Introduction
Multiple myeloma (MM) represents a hematologic
neoplasm characterized by the presence of aberrant clonal plasma
cells within the bone marrow environment. According to an annual
report from the American Cancer Society for 2023, an approximate
total of 35,730 novel cases of MM were predicted to be diagnosed in
the US, alongside a count of 12,590 fatalities attributed to this
condition (1). Consequently, a
profound comprehension of the intricate mechanisms underlying the
pathogenesis of MM is of significant importance.
Currently, treatment of patients newly diagnosed
with MM starts with induction therapy, followed by consolidation
therapy and finally maintenance therapy for those who are suitable
for hematopoietic stem cell transplantation (2-6).
The existing therapeutic approach during the induction therapy
phase involves the consideration of the impact of lenalidomide on
stem cell drug toxicity and the hepatotoxicity associated with
high-dose chemotherapy in the elderly demographic. The
incorporation of B-cell maturation antigen (BCMA) antibodies into
this phase of treatment prompts an inquiry into the potential
enhancement of accessibility to autologous stem cell
transplantation consolidation within this patient population
(2,7,8).
In consolidation treatment, stratified treatment is used to
evaluate whether the patients are suitable for autologous stem cell
transplantation according to their age, scores of the Eastern
Cooperative Oncology Group and the state of complications. Among
them, the combination of bortezomib, lenalidomide and dexamethasone
(VRD) has been selected as the preferred induction regimen if the
condition of primary MM allowed, and hematopoietic stem cell
collection was performed within 4 cycles to reduce the toxicity of
lenalidomide on hematopoietic stem cells and its effect on later
implantation (9,10). In addition, there are certain
reports addressing the potential to improve complete response (CR)
rates and achieve profound remissions through the combination of
BCMA-targeted agents with proteasome inhibitors and
immunomodulatory drugs. It is noteworthy that BCMA-targeted
therapeutics are presently predominantly used in patients with MM
who have relapsed, are refractory or have relapsed following
multiple lines of therapy (11).
During the maintenance phase of MM treatment, the National
Comprehensive Cancer Network and European Society for Medical
Oncology guidelines recommend lenalidomide as the preferred
maintenance therapy for MM, but certain patients are insensitive or
intolerant to it (11,12). In such cases, alternative
therapies are essential to maintain therapeutic efficacy (13).
BCMA is mostly expressed on mature B lymphocytes,
with its overexpression and activation associated with human MM
progression (14). BCMA is a
transmembrane glycoprotein of type III, with 184 amino acids and
possessing a molecular weight of 20.2 kDa, featuring an
extracellular N-terminus with a conserved 6-cysteine motif. This
glycoprotein is categorized as a constituent of the necrosis factor
family (10,15,16). BCMA interacts with the following
tumor necrosis factors (TNF) family members: B-cell activator of
the TNF family (BAFF) and a proliferation-inducing ligand (APRIL)
to induce plasma-cell survival. The progression of human MM is
associated with an increase in the expression of BAFF and APRIL in
serum instead of the bone marrow microenvironment. Upon binding to
APRIL or BAFF, BCMA influences B cells by promoting their
proliferation, survival, differentiation and maturation into plasma
cells; this modulation is essential for the prolonged survival of
plasma cells (17,18). In the context of MM, both BCMA
mRNA levels and the abundance of BCMA protein are markedly elevated
when compared to normal plasma cells (19). This substantial upregulation of
BCMA represents a pivotal factor in the initiation and progression
of MM, making it an essential target for treatment (20). Drug resistance in MM arises
through various mechanisms, including genetic and cellular changes.
Polymorphisms in multidrug resistance genes and the overexpression
of P-glycoprotein in MM cells contribute to resistance (21). Alterations in the tumor
microenvironment, such as increased cell adhesion and activation of
anti-apoptotic cytokine pathways like JAK/STAT and PI3K/AKT, also
play a significant role. Clonal evolution, including the
hyperexpression of proteasome 26S subunit ubiquitin receptor,
non-ATPase 4 due to chromosome 1q21 amplification, and the
post-treatment accumulation of
CD34+CD138+B7−H1+CD19−
plasma cells, further contributes to resistance. Additionally, the
dysregulation of various microRNAs impacts MM cell survival, the
cell cycle and the microenvironment, leading to resistance to
therapies such as bortezomib. Furthermore, programmed cell death 1
(PD-1) is upregulated on T cells in patients with MM, while PD-1
ligand 1 expression is elevated on MM cells, further contributing
to immune evasion and resistance (5,15,16,20,22).
A survey commissioned by the MM Patients Advocacy
Group revealed that 92% of patients with MM were aware of
BCMA-targeted therapies. The top three concerns include the
assessment of efficacy, consideration of potential side effects and
evaluation of eligibility criteria. Patients expressed their
willingness to embrace novel drugs and undergo monitoring for
potential side effects. This finding underscores the
open-mindedness of patients with MM towards adopting innovative
treatment modalities (21).
Currently, BCMA has emerged as a promising candidate
for diverse therapeutic interventions, encompassing targeted
therapies, and has additionally demonstrated potential as a
prognostic biomarker. The multifaceted roles played by BCMA
underscore its significance in the pathogenesis and clinical
management of MM. For instance, Dhakal et al (23) conducted a phase 3, randomized,
controlled trial on patients with 1-3 prior lines of therapy who
were refractory to lenalidomide, targeting BCMA. The trial yielded
promising results, with a CR rate of ≥73.1% and an overall response
of 84.6%, highlighting the potential efficacy of BCMA-targeted
therapies in a specific subset of patients with MM and underscores
the significance of ongoing research and clinical trials in this
area (14,24).
BCMA
As a biomarker
The National Institute of Health defines a biomarker
as an objectively measured indicator of biological processes,
whether normal, pathogenic or in response to a therapeutic agent
(25,26). Biomarkers, found in body fluids
or tissues, can be genomic, transcriptomic, proteomic or
clinicopathologic, and serve diagnostic, prognostic or predictive
purposes (27,28). Due to the small portion of the MM
plasma cells, more reliable biomarkers that can be widely used are
needed to guide treatment decisions (29).
Flow cytometry is a crucial tool for diagnosing MM
by determining the proportion of monoclonal plasma cells in bone
marrow and common markers including CD38, CD138 and CD56 are used
to distinguish neoplastic myeloma cells from reactive plasma cells
in clinical practice (30).
However, these markers have separate limitations: CD138 degrades
with temperature and time, CD38 can be influenced by other diseases
or medications and CD56 is a non-specific marker for MM (30-32). BCMA is highly expressed on
malignant plasma cells and high expression of BCMA in serum is
associated with the progression and the objective response rate
(ORR) in patients with MM and mouse models (33,34). Numerous studies have screened the
diagnostic utility of BCMA, predicting treatment responses and
assessing prognosis. Notably, a reduction in soluble BCMA (sBCMA)
has been consistently observed in various clinical trials,
correlating with an improved ORR (33). Furthermore, BCMA antigen is
overexpressed in abnormal plasma cells and it is stable and not
easily degraded by temperature (35,36). In addition, BCMA shows consistent
expression in newly diagnosed and relapsed/refractory (R/R) MM,
suggesting its potential as a biomarker across disease stages
(9).
Monitoring serum sBCMA levels and minimal residual
disease (MRD) levels of BCMA expression by flow cytometry also
provides valuable insight (37).
Lower sBCMA levels indicate effective treatment, while higher
levels are associated with the tumor burden (38,39). With a short half-life of 24-26 h,
sBCMA quickly reflects treatment effects, making it a sensitive
marker for monitoring disease status. This offers an advantage over
traditional M-protein monitoring, whose serum half-life typically
spans three to four weeks (40,41). In addition, anti-BCMA antibody
treatments are less prone to immune escape, making sBCMA a reliable
tumor marker (9,42).
As a prognostic indicator
Prognostic assessment tools for MM include ISS
staging, R-ISS staging, the 2014 IMWG criteria and Mayo staging,
alongside genetic analysis via fluorescence in situ
hybridization (FISH) or the detection of complex karyotypes via
high-resolution chromosome examination (43). In a cohort comprising 36 MM
patient samples, a hybridization technique was employed using three
distinct probes designed for chromosome 13q (43-45). The outcomes of this analysis
indicated that the utilization of FISH studies indicated not only
the translocation of chromosome 14q32 but also 17p13 yielded
notably meaningful findings during the clinical follow-up of
patients with MM. These results confirmed the precise detection of
chromosome mutation in patients with MM with FISH (38,46,47). However, these methods can be
costly, time-consuming and require specialized expertise,
highlighting the need for novel prognostic markers which can be
easily accessed.
sBCMA has emerged as a valuable predictor of
progression-free survival (PFS) in patients with MM (48). High sBCMA levels before treatment
are linked to poorer clinical outcomes and elevated levels during
early treatment phases suggest an unfavorable prognosis (33,49). Various methodologies employed for
the detection of BCMA in serum may contribute to the observed
association between sBCMA levels and clinical prognosis (50). sBCMA, shed from MM cells,
indicates tumor burden and quickly reflects changes in response to
treatment, offering a rapid prognostic tool (20,41).
BCMA-targeted therapies do not interfere with sBCMA
detection and BCMA can be used in high-throughput flow cytometry to
assess MRD. This method is currently more reliable for disease
monitoring than next-generation sequencing, though there is no
consensus on the definition of a negative MRD test (51).
As a target
Given the established efficacy of BCMA-targeted
therapy, numerous patients now have access to innovative drugs for
combatting R/R MM. Certain diagnostic tools and biomarkers have
demonstrated drug-targeted value, and the advent of flow cytometry
and sequencing technologies facilitates the monitoring of MRD
(50). These advances enable the
identification of specific high-risk groups and patients with R/R
MM. One of the primary objectives in the design of BCMA-targeting
drugs is to address these high-risk groups and improve the
prognosis of MM and R/R MM and the ultimate goal is to delay
disease recurrence and progression, with the intention of extending
overall survival (OS). Presently, there exist three predominant
strategies for the treatment of R/R MM and MM targeting BCMA
including monoclonal antibodies, antibody-drug conjugates and
emerging therapies.
Monoclonal antibodies
These agents target antigens upregulated in MM
cells, engaging immune mechanisms such as complement-dependent
cytotoxicity, antibody-dependent cellular cytotoxicity (ADCC) and
antibody-dependent cellular phagocytosis (ADCP) (52). For instance, GSK2857916, a
glycosylated IgG1 BCMA antibody drug (53,54), which enhances BCMA-targeted
therapy efficacy by improving ADCC through stronger binding to the
FC gamma receptor (FcgR) IIIa/CD16a receptor, has shown efficacy in
phase 2 trials with an ORR of 33%. Its cytotoxic payload was
activated upon targeted delivery and was demonstrated to be more
potent compared to non-targeted drugs; its hydrolysis-resistant
linker ensures stability and efficient transport (16,55).
Furthermore, GSK2857916 induces cell cycle arrest
and apoptosis in MM cells, triggers ADCC and ADCP through
interactions with natural killer (NK) cells, peripheral blood
mononuclear cells and macrophages, competes with BAFF and APRIL to
reduce NF-κB activation and diminishes NF-κB pathway support for
MM-cell proliferation and drug resistance (52,53,56,57) (Fig. 1A).
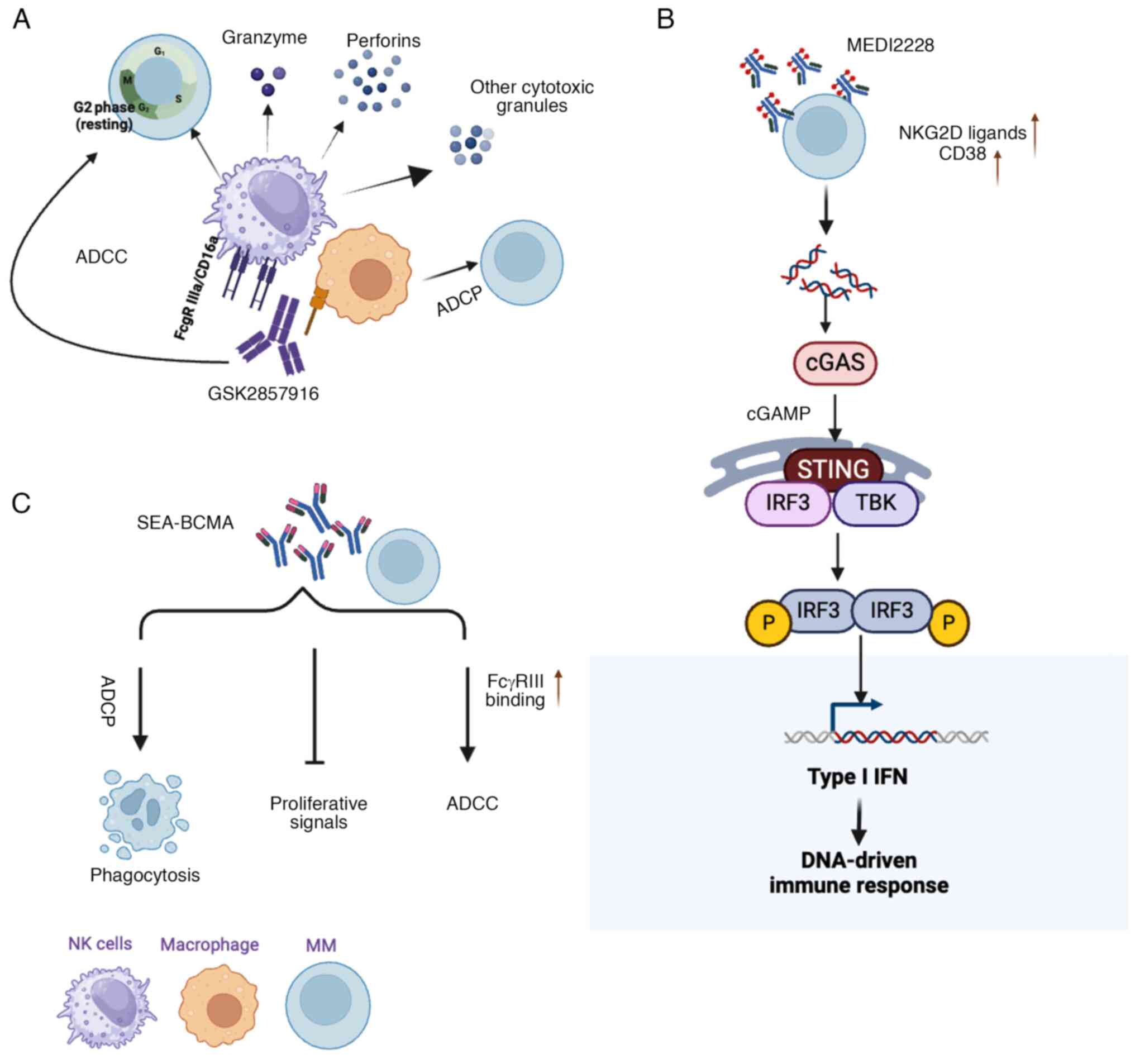 | Figure 1Mechanisms of action of three
BCMA-target drugs. (A) GSK2857916 induces MM-cell death via ADCC
and ADCP: Enhances efficacy by increasing NMM NMN pathway. (B)
SEA-BCMA inhibits BCMA through three primary mechanisms: Blocking
pro-survival signaling, enhancing ADCC and promoting phagocytosis.
triggers ADCP through interactions with macrophages. (C) MEDI2228
activates the IFN-regulated innate immune response via the
cGAS-STING-TBK1-IRF3 NMO MM, multiple myeloma; NK, natural killer;
BCMA, B-cell maturation antigen; ADCC, antibody-dependent cellular
cytotoxicity; ADCP, antibody-dependent cellular phagocytosis; cGAS,
cyclic GMP-AMP synthase; STING, stimulator of interferon genes;
TBK1, TANK-binding kinase-1; IRF3, interferon regulatory
factor-3. |
Antibody-drug conjugates (ADCs)
ADCs consist of monoclonal antibodies that can
specifically target antigens on tumor cells. Once ADCs bind to the
surface antigens expressed on tumor cells, toxic payloads are
released from ADCs, resulting in the microtubule inhibition or DNA
damage in the tumor cells. AMG224 and MEDI2228 are examples of ADCs
targeting BCMA. AMG224 achieved an ORR of 23% in phase 1 trials of
patients with MM and R/R MM with lower sBCMA (36,38,58).
MEDI2228 is a fully human ADC targeting BCMA, linked
to a DNA cross-linking pyrrolobenzodiazepine dimer through a
protease-cleavable linker, and it synergizes with DNA damage
response inhibitors to induce apoptotic cell death via DNA
cross-link formation (59).
Clinical studies have shown its superior efficacy compared to
monomethyl myricin F analogs, particularly in the presence of
elevated sBCMA levels, with ongoing Phase 1 trials assessing its
use as monotherapy in R/R MM conditions with the mechanism of
interferon (IFN)-stimulated gene enrichment following the DNA
damage-ATM/ATR-checkpoint kinase 1/2 pathway (60,61).
MEDI2228 also activates the IFN-regulated innate
immune response through the cyclic GMP-AMP synthase-stimulator of
interferon genes-TANK binding kinase 1-interferon regulatory
factors (IRF)3 and signal transducer and activator of transcription
1-IRF1 pathways in MM cells, demonstrating efficacy against both
proliferating and quiescent MM cells in vitro, though it is
less effective against BCMA-negative cells. In murine models, a
single low-dose intravenous administration of HDP-101, an analogous
ADC, induced tumor regression and achieved sustained complete
remission (61) (Fig. 1B).
Emerging therapies
There are also emerging therapies targeting BCMA to
enhance clinical outcomes. SEA-BCMA is a humanized IgG1 monoclonal
antibody targeting BCMA, showing promising early clinical results
with an ORR of 30%. It inhibits BCMA-mediated pro-survival
signaling, enhances ADCC and phagocytosis and has demonstrated
potential for durable anti-tumor activity, particularly in patients
with advanced, heavily pretreated MM (59,62). Preliminary data from the
SGN-BCMA-001 (NCT03582033) trial support its favorable safety
profile and suggest potential for synergistic combinations. Despite
the advantages over CAR-T therapy, such as accessibility and
applicability to elderly patients, BCMA-targeted therapies may pose
toxic side effects and economic burdens with prolonged use
(62,63) (Fig. 1C). However, it is essential to
acknowledge that these therapies may entail potential toxic side
effects and their efficacy when used as single agents tends to be
moderately effective. In addition, the prolonged use of these drugs
may impose a considerable economic burden.
BCMA-targeting strategies to modulate the
immune microenvironment (IME)
Having gained insight into the biological
characteristics of BCMA and its involvement in the pathways driving
tumor progression, this next chapter will delve into ongoing
clinical trials focusing on drugs that influence the IME to extend
survival through administration of the optimal doses with mitigated
adverse effects and maximal survival (8).
Bispecific T-cell engagers (BiTEs)
BiTEs represent a specialized form of targeted
immunotherapy that operates independently of major
histocompatibility complex restriction but the requirement for
intact antigen-presenting cell function (64). These BiTEs facilitate T
cell-mediated cytotoxicity by directly binding T cells to surface
antigens present on tumor cells. Upon interaction with both the
target tumor cell and T-cell lymphocyte, BiTEs initiate the
activation of the T-cell receptor and subsequent downstream
signaling events (65). BiTEs
are designed with one or more high-affinity domains capable of
binding the target protein on the tumor cell and a single
lower-affinity domain that binds a protein on immune cells,
particularly CD3 on T cells (64,65) (Fig. 2). Certain BiTEs also target other
T-cell receptors, such as CD16, NKG2D and, notably, G-protein
coupled receptor C family 5D (GPRC5D), which is a non-BCMA-directed
T-cell engager. GPRC5D is highly expressed on the surface of plasma
cells (66-68).
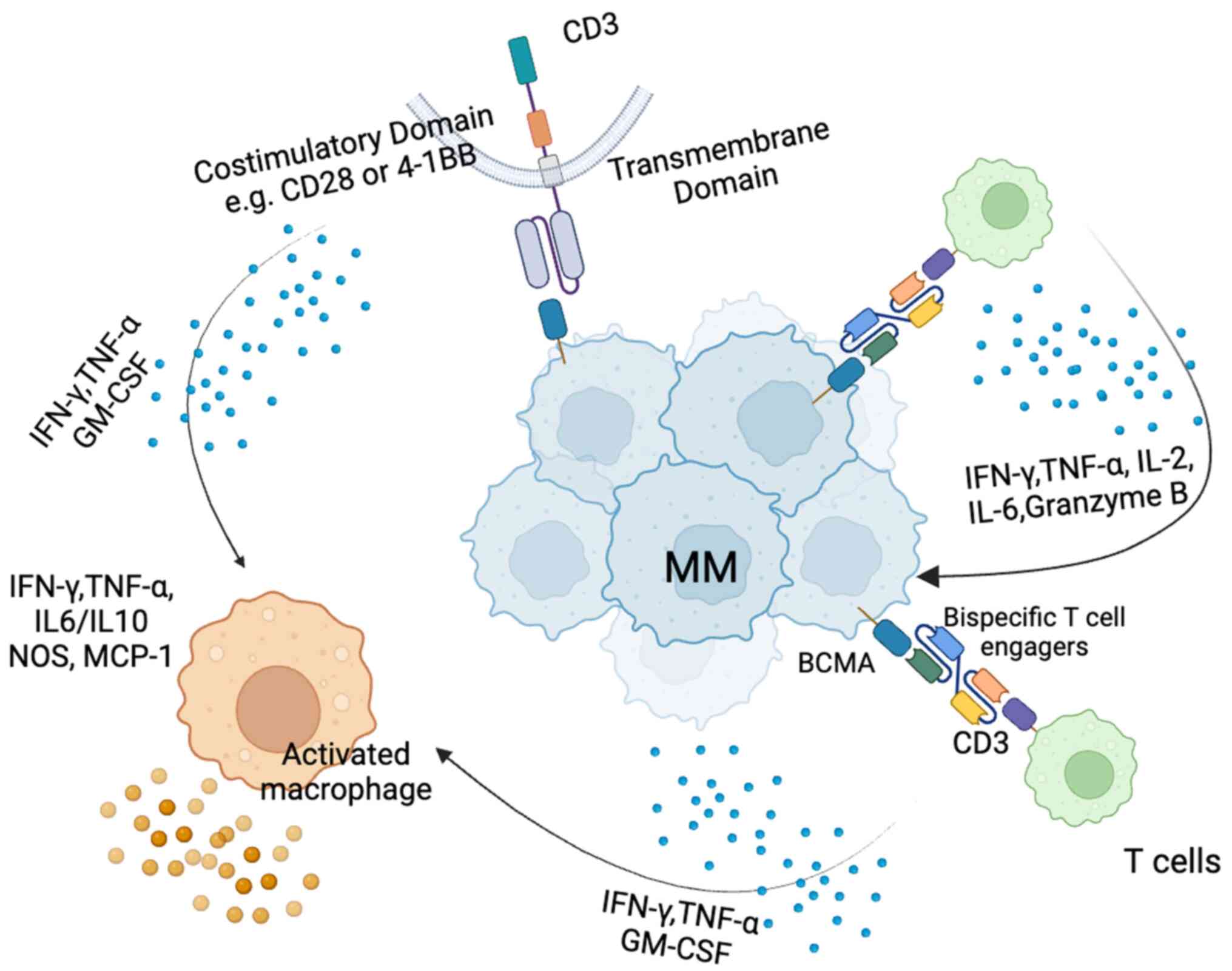 | Figure 2Mechanism of action of bispecific
T-cell engagers and CAR-T cells in MM. Upon binding to BCMA
expressed on MM cells, bispecific T cells release cytokines such as
IFN-γ and TNF-α, which can further activate macrophages. These
activated macrophages, also influenced by CAR T cells, release
additional cytokines (e.g., IFN-γ, TNF-α, IL-6 and IL-10) into the
TIME, thereby reshaping the TIME and enhancing the anti-tumor
response. CAR, chimeric antigen receptor; MM, multiple myeloma;
BCMA, B-cell maturation antigen; TIME, tumor immune
microenvironment; IFN, interferon; TNF, tumor necrosis factor; IL,
interleukin 2. |
This section reviews the latest clinical trial
results of BiTEs targeting BCMA in the treatment of MM,
highlighting their potential to improve outcomes for patients with
R/RMM, along with their advantages and disadvantages (Table I) (69).
 | Table ISummary of clinical trials for
bispecific T-cell engagers targeting BCMA. |
Table I
Summary of clinical trials for
bispecific T-cell engagers targeting BCMA.
| Product name | Antigen target | Clincial phase | Condition | Patients, n | ORR, % | ClinicalTrials
ID | Sponsor |
|---|
| Teclistamab | BCMA | Phase 1-2
study | R/R MM | 165 | ORR, 65; CR,
30 | NCT03145181 | Johnson &
Johnson |
| Teclistamab | BCMA | Phase 3 | R/R MM | 560 | Ongoing | NCT05083169 | Johnson &
Johnson |
| REGN5428 | BCMA | Phase 1 | R/R MM | 252 | ORR, 60; CR,
31.3 | NCT03761108 | Regeneron
Pharmaceuticals |
| Elranatamab | BCMA | Phase 1 | R/R MM | 123 | ORR, 68; | NCT03269136 CR,
38.2 | Pfizer |
| ABBV-383 | BCMA | Phase 1 | R/R MM | 124 | ORR, 68; | NCT03933735 CR,
N/A | TeneoOne Inc. |
| AMG 701 | BCMA | Phase 1 | R/R MM | 75 | At a dose | NCT03287908 of 9
mg: ORR, 83; CR, N/A | Amgen |
| CC93269 | BCMA | Phase 1 | R/R MM | 70 | ORR, 83.3; | NCT03486067 CR,
N/A; CR, N/A | Celgene |
Teclistamab
Teclistamab, a BCMA-CD3 bispecific antibody, is
effective in treating R/R MM and can be administered intravenously
(IV) or subcutaneously (S.C.). In the NCT03145181 trial,
teclistamab achieved an ORR of 65% in 165 patients, with responses
showing durability and an increasing depth over time (70). In addition, in the phase 3 trial
sponsored by Janssen Research Development, conducted in patients
with a median age of 64 years who had received more than three
prior therapies, an ORR of 64% was observed, with 30% achieving a
CR or partial response (PR) (63). The reduction in sBCMA levels
correlated with treatment response, indicating its potential as a
biomarker.
A reduction of 82% in BCMA was observed in 27
patients who had achieved a stringent CR (sCR), while an 87%
reduction was noted in the 8 patients exhibiting a CR. Furthermore,
a reduction of 90% was identified in 34 patients demonstrating a
very good PR (VGPR) (63,70).
The ongoing phase 3 NCT05083169 trial, launched by Janssen
Research, is evaluating the efficacy and safety of teclistamab in
combination with daratumumab, compared to other treatment options
in patients with R/R MM. This research provides valuable insights
into the optimal treatment for this patient population. The results
demonstrate substantial clinical activity of teclistamab in heavily
pretreated R/R MM, favorably comparing to existing therapies,
including CAR-T. While side effects such as cytokine release
syndrome (CRS) and neutropenia were observed, CRS events were
generally mild, with few grade 3 and no grade 4 occurrences. The
lower-grade CRS profile with teclistamab supports its potential for
outpatient administration. Although toxic effects were common, they
were mainly low-grade and reversible, indicating a significant
clinical benefit for a broader patient population (71).
REGN5428
REGN5428, a bispecific antibody targeting BCMA and
CD3, was evaluated in a Phase 1 clinical trial (NCT03761108) using
a 4+3 dose-escalation design, with IV weekly administration
followed by biweekly maintenance. At the highest dose level, the
trial achieved an ORR of 60% in 252 patients with a median age of
66 years. Among the participants, 81.3% achieved a VGPR, 31.3%
reached a CR and 43.8% maintained remission for over four months
(72).
Results from Phase 2 studies released this year
focused on dose optimization between 50 and 200 mg. The results
revealed that patients treated with 200 mg reached a higher
efficacy than those receiving 50 mg, with an ORR of 64 and 50%,
respectively. Of note, the 200-mg dose also correlated with fewer
AEs, with 95% of patients experiencing adverse events (AEs) vs.
100% in the 50-mg group. Common treatment-emergent AEs included CRS
in 37% of the 200-mg group (1% Grade 3) and 53% of the 50-mg group
(2% Grade 3). Fatigue occurred in 32% of the 200-mg group and 33%
of the 50-mg group, with no severe cases. Anemia was reported in
28% of the 200-mg group (24% severe) and 40% of the 50-mg group
(36% severe) (72-74). This clinical trial has recently
concluded enrollment and is currently in the follow-up phase.
Recently, an additional open-label, multi-cohort
clinical trial (NCT05137054) was initiated to evaluate the efficacy
of REGN5458 in conjunction with anti-CD38 antibody,
immunomodulatory drug (IMiD) and 1 proteasome inhibitor (PI) in
patients with R/R MM. Results indicate that when combining REGN5428
with IMiDs, PIs and anti-CD38 agents, the higher concentration of
200 mg may be a more effective option for treating R/R MM (74).
Elranatamab
Elranatamab, also known as PF-06863135, is a
humanized IgG2-type bispecific antibody that targets BCMA and CD3.
It can be administered weekly either IV or S.C. Administration
leads to slow absorption, with a half-life of 3 to 7 days (75-77). In the phase I NCT03269136 trial,
Elranatamab, a monoclonal antibody for treating R/R MM, was
administered at varying concentrations. Among those receiving S.C.
monotherapy, 55 patients were treated at efficacious dose levels
(≥215 μg/kg), while 10 patients treated with sub-efficacious
dose levels (80 or 130 μg/kg) did not achieve an
IMWG-confirmed PR or better. With a median follow-up of 12 months,
the ORR was 63.6, and 38.2% of patients achieved a CR or better,
with no grade ≥3 AEs observed (76,77). These results highlight how
immunotherapeutic approaches have expanded treatment options for
patients with R/R MM with a higher dose, underscoring the
importance of therapy to enhance efficacy while minimizing toxicity
for each patient. In the Phase II clinical trial (NCT04649359), 123
patients who had previously undergone non-BCMA-targeted therapies
were enrolled. Participants received S.C. elranatamab at a dose of
76 mg once weekly in 28-day cycles, following two step-up priming
doses of 12 and 32 mg administered on day 1 and 4 of cycle 1,
respectively. A CR or better was observed in 35.0% of the patients,
while a VGPR or better was achieved in 56.1% of the cohort. The
easy assessment of elranatamab posits it as a viable alternative
option to CAR-T-cell therapy. Further clinical trials should be
performed to observe the AEs and efficacy of elranatamab given at a
biweekly dose, aiming for it to maintain its high efficacy while
minimizing AEs (77,78).
ABBV-383
ABBV-383, a BCMA x CD3 bispecific T-cell-redirecting
antibody, is administered IV once every three weeks in patients
with R/RMM, showing low AEs. In the NCT03933735 Phase I trial,
which utilized a 3+3 design with backfilling for dose escalation,
the treatment demonstrated promising outcomes (79). The trial showed an impressive 68%
ORR in patients receiving doses of 40 mg or higher. As a
monotherapy, ABBV-383 demonstrated significant efficacy at both 40-
and 60-mg doses, with a median PFS of 13.7 and 11.2 months,
respectively. The 12-month duration of response (DOR) was 70% at 40
mg and 66% at 60 mg, with the median DOR not yet reached in
patients achieving a CR or better (80,81). However, the ORR at 60 mg was
slightly lower at 59%. Hematologic treatment-emergent AEs (TEAEs)
were mainly neutropenia (37%) and anemia (29%), while
non-hematologic TEAEs included CRS (57%) and fatigue (30%)
(82,83). ABBV-383 administration at doses
of 20, 40 and 60 mg can led to a rapid but transient induction of
key proinflammatory cytokines (IL-6, IL-8, IFN-γ and TNF-α) and a
gradual decrease in sBCMA levels, correlating with the therapeutic
response. In addition, ABBV-383 promoted T-cell activation and
proliferation, as indicated by increased expression of CD69 and
Ki67 markers on peripheral CD4 and CD8 T cells in evaluable
patients. These findings highlight ABBV-383's significant potential
to prolong OS in patients with R/R MM even with low doses (<60
mg) (81).
Furthermore, it is worth noting that a dose of 40 or
60 mg of ABBV-383 every three weeks has been selected for further
exploration as the optimal regimen. The low-dosing frequency and
off-the-shelf availability make ABBV-383 a convenient option for
patients with R/R MM compared to CAR-T therapy. In addition, its
high efficacy is comparable to that of ADC belantamab mafodotin and
CAR-T-cell therapies, which is 32 and 61.5-97%, respectively
(82).
AMG 701
AMG 701 represents a bispecific single-chain
variable fragment (scFv) antibody that incorporates a hexahistidine
tag for identification and purification. Its mode of administration
involves IV infusions administered weekly, organized within 4-week
cycles (84). The Phase I
clinical trial aimed to evaluate the safety, tolerability and
anti-myeloma activity of AMG701, as well as to estimate a
biologically active dose. The results indicate that the response to
AMG701 is dose-dependent. At doses ranging from 3 to 12 mg, the
response rate was 36% (16 out of 45 patients), while with an
earlier dose escalation to 9 mg, the response rate increased
significantly to 83%, including 3 PRs and 2 VGPRs. While the
underlying mechanisms require further exploration, studies in a
mouse model showed that AMG701 increases survival, potentially
through the induction of PD-1 expression on T cells. Furthermore,
combining AMG701 with a PD-1-blocking antibody enhanced its
efficacy, alongside increased concentrations of cytokines, such as
IFN-γ and IL-2. These findings suggest a promising potential for
combining anti-PD-1 therapy with AMG701(83,84).
CC-93269
CC-93269 is an asymmetric 2-arm humanized IgG T-cell
engager that binds bivalently to BCMA and monovalently to CD3ε in a
2+1 format. It can induce interaction between T cells and
BCMA-expressing myeloma cells and induces T-cell receptor/CD3
cross-linking leading to T-cell activation, and release of
proinflammatory cytokines and cytolytic enzymes, resulting in
MM-cell death (NCT03486067) (16,85).
The initial report released in 2019, involving 19
patients with a median age of 64 years who were resistant to their
last line of treatment, aimed to assess the safety and anti-tumor
response of CC-93269 at doses ranging from 0.15 to 10 mg. Among the
12 patients treated with ≥6 mg in Cycle 1, 10 achieved a PR or
better, yielding an ORR of 83.3%. This included 7 patients (58.3%)
who achieved a VGPR or better and 4 patients (33.3%) who attained
an sCR; 9 patients (75.0%) also achieved MRD negativity (86).
A subsequent clinical trial report from 2022 for
measuring the efficacy of CC-93269, which included 70 patients,
indicated that 39% (27/70) achieved an objective response, with a
median PFS of 13.3 weeks. All CRS events were limited to grade 1
(45%) or grade 2 (9%). Notably, none of the patients discontinued
treatment due to AEs and no treatment-related deaths occurred. At
the 30-mg target dose, observed trough concentrations by Cycle 2
Day 1, the predicted levels required for efficacy were exceeded
(85-87). The number of patients in the
clinical trial for CC-93269 was limited and additional participants
are needed to draw broader conclusions.
CAR-T BCMA drugs have shown promising
efficacy in the treatment of R/R MM
Over 20 CAR-T products targeting BCMA are currently
in clinical trials, with variations in design, BCMA epitopes,
co-stimulation thresholds and viral vectors leading to differences
in efficacy and challenges in production time and cost (88,89).
CAR-T therapy is the most effective BCMA-targeted
treatment for R/R MM, generally achieving a 50-93% ORR in patients
who have relapsed after three or more lines of therapy; the
structure and clinical efficacy of major second-generation
CAR-T-cell therapies are summarized in Table II (16,90,91). The therapy activates T-cells'
tumor-killing abilities through specific antigen recognition and
co-stimulatory signals, but the associated toxicities, including
CRS, immune effector cell-associated neurotoxicity syndrome
(ICANS), hematologic toxicity, infection and tumor lysis syndrome,
limit its application (Fig. 2).
CRS, triggered by cytokine release, affects >60% of patients,
with the severity influenced by disease type, CAR-T dose and
co-stimulatory molecules, while ICANS presents with neurological
symptoms and requires similar treatment (91-93). Hematological toxicity, mainly due
to lymphocyte depletion and off-target effects, increases infection
and bleeding risks, which can be managed with transfusions and
growth factors (94).
 | Table IIOverview of ongoing/completed
next-generation CAR-T cell therapy targeting B-cell maturation
antigen. |
Table II
Overview of ongoing/completed
next-generation CAR-T cell therapy targeting B-cell maturation
antigen.
| Product name | ScFv | Costimulatory
molecule | Platform of
virus | Outcome, % | CRS, % | Clinical trial
no. |
|---|
| bb2121 | Rat | 4-1BB | Lentivirus | ORR, 85; CR,
45 | 76 | NCT02658929 |
| bb21217 | Rat | 4-1BB | Lentivirus | ORR, 83; CR,
25 | 67 | NCT03274219 |
| BRD015 | Rat | CD28 | Lentivirus | ORR, 93; CR,
73 | 76 |
ChiCTR-OPC-16009113 |
| LCAR-B38M | UA | 4-1BB | Lentivirus | ORR, 88; CR,
68 | 89 | NCT03090659 |
| MCARH171 | Human | 4-1BB | Retrovirus | ORR, 64; CR, 0 | 60 | NCT03070327 |
| CT103A | human | 4-1BB | Lentivirus | ORR, 100; CR,
70 | 94 | ChiCTR
1800018137) |
| JCARH125 | Human | 4-1BB | Lentivirus | ORR, 82; CR,
27 | 80 | NCT03430011 |
| FCARH143 | Human | 4-1BB | Lentivirus | ORR, 100; CR,
36 | 91 | NCT03338972 |
In this review, recent advances in clinical trials
related to CAR-T therapies targeting BCMA were summarized and the
future directions for these drugs were outlined (Table III).
 | Table IIISummary of clinical trials for CAR-T
cells targeting BCMA. |
Table III
Summary of clinical trials for CAR-T
cells targeting BCMA.
| Product name | Antigen target | Clincial phase | Condition | Patients, n | Response, % | Clinical Trials
ID | Sponsor |
|---|
| bb2121 | BCMA | Phase 1 | R/R MM | 33 | ORR, 85; CR,
45 | NCT02658929 | Celgene |
| CT103A | BCMA | Phase 1b/2 | R/R MM | 18 | ORR, 96 CR,
74.3 | NCT05066646 | Nanjing IASO
Biotechnology Co., Ltd. |
| GC012F | BCMA and CD19 | Phase I | R/R MM | 29 | ORR, 93; CR,
82.8 | NCT04236011;
NCT04182581 | Gracell
Biotechnologies |
| MCARH171 | BCMA | Phase 1 | R/R MM | 11 | ORR, 64; CR,
N/A | NCT03070327 | Memorial Sloan
Kettering Cancer Center |
bb2121
b2121, also known as Idecabtagene vicleucel, a CAR
T-cell therapy targeting BCMA, shows significant potential in the
treatment of MM. It is the first CAR-T-cell therapy approved for
treating R/R MM after four or more prior therapies. bb2121 shows
reduced antigen-independent signaling and strong in vitro
cytotoxicity against myeloma cells with varying BCMA expression.
Previously, in an in vivo NOD scid gamma (NSG) mouse model
of human MM, bb2121 achieved rapid and sustained tumor elimination
(13,35).
In the Phase I clinical trial (NCT02658929), which
included 33 patients with MM who received CAR-T-cell infusions
ranging from 10-800×106 in the dose-escalation phase and
150 or 450×106 in the expansion phase, an impressive ORR
of 85% was achieved, with 9 patients attaining CR without any
relapse. Patients who received >150×106 demonstrated
a better response rate (35).
Additionally, the clearance of bone marrow plasma
cells occurs rapidly, typically within one month, and the incidence
of AEs is low, primarily restricted to grade 1 or 2. Importantly,
the efficacy of the treatment appears to be independent of BCMA
expression, as both high and low expression levels result in
similarly high response rates, indicating its potential
applicability for a broad range of MMs. However, there are
limitations to consider. The small sample size limits the
generalizability of the findings and future studies should involve
a larger patient cohort to better evaluate the treatment's efficacy
(35).
In the subsequent Phase II clinical trial conducted
in Japanese patients with R/R MM, an impressive ORR of 89% was
achieved with a median follow-up of 12.9 months. The treatment was
also associated with low AEs: Predominantly grade 1 or 2. Notably,
the regimen involving 450×106 CAR-positive T cells
offered the potential for a treatment-free period in this
challenging patient population. However, with only 9 patients
included to date, further studies with a larger cohort are needed
to validate these efficacy findings (95).
CT103A
CT103A constitutes a fully human CAR-T-cell product
designed to target BCMA. Clinical investigations of CT103A include
a phase 1, single-arm, open-label trial conducted at Tongji
Hospital in China (NCT05066646). The study enrolled 18 patients
with R/R MM, all of whom had received a minimum of three prior
lines, and the follow-up was 394 days. Patients with MM resistant
to CAR-T-cell therapy still benefited from CT103A, achieving an ORR
of 98% with low AEs including no observed immune effector
cell-associated neurotoxicity syndrome. After 1 year, the PFS rate
was 58.3% for all cohorts and 79.1% for the patients without
extramedullary myeloma. Furthermore, even at the lower dosage
levels (1 and 3×106 CAR-T cells/kg), CT103A demonstrated
continued activity and effectiveness with minimal side effects
(95,96).
Regarding safety outcomes, the predominant late AEs
observed 8 weeks post-CAR-T-cell infusion were hematologic
toxicities, notably leukopenia and neutropenia. In addition,
increasing the number of patients in the study would strengthen the
remarkably high-efficacy results (97).
GC012F
GC012F is a dual-targeting CAR-T-cell therapy that
targets both BCMA and CD19. It was developed using the innovative
FasTCAR-T platform within 22-36 h (98).
The overall response rates were 93.1% (27/29) and
most AEs were grade 2 or lower (79.3%), with only 6.9% of patients
experiencing grade 3 CRS. GC012F persisted for a median of 410 days
(range, 51-1,183 days) and notably remained detectable in 79.3% of
patients at 6 months and in 55.2% at 12 months post-infusion. sBCMA
plasma levels began to decline by day 4 in 80% of patients,
decreased sharply by day 10 in all patients and reached minimal
levels from 30 to 60 days post-infusion in all patients
(NCT04236011; NCT04182581) (99).
These robust response rates and extended durations
of response underscore the promising therapeutic efficacy of the
studied cohort but more R/R MM patients should be included for
future exploration (98).
MCARH171
MCARH171, a type of next-generation anti-BCMA CAR-T
cell, was investigated in patients with R/R MM (100). A total of 11 patients with R/R
MM were treated, with a median of six prior therapy lines,
achieving a 64% ORR and a median response duration of 106 days in
the NCT03070327 clinical trial. Peak expansion and persistence of
MCARH171, along with durable clinical responses, were
dose-dependent. Patients treated with ≤150×106 CAR-T
cells had lower peak expansion in their peripheral blood compared
to those receiving ≥450×106 CAR-T cells, showing
promising efficacy at higher doses. Peak expansion was associated
with response durability, independent of the dose level (91,100). The clinical trial of this drug
is constrained by a short follow-up duration and a limited number
of participants. However, while the drug shows significant efficacy
in treating R/R MM, its performance is not comparable to that of
other CAR-T therapies (100).
Conclusion and future perspective
The efficacy of BCMA as a therapeutic target in MM
or R/R MM has been demonstrated for common ADC drugs, bispecific
antibody T-cell activators and CAR-T-cell therapy. Overall, CAR-T
cell therapy and BiTEs activators were more efficacious than ADC
drugs alone, reflecting the superiority of antitumor immunotherapy
over traditional single-target ADC drugs. In addition,
non-BCMA-targeted CAR-T-cell therapies have shown promising
results, further contributing to options against the disease. CAR-T
cells can specifically target MM cells, either through a single
target or dual targets (e.g., BCMA and CD19), and a single dose has
been shown to provide sustained efficacy for >12 months with
lower AEs. Among the therapies, CAR-T-cell therapy demonstrates the
highest therapeutic efficacy on average. However, it is also the
most expensive compared to other treatments, including ADCs and
BiTEs. In addition, the low administration frequency of certain
BiTEs, such as ABBV-383, which is given every three weeks, offers
an economic advantage by potentially reducing the financial burden
on patients (81). Although
CAR-T and BCMA-targeted T-cell activators are not recommended as a
first- and second-line therapy, early remission needs to be
achieved in MM treatment to improve PFS and OS. From an individual
perspective, tumor cells have the ability to exist in the body for
a long period of time and even the current MRD test can not say for
sure that there are no tumor cells in a patient with CR. Further
expansion of CAR-T therapy and other immunotherapies can be used as
a supplement and assistance to the immune system to fight tumors.
The current means should be improved to achieve a longer PFS time
for patients, ulimately improving the quality of life. Furthermore,
there is still a need to explore the impact on the tumor
microenvironment, the process of the emergence of antigen escape
and how tumor cells repair damaged DNA when treating with
BCMA-targeted CAR-T or bispecific antibody T-cell activators.
Recent studies have shed light on immune-related
factors influencing the efficacy of CAR-T cell therapies,
particularly in R/R MM. One study on R/R MM demonstrated that
patients with higher numbers of activated/functional T cells and
fewer CD163+ macrophages prior to treatment were more likely to
experience a durable response to CD19 CAR-T-cell therapy (101). Similarly, resistance to
BCMA-targeted CAR-T-cell and BCMA-CD3 BiTE therapies in patients
with MM has been linked to a distinct immunological profile.
Single-cell sequencing of patient samples revealed an increased
proportion of terminally exhausted T cells and a low CD4 ratio, and
diminished populations of stem cell memory T cells and central
memory T cells are associated with CAR-T therapy resistance
(102). In addition,
upregulation of T cell immunoreceptor with Lg and ITIM domains + NK
cells and an increase in interferon-responsive dendritic cells were
identified as contributing factors to therapy resistance (103,104). These immune changes are thought
to impair the anti-tumor efficacy of CAR-T-cell therapies in MM by
promoting T-cell exhaustion and diminishing the overall immune
response (105).
In summary, general therapies targeting BCMA, such
as CAR-T cells, BiTEs and ADCs, have demonstrated significant
efficacy for MM and R/R MM treatment. While CAR-T therapy displays
significant therapeutic potential and durability, high cost and
accessibility remain major challenges, alongside the complexity of
managing resistance mechanisms. Future research should focus on
optimizing these therapies to improve patient access, reduce costs
and develop strategies to counteract tumor immune evasion,
including understanding the tumor microenvironment and resistance
pathways. Expanding on these areas may enhance treatment options,
potentially extending PFS and OS for patients with MM and R/R
MM.
Availability of data and materials
Not applicable.
Authors' contributions
RX, MW, LW and HZ contributed to the conception of
the study. RX, MW, LW, MP, YW and HZ contributed to the drafting of
the manuscript; RX, MW, MP, LW and HZ critically revised the
manuscript for important intellectual content. Data authentication
is not applicable. All authors read and approved the final version
of the manuscript and take responsibility for the decision to
submit for publication.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Acknowledgements
Not applicable.
Funding
The publication and research funding is supported by grant of
Jinhua Science and Technology Bureau (grant no. 2022-4-258 to RX),
Hengyang City Science and Technology Plan Projects in Hunan
Province, China (grant no. 202330046276 to HZ) and the Hunan
Provincial Department of Education Scientific Research Project
Fund, Hunan Province, China (grant no. 22C0209 to HZ).
References
|
1
|
Siegel RL, Giaquinto AN and Jemal A:
Cancer statistics, 2024. CA Cancer J Clin. 74:12–49. 2024.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Seckinger A, Delgado JA, Moser S, Moreno
L, Neuber B, Grab A, Lipp S, Merino J, Prosper F, Emde M, et al:
Target expression, generation, preclinical activity, and
pharmacokinetics of the BCMA-T cell bispecific antibody em801 for
multiple myeloma treatment. Cancer Cell. 31:396–410. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Bazarbachi AH, Al Hamed R, Malard F,
Bazarbachi A, Harousseau JL and Mohty M: Induction therapy prior to
autologous stem cell transplantation (ASCT) in newly diagnosed
multiple myeloma: An update. Blood Cancer J. 12:472022. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wang L, Luo R, Onyshchenko K, Rao X, Wang
M, Menz B, Gaedicke S, Grosu AL, Firat E and Niedermann G: Adding
liposomal doxorubicin enhances the abscopal effect induced by
radiation/αPD1 therapy depending on tumor cell mitochondrial DNA
and cGAS/STING. J Immunother Cancer. 11:e0062352023. View Article : Google Scholar
|
|
5
|
Rahmy S, Cheng X, Wang M, Feng H, Qiu W,
Zhao R and Lu X: Organ-specific regulation of CHD1 by acute PTEN
and p53 loss in mice. Biochem Biophys Res Commun. 525:614–619.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wang M, Rao X, Gaedicke S, Wang L, Menz B,
Multhoff G and Niedermann G: Adding a PPARα agonist enhances t
cell-mediated effects of RT in combination with anti-PD-1. Int J
Radiat Oncol Biol Phys. 120(Suppl 1): e409–e410. 2024. View Article : Google Scholar
|
|
7
|
Ali SA, Shi V, Maric I, Wang M, Stroncek
DF, Rose JJ, Brudno JN, Stetler-Stevenson M, Feldman SA, Hansen BG,
et al: T cells expressing an anti-B-cell maturation antigen
chimeric antigen receptor cause remissions of multiple myeloma.
Blood. 128:1688–1700. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hu M, Wang L, Su D, Yuan Q, Xiao C, Guo L,
Wang M, Kang C, Zhang J and Zhou T: Evaluation of mycotoxins,
mycobiota and toxigenic fungi in the traditional medicine Radix
Dipsaci. Front Microbiol. 15:14546832024. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Sanchez E, Li M, Kitto A, Li J, Wang CS,
Kirk DT, Yellin O, Nichols CM, Dreyer MP, Ahles CP, et al: Serum
B-cell maturation antigen is elevated in multiple myeloma and
correlates with disease status and survival. Br J Haematol.
158:727–738. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
X R, W M, W G, Z L, W X, C W and W C:
Chemotherapy-induced toxic epidermal necrolysis in a patient with
multiple myeloma, a case report and literature review. Front Oncol.
13:12274482023. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Salem DA, Maric I, Yuan CM, Liewehr DJ,
Venzon DJ, Kochenderfer J and Stetler-Stevenson M: Quantification
of B-cell maturation antigen, a target for novel chimeric antigen
receptor T-cell therapy in Myeloma. Leuk Res. 71:106–111. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Fabi A, Bhargava R, Fatigoni S, Guglielmo
M, Horneber M, Roila F, Weis J, Jordan K and Ripamonti CI; ESMO
Guidelines Committee: Electronic address
clinicalguidelines@esmo.org: Cancer-related fatigue: ESMO clinical
practice guidelines for diagnosis and treatment. Ann Oncol.
31:713–723. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Friedman KM, Garrett TE, Evans JW, Horton
HM, Latimer HJ, Seidel SL, Horvath CJ and Morgan RA: Effective
targeting of multiple B-cell maturation antigen-expressing
hematological malignances by anti-B-cell maturation antigen
chimeric antigen receptor T cells. Hum Gene Ther. 29:585–601. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kleber M, Ntanasis-Stathopoulos I and
Terpos E: BCMA in multiple myeloma-A promising key to therapy. J
Clin Med. 10:40882021. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Abramson HN: B-cell maturation antigen
(BCMA) as a target for new drug development in relapsed and/or
refractory multiple myeloma. Int J Mol Sci. 21:51922020. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yu B, Jiang T and Liu D: BCMA-targeted
immunotherapy for multiple myeloma. J Hematol Oncol. 13:1252020.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Tai YT, Acharya C, An G, Moschetta M,
Zhong MY, Feng X, Cea M, Cagnetta A, Wen K, van Eenennaam H, et al:
APRIL and BCMA promote human multiple myeloma growth and
immunosuppression in the bone marrow microenvironment. Blood.
127:3225–3236. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Coquery CM and Erickson LD: Regulatory
roles of the tumor necrosis factor receptor BCMA. Crit Rev Immunol.
32:287–305. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Novak AJ, Darce JR, Arendt BK, Harder B,
Henderson K, Kindsvogel W, Gross JA, Greipp PR and Jelinek DF:
Expression of BCMA, TACI, and BAFF-R in multiple myeloma: A
mechanism for growth and survival. Blood. 103:689–694. 2004.
View Article : Google Scholar
|
|
20
|
Shah N, Chari A, Scott E, Mezzi K and
Usmani SZ: B-cell maturation antigen (BCMA) in multiple myeloma:
Rationale for targeting and current therapeutic approaches.
Leukemia. 34:985–1005. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Oaknin A, Fariñas-Madrid L, García-Duran
C, Martin LP, O'Malley DM, Schilder RJ, Uyar D, Moroney JW, Diaz
JP, Spira AI, et al: Luveltamab tazevibulin (STRO-002), an
anti-folate receptor alpha (FolRα) antibody drug conjugate (ADC),
safety and efficacy in a broad distribution of FolRα expression in
patients with recurrent epithelial ovarian cancer (OC): Update of
STRO-002-GM1 phase 1 dose expansion cohort. J Clin Oncol. 41(Suppl
16): S55082023. View Article : Google Scholar
|
|
22
|
Rao X, Onyshchenko K, Wang L, Wang M and
Niedermann G: Comparison of two triple therapy regimens for
enhancing the abscopal effect in mice. Int J Radiat Oncol Biol
Phys. 117:e2562023. View Article : Google Scholar
|
|
23
|
Dhakal B, Yong K, Harrison SJ, Mateos MV,
Moreau P, van de Donk NWCJ, Sidana S, Popat R, Lendvai N, Lonardi
C, et al: First phase 3 results from CARTITUDE-4: Cilta-cel versus
standard of care (PVd or DPd) in lenalidomide-refractory multiple
myeloma. J Clin Oncol. 41:LBA1062023. View Article : Google Scholar
|
|
24
|
Dhakal B, Szabo A, Chhabra S, Hamadani M,
D'Souza A, Usmani SZ, Sieracki R, Gyawali B, Jackson JL,
Asimakopoulos F and Hari PN: Autologous transplantation for newly
diagnosed multiple myeloma in the era of novel agent induction: A
systematic review and meta-analysis. JAMA Oncol. 4:343–350. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Gopalakrishnan S, D'Souza A, Scott E,
Fraser R, Davila O, Shah N, Gale RP, Kamble R, Diaz MA, Lazarus HM,
et al: Revised international staging system is predictive and
prognostic for early relapse (<24 months) after autologous
transplantation for newly diagnosed multiple myeloma. Biol Blood
Marrow Transplant. 25:683–688. 2019. View Article : Google Scholar
|
|
26
|
Lin J, Zhang G, Lou B, Sun Y, Jia X, Wang
M, Zhou J and Xia Z: Identification of copper metabolism-related
markers in Parkinson's disease. Ann Med. 56:24250642024. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Strimbu K and Tavel JA: What are
biomarkers? Curr Opin HIV AIDS. 5:463–466. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Califf RM: Biomarker definitions and their
applications. Exp Biol Med (Maywood). 243:213–221. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Oliva S, Gambella M, Gilestro M, Muccio
VE, Gay F, Drandi D, Ferrero S, Passera R, Pautasso C, Bernardini
A, et al: Minimal residual disease after transplantation or
lenalidomide-based consolidation in myeloma patients: A prospective
analysis. Oncotarget. 8:5924–5935. 2017. View Article : Google Scholar :
|
|
30
|
Jeong TD, Park CJ, Shim H, Jang S, Chi HS,
Yoon DH, Kim DY, Lee JH, Lee JH, Suh C and Lee KH: Simplified flow
cytometric immunophenotyping panel for multiple myeloma,
CD56/CD19/CD138(CD38)/CD45, to differentiate neoplastic myeloma
cells from reactive plasma cells. Korean J Hematol. 47:260–266.
2012. View Article : Google Scholar
|
|
31
|
Bayer-Garner IB, Sanderson RD, Dhodapkar
MV, Owens RB and Wilson CS: Syndecan-1 (CD138) immunoreactivity in
bone marrow biopsies of multiple myeloma: Shed syndecan-1
accumulates in fibrotic regions. Mod Pathol. 14:1052–1058. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Van De Donk NWCJ, Richardson PG and
Malavasi F: CD38 antibodies in multiple myeloma: Back to the
future. Blood. 131:13–29. 2018. View Article : Google Scholar
|
|
33
|
Sanchez E, Gillespie A, Tang G, Ferros M,
Harutyunyan NM, Vardanyan S, Gottlieb J, Li M, Wang CS, Chen H and
Berenson JR: Soluble B-cell maturation antigen mediates
tumor-induced immune deficiency in multiple myeloma. Clin Cancer
Res. 22:3383–3397. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Flores-Montero J, de Tute R, Paiva B,
Perez JJ, Böttcher S, Wind H, Sanoja L, Puig N, Lecrevisse Q,
Vidriales MB, et al: Immunophenotype of normal vs myeloma plasma
cells: Toward antibody panel specifications for MRD detection in
multiple myeloma. Cytometry B Clin Cytom. 90:61–72. 2016.
View Article : Google Scholar
|
|
35
|
Raje N, Berdeja J, Lin Y, Siegel D,
Jagannath S, Madduri D, Liedtke M, Rosenblatt J, Maus MV, Turka A,
et al: Anti-BCMA CAR T-cell therapy bb2121 in relapsed or
refractory multiple myeloma. N Engl J Med. 380:1726–1737. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Chen H, Li M, Xu N, Ng N, Sanchez E, Soof
CM, Patil S, Udd K, Bujarski S, Cao J, et al: Serum B-cell
maturation antigen (BCMA) reduces binding of anti-BCMA antibody to
multiple myeloma cells. Leuk Res. 81:62–66. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Cohen AD, Garfall AL, Stadtmauer EA,
Melenhorst JJ, Lacey SF, Lancaster E, Vogl DT, Weiss BM, Dengel K,
Nelson A, et al: B cell maturation antigen-specific CAR T cells are
clinically active in multiple myeloma. J Clin Invest.
129:2210–2221. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Lee L, Bounds D, Paterson J, Herledan G,
Sully K, Seestaller-Wehr LM, Fieles WE, Tunstead J, McCahon L,
Germaschewski FM, et al: Evaluation of B cell maturation antigen as
a target for antibody drug conjugate mediated cytotoxicity in
multiple myeloma. Br J Haematol. 174:911–922. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Visram A, Soof C, Rajkumar SV, Kumar SK,
Bujarski S, Spektor TM, Kyle RA, Berenson JR and Dispenzieri A:
Serum BCMA levels predict outcomes in MGUS and smoldering myeloma
patients. Blood Cancer J. 11:1202021. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Bujarski S, Soof C, Li M, Wang CS, Sanchez
E, Emamy-Sadr M, Swift R, Rahbari K, Patil S, Spektor TM, et al:
Baseline and early changes in serum B-cell maturation antigen
levels predict progression free survival and response status for
multiple myeloma patients in a phase 1 trial evaluating
ruxolitinib, lenalidomide and methylprednisolone. Blood. 132(Suppl
1): 18942018. View Article : Google Scholar
|
|
41
|
Ghermezi M, Li M, Vardanyan S, Harutyunyan
NM, Gottlieb J, Berenson A, Spektor TM, Andreu-Vieyra C, Petraki S,
Sanchez E, et al: Serum B-cell maturation antigen: A novel
biomarker to predict outcomes for multiple myeloma patients.
Haematologica. 102:785–795. 2017. View Article : Google Scholar :
|
|
42
|
Mailankody S, Htut M, Lee KP, Bensinger W,
Devries T, Piasecki J, Ziyad S, Blake M, Byon J and Jakubowiak A:
JCARH125, anti-BCMA CAR T-cell Therapy for relapsed/refractory
multiple myeloma: Initial proof of concept results from a phase 1/2
multicenter study (EVOLVE). Blood. 132(Suppl 1): 9572018.
View Article : Google Scholar
|
|
43
|
Palumbo A, Avet-Loiseau H, Oliva S,
Lokhorst HM, Goldschmidt H, Rosinol L, Richardson P, Caltagirone S,
Lahuerta JJ, Facon T, et al: Revised international staging system
for multiple myeloma: A report from international myeloma working
group. J Clin Oncol. 33:2863–2869. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Dimopoulos MA, Kastritis E, Michalis E,
Tsatalas C, Michael M, Pouli A, Kartasis Z, Delimpasi S, Gika D,
Zomas A, et al: The international scoring system (ISS) for multiple
myeloma remains a robust prognostic tool independently of patients'
renal function. Ann Oncol. 23:722–729. 2012. View Article : Google Scholar
|
|
45
|
Landgren O and Rajkumar SV: New
developments in diagnosis, prognosis, and assessment of response in
multiple myeloma. Clin Cancer Res. 22:5428–5433. 2016. View Article : Google Scholar
|
|
46
|
Yuregir OO, Sahin FI, Yilmaz Z, Kizilkilic
E, Karakus S and Ozdogu H: Fluorescent in situ hybridization
studies in multiple myeloma. Hematology. 14:90–94. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Huber D, Voith Von Voithenberg L and
Kaigala GV: Fluorescence in situ hybridization (FISH): History,
limitations and what to expect from micro-scale FISH? Micro Nano
Eng. 1:15–24. 2018. View Article : Google Scholar
|
|
48
|
Laurent SA, Hoffmann FS, Kuhn PH, Cheng Q,
Chu Y, Schmidt-Supprian M, Hauck SM, Schuh E, Krumbholz M, Rübsamen
H, et al: γ-Secretase directly sheds the survival receptor BCMA
from plasma cells. Nat Commun. 6:73332015. View Article : Google Scholar
|
|
49
|
Coquery CM, Loo WM, Wade NS, Bederman AG,
Tung KS, Lewis JE, Hess H and Erickson LD: BAFF regulates
follicular helper t cells and affects their accumulation and
interferon-γ production in autoimmunity. Arthritis Rheumatol.
67:773–784. 2015. View Article : Google Scholar :
|
|
50
|
Oliva S, Bruinink DHO, Rihova L,
D'Agostino M, Pantani L, Capra A, van der Holt B, Troia R, Petrucci
MT, Villanova T, et al: Minimal residual disease assessment by
multiparameter flow cytometry in transplant-eligible myeloma in the
EMN02/HOVON 95 MM trial. Blood Cancer J. 11:1062021. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Sanchez E, Tanenbaum EJ, Patil S, Li M,
Soof CM, Vidisheva A, Waterman GN, Hekmati T, Tang G, Wang CS, et
al: The clinical significance of B-cell maturation antigen as a
therapeutic target and biomarker. Expert Rev Mol Diagn. 18:319–329.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Montes De Oca R, Alavi AS, Vitali N,
Bhattacharya S, Blackwell C, Patel K, Seestaller-Wehr L, Kaczynski
H, Shi H, Dobrzynski E, et al: Belantamab mafodotin (GSK2857916)
drives immunogenic cell death and immune-mediated antitumor
responses in vivo. Mol Cancer Ther. 20:1941–1955. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Trudel S, Lendvai N, Popat R, Voorhees PM,
Reeves B, Libby EN, Richardson PG, Hoos A, Gupta I, Bragulat V, et
al: Antibody-drug conjugate, GSK2857916, in relapsed/refractory
multiple myeloma: an update on safety and efficacy from dose
expansion phase I study. Blood Cancer J. 9:372019. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Touzeau C, Moreau P and Dumontet C:
Monoclonal antibody therapy in multiple myeloma. Leukemia.
31:1039–1047. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Lassiter G, Bergeron C, Guedry R, Cucarola
J, Kaye AM, Cornett EM, Kaye AD, Varrassi G, Viswanath O and Urits
I: Belantamab mafodotin to treat multiple myeloma: A comprehensive
review of disease, drug efficacy and side effects. Curr Oncol.
28:640–660. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Trudel S, Lendvai N, Popat R, Voorhees PM,
Reeves B, Libby EN, Richardson PG, Anderson LD Jr, Sutherland HJ,
Yong K, et al: Targeting B-cell maturation antigen with GSK2857916
antibody-drug conjugate in relapsed or refractory multiple myeloma
(BMA117159): A dose escalation and expansion phase 1 trial. Lancet
Oncol. 19:1641–1653. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Tai YT, Mayes PA, Acharya C, Zhong MY, Cea
M, Cagnetta A, Craigen J, Yates J, Gliddon L, Fieles W, et al:
Novel anti-B-cell maturation antigen antibody-drug conjugate
(GSK2857916) selectively induces killing of multiple myeloma.
Blood. 123:3128–3138. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Lee HC, Raje NS, Landgren O, Upreti VV,
Wang J, Avilion AA, Hu X, Rasmussen E, Ngarmchamnanrith G, Fujii H
and Spencer A: Phase 1 study of the anti-BCMA antibody-drug
conjugate AMG 224 in patients with relapsed/refractory multiple
myeloma. Leukemia. 35:255–258. 2021. View Article : Google Scholar
|
|
59
|
Taft D, Henderson C, O'Day C, Yu C, Li H,
Ho P and Van Epps H: Pharmacodynamics of SEA-BCMA, a nonfucosylated
antibody targeting BCMA, in patients with relapsed/refractory
multiple myeloma. Blood. 138(Suppl 1): 11972021. View Article : Google Scholar
|
|
60
|
Kumar SK, Migkou M, Bhutani M, Spencer A,
Ailawadhi S, Kalff A, Walcott F, Pore N, Gibson D, Wang F, et al:
Phase 1, first-in-human study of MEDI2228, a BCMA-targeted ADC in
patients with relapsed/refractory multiple myeloma. Blood.
136(Suppl 1): S26–S27. 2020. View Article : Google Scholar
|
|
61
|
Xing L, Wang S, Liu J, Yu T, Chen H, Wen
K, Li Y, Lin L, Hsieh PA, Cho SF, et al: BCMA-specific ADC MEDI2228
and daratumumab induce synergistic myeloma cytotoxicity via
IFN-driven immune responses and enhanced CD38 expression. Clin
Cancer Res. 27:5376–5388. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Hoffman JE, Lipe B, Melear J, Liedtke M,
Schroeder MA, Niesvizky R, Tomasson MH, Yasenchak CA, Green DJ, Li
H, et al: SEA-BCMA, an investigational nonfucosylated monoclonal
antibody: Ongoing results of a phase 1 study in patients with
relapsed/refractory multiple myeloma (SGNBCMA-001). Blood.
138(Suppl 1): S27402021. View Article : Google Scholar
|
|
63
|
Nooka AK, Moreau P, Usmani SZ, Garfall A,
van de Donk NWCJ, San-Miguel JF, Rocafiguera AO, Chari A, Karlin L,
Mateos MV, et al: Teclistamab, a B-cell maturation antigen (BCMA) x
CD3 bispecific antibody, in patients with relapsed/refractory
multiple myeloma (RRMM): Updated efficacy and safety results from
MajesTEC-1. J ClinOncol. 40(16Suppl): S80072022. View Article : Google Scholar
|
|
64
|
Huehls AM, Coupet TA and Sentman CL:
Bispecific T-cell engagers for cancer immunotherapy. Immunol Cell
Biol. 93:290–296. 2015. View Article : Google Scholar
|
|
65
|
Friedrich MJ, Neri P, Kehl N, Michel J,
Steiger S, Kilian M, Leblay N, Maity R, Sankowski R, Lee H, et al:
The pre-existing T cell landscape determines the response to
bispecific T cell engagers in multiple myeloma patients. Cancer
Cell. 41:711–725.e6. 2023. View Article : Google Scholar
|
|
66
|
Ellerman D: Bispecific T-cell engagers:
Towards understanding variables influencing the in vitro potency
and tumor selectivity and their modulation to enhance their
efficacy and safety. Methods. 154:102–117. 2019. View Article : Google Scholar
|
|
67
|
Verkleij CPM, Broekmans MEC, Van Duin M,
Frerichs KA, Kuiper R, de Jonge AV, Kaiser M, Morgan G, Axel A,
Boominathan R, et al: Preclinical activity and determinants of
response of the GPRC5DxCD3 bispecific antibody talquetamab in
multiple myeloma. Blood Adv. 5:2196–2215. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Atamaniuk J, Gleiss A, Porpaczy E, Kainz
B, Grunt TW, Raderer M, Hilgarth B, Drach J, Ludwig H, Gisslinger
H, et al: Overexpression of G protein-coupled receptor 5D in the
bone marrow is associated with poor prognosis in patients with
multiple myeloma. Eur J Clin Invest. 42:953–960. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Shah Z, Malik MN, Batool SS, Kotapati S,
Akhtar A, Rehman OU, Ghani M, Sadiq M, Akbar A, Ashraf A, et al:
Bispecific T-cell engager (BiTE) antibody based immunotherapy for
treatment of relapsed refractory multiple myeloma (RRMM): A
systematic review of preclinical and clinical trials. Blood.
134(Suppl 1): S55672019. View Article : Google Scholar
|
|
70
|
Usmani SZ, Garfall AL, Van De Donk NWCJ,
Nahi H, San-Miguel JF, Oriol A, Rosinol L, Chari A, Bhutani M,
Karlin L, et al: Teclistamab, a B-cell maturation antigen x CD3
bispecific antibody, in patients with relapsed or refractory
multiple myeloma (MajesTEC-1): A multicentre, open-label,
single-arm, phase 1 study. Lancet. 398:665–674. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Mateos MV, Bahlis NJ, Costa LJ, Perrot A,
Pei L, Rubin ML, Lantz K, Sun W, Jaffe M, Kobos R and Nooka AK:
MajesTEC-3: Randomized, phase 3 study of teclistamab plus
daratumumab versus investigator's choice of daratumumab,
pomalidomide, and dexamethasone or daratumumab, bortezomib, and
dexamethasone in patients with relapsed/refractory multiple
myeloma. J Clin Oncol. 40(16 Suppl): TPS80722022. View Article : Google Scholar
|
|
72
|
Madduri D, Rosko A, Brayer J, Zonder J,
Bensinger WI, Li J, Xu L, Adriaens L, Chokshi D, Zhang W, et al:
REGN5458, a BCMA x CD3 bispecific monoclonal antibody, induces deep
and durable responses in patients with relapsed/refractory multiple
myeloma (RRMM). Blood. 136(Suppl 1): S41–S42. 2020. View Article : Google Scholar
|
|
73
|
Lee HC, Bumma N, Richter JR, Dhodapkar MV,
Hoffman JE, Suvannasankha A, Zonder JA, Shah MR, Lentzsch S, Maly
JJ, et al: LINKER-MM1 study: Linvoseltamab (REGN5458) in patients
with relapsed/refractory multiple myeloma. J Clin Oncol. 41(Suppl
16): S80062023. View Article : Google Scholar
|
|
74
|
Rodríguez Otero P, Joseph NS, Kumar SK,
Lee HC, Leleu X, Manier S, Dimopoulos MA, Mateos MV, Oriol A, Bumma
N, et al: Trial in progress: REGN5458, a BCMAxCD3 bispecific
antibody, in a phase Ib multi-cohort study of combination regimens
for patients with relapsed/refractory multiple myeloma. Blood.
140(Suppl 1): S4444–S4446. 2022. View Article : Google Scholar
|
|
75
|
Solh M, Bahlis N, Raje N, Costello C,
Dholaria B, Levy M, Tomasson M, Dube H, Damore M, Lon H, et al:
Efficacy and safety of elranatamab (PF-06863135), A B-cell
maturation antigen (BCMA)-CD3 bispecific antibody, in patients with
relapsed or refractory multiple myeloma. Hematol Transfus Cell
Ther. 43(Suppl 1): S195–S196. 2021. View Article : Google Scholar
|
|
76
|
Raje NS, Jakubowiak A, Gasparetto C,
Cornell RF, Krupka HI, Navarro D, Forgie AJ, Navarro D, Forgie AJ,
Udata C, et al: Safety, clinical activity, pharmacokinetics, and
pharmacodynamics from a phase I study of PF-06863135, a B-cell
maturation antigen (BCMA)-CD3 bispecific antibody, in patients with
relapsed/refractory multiple myeloma (RRMM). Blood. 134(Suppl 1):
S18692019. View Article : Google Scholar
|
|
77
|
Bahlis NJ, Costello CL, Raje NS, Levy MY,
Dholaria B, Solh M, Tomasson MH, Damore MA, Jiang S, Basu C, et al:
Elranatamab in relapsed or refractory multiple myeloma: The
MagnetisMM-1 phase 1 trial. Nat Med. 29:2570–2576. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Lesokhin AM, Tomasson MH, Arnulf B, Bahlis
NJ, Miles Prince H, Niesvizky R, Rodrίguez-Otero P, Martinez-Lopez
J, Koehne G, Touzeau C, et al: Elranatamab in relapsed or
refractory multiple myeloma: Phase 2 MagnetisMM-3 trial results.
Nat Med. 29:2259–2267. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Weisel K, D'souza A, Hurd D, Voorhees P,
Teipel R, Chung A, Rodriguez C, Tuchman S, Korde N, Safah H, et al:
P862: A phase 1 first-in-human monotherapy study of ABBV-383, A
BCMA x CD3 bispecific T-CELL–redirecting antibody, in
relapsed/refractory multiple myeloma. Hemasphere. 7(Suppl):
e00363072023. View Article : Google Scholar :
|
|
80
|
D'Souza A, Shah N, Rodriguez C, Voorhees
PM, Weisel K, Bueno OF, Pothacamury RK, Freise KJ, Yue S, Ross JA,
et al: A phase I first-in-human study of ABBV-383, a B-cell
maturation antigen x CD3 bispecific T-cell redirecting antibody, in
patients with relapsed/refractory multiple myeloma. J Clin Oncol.
40:3576–3586. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Vij R, Kumar SK, D'Souza A, Mckay JT,
Voorhees PM, Chung A, Tuchman SA, Korde N, Weisel K, Teipel R, et
al: Updated safety and efficacy results of Abbv-383, a BCMA x CD3
bispecific T-cell redirecting antibody, in a first-in-human phase 1
study in patients with relapsed/refractory multiple myeloma. Blood.
142(Suppl 1): S33782023. View Article : Google Scholar
|
|
82
|
Kumar S, D'Souza A, Shah N, Rodriguez C,
Voorhees PM, Bueno OF, Buelow B, Freise KJ, Yue S, Pothacamury RK,
et al: A phase 1 first-in-human study of Tnb-383B, a BCMA x CD3
bispecific T-cell redirecting antibody, in patients with
relapsed/refractory multiple myeloma. Blood. 138(Suppl 1):
S9002021. View Article : Google Scholar
|
|
83
|
Harrison SJ, Minnema MC, Lee HC, Spencer
A, Kapoor P, Madduri D, Larsen J, Ailawadhi S, Kaufman JL, Raab MS,
et al: A phase 1 first in human (FIH) study of AMG 701, an
anti-B-cell maturation antigen (BCMA) half-life extended (HLE)
BiTE® (bispecific T-cell engager) molecule, in
relapsed/refractory (RR) multiple myeloma (MM). Blood. 136(Suppl
1): S28–S29. 2020. View Article : Google Scholar
|
|
84
|
Goldstein RL, Goyos A, Li CM, Deegen P,
Bogner P, Sternjak A, Thomas O, Klinger M, Wahl J, Friedrich M, et
al: AMG 701 induces cytotoxicity of multiple myeloma cells and
depletes plasma cells in cynomolgus monkeys. Blood Adv.
4:4180–4194. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Costa LJ, Wong SW, Bermúdez A, de la Rubia
J, Mateos MV, Ocio EM, Rodríguez-Otero P, San-Miguel L, Li S,
Sarmiento R, et al: First clinical study of the B-cell maturation
antigen (BCMA) 2+1 T cell engager (TCE) CC-93269 in patients (Pts)
with relapsed/refractory multiple myeloma (RRMM): Interim results
of a phase 1 multicenter trial. Blood. 134(Suppl 1): S1432019.
View Article : Google Scholar
|
|
86
|
Wong SW, Bar N, Victoria Mateos M, Ribas
P, Hansson M, Paris L, Hofmeister C, Rodriguez-Otero P, Bermúdez
MA, Santoro A, et al: P883: Alnuctamab (ALNUC; BMS-986349;
CC-93269), A BCMA x CD3 T-cell engager, in patients (PTS) with
relapsed/refractory multiple myeloma (RRMM): Latest results from a
phase 1 first-in-human clinical study. Hemasphere. 7(Suppl 1):
e12207452023. View Article : Google Scholar
|
|
87
|
Wong SW, Bar N, Paris L, Hofmeister CC,
Hansson M, Santoro A, Mateos MV, Rodríguez-Otero P, Lund J, Encinas
C, et al: Alnuctamab (ALNUC; BMS-986349; CC-93269), a B-cell
maturation antigen (BCMA) x CD3 T-cell engager (TCE), in patients
(pts) with relapsed/refractory multiple myeloma (RRMM): Results
from a phase 1 first-in-human clinical study. Blood. 140(Suppl 1):
S400–S402. 2022. View Article : Google Scholar
|
|
88
|
Roex G, Timmers M, Wouters K,
Campillo-Davo D, Flumens D, Schroyens W, Chu Y, Berneman ZN, Lion
E, Luo F and Anguille S: Safety and clinical efficacy of BCMA
CAR-T-cell therapy in multiple myeloma. J Hematol Oncol.
13:1642020. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Xu Y, Zhang C, Cai D, Zhu R and Cao Y:
Exosomal miR-155-5p drives widespread macrophage M1 polarization in
hypervirulent Klebsiella pneumoniae-induced acute lung injury via
the MSK1/p38-MAPK axis. Cell Mol Biol Lett. 28:922023. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Sperling AS, Derman BA, Nikiforow S, Im
SY, Ikegawa S, Prabhala RH, Rodriguez DH, Li Y, Quinn DS, Pearson
D, et al: Updated phase I study results of PHE885, a T-charge
manufactured BCMA-directed CAR-T cell therapy, for patients (pts)
with r/r multiple myeloma (RRMM). J Clin Oncol. 41(Suppl 16):
S80042023. View Article : Google Scholar
|
|
91
|
Teoh PJ and Chng WJ: CAR T-cell therapy in
multiple myeloma: More room for improvement. Blood Cancer J.
11:842021. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Wehrli M, Gallagher K, Chen YB, Leick MB,
McAfee SL, El-Jawahri AR, DeFilipp Z, Horick N, O'Donnell P,
Spitzer T, et al: Single-center experience using anakinra for
steroid-refractory immune effector cell-associated neurotoxicity
syndrome (ICANS). J Immunother Cancer. 10:e0038472022. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Lam N, Trinklein ND, Buelow B, Patterson
GH, Ojha N and Kochenderfer JN: Anti-BCMA chimeric antigen
receptors with fully human heavy-chain-only antigen recognition
domains. Nat Commun. 11:2832020. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Mehta P, Cron RQ, Hartwell J, Manson JJ
and Tattersall RS: Silencing the cytokine storm: The use of
intravenous anakinra in haemophagocytic lymphohistiocytosis or
macrophage activation syndrome. Lancet Rheumatol. 2:e358–e367.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Minakata D, Ishida T, Ando K, Suzuki R,
Tanaka J, Hagiwara S, Ananthakrishnan R, Kuwayama S, Nishio M,
Kanda Y and Suzuki K: Phase 2 results of idecabtagene vicleucel
(ide-cel, bb2121) in Japanese patients with relapsed and refractory
multiple myeloma. Int J Hematol. 117:729–737. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Wang D, Wang J, Hu G, Wang W, Xiao Y, Cai
H, Jiang L, Meng L, Yang Y, Zhou X, et al: A phase 1 study of a
novel fully human BCMA-targeting CAR (CT103A) in patients with
relapsed/refractory multiple myeloma. Blood. 137:2890–2901. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Li C, Wang D, Yu Q, Li Z, Wang W, Hu G, Mu
W, Li C, An N, Long X, et al: Long-term follow-up of fully human
BCMA-targeting CAR (CT103A) in patients with relapsed/refractory
multiple myeloma. Blood. 142(Suppl 1): S48542023. View Article : Google Scholar
|
|
98
|
Du J, Fu W, Jiang H, Dong B, Gao L, Liu L,
Ge J, He A, Li L, Lu J, et al: P869: Updated results of a phase I,
open-label study of bcma/cd19 dual-targeting fastcar-T GC012F for
patients with relapsed/refractory multiple myeloma (RRMM).
Hemasphere. 7(Suppl 1): e84060bf2023. View Article : Google Scholar :
|
|
99
|
Doucet L, Cailleteau A, Vaugier L,
Gourmelon C, Bureau M, Salaud C, Roualdes V, Samarut E, Aumont M,
Zenatri M, et al: Prognostic value of hPG80 (circulating
progastrin) in IDH-wild type glioblastoma treated with
radio-chemotherapy. J Clin Oncol. 40(Suppl 16): S20492022.
View Article : Google Scholar
|
|
100
|
Mailankody S, Ghosh A, Staehr M, Purdon
TJ, Roshal M, Halton E, Diamonte C, Pineda J, Anant P, Bernal Y, et
al: Clinical responses and pharmacokinetics of MCARH171, a
human-derived bcma targeted CAR T cell therapy in
relapsed/refractory multiple myeloma: Final results of a phase I
clinical trial. Blood. 132(Suppl 1): S9592018. View Article : Google Scholar
|
|
101
|
Yamaguchi Y, Gibson J, Ou K, Lopez LS, Ng
RH, Leggett N, Jonsson VD, Zarif JC, Lee PP, Wang X, et al: PD-L1
blockade restores CAR T cell activity through IFN-γ-regulation of
CD163+ M2 macrophages. J Immunother Cancer. 10:e0044002022.
View Article : Google Scholar
|
|
102
|
Ledergor G, Fan Z, Wu K, McCarthy E,
Hyrenius-Wittsten A, Starzinski A, Chang H, Bridge M, Kwek S,
Cheung A, et al: CD4+ CAR T-cell exhaustion associated with early
relapse of multiple myeloma after BCMA CAR T-cell therapy. Blood
Adv. 8:3562–3575. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Zwirner NW, Domaica CI and Fuertes MB:
Regulatory functions of NK cells during infections and cancer. J
Leukoc Biol. 109:185–194. 2021. View Article : Google Scholar
|
|
104
|
Padovan E, Terracciano L, Certa U, Jacobs
B, Reschner A, Bolli M, Spagnoli GC, Borden EC and Heberer M:
Interferon stimulated gene 15 constitutively produced by melanoma
cells induces e-cadherin expression on human dendritic cells.
Cancer Res. 62:3453–3458. 2002.PubMed/NCBI
|
|
105
|
Li W, Zhang B, Cao W, Zhang W, Li T, Liu
L, Xu L, Gao F, Wang Y, Wang F, et al: Identification of potential
resistance mechanisms and therapeutic targets for the relapse of
BCMA CAR-T therapy in relapsed/refractory multiple myeloma through
single-cell sequencing. Exp Hematol Oncol. 12:442023. View Article : Google Scholar : PubMed/NCBI
|
















