Introduction
Melanogenesis is the production of melanin pigments
by melanocytes in the epidermis and is a natural process that
determines skin color and provides protection against ultraviolet
(UV) radiation (1-3). When exposed to UVB radiation,
keratinocytes in the skin secrete α-melanocyte-stimulating hormone
(α-MSH) (4,5), which binds to the melanocortin 1
receptor (MC1R) on melanocytes, leading to intracellular cAMP
production (6,7). Elevation of intracellular cAMP
levels stimulates transcriptional factors, such as
microphthalmia-associated transcription factor (MITF) (8,9)
and cAMP response element-binding protein (CREB) through the
cAMP-dependent protein kinase (PKA) (9-12). MITF is a transcription factor
that binds to the promoter region of tyrosinase in melanogenic
genes and upregulates their expression. Tyrosinase is a key enzyme
that has a crucial role in regulating melanin synthesis (13,14). Inhibitors targeting tyrosinase
can modulate melanin production, and its expression and activity
have significant roles in regulating pigmentation levels (15). This mechanism is part of the
skin's natural defense system, aiming to protect the skin from UV
radiation by enhancing melanin production in response to UVB
exposure (16,17). Although production of melanin is
a natural and protective response to UVB exposure, excessive or
uneven melanin production can cause various effects, such as
hyperpigmentation, dark spots, and an uneven skin tone.
Hyperpigmentation may be a symptom of underlying skin conditions,
such as melasma or post-inflammatory hyperpigmentation. Specific
phytochemicals have been shown to modulate the melanogenesis
process, which offers a potential way to mitigate these effects. By
targeting key enzymes and signaling pathways involved in melanin
synthesis, these agents can help reduce the appearance of
hyperpigmentation and promote a more even skin tone. Thus,
increased understanding of the intricate molecular mechanisms of
how these agents modulate melanogenesis is essential for
comprehending melanin's role in skin coloration and its
overproduction, which can lead to such hyperpigmentation
disorders.
Among the various sources of herbal plants,
Centella asiatica (CA) has gained significant attention for
its potential benefits for skin health. CA is a tropical medicinal
plant belonging to the Apiaceae family that thrives in warm and
humid climates and is primarily found in Asia, India, Madagascar,
China, Malaysia and Indonesia (18). A myth says that tigers would roll
around in CA to heal their wounds when injured, leading to the
plant being called 'Tiger grass' (19). CA is a common medicinal herb with
a rich history in traditional medicine and has recently gained
attention for its potential effects on skin health. Previous
research trends in CA include improvements in skin health and in
antioxidant, anti-aging, anti-inflammatory and pharmacological
effects as well as integration with nanotechnology (19-21). CA's diverse applications in skin
care, aging, and other industries under-score its growing
significance and the expanding research interest in its benefits
for overall well-being since it has been widely used in traditional
medicine and cosmetics for its various skin benefits, including
wound healing, anti-aging and skin-whitening effects (22-24). These properties are mainly
attributed to the presence of triterpenoids, such as madecassoside
and asiaticoside, which are typically extracted into organic
solvents. Giant CA (GCA), recently registered by the Korea Forest
Service (2022), was developed to increase the content of the active
ingredients in CA, enhance its size, and improve its resistance to
environmental stresses, such as temperature changes and pests. Our
previous study (Seo et al, unpublished data) found that a
water extract of GCA showed potential results in modulating skin
troubles. Additionally, GCA has been found to possess antioxidant
abilities superior to those of CA. These findings prompted us to
investigate the potential skin-whitening effects of a GCA water
extract and compare it with those of the traditional CA.
The present study aimed to investigate the superior
skin-whitening properties of GCA relative to those of CA and
elucidate the underlying mechanisms of action. A comprehensive
screening of the anti-melanogenic efficacy of the GCA and CA
extracts was conducted, followed by an in-depth comparison of their
effects in both B16F10 melanoma cells and 3D human skin-equivalent
models. It was also intended to explore the molecular mechanisms
behind GCA's skin-whitening effects, focusing on key signaling
pathways and regulatory targets involved in melanogenesis. By
elucidating the specific molecular targets of GCA, it was aimed to
provide a strong foundation for its potential industrial
applications in the cosmetic and pharmaceutical sectors.
Materials and methods
Plant materials and extraction
methods
GCA and CA dried leaves were cultivated under
identical conditions for the same duration as previously described
(25) and preserved at -40°C
under regulated conditions. These dried samples were chopped into
tiny pieces that were pulverized into a fine powder with an RT-34
mixer from Rong Tsong Precision Technology. The powder was then
filtered through a ~20-mesh sieve. To perform the extraction, 300
mg of the powdered GCA and CA samples were immersed in 30 ml of
distilled water at 60°C for 30 min. Following extraction, the
solution was centrifuged at 3,000 × g for 10 min at room
temperature, filtered through Whatman™ filter paper No. 4 (Cytiva),
and then freeze dried.
Reagents
Dulbecco's modified eagle medium was purchased from
Hyclone; Cytiva. A solution of penicillin and streptomycin was
purchased from CellGro by Mediatech (http://www.cellgro.com). Fetal bovine serum was
purchased from Seradigm (https://us.vwr.com/cms/avantor_seradigm). The antibody
against tyrosinase (cat. no. AB170905) was obtained from Abcam.
Antibodies against MITF (cat. no. AB59201), phospho-PKA (p-PKA;
cat. no. 4781S), PKA (cat. no. 4782S), p-CREB (cat. no. 9198S) and
CREB (cat. no. 9197S) were purchased from Cell Signaling
Technology, Inc. The anti-body against β-actin (cat. no. SC-8432)
was obtained from Santa Cruz Biotechnology, Inc. A protein assay
reagent kit was purchased from Bio-Rad Laboratories, Inc. Arbutin
was purchased from MilliporeSigma and dissolved in dimethyl
sulfoxide.
Measurement of melanin content
Murine melanoma B16F10 cells were obtained from the
Korean Cell Line Bank (Seoul, Republic of Korea). B16F10 cells were
seeded at a density of 2×105 cells per 60 mm2
dish. After overnight incubation at 37°C, the cells were pretreated
with GCA and CA (25-100 µg/ml) for 1 h. Then, 200 nM of
α-MSH was added, and the cells were incubated for 72 h. The
conditioned media containing extracellular melanin was collected
and centrifuged at 13,572 × g for 10 min. Then, the supernatant was
transferred to a 96-well plate, and an Epoch microplate reader from
BioTek Instruments was used to measure the absorbance at 490 nm
(1).
A three-dimensional melanoma cell culture
system
The forced-floating method was used to establish a
three-dimensional melanoma cell-culture system. In the present
study, 1×104 B16F10 cells were cultured in an ultra-low
attachment (ULA) 96-well round plate (SPL Life Sciences) at 37°C.
The next day, the cells were co-treated with 200 nM α-MSH, GCA
(25-100 µg/ml), or arbutin (100 µg/ml). The ULA plate
was incubated for 3 days at 37°C. The melanin content in the 3D
cell cultures was analyzed by measuring the absorbance at 490 nm
(1).
Quantification of the cAMP level
To measure the intracellular cAMP concentration, the
cAMP assay was performed with cell lysate using a cAMP assay kit
(R&D Systems, Inc.) in accordance with the manufacturer's
protocol. B16F10 cells (2×105 cells per 60
mm2 dish) were incubated with the indicated
concentration of GCA (25-100 µg/ml) and α-MSH for 15 min.
Lysates were centrifuged 13,572 × g at 4°C, and then lysate was
used directly. The concentration of cAMP was observed by measuring
the absorbance at 450 nm in a plate reader.
Mushroom tyrosinase inhibition assay
To determine the tyrosinase inhibitory effect,
3,4-dihydroxy-L-phenylalanine (L-DOPA) was used as a substrate. To
assay the tyrosinase inhibition of GCA, 80 µl of distilled
water or 80 µl of L-DOPA, 80 µl of mushroom
tyrosinase (27.8 U/ml) and various concentrations of GCA (25-100
µg/ml) were added to each well of a 24-well plate. Arbutin
(100 µg/ml) was used as a positive control. The sample was
mixed with tyrosinase and a 1 mM L-DOPA substrate to react at 37°C
for 30 min. Tyrosinase activity was measured at 475 nm (2).
Western blot analysis
Cells were lysed in a RIPA lysis solution that
included 10 mM Tris (Ph 7.5), 150 mM NaCl, 5 mM EDTA, 0.1% Triton
X-100, 1 mM DTT, 0.1 mM PMSF, 10% glycerol, and a protease
inhibitor cocktail tablet from GenDEPOT, LLC. BCA protein assay
kits were used to quantify protein concentrations in the lysates
following the manufacturer's protocol. For protein separation, 10
µg of protein samples underwent electrophoresis in a 10%
sodium dodecyl sulfate-polyacrylamide gel electrophoresis system
and were then transferred to PVDF membranes supplied by
MilliporeSigma. Blocking of the membranes was achieved with 5% skim
milk for 1 h at room temperature, followed by overnight incubation
at 4°C with the designated primary antibody (1:1,000 dilution).
After attaching the horseradish peroxidase-conjugated with goat
anti-mouse IgG secondary antibody (cat. no. 1721019) or goat
anti-rabbit IgG (cat. no. 1721019) secondary antibody (both from
Bio-Rad Laboratories, Inc.) at a 1:5,000 dilution for 1 h at room
temperature, an E-9150 Ez-Capture II device by Atto Corporation was
used to detect protein bands (26). The ImageJ software version 1.53k
(National Institutes of Health) was used to measure the relative
density.
Analytical experimental procedures
GCA and CA extracts were applied in a Thermo
Vanquish HPLC system (Thermo Fisher Scientific Inc.) to identify
the phytochemicals. An HPLC Cortecs C18 column (2.1×50 mm, 1.6
µm) from Waters Corporation was used to perform the
chromatographic separations of the metabolites. The flow rate was
set to 0.3 ml/min. A 1-µl aliquot of a 1,000-ppm GCA and CA
extract was injected, and the column oven was set to 45°C. The
mobile phases were 0.1% formic acid in HPLC-grade water (Solvent A)
and 0.1% formic acid in HPLC-grade acetonitrile (Solvent B).
Gradient elution was achieved by running the following gradient
program: 0-0.5 min, 5% B; 0.5-3.5 min, 5-100% B; 3.5-4 min, 100% B;
4-4.1 min, 5% B; and a 2-min hold time followed by a 3-min
re-equilibration to the starting conditions. High-resolution mass
spectrometry (MS) data were obtained on a Thermo TSQ Altis
high-resolution mass spectrometer equipped with a hybrid
quadrupole-Orbitrap mass analyzer. Electrospray ionization (H-ESI)
in positive-ion mode was used to acquire all MS data. The optimized
MS parameters were as follows: Spray voltage maintained statically
at 3,500 V, sheath gas flow rate set at 50 arbitrary units,
auxiliary gas at 10 arbitrary units, and sweep gas at 1 arbitrary
unit. The ion-transfer tube and vaporizer temperatures were
maintained at 325°C and 350°C, respectively. Selected Reaction
Monitoring was conducted in positive-ion mode with a cycle time of
0.5 sec. The resolutions of quadrupole 1 (Q1) and quadrupole 3 (Q3)
were set to 0.7 and 1.2 full-width at half height, respectively,
without the use of a calibrated RF lens. Collision-induced
dissociation was performed with a gas pressure of 1.5 mTorr.
Molecular modeling and docking
simulation
Molecular docking simulations were conducted to
investigate the binding affinity and potential interactions between
the melanocortin-1 receptor (MC1R) and selected ligands. The 3D
structure of MC1R (PDB ID: 7F4D, α-MSH-bound melanocortin-1
receptor) was obtained from the Protein Data Bank (https://www.rcsb.org/), and the 3D structures of the
ligands were sourced from the Drug Bank. Autodock Vina in the
AMdock platform (27) was used
to conduct docking simulations between MC1R and four selected
ligands. A previous study identified α-MSH binding sites at GLU94
and LEU106 on MC1R (28);
therefore, a search space of 60 cubic Angstroms was centered on
these sites. AMdock generated simulations for the 10 most probable
binding configurations from which the results with the lowest
energy were selected. The two compounds were further analyzed using
the MolDock software (version 7.0.0, http://molexus.io/) to calculate their MolDock scores.
The same grid box parameters were applied, and the MolDock
optimizer algorithm was used to generate the scores (29).
In vitro pull-down assay and competition
assay of α-MSH with madecassoside/asiaticoside
B16F10 cellular supernatant (500 µg) was
incubated at 4°C with asiaticoside-Sepharose 4B beads,
madecassoside-Sepharose 4B beads (or Sepharose 4B alone as a
control) (100 µl, 50% slurry) in reaction buffer [50 mM Tris
(pH 7.5), 5 mM EDTA, 150 mM NaCl, 1 mM DTT, 0.01% Nonidet P-40, 2
µg/ml bovine serum albumin, 0.02 mM PMSF and 1X protease
inhibitor mixture] for 24 h at 4°C. For competition assays, α-MSH
(0.2, 2, 20, or 200 µM) was added to the reaction mixture to
a final volume of 500 µl and incubated at 4°C for an
additional 24 h. After incubation, the beads were washed five times
with buffer [50 mM Tris (pH 7.5), 5 mM EDTA, 150 mM NaCl, 1 mM DTT,
0.01% Nonidet P-40 and 0.02 mM PMSF]. The proteins bound to the
beads were then analyzed by immunoblotting.
3D human skin equivalent model
The 3D human skin-equivalent model,
Neoderm®-ME from Tego Science, Inc., consists of human
epidermal keratinocytes and human melanocytes.
Neoderm®-ME was transferred to a 12-well plate in a
maintenance medium (Tego Science, Inc.) containing GCA or arbutin
and incubated at 37°C in 5% CO2 for 7 days. The medium
was changed once every 2 days. On day 7, skin pigmentation was
observed by viewing with an Olympus AX70 light microscope (Olympus
Corporation). Lysate was collected and subsequently centrifuged at
4°C for 10 min at 13,572 × g. BCA assay reagent kits were used to
measure the protein concentration of the lysate following the
manufacturer's protocol to analyze tyrosinase expression.
Fontana-Masson staining
3D human skin-equivalent samples were fixed
overnight in 4% formaldehyde at room temperature, embedded in
paraffin, and then cut into 3-µm-thick sections with a
microtome. The slides were treated with an ammoniacal silver
solution at 56°C for 30 min, followed by rinsing in distilled
water. The slides were then incubated in a 0.2% gold chloride
solution at room temperature for 30 sec and then in a 5% sodium
thiosulfate solution for 2 min. The sections were incubated in
nuclear fast red solution for 5 min and dehydrated three times by
use of fresh absolute alcohol. An AX70 light microscope (Olympus
Corporation) was used to examine the staining results.
Statistical analyses
Statistical analyses were performed using the
SPSS-WIN 12.0K program (SPSS, Inc.). All data are presented as the
mean ± standard deviation or standard error of the mean. Unpaired
Student's t-test was used for single statistical comparisons and
one-way ANOVA followed by Tukey's Honest Significant Difference
(HSD) test for multiple comparisons. Differences were considered
statistically significant for values of P<0.05.
Results
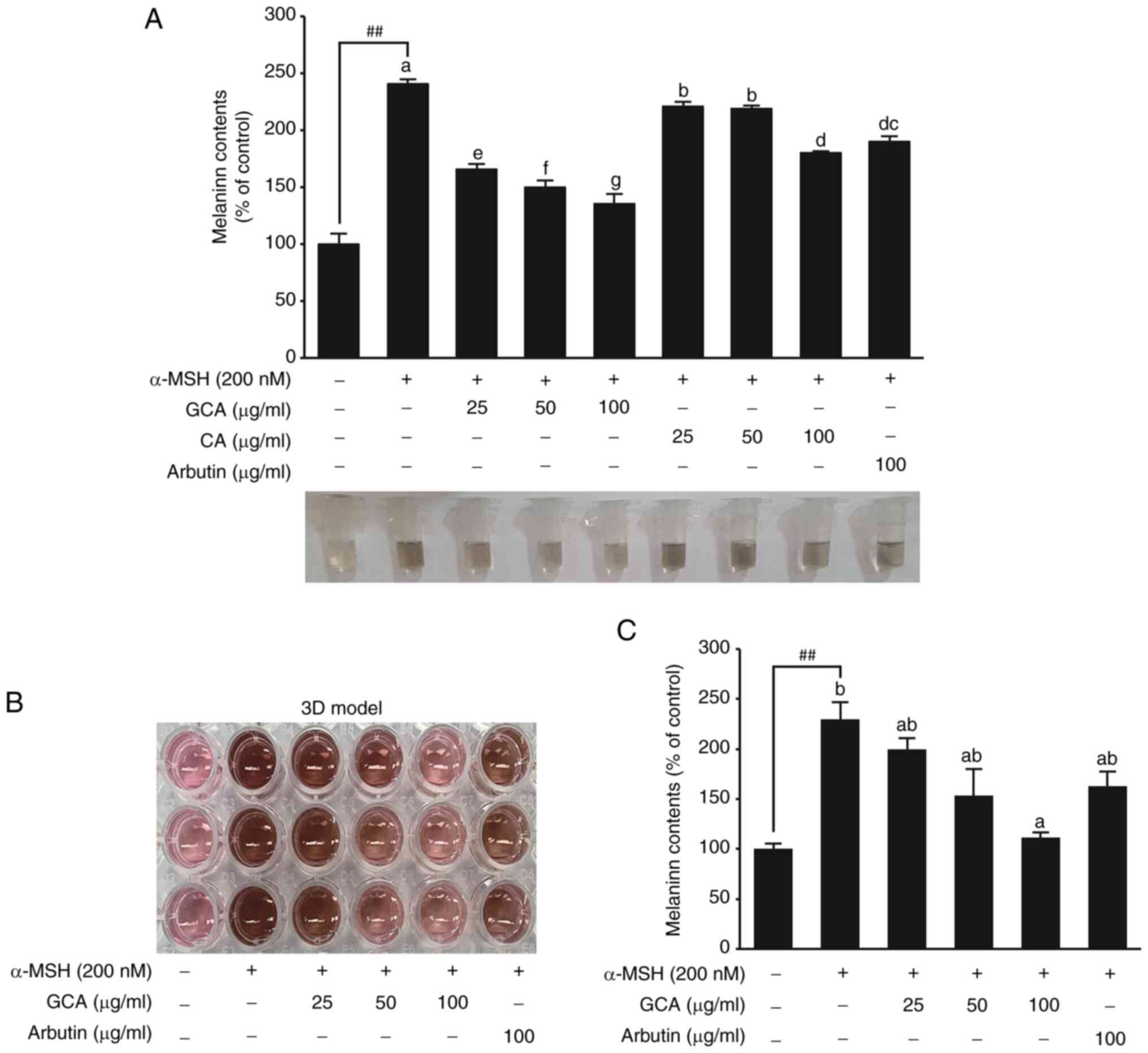
The GCA extract shows superior inhibition
of α-MSH-induced melanin secretion in both 2D and 3D models
Research on B16F10 cells has shown that α-MSH
increases melanin expression through various pathways (30,31). The concentration of α-MSH and
treatment time used in the present study were selected on the basis
of previous research results (32,33). It was found that α-MSH
effectively induced melanin expression up to 2.4-fold relative to
the control. Arbutin was used as a positive control (34,35). Melanin secretion was inhibited
more effectively by the GCA extract than by the CA extract
(Fig. 1A). Especially at a
concentration of 100 µg/ml, the GCA extract exhibited
superior efficacy in skin-whitening improvement, with a 77%
reduction relative to that of α-MSH induction, whereas the CA
extract showed a 45% reduction. To further confirm the
anti-melanogenic effect of GCA, a 3D cell-culture model was used,
which more closely mimics the in vivo skin environment
(1). The 3D model visually
demonstrated the skin-whitening effect of the GCA extract (Fig. 1B). Quantitative analysis of the
melanin content in the 3D model revealed that the GCA extract
reduced melanin production dose-dependently, with the 100
µg/ml concentration demonstrated efficacy comparable to that
of arbutin (Fig. 1C). These
results consistently demonstrated the superior melanin-inhibiting
effects of the GCA extract in both 2D and 3D cell-culture
systems.
The GCA extract inhibits melanogenesis by
modulating the cAMP-PKA-CREB-MITF signaling axis and tyrosinase
activity
These results suggested that GCA extract effectively
inhibits the PKA-CREB-MITF signaling cascade, which is critical for
melanin synthesis. In B16F10 cells, α-MSH significantly elevates
cAMP levels, triggering enhanced melanin synthesis (36). This increase in cAMP activates
PKA, phosphorylating critical proteins in the melanogenesis pathway
(9). The present results showed
that α-MSH treatment significantly increased intracellular cAMP
levels, which in turn activated PKA. In the presence of GCA
extract, a dose-dependent reduction in cAMP levels was observed,
suggesting that GCA modulates this signaling cascade by inhibiting
the α-MSH-induced cAMP increase (Fig. 2A). To further investigate the
effects observed in cells, additional experiments with L-DOPA were
performed to determine how the GCA extract affects tyrosinase
activity. The experiment targeted tyrosinase extracted from
mushrooms and evaluated the inhibitory effect of an GCA extract at
various concentrations. By using L-DOPA as a substrate to measure
the activity of tyrosinase, it was possible to gain a deeper
understanding of how the GCA extract regulates its enzyme's
activity, which is crucial in the melanin production process
(2,37). Particularly at concentrations of
50 µg/ml and 100 µg/ml (Fig. 2B), the GCA extract exhibited
pronounced effects in inhibiting tyrosinase activity. The
inhibitory effect of the GCA extract at 100 µg/ml was
comparable to that of arbutin, a known tyrosinase inhibitor,
indicating that the GCA extract effectively modulated the activity
of enzymes critical for melanin production. Tyrosinase is crucial
for melanin production, and its activity is regulated by various
factors and signaling pathways, including the
MC1R-cAMP-PKA-CREB-MITF axis (28,38). The current investigation focused
on assessing GCA extract's potential to modulate this pathway and
its downstream effects on melanogenesis. Treatment of B16F10
melanoma cells with GCA extract resulted in a dose-dependent
decrease in the phosphorylation of PKA and CREB, as well as reduced
expression levels of MITF and tyrosinase (Fig. 2C).
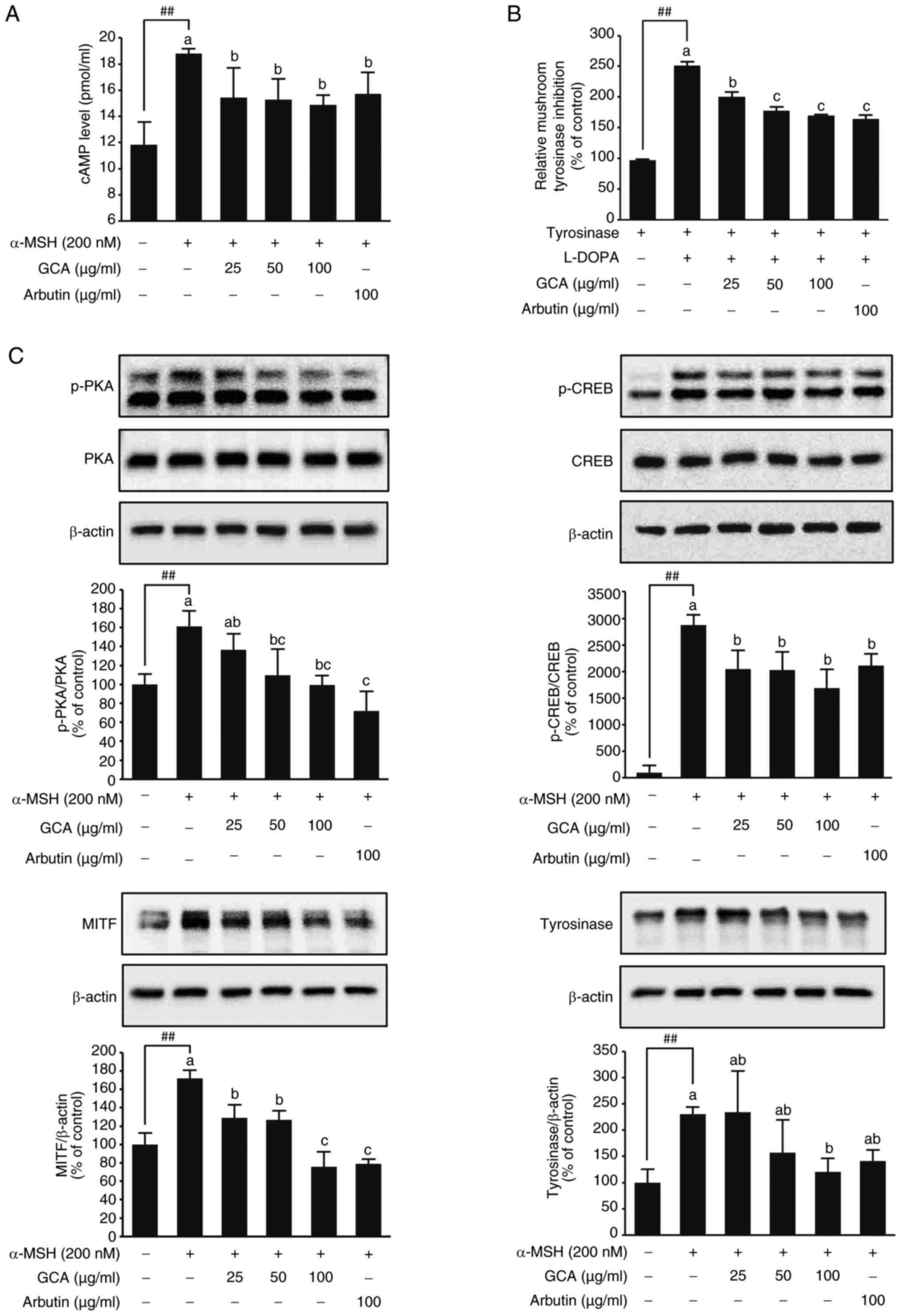 | Figure 2Inhibition of the
melanogenesis-related signaling and tyrosinase activity by the GCA
extract. (A) The GCA extract reduces cAMP levels, indicating its
potent modulatory effect on cAMP-dependent pathways critical for
melanogenesis. ##P<0.01 between the control and
α-MSH. Data are presented as mean values ± SD (n=3). Mean values
with different letters indicate statistically significant
differences among the treatment groups, including the α-MSH-induced
group, as determined by one-way ANOVA followed by Tukey's HSD test
(P<0.05). (B) Concentration-dependent inhibitory effect of the
GCA extract and arbutin on mushroom tyrosinase activity.
Significant differences were observed between the tyrosinase and
tyrosinase group + L-DOPA group (##P<0.01). Moreover,
the addition of GCA at concentrations of 25, 50 and 100
µg/ml and arbutin 100 µg/ml to the tyrosinase +
L-DOPA group significantly reduced the relative mushroom tyrosinase
activity compared with that in the tyrosinase + L-DOPA group, as
determined by one-way ANOVA followed by Tukey's HSD test
(P<0.05). (C) Effects of GCA extract on the expression and
phosphorylation of PKA, CREB, MITF and tyrosinase in B16F10 cells.
Protein expression levels were determined in cell lysates using
specific antibodies by immunoblotting. β-actin was used as a
loading control. Representative western blots from three
independent experiments are shown (n=3). Significant differences
between untreated control and α-MSH-induced group
(##P<0.01). Mean values with different letters
indicate statistically significant differences among the treatment
groups, including the α-MSH-induced group, as determined by one-way
ANOVA followed by Tukey's HSD test (P<0.05). α-MSH, α-melanocyte
stimulating hormone; CA, Centella asiatica; GCA, Giant CA;
PKA, cAMP-dependent protein kinase; CREB, cAMP response
element-binding protein; MITF, microphthalmia-associated
transcription factor. |
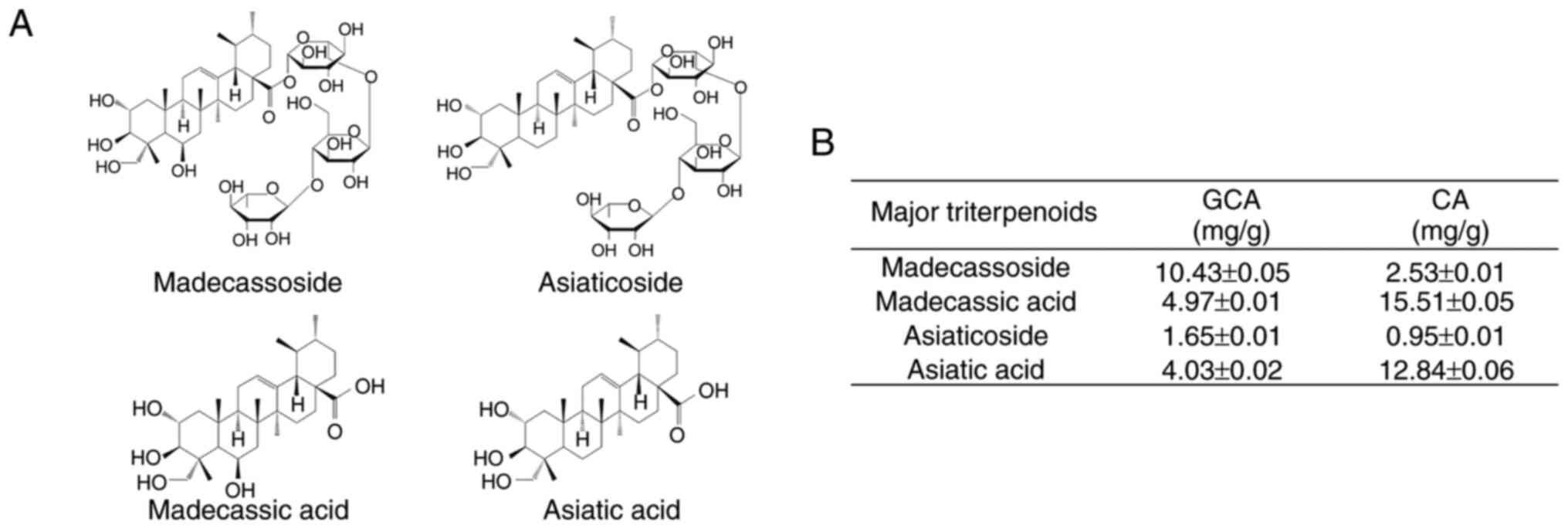
Comparative analysis of triterpenoid
content in GCA and CA extracts
To identify the key components contributing to the
melanin inhibition effect of GCA extract, HPLC-MS/MS analysis of
the major triterpenoids was performed in both the GCA and CA
extracts. The molecular structures of the four main triterpenoids
(madecassoside, asiaticoside, madecassic acid and asiatic acid) are
presented in Fig. 3A.
Quantitative analysis revealed significant differences in the
triterpenoid composition between the GCA and CA extracts (Fig. 3B). Notably, the GCA extract
contained substantially higher levels of madecassoside (10.43±0.05
mg/g) than those in the CA extract (2.53±0.01 mg/g). Similarly,
asiaticoside content was higher in the GCA extract (1.65±0.01 mg/g)
than in the CA extract (0.95±0.01 mg/g). These compounds have been
previously reported to possess anti-melanogenic properties
(39,40). Interestingly, the levels of
madecassic acid and asiatic acid were lower in the GCA extract than
in the CA extract. This differential composition suggests that the
enhanced melanogenesis inhibitory effect of the GCA extract is
primarily attributed to its higher content of madecassoside and
asiaticoside. These findings provide insight into the potential
active components responsible for the superior skin-whitening
effects of the GCA extract observed in the aforementioned
experiments.
Inhibitory effects of madecassoside and
asiaticoside on melanin production and their binding to MC1R
Treatment with asiaticoside and madecassoside
significantly reduced melanin production (Fig. 4A). Asiaticoside showed a more
potent inhibitory effect than that of madecassoside. Conversely,
madecassic acid and asiatic acid had minimal effects on inhibiting
melanin production, which may explain GCA's superior efficacy in
suppressing melanin production considering the higher content of
asiaticoside and madecassoside in GCA than in CA (Fig. 3B). Pull-down assay results
confirmed that asiaticoside and madecassoside directly bound to
MC1R (Fig. 4B and C).
Interestingly, increasing concentrations of α-MSH led to decreased
binding of asiaticoside and madecassoside to MC1R. This finding
suggests that asiaticoside and madecassoside compete with α-MSH for
binding to MC1R.
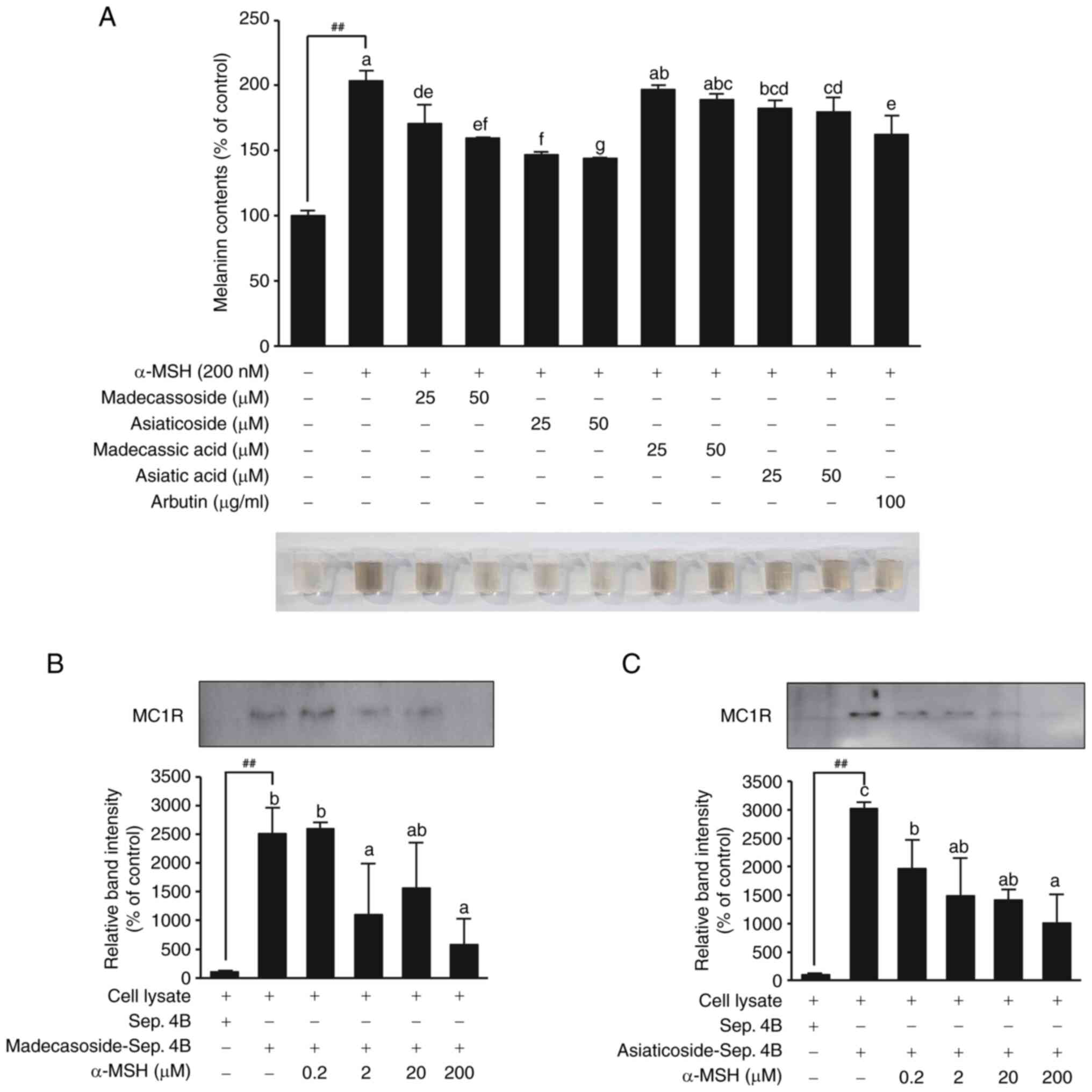 | Figure 4Inhibitory effects of madecassoside
and asiaticoside on melanin synthesis and their direct binding to
MC1R. (A) Inhibitory effects of madecassoside and asiaticoside on
α-MSH-induced melanin production in B16F10 cells. Significant
differences between the untreated control and α-MSH-induced group
(##P<0.01). Mean values with different letters
indicate statistically significant differences among the treatment
groups, including the α-MSH-induced group, as determined by one-way
ANOVA followed by Tukey's HSD test (P<0.05). (B and C)
Confirmation of direct binding between (B) madecassoside, (C)
asiaticoside and MC1R by pull-down assay. Sepharose 4B beads alone
were used as a negative control. Sepharose 4B beads conjugated with
madecassoside or asiaticoside were used to examine binding to MC1R.
Competitive binding between madecassoside, asiaticoside and MC1R
was assessed by treating with increasing concentrations of α-MSH.
The significant differences between the Sepharose 4B beads only
control (lane 1) and the madecassoside- or asiaticoside-conjugated
beads (lane 2) confirmed the binding of madecassoside and
asiaticoside to MC1R (##P<0.01). Evaluation of the
competitive binding between α-MSH and madecassoside or asiaticoside
showed significant differences in MC1R expression between the group
without α-MSH treatment (lane 2) and the groups treated with
increasing concentrations of α-MSH (0.2, 2, 20 and 200 µM;
lanes 3-6). Mean values with different letters indicate
statistically significant differences among the treatment groups,
as determined by one-way ANOVA followed by Tukey's HSD test
(P<0.05). Additionally, differences in MC1R expression at the
same α-MSH concentration were evaluated to compare the extent of
competitive binding with α-MSH between madecassoside and
asiaticoside. α-MSH, α-melanocyte stimulating hormone; MC1R,
melanocortin 1 receptor. |
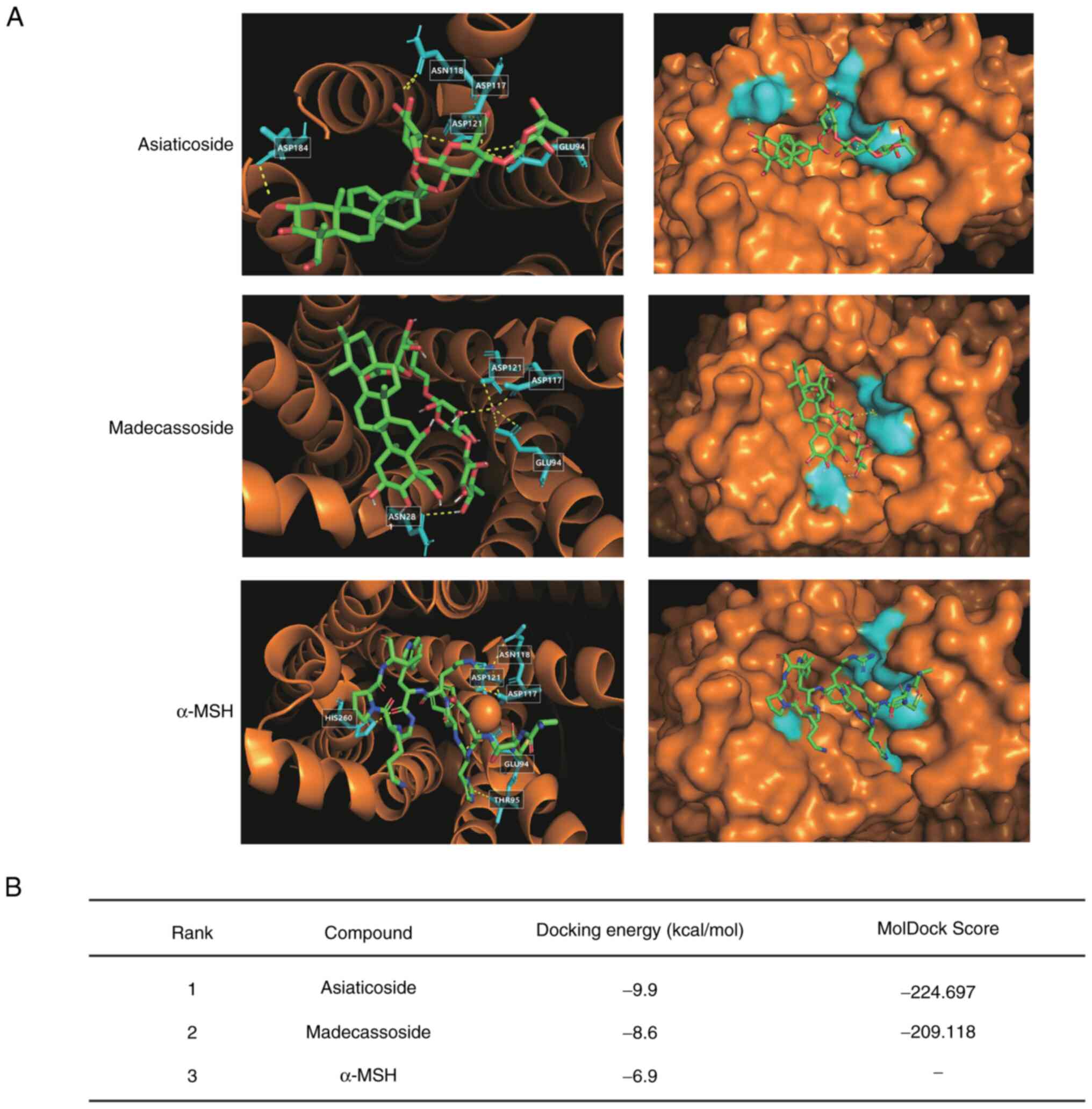
Molecular docking studies on the
interaction between MC1R and two major triterpenoids
To further investigate the binding of asiaticoside
or madecassoside to MC1R, molecular docking studies were performed
using the human MC1R structure. Previous studies have suggested
that the GLU94 of MC1R greatly influences ligand binding and
receptor function (41). The
present docking model results revealed that both asiaticoside and
madecassoside bound to GLU94 of MC1R, with docking energies of -9.9
and -8.6 kcal/mol, respectively (Fig. 5A and B). By contrast, the docking
energy of α-MSH was higher at -6.9 kcal/mol. These results
suggested that asiaticoside and madecassoside may bind more
strongly to MC1R than α-MSH, which is consistent with the findings
from the pull-down assay (Fig. 4B
and C). While the aforementioned Autodock results indicated
that asiaticoside has higher potency than madecassoside, further
analysis using MolDock confirmed this observation. The MolDock
binding scores for asiaticoside were -224.697, which were higher
than those for madecassoside, which scored -209.118. These results
provide stronger evidence of the higher binding affinity of
asiaticoside (Fig. 5B). However,
due to the large molecular weight of α-MSH, it was not possible to
obtain α-MSH MolDock results.
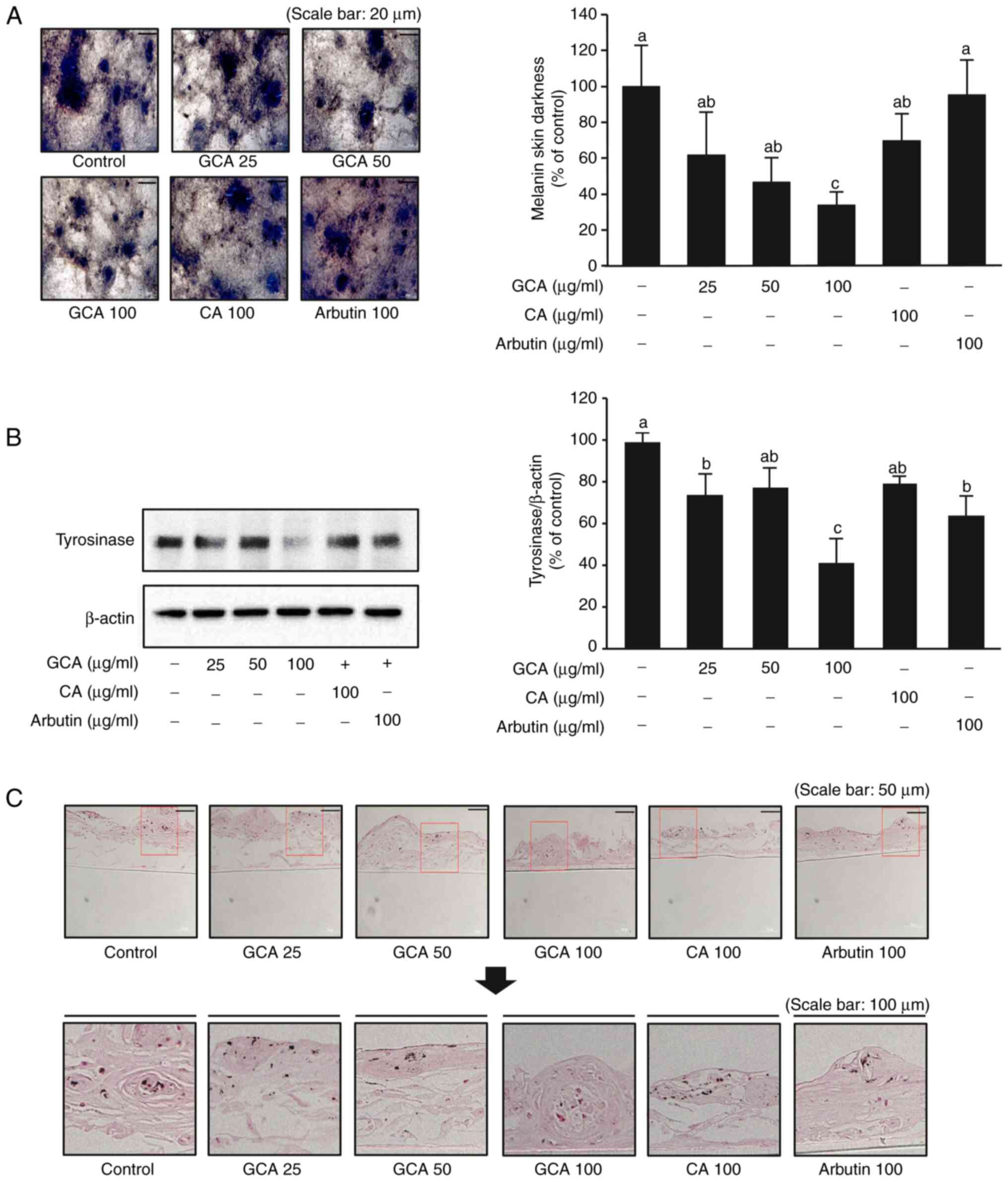
Inhibitory effect of the GCA extract on
melanin synthesis in a 3D human skin-equivalent model
To further validate the anti-melanogenic effects of
the GCA extract in a more physiologically relevant context, a 3D
human skin-equivalent model (Neoderm®-ME) was used. This
model incorporates human melanocytes and exhibits natural
melanogenesis over time, closely mimicking human skin (1). Neoderm®-ME was treated
with 25, 50 and 100 µg/ml GCA extract, 100 µg/ml CA
extract, and arbutin as a positive control. After 7 days of
treatment, the GCA extract at 100 µg/ml significantly
reduced melanin content by 64% relative to that in the control,
outperforming both the CA extract (30% reduction) and arbutin
(Fig. 6A). The expression of
tyrosinase, a critical enzyme in melanin synthesis, was also
markedly decreased in the presence of the GCA extract, particularly
at 100 µg/ml (Fig. 6B).
This reduction in tyrosinase expression is associated with the
observed decrease in melanin production (Fig. 6A). Furthermore, Fontana-Masson
staining, which enables visualization of melanin in tissue
sections, revealed a notable reduction in staining intensity in
samples treated with the GCA extract (Fig. 6C), corroborating the quantitative
data and tyrosinase expression patterns. Collectively, these
findings demonstrated that the GCA extract effectively inhibits
melanogenesis in a 3D human skin-equivalent model, suppressing both
melanin production and tyrosinase expression. These results, in
conjunction with the molecular docking studies and binding assays,
provide compelling evidence that the anti-melanogenic effects of
the GCA extract were superior to those of the CA extract,
highlighting its potential as a natural skin-whitening agent.
Discussion
The present study demonstrated that the GCA extract,
derived from a new species of CA, exhibited efficacy for inhibiting
α-MSH-induced melanin production superior to that of the
traditional CA extract. The enhanced inhibitory effect of the GCA
extract on melanogenesis can be attributed to its higher content of
key components, particularly madecassoside and asiaticoside. The
results of the present study identified that melanin production was
more effectively reduced by the GCA extract than by the CA extract
at various concentrations. HPLC-MS/MS analysis revealed that the
content of madecassoside and asiaticoside was significantly higher
in the GCA extract than in the CA extract. These findings suggest
that the higher concentrations of these active compounds in the GCA
extract contributed to its enhanced inhibition of melanin
production.
To further elucidate the mechanism of action, the
interaction between the two major active compounds, madecassoside
and asiaticoside, and MC1R, a key receptor in melanogenesis, were
investigated. Molecular docking simulations revealed that both
compounds bind to the GLU94 residue of MC1R, with binding
affinities higher than that of α-MSH. Interestingly, despite their
competitive binding to MC1R, madecassoside and asiaticoside did not
promote melanin production, unlike α-MSH. This finding suggests
that these compounds may act as antagonists of MC1R, inhibiting
α-MSH-induced receptor activation and downstream signaling pathways
leading to melanin synthesis. The potential antagonistic activity
of madecassoside and asiaticoside on MC1R is reminiscent of the
mechanism of action of agouti signaling protein (ASIP), a
well-known endogenous antagonist of MC1R. ASIP competes with α-MSH
for binding to MC1R and inhibits cAMP production, resulting in
decreased melanin formation (42). The similar binding patterns and
effects on melanin production observed with madecassoside and
asiaticoside suggest that these compounds may function as MC1R
antagonists, analogous to ASIP, but further studies are needed to
confirm their effect on MC1R signaling and to elucidate the
structural basis of their antagonistic activity. This consistency
between computational predictions and experimental results further
validates the reliability of molecular modeling approach in the
present study.
From an industrial perspective, GCA extracts show
significant potential for incorporation into various skincare
products, particularly in the growing market for natural and
plant-based cosmeceuticals. The use of water extraction methods in
obtaining GCA extracts offers a notable advantage, making the
production process more environmentally friendly and cost-effective
compared with organic solvent extractions (43). Furthermore, GCA contains higher
concentrations of active compounds, specifically madecassoside and
asiaticoside, compared with traditional CA. The present study aimed
to identify the most effective compounds for inhibiting
melanogenesis, and madecassoside and asiaticoside were selected due
to their high concentrations in GCA extract and their
anti-melanogenic properties, which were confirmed through
experimental data (Fig. 3B).
This higher potency could allow for more effective formulations or
the use of lower doses in skincare products. Additionally, the
higher yield of GCA cultivation compared with traditional CA
suggests a more efficient and economically viable production
process (25). These factors
combined could lead to more sustainable and cost-effective
production of skin-whitening ingredients, offering a promising
alternative in the cosmetics and skincare industry. However,
challenges such as maintaining compound stability in formulations,
ensuring consistent extract quality, and scaling up production will
need to be addressed in the product development process. Despite
these challenges, the industrial potential of GCA extracts remains
significant, presenting opportunities for innovation in natural
skincare products.
While the current study provides strong evidence for
the efficacy of GCA extracts in inhibiting melanogenesis, it is
important to note that these findings are primarily based on in
vitro and 3D skin model experiments. Future research directions
could include exploring synergistic effects with other
skin-whitening agents and investigating these compounds' potential
to address additional skin concerns. Further in vivo studies
and clinical trials are crucial to validate the efficacy and safety
of GCA on human skin, fully elucidate its mechanisms of action, and
optimize its application in skincare products. The promising
results from the present study provide a strong foundation for
future investigations and potential industrial applications in the
field of dermatology and cosmetics.
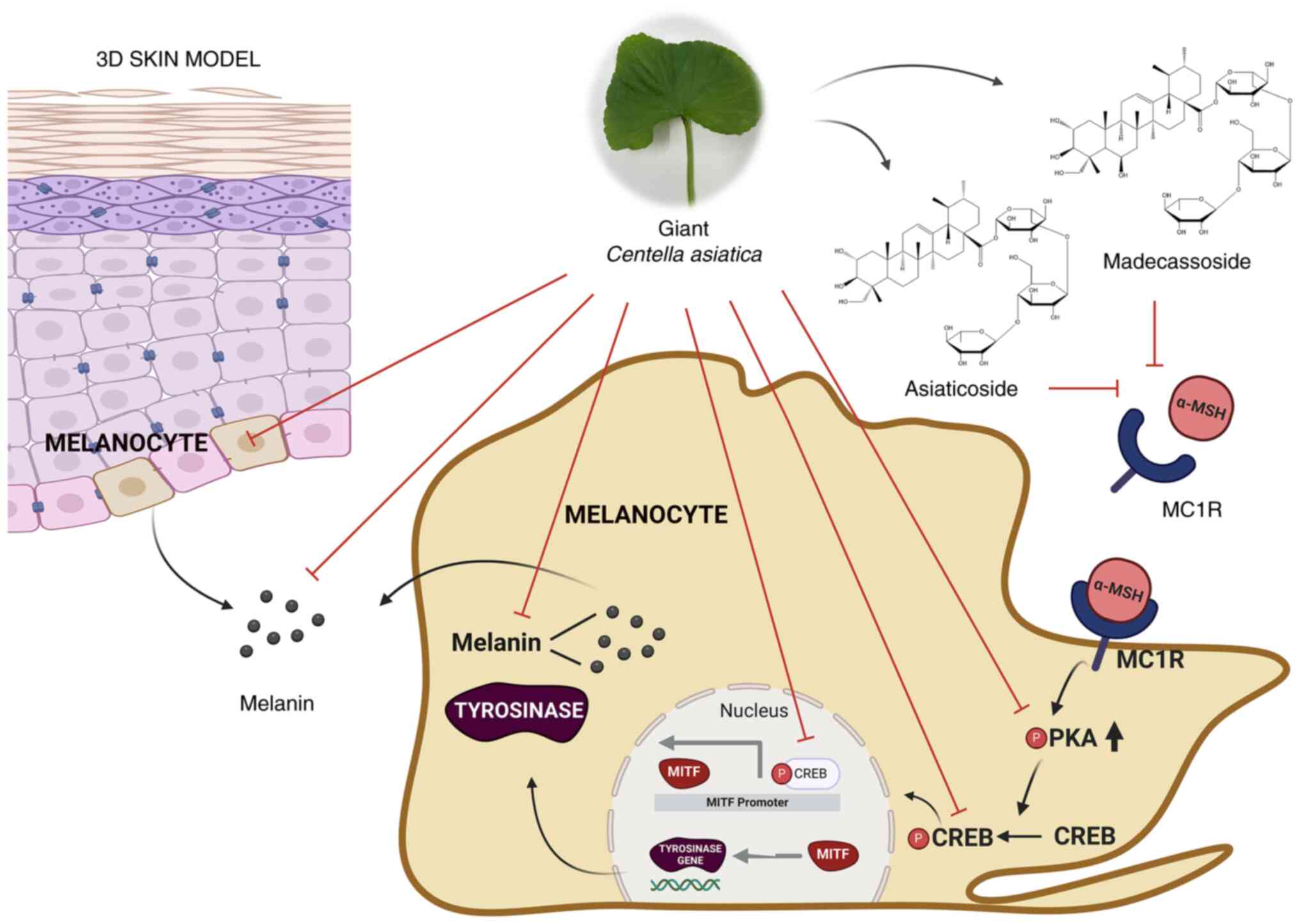
In conclusion, the present study established the GCA
extract to be a potent inhibitor of melanogenesis, with efficacy
superior to that of traditional CA extract. The higher content of
madecassoside and asiaticoside in GCA significantly contributed to
its enhanced skin-whitening effects. Additionally, to the best of
our knowledge, this is the first study to demonstrate that the two
principal compounds, madecassoside and asiaticoside, bind to MC1R
and contribute to the skin-whitening effect through their
interaction with this receptor. The proposed mechanism of action
for GCA's anti-melanogenic effects is summarized in Fig. 7. This discovery is of significant
academic importance as it highlights the unique mechanism by which
these compounds inhibit melanogenesis, thereby offering new
insights into their potential applications in dermatological
treatments. Further research, including clinical trials, is crucial
to validate the efficacy of GCA on human skin whitening and fully
elucidate its mechanisms of action.
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
JS, CJ, SL, HWP, DBS, DSY, TL, JHYP, CHL and KWL
conceived and designed the experiments. JS, CJ, SO and WS performed
the experiments. SL, HWP, DBS, DSY, CHL and KWL provided resources
and funding. JS, TL, JHYP, CHL and KWL prepared and revised the
manuscript. JS, CJ, HWP, DBS, DSY, TL, JHYP, CHL and KWL analyzed
the data and supervised the project. JS, CJ, SO, SL, HWP, DBS, DSY,
WS, JHYP, CHL and KWL confirm the authenticity of all the raw data.
All authors read and approved the final version of the
manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Authors' information
JS, 0000-0001-9680-7068; CJ, 0009-0007-3198-868X;
SO, 0009-0008-1909-8761; SL, 0000-0002-3057-3243;
WS,0000-0002-0903-3701; TL, 0000-0001-6930-9565; JHYP,
0000-0002-5518-4279; CHL, 0000-0002-1907-4400; KWL,
0000-0001-6302-2432.
Acknowledgments
Not applicable.
Funding
The present study was supported by BOBSNU Co. Ltd., ASK Company
Co. Ltd., Brain Korea 21 Plus Program of the Department of
Agricultural Biotechnology, Seoul National University and the Bio
and Medical Technology Development Program of the National Research
Foundation (NRF), funded by the Korean government (MSIT) (grant no.
2020M3H1A1073304). The present study was also supported by the NRF
of Korea (NRF) grant funded by the Korea government (MSIT) (grant
no. RS-2024-00333238).
References
|
1
|
Shon YJ, Kim WC, Lee SH, Hong S, Kim SY,
Park MH, Lee P, Lee J, Park KH, Lim W and Lim TG: Antimelanogenic
potential of brewer's spent grain extract through modulation of the
MAPK/MITF axis. Sustain. Mater. Technol. 38:e007212023.
|
|
2
|
Hong S, Lee S, Sim WJ, Kim WC, Kim SY,
Park MH, Lim W and Lim TG: Ultrasound-assisted pumpkin tendril
extracts inhibits melanogenesis by suppressing the CREB/MITF
signaling pathway in B16F10 melanoma cells, zebrafish, and a human
skin model. J Funct Foods. 109:1058132023. View Article : Google Scholar
|
|
3
|
Ito S; IFPCS: The IFPCS presidential
lecture: A chemist's view of melanogenesis. Pigment Cell Res.
16:230–236. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Videira IF, Moura DF and Magina S:
Mechanisms regulating melanogenesis. An Bras Dermatol. 88:76–83.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Böhm M, Wolff I, Scholzen TE, Robinson
SJ, Healy E, Luger TA, Schwarz T and Schwarz A:
alpha-Melanocyte-stimulating hormone protects from ultraviolet
radiation-induced apoptosis and DNA damage. J Biol Chem.
280:5795–5802. 2005. View Article : Google Scholar
|
|
6
|
García-Borrón JC, Abdel-Malek Z and
Jiménez-Cervantes C: MC1R, the cAMP pathway, and the response to
solar UV: Extending the horizon beyond pigmentation. Pigment Cell
Melanoma Res. 27:699–720. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Dessinioti C, Antoniou C, Katsambas A and
Stratigos AJ: Melanocortin 1 receptor variants: functional role and
pigmentary associations. Photochem Photobiol. 87:978–987. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Park HY, Wu C, Yonemoto L, Murphy-Smith M,
Wu H, Stachur CM and Gilchrest BA: MITF mediates cAMP-induced
protein kinase C-beta expression in human melanocytes. Biochem J.
395:571–578. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Shin H, Hong SD, Roh E, Jung SH, Cho WJ,
Park SH, Yoon DY, Ko SM, Hwang BY, Hong JT, et al: cAMP-dependent
activation of protein kinase A as a therapeutic target of skin
hyperpigmentation by diphenylmethylene hydrazinecarbothioamide. Br
J Pharmacol. 172:3434–3445. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhang H, Kong Q, Wang J, Jiang Y and Hua
H: Complex roles of cAMP-PKA-CREB signaling in cancer. Exp Hematol
Oncol. 9:322020. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Taylor SS, Kim C, Cheng CY, Brown SH, Wu J
and Kannan N: Signaling through cAMP and cAMP-dependent protein
kinase: Diverse strategies for drug design. Biochim Biophys Acta.
1784:16–26. 2008. View Article : Google Scholar :
|
|
12
|
Ahmed MB, Alghamdi AAA, Islam SU, Lee JS
and Lee YS: cAMP Signaling in Cancer: A PKA-CREB and EPAC-Centric
Approach. Cells. 11:20202022. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hartman ML and Czyz M: MITF in melanoma:
Mechanisms behind its expression and activity. Cell Mol Life Sci.
72:1249–1260. 2015. View Article : Google Scholar :
|
|
14
|
Gelmi MC, Houtzagers LE, Strub T, Krossa I
and Jager MJ: Mitf in normal melanocytes, cutaneous and uveal
melanoma: A delicate balance. Int J Mol Sci. 23:60012022.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Pillaiyar T, Manickam M and Namasivayam V:
Skin whitening agents: Medicinal chemistry perspective of
tyrosinase inhibitors. J Enzyme Inhib Med Chem. 32:403–425. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Mohania D, Chandel S, Kumar P, Verma V,
Digvijay K, Tripathi D, Choudhury K, Mitten SK and Shah D:
Ultraviolet Radiations: Skin defense-damage mechanism. Adv Exp Med
Biol. 996:71–87. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zamudio Díaz DF, Busch L, Kröger M, Klein
AL, Lohan SB, Mewes KR, Vierkotten L, Witzel C, Rohn S and Meinke
MC: Significance of melanin distribution in the epidermis for the
protective effect against UV light. Sci Rep. 14:34882024.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Torbati FA, Ramezani M, Dehghan R, Amiri
MS, Moghadam AT, Shakour N, Elyasi S, Sahebkar A and Emami SA:
Ethnobotany, phytochemistry and pharmacological features of
Centella asiatica: A comprehensive review. Adv Exp Med Biol.
1308:451–499. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Bylka W, Znajdek-Awiżeń P,
Studzińska-Sroka E and Brzezińska M: Centella asiatica in
cosmetology. Postepy Dermatol Alergol. 30:46–49. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Sun B, Wu L, Wu Y, Zhang C, Qin L, Hayashi
M, Kudo M, Gao M and Liu T: Therapeutic potential of Centella
asiatica and its triterpenes: A review. Front Pharmacol.
11:5680322020. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Park KS: Pharmacological effects of
Centella asiatica on skin diseases: Evidence and possible
mechanisms. Evid Based Complement Alternat Med. 2021:54626332021.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Idana F, Putra WAAG and Winarti NW:
Centella asiatica extract cream inhibited microphthalmia-associated
transcription factor (MITF) expression and prevented melanin amount
increase in Guinea pig skin exposed to ultraviolet-B. Neurologico
Spinale Medico Chirurgico. 5:27–31. 2022.
|
|
23
|
Bylka W, Znajdek-Awiżeń P,
Studzińska-Sroka E, Dańczak-Pazdrowska A and Brzezińska M: Centella
asiatica in dermatology: An overview. Phytother Res. 28:1117–1124.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Gohil KJ, Patel JA and Gajjar AK:
Pharmacological review on Centella asiatica: A potential herbal
cure-all. Indian J Pharm Sci. 72:546–556. 2010. View Article : Google Scholar
|
|
25
|
Oh S, Park S, Lee S, Park Y, Jang KI, Yu
KW, Kim D and Shin H: Comparison of growth characteristics and
physiological activity of two Centella asiatica cultivars in
greenhouse soil culture. J Bio-Environ Control. 30:351–358. 2021.
View Article : Google Scholar
|
|
26
|
Park H, Seo JW, Lee TK, Kim JH, Kim JE,
Lim TG, Park JHY, Huh CS, Yang H and Lee KW: Ethanol extract of
Yak-Kong fermented by lactic acid bacteria from a Korean infant
markedly reduces matrix metallopreteinase-1 expression induced by
solar ultraviolet irradiation in human keratinocytes and a 3D skin
model. Antioxidants (Basel). 10:2912021. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Valdés-Tresanco MS, Valdés-Tresanco ME,
Valiente PA and Moreno E: AMDock: A versatile graphical tool for
assisting molecular docking with Autodock Vina and Autodock4. Biol
Direct. 15:122020. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kim JH, Lee JE, Kim T, Yeom MH, Park JS,
di Luccio E, Chen H, Dong Z, Lee KW and Kang NJ: 7, 3′,
4′-trihydroxyisoflavone, a metabolite of the soy isoflavone
daidzein, suppresses α-melanocyte-stimulating hormone-induced
melanogenesis by targeting melanocortin 1 receptor. Front Mol
Biosci. 7:5772842020. View Article : Google Scholar
|
|
29
|
Javed A, Alam MB, Naznin M, Ahmad R, Lee
CH, Kim S and Lee SH: RSM-and ANN-based multifrequency ultrasonic
extraction of polyphenol-rich Sargassum horneri extracts exerting
antioxidative activity via the regulation of MAPK/Nrf2/HO-1
machinery. Antioxidants (Basel). 13:6902024. View Article : Google Scholar
|
|
30
|
Nam G, An SK, Park IC, Bae S and Lee JH:
Daphnetin inhibits α-MSH-induced melanogenesis via PKA and ERK
signaling pathways in B16F10 melanoma cells. Biosci Biotechnol
Biochem. 86:596–609. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Yoon Y, Bae S, Kim TJ, An S and Lee JH:
Nodakenin inhibits melanogenesis Via the ERK/MSK1 Signaling
Pathway. Pharmazie. 78:6–12. 2023.PubMed/NCBI
|
|
32
|
Bahraman AG, Jamshidzadeh A, Keshavarzi M,
Arabnezhad MR, Mohammadi H and Mohammadi-Bardbori A:
α-Melanocytestimulating hormone triggers melanogenesis via
activation of the aryl hydrocarbon receptor pathway in B16F10 mouse
melanoma cells. Int J Toxicol. 40:153–160. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Seo GY, Ha Y, Park AH, Kwon OW and Kim YJ:
Leathesia difformis extract inhibits α-MSH-induced melanogenesis in
B16F10 cells via down-regulation of CREB signaling pathway. Int J
Mol Sci. 20:5362019. View Article : Google Scholar
|
|
34
|
Kim DH, Shin DW and Lim BO: Fermented
Aronia melanocarpa inhibits melanogenesis through dual mechanisms
of the PI3K/AKT/GSK-3β and PKA/CREB pathways. Molecules.
28:29812023. View Article : Google Scholar
|
|
35
|
Kim MJ, Mohamed EA, Kim DS, Park MJ, Ahn
BJ, Jeung EB and An BS: Inhibitory effects and underlying
mechanisms of Artemisia capillaris essential oil on melanogenesis
in the B16F10 cell line. Mol Med Rep. 25:1132022. View Article : Google Scholar :
|
|
36
|
Wu PY, You YJ, Liu YJ, Hou CW, Wu CS, Wen
KC, Lin CY and Chiang HM: Sesamol inhibited melanogenesis by
regulating melanin-related signal transduction in B16F10 cells. Int
J Mol Sci. 19:11082018. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Lim YJ, Lee EH, Kang TH, Ha SK, Oh MS, Kim
SM, Yoon TJ, Kang C, Park JH and Kim SY: Inhibitory effects of
arbutin on melanin biosynthesis of alpha-melanocyte stimulating
hormone-induced hyperpigmentation in cultured brownish guinea pig
skin tissues. Arch Pharm Res. 32:367–373. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Otręba M, Rok J, Buszman E and Wrześniok
D: Regulation of melanogenesis: the role of cAMP and MITF. Postepy
Hig Med Dosw (Online). 66:33–40. 2012.In Polish.
|
|
39
|
Kwon KJ, Bae S, Kim K, An IS, Ahn KJ, An S
and Cha HJ: Asiaticoside, a component of Centella asiatica,
inhibits melanogenesis in B16F10 mouse melanoma. Mol Med Rep.
10:503–507. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Jung E, Lee JA, Shin S, Roh KB, Kim JH and
Park D: Madecassoside inhibits melanin synthesis by blocking
ultraviolet-induced inflammation. Molecules. 18:15724–15736. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Prusis P, Schiöth HB, Muceniece R, Herzyk
P, Afshar M, Hubbard RE and Wikberg JE: Modeling of the
three-dimensional structure of the human melanocortin 1 receptor,
using an automated method and docking of a rigid cyclic
melanocyte-stimulating hormone core peptide. J Mol Graph Model.
15:307–317.334. 1997. View Article : Google Scholar
|
|
42
|
Yang CW, Ran JS, Yu CL, Qiu MH, Zhang ZR,
Du HR, Li QY, Xiong X, Song XY, Xia B, et al: Polymorphism in MC1R,
TYR and ASIP genes in different colored feather chickens. 3
Biotech. 9:2032019. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Gallina L, Cravotto C, Capaldi G, Grillo G
and Cravotto G: Plant extraction in water: Towards highly efficient
industrial applications. Processes. 10:22332022. View Article : Google Scholar
|