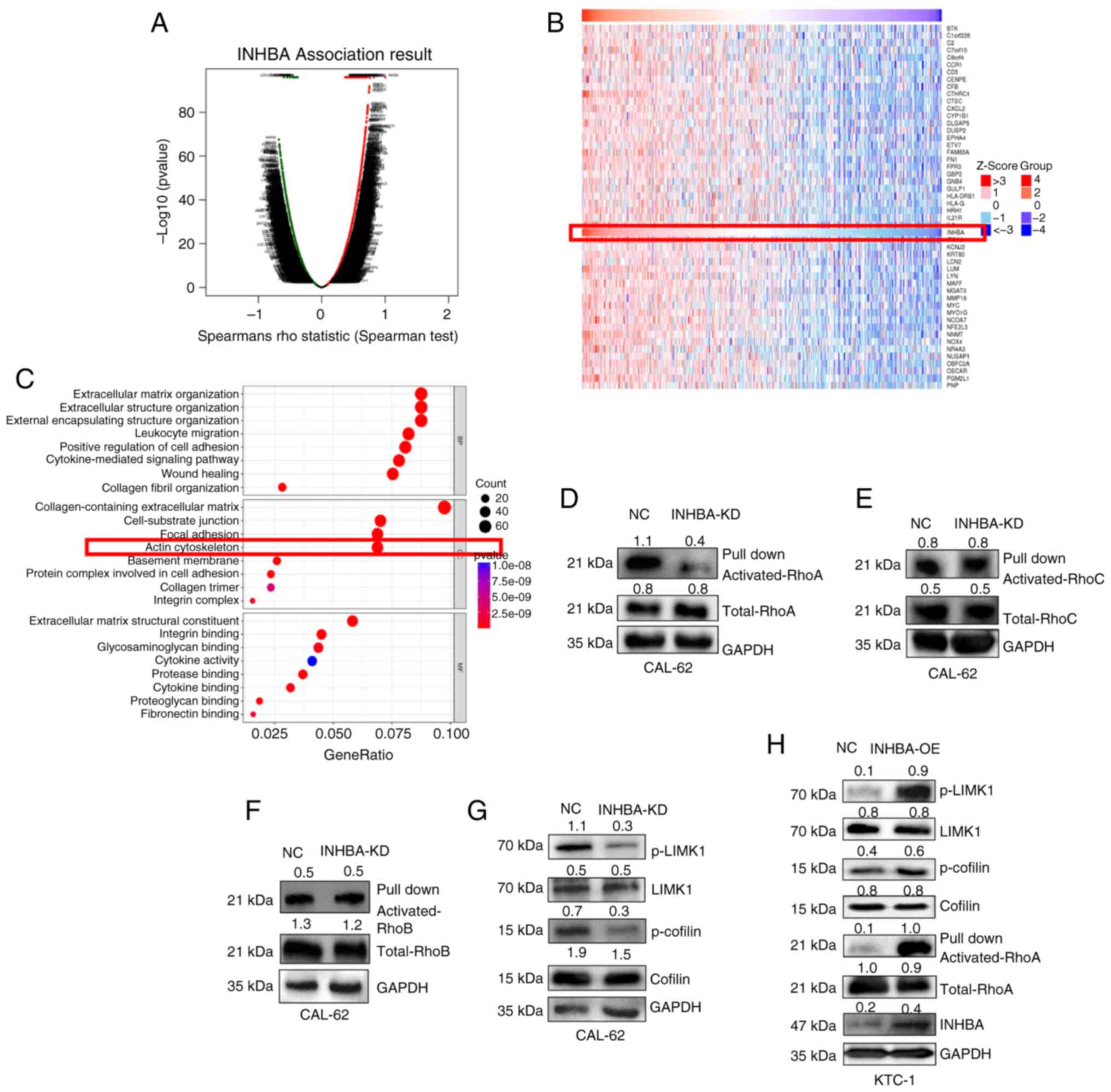
INHBA increases activation of the RhoA
pathway in TC
In the present study, the functional module of the
Linked Omics database was used to determine the molecular role of
INHBA in TC, and to analyze the co-expression patterns within the
TC cohort. As illustrated in Fig.
4A, genes marked with red dots exhibit those significantly
positively correlated with INHBA, whereas those marked with green
dots exhibit those significantly negatively correlated with INHBA
(false discovery rate <0.01). A heat map was used to display the
top 50 genes that were significantly positively associated with
INHBA (Fig. 4B). GO analysis
categorized the aforementioned proteins into three groups; namely,
cellular component, molecular function and biological process. The
results of the present study highlighted notable enrichment in the
terms 'extracellular matrix organization', 'wound healing' and
'actin cytoskeleton' (Fig. 4C).
These results suggested that INHBA may impact TC progression
through modulation of cytoskeleton-related pathways. Notably,
cancer cell migration is driven by regulation of the actin
cytoskeleton (31). Rho-GTPases
are pivotal in regulating cellular morphology, motility and
survival, and act as molecular switches through GTP binding and
hydrolysis (32). Activated Rho
family proteins, such as RhoA, are crucial in reshaping
cytoskeletal dynamics, facilitating essential transformations in
cell shape and movement that is crucial for tissue development and
wound healing (33). Moreover,
the results of previous studies highlighted the significant role of
Rho-GTPases in cancer cell migration and cell adhesion,
highlighting the importance of Rho-GTPases in cytoskeletal
regulation (34,35). To further elucidate the role of
INHBA in cytoskeletal regulation, western blot analysis was carried
out using CAL-62 cells. The results of the present study
highlighted that RhoA activation was decreased in the INHBA
knockdown group; however, the activation of RhoB and RhoC remained
unaltered (Fig. 4D-F). The role
of the RhoA/ROCK pathway in various types of cancer is
well-documented; for example, in gastric cancer, activation of the
RhoA/LIMK/Cofilin pathway has been shown to promote tumor
metastasis (36); in pancreatic
cancer, gastrin can induce focal adhesion formation and
cytoskeletal polarization through the RhoA/ROCK pathway, driving
invasive migration (37); and in
ovarian cancer, a stiffer matrix activates the RhoA/ROCK pathway,
enhancing tumor cell migration and invasion (38). However its involvement in TC
remains to be fully elucidated. Thus, downstream targets of the
RhoA/ROCK pathway, including LIMK and cofilin, and the
corresponding phosphorylation levels, were determined in TC cells.
The results revealed that INHBA knockdown led to decreased
phosphorylation levels of LIMK and cofilin, while INHBA
overexpression resulted in increased phosphorylation levels of
these proteins, further highlighting the impact of INHBA on the
LIMK/cofilin pathway (Fig. 4G and
H). Collectively, these findings further verify the role of
INHBA in modulating the cytoskeletal architecture through the
RhoA/ROCK signaling axis.
RhoA overexpression and treatment with a
RhoA/ROCK axis inhibitor rescue INHBA expression in TC cells
To assess the role of RhoA in INHBA-mediated cell
invasion and cytoskeletal protein regulation, the RhoA-OE plasmid
was used; its overexpression efficiency in untreated CAL-62 cells
was validated using western blotting (Fig. 5A and B). Subsequently, RhoA
overexpression was induced in CAL-62 cells with INHBA knockdown.
The results demonstrated that p-LIMK and p-cofilin levels were
rescued following RhoA overexpression (Fig. 5C). In addition, RhoA/ROCK
inhibition was carried out using the Y27632 inhibitor at a
concentration of 10 μM for 24 h in KTC-1 cells with INHBA
overexpression, and the results revealed that p-LIMK and p-cofilin
levels were decreased (Fig. 5C).
These results further highlighted the role of RhoA in this pathway.
In addition, the present study revealed that migration and invasion
was increased in CAL-62 cells with INHBA knockdown following RhoA
overexpression (Fig. 5D, F, H and
I), whereas migration and invasion was decreased in KTC-1 cells
with INHBA overexpression treated with the RhoA/ROCK inhibitor
Y27632 (Fig. 5E, G, H and J).
Since RhoA is a downstream molecule of INHBA, these results
suggested that INHBA may affect the phosphorylation and signaling
of RhoA-LIMK and cofilin, ultimately promoting a more aggressive
phenotype of TC.
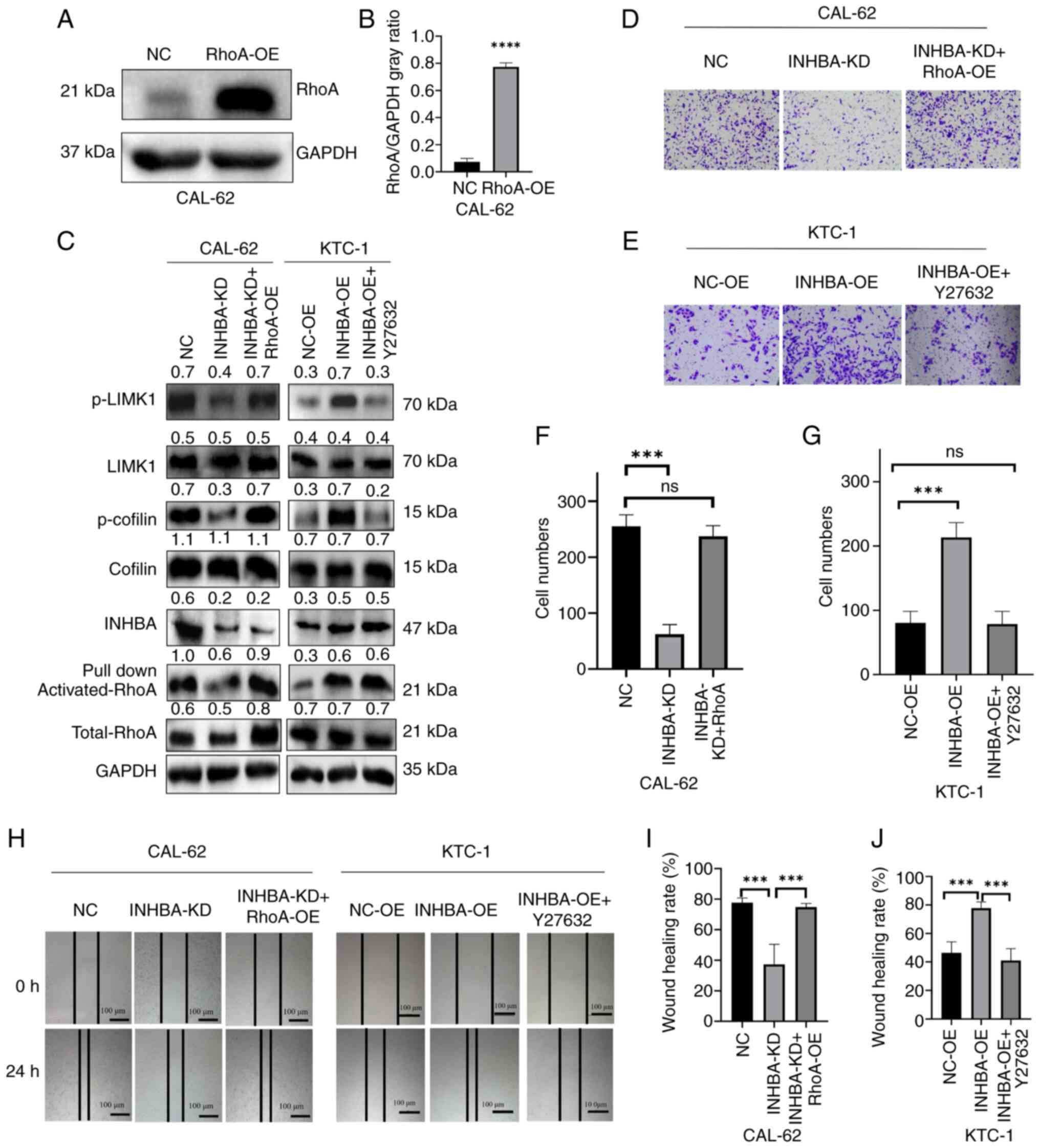 | Figure 5INHBA-mediated TC cell migration and
invasion were regulated by the RhoA/ROCK/LIMK/cofilin pathway. (A)
Transfection efficiency of RhoA-OE in CAL-62 cells; (B)
corresponding semi-quantitative analysis.
****P<0.0001 vs. NC. (C) RhoA OE or use of the RhoA
downstream inhibitor Y27632 altered proteins downstream of the
RhoA/ROCK axis in TC cell lines. (D) Rescue experiments were
carried out in CAL-62 cells and analyzed using Transwell assays.
(E) Statistical analysis of CAL-62 cell invasion from the rescue
experiments. (F) Rescue experiments were carried out in KTC-1 cells
and analyzed using Transwell assays. (G) Statistical analysis of
KTC-1 cell invasion from the rescue experiments. Magnification,
×200. (H) Rescue experiments were carried out in CAL-62 and KTC-1
cells and analyzed using wound healing assays. (I) Statistical
analysis of CAL-62 cell migration from the rescue experiments. (J)
Statistical analysis of KTC-1 cell migration from the rescue
experiments. ***P<0.001. Data are presented as the
mean ± SD. INHBA, inhibin βA; KD, knockdown; LIMK1, LIM kinase 1;
NC, negative control; NS, not significant; OE, overexpression; p-,
phosphorylated; ROCK, Rho-kinase; TC, thyroid cancer. |
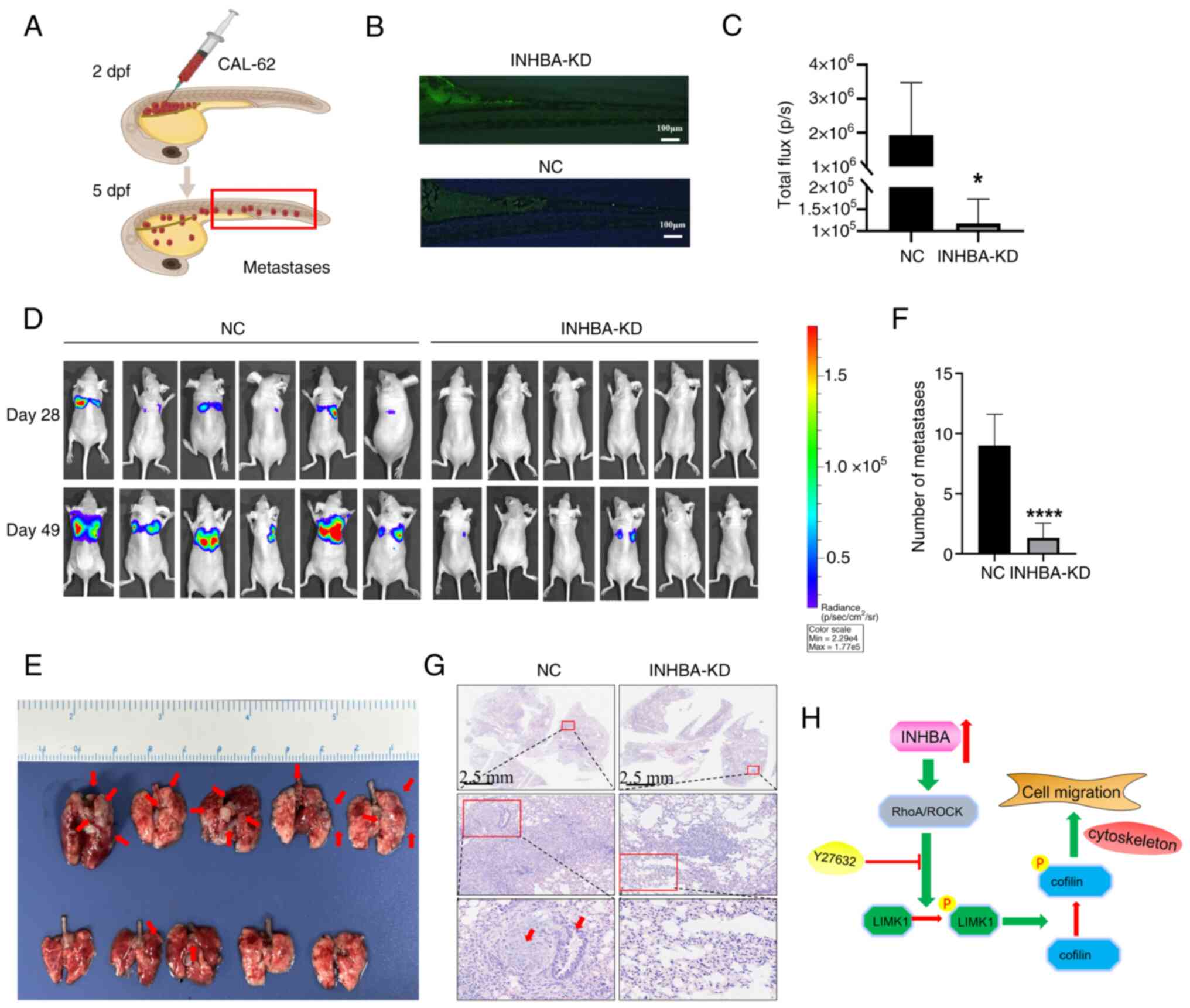
INHBA knockdown significantly suppresses
the metastasis of TC cells in vivo
To elucidate the biological function of INHBA in
vivo, zebrafish and nude mouse xenograft models were used in
the present study. INHBA knockdown significantly reduced the
metastatic ability of TC cell-derived xenografts in 5-dpf zebrafish
(Fig. 6A and B). Moreover, INHBA
knockdown notably decreased the metastasis of CAL-62 cells injected
into mice, resulting in fewer and smaller metastatic nodules,
compared with the control group (Fig. 6C-F). H&E staining further
confirmed the reduced metastatic ability of CAL-62 cells following
INHBA knockdown (Fig. 6G).
Collectively, these findings revealed that INHBA is a crucial
regulator of distant metastasis in TC.
Discussion
The management of TC has advanced in recent years.
Gene sequencing and mechanistic analysis have led to the
identification of effective molecules for the diagnosis and
treatment of TC. Multiple kinase inhibitors targeting key pathways
may prolong the progression-free survival of patients, and these
have been approved as a treatment option. For example, sorafenib
and lenvatinib target the VEGF receptor and platelet-derived growth
factor receptor pathways, and have been shown to be effective in
treating radioiodine-refractory differentiated thyroid cancer
(39,40). Additionally, dabrafenib and
trametinib specifically target the MAPK/ERK pathway, particularly
in patients with BRAF V600E mutations, helping to inhibit tumor
growth and proliferation (41).
Using the GEO database analysis, the results of the present study
revealed that INHBA was markedly upregulated in TC. Further
analysis of TC clinical data obtained from TCGA database
demonstrated that the expression of INHBA was significantly
positively associated with the T stage and N stage of patients with
TC, suggesting that INHBA may be a key regulator driving TC
aggressiveness. IHC and RT-qPCR analyses also revealed that INHBA
expression levels were increased in TC tissues.
Shibata et al (16) used the nCounter PanCancer
Progression panel to examine the expression levels of 740 genes
associated with tumor progression in samples obtained from six
low-risk and six high-risk patients with PTC. The results of this
previous study indicated a notable increase in INHBA expression in
patients with high-risk PTC, suggesting that INHBA exhibits
potential as a biomarker for advanced PTC. Notably, these results
are comparable with those of the present study, which demonstrated
that INHBA was associated with the N stage of patients with TC.
This association suggests that INHBA might serve a crucial role in
the metastatic process of TC. Previous studies have shown that
INHBA expression is dysregulated in some types of cancer. For
example, INHBA has been reported to be upregulated in colorectal
cancer, and may promote tumor growth and metastasis through
upregulating VCAN (42). A study
on pancreatic ductal adenocarcinoma highlighted that INHBA promotes
pancreatic cancer progression by enhancing tumor cell proliferation
through a SMAD3-dependent signaling pathway (43). Additionally, a study on upper
tract urothelial carcinoma emphasized that INHBA, via promoter
hypomethylation, can significantly promote tumor progression by
enhancing cell proliferation and migration (44). However, the specific roles and
mechanisms of INHBA in TC remain to be fully elucidated. In the
present study, CAL-62 and KTC-1 cell lines, which respectively
exhibit high and low INHBA expression levels, were used to
investigate the biological functions of INHBA. Notably, INHBA
knockdown reduced the migration of CAL-62 cells, whereas the
opposite effect was observed in KTC-1 cells following INHBA
overexpression. Moreover, in vivo studies confirmed that the
downregulation of INHBA expression in TC cell lines markedly
reduced tumor metastasis in xenograft models, including zebrafish
and nude mice. These findings are consistent with those of previous
reports on the pro-tumorigenic role of INHBA in other types of
cancer (43-46). These findings indicated a broad
relevance of INHBA in tumor progression and metastasis, and
underscore the critical need for further investigation into the
molecular mechanisms of the involvement INHBA of in TC biology and
its potential as a therapeutic target in cancer treatment.
Since INHBA has been shown to promote cell migration
in vitro and in vivo, further investigations into the
specific mechanisms underlying the effects of INHBA on TC cell
migration are required. In the present study, proteins associated
with actin cytoskeleton regulation were determined using TCGA
database and GO analysis, and the results of the present study
revealed that INHBA knockdown or overexpression altered the
morphology of TC cells.
Key processes in tumor metastasis, such as migration
and invasion, require cytoskeletal rearrangement (47). Invasive cancer cells migrate
along the basal membrane through a series of forward protrusions
and tail retractions, ultimately invading nearby tissues or distant
organs. This process relies on the rearrangement of the actin
cytoskeleton at the leading edge of the cell. The coordinated
action of various actin regulators is crucial for the
reorganization of the actin cytoskeleton (48,49). The cytoskeleton is composed of
actin, microtubules and intermediate filaments. In cancer cells,
mutations and the abnormal expression of cytoskeletal proteins
reduce the sensitivity of cells to chemotherapy, and affect cell
proliferation and migration. These aberrations disrupt regular
cellular functions, thus facilitating the aggressive behavior
typical of cancer cells (17,50,51). Activation of specific receptor
proteins on the plasma membrane initiates cytoskeletal
reorganization. These signals are mediated by the Rho GTPase family
and its downstream effector, ROCK, constituting the Rho/ROCK
signaling pathway (52,53). Notably, as a classical signaling
pathway implicated in cytoskeletal reorganization, the RhoA/ROCK
pathway impacts the metastasis of several types of cancer through
regulation of the cytoskeleton (54,55). RhoA, the most extensively studied
member of the Rho family (56),
activates ROCK, leading to the phosphorylation of LIMK1 and LIMK2,
at Thr508 and Thr505, respectively. LIMKs are also associated with
the progression and metastasis of various cancer types, such as
glioma and gastric cancer (57,58). Cofilin, a direct downstream
target of LIMK, is phosphorylated in its actin-binding domain,
inhibiting its interaction with actin filaments and therefore
regulating cytoskeletal dynamics. Previous studies have
demonstrated that LIMK promotes cancer cell migration through
regulating the phosphorylation of cofilin, affecting the stability
of actin filaments (59,60). The results of the present study
indicated that phosphorylation of LIMK/cofilin and TC cell
migration were induced by INHBA-mediated activation of the
RhoA/ROCK signaling pathway.
To further substantiate that RhoA/ROCK is the
downstream target influenced by altered INHBA expression, rescue
experiments were performed in the present study. Y27632 was applied
as an inhibitor of the RhoA/ROCK pathway. Notably, Y27632 is
capable of impeding cytoskeletal reorganization, cell migration and
proliferation, and it is well known that Y27632 can selectively
inhibit ROCK, with minimal effects on other kinases (26). In the field of drug discovery,
particularly in cancer research, Y27632 has demonstrated potential
in multiple areas (61). The
results of the rescue study revealed that Y27632 treatment reversed
the phosphorylation of LIMK and cofilin induced by INHBA
overexpression, leading to a significant reduction in TC cell
migration. This highlights the critical role of the RhoA/ROCK
pathway in mediating the effects of INHBA on TC; INHBA-mediated
activation of RhoA may be a key driver of cytoskeletal
reorganization and cellular motility in TC. Collectively, these
results confirmed the involvement of the RhoA/LIMK/cofilin pathway
in INHBA-induced TC cell migration. Activation of the
RhoA/LIMK/cofilin signaling pathway has been shown to promote tumor
progression through the cytoskeleton in various cancer types
(62,63); however, to the best of our
knowledge, the activation of RhoA induced by INHBA overexpression
has not been previously studied. The results of the present study
demonstrated that INHBA overexpression may lead to the activation
of RhoA, which in turn could impact phosphorylation of the
LIMK/cofilin pathway, thereby promoting TC cell migration.
In conclusion, the present study demonstrated that
INHBA is associated with TC metastasis by inducing RhoA activation,
leading to phosphorylation changes in the LIMK/cofilin pathway, as
illustrated in the hypothetical model (Fig. 6H). Clinical and cell line
analyses, alongside in vivo experiments in xenografted
zebrafish and nude mice, showed that INHBA knockdown could
significantly reduce metastasis. These findings suggested that
INHBA may enhance the aggressive phenotype of TC cells through the
RhoA/LIMK/cofilin signaling axis. However, the primary focus of the
present study was on elucidating the role of INHBA in promoting TC
metastasis at both cellular and animal levels. While functional
validation of Y27632 was conducted at the cellular level, its
effects on metastasis in mice were not within the scope of the
current research, which represents a limitation of the study.
Another limitation of the present study is the lack of information
on the histological type and stage of the cancer tissues used for
target validation. We did not further classify or stage the tissue
samples, partly due to the limited sample size. Future studies will
include this information to enhance the comprehensiveness and
accuracy of the present findings. Despite this, INHBA shows
potential as a biomarker and therapeutic target for aggressive
TC.
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
WZ, ZL and WX designed the present study. WZ and YZ
conducted the experiments. Statistical analysis was conducted by WW
and WZ wrote the manuscript. WX and ZL revised the manuscript. WX
and WZ confirm the authenticity of all the raw data. All authors
have read and approved the final version of the manuscript.
Ethics approval and consent to
participate
The present study was conducted in accordance with
ethical standards and was approved by the Ethics Committee of
Shandong Provincial ENT Hospital. The ethics committee separately
reviewed and approved the human studies and the animal studies as
part of this project. Each study was subject to its respective
ethical review standards. Despite the different ethical standards
for human and animal research, both were rigorously reviewed and
approved under the same project approval number (20220713). This
unified approval number reflects the comprehensive review and
approval by the ethics committee for the entire project. Written
informed consent was obtained from the patients.
Patient consent for publication
Written informed consent was obtained for
publication of the patient data and images.
Competing interests
The authors declare that they have no competing
interests.
Acknowledgements
Not applicable.
Funding
The present study was supported by the National Natural Science
Foundation of China (grant no. 82172961).
References
|
1
|
Siegel RL, Miller KD, Wagle NS and Jemal
A: Cancer statistics, 2023. CA Cancer J Clin. 73:17–48. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Pizzato M, Li M, Vignat J, Laversanne M,
Singh D, La Vecchia C and Vaccarella S: The epidemiological
landscape of thyroid cancer worldwide: GLOBOCAN estimates for
incidence and mortality rates in 2020. Lancet Diabetes Endocrinol.
10:264–272. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Araque DVP, Bleyer A and Brito JP: Thyroid
cancer in adolescents and young adults. Future Oncol. 13:1253–1261.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Miller KD, Fidler-Benaoudia M, Keegan TH,
Hipp HS, Jemal A and Siegel RL: Cancer statistics for adolescents
and young adults, 2020. CA Cancer J Clin. 70:443–459. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
American Cancer Society: Cancer facts
& figures 2024. Atlanta: American Cancer Society; 2024
|
|
6
|
Zheng R, Zhang S, Zeng H, Wang S, Sun K,
Chen R, Li L, Wei W and He J: Cancer incidence and mortality in
China, 2016. J Natl Cancer Cent. 2:1–9. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Molinaro E, Romei C, Biagini A, Sabini E,
Agate L, Mazzeo S, Materazzi G, Sellari-Franceschini S, Ribechini
A, Torregrossa L, et al: Anaplastic thyroid carcinoma: From
clinicopathology to genetics and advanced therapies. Nat Rev
Endocrinol. 13:644–660. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Durante C, Haddy N, Baudin E, Leboulleux
S, Hartl D, Travagli JP, Caillou B, Ricard M, Lumbroso JD, De
Vathaire F and Schlumberger M: Long-term outcome of 444 patients
with distant metastases from papillary and follicular thyroid
carcinoma: benefits and limits of radioiodine therapy. J Clin
Endocrinol Metab. 91:2892–2899. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Totsuka Y, Tabuchi M, Kojima I, Shibai H
and Ogata E: A novel action of activin A: Stimulation of insulin
secretion in rat pancreatic islets. Biochem Biophys Res Commun.
156:335–339. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Beattie GM, Lopez AD, Bucay N, Hinton A,
Firpo MT, King CC and Hayek A: Activin A maintains pluripotency of
human embryonic stem cells in the absence of feeder layers. Stem
Cells. 23:489–495. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Robson NC, Phillips DJ, McAlpine T, Shin
A, Svobodova S, Toy T, Pillay V, Kirkpatrick N, Zanker D, Wilson K,
et al: Activin-A: A novel dendritic cell-derived cytokine that
potently attenuates CD40 ligand-specific cytokine and chemokine
production. Blood. 111:2733–2743. 2008. View Article : Google Scholar
|
|
12
|
Kumar V, Ramnarayanan K, Sundar R,
Padmanabhan N, Srivastava S, Koiwa M, Yasuda T, Koh V, Huang KK,
Tay ST, et al: Single-cell atlas of lineage states, tumor
microenvironment, and subtype-specific expression programs in
gastric cancer. Cancer Discov. 12:670–691. 2022. View Article : Google Scholar
|
|
13
|
Zhang C, Liang Y, Ma MH, Wu KZ and Dai DQ:
KRT15, INHBA, MATN3, and AGT are aberrantly methylated and
differentially expressed in gastric cancer and associated with
prognosis. Pathol Res Pract. 215:893–899. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lyu S, Jiang C, Xu R, Huang Y and Yan S:
INHBA upregulation correlates with poorer prognosis in patients
with esophageal squamous cell carcinoma. Cancer Manag Res.
10:1585–1596. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Li X, Yu W, Liang C, Xu Y, Zhang M, Ding X
and Cai X: INHBA is a prognostic predictor for patients with colon
adenocarcinoma. BMC Cancer. 20:3052020. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Shibata M, Inaishi T, Ichikawa T, Shimizu
D, Soeda I, Takano Y, Takeuchi D, Tsunoda N and Kikumori T:
Identifying the tumor-progressive gene expression profile in
high-risk papillary thyroid cancer. Surg Today. 51:1703–1712. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hall A: The cytoskeleton and cancer.
Cancer Metastasis Rev. 28:5–14. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Hohmann T and Dehghani F: The
cytoskeleton-A complex interacting meshwork. Cells. 8:3622019.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yamazaki D, Kurisu S and Takenawa T:
Regulation of cancer cell motility through actin reorganization.
Cancer Sci. 96:379–386. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Hodge RG and Ridley AJ: Regulating Rho
GTPases and their regulators. Nat Rev Mol Cell Biol. 17:496–510.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Loirand G: Rho kinases in health and
disease: From basic science to translational research. Pharmacol
Rev. 67:1074–1095. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Sahai E and Marshall CJ: RHO-GTPases and
cancer. Nat Rev Cancer. 2:133–142. 2002. View Article : Google Scholar
|
|
23
|
Tomás G, Tarabichi M, Gacquer D, Hébrant
A, Dom G, Dumont JE, Keutgen X, Fahey TJ III, Maenhaut C and
Detours V: A general method to derive robust organ-specific gene
expression-based differentiation indices: Application to thyroid
cancer diagnostic. Oncogene. 31:4490–4498. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Tarabichi M, Saiselet M, Trésallet C,
Hoang C, Larsimont D, Andry G, Maenhaut C and Detours V: Revisiting
the transcriptional analysis of primary tumours and associated
nodal metastases with enhanced biological and statistical controls:
Application to thyroid cancer. Br J Cancer. 112:1665–1674. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Giordano TJ, Kuick R, Thomas DG, Misek DE,
Vinco M, Sanders D, Zhu Z, Ciampi R, Roh M, Shedden K, et al:
Molecular classification of papillary thyroid carcinoma: Distinct
BRAF, RAS, and RET/PTC mutation-specific gene expression profiles
discovered by DNA microarray analysis. Oncogene. 24:6646–6656.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Pernis AB, Ricker E, Weng CH, Rozo C and
Yi W: Rho Kinases in autoimmune diseases. Annu Rev Med. 67:355–374.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wang L, Luo X, Cheng C, Amos CI, Cai G and
Xiao F: A gene expression-based immune signature for lung
adenocarcinoma prognosis. Cancer Immunol Immunother. 69:1881–1890.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
29
|
National Research Council (US): Committee
for the Update of the Guide for the Care and Use of Laboratory
Animals. The National Academies Collection: Reports funded by
National Institutes of Health. Guide for the Care and Use of
Laboratory Animals. 8th edition. National Academies Press;
Washington, DC: 2011
|
|
30
|
American Veterinary Medical Association:
AVMA Guidelines for the Euthanasia of Animals. 2020 edition.
American Veterinary Medical Association; Schaumburg, IL: 2020
|
|
31
|
Seetharaman S and Etienne-Manneville S:
Cytoskeletal crosstalk in cell migration. Trends Cell Biol.
30:720–735. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Yamaguchi H and Condeelis J: Regulation of
the actin cytoskeleton in cancer cell migration and invasion.
Biochim Biophys Acta. 1773:642–652. 2007. View Article : Google Scholar
|
|
33
|
Arnold TR, Stephenson RE and Miller AL:
Rho GTPases and actomyosin: Partners in regulating epithelial
cell-cell junction structure and function. Exp Cell Res. 358:20–30.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Song S, Cong W, Zhou S, Shi Y, Dai W,
Zhang H, Wang X, He B and Zhang Q: Small GTPases: Structure,
biological function and its interaction with nanoparticles. Asian J
Pharm Sci. 14:30–39. 2019. View Article : Google Scholar
|
|
35
|
Strassheim D, Gerasimovskaya E, Irwin D,
Dempsey EC, Stenmark K and Karoor V: RhoGTPase in vascular disease.
Cells. 8:5512019. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Wang Y, Tu Z, Zhao W, Wang L, Jiang J, Gu
L, Li M, Jiang L, Wang Y and Bi Y: PLCB1 enhances cell migration
and invasion in gastric cancer via regulating actin cytoskeletal
remodeling and epithelial-mesenchymal transition. Biochem Genet.
61:2618–2632. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Mu G, Ding Q, Li H, Zhang L, Zhang L, He
K, Wu L, Deng Y, Yang D, Wu L, et al: Gastrin stimulates pancreatic
cancer cell directional migration by activating the
Gα12/13-RhoA-ROCK signaling pathway. Exp Mol Med. 50:1–14. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Wei X, Lou H, Zhou D, Jia Y, Li H, Huang
Q, Ma J, Yang Z, Sun C, Meng Y, et al: TAGLN mediated
stiffness-regulated ovarian cancer progression via RhoA/ROCK
pathway. J Exp Clin Cancer Res. 40:2922021. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Brose MS, Nutting CM, Jarzab B, Elisei R,
Siena S, Bastholt L, de la Fouchardiere C, Pacini F, Paschke R,
Shong YK, et al: Sorafenib in radioactive iodine-refractory,
locally advanced or metastatic differentiated thyroid cancer: A
randomised, double-blind, phase 3 trial. Lancet. 384:319–328. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Schlumberger M, Tahara M, Wirth LJ,
Robinson B, Brose MS, Elisei R, Habra MA, Newbold K, Shah MH, Hoff
AO, et al: Lenvatinib versus placebo in radioiodine-refractory
thyroid cancer. N Engl J Med. 372:621–630. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Subbiah V, Kreitman RJ, Wainberg ZA, Cho
JY, Schellens JHM, Soria JC, Wen PY, Zielinski C, Cabanillas ME,
Urbanowitz G, et al: Dabrafenib and trametinib treatment in
patients with locally advanced or metastatic BRAF V600-mutant
anaplastic thyroid cancer. J Clin Oncol. 36:7–13. 2018. View Article : Google Scholar
|
|
42
|
Guo J and Liu Y: INHBA promotes the
proliferation, migration and invasion of colon cancer cells through
the upregulation of VCAN. J Int Med Res. 49:30006052110149982021.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Yu SY, Luan Y, Tang S, Abazarikia A, Dong
R, Caffrey TC, Hollingsworth MA, Oupicky D and Kim SY: Uncovering
tumor-promoting roles of activin A in pancreatic ductal
adenocarcinoma. Adv Sci (Weinh). 10:e22070102023. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Kao CC, Chang YL, Liu HY, Wu ST, Meng E,
Cha TL, Sun GH, Yu DS and Luo HL: DNA hypomethylation is associated
with the overexpression of INHBA in upper tract urothelial
carcinoma. Int J Mol Sci. 23:20722022. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Wu Z, Chen J, Yang L, Sun K, Jiang Q, Dong
F, Lu W, Chen R and Chen Y: Elevated INHBA promotes tumor
progression of cervical cancer. Technol Cancer Res Treat.
23:153303382412347982024. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Hu C, Ye M, Bai J, Liu P, Lu F, Chen J, Xu
Y, Yan L, Yu P, Xiao Z, et al: FOXA2-initiated transcriptional
activation of INHBA induced by methylmalonic acid promotes
pancreatic neuroendocrine neoplasm progression. Cell Mol Life Sci.
81:502024. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Tang Y, He Y, Zhang P, Wang J, Fan C, Yang
L, Xiong F, Zhang S, Gong Z, Nie S, et al: LncRNAs regulate the
cytoskeleton and related Rho/ROCK signaling in cancer metastasis.
Mol Cancer. 17:772018. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Vilchez Mercedes SA, Bocci F, Levine H,
Onuchic JN, Jolly MK and Wong PK: Decoding leader cells in
collective cancer invasion. Nat Rev Cancer. 21:592–604. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Gao J and Nakamura F: Actin-associated
proteins and small molecules targeting the actin cytoskeleton. Int
J Mol Sci. 23:21182022. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Fife CM, McCarroll JA and Kavallaris M:
Movers and shakers: Cell cytoskeleton in cancer metastasis. Br J
Pharmacol. 171:5507–5523. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Jones MC, Zha J and Humphries MJ:
Connections between the cell cycle, cell adhesion and the
cytoskeleton. Philos Trans R Soc Lond B Biol Sci. 374:201802272019.
View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Burridge K and Wennerberg K: Rho and Rac
take center stage. Cell. 116:167–179. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Amano M, Nakayama M and Kaibuchi K:
Rho-kinase/ROCK: A key regulator of the cytoskeleton and cell
polarity. Cytoskeleton (Hoboken). 67:545–554. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Li X and Wang J: Mechanical tumor
microenvironment and transduction: Cytoskeleton mediates cancer
cell invasion and metastasis. Int J Biol Sci. 16:2014–2028. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Tuntithavornwat S, Shea DJ, Wong BS,
Guardia T, Lee SJ, Yankaskas CL, Zheng L,
Kontrogianni-Konstantopoulos A and Konstantopoulos K: Giant
obscurin regulates migration and metastasis via RhoA-dependent
cytoskeletal remodeling in pancreatic cancer. Cancer Lett.
526:155–167. 2022. View Article : Google Scholar :
|
|
56
|
Etienne-Manneville S and Hall A: Rho
GTPases in cell biology. Nature. 420:629–635. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
You T, Gao W, Wei J, Jin X, Zhao Z, Wang C
and Li Y: Overexpression of LIMK1 promotes tumor growth and
metastasis in gastric cancer. Biomed Pharmacother. 69:96–101. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Chen J, Ananthanarayanan B, Springer KS,
Wolf KJ, Sheyman SM, Tran VD and Kumar S: Suppression of LIM kinase
1 and LIM kinase 2 limits glioblastoma invasion. Cancer Res.
80:69–78. 2020. View Article : Google Scholar :
|
|
59
|
Maekawa M, Ishizaki T, Boku S, Watanabe N,
Fujita A, Iwamatsu A, Obinata T, Ohashi K, Mizuno K and Narumiya S:
Signaling from Rho to the actin cytoskeleton through protein
kinases ROCK and LIM-kinase. Science. 285:895–898. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Lee MH, Kundu JK, Chae JI and Shim JH:
Targeting ROCK/LIMK/cofilin signaling pathway in cancer. Arch Pharm
Res. 42:481–491. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
de Sousa GR, Vieira GM, das Chagas PF,
Pezuk JA and Brassesco MS: Should we keep rocking? Portraits from
targeting Rho kinases in cancer. Pharmacol Res. 160:1050932020.
View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Yang L, Hou Y, Du YE, Li Q, Zhou F, Li Y,
Zeng H, Jin T, Wan X, Guan S, et al: Mirtronic miR-4646-5p promotes
gastric cancer metastasis by regulating ABHD16A and metabolite
lysophosphatidylserines. Cell Death Differ. 28:2708–2727. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Fu L, Wang X, Yang Y, Chen M, Kuerban A,
Liu H, Dong Y, Cai Q, Ma M and Wu X: Septin11 promotes
hepatocellular carcinoma cell motility by activating RhoA to
regulate cytoskeleton and cell adhesion. Cell Death Dis.
14:2802023. View Article : Google Scholar : PubMed/NCBI
|