Introduction
Long interspersed nuclear element-1 (L1) is a
transposable element in vertebrate genomes, including zebrafish,
frogs and humans (1-4). Human and zebrafish L1 exhibit 70%
homology and both human and zebrafish L1 contain two open reading
frames (ORFs), ORF1 and ORF2 (3,5).
ORF2-encoded protein is evolutionarily conserved between zebrafish
and humans, containing both endonuclease and reverse transcriptase,
whereas no conserved domains are found in ORF1-encoded protein
(6-8). L1 is silenced in differentiated
cells to prevent uncontrolled mutagenesis (9). L1 is highly expressed in early
embryos of humans, rodents and fish (9-12). L1 elements exhibit active
expression prior to the 8-cell stage, which is subsequently
decreased in blastocysts during human embryo development. The
expression of zebrafish L1 is increased during the stage of 50%
epiboly [5.25 h post-fertilization (hpf)], although the expression
was reduced from the 4-somite (11 hpf) stage onwards (10,11). L1 serves a crucial role in early
embryonic development; inhibition of L1 transcription has been
suggested to impede embryo development in mice (1). Although numerous studies have
reported the processes associated with L1 activation during early
embryonic development, the underlying molecular mechanisms that
regulate L1 transcription have yet to be fully elucidated (10,11).
Lipovitellin (LV), constituting the majority of
vitellogenin, is an energy 'reservoir' that is gradually used
during embryonic development (13,14). Additionally, LV has antioxidant
activity and contributes to immune defense functions (15). However, whether LV serves a key
role in the high expression of L1 in early zebrafish embryos (EZEs)
has been unclear.
The present study aimed to elucidate the mechanisms
by which LV increasing the expression of EGFP gene induced by
L1-ORF2 (ORF2) and EZE expression genes, which is related with the
ability of LV binding with ORF2 DNA and increasing chromatin
accessibility.
Materials and methods
Plasmid construction
The expression vectors C1-ORF2, C1-Alux14 and
C1-LacZ were constructed as previously described (16). C1-delPolyA vector was obtained by
deleting the poly(A) DNA sequence (240 bp) from the C1-ORF2 vector
using the MluⅠ and ApaⅠ restriction enzymes.
ZE and plasmid microinjection
TU strain zebrafish were purchased from Nanjing
Yaoshunyu Biotechnology Co., Ltd. and maintained under a 14/10-h
light/dark cycle at 28.0±0.5°C, as previously described (17,18). Experimental procedures were
approved by the Institutional Animal Care and Use Committee of
Hebei Medical University (approval no. IACUC-Hebmu-2021009). Adult
fish were fed twice a day. A total of 10 pairs of adult male and
female (>16 weeks) zebrafish (weight, 0.36-0.44 g) were
maintained at 28°C and the embryos were obtained from breeding
pairs at 8-9 a.m. Fertilized embryos collected within 45 min
(1-cell stage embryos) were considered 0 hpf zebrafish embryos
(ZEs). Spawns for which the survival rate of the normal embryos up
to 24 h was >90% were considered good-quality embryos, whereas
survival rate of the normal embryos up to 24 h was <30% was
considered to contain poor-quality embryos.
The plasmids were diluted into a buffer of 0.1 mol/l
NaCl and 10 mmol/l Tris-HCl (pH 7.2); final concentration of
plasmids was 0.1 μg/μl. A total of 1.7 nl plasmid
solution was microinjected into ZE animal pole or yolk sac and the
embryos were then cultured in a Petri dish to 6, 12, 24, 48 or 72 h
at 28°C.
The expression of the EGFP reporter protein in ZEs
was observed under a fluorescence microscope (Leica Microsystems
GmbH) in 40X at an excitation wavelength of 490 nm. ImageJ software
1.53 (National Institutes of Health) was used to analyze the
fluorescence intensity.
Preparation of ZE lysate (ZEL) and LV
protein purification
The 0 hpf ZEs were collected and 10X embryo buffer
was added to the 0 hpf ZEs at a concentration of 1X (0.1 mol/l
NaCl, 0.5 mmol/l PMSF, 2 mmol/l dithiothreitol, 0.5 mmol/l EDTA and
10 mmol/l Tris-HCl, pH 7.4). The embryos were crushed using an
ultrasonic cell processor (VIBRA-Cell™ Sonics VCX105; Sonics &
Materials, Inc.). The homogenized embryos were centrifuged at
13,400 g for 5 min at 4°C and supernatant (ZEL) was collected. The
optimal density (OD) was measured by ultraviolet spectrophotometer
(UV-2800A; UNICO Instrument Co., Ltd. Shanghai) at 280 nm and the
ZEL was subsequently diluted using 1X embryo buffer (0.475, 0.95,
1.90, 3.80 and 7.60 μg/μl).
LV was purified from ZEL as previously described
(19). Purified LV was analyzed
using Easy-nLC1200 high-performance liquid chromatography (HPLC;
Shanghai Luming Biotechnology Co., Ltd.). The purified LV was
labeled using fluorescein isothiocyanate (FITC), as described by Li
et al (20). Aliquots of
1.7 nl FITC-LV (20 μg/μl) were injected into the
animal pole or yolk sac of 0 hpf or 3 hpf ZEs under the
stereomicroscope and fluorescence was observed at 72 h after
injection. Male zebrafish plasma (MZP) was isolated as described by
Medina-Gali et al (21).
Globin was extracted from MZP using a Micro Protein PAGE Recovery
kit [Real-Times (Beijing) Biotechnology Co., Ltd.] and identified
by SDS-PAGE (15% gel), as previously described (22).
In vitro chromatin reconstitution
A total of two chromatin reconstitution methods were
used: Incubation reconstitution, as described by Stein et al
(23), and the salt-dialysis
reconstitution method described by Lusser and Kadonaga (24).
In incubation reconstitution process (7,25), plasmids were diluted into plasmid
solution to a final concentration of 0.1 μg/μl. Each
plasmid was incubated with histone octamer (0.000, 0.005, 0.010,
0.020, 0.040, 0.080 and 0.160 μg/μl) and LV (0.000,
0.625, 1.250, 2.500, 5.000 and 10.000 μg/μl) or BSA
(0.000, 0.625, 1.250, 2.500 and 5.000 μg/μl) (Yuanye
Biotechnology Co., Ltd.) in buffer comprising 0.1 mol/l NaCl and 10
mmol/l Tris-HCl at room temperature for 30 min.
In the salt-dialysis reconstitution (24), each plasmid (at a final
concentration of 0.1 μg/μl) was mixed with histone
octamer and LV (or BSA) in initial buffer containing 2 mol/l NaCl,
1 mmol/l EDTA and 10 mmol/l Tris-HCl (pH 7.2). The chromatin was
placed into a dialysis bag (0.5 kDa; Beijing Solarbio Science &
Technology Co., Ltd.) for 4 h dialysis at 8°C. Finally, the volume
of the reconstituted chromatin was adjusted to 0.4 ml using buffer
containing 0.1 mol/l NaCl and 10 mmol/l Tris-HCl (pH 7.2).
SDS-PAGE
The samples loaded were as follows: Sample 1, 75
μl 1 mg/ml salmon sperm DNA (Beijing Solarbio Science &
Technology Co., Ltd.) was incubated with 75 μl ZEL (57
mg/ml) at 4°C for 15 min, and centrifuged for 5 min at 13,400 g at
4°C. For sample 2, the supernatant of sample 1 was incubated with
7.5 μl 10 mg/ml salmon sperm DNA at 4°C for 15 min, then
centrifuged for 5 min at 13,400 g at 4°C. Samples 3 and 4 were
obtained via repeating this procedure. The samples were added to 40
μl Tris-HCl containing 10 μl 10% SDS and 10 μl
loading buffer. Sample 5 contained 8 μl supernatant from the
fourth salmon-sperm DNA precipitate mixed with 8 μl
Tris-HCl, 4 μl 10% SDS and 4 μl loading buffer.
Sample 6 comprised 4 μl ZEL mixed with 12 μl
Tris-HCl, 4 μl 10% SDS and 4 μl loading buffer. For
sample 7, 75 μl 1 mg/ml salmon-sperm DNA was mixed with 75
μl ZEL (57 mg/ml); 750 μl 0.075 mol/l NaCl was then
added and the mixture was incubated at 4°C for 15 min and
centrifuged for 5 min at 13,400 g. The precipitate was dissolved in
40 μl Tris-HCl containing 10 μl 10% SDS and 10
μl loading buffer. Sample 9 (histone) was prepared in the
same way as sample 7 (histone) above. To generate sample 10, 175
μl FCS was incubated with 75 μl 1 mg/ml salmon-sperm
DNA at 4°C for 15 min and centrifuged for 5 min at 13,400 g. This
precipitate was dissolved in 40 μl Tris-HCl containing 10
μl 10% SDS and 10 μl loading buffer. Sample 11 was
generated by mixing 4 μl FCS with 12 μl Tris-HCl
containing 4 μl 10% SDS and 4 μl loading buffer. All
samples were boiled at 100°C for 2 min. A total of 5 μl each
sample was loaded onto each lane and subjected to SDS-PAGE (15%
gels).
Establishment of in vitro transcription
system
Nuclear Extraction kit (Beyotime Institute of
Biotechnology) was used to extract nuclei from 20 hpf ZEs using
0.42 mol/l NaCl for two sequential extractions. Each 1 ml
supernatant was mixed with 330 mg ammonium sulfate, centrifuged at
13,400 g for 5 min at 4°C and the resulting precipitate was
dissolved in 0.5X Tris-EDTA (TE) buffer (10 mmol/l Tris, 1 mmol/l
EDTA, pH 8.0) and loaded onto an equilibrated Sephadex G50 column
(1.5×20 cm height, Pharmacia) to obtain crude RNA polymerase II for
use in the following experiments.
In vitro transcription of chromatin was
performed as described by You et al (26). Chromatin from deyolked 3 hpf ZEs
(1,000 cells) was extracted as described by Purushothaman et
al (27). The chromatin was
dissolved in buffer at 4°C (0.1 mol/l NaCl and 10 mmol/l Tris-HCl;
pH 7.2).
A total of 10 nmol/l C1-ORF2 recombinant (or
chromatin) was incubated for 1 h at 30°C in reaction buffer with
crude RNA polymerase II for 1 h at 30°C in 400 mmol/l Tris-HCl
reaction buffer, which includes 200 μmol/l nucleotides, 20 U
RNase inhibitor, 200 mmol/l MgCl2, 25 mmol/l tris
(2-carboxyethyl) phosphine and 20 mmol/l spermidine.
The reaction was terminated by adding 125 mmol/l
Tris-HCl (pH 7.5) containing 12.5 mmol/l EDTA, 150 mmol/l NaCl, 1%
SDS and 2 μg/μl protease K at 55°C for 1 h. Reverse
transcription-quantitative (RT-q)PCR analysis was used to detect
the mRNA levels of EZE expression genes as previously described
(28). Table I shows primers for RT-qPCR
analysis.
 | Table IPrimers used for reverse
transcription-quantitative PCR. |
Table I
Primers used for reverse
transcription-quantitative PCR.
| Target | Sequence,
5′➔3′ | Length of product,
bp |
|---|
| EGFP | Forward:
GCACCATCTTCTTCAAGGAC | |
| Reverse:
TTGTCGGCCATGATATAGAC | 181 |
| GRHL3 | Forward:
AGACGAGCAGAGAGTCCT | |
| Reverse:
TTGCTGTAATGCTCGATGATG | 210 |
| SOX19A | Forward:
GAGGATGGACAGCTACGG | |
| Reverse:
CTATAGGACATGGGGTTGTAG | 179 |
| NNR | Forward:
GAGACATACCACAGGTGAAGC | |
| Reverse:
CCGCTCTGGTCTGTTGC | 234 |
RT-qPCR and RNA sequencing (RNA-seq)
Aliquots of 20 μl LV (50 μg/μl)
were injected into the abdominal cavity of adult male zebrafish
(weight, 0.37-0.43 g). Total RNA was extracted from zebrafish
livers 24 h after the zebrafish had been fed at 28°C in a
flowthrough system. The zebrafish were submersed in water
containing 100 mg/l 3-aminobenzoate methanesulfonate (Beijing Mreda
Technology, Inc.) for anesthesia. The zebrafish enterocoelia were
opened to obtain the zebrafish liver. Total RNA was extracted using
TRIzol® (Thermo Fisher Scientific, Inc.) from liver
tissues. Total RNA was reverse-transcribed into cDNA using
RevertAid First Strand cDNA Synthesis kit (Thermo Fisher
Scientific, Inc.) according to the manufacturer's protocol.
Expression levels of the EZE expression genes GRHL3
(grainyhead-like transcription factor 3), SOX19A (SRY-box
transcription factor 19a) and NNR (nanor) were detected using qPCR
kit (cat. no. QP031, GeneCopoeia). qPCR was performed using the
following thermocycling conditions: Initial denaturation at 95°C
for 2 min, followed by 40 cycles at 95°C for 10 sec and 56°C for 40
sec. The 2−∆∆Cq method was used to calculate the
relative mRNA expression levels (29). The primer pairs utilized for
RT-qPCR analysis are shown in Table
I.
Zebrafish were placed into water containing 0.5 g/l
3-aminobenzoate methanesulfonate for ≥20 min to ensure death, prior
to storage at −20°C.
RNA-seq analysis was performed by Shanghai OE
Biotech. Co., Ltd., as previously reported (28). Total RNA was extracted from
zebrafish liver tissue using TRIzol®. The purity and
quantification of the RNA was evaluated using a NanoDrop™ 2000
spectrophotometer (Thermo Fisher Scientific, Inc.), whereas RNA
integrity was assessed using Agilent 2100 Bioanalyzer (Agilent
Technologies, Inc.). Subsequently, the libraries were constructed
using a TruSeq® Stranded mRNA LT Sample Prep kit (cat.
no. NR616-02, Illumina, Inc.). Gene Ontology (GO; geneontology.org/) enrichment analysis were
performed.
Electrophoretic mobility-shift assay
(EMSA)
Fragments were obtained by digesting C1-ORF2
(XhoI/PstI), C1-Alux14 (HindⅢ/NheⅠ),
C1-LacZ (ApaI/XhoI) and C1 (XhoI/PstI).
These fragments were precipitated with ethanol, centrifuged at
13,400 g for 5 min at 4°C and precipitate was dissolved in double
distilled water. All restriction enzymes were purchased from Takara
Biotechnology Co., Ltd. The final concentration of fragments was 20
ng/μl and the concentration determination method was
measured by ultraviolet spectrophotometer (UV-2800A; UNICO
Instrument Co., Ltd. Shanghai) at 280 nm; these fragments were
incubated with histone or LV for 15 min at room temperature in 10
mmol/l Tris-100 mmol/l NaCl buffer. LV concentrations were 0.0000,
0.0625, 0.1250, 0.2500, 0.5000, 0.7500, 1.0000, 1.2500, 1.5000 and
1.7500 μg/μl in the experiments that ORF2, Alux14, C1
fragments were incubated with LV. The LV concentrations were
0.0000, 0.0125, 0.0625 and 0.3125 μg/μl and histone
concentrations were 0.025 μg/μl in the experiments of
the effects of incubation order. The LV concentrations were 0.0000,
0.0625, 0.1250, 0.2500, 0.5000, 1.0000 and 2.0000
μg/μl, the histone concentrations were 0.015, 0.020,
0.025, 0.030 μg/μl to assess effects of histone
concentrations. A total of 10 μl of each sample was loaded
into a well with the gel; all experiments were using 1.5% agarose
gel with 5% ethidium bromide (cat. no.1239-45-8; Shanghai Yien
Chemical Technology Co., Ltd.). Finally, images were captured using
an Alpha Innotech gel-imaging analyzer (Alpha Innotech
Corporation).
Chromatin immunoprecipitation (ChIP)-PCR
assay
ChIP assay was employed to validate the affinity of
LV with ORF2. ZEs (25 mg, 0 hpf) were microinjected using C1-ORF2
or C1-Alux14 (control) plasmids. Subsequently, the injected embryos
were incubated at 28°C for 2 h and frozen at −20°C for processing.
Samples were prepared using the SimpleChIP® Plus
Enzymatic chromatin IP kit (Cell Signaling Technology, Inc.; cat
no. #9005), following the manufacturer's protocol. A total of 1.5%
formaldehyde was added into the embryos for 30 min at room
temperature to produce cross-linked protein and DNA and then
chromatin fragments were obtained using sonication (20 sec per
time; 800 Hz) at intervals of 30 sec (20 cycles) at 4°C, followed
by centrifugation at 13,400 × g for 5 min at 4°C. The chromatin
fragments were incubated with 0.03 μg/μl anti-LV
antibody at 4°C for overnight. PCR was performed using the
following EGFP primers: Forward, 5′-ACATCCTGGGGCACAAGC-3′ and
reverse, 5′-CTTGTACAGCTCGTCCATGC-3′. PCR kit [cat. no. ET101,
TIANGEN BIOTECH (BEIJING) CO., LTD.] was performed using the
following thermocycling conditions: Initial denaturation at 94°C
for 3 min, followed by 30 cycles of 94°C for 30 sec, 55°C for 30
sec and 72°C for 60 sec and final extension at 72°C for 5 min. PCR
products (311 bp) were separated by 1% agarose gels and visualized
using a UV transilluminator (Alpha Innotech Corp. USA).
Assay for transposase-accessible
chromatin with sequencing (ATAC-Seq)
HeLa cells (Shanghai Tongpai Biotechnology Co.,
Ltd.) were seeded (1×105 cells/well) in 24-well plates
and cultured in Dulbecco's Modified Eagle Medium (cat no. 10566016,
Thermo Fisher Scientific, Inc.) with 10% fetal bovine serum (cat
no. ZF181FBS-500, Zeta Life Co., Inc.) at 37°C with 5%
CO2. The cells (2×105 cells/well) were
transfected with C1-ORF2 vector (0.2 μg/μl) using
Lipofectamine®2000 (Thermo Fisher Scientific, Inc.) at
room temperature for 5 min, following the manufacturer's protocol.
After 48 h, cells were grown in selective medium containing 1 mg/ml
G418 (Beijing Biotopped Life Sciences, Inc.) for approximately 3
weeks. Then, the cells stably transfected with C1-ORF2 were
obtained. LV (1.18 μg/μl) was added into stably
transfected HeLa cells for 48 h. Same volume saline was added into
stably transfected HeLa cells as a control. ATAC-seq was performed
by Shanghai Jiayin Biotechnology Ltd. using a Novaseq 6000
Sequencing System (Illumina, Inc.) as previously described
(30,31). Trimmomatic software (version
0.36, usadellab.org/cms/?page=trimmomatic) was used for
quality control. Burrows Wheeler Aligner-maximal exact matches
software (version 0.7.13-r1126, bio-bwa.sourceforge.net/) compared the clean data to
the reference (CMV-EGFP-ORF2-PolyA) (32). MACS2 software (version 2.1.2,
pypi.org/project/MACS2/) was used for
analysis, and Q<0.05 was used as the screening threshold.
DeepTools software (3.4.3, deeptools.readthedocs.io/en/develop/) was used to
analyze signal distribution near to the CMV-EGFP-ORF2-PolyA
reference.
DNase I digestion
C1-OR F 2-h istone-LV a nd C1-Alux14-histone-LV
recombinants were obtained by the incubation reconstitution (see
subsection: In vitro chromatin reconstitution). The
recombinants were digested with 0.045 U/μl DNase Ⅰ for 0
sec, 30 sec, 2 min or 4 min at room temperature. Next, the
recombinants of C1-ORF2-histone-LV and C1-ORF2-histone-BSA were
obtained using the dialysis recombination. Briefly, 100 μl
of four different recombinant solutions were digested with DNase I
(0.02, 0.04 or 0.08 U/μl) at room temperature for 1 min.
Aliquots 10 μl termination solution (0.1 % SDS-10 mmol/l
EDTA) was added and then precipitated with absolute ethyl alcohol.
The precipitates were washed twice with 75% ethanol, dried,
dissolved with 50 μl 2.5% SDS and 1/10 volume of xylene
cyanol FF loading buffer. Each 10 μl sample was loaded into
one lane and subject 1.5% agarose gel electrophoresis.
Statistical analysis
Each experiment was repeated at least three times.
Data are presented as the mean ± standard deviations (SD).
Statistical analysis was performed using SPSS software (version
17.0; IBM Corporation) and GraphPad Prism (version 6.0; Dotmatics).
Group differences were assessed using one-way ANOVA with Tukey's
post hoc test. P<0.05 was considered to indicate a statistically
significant difference.
Results
L1-ORF2 enhances EGFP expression in
EZEs
To determine whether human L1-ORF2 enhances EGFP
expression in EZEs, C1-ORF2, C1-Alux14, C1-LacZ and pEGFP-C1 (C1)
expression vectors were injected into 0 hpf ZEs and subsequently
the EGFP fluorescence intensity was observed at 24 h
post-injection. Brightest EGFP fluorescence was observed in the
C1-ORF2 group (Fig. 1A and B).
C1-ORF2 was injected into 0 hpf ZEs and the EGFP fluorescence
intensity was observed at 6, 12, 24 and 48 h post-injection. EGFP
fluorescence intensity induced by C1-ORF2 increased with time
(Fig. 1C and D). However, when
C1-ORF2 was injected into 0, 2 and 4 hpf ZEs, EGFP fluorescence
intensity of 0 hpf was higher than of 2 hpf and 4 hpf groups, which
indicated that EGFP expression is related to embryo development
stage (Fig. 1E and F). Taken
together, these results suggested that the developmental stage
affected expression of EGFP induced by C1-ORF2 in EZEs. To
determine whether ORF2 activates EGFP via transcriptional changes,
RT-qPCR was used to quantify EGFP mRNA. C1-ORF2 significantly
increased expression of EGFP mRNA compared with C1-Alux14 or
C1-LacZ vectors (Fig. 1G). These
results suggested that ORF2 could activate EGFP gene
expression at the transcriptional level.
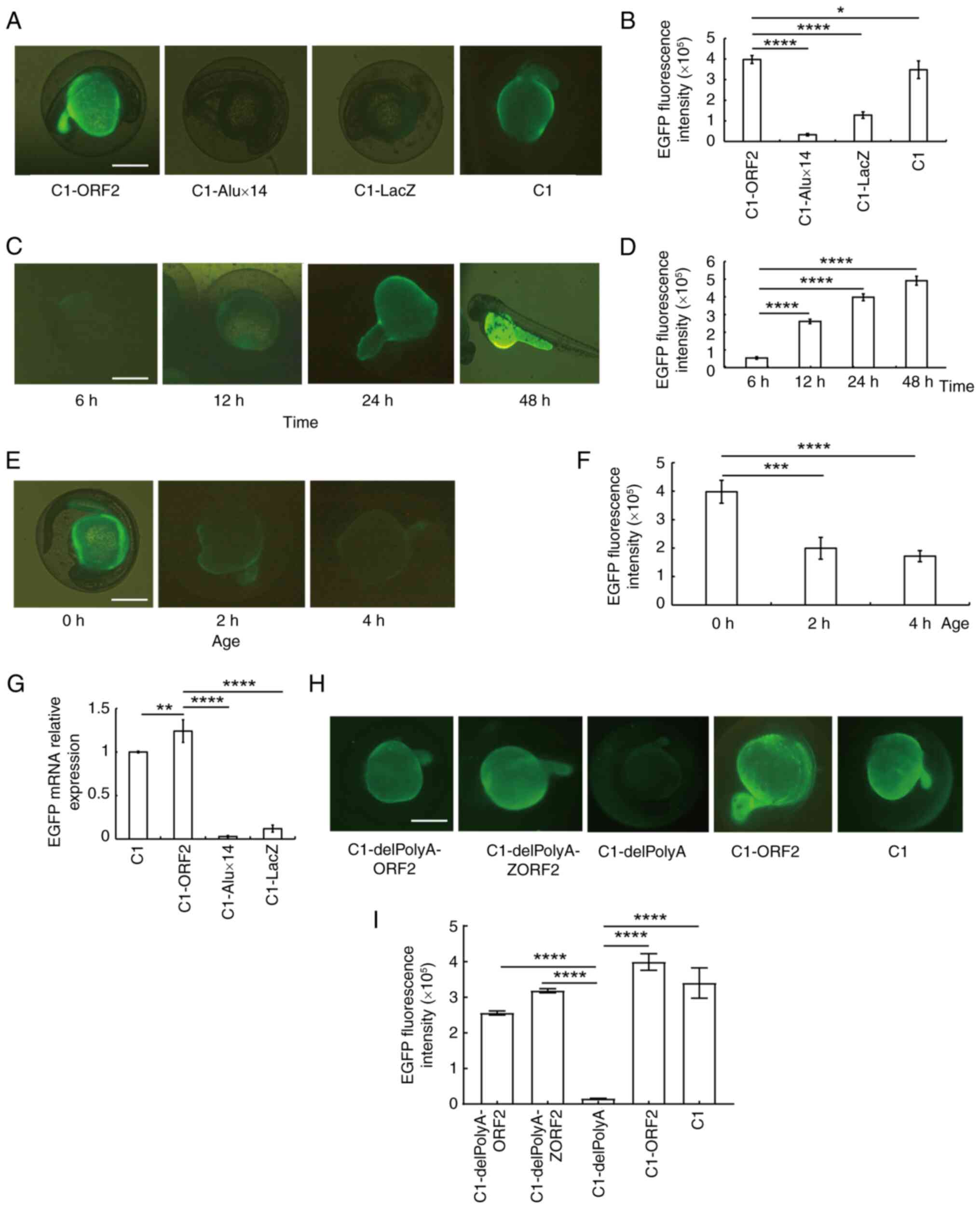 | Figure 1ORF2 activates EGFP expression in 0 h
zebrafish embryos. (A) EGFP fluorescence (B) intensity following
injection of the C1-ORF2, C1-Alux14, C1-LacZ or C1 (pEGFP-C1)
vectors. (C) EGFP fluorescence (D) intensity after C1-ORF2
injection. (E) EGFP fluorescence (F) intensity induced by C1-ORF2
injection into embryos at different developmental stages, observed
after 24 h. (G) ORF2 activates EGFP expression at the
transcriptional level. (H) EGFP fluorescence (I) intensity at 24 h
after injection of C1-delPolyA-ORF2, C1-delPolyA-ZORF2,
C1-delPolyA, C1-ORF2 and C1. Scale bar, 200 μm.
*P<0.05, **P<0.01, ***P<0.001,
****P<0.0001. EGFP, enhanced green fluorescent
protein; ORF2, open reading frame 2. |
To exclude any enhancer role of poly(A) on
EGFP activation, poly(A) was removed from the C1 plasmid,
generating C1-delPolyA. The C1, C1-ORF2, C1-delPolyA,
C1-delPolyA-ORF2 and C1-delPolyA-ZORF2 vectors were injected into
EZEs and the lowest EGFP fluorescence intensity was observed in the
C1-delPolyA group, whereas the EGFP fluorescence intensity induced
by C1-delPolyA-ORF2 and C1-delPolyA-ZORF2 were significantly higher
compared with that induced by C1-delPolyA (Fig. 1H and I). These results further
demonstrated that ORF2 activated EGFP gene expression in EZEs.
Histone inhibits EGFP expression induced
by ORF2, which is negated by ZEL
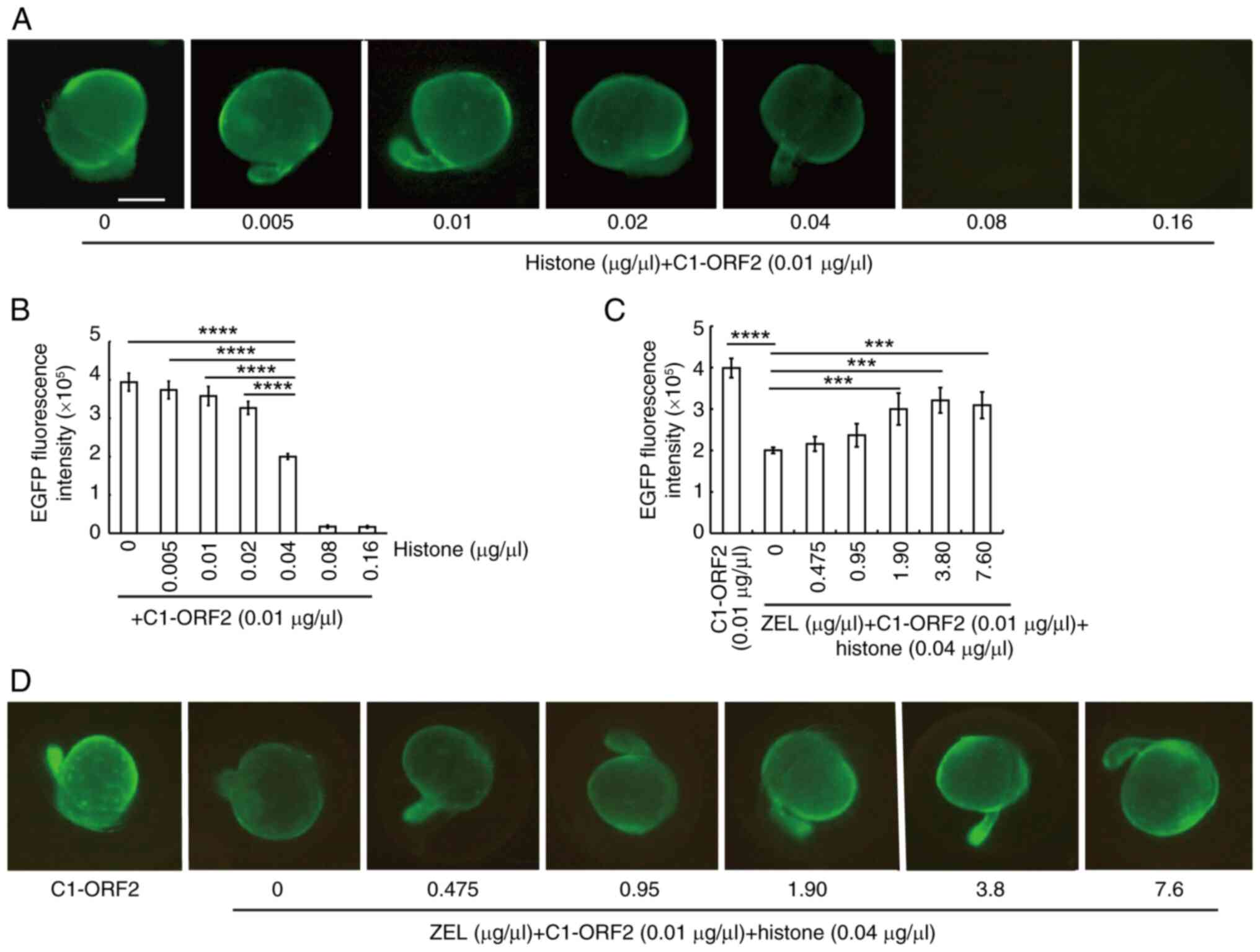
To observe the effects of histone on ORF2-induced
EGFP expression in EZEs, C1-ORF2 was incubated with histone and
injected into 0 hpf ZEs. EGFP expression was then observed at 24 h
post-injection. EGFP fluorescence decreased as histone
concentration increased (Fig.
2A). Upon incubation with 0.04 μg/μl histone, the
intensity of EGFP induced by ORF2 decreased by almost 50% compared
with 0 μg/μl histone (Fig. 2B).
Based on relative levels of histones and
transcription factors, which both regulate the onset of
transcription in the EZEs (33),
it was hypothesized that ZEL contains certain components that
eliminate the inhibitory effect of histones on C1-ORF2. As the
concentration of ZEL increased, the inhibitory effect induced by
histones decreased (Fig. 2D);
3.8 μg/μl ZEL could eliminate 80.49% of the
histone-mediated inhibition of EGFP expression (Fig. 2C). These findings indicated that
ZEL attenuates the suppression of ORF2-dependent EGFP expression by
histone.
LV ameliorates histone-induced inhibition
and enhances expression of EZE genes
It was hypothesized that DNA-binding proteins of the
ZEL could activate EGFP fluorescence via interaction with C1-ORF2
in 0 hpf ZEs. Salmon sperm DNA was incubated with ZEL and the key
proteins were divided according to their molecular mass (~115, ~100
and ~25 kDa; Fig. 3A and B).
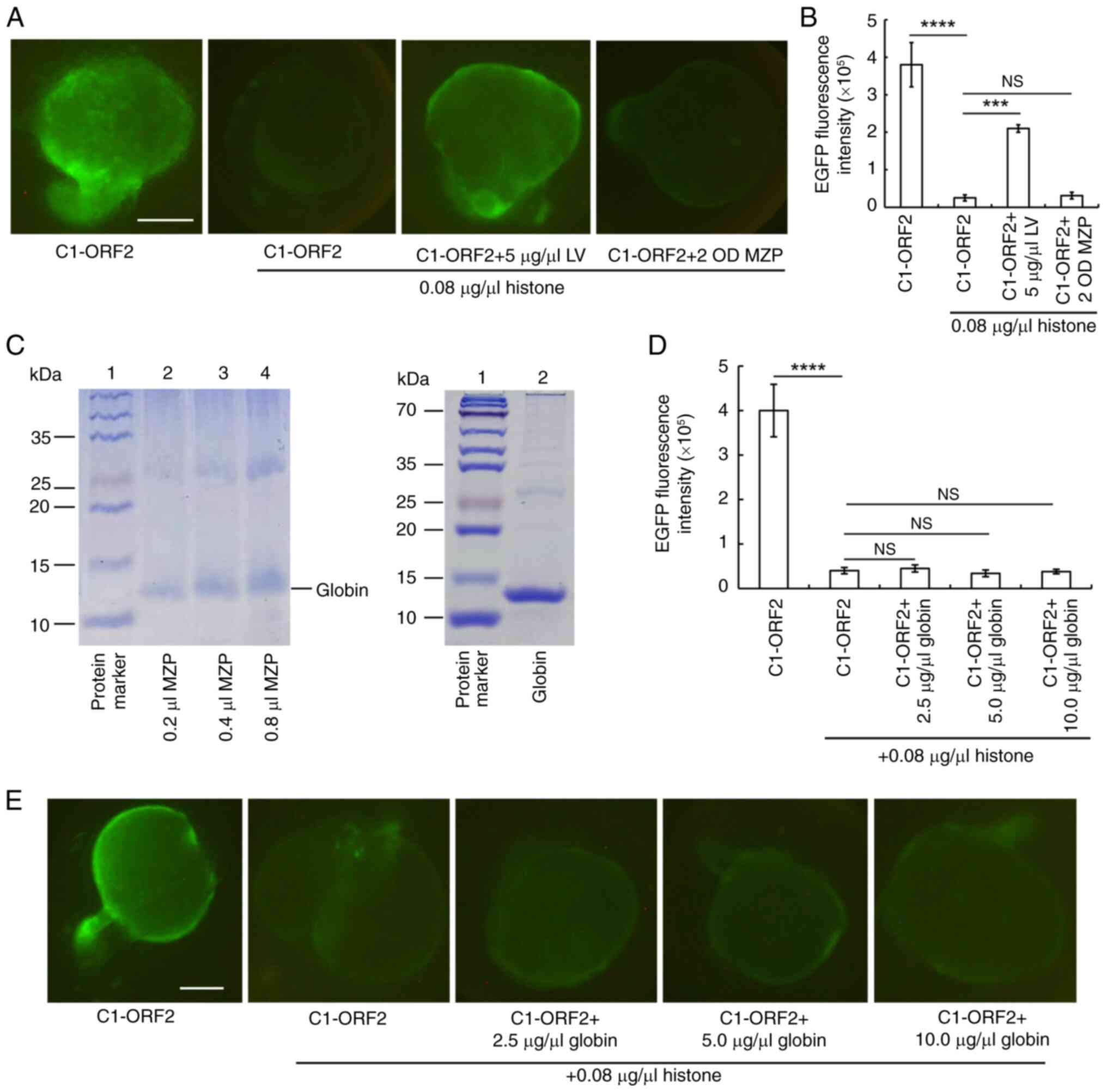
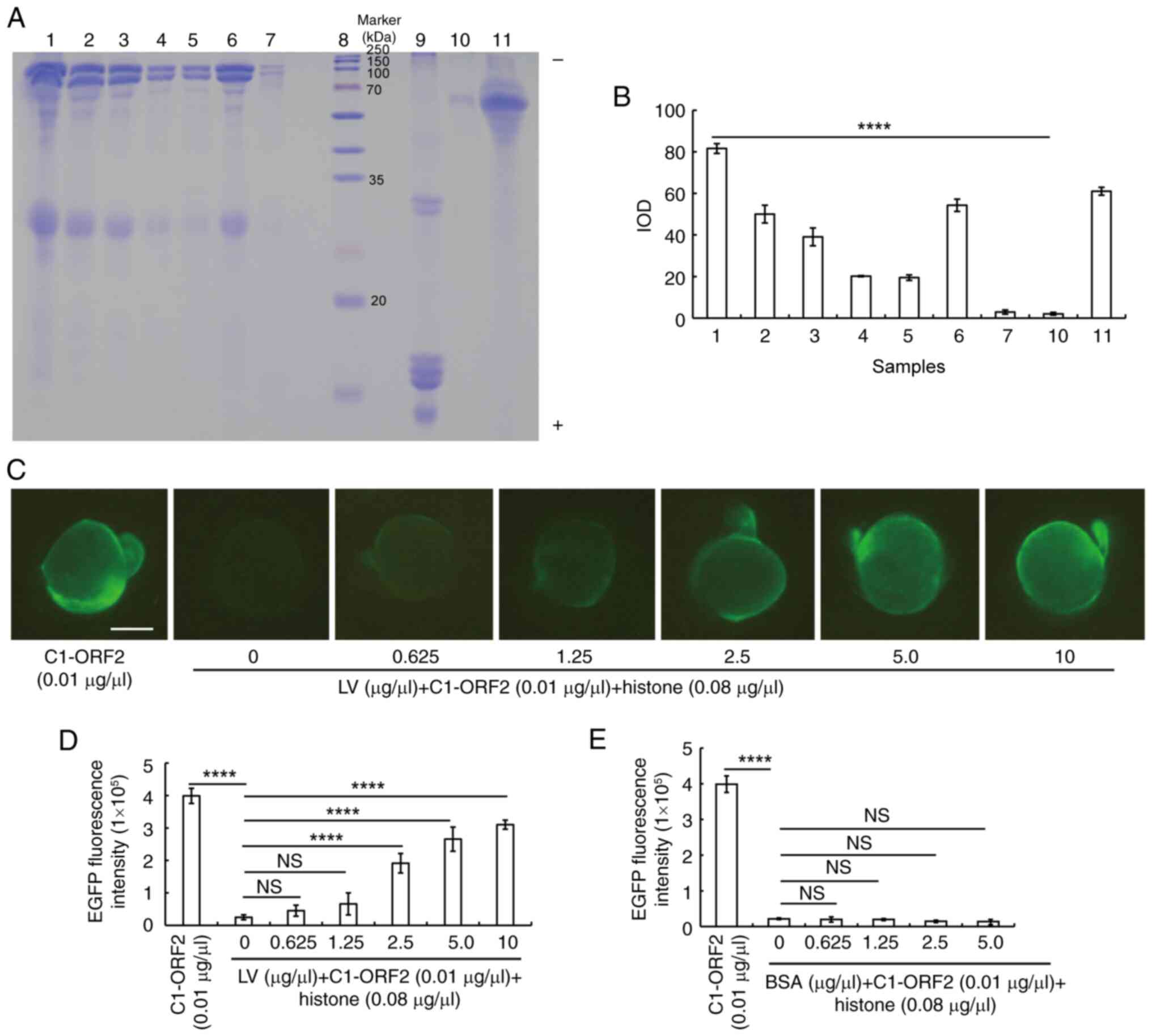 | Figure 3LV alleviates histone-induced
inhibition. (A) SDS-PAGE of ZEL incubated with salmon-sperm DNA.
Lanes 1-4, First to fourth precipitate of salmon-sperm DNA and ZEL,
the supernatant of the fourth precipitate (lane 5), ZEL (lane 6),
diluent (lane 7), Marker (lane 8), histone (lane 9), precipitate of
salmon-sperm DNA and FCS (lane 10) and FCS (lane 11). (B) IOD. (C)
EGFP fluorescence (D) intensity induced by C1-ORF2 incubated with
histone and LV (scale bar, 200 μm). (E) Embryos showing EGFP
fluorescence induced by C1-ORF2 incubated with histone and BSA.
****P<0.0001. NS, not significant; LV, lipovitellin;
ZEL, zebrafish embryo lysate; EGFP, enhanced green fluorescent
protein; ORF2, open reading frame 2; IOD, integrated optical
density. |
LV was purified from ZEL. The purified LV was
analyzed using Easy-nLC1200 high-performance liquid chromatography
(Table SI). The different
concentrations of LV (0, 0.625, 1.25, 2.5, 5.0 and 10
μg/μl) were incubated with histone and C1-ORF2. LV
could attenuate the inhibitory effect of histone on C1-ORF2-induced
EGFP expression (Fig. 3C and D);
by contrast, BSA failed to alleviate the inhibitory effect of
histone on EGFP expression at any concentration (Fig. 3E).
MZP, which does not contain LV, was used as an
appropriate control for demonstrating the specificity of LV. MZP
was found not to attenuate histone-induced inhibition on EGFP
expression (Fig. 4A and B).
Globin is an abundant protein in MZP, with a molecular weight of
~13 kDa (34,35). SDS-PAGE showed that globin was
one of the abundant proteins in MZP (Fig. 4C). Different concentrations of
globin did not weaken histone-induced inhibition (Fig. 4D and E).
In a previous study, the concentration of LV was
higher in good-compared with poor-quality eggs (36). To explore the role of LV in EGFP
gene activation, C1-delPolyA, C1-delPolyA-ORF2 and
C1-delPolyA-ZORF2 were injected into 0 hpf ZEs with poor or good
quality, and EGFP fluorescence was observed at 24 h post-injection.
ORF2 and ZORF2 could induce strong EGFP fluorescence in good-but
not in poor-quality embryos (Fig. 5A
and B).
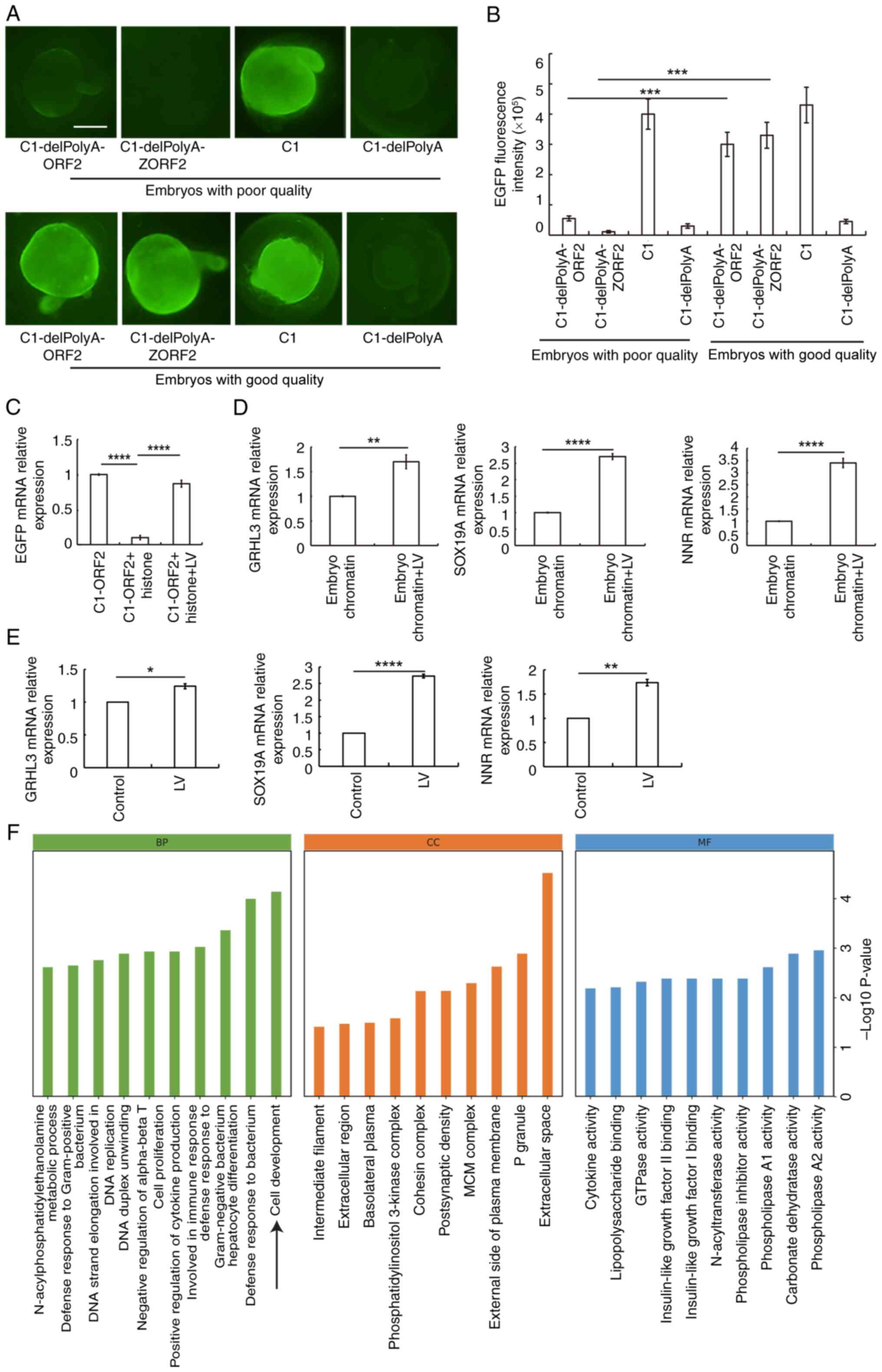 | Figure 5LV activates EGFP gene expression in
good-quality 0 h ZEs and promotes expression both of EGFP reporter
gene and genes of EZEs. (A) Embryos injected with C1-delPolyA-ORF2,
C1-delPolyA-ZORF2, C1 and C1-delPolyA expression vectors. The scale
bar represents 200 μm. (B) EGFP fluorescence intensity. (C)
LV attenuates the inhibitory effect of histone on EGFP mRNA. LV
promotes expression of GRHL3, SOX19A and NNR genes in (D) EZEs and
(E) adult male zebrafish liver. *P<0.01,
**P<0.01, ***P<0.001,
****P<0.0001. (F) GO gene function classification.
GO, Gene Ontology; GRHL3, grainyhead-like transcription factor 3;
SOX19A, SRY-box transcription factor 19a; NNR, nanor. BP,
biological process; CC, cell component; MF, molecular function; LV,
lipovitellin; EGFP, enhanced green fluorescent protein; ORF2, open
reading frame 2. |
An in vitro transcription assay was employed
to explore whether LV serves a role in gene expression regulation.
Histone inhibited EGFP transcription, whereas LV attenuated this
inhibitory effect (Fig. 5C). LV
increased the mRNA levels of the GRHL3, SOX19A and NNR (Fig. 5D).
To verify whether results were reflective of the
role of LV in terms of regulating transcription of ZEs, LV was
injected into the abdominal cavity of adult male zebrafish. RT-qPCR
was then used to detect the expression levels of the GRHL3, SOX19A
and NNR genes. LV could increase transcription of the GRHL3, SOX19A
and NNR genes (Fig. 5E).
Furthermore, RNA-seq method was used to detect the influence of LV
on the gene expression profile of liver tissue. LV increased the
transcription of SOX19A by 4.89-fold; RT-qPCR shows that LV
increased the transcription level of SOX19A by 2.73-fold (Fig. 5E). However, GRHL3 and NNR genes
were not detected by RNA-seq. Sensitivity of RNA-seq is lower than
that of RT-qPCR (35). GO
enrichment analyses showed that the upregulated genes induced by LV
injection were significantly enriched in 'cell development'
(Fig. 5F). These results
demonstrated that LV increased the expression of genes associated
with early embryo development.
LV binds ORF2 to loosen the structure of
chromatin, subsequently regulating gene expression
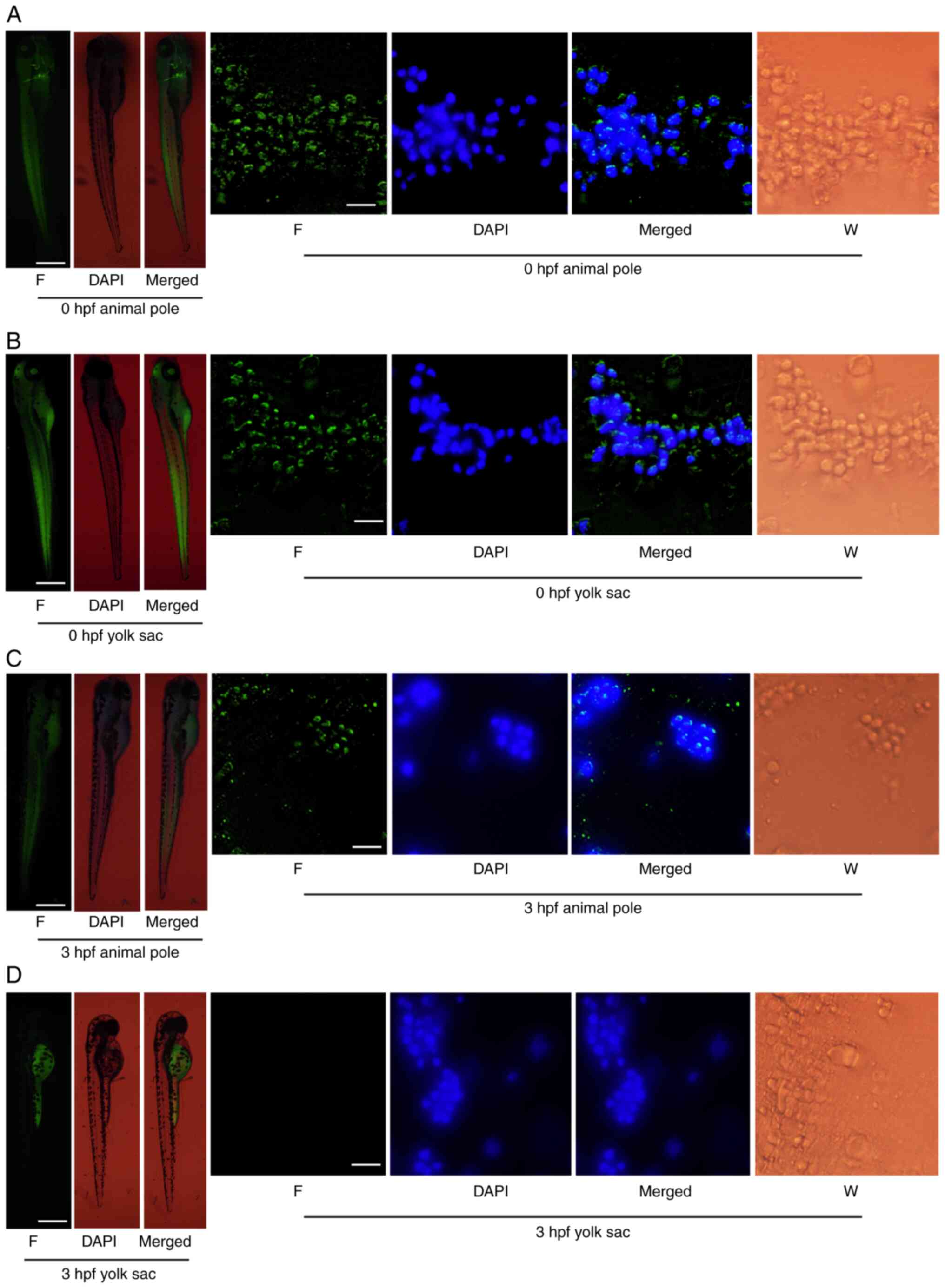
As LV is primarily found in the yolk sac of embryo,
it was important to ensure that LV could move into cell nucleus.
FITC-LV was injected into the animal pole or yolk sac of ZEs at 0
or 3 hpf and fluorescence was observed after 72 h. When FITC-LV was
injected into the animal pole, green fluorescence was found in both
zebrafish and cells, including the nucleus. This indicated that
FITC-LV could cross the zebrafish cell and nuclear membrane
(Fig. 6A and C). When FITC-LV
was injected into the yolk sac of zebrafish embryo at 0 hpf, both
zebrafish and cells (including nuclei) showed green fluorescence
(Fig. 6B). However, when FITC-LV
was injected into yolk sac of 3 hpf embryos, the fluorescence was
only found in yolk sac (Fig.
6D). These findings indicated that FITC-LV was unable to
penetrate the syncytial layer at 3 hpf of embryonic development.LV
was passed through the cell and nuclear membrane and might
therefore have an important role in early embryos.
Since LV serves an important role in gene
regulation, the present study explored which DNA fragment best
binds LV. ChIP analysis of the embryo tissue revealed LV bound ORF2
more readily than it did Alux14 (Fig. 7A). In addition, ORF2, Alux14 and
C1 fragments were incubated with LV. EMSAs showed that the binding
ability of ORF2 and LV was greater compared with that of LV with C1
or Alux14 (Fig. 7B and C).
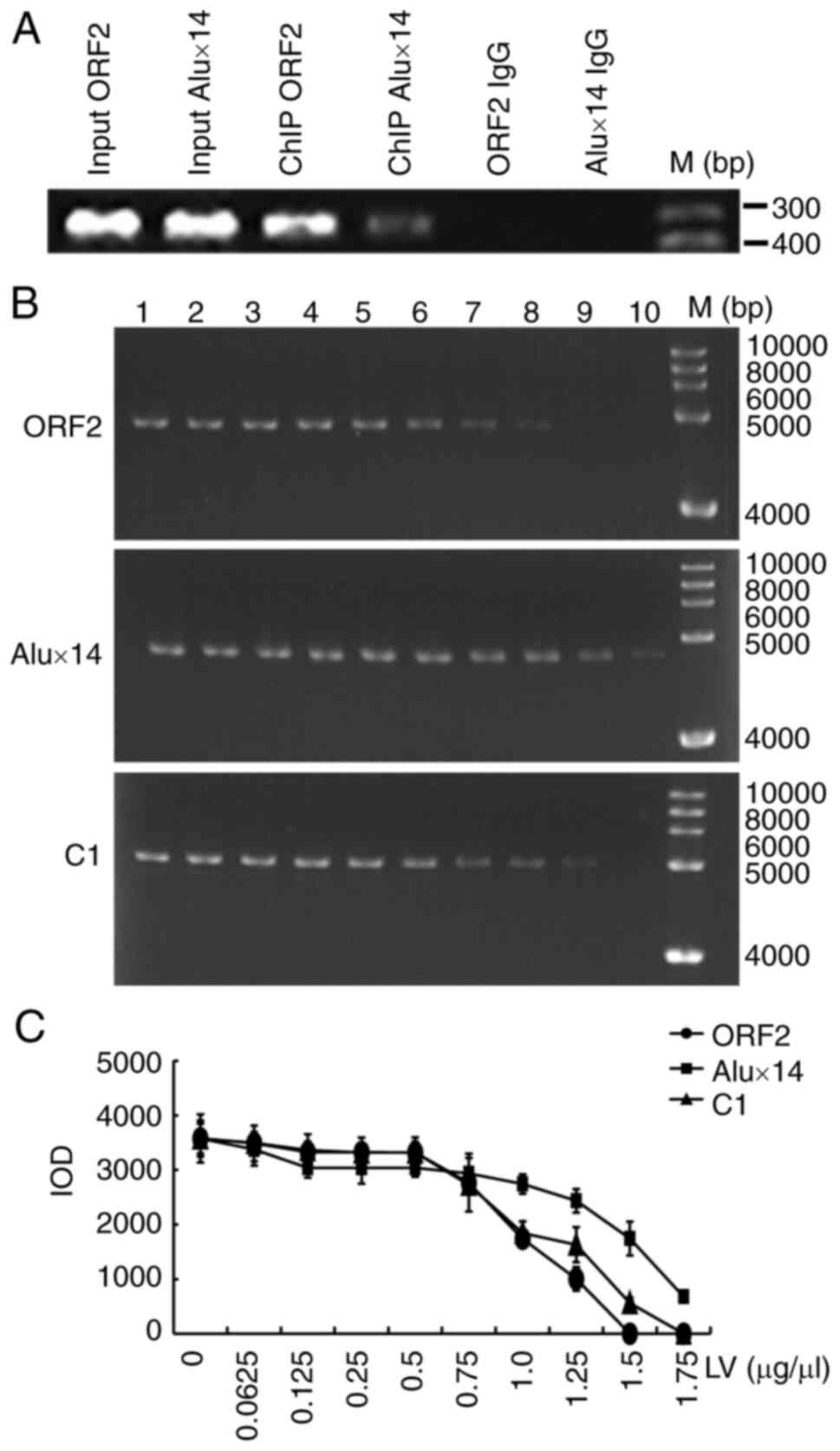 | Figure 7LV binds ORF2 more readily than with
Alux14. (A) ChIP analysis of LV binding to ORF2 or Alux14.
Rabbit-IgG immunoprecipitate was used as the negative control. (B)
Purified ORF2, Alux14 or C1 fragments were incubated with LV in
EMSA. 1-10 lanes: 0, 0.0625, 0.125, 0.25, 0.5, 0.75, 1.0, 1.25, 1.5
and 1.75 μg/μl LV.M, Marker. (C) IOD value. IOD,
integrated optical density; LV, lipovitellin; ORF2, open reading
frame 2; ChIP, chromatin immunoprecipitation assay; EGFP, enhanced
green fluorescent protein. |
LV could ameliorate the inhibitory effect on C1-ORF2
mediated by histone, To determine the underlying mechanism,
histone, C1/ORF2 fragments and LV were mixed. Histone was incubated
with LV and C1/ORF2 fragments. EMSA showed that C1/ORF2 fragments
underwent complete hysteresis at 0 μg/μl LV (Fig. 8A, lane 2). With increasing LV
concentrations (0.0125 and 0.0625 μg/μl), both C1 and
ORF2 underwent partial gel shifts (Fig. 8A; lanes 3 and 4); but when LV
concentration was high (0.03125 μg/μl), the C1 and
ORF2 fragments underwent complete shifts (Fig. 8A, lane 5). When C1/ORF2 fragments
were incubated with histone first and subsequently with LV, all
concentrations of LV caused complete gel retardation of the C1 and
ORF2 fragments (Fig. 8A).
Similarly, when C1/ORF2 fragments were incubated with LV first and
with histone, all concentrations of LV caused complete gel
retardation of the C1 and ORF2 fragments (Fig. 8A). Fig. 8B shows the sum of the IOD values
of the C1 and ORF2 fragments in each lane. Subsequently, effects of
histone concentration on gel retardation when histone was incubated
with LV prior to addition of C1/ORF2 were examined. LV induced the
partial shift of C1/ORF2 with 0.015 μg/μl, 0.02 and
0.025, but not 0.03 μg/μl histone (Fig. 8C and D).
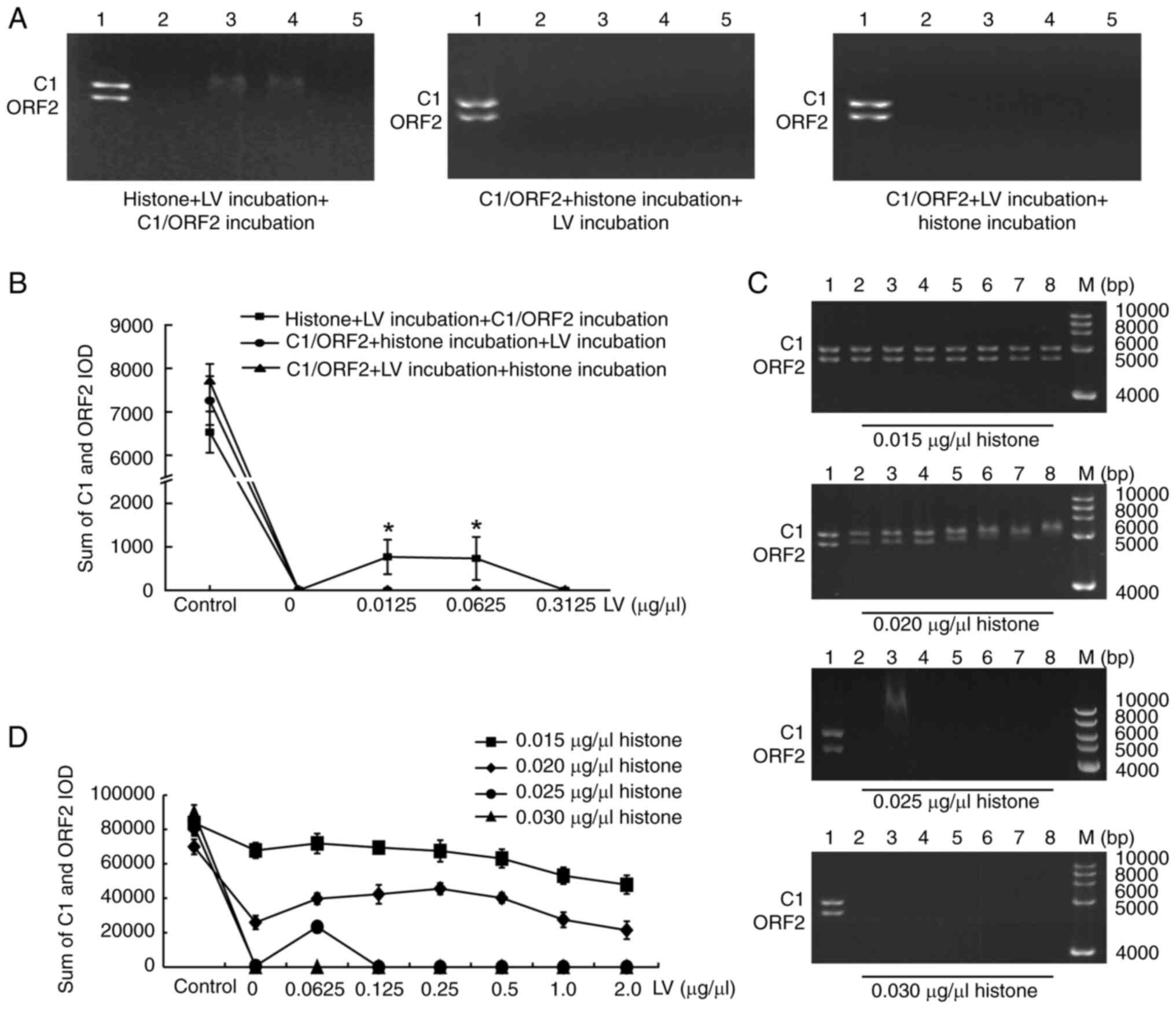 | Figure 8LV interferes with histone binding to
C1 and ORF2 sequence. (A) EMSA following incubation of LV with
histone and C1-ORF2. Left, histone was incubated with LV, and
incubated with C1/ORF2 fragments; middle, histone was incubated
with C1/ORF2 fragments, and with LV; right, LV was incubated with
C1/ORF2 fragments, then with histone. 1, control group, 2-5 lanes:
0, 0.0125, 0.0625 and 0.3125 μg/μl LV. (B) IOD values
of the C1 and ORF2 fragments in each lane. (C) Effect and (D) IOD
of histone concentrations on gel retardation when histone was first
incubated with LV, and with C1/ORF2. 1-8 lanes: 0, 0.0625, 0.125,
0.25, 0.5, 1.0 and 2.0 μg/μl LV, lipovitellin; ORF2,
open reading frame 2; IOD, integrated optical density; M,
Marker. |
Transcription factors act on specific regions of
chromatin to improve chromatin accessibility, manifested as an
increased sensitivity to DNase I digestion (37). The present study investigated
whether LV was able to promote the sensitivity of C1-ORF2 to DNase
I digestion. C1-ORF2 DNA was more easily digested compared with
C1-Alux14 (Fig. 9A and B). LV or
BSA was incubated with C1-ORF2 and histone by salt-dialysis
reconstitution and digested with different concentrations of DNase
I. The brightness of the C1-ORF2 fragments incubated with LV was
significantly lower compared with BSA when digested using the same
concentration of DNase I (Fig. 9C
and D). Taken together, these results suggested that LV
promoted sensitivity of C1-ORF2 to DNase I digestion, which
indicated that LV increased the chromatin accessibility of C1-ORF2
recombinant.
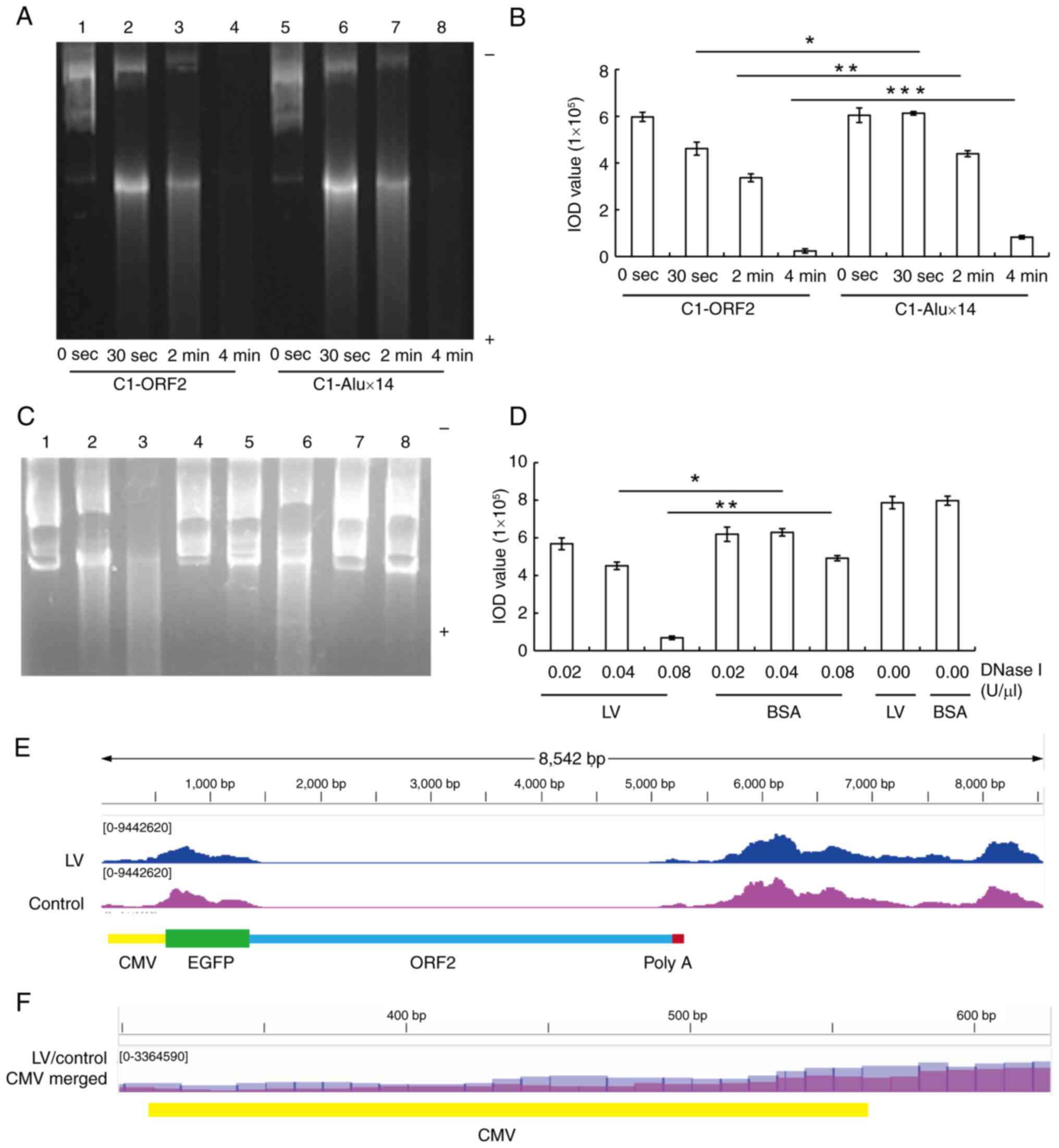 | Figure 9LV increases DNase I
digestion-sensitivity of C1-ORF2. (A) Representative DNase I
digestion of C1-ORF2 + histone + LV (C1-ORF2) or C1-Alux14 +
histone + LV (C1-Alux14) recombinants. (B) IOD values of each lane.
(C) Representative image showing DNase I digestion according to the
salt-dialysis reconstitution method. C1-ORF2 (0.06 μg),
histone (0.18 μg) and 3 μg LV (or BSA) were
recombined using the salt-dialysis reconstitution method. The
recombinants were digested for 1 min with 0.02, 0.04 or 0.08
U/μl DNase I. 1-3 lanes: 0.02, 0.04 and 0.08
μg/μl LV; 4-6 lanes: 0.02, 0.04 and 0.08
μg/μl BSA; 7 lane: 0.00 μg/μl LV; 8
lane: 0.00 μg/μl BSA. (D) IOD values of each lane.
(E) C1-ORF2 ATAC-seq signals. CMV area (CMV enhancer and promoter)
ATAC-seq signals in the LV treatment group were stronger than those
in control group. (F) Amplified CMV ATAC-seq signals in the LV
(blue) and control (purple) group. CMV ATAC-seq signals in the LV
group were more numerous than those in the control group.
*P<0.01, **P<0.01,
***P<0.001. LV, lipovitellin; ORF2, open reading
frame 2; IOD, integrated optical density, ATAC-seq, assay for
transposase-accessible chromatin with sequencing; CMV,
cytomegalovirus enhancer and promoter. |
To verify that LV was able to improve chromatin
accessibility of the cytomegalovirus (CMV) region (enhancer and
promoter) of the EGFP gene in the C1-ORF2 plasmid, HeLa cells were
stably transfected with C1-ORF2 plasmid and treated with LV.
ATAC-seq was performed to measure the degree of chromatin opening
in the CMV region of the EGFP gene in stably transfected plasmids.
There were more CMV-area ATAC-seq signals in the LV compared with
the control group (Fig. 9E and
F). Taken together, the results of ATAC-seq confirmed that LV
enhanced the CMV chromatin accessibility of C1-ORF2, consistent
with the DNase I digestion experiments.
Discussion
L1 is dynamically expressed in early embryos,
although its expression decreases with embryonic development
(10,38,39). Here, C1-ORF2 could induce the
high expression of EGFP gene in 0 hpf ZEs, whereas C1-Alux14 or
C1-LacZ could not. However, when C1-ORF2 was transfected into HeLa
cells, the EGFP gene was almost non-expressed (16,40). To exclude the enhancer role of
poly(A) on EGFP gene expression, poly(A) was removed from the C1
plasmid to generate C1-delPolyA expression vector and both human
ORF2 and zebrafish ORF2 (ZORF2) were inserted into C1-delPolyA. In
the absence of poly(A), both ORF2 and ZORF2 induce EGFP expression
in 0 hpf ZEs.
The relative levels of histone and transcription
factors regulate onset of transcription in embryos (33). Methylcytosine-modifying
10-11-translocation 1 activates L1 by attenuating histone
repression (41,42). In the present study, histone
decreased EGFP expression and ZEL eliminated histone inhibition in
0 hpf ZEs. Therefore, it was hypothesized that a component of the
ZEL could reduce histone inhibition.
EZEs contain various DNA-binding proteins that
fulfill important roles in the zygotic genome activation (43). It has been reported that purified
zebrafish LV is a phospholipoglycoprotein with molecular mass of
~445 kDa, which can be resolved into polypeptides corresponding to
~117, ~102 and ~23.8 kDa by SDS-PAGE (44). Therefore, the DNA-binding
proteins in the ZEL were identified by SDS-PAGE following
precipitation of DNA. LV was the most abundant protein in the
precipitate. As a predominant DNA-binding protein in embryos, LV is
involved in lipid and metal storage and is utilized gradually
during embryonic development (13,45); several proteins have been shown
to regulate L1 expression (46);
therefore, LV may serve an important role in regulating L1
expression. Furthermore, purified LV was shown to attenuate
histone-induced inhibition similarly to ZEL. In addition, it was
shown that neither ORF2 nor ZORF2 induced EGFP expression in
poor-quality embryos, which were similar to embryos featuring LV
knockout (36). Therefore, LV
might affect ORF2 expression.
Furthermore, LV increased the EGFP mRNA levels when
incubated with the C1-ORF2. The GRHL3, SOX19A and NNR genes have
been shown to be EZE expression genes (47,48). Both in vitro transcription
system and in vivo LV injection experiments showed that LV
increased the mRNA expression levels of the EZE genes GRHL3, SOX19A
and NNR. Therefore, LV may be the regulating factor of ORF2 in
early embryos.
When FITC-LV injected into yolk sac of zebrafish,
FITC-LV could penetrate into the cytoplasm and nucleus in 0 but not
3 hpf embryos. In 3 hpf embryos, the embryo genome is
transcriptionally activated and cells were able to synthesize the
required proteins by themselves, so LV may regulate gene expression
only in the early embryos (49).
Multiple DNA-binding proteins affect gene
transcription, and several proteins regulate L1 expression
(46). ChIP analysis and EMSAs
showed that the affinity between ORF2 DNA and LV was higher
compared with between Alux14 and LV. These results suggested that
LV may be an activator of ORF2; however, the underlying mechanism
governing how LV regulates ORF2 transcription and acts as a
trans-acting factor has yet to be elucidated.
Competition between histone and transcription
factor binding regulates the onset of transcription in ZEs
(33). The present study
identified a decrease in C1/ORF2 gel retardation when LV was
incubated with histone before addition of ORF2. Histone is a
universal DNA-binding protein that forms octamers to package DNAs.
Histone has a higher binding stability compared with other
DNA-binding proteins; therefore, transcription factors activate
gene expression only prior to histone binding to DNA (50). Theoretically, binding of proteins
to histone could also delay histone from packaging the DNA. In the
present study, only the pre-incubation of LV with histone could
reduce C1/ORF2 gel retardation that was caused by histone,
indicating that LV had the capability of binding to histone. The
histone in the ZEL is bound to the histone chaperone molecule,
rather than being isolated (51), suggesting that LV is also a type
of chaperone molecule. LV therefore exerts an important role in
balancing the binding of histone to DNA.
Chromatin accessibility is directly associated with
transcription in eukaryotes (52). Accessible regions associated with
regulatory proteins are highly sensitive to DNase I digestion
(37). The present study
demonstrated that LV attenuated inhibition of histone-induced EGFP
expression in the C1-ORF2 vector. Chromatin was reconstituted using
LV, histones and C1-ORF2 (or C1-Alux14) and the DNase I digestion
showed that LV, but not BSA, promoted DNase I digestion sensitivity
of recombinant C1-ORF2. ATAC-seq is a novel method to detect
chromatin accessibility (53).
ATAC-seq was used to verify whether LV enhanced chromatin
accessibility; LV induced more ATAC-seq signals in the CMV area
when LV was added to C1-ORF2 stably transfected HeLa cells.
The present study demonstrated that LV attenuates
the inhibitory effect of histone on ORF2-induced EGFP reporter gene
expression, upregulated expression of EZE genes both in vivo
and in vitro, bound ORF2 DNA and histones and increased
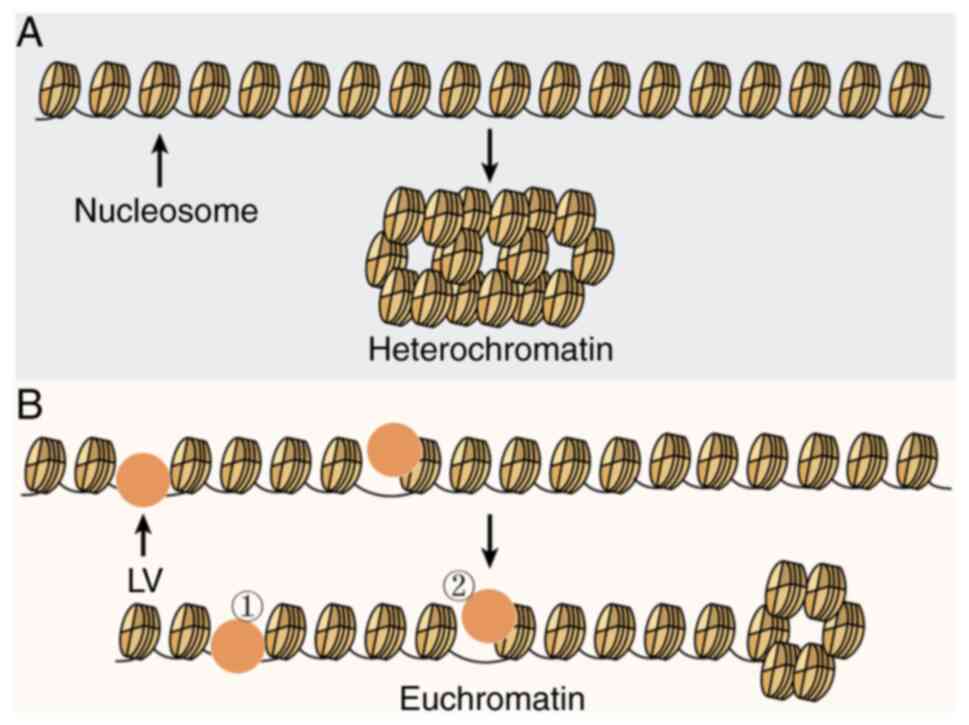
accessibility of C1-ORF2 DNA. Binding of DNA with histones forms
nucleosomes, which are packaged into heterochromatin, inhibiting
gene expression (54,55). LV interferes with nucleosome
packaging by binding ORF2 DNA and histones, which prevents the
formation of heterochromatin, which promotes DNA transcription. The
present study has suggested that LV interfered with the binding of
histones to ORF2 DNA through binding with both ORF2 and histones.
Dissociation of histones from the ORF2 DNA results in a loosening
of ORF2 DNA (56). When histone
binds to LV, a complex of histone and LV is formed (histone-LV),
which decreases and interferes with binding between histone and DNA
(57).
Fig. 10 shows
potential molecular mechanisms that may explain how LV increases
expression of the ORF2-induced EGFP gene and EZE-associated genes
in ZEs. The packaging of histone and L1-ORF2 into heterochromatin
in the absence of LV caused the decreased L1-ORF2 accessibility,
however the presence of LV increased L1-ORF2 accessibility due to
LV interfering with the tight packaging of ORF2 DNA by histones. To
the best of our knowledge, the present study is the first to
demonstrate that LV is the main DNA-binding protein in ZEL, acting
as a trans-acting factor. The binding of LV to DNAs had sequence
specificity and LV had greater affinity for the ORF2 fragment. LV
bound histone to interfere with binding between histone and DNA and
promoted ORF2-induced high expression of EGFP gene by increasing
the accessibility of ORF2-containing DNA constructs and expression
of development-associated genes.
The present study found that LV is a regulating
factor of ORF2; however, the present study had limitations. During
early embryonic development, gene expression regulation involves
proteins, protein and DNA modifications and RNAs (58); therefore, further studies should
consider the potential synergistic effect of LV with other gene
expression factors. LV affected the expression of the EGFP gene
induced by both the ZORF2 and ORF2. There is no LV component in
human embryos, although human ORF2 is also highly expressed in
early human embryos, suggesting that equivalent components of LV
are also present in human early embryos (59). These should be identified in
future.
Supplementary Data
Availability of data and materials
The data generated in the present study may be
found in the Science Data Bank under accession number (31253.11.
sciencedb.12153 and 31253.11. sciencedb.13038) or at the following
URL: doi.org/10.57760/sciencedb.12153 and
doi.org/10.57760/sciencedb.13038).
Authors' contributions
NJ, CGW and WXW conceived and designed the study
and performed experiments. XDW, YZ, LA, ZXS performed experiments
and analyzed and interpretation of data. GZ, XF and YW analyzed and
interpreted the data and reviewed the manuscript. ZJL and XW
designed experiments, wrote the manuscript and analysis data. ZJL
and XW confirm the authenticity of all the raw data. All authors
have read and approved the final manuscript.
Ethics approval and consent to
participate
Experiments were approved by the Committee on
Ethics of Animal Experiments of Hebei Medical University (approval
no. IACUC-Hebmu-2021009).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Acknowledgements
Not applicable.
Funding
The present study was supported by National Natural Science
Foundation of China (grant no. 81771499), Natural Science
Foundation of Hebei Province, China (grant nos. H2018206099 and
H2021206460) and Science and Technology Research Project of
Colleges and Universities in Hebei Province (grant no.
ZC2016057).
References
|
1
|
Percharde M, Lin CJ, Yin Y, Guan J,
Peixoto GA, Bulut-Karslioglu A, Biechele S, Huang B, Shen X and
Ramalho-Santos M: A LINE1-nucleolin partnership regulates early
development and ESC identity. Cell. 174:391–405.e19. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Milioto V, Perelman PL, Paglia L, Biltueva
L, Roelke M and Dumas F: Mapping retrotransposon LINE-1 sequences
into two cebidae species and homo sapiens genomes and a short
review on primates. Genes (Basel). 13:17422022. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Otsu M and Kawai G: Distinct RNA
recognition mechanisms in closely related LINEs from zebrafish.
Nucleosides Nucleotides Nucleic Acids. 38:294–304. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wang F, Chamani IJ, Luo D, Chan K, Navarro
PA and Keefe DL: Inhibition of LINE-1 retrotransposition represses
telomere reprogramming during mouse 2-cell embryo development. J
Assist Reprod Genet. 38:3145–3153. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Tiwari B, Jones AE, Caillet CJ, Das S,
Royer SK and Abrams JM: p53 directly represses human LINE1
transposons. Genes Dev. 34:1439–1451. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kajikawa M, Sugano T, Sakurai R and Okada
N: Low dependency of retrotransposition on the ORF1 protein of the
zebrafish LINE, ZfL2-1. Gene. 499:41–47. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Peterson CL and Hansen JC: Chicken
erythrocyte histone octamer preparation. CSH Protoc.
2008:pdb.prot51122008.PubMed/NCBI
|
|
8
|
Wehbi SS and Zu Dohna H: A comparative
analysis of L1 retrotransposition activities in human genomes
suggests an ongoing increase in L1 number despite an evolutionary
trend towards lower activity. Mob DNA. 12:262021. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Garcia-Cañadas M, Sanchez-Luque FJ,
Sanchez L, Rojas J and Garcia Perez JL: LINE-1 retrotransposition
assays in embryonic stem cells. Methods Mol Biol. 2607:257–309.
2023. View Article : Google Scholar
|
|
10
|
Chang NC, Rovira Q, Wells J, Feschotte C
and Vaquerizas JM: Zebrafish transposable elements show extensive
diversification in age, genomic distribution, and developmental
expression. Genome Res. 32:1408–1423. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kohlrausch FB, Berteli TS, Wang F, Navarro
PA and Keefe DL: Control of LINE-1 expression maintains genome
integrity in germline and early embryo development. Reprod Sci.
29:328–340. 2022. View Article : Google Scholar
|
|
12
|
Lee HJ, Hou Y, Maeng JH, Shah NM, Chen Y,
Lawson HA, Yang H, Yue F and Wang T: Epigenomic analysis reveals
prevalent contribution of transposable elements to cis-regulatory
elements, tissue-specific expression, and alternative promoters in
zebrafish. Genome Res. 32:1424–1436. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Liang X, Hu Y, Feng S, Zhang S, Zhang Y
and Sun C: Heavy chain (LvH) and light chain (LvL) of lipovitellin
(Lv) of zebrafish can both bind to bacteria and enhance
phagocytosis. Dev Comp Immunol. 63:47–55. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Romero S, Laino A, Molina G, Cunningham M
and Garcia CF: Embryonic and post-embryonic development of the
spider Polybetes pythagoricus (Sparassidae): A biochemical point of
view. An Acad Bras Cienc. 94:e202101592022. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Li H and Zhang S: Functions of
vitellogenin in eggs. Results Probl Cell Differ. 63:389–401. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wang H, Sun W, Li Z, Wang X and Lv Z:
Identification and characterization of two critical sequences in
SV40PolyA that activate the green fluorescent protein reporter
gene. Genet Mol Biol. 34:396–405. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Dang Y, Wang F and Liu C: Real-time PCR
array to study the effects of chemicals on the growth
hormone/insulin-like growth factors (GH/IGFs) axis of zebrafish
embryos/larvae. Chemosphere. 207:365–376. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Dong M, Ding Y, Liu Y, Xu Z, Hong H, Sun
H, Huang X, Yu X and Chen Q: Molecular insights of
2,6-dichlorobenzoquinone-induced cytotoxicity in zebrafish embryo:
Activation of ROS-mediated cell cycle arrest and apoptosis. Environ
Toxicol. 38:694–700. 2023. View Article : Google Scholar
|
|
19
|
Holbech H, Andersen L, Petersen GI,
Korsgaard B, Pedersen KL and Bjerregaard P: Development of an ELISA
for vitellogenin in whole body homogenate of zebrafish (Danio
rerio). Comp Biochem Physiol C Toxicol Pharmacol. 130:119–131.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Li Z, Zhang S and Liu Q: Vitellogenin
functions as a multivalent pattern recognition receptor with an
opsonic activity. PLoS One. 3:e19402008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Medina-Gali R, Belló-Pérez M, Ciordia S,
Mena MC, Coll J, Novoa B, Ortega-Villaizán MDM and Perez L: Plasma
proteomic analysis of zebrafish following spring viremia of carp
virus infection. Fish Shellfish Immunol. 86:892–899. 2019.
View Article : Google Scholar
|
|
22
|
Kielkopf CL, Bauer W and Urbatsch IL:
Sodium dodecyl sulfate-polyacrylamide gel electrophoresis of
proteins. Cold Spring Harb Protoc. 2021:pdb. prot1022282021.
View Article : Google Scholar
|
|
23
|
Stein A, Whitlock JP Jr and Bina M: Acidic
polypeptides can assemble both histones and chromatin in vitro at
physiological ionic strength. Proc Natl Acad Sci USA. 76:5000–5004.
1979. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Lusser A and Kadonaga JT: Strategies for
the reconstitution of chromatin. Nat Methods. 1:19–26. 2004.
View Article : Google Scholar
|
|
25
|
Athanikar JN, Badge RM and Moran JV: A
YY1-binding site is required for accurate human LINE-1
transcription initiation. Nucleic Acids Res. 32:3846–3855. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
You C, Ji D, Dai X and Wang Y: Effects of
Tet-mediated oxidation products of 5-methylcytosine on DNA
transcription in vitro and in mammalian cells. Sci Rep. 4:70522014.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Purushothaman K, Das PP, Presslauer C, Lim
TK, Johansen SD, Lin Q and Babiak I: Proteomics analysis of early
developmental stages of zebrafish embryos. Int J Mol Sci.
20:63592019. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ji N, Wu CG, Wang XD, Song ZX, Wu PY, Liu
X, Feng X, Zhang XM, Wang XF and Lv ZJ: Anti-aging effects of Alu
antisense RNA on human fibroblast senescence through the MEK-ERK
pathway mediated by KIF15. Curr Med Sci. 43:35–47. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Elshafie NO, Gribskov M, Lichti NI,
Sayedahmed EE, Childress MO and Dos Santos AP: miRNome expression
analysis in canine diffuse large B-cell lymphoma. Front Oncol.
13:12386132023. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Guo M, Yang F, Zhu L, Wang L, Li Z, Qi Z,
Fotopoulos V, Yu J and Zhou J: Loss of cold tolerance is conferred
by absence of the WRKY34 promoter fragment during tomato evolution.
Nat Commun. 15:66672024. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Wei W, Cheng B, Yang X, Chu X, He D, Qin
X, Zhang N, Zhao Y, Shi S, Cai Q, et al: Single-cell multiomics
analysis reveals cell/tissue-specific associations in bipolar
disorder. Transl Psychiatry. 14:3232024. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Langmead B and Salzberg SL: Fast
gapped-read alignment with Bowtie 2. Nat Methods. 9:357–359. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Zhan Y, Yin A, Su X, Tang N, Zhang Z, Chen
Y, Wang W and Wang J: Interpreting the molecular mechanisms of
RBBP4/7 and their roles in human diseases (Review). Int J Mol Med.
53:482024. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Farhana R, Lei R, Pham K, Derrien V,
Cedeño J, Rodriquez V, Bernad S, Lima FF and Miksovska J: Globin X:
A highly stable intrinsically hexacoordinate globin. J Inorg
Biochem. 236:1119762022. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Li C, Tan XF, Lim TK, Lin Q and Gong Z:
Comprehensive and quantitative proteomic analyses of zebrafish
plasma reveals conserved protein profiles between genders and
between zebrafish and human. Sci Rep. 6:243292016. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Yilmaz O, Patinote A, Nguyen TV, Com E,
Lavigne R, Pineau C, Sullivan CV and Bobe J: Scrambled eggs:
Proteomic portraits and novel biomarkers of egg quality in
zebrafish (Danio rerio). PLoS One. 12:e01880842017. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Moyano TC, Gutiérrez RA and Alvarez JM:
Genomic footprinting analyses from DNase-seq data to construct gene
regulatory networks. Methods Mol Biol. 2328:25–46. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Carmignac V, Barberet J, Iranzo J, Quéré
R, Guilleman M, Bourc'his D and Fauque P: Effects of assisted
reproductive technologies on transposon regulation in the mouse
pre-implanted embryo. Hum Reprod. 34:612–622. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Navarro PA, Wang F, Pimentel R, Robinson
LG Jr, Berteli TS and Keefe DL: Zidovudine inhibits telomere
elongation, increases the transposable element LINE-1 copy number
and compromises mouse embryo development. Mol Biol Rep.
48:7767–7773. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Han JS and Boeke JD: A highly active
synthetic mammalian retrotransposon. Nature. 429:314–318. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Zhang P, Ludwig AK, Hastert FD, Rausch C,
Lehmkuhl A, Hellmann I, Smets M, Leonhardt H and Cardoso MC: L1
retrotransposition is activated by Ten-eleven-translocation protein
1 and repressed by methyl-CpG binding proteins. Nucleus. 8:548–562.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Zhang S, Dong Y and Cui P: Vitellogenin is
an immunocompetent molecule for mother and offspring in fish. Fish
Shellfish Immunol. 46:710–715. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Veil M, Yampolsky LY, Grüning B and
Onichtchouk D: Pou5f3, SoxB1, and Nanog remodel chromatin on high
nucleosome affinity regions at zygotic genome activation. Genome
Res. 29:383–395. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Wang J, Zhang X, Shan R, Ma S, Tian H,
Wang W and Ru S: Lipovitellin as an antigen to improve the
precision of sandwich ELISA for quantifying zebrafish (Danio rerio)
vitellogenin. Comp Biochem Physiol C Toxicol Pharmacol.
185-186:87–93. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Thompson JR and Banaszak LJ: Lipid-protein
interactions in lipovitellin. Biochemistry. 41:9398–9409. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Ramos KS, Bojang P and Bowers E: Role of
long interspersed nuclear element-1 in the regulation of chromatin
landscapes and genome dynamics. Exp Biol Med (Maywood).
246:2082–2097. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Tian T, Wang L, Shen Y, Zhang B, Finnell
RH and Ren A: Hypomethylation of GRHL3 gene is associated with the
occurrence of neural tube defects. Epigenomics. 10:891–901. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Desai K, Spikings E and Zhang T: Effect of
chilling on sox2, sox3 and sox19a gene expression in zebrafish
(Danio rerio) embryos. Cryobiology. 63:96–103. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Fang F, Chen D, Basharat AR, Poulos W,
Wang Q, Cibelli JB, Liu X and Sun L: Quantitative proteomics
reveals the dynamic proteome landscape of zebrafish embryos during
the maternal-to-zygotic transition. iScience. 27:1099442024.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Lindeman LC, Winata CL, Aanes H, Mathavan
S, Alestrom P and Collas P: Chromatin states of
developmentally-regulated genes revealed by DNA and histone
methylation patterns in zebrafish embryos. Int J Dev Biol.
54:803–813. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Sokolova M and Vartiainen MK: Chromatin
immunoprecipitation experiments from Drosophila ovaries. Methods
Mol Biol. 2626:335–351. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Popchock AR, Larson JD, Dubrulle J, Asbury
CL and Biggins S: Direct observation of coordinated assembly of
individual native centromeric nucleosomes. EMBO J. 42:e1145342023.
View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Pallarès-Albanell J, Ortega-Flores L,
Senar-Serra T, Ruiz A, Abril JF, Rossello M and Almudi I: Gene
regulatory dynamics during the development of a paleopteran insect,
the mayfly Cloeon dipterum. bioRxiv. May 17–2024.Epub ahead of
print.
|
|
54
|
Muto Y, Wilson PC, Ledru N, Wu H, Dimke H,
Waikar SS and Humphreys BD: Single cell transcriptional and
chromatin accessibility profiling redefine cellular heterogeneity
in the adult human kidney. Nat Commun. 12:21902021. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Jiang Z and Zhang B: On the role of
transcription in positioning nucleosomes. PLoS Comput Biol.
17:e10085562021. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Zhao M, Wang Z, Yung S and Lu Q:
Epigenetic dynamics in immunity and autoimmunity. Int J Biochem
Cell Biol. 67:65–74. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Hocher A, Laursen SP, Radford P, Tyson J,
Lambert C, Stevens KM, Montoya A, Shliaha PV, Picardeau M, Sockett
RE, et al: Histones with an unconventional DNA-binding mode in
vitro are major chromatin constituents in the bacterium
Bdellovibrio bacteriovorus. Nat Microbiol. 8:2006–2019. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Wang SH, Liu L, Bao KY, Zhang YF, Wang WW,
Du S, Jia NE, Suo S, Cai J, Guo JF and Lv G: EZH2 contributes to
anoikis resistance and promotes epithelial ovarian cancer
peritoneal metastasis by regulating m6A. Curr Med Sci. 43:794–802.
2023. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Guo H, Zhu P, Yan L, Li R, Hu B, Lian Y,
Yan J, Ren X, Lin S, Li J, et al: The DNA methylation landscape of
human early embryos. Nature. 511:606–610. 2014. View Article : Google Scholar : PubMed/NCBI
|