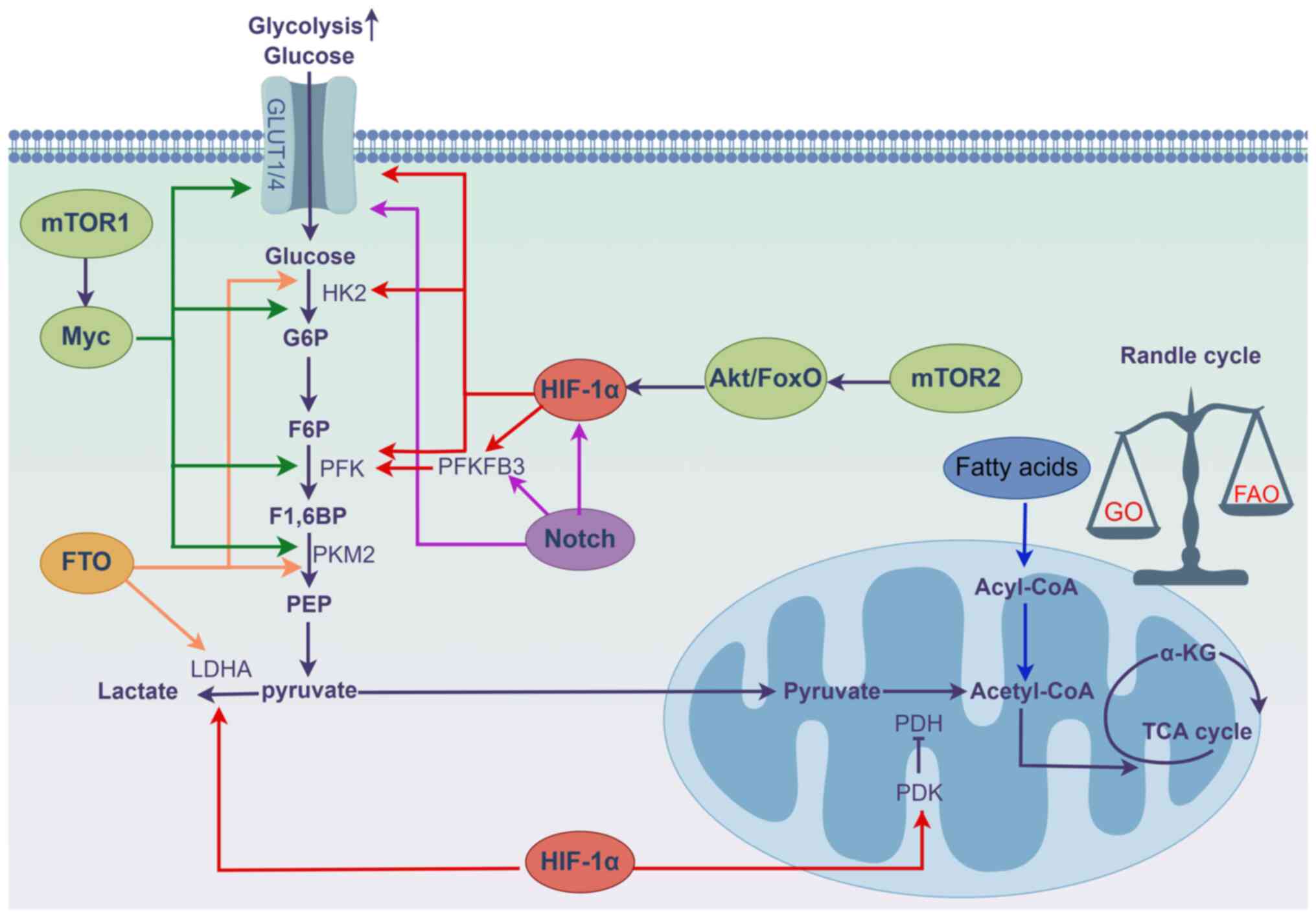
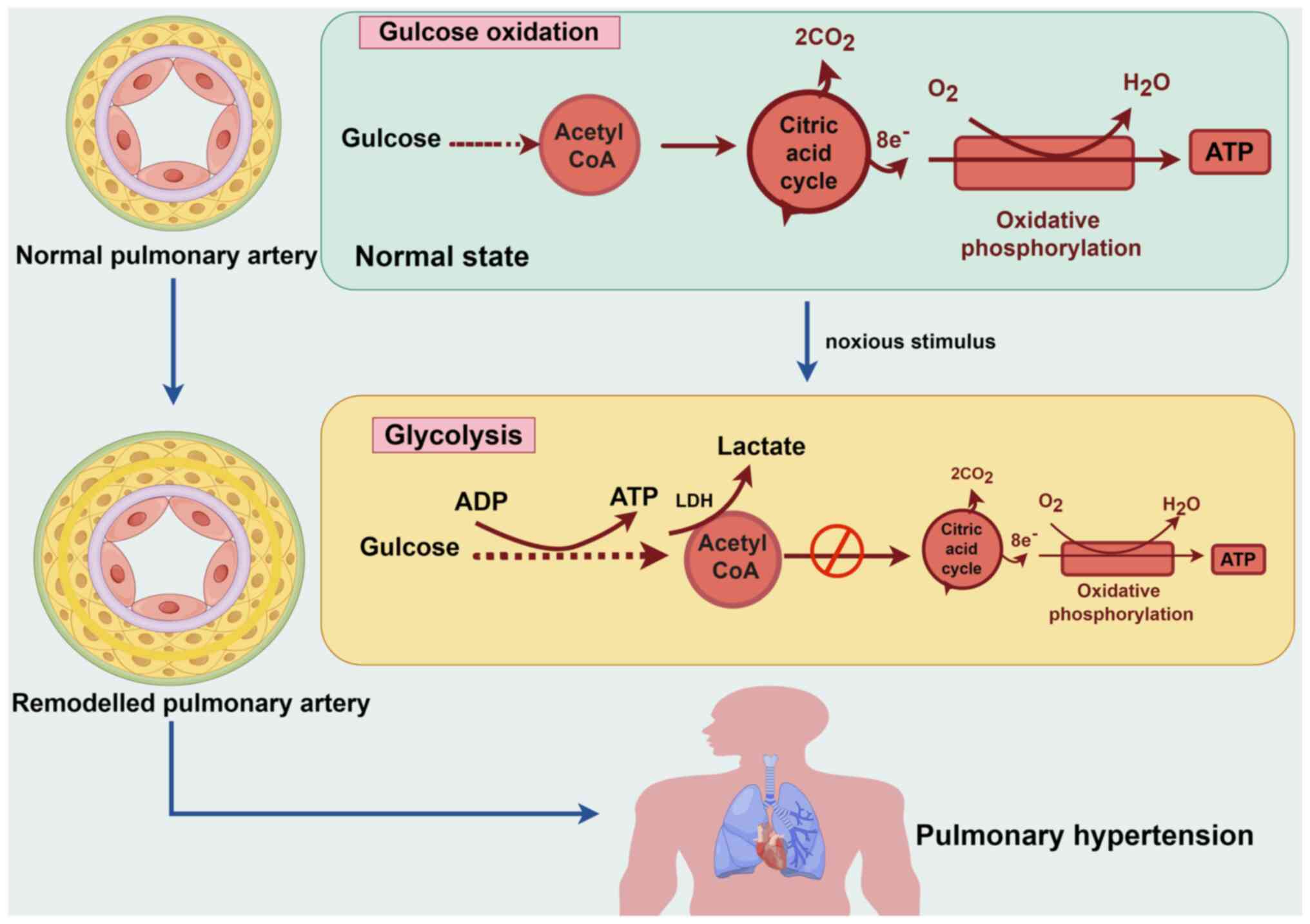
|
1
|
Humbert M, Kovacs G, Hoeper MM,
Badagliacca R, Berger RMF, Brida M, Carlsen J, Coats AJS,
Escribano-Subias P, Ferrari P, et al: 2022 ESC/ERS guidelines for
the diagnosis and treatment of pulmonary hypertension. Eur Respir
J. 61:22008792023. View Article : Google Scholar
|
|
2
|
Jia Z, Wang S, Yan H, Cao Y, Zhang X, Wang
L, Zhang Z, Lin S, Wang X and Mao J: Pulmonary vascular remodeling
in pulmonary hypertension. J Pers Med. 13:3662023. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kang M, Hart CM, Kempker JA, Veeraraghavan
S and Trammell AW: Pulmonary hypertension mortality trends in
United States 1999-2019. Ann Epidemiol. 75:47–52. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hoeper MM, Humbert M, Souza R, Idrees M,
Kawut SM, Sliwa-Hahnle K, Jing ZC and Gibbs JSR: A global view of
pulmonary hypertension. Lancet Respir Med. 4:306–322. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
George MP, Gladwin MT and Graham BB:
Exploring new therapeutic pathways in pulmonary hypertension.
metabolism, proliferation, and personalized medicine. Am J Respir
Cell Mol Biol. 63:279–292. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Culley MK and Chan SY: Mitochondrial
metabolism in pulmonary hypertension: Beyond mountains there are
mountains. J Clin Invest. 128:3704–3715. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Smolders VFED, Rodríguez C, Morén C,
Blanco I, Osorio J, Piccari L, Bonjoch C, Quax PHA, Peinado VI,
Castellà M, et al: Decreased glycolysis as metabolic fingerprint of
endothelial cells in chronic thromboembolic pulmonary hypertension.
Am J Respir Cell Mol Biol. 63:710–713. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Goncharov DA, Kudryashova TV, Ziai H,
Ihida-Stansbury K, DeLisser H, Krymskaya VP, Tuder RM, Kawut SM and
Goncharova EA: Mammalian target of rapamycin complex 2 (mTORC2)
coordinates pulmonary artery smooth muscle cell metabolism,
proliferation, and survival in pulmonary arterial hypertension.
Circulation. 129:864–874. 2014. View Article : Google Scholar
|
|
9
|
Singh N, Manhas A, Kaur G, Jagavelu K and
Hanif K: Inhibition of fatty acid synthase is protective in
pulmonary hypertension. Br J Pharmacol. 173:2030–2045. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Bertero T, Perk D and Chan SY: The
molecular rationale for therapeutic targeting of glutamine
metabolism in pulmonary hypertension. Expert Opin Ther Targets.
23:511–524. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hantzidiamantis PJ and Lappin SL:
StatPearls; Treasure Island (FL): 2023
|
|
12
|
Warburg O, Wind F and Negelein E: The
metabolism of tumors in the body. J Gen Physiol. 8:519–530. 1927.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Vaupel P and Multhoff G: Revisiting the
Warburg effect: Historical dogma versus current understanding. J
Physiol. 599:1745–1757. 2021. View Article : Google Scholar
|
|
14
|
Archer SL: Pyruvate kinase and warburg
metabolism in pulmonary arterial hypertension: Uncoupled Glycolysis
and the cancer-like phenotype of pulmonary arterial hypertension.
Circulation. 136:2486–2490. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Archer SL: Acquired mitochondrial
abnormalities, including epigenetic inhibition of superoxide
dismutase 2, in pulmonary hypertension and cancer: Therapeutic
implications. Adv Exp Med Biol. 903:29–53. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Vaupel P, Schmidberger H and Mayer A: The
Warburg effect: Essential part of metabolic reprogramming and
central contributor to cancer progression. Int J Radiat Biol.
95:912–919. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Condon D, Agarwal S, Chakraborty A and de
Jesus Perez VA: The cancer hypothesis of pulmonary arterial
hypertension: The next ten years. Am J Physiol Lung Cell Mol
Physiol. 318:L1138–L1139. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Christou H and Khalil RA: Mechanisms of
pulmonary vascular dysfunction in pulmonary hypertension and
implications for novel therapies. Am J Physiol Heart Circ Physiol.
322:H702–H724. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ryanto GRT, Suraya R and Nagano T:
Mitochondrial dysfunction in pulmonary hypertension. Antioxidants
(Basel). 12:3722023. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Arai MA, Sakuraba K, Makita Y, Hara Y and
Ishibashi M: Evaluation of naturally occurring HIF-1 inhibitors for
pulmonary arterial hypertension. Chembiochem. 22:2799–2804. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ahmadi A, Ohira H and Mielniczuk LM: FDG
PET imaging for identifying pulmonary hypertension and right heart
failure. Curr Cardiol Rep. 17:5552015. View Article : Google Scholar
|
|
22
|
Abikhzer Y, Probst S and Rush C: Pulmonary
hypertension findings detected by F-18 FDG PET scan. Clin Nucl Med.
33:405–406. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Marsboom G, Wietholt C, Haney CR, Toth PT,
Ryan JJ, Morrow E, Thenappan T, Bache-Wiig P, Piao L, Paul J, et
al: Lung 18F-fluorodeoxyglucose positron emission tomography for
diagnosis and monitoring of pulmonary arterial hypertension. Am J
Respir Crit Care Med. 185:670–679. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zhao L, Ashek A, Wang L, Fang W, Dabral S,
Dubois O, Cupitt J, Pullamsetti SS, Cotroneo E, Jones H, et al:
Heterogeneity in lung (18)FDG uptake in pulmonary arterial
hypertension: Potential of dynamic (18)FDG positron emission
tomography with kinetic analysis as a bridging biomarker for
pulmonary vascular remodeling targeted treatments. Circulation.
128:1214–1224. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Caruso P, Dunmore BJ, Schlosser K, Schoors
S, Dos Santos C, Perez-Iratxeta C, Lavoie JR, Zhang H, Long L,
Flockton AR, et al: Identification of MicroRNA-124 as a major
regulator of enhanced endothelial cell glycolysis in pulmonary
arterial hypertension via PTBP1 (polypyrimidine tract binding
protein) and pyruvate kinase M2. Circulation. 136:2451–2467. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Akagi S, Nakamura K, Kondo M, Hirohata S,
Udono H, Nishida M, Saito Y, Yoshida M, Miyoshi T and Ito H:
Evidence for hypoxia-induced shift in ATP production from
glycolysis to mitochondrial respiration in pulmonary artery smooth
muscle cells in pulmonary arterial hypertension. J Clin Med.
12:50282023. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Fijalkowska I, Xu W, Comhair SAA, Janocha
AJ, Mavrakis LA, Krishnamachary B, Zhen L, Mao T, Richter A,
Erzurum SC and Tuder RM: Hypoxia inducible-factor1alpha regulates
the metabolic shift of pulmonary hypertensive endothelial cells. Am
J Pathol. 176:1130–1138. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Cao Y, Zhang X, Wang L, Yang Q, Ma Q, Xu
J, Wang J, Kovacs L, Ayon RJ, Liu Z, et al: PFKFB3-mediated
endothelial glycolysis promotes pulmonary hypertension. Proc Natl
Acad Sci USA. 116:13394–13403. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Boehme J, Sun X, Tormos KV, Gong W,
Kellner M, Datar SA, Kameny RJ, Yuan JXJ, Raff GW, Fineman JR, et
al: Pulmonary artery smooth muscle cell hyperproliferation and
metabolic shift triggered by pulmonary overcirculation. Am J
Physiol Heart Circ Physiol. 311:H944–H957. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Wujak M, Veith C, Wu CY, Wilke T, Kanbagli
ZI, Novoyatleva T, Guenther A, Seeger W, Grimminger F, Sommer N, et
al: Adenylate kinase 4-A Key regulator of proliferation and
metabolic shift in human pulmonary arterial smooth muscle cells via
Akt and HIF-1α signaling pathways. Int J Mol Sci. 22:103712021.
View Article : Google Scholar
|
|
31
|
Xu W and Erzurum SC: Endothelial cell
energy metabolism, proliferation, and apoptosis in pulmonary
hypertension. Compr Physiol. 1:357–372. 2011.PubMed/NCBI
|
|
32
|
Pullamsetti SS, Mamazhakypov A, Weissmann
N, Seeger W and Savai R: Hypoxia-inducible factor signaling in
pulmonary hypertension. J Clin Invest. 130:5638–5651. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Semenza GL: Hypoxia-inducible factors in
physiology and medicine. Cell. 148:399–408. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Paredes F, Williams HC and San Martin A:
Metabolic adaptation in hypoxia and cancer. Cancer Lett.
502:133–142. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Yu B, Wang X, Song Y, Xie G, Jiao S, Shi
L, Cao X, Han X and Qu A: The role of hypoxia-inducible factors in
cardiovascular diseases. Pharmacol Ther. 238:1081862022. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Mobasheri A, Richardson S, Mobasheri R,
Shakibaei M and Hoyland JA: Hypoxia inducible factor-1 and
facilitative glucose transporters GLUT1 and GLUT3: Putative
molecular components of the oxygen and glucose sensing apparatus in
articular chondrocytes. Histol Histopathol. 20:1327–1338.
2005.PubMed/NCBI
|
|
37
|
Mamun AA, Hayashi H, Yamamura A, Nayeem MJ
and Sato M: Hypoxia induces the translocation of glucose
transporter 1 to the plasma membrane in vascular endothelial cells.
J Physiol Sci. 70:442020. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Kim JW, Tchernyshyov I, Semenza GL and
Dang CV: HIF-1-mediated expression of pyruvate dehydrogenase
kinase: A metabolic switch required for cellular adaptation to
hypoxia. Cell Metab. 3:177–185. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Menendez MT, Teygong C, Wade K, Florimond
C and Blader IJ: siRNA screening identifies the host hexokinase 2
(HK2) gene as an important hypoxia-inducible transcription factor 1
(HIF-1) target gene in toxoplasma gondii-infected cells. mBio.
6:e004622015. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Cui XG, Han ZT, He SH, Wu XD, Chen TR,
Shao CH, Chen DL, Su N, Chen YM, Wang T, et al: HIF1/2α mediates
hypoxia-induced LDHA expression in human pancreatic cancer cells.
Oncotarget. 8:24840–24852. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Samec M, Liskova A, Koklesova L, Mersakova
S, Strnadel J, Kajo K, Pec M, Zhai K, Smejkal K, Mirzaei S, et al:
Flavonoids targeting HIF-1: Implications on cancer metabolism.
Cancers (Basel). 13:1302021. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Michelakis ED, Gurtu V, Webster L, Barnes
G, Watson G, Howard L, Cupitt J, Paterson I, Thompson RB, Chow K,
et al: Inhibition of pyruvate dehydrogenase kinase improves
pulmonary arterial hypertension in genetically susceptible
patients. Sci Transl Med. 9:eaao45832017. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Chen J, Zhang M, Liu Y, Zhao S, Wang Y,
Wang M, Niu W, Jin F and Li Z: Histone lactylation driven by
mROS-mediated glycolytic shift promotes hypoxic pulmonary
hypertension. J Mol Cell Biol. 14:mjac0732023. View Article : Google Scholar :
|
|
44
|
Cotroneo E, Ashek A, Wang L, Wharton J,
Dubois O, Bozorgi S, Busbridge M, Alavian KN, Wilkins MR and Zhao
L: Iron homeostasis and pulmonary hypertension: Iron deficiency
leads to pulmonary vascular remodeling in the rat. Circ Res.
116:1680–1690. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Wedgwood S, Lakshminrusimha S, Schumacker
PT and Steinhorn RH: Hypoxia inducible factor signaling and
experimental persistent pulmonary hypertension of the newborn.
Front Pharmacol. 6:472015. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Bonnet S, Michelakis ED, Porter CJ,
Andrade-Navarro MA, Thébaud B, Bonnet S, Haromy A, Harry G, Moudgil
R, McMurtry MS, et al: An abnormal mitochondrial-hypoxia inducible
factor-1alpha-Kv channel pathway disrupts oxygen sensing and
triggers pulmonary arterial hypertension in fawn hooded rats:
Similarities to human pulmonary arterial hypertension. Circulation.
113:2630–2641. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Archer SL, Gomberg-Maitland M, Maitland
ML, Rich S, Garcia JGN and Weir EK: Mitochondrial metabolism, redox
signaling, and fusion: A mitochondria-ROS-HIF-1alpha-Kv1.5
O2-sensing pathway at the intersection of pulmonary hypertension
and cancer. Am J Physiol Heart Circ Physiol. 294:H570–H578. 2008.
View Article : Google Scholar
|
|
48
|
Boucherat O, Vitry G, Trinh I, Paulin R,
Provencher S and Bonnet S: The cancer theory of pulmonary arterial
hypertension. Pulm Circ. 7:285–299. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Rhodes CJ, Howard LS, Busbridge M, Ashby
D, Kondili E, Gibbs JS, Wharton J and Wilkins MR: Iron deficiency
and raised hepcidin in idiopathic pulmonary arterial hypertension:
Clinical prevalence, outcomes, and mechanistic insights. J Am Coll
Cardiol. 58:300–309. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Lanspa SJ, Liu MW and Jenkins HJ Jr: Giant
bulla in pneumatosis cystoides intestinalis. J Clin Gastroenterol.
10:437–440. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Li M, Liu Y, Jin F, Sun X, Li Z, Liu Y,
Fang P, Shi H and Jiang X: Endothelin-1 induces hypoxia inducible
factor 1α expression in pulmonary artery smooth muscle cells. FEBS
Lett. 586:3888–3893. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Alqarni AA, Aldhahir AM, Alghamdi SA,
Alqahtani JS, Siraj RA, Alwafi H, AlGarni AA, Majrshi MS, Alshehri
SM and Pang L: Role of prostanoids, nitric oxide and endothelin
pathways in pulmonary hypertension due to COPD. Front Med
(Lausanne). 10:12756842023. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Jeffrey Man HS, Tsui AKY and Marsden PA:
Nitric oxide and hypoxia signaling. Vitam Horm. 96:161–192. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Dabral S, Muecke C, Valasarajan C,
Schmoranzer M, Wietelmann A, Semenza GL, Meister M, Muley T,
Seeger-Nukpezah T, Samakovlis C, et al: A RASSF1A-HIF1α loop drives
Warburg effect in cancer and pulmonary hypertension. Nat Commun.
10:21302019. View Article : Google Scholar
|
|
55
|
Siebel C and Lendahl U: Notch signaling in
development, tissue homeostasis, and disease. Physiol Rev.
97:1235–1294. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Gozlan O and Sprinzak D: Notch signaling
in development and homeostasis. Development. 150:dev2011382023.
View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Fernández-Chacón M, García-González I,
Mühleder S and Benedito R: Role of Notch in endothelial biology.
Angiogenesis. 24:237–250. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Dabral S, Tian X, Kojonazarov B, Savai R,
Ghofrani HA, Weissmann N, Florio M, Sun J, Jonigk D, Maegel L, et
al: Notch1 signalling regulates endothelial proliferation and
apoptosis in pulmonary arterial hypertension. Eur Respir J.
48:1137–1149. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Wang Y, Dai S, Cheng X, Prado E, Yan L, Hu
J, He Q, Lv Y, Lv Y and Du L: Notch3 signaling activation in smooth
muscle cells promotes extrauterine growth restriction-induced
pulmonary hypertension. Nutr Metab Cardiovasc Dis. 29:639–651.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Tien PC, Chen X, Elzey BD, Pollock RE and
Kuang S: Notch signaling regulates a metabolic switch through
inhibiting PGC-1α and mitochondrial biogenesis in dedifferentiated
liposarcoma. Oncogene. 42:2521–2535. 2023. View Article : Google Scholar :
|
|
61
|
Landor SKJ, Mutvei AP, Mamaeva V, Jin S,
Busk M, Borra R, Grönroos TJ, Kronqvist P, Lendahl U and Sahlgren
CM: Hypoand hyperactivated Notch signaling induce a glycolytic
switch through distinct mechanisms. Proc Natl Acad Sci USA.
108:18814–18819. 2011. View Article : Google Scholar
|
|
62
|
Sellers K, Allen TD, Bousamra M II, Tan J,
Méndez-Lucas A, Lin W, Bah N, Chernyavskaya Y, MacRae JI, Higashi
RM, et al: Metabolic reprogramming and Notch activity distinguish
between non-small cell lung cancer subtypes. Br J Cancer.
121:51–64. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Miyagawa K, Shi M, Chen PI, Hennigs JK,
Zhao Z, Wang M, Li CG, Saito T, Taylor S, Sa S, et al: Smooth
muscle contact drives endothelial regeneration by
BMPR2-Notch1-mediated metabolic and epigenetic changes. Circ Res.
124:211–224. 2019. View Article : Google Scholar :
|
|
64
|
Moriyama H, Moriyama M, Isshi H, Ishihara
S, Okura H, Ichinose A, Ozawa T, Matsuyama A and Hayakawa T: Role
of notch signaling in the maintenance of human mesenchymal stem
cells under hypoxic conditions. Stem Cells Dev. 23:2211–2224. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Moriyama H, Moriyama M, Ozawa T, Tsuruta
D, Iguchi T, Tamada S, Nakatani T, Nakagawa K and Hayakawa T: Notch
signaling enhances stemness by regulating metabolic pathways
through modifying p53, NF-κB, and HIF-1α. Stem Cells Dev.
27:935–947. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Liu GY and Sabatini DM: mTOR at the nexus
of nutrition, growth, ageing and disease. Nat Rev Mol Cell Biol.
21:183–203. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Saxton RA and Sabatini DM: mTOR signaling
in growth, metabolism, and disease. Cell. 169:361–371. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Sangüesa G, Roglans N, Baena M, Velázquez
AM, Laguna JC and Alegret M: mTOR is a key protein involved in the
metabolic effects of simple sugars. Int J Mol Sci. 20:11172019.
View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Wang AP, Li XH, Yang YM, Li WQ, Zhang W,
Hu CP, Zhang Z and Li YJ: A critical role of the mTOR/eIF2α pathway
in hypoxia-induced pulmonary hypertension. PLoS One.
10:e01308062015. View Article : Google Scholar
|
|
70
|
Krymskaya VP, Snow J, Cesarone G, Khavin
I, Goncharov DA, Lim PN, Veasey SC, Ihida-Stansbury K, Jones PL and
Goncharova EA: mTOR is required for pulmonary arterial vascular
smooth muscle cell proliferation under chronic hypoxia. FASEB J.
25:1922–1933. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Szwed A, Kim E and Jacinto E: Regulation
and metabolic functions of mTORC1 and mTORC2. Physiol Rev.
101:1371–1426. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Zhu Y, Shu D, Gong X, Lu M, Feng Q, Zeng
XB, Zhang H, Gao J, Guo YW, Liu L, et al: Platelet-derived TGF
(transforming growth factor)-β1 enhances the aerobic glycolysis of
pulmonary arterial smooth muscle cells by PKM2 (pyruvate kinase
muscle isoform 2) upregulation. Hypertension. 79:932–945. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Hudson CC, Liu M, Chiang GG, Otterness DM,
Loomis DC, Kaper F, Giaccia AJ and Abraham RT: Regulation of
hypoxia-inducible factor 1alpha expression and function by the
mammalian target of rapamycin. Mol Cell Biol. 22:7004–7014. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Lu H, Forbes RA and Verma A:
Hypoxia-inducible factor 1 activation by aerobic glycolysis
implicates the Warburg effect in carcinogenesis. J Biol Chem.
277:23111–23115. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Feng Y and Wu L: mTOR up-regulation of
PFKFB3 is essential for acute myeloid leukemia cell survival.
Biochem Biophys Res Commun. 483:897–903. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Wang C, Jiang J, Ji J, Cai Q, Chen X, Yu
Y, Zhu Z and Zhang J: PKM2 promotes cell migration and inhibits
autophagy by mediating PI3K/AKT activation and contributes to the
malignant development of gastric cancer. Sci Rep. 7:28862017.
View Article : Google Scholar : PubMed/NCBI
|
|
77
|
He L, Gomes AP, Wang X, Yoon SO, Lee G,
Nagiec MJ, Cho S, Chavez A, Islam T, Yu Y, et al: mTORC1 promotes
metabolic reprogramming by the suppression of GSK3-dependent Foxk1
phosphorylation. Mol Cell. 70:949–960.e4. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Dodd KM, Yang J, Shen MH, Sampson JR and
Tee AR: mTORC1 drives HIF-1α and VEGF-A signalling via multiple
mechanisms involving 4E-BP1, S6K1 and STAT3. Oncogene.
34:2239–2250. 2015. View Article : Google Scholar
|
|
79
|
Chi H: Sin1-mTORC2 signaling drives
glycolysis of developing thymocytes. J Mol Cell Biol. 11:91–92.
2019. View Article : Google Scholar
|
|
80
|
Lan N, Lu Y, Zhang Y, Pu S, Xi H, Nie X,
Liu J and Yuan W: FTO-a common genetic basis for obesity and
cancer. Front Genet. 11:5591382020. View Article : Google Scholar
|
|
81
|
Frayling TM, Timpson NJ, Weedon MN,
Zeggini E, Freathy RM, Lindgren CM, Perry JRB, Elliott KS, Lango H,
Rayner NW, et al: A common variant in the FTO gene is associated
with body mass index and predisposes to childhood and adult
obesity. Science. 316:889–894. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Jia G, Yang CG, Yang S, Jian X, Yi C, Zhou
Z and He C: Oxidative demethylation of 3-methylthymine and
3-methyluracil in single-stranded DNA and RNA by mouse and human
FTO. FEBS Lett. 582:3313–3319. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Jia G, Fu Y, Zhao X, Dai Q, Zheng G, Yang
Y, Yi C, Lindahl T, Pan T, Yang YG and He C: N6-methyladenosine in
nuclear RNA is a major substrate of the obesity-associated FTO. Nat
Chem Biol. 7:885–887. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Azzam SK, Alsafar H and Sajini AA: FTO m6A
demethylase in obesity and cancer: Implications and underlying
molecular mechanisms. Int J Mol Sci. 23:38002022. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Li W, Xing C, Bao L, Han S, Luo T, Wang Z
and Fan H: Comprehensive analysis of RNA m6A methylation in
pressure overload-induced cardiac hypertrophy. BMC Genomics.
23:5762022. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Zhang B, Jiang H, Wu J, Cai Y, Dong Z,
Zhao Y, Hu Q, Hu K, Sun A and Ge J: m6A demethylase FTO attenuates
cardiac dysfunction by regulating glucose uptake and glycolysis in
mice with pressure overload-induced heart failure. Signal Transduct
Target Ther. 6:3772021. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Hu L, Wang J, Huang H, Yu Y, Ding J, Yu Y,
Li K, Wei D, Ye Q, Wang F, et al: YTHDF1 regulates pulmonary
hypertension through translational control of MAGED1. Am J Respir
Crit Care Med. 203:1158–1172. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Zeng Y, Huang T, Zuo W, Wang D, Xie Y,
Wang X, Xiao Z, Chen Z, Liu Q, Liu N and Xiao Y: Integrated
analysis of m6A mRNA methylation in rats with
monocrotaline-induced pulmonary arterial hypertension. Aging
(Albany NY). 13:18238–18256. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Liu Y, Wang R, Zhang L, Li J, Lou K and
Shi B: The lipid metabolism gene FTO influences breast cancer cell
energy metabolism via the PI3K/AKT signaling pathway. Oncol Lett.
13:4685–4690. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Liu Y, Liang G, Xu H, Dong W, Dong Z, Qiu
Z, Zhang Z, Li F, Huang Y, Li Y, et al: Tumors exploit FTO-mediated
regulation of glycolytic metabolism to evade immune surveillance.
Cell Metab. 33:1221–1233.e11. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Fang YH, Piao L, Hong Z, Toth PT, Marsboom
G, Bache-Wiig P, Rehman J and Archer SL: Therapeutic inhibition of
fatty acid oxidation in right ventricular hypertrophy: Exploiting
Randle's cycle. J Mol Med (Berl). 90:31–43. 2012. View Article : Google Scholar
|
|
92
|
Randle PJ, Priestman DA, Mistry SC and
Halsall A: Glucose fatty acid interactions and the regulation of
glucose disposal. J Cell Biochem. 55(Suppl 1): S1–S11. 1994.
View Article : Google Scholar
|
|
93
|
Archer SL, Fang YH, Ryan JJ and Piao L:
Metabolism and bioenergetics in the right ventricle and pulmonary
vasculature in pulmonary hypertension. Pulm Circ. 3:144–152. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Lee MH, Sanders L, Kumar R,
Hernandez-Saavedra D, Yun X, Ford JA, Perez MJ, Mickael C, Gandjeva
A, Koyanagi DE, et al: Contribution of fatty acid oxidation to the
pathogenesis of pulmonary hypertension. Am J Physiol Lung Cell Mol
Physiol. 323:L355–L371. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Mey JT, Hari A, Axelrod CL, Fealy CE,
Erickson ML, Kirwan JP, Dweik RA and Heresi GA: Lipids and ketones
dominate metabolism at the expense of glucose control in pulmonary
arterial hypertension: A hyperglycaemic clamp and metabolomics
study. Eur Respir J. 55:19017002020. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Sutendra G, Bonnet S, Rochefort G, Haromy
A, Folmes KD, Lopaschuk GD, Dyck JRB and Michelakis ED: Fatty acid
oxidation and malonyl-CoA decarboxylase in the vascular remodeling
of pulmonary hypertension. Sci Transl Med. 2:44ra582010. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Greenberger LM, Horak ID, Filpula D, Sapra
P, Westergaard M, Frydenlund HF, Albaek C, Schrøder H and Ørum H: A
RNA antagonist of hypoxia-inducible factor-1alpha, EZN-2968,
inhibits tumor cell growth. Mol Cancer Ther. 7:3598–3608. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Zhao H, Jiang H, Li Z, Zhuang Y, Liu Y,
Zhou S, Xiao Y, Xie C, Zhou F and Zhou Y: 2-Methoxyestradiol
enhances radiosensitivity in radioresistant melanoma MDA-MB-435R
cells by regulating glycolysis via HIF-1α/PDK1 axis. Int J Oncol.
50:1531–1540. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Fallah J and Rini BI: HIF inhibitors:
Status of current clinical development. Curr Oncol Rep. 21:62019.
View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Bruce JY, Eickhoff J, Pili R, Logan T,
Carducci M, Arnott J, Treston A, Wilding G and Liu G: A phase II
study of 2-methoxyestradiol nanocrystal colloidal dispersion alone
and in combination with sunitinib malate in patients with
metastatic renal cell carcinoma progressing on sunitinib malate.
Invest New Drugs. 30:794–802. 2012. View Article : Google Scholar
|
|
101
|
Jabs M, Rose AJ, Lehmann LH, Taylor J,
Moll I, Sijmonsma TP, Herberich SE, Sauer SW, Poschet G, Federico
G, et al: Inhibition of endothelial notch signaling impairs fatty
acid transport and leads to metabolic and vascular remodeling of
the adult heart. Circulation. 137:2592–2608. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Chen T, Zhou Q, Tang H, Bozkanat M, Yuan
JXJ, Raj JU and Zhou G: miR-17/20 controls prolyl hydroxylase 2
(PHD2)/hypoxia-inducible factor 1 (HIF1) to regulate pulmonary
artery smooth muscle cell proliferation. J Am Heart Assoc.
5:e0045102016. View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Wang S, Zeng H, Xie XJ, Tao YK, He X,
Roman RJ, Aschner JL and Chen JX: Loss of prolyl hydroxylase domain
protein 2 in vascular endothelium increases pericyte coverage and
promotes pulmonary arterial remodeling. Oncotarget. 7:58848–58861.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Han XJ, Zhang WF, Wang Q, Li M, Zhang CB,
Yang ZJ, Tan RJ, Gan LJ, Zhang LL, Lan XM, et al: HIF-1α promotes
the proliferation and migration of pulmonary arterial smooth muscle
cells via activation of Cx43. J Cell Mol Med. 25:10663–10673. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Dessouroux A, Akwa Y and Baulieu EE: DHEA
decreases HIF-1alpha accumulation under hypoxia in human pulmonary
artery cells: Potential role in the treatment of pulmonary arterial
hypertension. J Steroid Biochem Mol Biol. 109:81–89. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Ball MK, Waypa GB, Mungai PT, Nielsen JM,
Czech L, Dudley VJ, Beussink L, Dettman RW, Berkelhamer SK,
Steinhorn RH, et al: Regulation of hypoxia-induced pulmonary
hypertension by vascular smooth muscle hypoxia-inducible factor-1α.
Am J Respir Crit Care Med. 189:314–324. 2014. View Article : Google Scholar :
|
|
107
|
Docherty CK, Nilsen M and MacLean MR:
Influence of 2-methoxyestradiol and sex on hypoxia-induced
pulmonary hypertension and hypoxia-inducible factor-1-α. J Am Heart
Assoc. 8:e0116282019. View Article : Google Scholar
|
|
108
|
He Y, Fang X, Shi J, Li X, Xie M and Liu
X: Apigenin attenuates pulmonary hypertension by inducing
mitochondria-dependent apoptosis of PASMCs via inhibiting the
hypoxia inducible factor 1α-KV1.5 channel pathway. Chem Biol
Interact. 317:1089422020. View Article : Google Scholar
|
|
109
|
Jiang Y, Zhou Y, Peng G, Liu N, Tian H,
Pan D, Liu L, Yang X, Li C, Li W, et al: Topotecan prevents
hypoxia-induced pulmonary arterial hypertension and inhibits
hypoxia-inducible factor-1α and TRPC channels. Int J Biochem Cell
Biol. 104:161–170. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Koulmann N, Novel-Chaté V, Peinnequin A,
Chapot R, Serrurier B, Simler N, Richard H, Ventura-Clapier R and
Bigard X: Cyclosporin A inhibits hypoxia-induced pulmonary
hypertension and right ventricle hypertrophy. Am J Respir Crit Care
Med. 174:699–705. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Kurosawa R, Satoh K, Kikuchi N, Kikuchi H,
Saigusa D, Al-Mamun ME, Siddique MAH, Omura J, Satoh T, Sunamura S,
et al: Identification of celastramycin as a novel therapeutic agent
for pulmonary arterial hypertension. Circ Res. 125:309–327. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
112
|
Abud EM, Maylor J, Undem C, Punjabi A,
Zaiman AL, Myers AC, Sylvester JT, Semenza GL and Shimoda LA:
Digoxin inhibits development of hypoxic pulmonary hypertension in
mice. Proc Natl Acad Sci USA. 109:1239–1244. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
113
|
Kovacs L, Cao Y, Han W, Meadows L,
Kovacs-Kasa A, Kondrikov D, Verin AD, Barman SA, Dong Z, Huo Y and
Su Y: PFKFB3 in smooth muscle promotes vascular remodeling in
pulmonary arterial hypertension. Am J Respir Crit Care Med.
200:617–627. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
114
|
Jiang L, Goncharov DA, Shen Y, Lin D,
Chang B, Pena A, DeLisser H, Goncharova EA and Kudryashova TV:
Akt-dependent glycolysis-driven lipogenesis supports proliferation
and survival of human pulmonary arterial smooth muscle cells in
pulmonary hypertension. Front Med (Lausanne). 9:8868682022.
View Article : Google Scholar : PubMed/NCBI
|
|
115
|
Yan N: A glimpse of membrane transport
through structures-advances in the structural biology of the GLUT
glucose transporters. J Mol Biol. 429:2710–2725. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
116
|
Thorens B and Mueckler M: Glucose
transporters in the 21st century. Am J Physiol Endocrinol Metab.
298:E141–E145. 2010. View Article : Google Scholar :
|
|
117
|
Ismail A and Tanasova M: Importance of
GLUT transporters in disease diagnosis and treatment. Int J Mol
Sci. 23:86982022. View Article : Google Scholar : PubMed/NCBI
|
|
118
|
Broderick TL and King TM: Upregulation of
GLUT-4 in right ventricle of rats with monocrotaline-induced
pulmonary hypertension. Med Sci Monit. 14:BR261–BR264.
2008.PubMed/NCBI
|
|
119
|
Li W, Chen W, Peng H, Xiao Z, Liu J, Zeng
Y, Huang T, Song Q, Wang X and Xiao Y: Shikonin improves pulmonary
vascular remodeling in monocrotaline-induced pulmonary arterial
hypertension via regulation of PKM2. Mol Med Rep. 27:602023.
View Article : Google Scholar
|
|
120
|
Liu A, Li B, Yang M, Shi Y and Su J:
Targeted treprostinil delivery inhibits pulmonary arterial
remodeling. Eur J Pharmacol. 923:1747002022. View Article : Google Scholar : PubMed/NCBI
|
|
121
|
Chowdhury B, Luu AZ, Luu VZ, Kabir MG, Pan
Y, Teoh H, Quan A, Sabongui S, Al-Omran M, Bhatt DL, et al: The
SGLT2 inhibitor empagliflozin reduces mortality and prevents
progression in experimental pulmonary hypertension. Biochem Biophys
Res Commun. 524:50–56. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
122
|
Tang Y, Tan S, Li M, Tang Y, Xu X, Zhang
Q, Fu Q, Tang M, He J, Zhang Y, et al: Dapagliflozin, sildenafil
and their combination in monocrotaline-induced pulmonary arterial
hypertension. BMC Pulm Med. 22:1422022. View Article : Google Scholar : PubMed/NCBI
|
|
123
|
Kayano H, Koba S, Hirano T, Matsui T,
Fukuoka H, Tsuijita H, Tsukamoto S, Hayashi T, Toshida T, Watanabe
N, et al: Dapagliflozin influences ventricular hemodynamics and
exercise-induced pulmonary hypertension in type 2 diabetes
patients-a randomized controlled trial. Circ J. 84:1807–1817. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
124
|
Zapater JL, Lednovich KR, Khan MW, Pusec
CM and Layden BT: Hexokinase domain-containing protein-1 in
metabolic diseases and beyond. Trends Endocrinol Metab. 33:72–84.
2022. View Article : Google Scholar
|
|
125
|
Wilson JE: Isozymes of mammalian
hexokinase: Structure, subcellular localization and metabolic
function. J Exp Biol. 206:2049–2057. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
126
|
Chen F, Wang H, Lai J, Cai S and Yuan L:
3-Bromopyruvate reverses hypoxia-induced pulmonary arterial
hypertension through inhibiting glycolysis: In vitro and in vivo
studies. Int J Cardiol. 266:236–241. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
127
|
Zhang YL, Zhang R, Shen YF, Huang KY, He
YY, Zhao JH and Jing ZC: 3-Bromopyruvate attenuates experimental
pulmonary hypertension via inhibition of glycolysis. Am J
Hypertens. 32:426–432. 2019. View Article : Google Scholar
|
|
128
|
Liu J, Wang W, Wang L, Qi XM, Sha YH and
Yang T: 3-Bromopyruvate alleviates the development of
monocrotaline-induced rat pulmonary arterial hypertension by
decreasing aerobic glycolysis, inducing apoptosis, and suppressing
inflammation. Chin Med J (Engl). 133:49–60. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
129
|
Gao S, Chen X, Jin H, Ren S, Liu Z, Fang X
and Zhang G: Overexpression of ErbB2 renders breast cancer cells
susceptible to 3-BrPA through the increased dissociation of
hexokinase II from mitochondrial outer membrane. Oncol Lett.
11:1567–1573. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
130
|
Mathupala SP, Ko YH and Pedersen PL:
Hexokinase II: Cancer's double-edged sword acting as both
facilitator and gatekeeper of malignancy when bound to
mitochondria. Oncogene. 25:4777–4786. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
131
|
Pajak B, Siwiak E, Sołtyka M, Priebe A,
Zieliński R, Fokt I, Ziemniak M, Jaśkiewicz A, Borowski R,
Domoradzki T and Priebe W: 2-Deoxy-d-glucose and its analogs: From
diagnostic to therapeutic agents. Int J Mol Sci. 21:2342019.
View Article : Google Scholar
|
|
132
|
Laussel C and Léon S: Cellular toxicity of
the metabolic inhibitor 2-deoxyglucose and associated resistance
mechanisms. Biochem Pharmacol. 182:1142132020. View Article : Google Scholar : PubMed/NCBI
|
|
133
|
Lu Y, Chen R, Ma JY, Wang LP, Qiu LL, Wang
CP, Yan JC and Liu PJ: Platelet derived growth factor-BB regulates
phenotype transformation of pulmonary artery smooth muscle cells
via SIRT3 affecting glycolytic pathway. Zhonghua Xin Xue Guan Bing
Za Zhi. 47:993–999. 2019.In Chinese. PubMed/NCBI
|
|
134
|
Maier A, Liao SL, Lescure T, Robson PM,
Hirata N, Sartori S, Narula N, Vergani V, Soultanidis G, Morgenthau
A, et al: Pulmonary artery 18F-fluorodeoxyglucose uptake
by PET/CMR as a marker of pulmonary hypertension in sarcoidosis.
JACC Cardiovasc Imaging. 15:108–120. 2022. View Article : Google Scholar
|
|
135
|
Frille A, Steinhoff KG, Hesse S, Grachtrup
S, Wald A, Wirtz H, Sabri O and Seyfarth HJ: Thoracic
[18F]fluorodeoxyglucose uptake measured by positron emission
tomography/computed tomography in pulmonary hypertension. Medicine
(Baltimore). 95:e39762016. View Article : Google Scholar : PubMed/NCBI
|
|
136
|
Van Schaftingen E, Jett MF, Hue L and Hers
HG: Control of liver 6-phosphofructokinase by fructose
2,6-bisphosphate and other effectors. Proc Natl Acad Sci USA.
78:3483–3486. 1981. View Article : Google Scholar : PubMed/NCBI
|
|
137
|
Rider MH, Bertrand L, Vertommen D, Michels
PA, Rousseau GG and Hue L:
6-Phosphofructo-2-kinase/fructose-2,6-bisphosphatase: Head-to-head
with a bifunctional enzyme that controls glycolysis. Biochem J.
381:561–579. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
138
|
Wang L, Zhang X, Cao Y, Ma Q, Mao X, Xu J,
Yang Q, Zhou Y, Lucas R, Fulton DJ, et al: Mice with a specific
deficiency of Pfkfb3 in myeloid cells are protected from
hypoxia-induced pulmonary hypertension. Br J Pharmacol.
178:1055–1072. 2021. View Article : Google Scholar
|
|
139
|
Zahra K, Dey T, Ashish, Mishra SP and
Pandey U: Pyruvate kinase M2 and cancer: The role of PKM2 in
promoting tumorigenesis. Front Oncol. 10:1592020. View Article : Google Scholar : PubMed/NCBI
|
|
140
|
Dasgupta A, Wu D, Tian L, Xiong PY,
Dunham-Snary KJ, Chen KH, Alizadeh E, Motamed M, Potus F, Hindmarch
CCT and Archer SL: Mitochondria in the pulmonary vasculature in
health and disease: Oxygen-sensing, metabolism, and dynamics. Compr
Physiol. 10:713–765. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
141
|
Shimauchi T, Boucherat O, Yokokawa T,
Grobs Y, Wu W, Orcholski M, Martineau S, Omura J, Tremblay E,
Shimauchi K, et al: PARP1-PKM2 axis mediates right ventricular
failure associated with pulmonary arterial hypertension. JACC Basic
Transl Sci. 7:384–403. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
142
|
Arora S, Joshi G, Chaturvedi A, Heuser M,
Patil S and Kumar R: A perspective on medicinal chemistry
approaches for targeting pyruvate kinase M2. J Med Chem.
65:1171–1205. 2022. View Article : Google Scholar
|
|
143
|
Chhipa AS and Patel S: Targeting pyruvate
kinase muscle isoform 2 (PKM2) in cancer: What do we know so far?
Life Sci. 280:1196942021. View Article : Google Scholar : PubMed/NCBI
|
|
144
|
Hua Q, Mi B, Xu F, Wen J, Zhao L, Liu J
and Huang G: Hypoxia-induced lncRNA-AC020978 promotes proliferation
and glycolytic metabolism of non-small cell lung cancer by
regulating PKM2/HIF-1α axis. Theranostics. 10:4762–4778. 2020.
View Article : Google Scholar :
|
|
145
|
Chen D, Wei L, Liu ZR, Yang JJ, Gu X, Wei
ZZ, Liu LP and Yu SP: Pyruvate kinase M2 increases angiogenesis,
neurogenesis, and functional recovery mediated by upregulation of
STAT3 and focal adhesion kinase activities after ischemic stroke in
adult mice. Neurotherapeutics. 15:770–784. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
146
|
Pei L, Le Y, Chen H, Feng J, Liu Z, Zhu J,
Wang C, Chen L, Dou X and Lu D: Cynaroside prevents macrophage
polarization into pro-inflammatory phenotype and alleviates cecal
ligation and puncture-induced liver injury by targeting PKM2/HIF-1α
axis. Fitoterapia. 152:1049222021. View Article : Google Scholar
|
|
147
|
Xiong PY, Motamed M, Chen KH, Dasgupta A,
Potus F, Tian L, Martin A, Mewburn J, Jones O, Thébaud A and Archer
SL: Inhibiting pyruvate kinase muscle isoform 2 regresses group 2
pulmonary hypertension induced by supra-coronary aortic banding.
Acta Physiol (Oxf). 234:e137642022. View Article : Google Scholar : PubMed/NCBI
|
|
148
|
Piao L, Sidhu VK, Fang YH, Ryan JJ, Parikh
KS, Hong Z, Toth PT, Morrow E, Kutty S, Lopaschuk GD and Archer SL:
FOXO1-mediated upregulation of pyruvate dehydrogenase kinase-4
(PDK4) decreases glucose oxidation and impairs right ventricular
function in pulmonary hypertension: Therapeutic benefits of
dichloroacetate. J Mol Med (Berl). 91:333–346. 2013. View Article : Google Scholar
|
|
149
|
Michelakis ED, McMurtry MS, Wu XC, Dyck
JRB, Moudgil R, Hopkins TA, Lopaschuk GD, Puttagunta L, Waite R and
Archer SL: Dichloroacetate, a metabolic modulator, prevents and
reverses chronic hypoxic pulmonary hypertension in rats: Role of
increased expression and activity of voltage-gated potassium
channels. Circulation. 105:244–250. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
150
|
McMurtry MS, Bonnet S, Wu X, Dyck JRB,
Haromy A, Hashimoto K and Michelakis ED: Dichloroacetate prevents
and reverses pulmonary hypertension by inducing pulmonary artery
smooth muscle cell apoptosis. Circ Res. 95:830–840. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
151
|
Guignabert C, Tu L, Izikki M, Dewachter L,
Zadigue P, Humbert M, Adnot S, Fadel E and Eddahibi S:
Dichloroacetate treatment partially regresses established pulmonary
hypertension in mice with SM22alpha-targeted overexpression of the
serotonin transporter. FASEB J. 23:4135–4147. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
152
|
Ciapponi A, Pizarro R and Harrison J:
WITHDRAWN: Trimetazidine for stable angina. Cochrane Database Syst
Rev. 3:CD0036142017.PubMed/NCBI
|
|
153
|
Wu Z, Yu L and Li X and Li X: Protective
mechanism of trimetazidine in myocardial cells in myocardial
infarction rats through ERK signaling pathway. Biomed Res Int.
2021:99245492021. View Article : Google Scholar : PubMed/NCBI
|
|
154
|
Ferrari R, Ford I, Fox K, Challeton JP,
Correges A, Tendera M, Widimský P and Danchin N; ATPCI
investigators: Efficacy and safety of trimetazidine after
percutaneous coronary intervention (ATPCI): A randomised,
double-blind, placebo-controlled trial. Lancet. 396:830–838. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
155
|
Shu H, Peng Y, Hang W, Zhou N and Wang DW:
Trimetazidine in heart failure. Front Pharmacol. 11:5691322021.
View Article : Google Scholar : PubMed/NCBI
|
|
156
|
Parra V, Bravo-Sagua R, Norambuena-Soto I,
Hernández-Fuentes CP, Gómez-Contreras AG, Verdejo HE, Mellado R,
Chiong M, Lavandero S and Castro PF: Inhibition of mitochondrial
fission prevents hypoxia-induced metabolic shift and cellular
proliferation of pulmonary arterial smooth muscle cells. Biochim
Biophys Acta Mol Basis Dis. 1863:2891–2903. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
157
|
Dobbins RL, Szczepaniak LS, Bentley B,
Esser V, Myhill J and McGarry JD: Prolonged inhibition of muscle
carnitine palmitoyltransferase-1 promotes intramyocellular lipid
accumulation and insulin resistance in rats. Diabetes. 50:123–130.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
158
|
Leamy AK, Egnatchik RA and Young JD:
Molecular mechanisms and the role of saturated fatty acids in the
progression of non-alcoholic fatty liver disease. Prog Lipid Res.
52:165–174. 2013. View Article : Google Scholar
|
|
159
|
Ma Y, Temkin SM, Hawkridge AM, Guo C, Wang
W, Wang XY and Fang X: Fatty acid oxidation: An emerging facet of
metabolic transformation in cancer. Cancer Lett. 435:92–100. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
160
|
Verdejo HE, Rojas A, López-Crisosto C,
Baraona F, Gabrielli L, Maracaja-Coutinho V, Chiong M, Lavandero S
and Castro PF: Effects of trimetazidine on right ventricular
function and ventricular remodeling in patients with pulmonary
artery hypertension: A randomised controlled trial. J Clin Med.
12:15712023. View Article : Google Scholar : PubMed/NCBI
|
|
161
|
Cavallino C, Facchini M, Veia A, Bacchni
S, Rosso R, Rognoni A, Rametta F, Lupi A and Bongo AS: New
anti-anginal drugs: Ranolazine. Cardiovasc Hematol Agents Med Chem.
13:14–20. 2015. View Article : Google Scholar
|
|
162
|
McKelvey KJ, Wilson EB, Short S, Melcher
AA, Biggs M, Diakos CI and Howell VM: Glycolysis and fatty acid
oxidation inhibition improves survival in glioblastoma. Front
Oncol. 11:6332102021. View Article : Google Scholar : PubMed/NCBI
|
|
163
|
McCormack JG, Barr RL, Wolff AA and
Lopaschuk GD: Ranolazine stimulates glucose oxidation in normoxic,
ischemic, and reperfused ischemic rat hearts. Circulation.
93:135–142. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
164
|
Clarke B, Wyatt KM and McCormack JG:
Ranolazine increases active pyruvate dehydrogenase in perfused
normoxic rat hearts: Evidence for an indirect mechanism. J Mol Cell
Cardiol. 28:341–350. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
165
|
Han QJ, Forfia P, Vaidya A, Ramani G,
deKemp RA, Mach RH, Mankoff DA, Bravo PE, DiCarli M, Chan SY, et
al: Effects of ranolazine on right ventricular function, fluid
dynamics, and metabolism in patients with precapillary pulmonary
hypertension: Insights from a longitudinal, randomized,
double-blinded, placebo controlled, multicenter study. Front
Cardiovasc Med. 10:11187962023. View Article : Google Scholar : PubMed/NCBI
|
|
166
|
Wu J, Liu T, Shi S, Fan Z, Hiram R, Xiong
F, Cui B, Su X, Chang R, Zhang W, et al: Dapagliflozin reduces the
vulnerability of rats with pulmonary arterial hypertension-induced
right heart failure to ventricular arrhythmia by restoring calcium
handling. Cardiovasc Diabetol. 21:1972022. View Article : Google Scholar : PubMed/NCBI
|
|
167
|
Luo L, Xiao L, Lian G, Wang H and Xie L:
miR-125a-5p inhibits glycolysis by targeting hexokinase-II to
improve pulmonary arterial hypertension. Aging (Albany NY).
12:9014–9030. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
168
|
Kassa B, Kumar R, Mickael C, Sanders L,
Vohwinkel C, Lee MH, Gu S, Poth JM, Stenmark KR, Zhao YY, et al:
Endothelial cell PHD2-HIF1α-PFKFB3 contributes to right ventricle
vascular adaptation in pulmonary hypertension. Am J Physiol Lung
Cell Mol Physiol. 321:L675–L685. 2021. View Article : Google Scholar
|
|
169
|
Qi L, Lv T, Cheng Y, Yu M, Han H, Kong H,
Xie W, Wang H, Zhang Y and Huang Z: Fasudil dichloroacetate (FDCA),
an orally available agent with potent therapeutic efficiency on
monocrotaline-induced pulmonary arterial hypertension rats. Bioorg
Med Chem Lett. 29:1812–1818. 2019. View Article : Google Scholar : PubMed/NCBI
|