Introduction
Severe acute pancreatitis (SAP) is one of the most
common and serious gastrointestinal inflammatory diseases, which is
associated with high rates of morbidity and mortality. Notably, it
has been reported that 20-30% of patients who develop SAP have a
mortality rate as high as 20-40% globally (1,2).
The basis of AP is inflammation, in which pancreatic
enzymes are activated and then cause inflammation of the pancreatic
tissue, which locally manifests as pancreatic digestion, edema,
bleeding and pancreatic necrosis. The injured pancreatic tissue
releases a large amount of pro-inflammatory cytokines, including
tumor necrosis factor α (TNF-α), interleukin (IL)-6 and IL-1β,
triggering a cascade of inflammatory mediators, which develops
rapidly from local inflammation into systemic inflammatory response
syndrome (SIRS) (3).
Inflammation is present throughout the pathophysiological course of
AP; therefore, if SIRS is not controlled, sepsis, septic shock and
multiple organ dysfunction syndrome (MODS), resulting from the
presence of infectious factors (such as surgical infection), can
occur (4,5).
Autophagy is an evolutionarily conserved
self-protection mechanism that is closely related to the occurrence
and development of inflammation. Under normal conditions, autophagy
is maintained at a low level; however, starvation, stress,
infection, immune disorders and other conditions can induce
increased autophagy (6,7). Previous studies have shown that
autophagy is involved in the inflammatory process of SAP (8,9),
and the inhibition of key molecules in the process of autophagy can
reduce the degree of inflammation in model animals with SAP
(10).
Although the activation of trypsin (TP) in
pancreatic tissue is considered a key cause of pancreatic tissue
inflammation, experimental results have suggested that the
activation of TP alone is insufficient, and lipopolysaccharide
(LPS) from the gut may be involved in the development of
pancreatitis (11-13). However, whether there is a
synergistic effect between TP and LPS remains unclear.
Artesunate (AS) is a World Health
Organization-approved first-line drug for the treatment of severe
malaria in adults and children (14). In addition to its anti-malarial
effects, in recent years, it has been shown that AS can exert
notable anti-inflammatory and antitumor effects (15). In our previous study, AS was
revealed to inhibit the Toll-like receptor 4 (TLR4)/nuclear
factor-κB (NF-κB) signaling pathway, which could decrease digestive
enzyme activity and pro-inflammatory cytokine expression, thereby
improving survival in rats with SAP (16). However, the detailed molecular
mechanism of AS in AP is not yet clear.
In the present study, the effects of AS on an animal
model of AP induced by cerulein (CR) and LPS were investigated.
Subsequently, the synergistic effects of TP and LPS on mouse
macrophages, and CR combined with LPS on acinar cells were
investigated, as well as the synergistic effects and molecular
mechanisms of TP and LPS on mouse macrophages.
Materials and methods
Reagents
AS for injection was purchased from Guilin
Pharmaceutical Co., Ltd. For the in vivo experiments, AS was
dissolved in dimethyl sulfoxide (100%; 0.02 ml/10 g administration
volume, determined to have no negative effect in preliminary
experiments); for the in vitro experiments, AS was dissolved
in 1 ml 5% sodium bicarbonate and then diluted in 0.9% sterile
normal saline (NS).
LPS (from Escherichia coli O55:B5) was
purchased from MilliporeSigma. CR was purchased from MedChemExpress
(cat. no. HY-A0190). LY294002 were purchased from MedChemExpress
(cat. no. HY-10108). TP was purchased from Nanjing Jiancheng
Bioengineering Institute (cat. no. I015-1-1). The primary
antibodies used for western blotting (WB) and immunofluorescence
staining were: Anti-Beclin-1 (cat. no. 3738), anti-TNF receptor
associated factor 6 (TRAF6; cat. no. 8028), anti-microtubule
associated protein 1 light chain 3 (LC3; cat. no. 2775),
anti-myeloid differentiation primary response 88 (MyD88; cat. no.
4283), anti-TLR4 (cat. no. 38519), anti-ATG16L1 (cat. no. 8089),
anti-β-actin (cat. no. 4967) and anti-GAPDH (cat. no. 2118) (all
from Cell Signaling Technology, Inc.). The secondary antibodies
were horseradish peroxidase (HRP)-conjugated goat anti-rabbit (cat.
no. SA00001-2) or anti-mouse (cat. no. SA00001-1) (both Proteintech
Group, Inc.). The primary antibodies used for immunohistochemistry
were: Anti-Beclin-1 (cat. no. A7353; ABclonal Biotech Co., Ltd.),
anti-LC3 (cat. no. 2775; Cell Signaling Technology, Inc.) and
anti-TLR4 (cat. no. ab22048; Abcam).
The enzyme-linked immunosorbent assay (ELISA) kits
used to detect cytokines, including TNF-α (cat. no. BMS607-3TEN)
and IL-6 (cat. no. KMC0061), were purchased from Thermo Fisher
Scientific, Inc. The amylase (cat. no. C016-1-1), TP (cat. no.
A080-2-2) and superoxide dismutase (SOD; cat. no. A001-3-2) assay
kits were purchased from Nanjing Jiancheng Bioengineering
Institute. The total protein extraction kit (cat. no. BB-3101) was
purchased from BestBio Ltd. The 2-step plus Poly-HRP Anti
Mouse/Rabbit IgG Detection System (with DAB solution) (cat. no.
E-IR-R-217) was purchased from Elabscience Biotechnology Co., Ltd.
Anti-rabbit Alexa Fluor 555 immunofluorescent staining kit (cat.
no. P0179) was purchased from Beyotime Institute of Biotechnology.
Autophagic double-labeled adenovirus HBAD-monomeric red fluorescent
protein (mRFP)-green fluorescent protein (GFP)-LC3 (cat. no.
HB-AP210 001) was purchased from Hanheng Biotechnology (Shanghai)
Co., Ltd.
Animals
Kunming (KM) mice (age, 3-4 weeks; weight, 18-22 g)
were obtained from Hunan SJA Laboratory Animal Co., Ltd. All mice
were housed in a pathogen-free facility (temperature, 20-25°C;
humidity, 50-65%) at Zunyi Medical University (Zunyi, China) under
a 12-h artificial light-dark cycle, and with ad libitum
access to food and purified water. All protocols and experimental
procedures involving live animals were approved by the Animal Care
Welfare Committee of Zunyi Medical University [approval no. ZMU
(2020) 2-296]. All of the experiments were conducted in compliance
with the National and Institutional Guidelines for the Care and Use
of Experimental Animals (17).
Cell lines, cell culture and mouse
peritoneal macrophage (PM) purification
The mouse macrophage cell line RAW264.7 and the rat
pancreatic exocrine cell line AR42J were obtained from American
Type Culture Collection. RAW264.7 cells were cultured in Dulbecco's
modified Eagle's medium (DMEM; cat. no. 11965092; Gibco; Thermo
Fisher Scientific, Inc.) supplemented with 10% (v/v) fetal bovine
serum (FBS; Biological Industries; Sartorius AG) at 37°C in 5%
CO2. AR42J cells were cultured in Ham's F-12K (Kaighn's)
medium (F12K; cat. no. 21127022; Gibco; Thermo Fisher Scientific,
Inc.) containing 10% FBS at 37°C in 5% CO2.
Murine PMs from normal male KM mice were isolated
and cultured according to previously published methods (18,19). After each mouse was
intraperitoneally injected with 10 ml NS, PMs were collected by
aspiration, suspended in DMEM, added to a cell culture dish and
incubated for 3 h at 37°C in a humidified incubator containing 5%
CO2. Thereafter, the cells were washed with
phosphate-buffered saline (PBS) to remove floating cells. Adherent
cells were considered to be PMs with ~90% purity. All reagents and
appliances used in the experiments were endotoxin-free.
Establishment of an animal model of AP
and treatment
The mouse model of AP was established by the
intraperitoneal injection of CR (100 μg/kg) once every hour
for 6 h, followed immediately by the intraperitoneal injection of
LPS (10 mg/kg) (20). Briefly,
28 KM mice were divided into four groups (n=6/group): i) The NS
group was intraperitoneally injected with NS (0.1 mg/10 g); ii) the
AP group received CR (100 μg/kg) intraperitoneally once an
hour for 6 h, and the mouse model of AP was successfully
established by intraperitoneal injection of LPS (10 mg/kg)
immediately after the last CR injection; and the iii) AS5 + AP and
iv) AS15 + AP groups, in which the mice were intraperitoneally
injected with 5 or 15 mg/kg AS, respectively, immediately after the
last injection of LPS.
The whole experiment lasted 24 h. During the
experimental period, the health status of mice was observed every 2
h and the mortality rate of mice was 0%. The mice were euthanized
through cervical dislocation at 2, 8 (28 mice each time) and 18 h
(only 4 mice in the NS group with AP + AS15 and 5 mice in the AP
group with AP + AS5) after model establishment to minimize the time
required for loss of consciousness. In addition, predetermined
humane endpoints were set, which if reached meant the animal
experiments could not continue (21). The experiment was terminated when
the mice appeared weak, and lost the ability to eat and drink on
their own. Death was verified by the loss of heartbeat. No mice
reached the humane endpoints.
Collection of serum, pancreatic tissue
and peritoneal lavage fluid
A total of 2, 8 and 18 h after establishment of the
AP model, serum was collected by removing the eyeballs from the
sacrificed mice at the 2-h time point and leaving them for 2 h at
room temperature. After centrifugation of the blood from the eye
socket after eyeball removal was performed at 4°C and 1,008 × g for
10 min, the supernatant was collected to detect pro-inflammatory
cytokine levels and amylase activity.
In addition, mice were sacrificed by cervical
dislocation at the 2-, 8- and 18-h time points. Mice were injected
intraperitoneally with 5 ml PBS and rubbed gently for 2 min at the
2 h time point. The peritoneal lavage fluid was then aspirated
using a pipette, centrifuged at 178 × g for 3 min at room
temperature, and the pellet and supernatant were separated to
detect the number of PMs and pro-inflammatory cytokine levels,
respectively.
The pancreas was excised by laparotomy, observed
visually to detect the presence or absence of edema, hyperemia and
necrosis, and then images of the pancreatic tissue were captured at
the 2-h time point. Detection of the pancreatic coefficients at the
8-h time point, and the pancreatic coefficient was calculated as
follows: Wet gland weight (mg)/mouse weight (g). Subsquently,
one-third of the pancreatic tissue (pancreatic tail) of each mouse
was cut off for homogenization (45 Hz, 50 sec, three times) at the
2-h time point and the 18-h time point. Since the volumes and
masses of the aforementioned pancreatic tissues from each mouse
were different, different volumes of saline were added for
homogenization according to the mass, in order to obtain the same
mass concentration of the pancreatic tissue homogenate. Finally,
the homogenates were centrifuged at 4°C and 1,008 × g for 10 min,
and the supernatant was obtained to detect pro-inflammatory
cytokine levels (2-h time point), TP activity (18-h time point) and
SOD activity (2-h time point).
The specific activity of an enzyme is
internationally expressed as U/mg protein, which indicates the
number of units of enzyme activity per unit weight (mg) of protein.
Therefore, U/mg protein was used to express TP and SOD activities,
according to the instructions of the kit manufacturer. Amylase
activity, on the other hand, since there is no protein involved,
was expressed in U/dl according to the kit manufacturer's
instructions. The international unit of pro-inflammatory cytokines
was formulated by the International Organization for
Standardization (22) and is
expressed in the form of international unit (IU). Different
inflammatory factors have different IUs, for example, the IU of
IL-1β is pg/ml (not used here), the IU of TNF-α is pg/ml or
pg/106 cells and the IU of IL-6 is pg/ml or
pg/106 cells. Therefore, the use of pg/ml was
standardized when indicating pro-inflammatory cytokine levels
according to the instructions provided with the ELISA kits.
Therefore, the units of pro-inflammatory cytokines were different
from those of digestive enzyme activity.
PM count in ascites
After centrifuging the mouse peritoneal fluid, the
supernatant was poured into an Eppendorf tube and retained.
Subsequently, 1 ml PBS was added to the pellet and mixed, after
which, 20 μl of the suspension was added to an automated
cell counter (Countstar; Shanghai Ruiyu Biotechnology Co., Ltd.),
and the total number of PMs was counted.
Histological examination of pancreatic
tissue
The pancreatic tissue was fixed in 4%
paraformaldehyde at 4°C for 24 h, embedded in paraffin and cut into
3- to 4-μm sections. The sections were stained with in
hematoxylin solution for 10 min, with color separation in acid
water and ammonia for 3 sec each, rinsed in running water for 1 h
and then put into distilled water. Sections were dehydrated in 70
and 90% alcohol for 10 min each, and then stained with eosin
staining solution for 3 min at room temperature using an ST5010
AutoStainer (Leica Microsystems GmbH). Finally, the changes in
pulmonary histopathology were observed under a BX43 light
microscope (Olympus Corporation).
Immunohistochemistry
Pancreatic sections were deparaffinized using xylene
and hydrated in different concentrations of ethanol, 100, 95, 90,
85 and 70% for 5 min each, and then rinsed in tap water for 10 min,
before being treated with citrate antigen repair solution (pH 6.0)
at 100°C for 18 min. The sections were then treated with 3%
H2O2 at 37°C for 10 min to block endogenous
peroxidase, blocked with goat serum (from cat. no. E-IR-R-217 kit)
for 30 min at 37°C, and then incubated with anti-TLR4 (1:50),
anti-LC3 (1:100) and anti-Beclin-1 (1:50) antibodies at 4°C for 18
h. Subsequently, the sections were incubated with
polyperoxidase-anti-mouse/rabbit IgG (from cat. no. E-IR-R-217 kit)
at 37°C for 30 min, with DAB for 30 sec and with hematoxylin at
room temperature for 10 min. After 10 sec of differentiation
solution fractionation configured from 5% ethanol hydrochloride,
the sections were dehydrated through a concentration gradient of
ethanol and xylene, sealed with neutral resin and images were
captured under a light microscope. Semi-quantification of protein
expression was performed in three randomly selected regions using
ImageJ software version 1.54d (National Institutes of Health). The
average optical density value is obtained by dividing the
cumulative optical density by the area of the effective target
distribution. Dark brown staining of cells indicated a positive
signal.
Transmission electron microscopy
The pancreatic tissues were fixed in 2.5%
glutaraldehyde fixative at 4°C for 24 h, dehydrated through a
graded ethanol series and embedded in epoxy resin for
pre-polymerization at 45°C for 12 h, then polymerized at 60°C for
24 h. After staining with toluidine blue, the pancreatic tissues
were sectioned to a thickness of 1-3 μm using an ultrathin
sectioning machine. The obtained sections were double stained with
uranyl acetate and lead nitrate for 30 min at room temperature, and
were then observed using transmission electron microscopy
(JEM-1400Plus; Japan Electronics Co., Ltd.).
Treatment of macrophages with AS
Mouse PMs were isolated, grown and adhered to
96-well plates (5.0×104 cells/well) at 37°C in 5%
CO2 for 4 h. AS (20 μg/ml) in serum-free DMEM was
added after attachment, and after 2 h of incubation at 37°C in 5%
CO2, the cells in the 96-well plates were stimulated
with TP (1, 5 and 20 μg/ml) or LPS (1, 3 and 9 ng/ml). After
4 h of incubation at 37°C in 5% CO2, the supernatants
were collected to detect TNF-α and IL-6 levels.
RAW264.7 cells were grown to confluence in 96-well
plates (5.0×104 cells/well), washed twice and incubated
with serum-free DMEM for 12 h. AS (35 μg/ml) in serum-free
DMEM was added after attachment, and after 2 h of incubation at
37°C in 5% CO2, the cells in the 96-well plates were
stimulation with TP (10 μg/ml) or different concentrations
of LPS (1, 3 and 9 μg/ml). After 4 h of incubation, the
supernatants were collected to detect TNF-α levels (TP10 + LPS9
only made two independent samples, while the other groups had three
independent samples).
Treatment of macrophages with autophagy
inhibitor
Mouse PMs (5.0×104 cells/well) were grown
on 96-well plates at 37°C in 5% CO2 for 4 h. LY294002
(10 μM) or LY294002 (10 μM) combined with AS (20
μg/ml) in serum-free DMEM was added after attachment, and
after 2 h of incubation at 37°C in 5% CO2, the cells
were stimulated with TP (5 μg/ml) or LPS (1 ng/ml) for 4 h
at 37°C in 5% CO2. Subsquently, the supernatants were
collected to detect TNF-α and IL-6 levels.
AS treatment of AR42J cells
AR42J cells (5.0×104 cells/well) were
grown to confluence in 96-well plates, washed twice and incubated
with F12K culture medium (containing 10% FBS) for 24 h at 37°C in
5% CO2. After CR (0.5 nM) or LPS (0.1, 0.3 and 0.9
μg/ml) were added (only three independent samples for the
Medium, LPS0.1 and LPS0.3 groups, while the other groups had four
independent samples), the cells were treated with AS (0.5
μg/ml) for 24 h at 37°C in 5% CO2 (only three
independent samples for the Medium group, while the other groups
had four independent samples). Thereafter, the supernatants were
collected to detect amylase activity.
Immunof luorescence staining
R AW264.7 cells (1.0×105 cells/well) in
24-well plates (each well plus coverslips) were treated with AS (35
μg/ml) for 2 h at 37°C in 5% CO2, and then
treated with TP (10 μg/ml) and/or LPS (3 ng/ml) for 1 h at
37°C in 5% CO2. The cells were collected and fixed in 4%
paraformaldehyde for 1 h at room temperature, after which, blocking
was performed with goat serum for 1 h at room temperature. After
incubating the slides with polyclonal antibodies against LC3
(1:100) at 4°C for 24 h, the slides were washed with PBS, and then
incubated with fluorescein isothiocyanate-labeled goat anti-rabbit
IgG antibody (1:200; from cat. no. P0179 kit) for 24 h at 4°C.
Finally, the slides were washed with PBS and the cell nuclei were
stained with 1 mg/ml DAPI for 5 min at room temperature. After PBS
washing and glycerol mounting, the LC3 levels in the cells were
observed using fluorescence microscopy (BX43; Olympus
Corporation).
HBAD-mRFP-GFP-LC3 infection analysis
RAW264.7 cells (1.0×104/well) in 24-well
plates (each well plus coverslips) were infected with autophagic
double-labeled adenovirus mRFP-GFP-LC3 at 1×108 PFU/ml
for 8 h at 37°C in 5% CO2 according to the
manufacturer's instructions, and then the virus-containing medium
was aspirated and the cells were washed twice with PBS.
Subsequently, RAW264.7 cells in 24-well plates were treated with AS
(35 μg/ml) for 2 h at 37°C in 5% CO2, and then
treated with TP (10 μg/ml) and/or LPS (3 ng/ml) for 1 h at
37°C in 5% CO2. After the cells were collected, PBS was
added and the cells were washed twice, and then 400 μl
paraformaldehyde was added and fixed for 20 min at room
temperature. A small amount of fluorescence quencher was added to
the slide in advance. After fixation with paraformaldehyde, the
slides were washed with PBS, and the plates were observed under a
laser confocal microscope and images were captured (STELLARIS5;
Leica Microsystems GmbH).
Assays of pro-inflammatory cytokine
levels and enzyme activities
The TNF-α and IL-6 levels in the mouse serum,
pancreatic homogenates, lavage fluid and cellular supernatant were
measured using ELISA kits according to the manufacturer's
protocols. The activities of amylase in the mouse serum and
cellular supernatant, and TP and SOD in the pancreatic homogenates,
were determined using their respective enzyme activity assay
kits.
WB
Total protein was extracted from RAW264.7 cells and
mouse pancreatic tissues using the Total Protein Extraction Kit,
and the protein concentration was determined by BCA Protein
Concentration Measurement Kit (Enhanced) (cat. no. P0009; Beyotime
Institute of Biotechnology). Equal amounts (20 μg) of
proteins were supersampled. LC3 was separated by 15% sodium dodecyl
sulfate polyacrylamide gel electrophoresis, and the rest of the
proteins were separated by 10% sodium dodecyl sulfate
polyacrylamide gel electrophoresis, followed by transfer to
polyvinylidene fluoride membranes and blocked with 5% skimmed milk
for 2 h at room temperature. After cutting the membranes according
to different molecular weights, anti-LC3 (1:1,000), anti-ATG16L1
(1:1,000), anti-TLR4 (1:1,000), anti-MyD88 (1:1,000), anti-TRAF6
(1:1,000), anti-Bcelin-1 (1:1,000), anti-β-actin (1:1,000) and
anti-GAPDH (1:1,000) antibodies were incubated at 4°C for 18 h.
Subsequently, the chemiluminescent substrates were enhanced with
HRP-conjugated goat anti-rabbit secondary antibody (1:2,000) or
anti-mouse secondary antibody (1:2,000) for 1 h at room
temperature. Subsequently, the membrane regeneration solution (cat.
no. SW3020; Beijing Solarbio Science & Technology Co., Ltd.)
was used to elute the antibodies and the membranes were incubated
again with alternative primary antibodies, followed by further
incubation with horseradish peroxidase-conjugated secondary
antibodies. Chemiluminescent-labeled immunoreactive protein bands
were visualized using the ChemiDoc™ Touch imaging system and the
SuperSignal chemiluminescent substrate (both from Bio-Rad
Laboratories, Inc.), and were analyzed using the ImageJ software
package.
Statistical analysis
Data are presented as the mean ± SD and each
experiment was performed in triplicate. Data from each group were
statistically analyzed using one-way ANOVA and Tukey's post hoc
test using GraphPad Prism 8.4.2 software (Dotmatics). P<0.05 was
considered to indicate a statistically significant difference.
Results
AS significantly alleviates damage to the
pancreatic tissue in AP model mice
To investigate the therapeutic effect of AS on a
mouse model of AP induced by CR combined with LPS, the general
condition of the pancreatic tissue, the pancreatic coefficient and
pathological changes in the pancreatic tissue were observed.
Gross observation showed that the surface of the
pancreatic tissue in the NS group was smooth, with no bleeding
spots; however, the pancreatic tissue from the mice in the AP group
was swollen, gray-colored, and there were a number of saponified
and bleeding points on the tissue surface (Fig. 1A). AS treatment (5 and 15 mg/kg)
did not reduce the edema of the pancreatic tissues but reduced the
number of bleeding points, and adhesion between pancreatic tissues
and surrounding tissues could be observed to be markedly reduced
during the sampling process. Furthermore, the pancreatic
coefficient was high in the AP group compared with NS group but was
decreased in the AS treatment groups (Fig. 1B).
Histopathological observation of pancreatic tissue
showed that in the NS group, the pancreatic lobular septum was
clear, the acinar arrangement was regular and no obvious changes
were observed (Fig. 1C). In the
AP group, the pancreatic tissue structure was disorganized, the
septa of the lobules were markedly enlarged, large coagulated
necrosis was observed in the glandular parenchyma, which was
accompanied by bleeding, and a large number of inflammatory cells
infiltrated around the necrotic lesion. In the AP + AS5 and AP +
AS15 groups, pancreatic hemorrhage, necrosis and inflammatory cell
infiltration were reduced compared with those in the AP group. The
effect in the AP + AS15 group was more obvious than that in the AP
+ AS5 group. These results indicated that AS reduced the degree of
pancreatic tissue damage in a mouse model of AP.
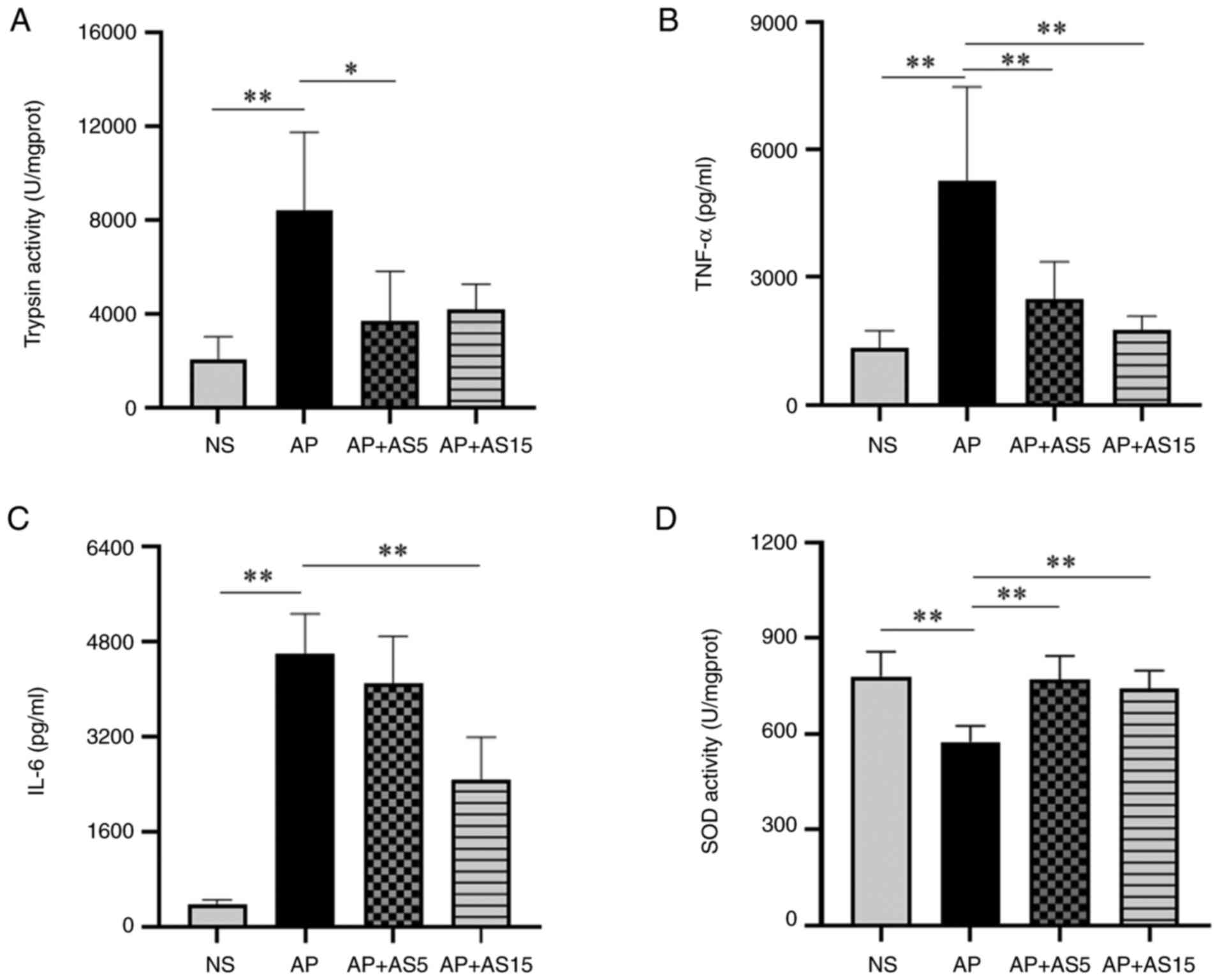
AS reduces both the activation of enzymes
and the levels of pro-inflammatory cytokines in pancreatic
tissue
Enzyme activation is a hallmark of AP (23); in particular, TP activity is
closely related to the severity of pancreatitis. The activation of
pancreatic enzymes and the levels of pro-inflammatory cytokines in
pancreatic tissue are closely related to the degree of local
inflammatory response in the pancreatic tissue, as well as the
subsequent systemic inflammatory response (24).
The results of the present study showed that
pancreatic TP activity was significantly increased in the AP group
compared with that in the NS group, whereas AS (5 and 15 mg/ml)
significantly decreased its activity (Fig. 2A). Furthermore, compared with
those in the NS group, pancreatic TNF-α and IL-6 levels were
significantly increased in the AP group, but were significantly
decreased in the AP + AS15 group (Fig. 2B and C).
Excessive production of oxygen free radicals is
closely related to the inflammatory state of the human body, and
SOD serves an important role in scavenging oxygen free radicals
(25,26). Therefore, SOD activity was
assessed in pancreatic tissue. The results showed that the SOD
activity was significantly decreased in the AP group compared with
that in the NS group, but was significantly increased in the AS
groups (Fig. 2D).
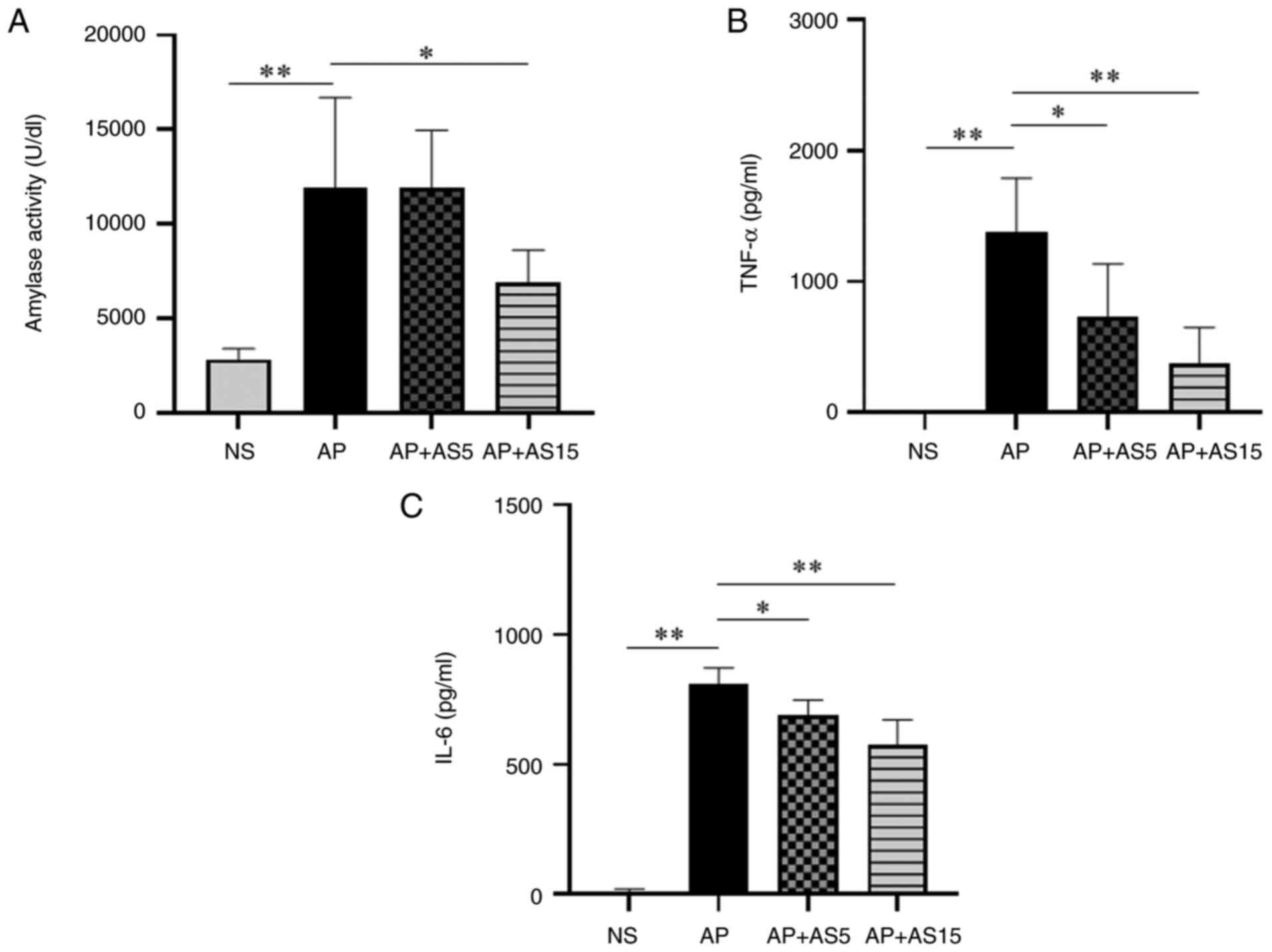
AS reduces pancreatic enzyme activity and
pro-inflammatory cytokine levels in the blood
Pancreatic enzymes that are released in response to
pancreatic tissue damage enter the bloodstream; therefore, the
serum levels of pancreatic enzymes are closely related to the
severity of pancreatitis (27).
In particular, the activity of pancreatic amylase in the blood is
closely related to the severity of pancreatitis (27). In the present study, pancreatic
amylase activity was investigated. The results showed that the
activity of serum amylase was significantly increased in the AP
group compared with that in the NS group, but was significantly
decreased in the AP + AS group (Fig.
3A).
The local inflammatory response in pancreatitis can
trigger a systemic inflammatory response known as SIRS; therefore,
pro-inflammatory cytokine levels are strongly associated with local
and systemic inflammation during AP (28,29). In the present study, the serum
levels of TNF-α and IL-6 were investigated. The results showed the
levels of TNF-α and IL-6 in the AP group were significantly
increased compared with those in the NS group, but were
significantly decreased in the AS groups (Fig. 3B and C).
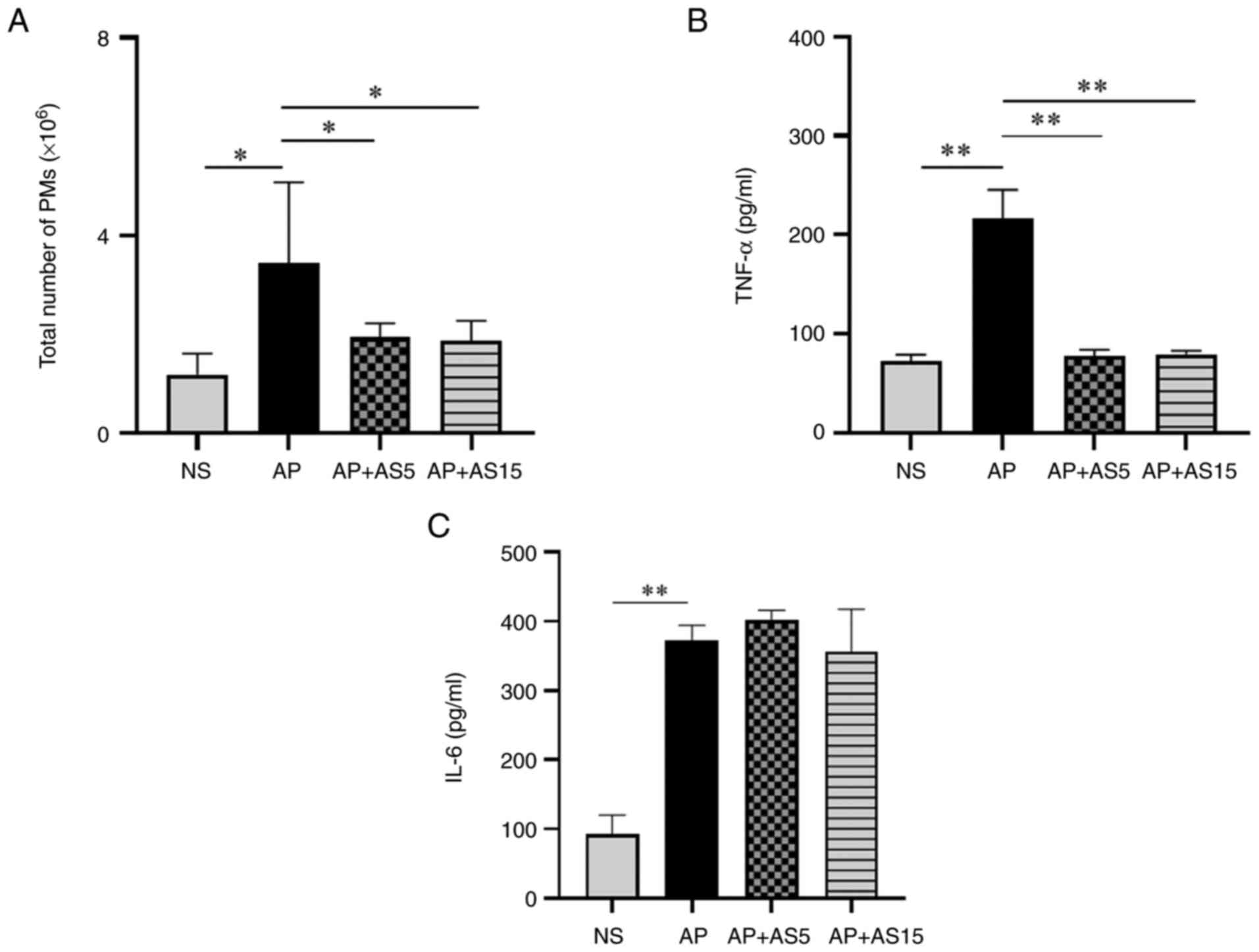
AS significantly reduces the levels of
pro-inflammatory cytokines and the number of PMs in the peritoneal
lavage fluid
Macrophages are a major source of pro-inflammatory
cytokine production. The peritoneal cavity contains a large number
of macrophages, and the number and function of PMs are closely
related to the severity of pancreatitis (30,31).
The results of the present study showed that
compared with that in the NS group, the total number of PMs in the
AP group was significantly increased; however, AS treatment
significantly decreased the total number of PMs (Fig. 4A). The levels of TNF-α and IL-6
in the peritoneal lavage fluid were significantly higher in the AP
group than those in the NS group, whereas AS significantly
decreased TNF-α levels (Fig. 4B and
C).
AS inhibits autophagy and TLR4 signaling
pathway-related proteins in vivo
Autophagy serves an important role in the
pathophysiological process of AP. In the late stage of autophagy,
autophagic lysosomes with a single membrane are present (32). Transmission electron microscopy
showed no autophagic abnormalities in the NS group, whereas a large
number of autophagic lysosomes were apparent in the AP group
compared with that in the NS group; however, almost no autophagic
lysosomes were observed in the AP + AS15 group (Fig. 5A).
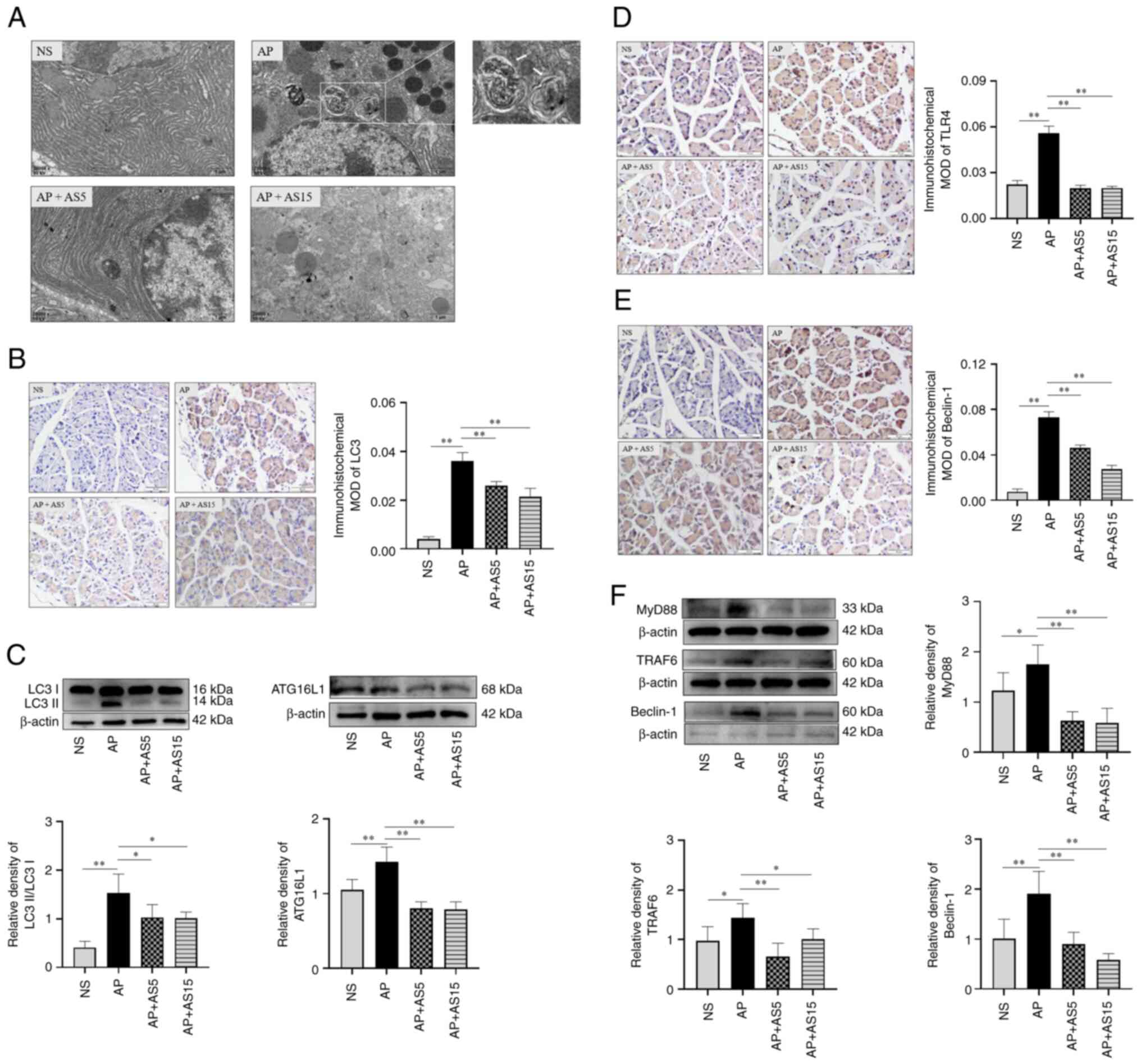 | Figure 5AS inhibits autophagy and TLR4
signaling pathway-related protein expression in AP model mice. (A)
Representative transmission electron microscopy images of
pancreatic tissues at the 2 h time point. White arrows indicate
autophagic lysosomes. Magnification, ×20,000. (B)
Immunohistochemical analysis of LC3 protein expression in
pancreatic tissues at the 2 h time point (n=3). A dark brown color
in the cells indicates a positive signal. (C) Western blot analysis
of LC3II and ATG16L1 protein expression in the pancreatic tissues
of mice with AP at the 2 h time point (n=6). Immunohistochemical
analysis of (D) TLR4 and (E) Beclin-1 protein expression in
pancreatic tissues at the 2 h time point (n=3). A dark brown color
in the cells indicates a positive signal. Magnification, ×40. (F)
Western blotting of MyD88, TRAF6 and Beclin-1 protein expression in
the pancreatic tissues of mice with AP at the 2 h time point (n=6).
Data are presented as the mean ± SD and were analyzed by one-way
ANOVA and Tukey's test. *P<0.05,
**P<0.01. AP, acute pancreatitis; AS, artesunate;
ATG, autophagy-related gene; LC3, microtubule associated protein 1
light chain 3; MOD, mean optical density; MyD88, myeloid
differentiation primary response 88; NS, normal saline; TLR4,
Toll-like receptor 4; TRAF6, tumor necrosis factor receptor
associated factor 6. |
LC3 is a molecular hallmark for the occurrence of
autophagy in cells (33) and
autophagy-related gene (ATG) is also involved in regulating the
formation of cellular autophagosomes (34). The present study observed the
changes in LC3 and ATG16L1 in the pancreatic tissues of mice with
AP. Immunohistochemistry results showed that LC3 protein expression
was significantly increased in the AP group compared with that in
the NS group, whereas it was significantly decreased in the AS
groups (Fig. 5B). WB results
showed that LC3II and ATG16L1 protein expression levels were
significantly increased in the AP group compared with those in the
NS group, whereas they were significantly decreased in the AS
groups (Fig. 5C). These results
indicated that AS may inhibit the expression of key molecules in
the autophagy pathway in the pancreatic tissues of mice with
AP.
The LPS-induced autophagy process is closely related
to the TLR4/TRAF6/Beclin-1 signaling pathway (35); therefore, immunohistochemistry
and WB were used to detect changes in the expression levels of
important molecules in the TLR4 signaling pathway in the pancreatic
tissues of mice with AP, and further observed the effects of AS on
inflammation and autophagy. Immunohistochemistry showed that the
protein expression levels of TLR4 and Beclin-1 were significantly
increased in the AP group compared with those in the NS group,
whereas they were significantly decreased in the AS groups
(Fig. 5D and E). The results of
WB showed that the expression levels of MyD88, TRAF6 and Beclin-1
were significantly increased in the AP group compared with those in
the NS group, whereas they were significantly decreased in the AS
groups (Fig. 5F). These findings
indicated that AS inhibited the expression of molecules related to
the TLR4/TRAF6/Beclin-1 signaling pathway in the pancreatic tissues
of mice with AP.
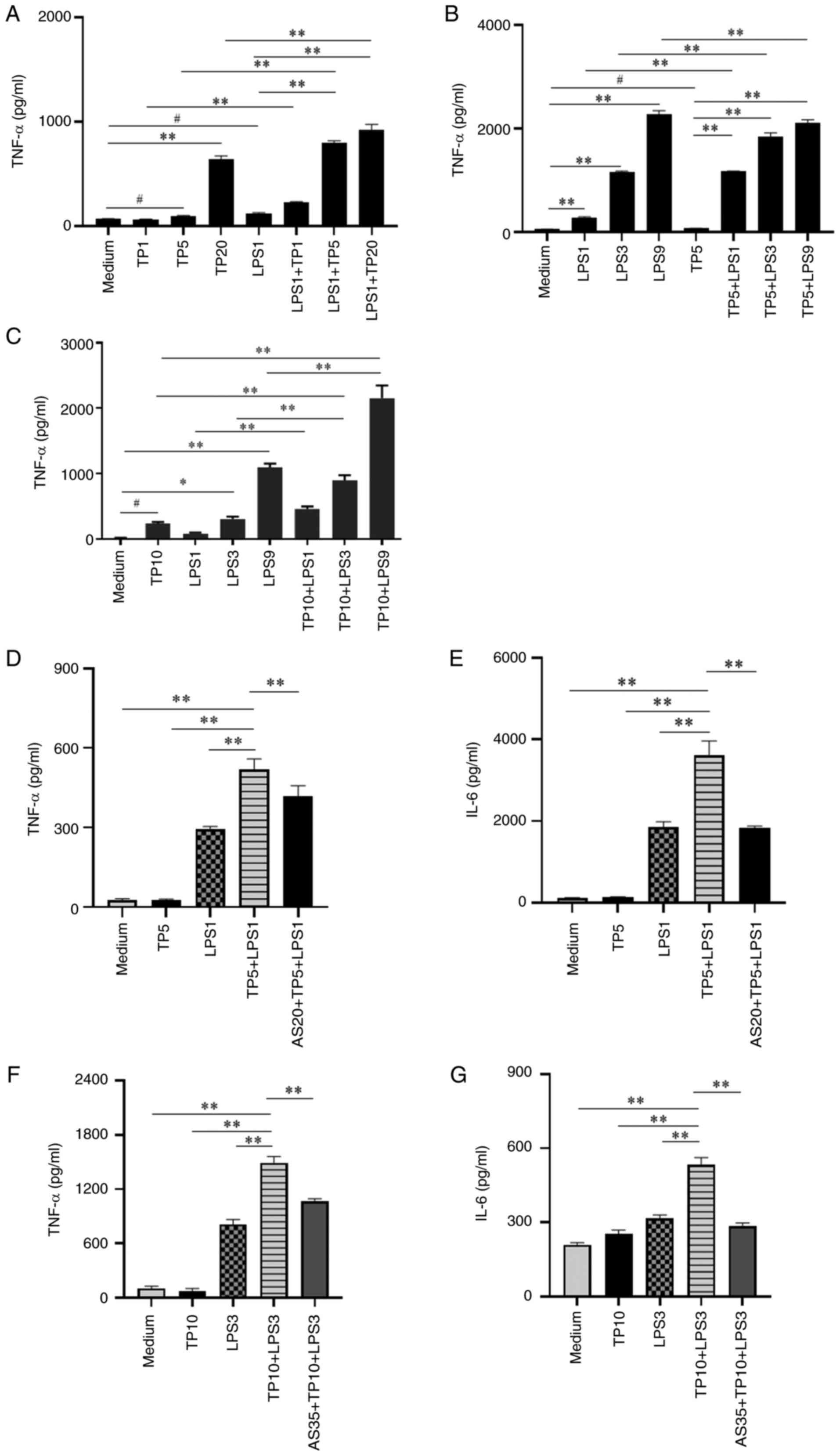
AS inhibits the release of
pro-inflammatory cytokines from mouse macrophages treated with TP
and LPS in vitro
Macrophages serve important roles in the occurrence
and development of AP (36,37), and TP and LPS can induce
macrophages to release pro-inflammatory cytokines (19,38); however, whether TP combined with
LPS could synergistically induce the release of more
pro-inflammatory cytokines than TP or LPS alone is unclear. In the
present study, both the mouse RAW264.7 cell line and PMs were used
to determine the effect of TP combined with LPS.
Firstly, PMs were treated with a low concentration
of LPS (1 ng/ml) combined with different concentrations of TP (1, 5
and 20 μg/ml). After 4 h, the cell supernatant was collected
and the levels of TNF-α in the supernatant were detected. The
results showed that LPS and different concentrations of TP could
induce TNF-α release; however, TP5 + LPS1 group could induce
significantly higher TNF-α release (Fig. 6A). In addition, PMs were treated
with TP (5 μg/ml) combined with different concentrations of
LPS (1, 3 and 9 ng/ml). The results also showed that the TP5 + LPS1
group could induce significantly more TNF-α release (Fig. 6B). The TNF-α levels induced by
the combination of LPS (1 ng/ml) and TP (5 μg/ml) were
significantly higher than those using either factor alone;
therefore, 1 ng/ml LPS plus 5 μg/ml TP were used in
subsequent experiments in PMs.
Secondly, the effects of TP plus LPS on TNF-α were
validated in RAW264.7 cells. RAW264.7 cells were treated with TP
(10 μg/ml) combined with different concentrations of LPS (1,
3 and 9 ng/ml) for 4 h. The RAW264.7 cell validation results were
consistent with those detected using PMs (Fig. 6C). Based on the aforementioned
results, a combination of 10 μg/ml TP with 3 ng/ml LPS was
selected for subsequent experiments using RAW264.7 cells.
Furthermore, the effects of AS (20 and 35
μg/ml) on PMs and RAW264.7 cells treated with TP and LPS
were observed. The results showed that AS significantly inhibited
the release of TNF-α and IL-6 induced by TP combined with LPS in
PMs (Fig. 6D and E). The results
in RAW264.7 cells were consistent with those in PMs (Fig. 6F and G). These results
demonstrated that TP combined with LPS could significantly increase
the release of pro-inflammatory cytokines from mouse macrophages,
whereas AS could markedly inhibit this pro-inflammatory cytokine
release.
AS decreases the amylase activity induced
by CR combined with LPS in vitro
Parenchymal cells release large amounts of
pancreatic enzymes, but less pro-inflammatory cytokines, to
participate in inflammation (39). Cholecystokinin can induce
pancreatic acinar cells to produce amylase (40). CR is a gastric regulatory
molecule similar to cholecystokinin in function and composition,
which can stimulate the secretion of amylase from the stomach, bile
duct and pancreas; therefore, CR is often used in animal and cell
experiments to replace cholecystokinin (41-43). In the present study, CR combined
with LPS was used to treat the AR42J acinar cell line, and the
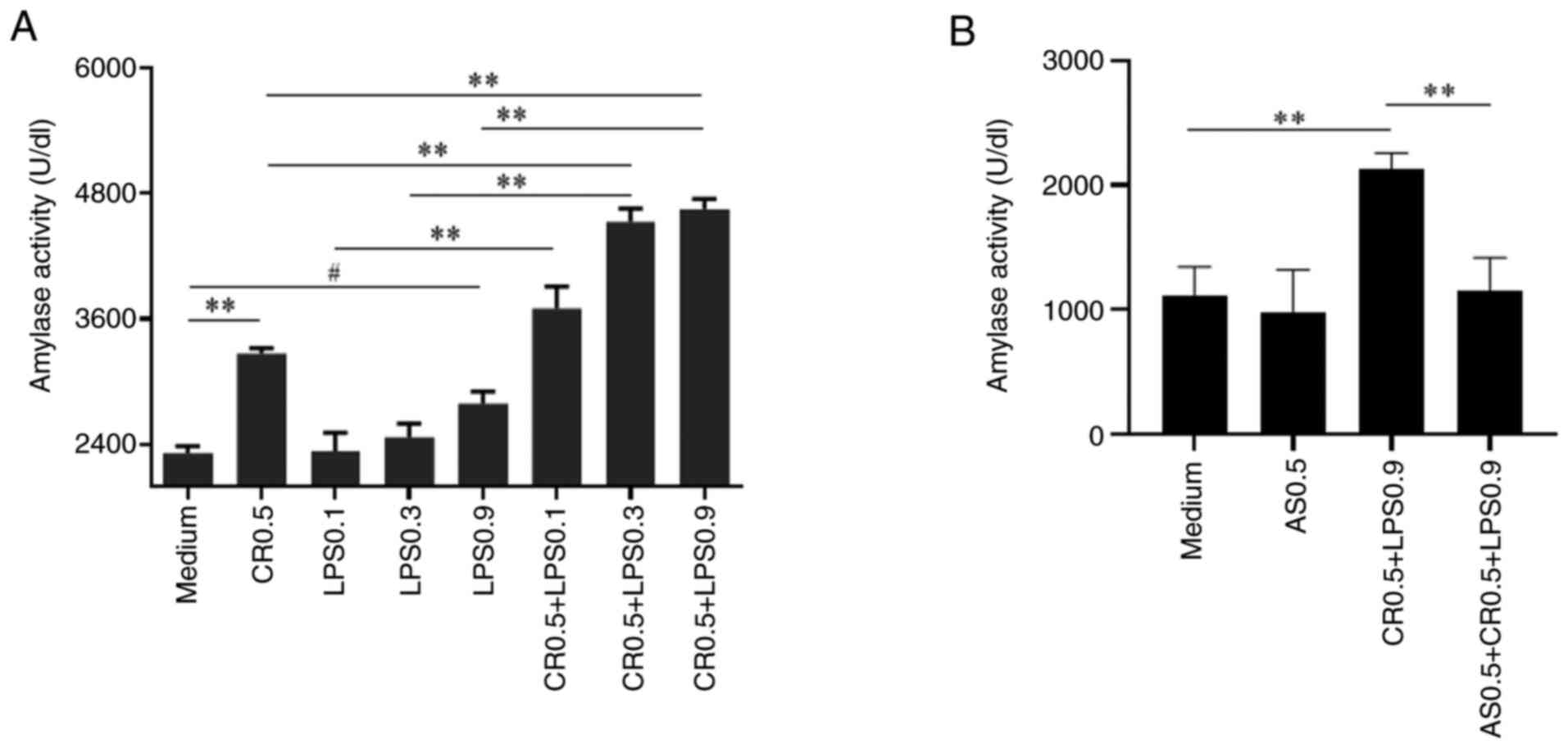
amylase activity in acinar cells was measured.
The results showed that either 0.5 nM CR or 0.9
μg/ml LPS alone could significantly increase amylase
activity compared with that in the control (Medium) group, whereas
0.9 μg/ml LPS combined with 0.5 nM CR significantly
increased the amylase activity compared with that induced by either
factor alone (Fig. 7A). Thus,
the combination of 0.5 nM CR and 0.9 μg/ml LPS was selected
for subsequent experiments. Based on the aforementioned
experiments, the effect of AS (0.5 μg/ml) on amylase
activity was investigated. The results showed that AS significantly
decreased the amylase activity induced by CR combined with LPS
(Fig. 7B).
Autophagy serves an important role in the
release of pro-inflammatory cytokines, and AS inhibits autophagy
and TLR4 signaling pathway-related proteins in vitro
To detect whether macrophages treated with TP
combined with LPS induced excessive autophagy in AP, the effects of
the autophagy inhibitor LY294002 (10 μM) on the release of
TNF-α and IL-6 induced by LPS (1 ng/ml) and TP (5 μg/ml)
were observed in PMs. The results showed that LY294002 could
significantly decrease the levels of TNF-α and IL-6 released by PMs
treated with TP combined with LPS (Fig. 8A and B), Subsequently, the
effects of AS (20 μg/ml) combined with the autophagy
inhibitor LY294002 (10 μM) were observed on the release of
TNF-α and IL-6 induced by LPS (1 ng/ml) and TP (5 μg/ml) in
PMs. The results showed that both AS and LY294002 could
significantly decrease the levels of TNF-α and IL-6 released by PMs
treated with TP combined with LPS (Fig. 8C and D). However, compared with
in the group treated with AS or LY294002 alone, the combination of
AS and LY294002 did not further reduce the levels of TNF-α and IL-6
induced by LPS and TP.
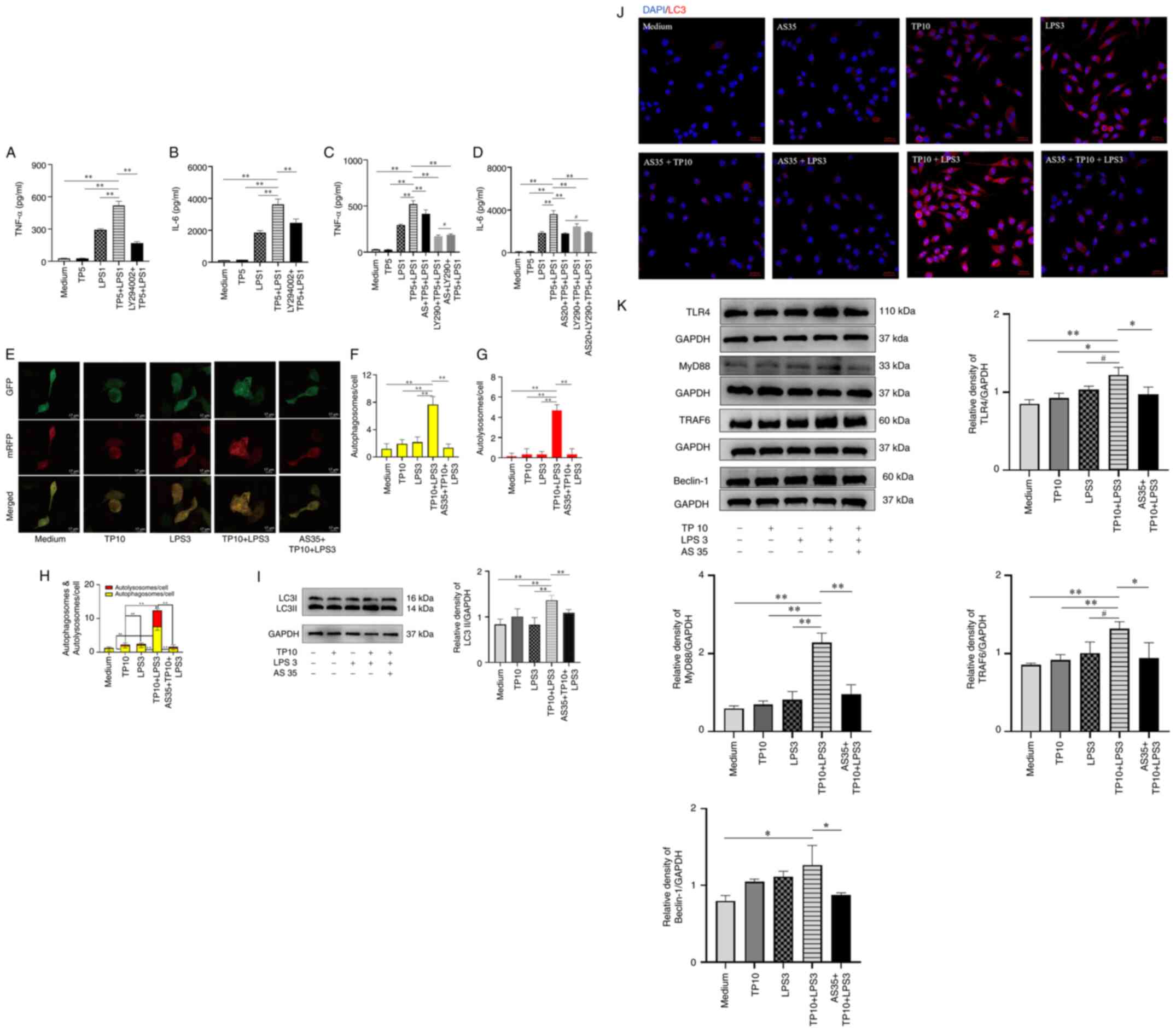 | Figure 8AS inhibits the protein expression
levels of key molecules associated with TLR4-dependent autophagy in
PMs and RAW264.7 cells. Autophagy inhibitor LY294002 significantly
inhibited (A) TNF-α and (B) IL-6 release from PMs induced by TP
combined with LPS (n=3). AS combined with autophagy inhibitor
LY294002 had no significant effect on the release of (C) TNF-α and
(D) IL-6 from PMs induced by TP combined with LPS (n=3). (E) AS
inhibited autophagic flow induced by TP combined with LPS in
RAW264.7 cells (n=3). Autophagosomes (yellow) and autolysosomes
(red) were observed and counted. Scale bar, 17 μm. Number of
(F) autophagosomes, (G) autolysosomes, and (H) autophagosomes and
autolysosomes. AS inhibited LC3II protein expression induced by TP
combined with LPS in RAW264.7 cells, as determined by (I) western
blotting and (J) laser scanning confocal microscope. Magnification,
×400. (K) AS inhibited the protein expression levels of TLR4,
MyD88, TRAF6 and Beclin-1 in RAW264.7 cells treated with TP
combined with LPS (n=3). Data are presented as the mean ± SD and
were analyzed by one-way ANOVA and Tukey's test.
*P<0.05, **P<0.01;
#P>0.05. AS, artesunate; GFP, green fluorescent
protein; IL, interleukin; LC3, microtubule associated protein 1
light chain 3; LPS, lipopolysaccharide; mRFP, monomeric red
fluorescent protein; MyD88, myeloid differentiation primary
response 88; NS, normal saline; TLR4, Toll-like receptor 4; TNF-α,
tumor necrosis factor α; TRAF6, TNF receptor associated factor 6;
TP, trypsin. |
To observe the effects of AS on autophagy, the
HBDA-mRFP-GFP-LC3 adenovirus was used to monitor autophagic flow in
real-time; mRFP can be used to label and track LC3, and the
attenuation of GFP indicates the fusion of lysosomes and
autophagosomes to form autolysosomes. The results showed that the
number of autophagosomes and autolysosomes was significantly
increased after TP (10 μg/ml) combined with LPS (3 ng/ml)
treatment in RAW264.7 cells; however, AS (35 μg/ml)
significantly reduced the number of autophagosomes and
autolysosomes (Fig. 8E-H),
suggesting that AS could inhibit the excessive autophagy stimulated
by TP combined with LPS in RAW264.7 cells.
Secondly, WB results showed that the protein
expression levels of LC3II were significantly increased after TP
(10 μg/ml) combined with LPS (3 ng/ml) treatment in RAW264.7
cells compared with that in the control (Medium) group, whereas AS
(35 μg/ml) significantly decreased the protein expression
levels of LC3II (Fig. 8I).
Furthermore, immunofluorescence microscopy showed
that TP (10 μg/ml) and LPS (3 ng/ml), alone or in
combination, increased the expression of LC3 compared with that in
the control group, whereas AS (35 μg/ml) notably decreased
the expression of LC3 in RAW264.7 cells (Fig. 8J). These results demonstrated
that the effect of AS on the release of pro-inflammatory cytokines
may be closely related to the inhibition of autophagy.
Subsequently, the present study observed whether TP (10
μg/ml) combined with LPS (3 ng/ml) induced changes in
important molecules (TLR4, Myd88, TRAF6 and Beclin-1) in the TLR4
signaling pathway in RAW264.7 cells. The results showed that TP
combined with LPS increased the protein expression levels of TLR4,
Myd88, TRAF6 and Beclin-1 in RAW264.7 cells compared with those in
the control (Medium) group, whereas AS (35 μg/ml)
significantly decreased their protein expression levels (Fig. 8K).
Discussion
The results of the present study suggested that AS
has a protective effect on AP in mice induced by LPS combined with
CR, in which it reduces pro-inflammatory cytokine release and
pancreatic enzyme levels, and attenuates pancreatic tissue damage.
Furthermore, it was indicated that the molecular mechanism of AS
treatment of AP may be closely related to inhibition of the
TLR4/NF-κB signaling pathway and autophagic signaling.
Most AP models have been performed in rodents, and
the commonly used methods are pancreatic duct ligation (44), intraperitoneal injection of an
L-arginine inducer (45),
retrograde injection of sodium taurocholate into the
biliopancreatic duct (46), and
intraperitoneal injection of CR into the pancreatic duct (47). Our previous study established a
rat model of AP using retrograde injection of sodium taurocholate
into the biliopancreatic duct, which produced AP that was similar
in severity to the human disease (16). However, it is not an ideal model
of AP-MODS because it requires surgery and delicate manipulation,
and is not easily replicated (48). Therefore, an AP model that has
the advantages of being noninvasive, easy to perform and
reproducible was chosen for the present study, namely
intraperitoneal injection of LPS combined with CR, which also
increases the severity of AP and MODS compared with the AP model
established using CR alone (49,50), and mimics AP-related sepsis
(51).
The present results showed that the mouse pancreatic
tissues developed lesions characterized by interstitial edema,
hemorrhage and necrotic acinar cells in response to LPS combined
with CR. In addition, enhanced amylase and TP activities,
significantly elevated levels of pro-inflammatory cytokines and PM
counts, and significantly decreased SOD activity suggested that LPS
combined with CR could be used to successfully establish a mouse
model of AP.
AP is an acute gastrointestinal disorder with high
morbidity and mortality rates (52,53). The severe systemic inflammation
induced by AP frequently leads to the development of MODS and
subsequent death (54,55). The pathogenesis of AP is complex,
and although numerous studies have been conducted to gain a better
understanding of its pathophysiology (56,57), and several clinical drug trials
have been performed, including those applying protease inhibitors,
such as ulinastatin and somatostatin (58), their therapeutic efficacy remains
questionable (59). There is no
effective treatment for AP; therefore, the search for effective and
safe drugs has become an important research goal in the treatment
of AP.
AS, a sesquiterpene lactone obtained from the plant
Artemisia annua (60), is
a stable derivative of artemisinin and is an effective drug used to
treat malaria, improve inflammation and treat tumors (such as lung
and liver cancer) (61,62). Our previous study showed that AS
can inhibit the TLR4/NF-κB signaling pathway to reduce digestive
enzyme activity and pro-inflammatory cytokine expression, thereby
improving the survival rate of rats with SAP (16). Therefore, the role of AS was
investigated in the present mouse model of AP, and the results
suggested that AS may produce significant inflammatory protection
in mice with AP.
In the diagnosis of AP, digestive enzymes are
highly sensitive and specific markers (63). In addition, oxygen radicals are
involved in the process of pancreatic necrosis (25). Therefore, the activities of
amylase, TP and SOD in mice can reflect the severity of AP in the
model and the therapeutic effect of drugs. In addition to digestive
enzymes, pro-inflammatory cytokines released by local macrophages,
alveolar cells and distal macrophages are markers of AP severity
(64). The mononuclear
macrophage system serves an important role in maintaining internal
environment stability. PMs are mononuclear macrophages, the number
and functional status of which can reflect the state of the
macrophage system; therefore, the total number of PMs is important
in AP (65).
In the present study, the results indicated that
although the edema of pancreatic tissues in mice with AP was not
reduced after the administration of AS, the adhesion between
pancreatic tissues and surrounding tissues could be observed to be
markedly reduced during the sampling process, and the number of
hemorrhagic spots was reduced. Moreover, AS could reduce the
pancreatic coefficient, pancreatic hemorrhage, necrosis and
inflammatory cell infiltration in mice; all of these results
indicated that AS could reduce the degree of pancreatic injury in
mice with AP. Moreover, AS not only significantly decreased serum
amylase and TP activities, and increased SOD activity, in mice with
AP, but also significantly decreased TNF-α and IL-6 levels in the
serum, pancreatic tissue and peritoneal lavage fluid. In addition,
the number of PMs was significantly reduced after AS treatment.
This suggested that PMs are important in AP and that AS could
reduce the inflammatory response in mice with AP by reducing the
levels of pro-inflammatory cytokines and digestive enzymes, and
increasing the activity of SOD.
During the development of SAP, LPS induces the
release of large amounts of pro-inflammatory cytokines from
macrophages and acinar cells, which are involved in the
pathophysiological process of pancreatitis development and promote
the progression from local to systemic inflammation (13,66). AR42J cells treated with a
combination of CR and LPS exhibited markers of more severe
pancreatitis, including enhanced secretion of digestive enzymes and
pro-inflammatory cytokines, compared with CR stimulation alone, as
well as less apoptosis and substantial evidence of necrosis
(67), which is more conducive
to the therapeutic effect of drugs in AP. Therefore, the present
study established a cellular model of AP by treating AR42J cells
with CR combined with LPS, which was consistent with models
generated in national and international studies, and previous
results from our laboratory (68,69). The present results showed that CR
combined with LPS could induce a further increase in the level of
secreted amylase activity in AR42J cells compared with CR and LPS
alone, with a significant synergistic effect. These findings
suggested that CR combined with LPS could successfully establish a
cell model of AP.
Both TP and NF-κB activation, which are independent
events (70), can be observed in
the early stages of AP, and can further exacerbate pancreatic
tissue damage and the systemic inflammatory response. Activated TP
and intestinal-transported LPS coexist during AP; therefore, LPS
can amplify the TP-induced inflammatory response, and the same TP
can also amplify the LPS-induced inflammatory response, which could
better mimic the pathological process of AP. Therefore, the present
study established a cellular model of AP by treating macrophages
with LPS combined with TP. The results showed that small doses of
LPS combined with TP could induce a large release of TNF-α and IL-6
from PMs, with a significant synergistic effect, which was more
effective than that induced by LPS or TP alone. The synergistic
effect was also verified in RAW264.7 cells. This result suggested
that LPS combined with TP could successfully establish a cell model
of AP.
The levels of pro-inflammatory cytokines and
digestive enzyme activity in the supernatants of the three cell
models were observed, the results showed that AS significantly
reduced the levels of TNF-α and IL-6, and amylase activity,
suggesting that AS could exert its anti-inflammatory effects by
inhibiting digestive enzyme activity and the release of
pro-inflammatory cytokines.
It has been reported that activation of trypsinogen
may be associated with abnormal autophagy in pancreatic cells.
Moreover, excessive autophagy could be involved in, or induced by,
the development of an excessive inflammatory response (71,72). During AP, autophagy is activated,
but is impaired and incomplete. It has been shown that aberrant
autophagy in pancreatic cells caused by excessive activation of
autophagy or blockade of the autophagic pathway promotes the
development and progression of AP (73). Therefore, the present study
determined the effects of the autophagy inhibitor LY294002 on TP
combined with LPS-stimulated PMs, and showed that it could inhibit
the release of pro-inflammatory cytokines from PMs. This suggested
that autophagy may be aberrantly activated during AP and that AP is
closely related to autophagy. However, after treatment with a
combination of AS and the autophagy inhibitor, the levels of TNF-α
and IL-6 were not further reduced, thus it was hypothesized that
the effects of AS and autophagy inhibitors may be the same, and
both inhibit excessive autophagy.
TLR4 is an important component of the innate immune
response that has an important role in the recognition of and
defense against invading pathogens. LPS activates TLR4 in a
MyD88-dependent manner, triggering a classical inflammatory cascade
response, leading to the activation of NF-κB and the release of
pro-inflammatory cytokines (74). The TLR4/NF-κB signaling pathway
and autophagic pathways serve important roles not only in immune
cells, but also in AR42J acinar cells (75-77). The current gold standard for the
detection of autophagy is the observation of autophagosomes by
electron microscopy and the detection of the autophagy marker LC3
(78). During autophagy, LC3I is
modified and processed to produce LC3II, which is localized to
autophagic vesicles. Thus, both LC3 and LC3II present in autophagic
vesicles are used as molecular markers for the occurrence of
autophagy in cells, and the amount of LC3II is proportional to the
degree of autophagy (79).
Beclin-1 is also a key molecular marker of autophagy (80). The present results showed that AS
not only decreased LC3 and ATG16L1 protein levels, and reduced
autophagic lysosome production, but also significantly inhibited
the protein expression levels of TLR4, MyD88, TRAF6 and Beclin-1 in
the LPS combined with CR-induced mouse model of AP, suggesting that
the therapeutic effects of AS on the AP model mice are closely
related to the TLR4/NF-κB signaling pathway and autophagy. In the
LPS combined with TP-induced RAW264.7 cell inflammation model, AS
was observed to reduce the number of autophagosomes and
autolysosomes under laser confocal microscopy; and AS reduced the
protein expression levels of LC3II, TLR4, MyD88, TRAF6 and
Beclin-1. The immunofluorescence results also showed that AS
significantly reduced the excessive elevation of LC3 induced by TP
combined with LPS stimulation in RAW264.7 cells. In combination,
these results suggested that the mechanism by which AS exerts its
anti-inflammatory effects on AP might be closely related to
inhibition of TLR4/NF-κB and autophagic signaling pathways.
In conclusion, AS exhibited a significant
protective effect toward mice with AP via a mechanism that could be
related to inhibition of the TLR4/NF-κB and autophagy signaling
pathways, and reduction in digestive enzyme activity and
pro-inflammatory cytokine expression. Therefore, AS may be
considered as a valuable therapeutic agent for AP.
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
DL, CL, FD and HZ substantially contributed to the
experimental conception and design of the study. FO and RQ
contributed to the acquisition of data and analysis of in
vitro experiments. DL, CL, FD, ZZ, YW, YZ and ML contributed to
the animal experiments. HZ, XL, XP, YH and YC contributed to
statistical analysis and visualization. DL, XL and HZ are
responsible for manuscript writing. HZ supervised all experiments.
DL, CL and FD confirm the authenticity of all the raw data. All
authors have read and approved the final version of the manuscript.
All authors took responsibility for the integrity and accuracy of
the study.
Ethics approval and consent to
participate
Ethics approval for the animal experiments was
obtained from the Ethical Committee of Zunyi Medical University
[approval no. ZMU (2020) 2-296]. All animal experiments conducted
in the present study followed the guidelines and regulations
specified in this ethical approval.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Acknowledgements
Not applicable.
Funding
This work was supported by grants from the National Natural
Science Foundation of China (grant no. 81673495), the Major
National Science and Technology Program of China for Innovative
Drug (grant no. 2017ZX09101002-002-009), the National Natural
Science Foundation of China-Guizhou Provincial People's Government
Joint Fund Project (grant no. NSFC-U1812403-4-1) and the Fourth
Batch of 'Thousand People Innovation and Entrepreneurship Talents
Fund' in Guizhou Province.
References
|
1
|
Greenberg JA, Hsu J, Bawazeer M, Marshall
J, Friedrich JO, Nathens A, Coburn N, May GR, Pearsall E and McLeod
RS: Clinical practice guideline: Management of acute pancreatitis.
Can J Surg. 59:128–140. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Tenner S, Baillie J, Dewitt J and Vege SS;
American College of Gastroenterology: American college of
gastroenterology guideline: Management of acute pancreatitis. Am J
Gastroenterol. 108:1400–1416. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Meher S, Mishra TS, Sasmal PK, Rath S,
Sharma R, Rout B and Sahu MK: Role of biomarkers in diagnosis and
prognostic evaluation of acute pancreatitis. J Biomark.
2015:5195342015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Habtezion A, Gukovskaya AS and Pandol SJ:
Acute pancreatitis: A multifaceted set of organelle and cellular
interactions. Gastroenterology. 156:1941–1950. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ge P, Luo Y, Okoye CS and Chen H, Liu J,
Zhang G, Xu C and Chen H: Intestinal barrier damage, systemic
inflammatory response syndrome, and acute lung injury: A
troublesome trio for acute pancreatitis. Biomed Pharmacother.
32:1107702020. View Article : Google Scholar
|
|
6
|
Yuan X, Wu J, Guo X, Li W, Luo C, Li S,
Wang B, Tang L and Sun H: Autophagy in acute pancreatitis:
Organelle interaction and microRNA regulation. Oxid Med Cell
Longev. 2021:88119352021. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Banks PA, Bollen TL, Dervenis C, Gooszen
HG, Johnson CD, Sarr MG, Tsiotos GG and Vege SS; Acute Pancreatitis
Classification Working Group: Classification of acute
pancreatitis-2012: Revision of the Atlanta classification and
definitions by international consensus. Gut. 62:102–111. 2013.
View Article : Google Scholar
|
|
8
|
Vege SS, Dimagno MJ, Forsmark CE, Martel M
and Barkun AN: Initial medical treatment of acute pancreatitis:
American gastroenterological association institute technical
review. Gastroenterology. 154:1103–1139. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Dumnicka P, Maduzia D, Ceranowicz P,
Olszanecki R, Drożdż R and Kuśnierz-Cabala B: The interplay between
inflammation, coagulation and endothelial injury in the early phase
of acute pancreatitis: Clinical implications. Int J Mol Sci.
18:3542017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kuo CEA, Wu SY, Lee CH, Lai YR, Lu CH,
Chen PC, Cheng JH, Tsai LY, Yen KT, Tsao Y and Tsai SM: Toona
sinensis modulates autophagy and cytokines in
lipopolysaccharide-induced RAW 264.7 macrophages. Biomed
Pharmacother. 129:1103862020. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Dawra R, Sah RP, Dudeja V, Rishi L,
Talukdar R, Garg P and Saluja AK: Intra-acinar trypsinogen
activation mediates early stages of pancreatic injury but not
inflammation in mice with acute pancreatitis. Gastroenterology.
141:2210–2217.e2. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Liu J, Huang L, Luo M and Xia X: Bacterial
translocation in acute pancreatitis. Crit Rev Microbiol.
45:539–547. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Li J, Wu Y, Zhang S, Zhang J, Ji F, Bo W,
Guo X and Li Z: Baicalein protect pancreatic injury in rats with
severe acute pancreatitis by inhibiting pro-inflammatory cytokines
expression. Biochem Biophys Res Commun. 466:664–669. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Roussel C, Caumes E, Thellier M, Ndour PA,
Buffet PA and Jauréguiberry S: Artesunate to treat severe malaria
in travellers: Review of efficacy and safety and practical
implications. J Travel Med. 24:taw0932017. View Article : Google Scholar
|
|
15
|
Lei XY, Tan RZ, Jia J, Wu SL, Wen CL, Lin
X, Wang H, Shi ZJ, Li B, Kang Y and Wang L: Artesunate relieves
acute kidney injury through inhibiting macrophagic Mincle-mediated
necroptosis and inflammation to tubular epithelial cell. J Cell Mol
Med. 25:8775–8788. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Cen Y, Liu C, Li X, Yan Z, Kuang M, Su Y,
Pan X, Qin R, Liu X, Zheng J and Zhou H: Artesunate ameliorates
severe acute pancreatitis (SAP) in rats by inhibiting expression of
pro-inflammatory cytokines and Toll-like receptor 4. Int
Immunopharmacol. 38:252–260. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
National Research Council (US): Committee
for the Update of the Guide for the Care and Use of Laboratory
Animals: Guide for the care and use of laboratory animals. 8th
edition. National Academies Press; Washington, DC: 2011
|
|
18
|
Zhang W, Wang G, Xu ZG, Tu H, Hu F, Dai J,
Chang Y, Chen Y, Lu Y, Zeng H, et al: Lactate is a natural
suppressor of RLR signaling by targeting MAVS. Cell.
178:176–189.e15. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Luo J, Wang N, Hua L, Deng F, Liu D, Zhou
J, Yuan Y, Ouyang F, Chen X, Long S, et al: The anti-sepsis effect
of isocorydine screened from guizhou ethnic medicine is closely
related to upregulation of vitamin d receptor expression and
inhibition of NFκB p65 translocation into the nucleus. J Inflamm
Res. 15:5649–5664. 2022. View Article : Google Scholar :
|
|
20
|
Li X, He C, Li N, Ding L, Chen H, Wan J,
Yang X, Xia L, He W, Xiong H, et al: The interplay between the gut
microbiota and NLRP3 activation affects the severity of acute
pancreatitis in mice. Gut Microbes. 11:1774–1789. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhang W, Xie Z, Fang X, Wang Z, Li Z, Shi
Y and Wang X, Li L and Wang X: Laboratory animal ethics education
improves medical students' awareness of laboratory animal ethics.
BMC Med Educ. 24:7092024. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Nomenclature Committee of the
International Union of Biochemistry (NC-IUB): 'Units of Enzyme
Activity'. Eur J Biochem. 97:319–320. 1979. View Article : Google Scholar
|
|
23
|
Geokas MC, Baltaxe HA, Banks PA, Silva J
Jr and Frey CF: Acute pancreatitis. Ann Intern Med. 103:86–100.
1985. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wang GJ, Gao CF, Wei D, Wang C and Ding
SQ: Acute pancreatitis: Etiology and common pathogenesis. World J
Gastroenterol. 15:1427–1430. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Armstrong JA, Cash N, Soares PMG, Souza
MHLP, Sutton R and Criddle DN: Oxidative stress in acute
pancreatitis: Lost in translation? Free Radic Res. 47:917–933.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Fernández-Sánchez A, Madrigal-Santillán E,
Bautista M, Esquivel-Soto J, Morales-González A, Esquivel-Chirino
C, Durante-Montiel I, Sánchez-Rivera G, Valadez-Vega C and
Morales-González JA: Inflammation, oxidative stress, and obesity.
Int J Mol Sci. 12:3117–3132. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Sheehan SJ, Lee JH, Wells CK and Topazian
M: Serum amylase, pancreatic stents, and pancreatitis after
sphincter of Oddi manometry. Gastrointest Endosc. 62:260–265. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Jang DI, Lee AH, Shin HY, Song HR, Park
JH, Kang TB, Lee SR and Yang SH: The role of tumor necrosis factor
alpha (TNF-α) in autoimmune disease and current TNF-α Inhibitors in
therapeutics. Int J Mol Sci. 22:27192021. View Article : Google Scholar
|
|
29
|
Shapouri-Moghaddam A, Mohammadian S,
Vazini H, Taghadosi M, Esmaeili SA, Mardani F, Seifi B, Mohammadi
A, Afshari JT and Sahebkar A: Macrophage plasticity, polarization,
and function in health and disease. J Cell Physiol. 233:6425–6440.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Dijkstra CD, Döpp EA, Joling P and Kraal
G: The heterogeneity of mononuclear phagocytes in lymphoid organs:
Distinct macrophage subpopulations in rat recognized by monoclonal
antibodies ED1, ED2 and ED3. Adv Exp Med Biol. 186:409–419.
1985.PubMed/NCBI
|
|
31
|
Mikami Y, Takeda K, Shibuya K, Qiu-Feng H,
Shimamura H, Yamauchi J, Egawa S, Sunamura M, Yagi H, Endo Y and
Matsuno S: Do peritoneal macrophages play an essential role in the
progression of acute pancreatitis in rats? Pancreas. 27:253–260.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Gros F and Muller S: The role of lysosomes
in metabolic and autoimmune diseases. Nat Rev Nephrol. 19:366–383.
2023. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Mareninova OA, Jia W, Gretler SR, Holthaus
CL, Thomas DDH, Pimienta M, Dillon DL, Gukovskaya AS, Gukovsky I
and Groblewski GE: Transgenic expression of GFP-LC3 perturbs
autophagy in exocrine pancreas and acute pancreatitis responses in
mice. Autophagy. 16:2084–2097. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Li X, He S and Ma B: Autophagy and
autophagy-related proteins in cancer. Mol Cancer. 19:122020.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Ngowi EE, Sarfraz M, Afzal A, Khan NH,
Khattak S, Zhang X, Li T, Duan SF, Ji XY and Wu DD: Roles of
hydrogen sulfide donors in common kidney diseases. Front Pharmacol.
11:5642812020. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Hu F, Lou N, Jiao J, Guo F, Xiang H and
Shang D: Macrophages in pancreatitis: Mechanisms and therapeutic
potential. Biomed Pharmacother. 131:1106932020. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Sendler M, Weiss FU, Golchert J, Homuth G,
Van Den Brandt C, Mahajan UM, Partecke LI, Döring P, Gukovsky I,
Gukovskaya A, et al: Cathepsin B-mediated activation of trypsinogen
in endocytosing macrophages increases severity of pancreatitis in
mice. Gastroenterology. 154:704–718.e10. 2018. View Article : Google Scholar
|
|
38
|
Saluja A, Dudeja V, Dawra R and Sah RP:
Early intra-acinar events in pathogenesis of pancreatitis.
Gastroenterology. 156:1979–1993. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Yasunaga K, Ito T, Miki M, Ueda K,
Fujiyama T, Tachibana Y, Fujimori N, Kawabe K and Ogawa Y: Using
CRISPR/Cas9 to knock out amylase in acinar cells decreases
pancreatitis-induced autophagy. Biomed Res Int. 2018:87193972018.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Selig L, Sack U, Gaiser S, Klöppel G,
Savkovic V, Mössner J, Keim V and Bödeker H: Characterisation of a
transgenic mouse expressing R122H human cationic trypsinogen. BMC
Gastroenterol. 6:302006. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Singh VK, Wu BU, Bollen TL, Repas K,
Maurer R, Mortele KJ and Banks PA: Early systemic inflammatory
response syndrome is associated with severe acute pancreatitis.
Clin Gastroenterol Hepatol. 7:1247–1251. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Lankisch PG, Assmus C, Lehnick D,
Maisonneuve P and Lowenfels AB: Acute pancreatitis: Does gender
matter? Dig Dis Sci. 46:2470–2474. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Mukherjee R, Mareninova OA, Odinokova IV,
Huang W, Murphy J, Chvanov M, Javed MA, Wen L, Booth DM, Cane MC,
et al: Mechanism of mitochondrial permeability transition pore
induction and damage in the pancreas: Inhibition prevents acute
pancreatitis by protecting production of ATP. Gut. 65:1333–1346.
2016. View Article : Google Scholar
|
|
44
|
Zhang L, Wu Z, Tong Z, Yao Q, Wang Z and
Li W: Vagus nerve stimulation decreases pancreatitis severity in
mice. Front Immunol. 11:5959572021. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Qi-Xiang M, Yang F, Ze-Hua H, Nuo-Ming Y,
Rui-Long W, Bin-Qiang X, Jun-Jie F, Chun-Lan H and Yue Z:
Intestinal TLR4 deletion exacerbates acute pancreatitis through gut
microbiota dysbiosis and Paneth cells deficiency. Gut Microbes.
14:21128822022. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Li H, Xie J, Guo X, Yang G, Cai B, Liu J,
Yue M, Tang Y, Wang G, Chen S, et al: Bifidobacterium spp. and
their metabolite lactate protect against acute pancreatitis via
inhibition of pancreatic and systemic inflammatory responses. Gut
Microbes. 14:21274562022. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Baron TH, DiMaio CJ, Wang AY and Morgan
KA: American gastroenterological association clinical practice
update: Management of pancreatic necrosis. Gastroenterology.
158:67–75.e1. 2020. View Article : Google Scholar
|
|
48
|
Sah RP, Garg P and Saluja AK: Pathogenic
mechanisms of acute pancreatitis. Curr Opin Gastroenterol.
28:507–515. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Biczo G, Vegh ET, Shalbueva N, Mareninova
OA, Elperin J, Lotshaw E, Gretler S, Lugea A, Malla SR, Dawson D,
et al: Mitochondrial dysfunction, through impaired autophagy, leads
to endoplasmic reticulum stress, deregulated lipid metabolism, and
pancreatitis in animal models. Gastroenterology. 154:689–703. 2018.
View Article : Google Scholar
|
|
50
|
Wu JS, Li WM, Chen YN, Zhao Q and Chen QF:
Endoplasmic reticulum stress is activated in acute pancreatitis. J
Dig Dis. 17:295–303. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Lu G, Tong Z, Ding Y, Liu J, Pan Y, Gao L,
Tu J, Wang Y, Liu G and Li W: Aspirin protects against acinar cells
necrosis in severe acute pancreatitis in mice. Biomed Res Int.
2016:60894302016. View Article : Google Scholar
|
|
52
|
Sendler M, Dummer A, Weiss FU, Krüger B,
Wartmann T, Scharffetter-Kochanek K, Van Rooijen N, Malla SR,
Aghdassi A, Halangk W, et al: Tumour necrosis factor α secretion
induces protease activation and acinar cell necrosis in acute
experimental pancreatitis in mice. Gut. 62:430–439. 2013.
View Article : Google Scholar
|
|
53
|
Munir F, Jamshed MB, Shahid N, Hussain HM,
Muhammad SA, Mamun AA and Zhang Q: Advances in immunomodulatory
therapy for severe acute pancreatitis. Immunol Lett. 217:72–76.
2020. View Article : Google Scholar
|
|
54
|
Mayer J, Rau B, Gansauge F and Beger HG:
Inflammatory mediators in human acute pancreatitis: Clinical and
pathophysiological implications. Gut. 47:546–552. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Mentula P, Kylänpää ML, Kemppainen E,
Jansson SE, Sarna S, Puolakkainen P, Haapiainen R and Repo H: Early
prediction of organ failure by combined markers in patients with
acute pancreatitis. Br J Surg. 92:68–75. 2005. View Article : Google Scholar
|
|
56
|
Noh KW, Pungpapong S, Wallace MB, Woodward
TA and Raimondo M: Do cytokine concentrations in pancreatic juice
predict the presence of pancreatic diseases? Clin Gastroenterol
Hepatol. 4:782–789. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Shimizu K: Pancreatic stellate cells:
Molecular mechanism of pancreatic fibrosis. J Gastroenterol
Hepatol. 23(Suppl 1): S119–S121. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Horváth IL, Bunduc S, Fehérvári P, Váncsa
S, Nagy R, Garmaa G, Kleiner D, Hegyi P, Erőss B and Csupor D: The
combination of ulinastatin and somatostatin reduces complication
rates in acute pancreatitis: A systematic review and meta-analysis
of randomized controlled trials. Sci Rep. 12:179792022. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Cohen J, Vincent JL, Adhikari NKJ, Machado
FR, Angus DC, Calandra T, Jaton K, Giulieri S, Delaloye J, Opal S,
et al: Sepsis: A roadmap for future research. Lancet Infect Dis.
15:581–614. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Bhattacharjee S, Coppens I, Mbengue A,
Suresh N, Ghorbal M, Slouka Z, Safeukui I, Tang HY, Speicher DW,
Stahelin RV, et al: Remodeling of the malaria parasite and host
human red cell by vesicle amplification that induces artemisinin
resistance. Blood. 131:1234–1247. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Zhao D, Zhang J, Xu G and Wang Q:
Artesunate protects LPS-induced acute lung injury by inhibiting
TLR4 expre-ssion and inducing Nrf2 activation. Inflammation.
40:798–805. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Gugliandolo E, D'amico R, Cordaro M, Fusco
R, Siracusa R, Crupi R, Impellizzeri D, Cuzzocrea S and Di Paola R:
Neuroprotective effect of artesunate in experimental model of
traumatic brain injury. Front Neurol. 9:5902018. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Yang RW, Shao ZX, Chen YY, Yin Z and Wang
WJ: Lipase and pancreatic amylase activities in diagnosis of acute
pancreatitis in patients with hyperamylasemia. Hepatobiliary
Pancreat Dis Int. 4:600–603. 2005.PubMed/NCBI
|
|
64
|
Schneider L, Jabrailova B, Strobel O,
Hackert T and Werner J: Inflammatory profiling of early
experimental necrotizing pancreatitis. Life Sci. 126:76–80. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Zhou X, Li W, Wang S, Zhang P, Wang Q,
Xiao J, Zhang C, Zheng X, Xu X, Xue S, et al: YAP aggravates
inflammatory bowel disease by regulating M1/M2 macrophage
polarization and gut microbial homeostasis. Cell Rep.
27:1176–1189.e5. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Zhang X, Kang Y, Li X, Huang Y, Qi R, Han
Y, Cai R, Gao Y and Qi Y: Potentilla discolor ameliorates
LPS-induced inflammatory responses through suppressing NF-κB and
AP-1 pathways. Biomed Pharmacother. 144:1123452021. View Article : Google Scholar
|
|
67
|
Liu Y, Yang L, Chen KL, Zhou B, Yan H,
Zhou ZG and Li Y: Knockdown of GRP78 promotes apoptosis in
pancreatic acinar cells and attenuates the severity of cerulein and
LPS induced pancreatic inflammation. PLoS One. 9:e923892014.
View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Liu Y, Zhou ZG, Chen KL, Zhou B, Yang L,
Yan H and Li Y: The ER chaperone GRP78 is associated with the
severity of cerulein-induced pancreatic inflammation via regulating
apoptosis of pancreatic acinar cells. Hepatogastroenterology.
59:1670–1676. 2012.PubMed/NCBI
|
|
69
|
Wu L, Cai B, Liu X and Cai H: Emodin
attenuates calcium overload and endoplasmic reticulum stress in
AR42J rat pancreatic acinar cells. Mol Med Rep. 9:267–272. 2014.
View Article : Google Scholar
|
|
70
|
Mayerle J, Sendler M, Hegyi E, Beyer G,
Lerch MM and Sahin-Tóth M: Genetics, cell biology, and
pathophysiology of pancreatitis. Gastroenterology.
156:1951–1968.e1. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Banks PA and Freeman ML; Practice
Parameters Committee of the American College of Gastroenterology:
Practice guidelines in acute pancreatitis. Am J Gastroenterol.
101:2379–2400. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Gloor B, Müller CA, Worni M, Martignoni
ME, Uhl W and Büchler MW: Late mortality in patients with severe
acute pancreatitis. Br J Surg. 88:975–979. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Wang X, Zhou G, Liu C, Wei R, Zhu S, Xu Y,
Wu M and Miao Q: Acanthopanax versus 3-methyladenine ameliorates
sodium taurocholate-induced severe acute pancreatitis by inhibiting
the autophagic pathway in rats. Mediators Inflamm.
2016:83697042016. View Article : Google Scholar
|
|
74
|
Hoque R, Farooq A, Ghani A, Gorelick F and
Mehal WZ: Lactate reduces liver and pancreatic injury in Toll-like
receptor- and inflammasome-mediated inflammation via GPR81-mediated
suppression of innate immunity. Gastroenterology. 146:1763–1774.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Wang Y, Wang G, Cui L, Liu R, Xiao H and
Yin C: Angiotensin 1-7 ameliorates caerulein-induced inflammation
in pancreatic acinar cells by downregulating Toll-like receptor
4/nuclear factor-κB expression. Mol Med Rep. 17:3511–3518.
2018.
|
|
76
|
Chang RJ, Wang HL, Qin MB, Liang ZH, He
JP, Wei YL, Fu HZ and Tang GD: Ghrelin inhibits IKKβ/NF-κB
activation and reduces pro-inflammatory cytokine production in
pancreatic acinar AR42J cells treated with cerulein. Hepatobiliary
Pancreat Dis Int. 20:366–375. 2021. View Article : Google Scholar
|
|
77
|
Sun H, Tian J and Li J: MiR-92b-3p
ameliorates inflammation and autophagy by targeting TRAF3 and
suppressing MKK3-p38 pathway in caerulein-induced AR42J cells. Int
Immunopharmacol. 88:1066912020. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Wang S, Ni HM, Chao X, Wang H, Bridges B,
Kumer S, Schmitt T, Mareninova O, Gukovskaya A, De Lisle RC, et al:
Impaired TFEB-mediated lysosomal biogenesis promotes the
development of pancreatitis in mice and is associated with human
pancreatitis. Autophagy. 15:1954–1969. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Han F, Xiao QQ, Peng S, Che XY, Jiang LS,
Shao Q and He B: Atorvastatin ameliorates LPS-induced inflammatory
response by autophagy via AKT/mTOR signaling pathway. J Cell
Biochem. 119:1604–1615. 2018. View Article : Google Scholar
|
|
80
|
Cicchini M, Karantza V and Xia B:
Molecular pathways: Autophagy in cancer-a matter of timing and
context. Clin Cancer Res. 21:498–504. 2015. View Article : Google Scholar
|