Introduction
Regulatory B cells (Bregs) represent a distinct
subset within the B lymphocyte population and serve a key role in
maintaining immune homeostasis, modulating intensity of immune
response and suppressing excessive inflammatory reactions. Unlike
regulatory T cells (Tregs), which are defined by specific
transcription factors such as Foxp3, Bregs are primarily
characterized by the regulatory elements they secrete (1). Bregs exert their immunomodulatory
functions mainly through the secretion of inhibitory cytokines,
including IL-10, transforming growth factor (TGF)-β and IL-35
(2-5). Among these, IL-10-secreting Bregs
(B10 cells) suppress autoimmune and inflammatory responses
(6). Yanaba (7) identified the cellular phenotype of
Bregs as expressing
CD1dhighCD5+CD19+. Secretion of
IL-10 inhibits the activity of co-stimulatory molecules such as B7,
thereby impeding T cell activation, which underscores the intricate
mechanisms underlying their function (7).
Bregs are primarily categorized by their cytokine
profiles, as specific phenotypes and transcription factors have yet
to be established (8). This lack
of distinct phenotypes presents a challenge for the expansion of
specific Bregs. Moreover, Bregs play a critical role in various
immune-associated diseases, including autoimmune and inflammatory
conditions, tumor immune escape and organ transplantation (9-11). However, the in vivo
numbers of B10 cells are often insufficient to harness their
therapeutic potential.
Numerous signaling pathways and transcription
factors have been shown to regulate the development and function of
B10 cells (12). Key pathways
include toll-like receptor (TLR) signaling pathway, B cell receptor
(BCR) signaling, CD40-CD40L interactions and B
lymphocyte-activating factor (BAFF), alongside critical
transcription factors such as signal transducer and activator of
transcription 3 (STAT3), nuclear factor-κB (NF-κB) and
interferon-regulatory factors (IRFs) (13). Under appropriate stimuli, such as
cytokines IL-10 and TGF-β, pathogen-associated molecular patterns
(PAMPs) cytosine-phosphate-guanine (CpG)-containing
oligodeoxynucleotide (ODN) and lipopolysaccharide (LPS), immune
checkpoint signals CD40 activation and PD-1/PD-L1 interactions, and
cytokines like IL-21, B cells differentiate into Bregs, facilitated
by these pathways and factors that govern their development and
immunomodulatory functions (8,14).
Furthermore, the ex vivo expansion of B10
cells presents a broader array of cellular therapeutic options in
immune transplantation. The adoptive transfer of B10 cells can
exert immunomodulatory functions, suppress transplant rejection,
promote transplant tolerance and decrease the reliance on
immunosuppressive agents and their associated side effects.
Additionally, B10 cells can be employed to investigate cellular
immune regulatory mechanisms (15). While Tregs have been the primary
focus of adoptive cell therapies due to their established expansion
protocols and widespread clinical applications in transplant
tolerance research (16), Bregs
are gaining recognition for their ability to enhance regulatory
functions of various immune cells, including Tregs (17).
Although both Bregs and Tregs contribute to
promoting transplantation tolerance (18), Bregs possess advantages in terms
of antigen recognition. Bregs are involved in the immune response
at an earlier stage and respond more rapidly than Tregs; B cells
can directly recognize naïve antigens via BCR, while T cells
require antigen processing before responding (8). Despite ongoing debates regarding
the role of IL-10 in transplant immunization, induction of graft
tolerance with anti-CD45RB antibodies depends solely on the
presence of B cells, independent of IL-10 (19-21). Regardless of the role of IL-10,
the contribution of B10 cells in establishing a tolerogenic
environment is key (22).
The present review discusses the significance of the
TLR, BCR and CD40-CD40L signaling pathways in the generation of B10
cells, focusing on their expansion capacity and inhibitory
functions. Given the lack of comprehensive literature addressing
the mechanisms involved in B cell differentiation into B10 cells
in vitro, the present review aims to elucidate the
mechanisms underlying their expansion to facilitate advances in
in vitro expansion technologies and cellular therapies.
Mechanisms involved in the classical
signaling pathways for B10 expansion in vitro
TLR signaling pathways
At present, the prevailing method of expansion
entails in vitro activation of naive B cells, with the TLR
signaling pathway serving a crucial role in their secretion of
cytokines such as IL-10 (23,24). TLRs are highly conserved pattern
recognition receptors, primarily responsible for recognizing PAMPs
and initiating associated signaling pathways (25,26). Consequently, TLRs elicit the
inflammatory immune response, activate immune cells and exert
anti-infection functions (27-29). In B cells, the TLR/myeloid
differentiation primary response 88 (MyD88) pathway governs the
production of immunosuppressive factors such as IL-10 (30-33) and represents a pivotal pathway
for in vitro expansion. TLRs are type I transmembrane
glycoproteins with a transmembrane helix (34). The extracellular region features
a leucine-rich repeat sequence that binds to PAMPs (34). TLR signaling occurs when the
toll/IL-1 receptor (TIR) structural domain interacts with MyD88, an
adaptor protein located within the TIR receptor region (34). Typically, stimulation of TLR is
followed by activation of B cells and production of antibodies
(35). Nevertheless, previous
experiments have demonstrated that B10 cells are produced (32,36-38) upon appropriate stimulation such
as LPS, a component of the outer membrane of Gram-negative
bacteria, and CpG ODN, which are synthetic DNA sequences containing
unmethylated CpG motifs that can stimulate the immune system via
Toll-like receptor 9 (TLR9).
Following TLR stimulation, signaling pathways, such
as NF-κB, MAPK and PI-3K/AKT pathways, are activated in B cells in
response to inflammatory factors (39). Downstream, STAT3 and ERK are key
for B cells to produce IL-10 after TLR agonist stimulation
(33,40-42).
TLR9 in endosomes: Ongoing signaling
Currently, in vitro expansion of Bregs is
primarily achieved through TLR9 stimulation. TLR9 recognizes
sequences in bacterial and viral double-stranded DNA that encompass
CpG sequences (43). As a
non-ligand crystal, TLR9 can bind CpG-DNA (44). Clinical trials have shown that
CpG ODN activates B cells via TLR9, thereby establishing the in
vitro activation and generation of Bregs (45,46). This recognition initiates the
downstream signaling pathway and activates B cells.
TLR9 is primarily located in the endoplasmic
reticulum, endosome and lysosomes. Following uptake by lyosomal
vesicles, CpG ODN binds to TLR9 and give rise to TLR9-CpG
complexes. In general, agonist CpG-DNA binds to TLR9 in a 2:2
ratio, forming a symmetrical TLR9-CpG-DNA complex (47). Unlike natural TLR9 agonists,
artificially designed agonists possess a structure that resists
nuclease degradation and leads to more robust activation (48). Due to differences in TLR9
expression across species, immune stimulation in humans and large
animals is less pronounced, and higher doses of CpG ODN are
required to achieve an effect in larger species. Additionally, to
ensure the effective translation of mouse experimental results to
human clinical applications, it is essential to design TLR9 ligands
tailored to the immune responses of each species. In addition, to
fulfill the research requirements of TLR9 agonists, it is necessary
to design separate TLR9 agonists for humans and mice (49).
The formation of the TLR9-CpG complex triggers TLR9
oligomerization, entailing the binding of multiple TLR9 molecules.
Following TLR9 oligomerization, the ligand-bound oligomers recruit
the appropriate adaptor protein to form the TLR9-MyD88 complex
(50). TLR9 interacts with
MyD88, forming the TLR9/MyD88 pathway which activates downstream
signaling molecules through TIR structural domain-mediated
signaling (51). The activation
involves proteins from the IL-1 receptor-associated kinase (IRAK)
family, as well as TNF receptor-associated factor (TRAF) and
TGF-β-activated kinase 1 (TAK1)-binding protein (52). IRAK1 and IRAK4 are key components
of the TLR9 signaling pathway (51). These are phosphorylated and
activated to form a complex that activates TAK1 (52). The activated TAK1 triggers a
series of signaling molecules, including MAPK, IκB kinase (IKK) and
NF-κB (53).
The MAPK pathway encompasses downstream molecules
such as ERK, JNK and p38. The activated MAPK has the potential to
activate the transcription factor activator protein (AP)-1
(54), a key effector molecule
downstream of the JNK pathway. AP-1 may bind to the IL-10 gene
promoter, participating in transcriptional regulation of this gene.
Microarray analysis demonstrated BAFF operates through AP-1,
binding to the IL-10 promoter region, thereby enhancing IL-10
secretion (55). This suggests
that the products of the MAPK pathway influence the differentiation
and proliferation of B cells into Bregs.
By contrast, the activation of IKK leads to the
phosphorylation of IκB (an intracellular inhibitory factor),
resulting in the degradation of IκB and release of NF-κB. NF-κB in
B cells then enters the nucleus and binds to specific DNA sequences
(56), facilitating the
transcription of Breg-related genes.
Recent study have indicated an upregulation in the
expression of tyrosine hydroxylase (TH), a key enzyme for
catecholamine production, following TLR9 activation by CpG ODN
(57). This upregulation is
associated with increased production of IL-10 (58). Additionally, B cells produce
catecholamines in a time- and stimulus-dependent manner, further
inducing the production of IL-10 (57). To the best of our knowledge,
non-classical pathways of TLR9-induced Breg production have not
been identified.
In general, TLR9 is a pathway that requires further
investigation to facilitate development of more effective agonists.
By studying the structure and stability of TLR9 agonists to resist
nuclease degradation, while also exploring other signaling axes, a
deeper understanding of the impact mechanisms of TLR9 agonists on
B10 ex vivo expansion can be achieved, optimizing the
expansion outcomes.
TLR4 on the membrane: Key signaling in
B10 expansion
TLR4 is primarily located on the cell membrane
surface and activates B cells by recognizing ligands such as LPS.
Inflammatory stimuli activate the TLR4-MyD88 axis of B cells, which
ultimately indirectly phosphorylates STAT3 (31), regulating key cellular processes
such as cell differentiation, proliferation and survival (59-62). STAT3 interacts with JAK to
promote formation of phosphorylated (p)STAT3 dimers (59-61) and the translocation of pSTAT3
protein from the cytoplasm to the nucleus. In the nucleus, STAT3
binds specific DNA sequences to promote transcription of target
genes and the differentiation of B cells to Bregs (63). Furthermore, STAT3 can regulate
the transcription of key genes, such as B lymphocyte-induced
differentiation protein (63,64), which is essential for driving B
cell differentiation and modulating immune responses. This
regulation initiates a cascade of responses in downstream signaling
molecules, including MAPK and NF-κB pathways (40).
Resveratrol inhibits phosphorylation of STAT3,
thereby reducing the production of Breg-derived TGF-β and
decreasing Treg formation (65).
Similarly, in CD1dhiCD5+ B10 cells,
activation of TLR4 by LPS leads to the interaction of pB cell
linker protein with Bruton's tyrosine kinase (Btk), resulting in
phosphorylation and subsequent nuclear translocation of STAT3 to
transduce IL-10 gene (66).
Similar to the TLR9-MyD88 axis, this process ultimately leads to
the activation of ERK, JNK, p38 and NF-κB (54). Activated NF-κB enters the nucleus
and binds to the promoter regions of Breg-related genes,
facilitating the transcription and expression of these genes
(40).
In the TLR pathway, the activation of NF-κB is
essential for inducing IL-10 production (67). Blocking experiments have shown
that NF-κB serves a vital role in Breg formation (66,68). Lee et al (69), using bromodomain and
extra-terminal domain protein blocker JQ1, demonstrated that
bromodomain protein 4 (BRD4), which produces the promoter of the
IL-10 gene, promotes IL-10 production in Bregs when BRD4 interacts
with NF-κB. Further research is still needed to determine the role
NF-κB plays and how it can be utilized for in vitro
amplification.
TLR3, TLR4 and TLR5 activate the Myd88-independent
pathway, with TLR4 recruiting TIR domain-containing adaptor
inducing IFN-β (TRIF), which recruits TRAF3 and the kinases TBK
(TANK-binding kinase 1) and IKKε/IKKi. TBK, which plays a key role
in regulating immune responses, leads to the activation of NF-κB
and the phosphorylation of IRF-3, which undergoes nuclear
translocation and induces expression of type I interferons.
Previous study of B cell dephosphorylation using small molecule
inhibitor ezrin have shown that in the TLR-Myd88-independent
pathway, a key TRIF-TBK1-IKK-IRF3 axis, as well as a robust NF-κB
signaling, leads to higher levels of IL-10 secretion (70). Current studies have focused on
the Myd88-dependent pathway (30,71); the Myd88-independent pathway may
be an alternative mode of action for in vitro expansion of
Bregs (Fig. 1).
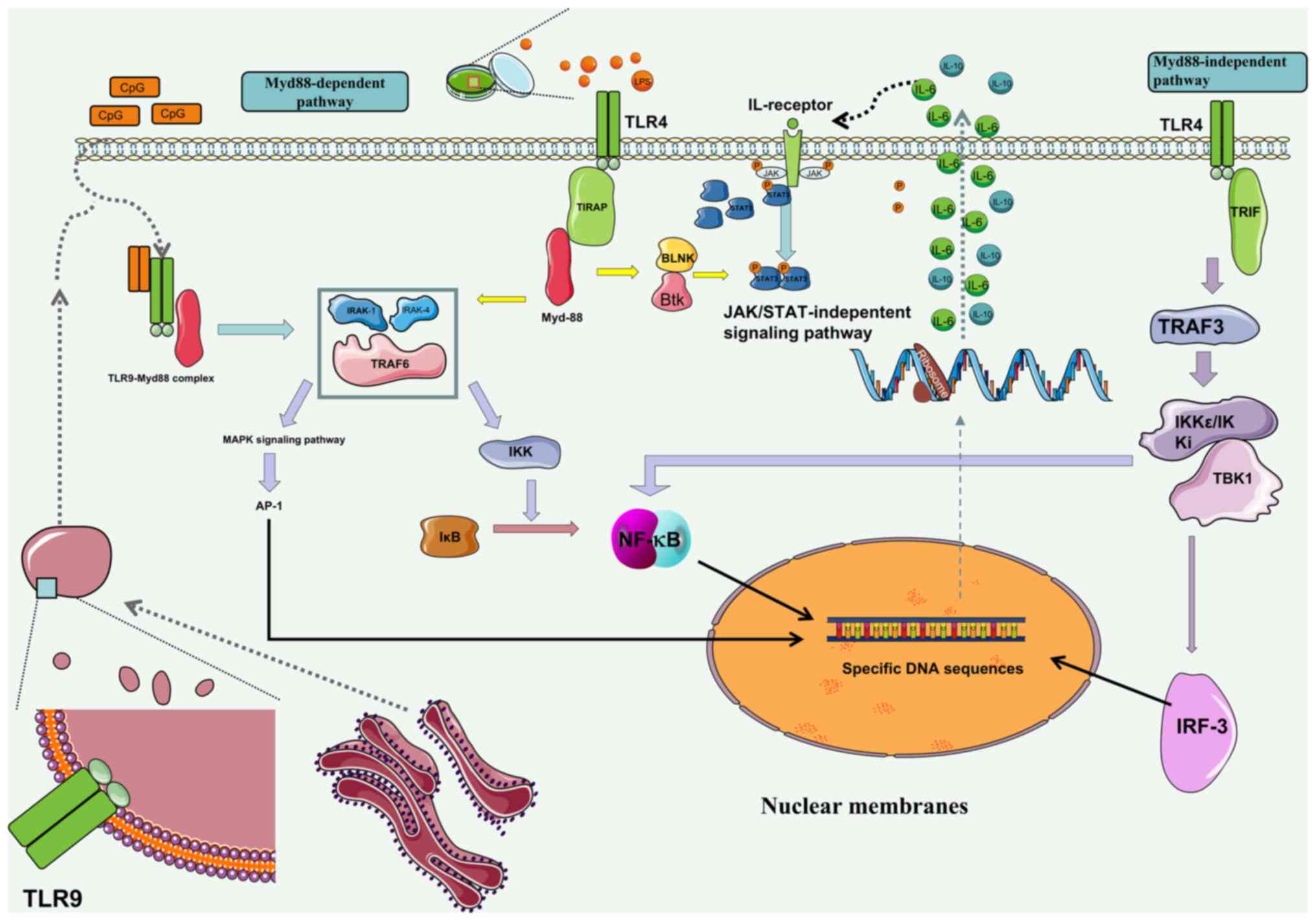 | Figure 1Generation of B10 cells via in
vitro stimulation of TLR pathways. LPS, CpG ODN and other
stimulants added in vitro activate the TLR/MyD88 signaling
pathway through TLR4 and TLR9 receptors, promoting the activation
of MAPK pathway, NF-κB and STAT3 phosphorylation, causing
transcription factors to enter the nucleus. After binding promoter
sequences of suppressor genes such as IL-10, molecules such as
NF-κB and AP-1, can promote their expression and exert
immunosuppressive function. Cytokines such as IL-6, which are
expressed as a result of TLR activation, cause STAT3
phosphorylation through the JAK/STAT pathway. Phosphorylated STAT3
promotes the expression of Blimp-1 and other factors, leading to
the differentiation of B cells into Bregs. In addition, TLR3, 4 and
5 activate the Myd88-independent pathway and cause B cell
differentiation toward Bregs. Futher, BCR and CD19 signaling
pathways work together to promote production of IL-10. LPS,
lipopolysaccharide; CpG ODN, Cytosine-phosphate-Guanine
oligodeoxynucleotide; TLR, toll-like receptor; Blimp, B
lymphocyte-induced maturation protein; Breg, regulatory B cell;
BCR, B cell receptor; IRAK, Interleukin-1 receptor-associated
kinase; TRAF, TNF receptor-associated factor; BLNK, B cell linker
protein; Btk, Bruton's tyrosine kinase; TRIF, TIR-domain-containing
adapter-inducing interferon-β; AP, activator protein; IRF,
interferon regulatory factor; IKK, IκB kinase; TBK, TANK-binding
kinase. |
Together, the aforementioned mechanisms lead to
in vitro expansion of Bregs. Further research is still
needed on the biological properties of Bregs, the TLR pathways
involved in their expansion and transcription factors such as
STAT3, IRF7 and NF-κB.
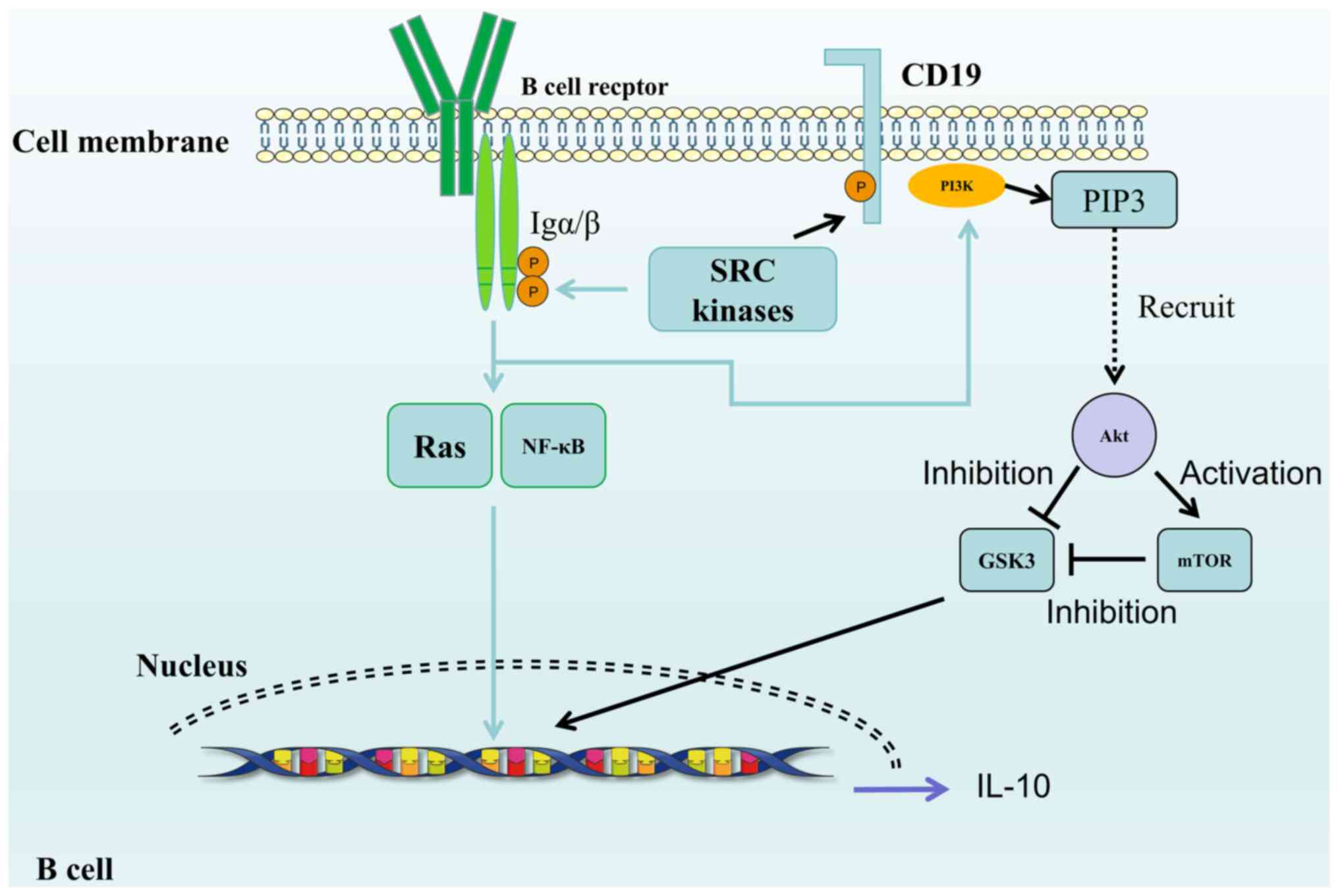
BCR signaling pathway
BCR is an antigen recognition receptor on the
surface of B cells (72). Each B
cell expresses a specific BCR, consisting of two primary
components: A membrane-anchored immunoglobulin (mIg) and a signal
molecule tightly binding to it (Fig.
2). The Ig portion of the BCR comprises two heavy and two light
chains, linked together by disulfide bonds to form an
antigen-binding unit. Signaling transduction molecules heterodimers
associated with BCR Igs through noncovalent bonds, including Igα
(CD79a) and Igβ (CD79b), which contain immunoreceptor
tyrosine-activated motifs (ITAM) (73-76). ITAM binds to SRC kinases such as
LYN, FYN kinase and B-lymphoid tyrosine kinase. Cross-linking of
BCR to specific antigens induces phosphorylation of ITAM tyrosine
by these SRC kinases (77).
CD19, which acts as a co-receptor, synergistically lowers the
threshold for B cell activation; tyrosines in CD19 structural
domains are phosphorylated by SRC kinases, serving as docking sites
for SH2-containing proteins (Src Homology 2), such as PI3K
(78).
BCR serves an essential role in B cell
differentiation. When activated, BCR can generate a prolonged
activation signal within the B cell (79-81), thereby prompting Bregs to
proliferate and transmit signals to the cell via signal molecules.
These signals will trigger cellular responses, enabling B cells to
differentiate into regulatory or antibody-producing cells (82,83). B cells activated by BCR protect
NOD mice from type 1 diabetes in an IL-10-dependent manner
(84). LPS-induced Breg
proliferation is reduced in inhibitor of κB, NF-κB essential
modulator)-deficient mice (85).
However, BCR stimulation compensates for this reduction by
promoting Breg expansion (85).
This further suggests that the BCR complex promotes Breg
proliferation and differentiation.
Although antigen and BCR signaling serve a vital
role in the early development of Bregs, the expansion of Bregs can
be further optimized by activation of TLRs (8,36,86,87). BCR and TLR (88,89) activate the PI3K/Akt/glycogen
synthase kinase (GSK)-3 pathway. Following activation, PI3K is
recruited to the membrane by binding to sequences containing
p-tyrosine on membrane-associated scaffold proteins (such as CD19)
through its Src homology 2 domain (90-93). Once at the membrane, PI3K
generates phosphatidylinositol 3,4,5-trisphosphate (PIP3) by
phosphorylating membrane phosphatidylinositol 4,5-bisphosphate
(94,95). PIP3 is dephosphorylated to
produce PI(3,4) P2. Acting as anchors, PIP3 and
PI(3,4)P2 recruit proteins containing PH
homology domains to the membrane (96). This leads to recruitment and
activation of signaling enzymes containing the PH domain (such as
Btk, phospholipase C-2 and Akt) at the membrane. Akt is the primary
mediator of the anti-apoptotic signal produced by PI3K (97), and phosphorylates proteins that
regulate cell survival. Additionally, Akt can phosphorylate
serine/threonine (Ser/Thr) kinase GSK-3α and GSK-3β (98). GSK-3 is a multifunctional kinase
involved in regulation of various physiological and pathological
processes (99-101), such as glycogen synthesis, cell
death and survival. These negative regulatory sites on GSK-3α and
GSK-3β are phosphorylated by Akt. GSK-3β is a multifunctional
Ser/Thr protein kinase that plays a role in B cell metabolic
activity and proliferation. During differentiation of B cells into
Bregs, phosphorylation of the Ser residues of GSK-3 leads to its
inactivation (99), resulting in
phosphorylation of the negative regulatory sites on GSK by BCR,
inhibiting GSK-3 activity (102). In vitro use of GSK-3
inhibitors significantly increases its phosphorylation, thereby
inhibiting GSK-3 activity and resulting in a significant increase
in the common Breg subtypes
(CD19+CD24+CD27+,
CD19+CD24+CD38high,
CD19+CD27intCD38hi,
CD19+CD39+CD73+). Expression of
mBreg (CD19+CD24hiCD27+) is most
significantly increased, and subsequent adoptive transfer
experiment, where mBreg cells were transferred into murine GVHD
model, have confirmed the enhancement of its inhibitory function
(99). Following mTOR
activation, GSK-3 is inhibited, specifically leading to secretion
of IL-10 by B cells and driving the generation of Tregs (103). This can also account for the
reduction in regulatory cells observed after use of the mTOR
inhibitor rapamycin (104).
GSK-3, downstream of mTORC1, serves as a key metabolic regulatory
factor for IL-10 secretion in B cells (104).
The activation of BCR triggers signaling pathways,
including PI3K/Akt, Ras/MAPK and NF-κB pathways (79). Since Bregs control inflammation
and regulate immunity, their amplified signaling pathways partially
overlap with pathways involved in the immune response of B cells
(84). Under specific
conditions, such as mTOR agonist and GSK-3 inhibitor (104), these signaling pathways promote
proliferation of Bregs, so in vitro expansion methods mimic
the stimulation of inflammation.
CD40 signaling: Working with TLRs and
BCR
CD40 is a membrane-bound molecule on the surface of
B cells and is part of the TNF receptor family. This molecule is
activated by membrane-bound ligands through direct cell-to-cell
contact or by soluble ligands in solution (105).
During the adaptive immune response, CD40 interacts
with CD40 ligand (L) on the surface of antigen-activated
CD4+ T cells, stimulating B cell proliferation and
activation (106,107). CD40 agonist antibody mimics
CD40L binding to CD40, thus simulating T cell-dependent signaling
that activates B cells. CD40 signaling pathway can activate various
downstream signaling molecules, such as TRAF, NF-κB and MAPK, which
influence B cell proliferation and function. As a co-stimulatory
receptor, CD40 can work synergistically with cytokines, BCR and
TLRs to elevate STAT3 phosphorylation levels (108). Moreover, CD40 promotes
intracellular signal by recruiting TRAF in intracellular membranes,
activating different signaling pathways, including the classical
and non-classical NF-κB, MAPK, PI3K and PLCγ pathways (109).
The aforementioned findings illustrate the
importance of the CD40 pathway in efficient amplification of Bregs.
Previous study have found that in vitro stimulation of CD40
and IL-21 receptor signaling can drive the development and
proliferation of B10 cells four million-fold and that the IL-10
produced is effective in suppressing the inflammatory response
in vivo (36). Splenic
CD1dhiCD5+ Bregs are significantly amplified
in vivo through an intrinsic genetic mechanism (involving
highly reactive CD40) that can suppress autoantibody and
T-dependent antibody responses and inhibit germinal centers
(110). In addition, recent
tumor study have found that PPARδ in Bregs may bind agonistic
anti-CD40 antibodies, thereby stimulating B10 production (111). Advances in in vitro
amplication technology may be facilitated by simulating changes in
Bregs within tumors in vitro.
In summary, inflammatory factors are responsible for
generation of the immune response and regulatory cells. In
addition, in the absence of TLR agonists, stimulation of BCR and
CD40 only marginally increases the expression of IL-10 (33). This suggests that effective
stimulation of TLRs is more key for IL-10 production than BCR and
CD40. However, it has also been shown that by blocking CD40, more
granzyme (GZM)B is produced, serving a regulatory role in
transplantation (112). Hence,
the effects of stimulation or blocking of the CD40 signaling
pathway on Bregs are complex and varied.
B10 in vitro amplification
technology
Immune activation is required for Bregs to exhibit
suppressive functions. Bregs are present in B cell populations at
different stages of maturation and differentiation (8), suggesting several lineages can be
induced to acquire regulatory capacity in response to environmental
factors (113). When exposed to
specific antigens or appropriate stimuli, subpopulations of B
cells, such as those triggered by TLR ligands and anti-CD40
stimulators, have the capacity to differentiate into regulatory B
cells (8,14). In vitro, specifically act
on TLRs, CD40 and BCR to promote activation and expansion of B10
cells. In addition to the addition of stimulus, the use of drugs
and cell coculture used to study the expansion of Bregs. Selecting
appropriate stimulants and culture conditions is necessary
according to the specific experimental design and research purpose.
At the same time, care should be taken to control the concentration
of the stimuli and treatment time to avoid excessive or incomplete
activation affecting generation and function of Bregs (68,104).
Breg expansion induced by stimulants
Currently, Bregs are primarily expanded in
vitro by stimulating TLR4, TLR9 and CD40 (68,83,114). The chronology and location of
the aforementioned events in vivo remain to be clarified.
TLR agonists, such as LPS from gram-negative bacteria,
peptidoglycan from gram-positive bacteria or CpG-containing
oligonucleotides that mimic bacterial DNA (115), are potent factors in inducing
IL-10 production by naïve B cells (32,37). In addition, IFN-α, an important
cytokine that plays a crucial role in the regulation of the immune
system and antiviral responses, also increases TLR7- or
TLR8-induced IL-10 production in B cells (33). The differentiation and activation
of Bregs necessitate the engagement of various molecules for
binding, such as TLR-2, TLR-4, TLR-9, BCR signaling, and
co-stimulatory factors CD40, CD80/CD86, BAFF, as well as cytokines
(IL-1β, IL-2, IL-6, IL-21, IL-35, IFN-α) (36,116-119) (Table I).
 | Table IMethods of IL-10-secreting B cell
stimulation in vitro. |
Table I
Methods of IL-10-secreting B cell
stimulation in vitro.
| Species | Stimulus | Phenotype | Disease | Effect | (Refs.) |
|---|
| C57BL/6 mice | Astilbin + LPS |
CD19+CD1dhi and
CD19+TIM-1+ | Colonitis | | (31) |
| LPS |
CD5+CD1dhiFasL+ | Contact
hypersensitivity to oxazolone; autoimmune disease | Pro-inflammatory
factors increased | (36,68) |
| IL-21 |
CD1dhiCD5+ | Autoimmune
disease | Effect on B cells
is poor | (36,117) |
| NIH-3T3-CD154/BLyS
cells + IL-21 |
CD1dhiCD5+ and
CD1dloCD5- | Autoimmune
disease | Amplifcation of B10
cells ~4000000-fold | (36) |
| CpG ODN + β-ADR
agonist |
CD19+CD1d+,
TIM-1+, CD5+ and CD5− | Autoimmune
disease | Expression of
enzyme tyrosine hydroxylase induced in B cells upon activation of
TLR9 | (57) |
| Anti-CD40 mAb +
CpG |
CD5+CD1dhi | Contact
hypersensitivity to oxazolone | | (68) |
| LPS + anti-CD40
mAb |
CD1dhiCD5+CD19+ | Autoimmune
disease | | (83) |
| LPS + CpG ODN +
ionomycin |
CD19+CD25+TIM-1+LAP+PD-L1+ |
Transplantation | | (114) |
| CpG ODN |
CD19+ |
Transplantation | | (114) |
| LPS + MSC | | Inflammatory
disease | Greater effect on B
cells than that of LPS alone; IL-10 expression increased by 18.4
times and pro-inflammatory factor levels decreased | (139) |
| Human | CD154+
Chinese hamster ovary |
CD73−CD25+CD71+ |
Transplantation | Amplified B10
~900-fold and for >14 days | (42) |
| mTOR agonist |
CD19+IL-10+ | Inflammatory
disease | | (104) |
| GSK-3
inhibitor |
CD19+CD24hiCD27+ | Inflammatory
disease | | (104) |
| CD40L + CpG +
IL-21 |
CD19+ | Autoimmune
disease | | (129) |
| TLR7 agonist +
Tα-1 |
CD19+CD24+CD38hi
and CD24low/negCD38hi | Multiple
sclerosis | | (138) |
| Sirolimus |
CD19+CD24+CD38+
and | Organ | | (141) |
|
CD19+CD24+CD38+TGF-β+ |
transplantation | | |
| CD40L+
ILCs |
CD19+CD27− | Allergic
disease | | (149) |
| Abatacept |
IgD+IgM+CD24highCD38highCD1dhigh | Rheumatoid
arthritis | | (155) |
| Spragye-Dawley
rats | LPS + CpG ODN |
IL-35+TGF-β+ | Periodontitis | | (127) |
| MSC |
CD19+CD24highCD38high | Autoimmune
disease | | (150,151) |
| BALB/c, C57BL/6,
TLR5−/−, and MyD88−/− mice | Recombinant fusion
protein (rFlaA:Betv1) |
CD19+CD24+CD1d+IgM+CD38+ | Allergic
disease | | (138) |
Stimulation of signaling pathways
LPS stimulates the production of B10 cells. B10
cells exert immunomodulatory functions that rely not only on
secretion of cytokines such as IL-10 but also on the involvement of
surface ligands such as FasL. In mice, LPS was found to rapidly
increase FasL expression in CD1dhiCD5+ B
cells; this subpopulation was able to inhibit CD4+ T
cell proliferation (68).
Therefore, when inducing Breg generation, it is insufficient to
solely focus on the ability of cells to secrete IL-10. Furthermore,
ultraviolet irradiation induces upregulation of TLR4 on the surface
of B cells, enhancing regulatory capacity in contact
hypersensitivity reactions (120). Earlier study found that splenic
CD1dhiCD5+ Bregs producing IL-10 are notably
induced in mice by LPS and anti-CD40 antibodies (83). The use of radiation in
combination with stimulants can be considered for in vitro
expansion.
Sole stimulation of TLR9 is insufficient and
typically necessitates a combination of other agonists for optimal
efficacy. In experimental autoimmune encephalomyelitis (EAE), use
of the TLR9 agonist ODN 1826 in conjunction with irradiation
results in the enhanced generation of B10 cells (10). The potency of the TLR9 stimulator
ODN is augmented through various means, such as co-formulation with
deposit-forming adjuvants and use of polyphosphate polyelectrolyte
analogs to enhance the immunoreactivity of the CpG ODN (49).
The upregulation of IL-10 expression in B cells is
evident upon stimulation with CD40 monoclonal antibodies (83), thus affirming an association
between CD40 activation and B10 amplification. B cells derived from
CD22−/− mice exhibit a noteworthy degree of
hyperresponsiveness to CD40 signaling (121). In subsequent study, CD40
stimulation led to a 16-fold increase in the population of B10
cells compared with wild-type mice (110). The existing methods for
activating CD40 signaling have evolved beyond the sole reliance on
antibodies and use foster cells for coculture-based stimulation
(36).
Previous studies have reported IL-10 production and
regulatory functions when human B cells or Breg subpopulations are
activated by CpG and anti-CD40 antibodies (122-124). Previous experiment have
reinforced these findings, noting increased expression of IL-10
compared with that induced by the stimulation of TLR4 alone
(68). These observations
support the potential for a more robust immunosuppressive effect of
Bregs, particularly via enhanced IL-10 secretion, which plays a
critical role in dampening inflammatory responses and promoting
immune tolerance, warranting further exploration.
The immunosuppressive effect of cells obtained by
stimulating TLR9 with CpG alone is inferior to that of cells
obtained following combined stimulation with TLR4, TLR9 and PMA
(Phorbol 12-myristate-13-acetate (PMA), a synthetic compound that
activates protein kinase C (PKC) (114,125). PMA serves as a mimetic of
diacylglycerol (DAG), while ionomycin acts as a Ca2+
transporter, facilitating the transfer of Ca2+ from
organelles to the cytosol. PKC can be activated by the concerted
action of DAG and Ca2+. Intracellularly, activation of
PKC instigates the phosphorylation of numerous downstream protein
kinases, initiating a cascade reaction that leads to the expression
of multiple proteins, such as PI3K, NF-κB, thereby inducing
cellular activation. Consequently, the combined influence of PMA
and ionomycin activates PKC, thereby triggering downstream
responses (83,126). The principal outcome of this
activation is stimulation of cytokine production, providing a
plausible explanation for the observed increase in IL-10 production
in response to TLR agonists and PMA activation in B cells. In
periodontitis, CD25+ B cells induced in vitro
with Breg functionality exhibit augmented production of IL-10,
IL-35 and TGF-β when subjected to co-stimulation with both LPS and
CpG compared with stimulation with LPS or CpG alone (127). Moreover, the stimulation of
TLR4 and TLR9, combined with the addition of PMA and ionomycin,
significantly upregulates expression of T cell immunoglobulin and
mucin domain-1 (TIM-1) in the resulting Breg population. TIM-1 is a
protein that plays a crucial regulatory role in the immune system,
promoting proliferation and differentiation of Bregs by enhancing
the expression of STAT3 (42).
IL-21 is necessary for B10 cells to mature into
IL-10-secreting effector cells (36,128), however, IL-21 alone has little
effect (36,117). In a study conducted by
Zheremyan (129), B cells were
isolated from human blood and cultured in vitro; CD40L + CpG
led to the highest production of IL-10 by Bregs (129). The combination of CD40L + CpG +
IL-21 demonstrated the strongest immunosuppressive ability
(129). In a previous study by
Chesneau (130), the
combination of CD40L + CpG + IL-21 + F(ab')2 anti-BCR Abs + IL-2
resulted in generation of Bregs secreting granzyme B (GZMB). GZMB
is a cytotoxic protease belonging to the GZM family, primarily
produced by cytotoxic T and natural killer cells. GZMB plays a key
role in the immune system, particularly in the immune response
against viral infections and tumor cells (131). Secreted GZMB promotes the
proliferation and expansion of GZMB+ B cells in an
ERK1/2-dependent manner, while concurrently inhibiting
proliferation of Teff (effector T cells) cells in a
contact-dependent manner (130). However, GZMB+ B
cells are susceptible to apoptosis, possibly due to relatively high
caspase-3 activity, which limits application of GZMB+ B
cells (130).
Furthermore, the combination of CD40 with cytokines
has been shown to induce generation of B10 cells (129). Yoshizaki et al (36) devised a method involving feeder
cells (NIH-3T3-CD154/BLyS cells) expressing CD40L and BAFF, which
were cocultured with IL-4 and IL-21 cytokines; they observed
notable expansion of IL-10+ B cells in mice of up to
4×106 times. These in vitro expanded eBregs, were
subsequently infused into mice with EAE, resulting in significant
alleviation of EAE symptoms (36). This demonstrates that these
expanded eBregs retain their regulatory function in vivo,
representing the highest expansion efficiency documented thus far.
Additionally, Menon et al (132) conducted a study in which B
cells from both healthy individuals and patients with systemic
lupus erythematosus (SLE) were expanded in vitro through
stimulation with IFN-α and CpG-C; there was notable proliferation
of B10 cells while maintaining their cellular phenotype and
immunomodulatory capabilities in the healthy group. Conversely, no
comparable effect was observed in the SLE group (132).
Stimulation of adrenergic receptors on B cells, in
conjunction with the activation of TLR9 receptors, induces the
generation of B10 cells (57). A
total of >80% of IL-10-producing B cells concurrently express
the enzyme tyrosine hydroxylase (TH), suggesting TH as a potential
biomarker for Bregs generated by TLR9 activation (57). This diverges from the perspective
maintained by certain scholars regarding TIM-1 as a biomarker of
B10 cells as TIM-1 is involved in the maintenance and induction of
B10 cells, identifying over 70% of B10 cells (42,133).
It is unclear which optimal stimulatory signals are
required to achieve maximal inhibition of Teff. It is unclear
whether TLR-9 or TLR-4 stimuli are superior. Moreover, while CpG
ODNs are known to stimulate TLR9, there are multiple types, and it
remains unclear which type most effectively stimulates TLR9 to
induce the production of Bregs. Additionally, although
IL-10+ Bregs are predominantly utilized for cellular
therapy, selection of phenotypical markers or Bregs for therapeutic
purposes is still undetermined. Overall, none of the aforementioned
methods have been proven safe and effective by large-scale clinical
trials and the therapeutic mechanism still needs to be clarified,
which limits amplification tecniques. Exploration of novel methods
and techniques to expand Bregs to serve a broader range of
therapies is key.
Combining pharmacotherapy or cell-based
approaches in in vitro cultivation
Certain immunosuppressants, such as mTOR inhibitors
(e.g., sirolimus), abatacept (134,135), can stimulate the production of
B10 cells. Besides the addition of agonists, experiments using
drugs for in vitro expansion have been conducted (135,136). Sirolimus, an immunosuppressant
drug used to prevent organ transplant rejection, amplifies Bregs in
peripheral blood mononuclear cell in vitro, which secrete
IL-10 as well as TGF-β (135).
The use of astilbin, a flavonoid compound that has an
anti-inflammatory activity, in combination with LPS induces the
production of IL-10+ Bregs by promoting STAT3
phosphorylation in TIM-1+ B cells and they suppresses
the development and symptoms of dextran sulphate sodium-induced
colitis (31).
GSK-3 inhibition selectively increases IL-10
production by B cells, without increasing TNF-α or IL-6, and can
rescue IL-10 production even in the absence of mTOR activity
following rapamycin treatment. Additionally, GSK-3 inhibition,
selectively enhances IL-10 production, leading to enhanced ability
of B cells to induce the differentiation of
CD4+CD25− T cells into IL-10-producing Type 1
regulatory T cells (Tr1) in coculture experiments, even in the
absence of TLR activation (104,137). In addition, a recombinant
fusion protein composed of the TLR5 ligand flagellin A from
Listeria monocytogenes (rFlaA) and the major birch pollen
allergen Bet v 1 (rFlaA:Betv1) promotes an increase in B10 cells
via a similar mTOR agonist (138).
The addition of TLR stimulants promotes
differentiation of Bregs and leads to the production of
pro-inflammatory factors, so appropriate drugs are required to
increase B10 cells production and functionality. In an in
vitro experiment stimulating TLR7, the addition of Thymosin-α1
(Tα-1 can promote Breg while decreasing production of
pro-inflammatory factors, but its function is dependent on the
stimulation of TLR7 (139).
As dendritic cells have a proliferation-promoting
effect on Bregs via type I IFNs, exploring the feasibility of their
coculture is necessary (33,140). BAFF secreted by immune cells,
as well as a proliferation-inducing ligand (APRIL), have a role in
promoting Breg production and enhanced function by binding to BAFF
receptor on the surface of the B cells, as well as to transmembrane
activator and CAML interactor (TACI) (55,141-147). TACI is a receptor protein that
serves a key role in the immune system. It is a member of the BAFF
receptor family, binding to BAFF and APRIL, and is key in B cell
activation and immune responses. Investigating BAFF receptor may
provide valuable insights into the interaction between B cells and
other immune cell populations. For example, in in vitro cell
coculture experiments, the high expression of CD40L in palatine
tonsil lymphocytes sustains the survival of B cells and facilitates
differentiation of B cells into Bregs via BAFF receptor, ultimately
leading to an increase in Bregs expressing IL-10 and PD-L1, when
ratio of B cells to palatine tonsil lymphocytes is 1:1 (148). As the earliest stem cells used
in clinical practice, mesenchymal stem cells (149,150) with immunomodulatory properties
promote the production of Tregs and alleviate autoimmune diseases
such as SLE by inhibiting the activation, proliferation, and
cytokine production of B and T lymphocytes. Mesenchymal stem cells
expand Bregs in vitro in a dose-dependent manner; this
effect is increased by addition of LPS (151).
To the best of our knowledge, the efficacy of in
vitro amplification of Bregs using novel drugs has not been
compared with traditional stimulants, nor has the potential for
synergistic stimulation been explored. Additionally, in
vitro cell co-culture represents another research avenue beyond
drug-mediated expansion of Bregs. This includes co-culture with
tumor cells, although such approaches must address safety and
ethical considerations (152).
Engineering Bregs
The purification and amplification of Bregs face
limitations due to their relatively low abundance in human blood
and the slow rate of in vitro expansion (42). The number and function of
specific Bregs isolated and expanded alone may not be sufficient
for clinical applications. However, modifying cells may solve this
problem (153,154). As a new technological tool,
cell engineering promotes the expansion of regulatory B cells
through different strategies, enhancing immunosuppressive functions
of Bregs more robustly. In a previous study, lentiviral
transfection of B cells specifically enhanced expression of their
suppressor genes, inducing the generation of cells capable of
specifically secreting IL-10 in vitro (155). Naïve B cells, as an alternative
to activated B cells, do not secrete antibodies, express low levels
of co-stimulatory molecules and exhibit poor immunogenicity
(155). Alonso-Guallart et
al engineered CD40L-stimulated B cells (CD40L-sBc) by inducing
the CD40L K562 leukemia cell line to produce CD40L-sBc (used as
immunostimulatory antigen-presenting cells, for ex vivo
expansion of Tregs and inhibition of pro-inflammatory factor
formation, providing strategies for modulating immune responses in
therapeutic applications (156). There is need to modify Bregs to
produce cells with enhanced immunosuppressive functions and antigen
specificity, which could improve targeted immune regulation. This
approach could help treat autoimmune diseases, prevent transplant
rejection, and reduce side effects of broad immunosuppressive
therapies.
Chimeric antigen receptor (CAR)
CAR technology is a cell immunotherapy approach
primarily used in cancer treatment. This technique leverages
genetic engineering to introduce specific receptor genes into T
cells, enabling them to recognize and attack tumor cells (157,158). In recent years, CAR has been
applied to treatment of autoimmune diseases (153). For example, CD19 CAR-T cells
that specifically eliminate B cells have been studied for their
potential to alleviate symptoms of SLE (159). CAR-Tregs enhance the ability of
Tregs to modulate immune responses, effectively suppressing
excessive immune reactions and autoimmune diseases (154,160,161).
Furthermore, with advancements in CAR-Treg
(131), which involves the
expression of fragment antigen-binding regions specific to
alloantigens or autoantigens, robust suppression of alloimmune or
autoimmune responses can be achieved (132-134). Human leukocyte antigen (HLA) is
a component of the major histocompatibility complex in humans,
responsible for regulating immune system response. Since the
primary cause of transplant rejection is incompatibility of HLA
receptors between the donor and recipient, these molecules are
considered suitable targets for Tregs to enhance protection of
transplanted organs (162,163). The mouse models of heart and
skin transplantation have revealed that Tregs from mice carrying
anti-HLA-A2 CAR significantly suppress proliferation of
CD4+ T cells in response to specific allogeneic antigens
(160,164). Moreover, combination with
immunosuppressants significantly prolongs graft survival time
(160,165). In this context, exploration of
CAR-Breg holds promising prospects for future research. The
integration of CD19, CD40, BCR and TLR components may facilitate
the development of an effective CAR-Breg design. By designing
CAR-Bregs targeting transplant rejection-related markers such as
HLA-G, it may be possible to enhance immunosuppressive functions
(166). The design of CAR-Bregs
aims to amplify the regulatory functions of B cells, particularly
in inhibiting abnormal immune response and promoting immune
tolerance. This highlights the potential of CAR-Bregs for enhancing
immune regulation in clinical settings. Currently, the design of
CAR-Bregs remains largely theoretical, and there is need for
further breakthroughs regarding potential application in
transplantation immunization.
Applications of cellular therapy in
transplantation
Immunotherapy and in vitro expansion
techniques are complementary. Cellular therapy, originating in
tumor treatment, involves transplanting or infusing normal or
bioengineered human cells into a patient, where the newly
introduced cells replace damaged ones (167,168). With the continuous development
of cellular therapies, adoptive regulatory cell therapy has been
recognized as a potential strategy to enhance graft tolerance
(169-171). The numbers of Bregs in tolerant
patients are similar to those in healthy individuals, whereas Breg
populations are impaired in patients with chronic rejection
(172,173). Bregs serve an important role in
maintaining organ transplantation tolerance. Thus, adoptive Breg
therapy may provide a new immunomodulatory therapeutic technique
aimed at fostering transplantation tolerance by interacting with
other immune cells (Fig. 3).
However, research in this area remains largely in the preclinical
stage, facing challenges such as insufficient expansion of
regulatory cells and low efficiency (168).
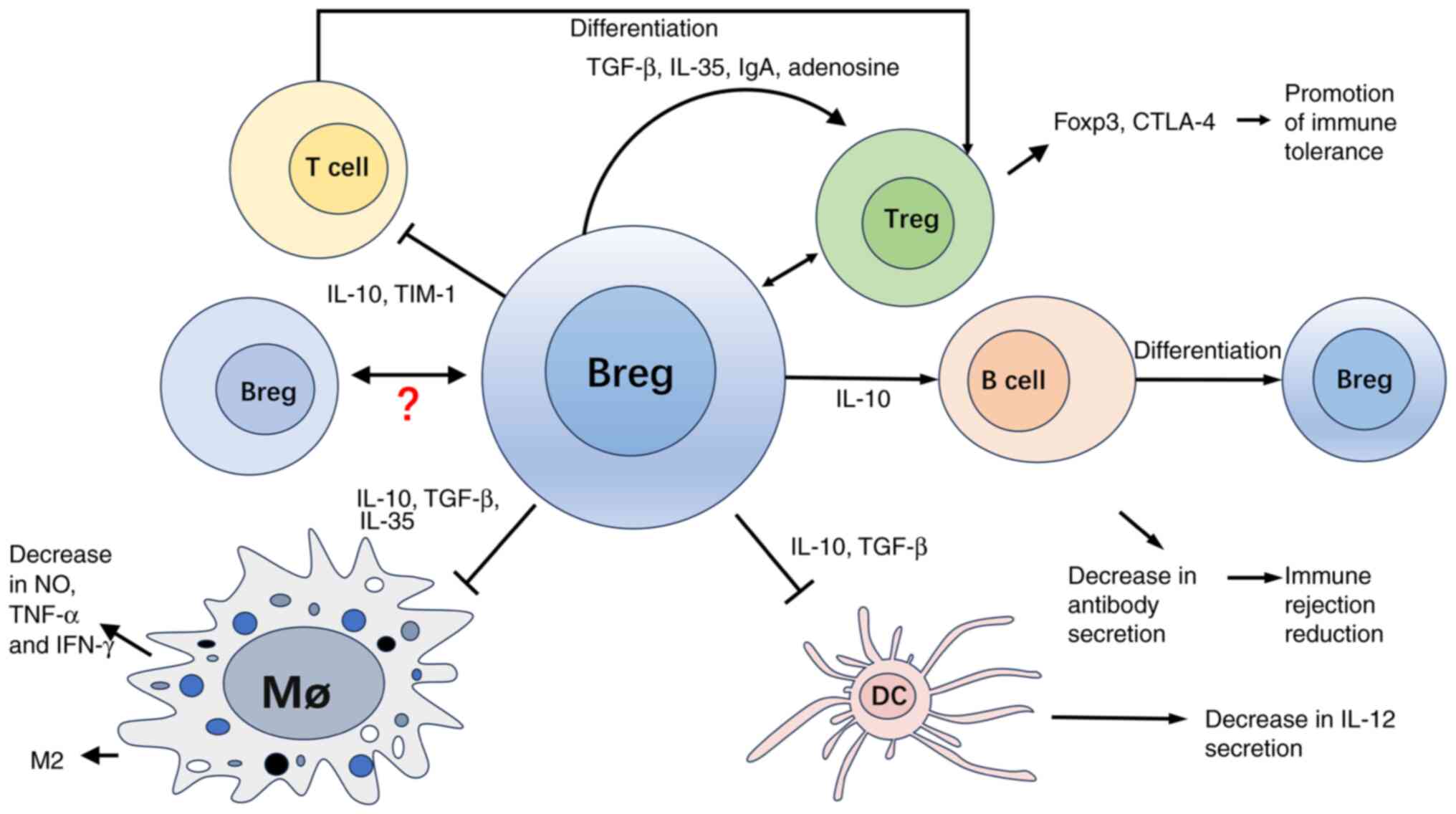 | Figure 3Expanded Bregs in vitro may
play a role in surrounding immune cells and microenvironment by
secreting cytokines and cell-to-cell contact and inducing graft
tolerance. Following adoptive transfer, Bregs secreted cytokines
such as IL-10 and engaged in intercellular interactions to inhibit
pro-inflammatory Teff cells. Additionally, Bregs promoted the
differentiation of B cells into Bregs, macrophages (Mø) into the M2
inhibitory phenotype, and T cells into Tregs, leading to increased
expression of Foxp3 and CTLA-4. Moreover, TGF-β, an
anti-inflammatory cytokine secreted by Bregs, supported the
survival of these anti-inflammatory cells. Collectively, these
mechanisms contribute to establishment of an immune-tolerant
environment in transplant recipients. TIM, T-cell immunoglobulin
and Mucin-domain-containing molecule; Breg, regulatory B cell;
Treg, regulatory T cell; M2, M2 macrophage; Mø, macrophage; CTLA,
cytotoxic T-lymphocyte antigen; DC, dendritic cell; Foxp, forkhead
box protein. |
Cell therapy in transplantation
B cells are involved in development of immune
tolerance in transplant recipients. A previous study have shown
that deletion of B cells in transplant patients using CD20
antibodies results in acute rejection, which is most likely due to
the elimination of Bregs (174). Although there is evidence that
Bregs play a central role in the induction and maintenance of
tolerance in various immunopathologies, tolerant kidney transplant
patients exhibit an increase in B cell numbers in vivo
(175). However, due to
interspecies differences, the predictive value of animal models is
limited, highlighting the need to regulate both the quantity and
functional activity of B10 cells. The in vitro expansion of
human Bregs is challenging due to the rarity of Bregs in peripheral
blood, the complex and specific stimulatory signals required for
their differentiation, and difficulties in maintaining their
functional properties, such as IL-10 production (175). Additionally, certain phenotypes
are exclusively expressed in humans, which necessitates targeted
strategies for the expansion of specific human Breg phenotypes.
During formation of immune tolerance in transplant
recipients, B10 cells serve a major role. In our previous study on
an immune tolerance model for islet transplantation in diabetic
mice (21), an increase in B10
cell levels in vivo was associated with disease alleviation.
Similar findings were noted in on graft-vs.-host disease (GVHD) and
kidney transplantation (176,177), where increased levels of B10
cells are linked to improved disease outcomes. These results
suggest that B10 serve a key role in immunoregulation during
transplantation.
Following in vitro expansion, B10 cells can
be reinfused into transplant recipients to extend graft survival
time. Shankar et al (42)
reported adoptive transfer of human-derived Bregss after in
vitro expansion into mice, extending the survival time of skin
grafts. By contrast, a previous study focused on murine Breg cell
expansion in vitro (42).
Mouse model studies have demonstrated that the adoptive transfer of
B cells with induced regulatory function can effectively mitigate
the disease process (11,177-182).
For example, in a murine cardiac transplantation model,
antibody-induced tolerance is disrupted following B cell depletion
and is associated with IL-10+ B cells (22,183). In a murine model of GVHD,
allogeneic bone marrow transplantation (BMT) followed by adoptive
transfer of B10 cells revealed that GVHD is more severe in mice
transplanted with CD19−/− donor cells compared with
those receiving CD19+/+ cells; this is associated with a
significant increase in CD8+ T cells, as well as TNF-α-
and IFN-γ-producing CD4+ T cells in the spleen 14 days
post-BMT. However, when B cells are cultured in vitro with
PMA, ionomycin, LPS and brefeldin A to generate B10 cells, adoptive
transfer of these cells into CD19−/− donor mice resultes
in a significant improvement in GVHD severity, with a marked
reduction in skin scores at time of transplantation compared with
14 days post-transplant (176).
In a model of allogeneic cardiac transplantation, transplant
tolerance induced by anti-CD40L mAb is disrupted following
treatment with anti-CD20 mAb. However, adoptive transfer of B10
from IL-10-sufficient littermate mice rescues graft survival.
Compared with the adoptive transfer of B cells from
IL-10−/− or IL-10-deficient mice, this approach
significantly prolongs cardiac graft survival beyond 40 days and
notably decreases histopathological rejection scores in allogeneic
transplants (22). Further
transplantation experiments in small animal models are needed to
validate the feasibility and safety of the adoptive transfer of B10
cells in transplantation across different organ sites.
In clinical investigations, it has been
hypothesized that B10 cells negatively regulate transplant immunity
following solid organ transplantation and adoptive transfer of
autologous B10 cells could serve as a novel therapeutic approach
for transplant recipients to manage persistent chronic rejection
(184). Shankar (42) demonstrated in a humanized mouse
model of skin transplantation that in vitro stimulation with
CD154 and expansion of human IL-10+ B cells
significantly prolongs graft survival (>80 days). Notably, the
frequency of B10 cells in the graft and spleen markedly increase
and production of TNF-α and IFN-γ by CD4+ T cells is
significantly suppressed. These findings indirectly support the
potential of adoptive transfer of B10 to improve human solid organ
transplantation outcomes, providing a basis for further clinical
translation (42).
Adoptively transferred B10 cells alter induction
and localization of T follicular helper and regulatory cells in
vivo and secrete the anti-inflammatory cytokine IL-10. These
effects lead to an indirect increase in the number and function of
Tregs in the recipient, thereby modulating immune response and
inflammation to exert anti-inflammatory effects, which extends
graft survival time (183,185). Although B10 exhibits a
broad-spectrum effect, in islet transplantation experiment,
stimulating B cells in vitro with pro-inflammatory factors
generates regulatory cells, some of which secrete TGF-β (125). Bregs that secrete TGF-β
significantly prolong graft survival compared with B10, suggesting
phenotypes of Bregs are adapted to specific transplanted organs
(114,125).
Potential problems and improvement
strategies
There are numerous issues and challenges of Breg
therapy. One issue is the difficulty in expanding Breg cells in
vitro (42), with
improvements in expansion efficiency being a major focus of ongoing
research. Addressing this challenge requires continuous exploration
of novel stimulation methods, including adjustments to duration and
types of stimulants employed (68,114). In transplantation, B10 cell
therapy requires identification of the most suitable in
vitro expansion methods to balance both quantity and quality.
Additionally, maintaining purity and activity of Bregs is
challenging (42), as prolonged
culture periods can result in phenotypical instability, which may
compromise therapeutic efficacy. This necessitates the exploration
of standardized expansion protocols to achieve a stable B10 cell
phenotype. Moreover, there is an increased risk of infections and
tumor development associated with cell therapy, particularly in
immunosuppressed patients. This underscores the need for
large-scale animal studies to ensure the safety and efficacy of
cell therapy. Furthermore, to enhance therapeutic effects, there is
need for further exploration of effective delivery routes and
targeted technologies to enable Bregs to reach disease sites
directly; this may be facilitated by CAR technology (125). Based on patient conditions and
the organ transplanted, different B10 cell phenotypes may need to
be reinfused, allowing development of personalized treatment
plans.
In summary, adoptively transferred B10 cells
balance Treg/Teff dynamics in vivo, resulting in prolonged
organ or tissue transplants survival times. Ongoing exploration of
endogenous Breg expansion and harvesting, optimization of in
vitro expansion methods and improvement of in vivo
injection methods provide a basis for the theoretical and practical
use of Breg therapy in the clinical phase.
Conclusion
Bregs serve a crucial immunosuppressive role in
promoting the long-term survival of grafts (42). Various methods have successfully
achieved efficient in vitro expansion of certain Breg
phenotypes and there is a relatively comprehensive understanding of
the signaling pathways involved in in vivo expansion
mechanisms (36,139). However, the development of Breg
therapy is still hindered by several challenges, including lack of
clear definitions for specific cell phenotypes, inadequate in
vitro expansion technologies, the absence of standardized
methodology and no definitive conclusions regarding the types of
stimulant to be utilized (42,57,133).
Enhancing in vitro expansion technologies be
facilitated by studying and simulating Breg expansion under in
vivo inflammatory and tumor conditions (67,119,186). Future advancements in Breg
expansion strategies should integrate both in vitro
expansion and in vivo facilitation to maximize cell yield
and therapeutic effectiveness for the patient. This may involve the
combined action of pharmacological agents or microorganisms
(140,187).
Beyond transplantation immunology, expansion
technologies hold potential for the treatment of autoimmune
diseases and other immune-associated disorders. Individualized
treatment approaches could emerge by expanding Bregs tailored to
meet specific immunomodulatory needs of patients. Different
stimulation methods yield distinct subtypes of Breg cells, paving
the way for their application in a variety of diseases to fulfill
specific therapeutic roles. Additionally, these technologies serve
as valuable research tools to elucidate the biological properties
and functional mechanisms of Bregs.
Availability of data and materials
Not applicable.
Authors' contributions
GZ and DZ conceived the study and wrote the
manuscript. GH constructed figures. YuY and YC revised the
manuscript. YiY, GH, YuY, GZ, DZ and YC edited the manuscript. Data
authentication is not applicable. All authors have read and
approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Acknowledgements
Not applicable.
Funding
The present study was supported by National Natural Science
Foundation of China (grant no. 81771723) and Department of Science
and Technology of Sichuan Province (grant no. 2022ZYFS0157).
References
|
1
|
Neu SD and Dittel BN: Characterization of
definitive regulatory B cell subsets by cell surface phenotype,
function and context. Front Immunol. 12:7874642021. View Article : Google Scholar
|
|
2
|
Mohib K, Cherukuri A, Zhou Y, Ding Q,
Watkins SC and Rothstein DM: Antigen-dependent interactions between
regulatory B cells and T cells at the T:B border inhibit subsequent
T cell interactions with DCs. Am J Transplant. 20:52–63. 2020.
View Article : Google Scholar
|
|
3
|
Amu S, Saunders SP, Kronenberg M, Mangan
NE, Atzberger A and Fallon PG: Regulatory B cells prevent and
reverse allergic airway inflammation via FoxP3-positive T
regulatory cells in a murine model. J Allergy Clin Immunol.
125:1114–1124.e8. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Vomhof-DeKrey EE, Yates J, Hägglöf T,
Lanthier P, Amiel E, Veerapen N, Besra GS, Karlsson MC and
Leadbetter EA: Cognate interaction with iNKT cells expands
IL-10-producing B regulatory cells. Proc Natl Acad Sci USA.
112:12474–12479. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Boldison J, Da Rosa LC, Davies J, Wen L
and Wong FS: Dendritic cells license regulatory B cells to produce
IL-10 and mediate suppression of antigen-specific CD8 T cells. Cell
Mol Immunol. 17:843–855. 2020. View Article : Google Scholar :
|
|
6
|
Geladaris A, Häusser-Kinzel S, Pretzsch R,
Nissimov N, Lehmann-Horn K, Häusler D and Weber MS: IL-10-providing
B cells govern pro-inflammatory activity of macrophages and
microglia in CNS autoimmunity. Acta Neuropathol. 145:461–477. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yanaba K, Bouaziz JD, Haas KM, Poe JC,
Fujimoto M and Tedder TF: A regulatory B cell subset with a unique
CD1dhiCD5+ phenotype controls T cell-dependent inflammatory
responses. Immunity. 28:639–650. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Rosser EC and Mauri C: Regulatory B cells:
Origin, phenotype, and function. Immunity. 42:607–612. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Shang J, Zha H and Sun Y: Phenotypes,
Functions, and Clinical Relevance of Regulatory B Cells in Cancer.
Front Immunol. 11:5826572020. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hong J, Fang J, Lan R, Tan Q, Tian Y,
Zhang M, Okunieff P, Zhang L, Lin J and Han D: TLR9 mediated
regulatory B10 cell amplification following sub-total body
irradiation: Implications in attenuating EAE. Mol Immunol.
83:52–61. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zheremyan EA, Ustiugova AS, Karamushka NM,
Uvarova AN, Stasevich EM, Bogolyubova AV, Kuprash DV and Korneev
KV: Breg-Mediated Immunoregulation in the Skin. Int J Mol Sci.
25:5832024. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Mauri C and Menon M: Human regulatory B
cells in health and disease: therapeutic potential. J Clin Invest.
127:772–779. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yu CR, Choi JK, Uche AN and Egwuagu CE:
Production of IL-35 by Bregs is mediated through binding of
BATF-IRF-4-IRF-8 complex to il12a and ebi3 promoter elements. J
Leukoc Biol. 104:1147–1157. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Oleinika K, Mauri C and Salama AD:
Effector and regulatory B cells in immune-mediated kidney disease.
Nat Rev Nephrol. 15:11–26. 2019. View Article : Google Scholar
|
|
15
|
Elias C, Chen C and Cherukuri A:
Regulatory B cells in solid organ transplantation: From immune
monitoring to immunotherapy. Transplantation. 108:1080–1089.
2024.
|
|
16
|
Juneja T, Kazmi M, Mellace M and Saidi RF:
Utilization of Treg Cells in Solid Organ Transplantation. Front
Immunol. 13:7468892022. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kessel A, Haj T, Peri R, Snir A, Melamed
D, Sabo E and Toubi E: Human CD19(+)CD25(high) B regulatory cells
suppress proliferation of CD4(+) T cells and enhance Foxp3 and
CTLA-4 expression in T-regulatory cells. Autoimmun Rev. 11:670–677.
2012. View Article : Google Scholar
|
|
18
|
Lee KM, Stott RT, Zhao G, SooHoo J, Xiong
W, Lian MM, Fitzgerald L, Shi S, Akrawi E, Lei J, et al:
TGF-β-producing regulatory B cells induce regulatory T cells and
promote transplantation tolerance. Eur J Immunol. 44:1728–1736.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Deng S, Moore DJ, Huang X, Lian MM,
Mohiuddin M, Velededeoglu E, Lee MK IV, Sonawane S, Kim J, Wang J,
et al: Cutting edge: Transplant tolerance induced by anti-CD45RB
requires B lymphocytes. J Immunol. 178:6028–6032. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Singh A, Carson WF IV, Secor ER Jr,
Guernsey LA, Flavell RA, Clark RB, Thrall RS and Schramm CM:
Regulatory role of B cells in a murine model of allergic airway
disease. J Immunol. 180:7318–7326. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhao G, Moore DJ, Lee KM, Kim JI, Duff PE,
O'Connor MR, Hirohashi T, Lei J, Yang M, Markmann JF and Deng S: An
unexpected counter-regulatory role of IL-10 in
B-lymphocyte-mediated transplantation tolerance. Am J Transplant.
10:796–801. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Lal G, Nakayama Y, Sethi A, Singh AK,
Burrell BE, Kulkarni N, Brinkman CC, Iwami D, Zhang T and Bromberg
JS: Interleukin-10 From Marginal Zone Precursor B-Cell Subset Is
Required for Costimulatory Blockade-Induced Transplantation
Tolerance. Transplantation. 99:1817–1828. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Fillatreau S, Gray D and Anderton SM: Not
always the bad guys: B cells as regulators of autoimmune pathology.
Nat Rev Immunol. 8:391–397. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Anderton SM and Fillatreau S: Activated B
cells in autoimmune diseases: the case for a regulatory role. Nat
Clin Pract Rheumatol. 4:657–666. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Lanzavecchia A and Sallusto F: Toll-like
receptors and innate immunity in B-cell activation and antibody
responses. Curr Opin Immunol. 19:268–274. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zindel J and Kubes P: DAMPs, PAMPs, and
LAMPs in immunity and sterile inflammation. Annu Rev Pathol.
15:493–518. 2020. View Article : Google Scholar
|
|
27
|
Romani L: Immunity to fungal infections.
Nat Rev Immunol. 11:275–288. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kumar V and Sharma A: Innate immunity in
sepsis pathogenesis and its modulation: New immunomodulatory
targets revealed. J Chemother. 20:672–683. 2008. View Article : Google Scholar
|
|
29
|
Iwasaki A and Medzhitov R: Regulation of
adaptive immunity by the innate immune system. Science.
327:291–295. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Sîrbulescu RF, Mamidi A, Chan SY, Jin G,
Boukhali M, Sobell D, Ilieş I, Chung JY, Haas W, Whalen MJ, et al:
B cells support the repair of injured tissues by adopting
MyD88-dependent regulatory functions and phenotype. FASEB J.
35:e220192021. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Xu Y, Wu K, Han S, Ding S, Lu G, Lin Z,
Zhang Y, Xiao W, Gong W, Ding Y and Deng B: Astilbin combined with
lipopolysaccharide induces IL-10-producing regulatory B cells via
the STAT3 signalling pathway. Biomed Pharmacother. 129:1104502020.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Lampropoulou V, Hoehlig K, Roch T, Neves
P, Calderón Gómez E, Sweenie CH, Hao Y, Freitas AA, Steinhoff U,
Anderton SM and Fillatreau S: TLR-activated B cells suppress T
cell-mediated autoimmunity. J Immunol. 180:4763–4773. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Liu BS, Cao Y, Huizinga TW, Hafler DA and
Toes RE: TLR-mediated STAT3 and ERK activation controls IL-10
secretion by human B cells. Eur J Immunol. 44:2121–2129. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Kawai T and Akira S: The role of
pattern-recognition receptors in innate immunity: update on
Toll-like receptors. Nat Immunol. 11:373–384. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Pasare C and Medzhitov R: Control of
B-cell responses by Toll-like receptors. Nature. 438:364–368. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Yoshizaki A, Miyagaki T, DiLillo DJ,
Matsushita T, Horikawa M, Kountikov EI, Spolski R, Poe JC, Leonard
WJ and Tedder TF: Regulatory B cells control T-cell autoimmunity
through IL-21-dependent cognate interactions. Nature. 491:264–268.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Barr TA, Brown S, Ryan G, Zhao J and Gray
D: TLR-mediated stimulation of APC: Distinct cytokine responses of
B cells and dendritic cells. Eur J Immunol. 37:3040–3053. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Hao Y, O'Neill P, Naradikian MS, Scholz JL
and Cancro MP: A B-cell subset uniquely responsive to innate
stimuli accumulates in aged mice. Blood. 118:1294–1304. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Takeda K and Akira S: TLR signaling
pathways. Semin Immunol. 16:3–9. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Tian F, Xian K, Yang B, Duan Q, Qian L and
Shi C: Deficiency in TLR4 impairs regulatory B cells production
induced by Schistosome soluble egg antigen. Mol Biochem Parasitol.
253:1115322023. View Article : Google Scholar
|
|
41
|
Oladipupo FO, Yu CR, Olumuyide E,
Jittaysothorn Y, Choi JK and Egwuagu CE: STAT3 deficiency in B
cells exacerbates uveitis by promoting expansion of pathogenic
lymphocytes and suppressing regulatory B cells (Bregs) and Tregs.
Sci Rep. 10:161882020. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Shankar S, Stolp J, Juvet SC, Beckett J,
Macklin PS, Issa F, Hester J and Wood KJ: Ex vivo-expanded human
CD19+TIM-1+ regulatory B cells suppress immune responses in vivo
and are dependent upon the TIM-1/STAT3 axis. Nat Commun.
13:31212022. View Article : Google Scholar :
|
|
43
|
Bauer S, Kirschning CJ, Häcker H, Redecke
V, Hausmann S, Akira S, Wagner H and Lipford GB: Human TLR9 confers
responsiveness to bacterial DNA via species-specific CpG motif
recognition. Proc Natl Acad Sci USA. 98:9237–9242. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Ohto U, Shibata T, Tanji H, Ishida H,
Krayukhina E, Uchiyama S, Miyake K and Shimizu T: Structural basis
of CpG and inhibitory DNA recognition by Toll-like receptor 9.
Nature. 520:702–705. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Akkaya M, Akkaya B, Kim AS, Miozzo P, Sohn
H, Pena M, Roesler AS, Theall BP, Henke T, Kabat J, et al:
Toll-like receptor 9 antagonizes antibody affinity maturation. Nat
Immunol. 19:255–266. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Alaqla A, Hu Y, Huang S, Ruiz S, Kawai T
and Han X: TLR9 signaling is required for the porphyromonas
gingivalis-induced activation of IL-10-expressing B cells. Int J
Mol Sci. 24:66932023. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Ohto U and Shimizu T: Structural aspects
of nucleic acid-sensing Toll-like receptors. Biophys Rev. 8:33–43.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Hu Q, Li H, Wang L, Gu H and Fan C: DNA
Nanotechnology-Enabled drug delivery systems. Chem Rev.
119:6459–6506. 2019. View Article : Google Scholar
|
|
49
|
Mutwiri G, van Drunen Littel-van den Hurk
S and Babiuk LA: Approaches to enhancing immune responses
stimulated by CpG oligodeoxynucleotides. Adv Drug Deliv Rev.
61:226–232. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Kawai T and Akira S: Signaling to
NF-kappaB by Toll-like receptors. Trends Mol Med. 13:460–469. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Uematsu S, Sato S, Yamamoto M, Hirotani T,
Kato H, Takeshita F, Matsuda M, Coban C, Ishii KJ, Kawai T, et al:
Interleukin-1 receptor-associated kinase-1 plays an essential role
for Toll-like receptor (TLR)7- and TLR9-mediated interferon-{alpha}
induction. J Exp Med. 201:915–923. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Walsh MC, Lee J and Choi Y: Tumor necrosis
factor receptor-associated factor 6 (TRAF6) regulation of
development, function, and homeostasis of the immune system.
Immunol Rev. 266:72–92. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
O'Neill LA and Bowie AG: The family of
five: TIR-domain-containing adaptors in Toll-like receptor
signalling. Nat Rev Immunol. 7:353–364. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Sato S, Sanjo H, Takeda K, Ninomiya-Tsuji
J, Yamamoto M, Kawai T, Matsumoto K, Takeuchi O and Akira S:
Essential function for the kinase TAK1 in innate and adaptive
immune responses. Nat Immunol. 6:1087–1095. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Yang M, Sun L, Wang S, Ko KH, Xu H, Zheng
BJ, Cao X and Lu L: Novel function of B cell-activating factor in
the induction of IL-10-producing regulatory B cells. J Immunol.
184:3321–3325. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Huang X and Yang Y: Targeting the
TLR9-MyD88 pathway in the regulation of adaptive immune responses.
Expert Opin Ther Targets. 14:787–796. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Honke N, Lowin T, Opgenoorth B, Shaabani
N, Lautwein A, Teijaro JR, Schneider M and Pongratz G: Endogenously
produced catecholamines improve the regulatory function of
TLR9-activated B cells. PLoS Biol. 20:e30015132022. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Pongratz G, Melzer M and Straub RH: The
sympathetic nervous system stimulates anti-inflammatory B cells in
collagen-type II-induced arthritis. Ann Rheum Dis. 71:432–439.
2012. View Article : Google Scholar
|
|
59
|
Darnell JE Jr: STATs and gene regulation.
Science. 277:1630–1635. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Levy DE and Darnell JE Jr: Stats:
Transcriptional control and biological impact. Nat Rev Mol Cell
Biol. 3:651–662. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
61
|
Bromberg J: Stat proteins and oncogenesis.
J Clin Invest. 109:1139–1142. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Bromberg JF, Wrzeszczynska MH, Devgan G,
Zhao Y, Pestell RG, Albanese C and Darnell JE Jr: Stat3 as an
oncogene. Cell. 98:295–303. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Wang YH, Tsai DY, Ko YA, Yang TT, Lin IY,
Hung KH and Lin KI: Blimp-1 Contributes to the Development and
Function of Regulatory B Cells. Front Immunol. 10:19092019.
View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Diehl SA, Schmidlin H, Nagasawa M, van
Haren SD, Kwakkenbos MJ, Yasuda E, Beaumont T, Scheeren FA and
Spits H: STAT3-mediated up-regulation of BLIMP1 Is coordinated with
BCL6 down-regulation to control human plasma cell differentiation.
J Immunol. 180:4805–4815. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Michée-Cospolite M, Boudigou M, Grasseau
A, Simon Q, Mignen O, Pers JO, Cornec D, Le Pottier L and Hillion
S: Molecular Mechanisms Driving IL-10- Producing B Cells Functions:
STAT3 and c-MAF as Underestimated Central Key Regulators? Front
Immunol. 13:8188142022. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Jin G, Hamaguchi Y, Matsushita T, Hasegawa
M, Le Huu D, Ishiura N, Naka K, Hirao A, Takehara K and Fujimoto M:
B-cell linker protein expression contributes to controlling
allergic and autoimmune diseases by mediating IL-10 production in
regulatory B cells. J Allergy Clin Immunol. 131:1674–1682. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Zhou M, Wen Z, Cheng F, Ma J, Li W, Ren H,
Sheng Y, Dong H, Lu L, Hu HM and Wang LX: Tumor-released
autophagosomes induce IL-10-producing B cells with suppressive
activity on T lymphocytes via TLR2-MyD88-NF-κB signal pathway.
Oncoimmunology. 5:e11804852016. View Article : Google Scholar
|
|
68
|
Wang K, Tao L, Su J, Zhang Y, Zou B, Wang
Y, Zou M, Chen N, Lei L and Li X: TLR4 supports the expansion of
FasL + CD5 + CD1d hi regulatory B cells, which decreases in contact
hypersensitivity. Mol Immunol. 87:188–199. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Lee MB, Lee JH, Hong SH, You JS, Nam ST,
Kim HW, Park YH, Lee D, Min KY, Park YM, et al: JQ1, a BET
inhibitor, controls TLR4-induced IL-10 production in regulatory B
cells by BRD4-NF-κB axis. BMB Rep. 50:640–646. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Pore D, Matsui K, Parameswaran N and Gupta
N: Cutting Edge: Ezrin Regulates Inflammation by Limiting B Cell
IL-10 Production. J Immunol. 196:558–562. 2016. View Article : Google Scholar
|
|
71
|
Mishima Y, Oka A, Liu B, Herzog JW, Eun
CS, Fan TJ, Bulik-Sullivan E, Carroll IM, Hansen JJ, Chen L, et al:
Microbiota maintain colonic homeostasis by activating
TLR2/MyD88/PI3K signaling in IL-10-producing regulatory B cells. J
Clin Invest. 129:3702–3716. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Lanzavecchia A: Antigen-specific
interaction between T and B cells. Nature. 314:537–539. 1985.
View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Clark MR, Campbell KS, Kazlauskas A,
Johnson SA, Hertz M, Potter TA, Pleiman C and Cambier JC: The B
cell antigen receptor complex: Association of Ig-alpha and Ig-beta
with distinct cytoplasmic effectors. Science. 258:123–126. 1992.
View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Reth M: Antigen receptor tail clue.
Nature. 338:383–384. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Niiro H and Clark EA: Regulation of B-cell
fate by antigen-receptor signals. Nat Rev Immunol. 2:945–956. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Dal Porto JM, Gauld SB, Merrell KT, Mills
D, Pugh-Bernard AE and Cambier J: B cell antigen receptor signaling
101. Mol Immunol. 41:599–613. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Saijo K, Schmedt C, Su IH, Karasuyama H,
Lowell CA, Reth M, Adachi T, Patke A, Santana A and Tarakhovsky A:
Essential role of Src-family protein tyrosine kinases in NF-kappaB
activation during B cell development. Nat Immunol. 4:274–279. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Carter RH and Fearon DT: CD19: lowering
the threshold for antigen receptor stimulation of B lymphocytes.
Science. 256:105–107. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Baracho GV, Miletic AV, Omori SA, Cato MH
and Rickert RC: Emergence of the PI3-kinase pathway as a central
modulator of normal and aberrant B cell differentiation. Curr Opin
Immunol. 23:178–183. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Fujimoto M, Fujimoto Y, Poe JC, Jansen PJ,
Lowell CA, DeFranco AL and Tedder TF: CD19 regulates Src family
protein tyrosine kinase activation in B lymphocytes through
processive amplification. Immunity. 13:47–57. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Gold MR, Law DA and DeFranco AL:
Stimulation of protein tyrosine phosphorylation by the B-lymphocyte
antigen receptor. Nature. 345:810–813. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Fillatreau S, Sweenie CH, McGeachy MJ,
Gray D and Anderton SM: B cells regulate autoimmunity by provision
of IL-10. Nat Immunol. 3:944–950. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Yanaba K, Bouaziz JD, Matsushita T,
Tsubata T and Tedder TF: The development and function of regulatory
B cells expressing IL-10 (B10 cells) requires antigen receptor
diversity and TLR signals. J Immunol. 182:7459–7472. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Hussain S and Delovitch TL: Intravenous
transfusion of BCR-activated B cells protects NOD mice from type 1
diabetes in an IL-10-dependent manner. J Immunol. 179:7225–7232.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Adori M, Khoenkhoen S, Zhang J, Dopico XC
and Karlsson Hedestam GB: Enhanced B Cell Receptor Signaling
Partially Compensates for Impaired Toll-like Receptor 4 Responses
in LPS-Stimulated IκBNS-Deficient B Cells. Cells. 12:12292023.
View Article : Google Scholar
|
|
86
|
Tedder TF: B10 cells: A functionally
defined regulatory B cell subset. J Immunol. 194:1395–1401. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Parekh VV, Prasad DVR, Banerjee PP, Joshi
BN, Kumar A and Mishra GC: B cells activated by lipopolysaccharide,
but not by anti-Ig and anti-CD40 antibody, induce anergy in CD8+ T
cells: role of TGF-beta 1. J Immunol. 170:5897–5911. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Hazeki K, Nigorikawa K and Hazeki O: Role
of phosphoinositide 3-kinase in innate immunity. Biol Pharm Bull.
30:1617–1723. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Ruse M and Knaus UG: New players in
TLR-mediated innate immunity: PI3K and small Rho GTPases. Immunol
Res. 34:33–48. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Tuveson DA, Carter RH, Soltoff SP and
Fearon DT: CD19 of B cells as a surrogate kinase insert region to
bind phosphatidylinositol 3-kinase. Science. 260:986–989. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Ingham RJ, Holgado-Madruga M, Siu C, Wong
AJ and Gold MR: The Gab1 protein is a docking site for multiple
proteins involved in signaling by the B cell antigen receptor. J
Biol Chem. 273:30630–30637. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Okada T, Maeda A, Iwamatsu A, Gotoh K and
Kurosaki T: BCAP: The tyrosine kinase substrate that connects B
cell receptor to phosphoinositide 3-kinase activation. Immunity.
13:817–827. 2000. View Article : Google Scholar
|
|
93
|
Panchamoorthy G, Fukazawa T, Miyake S,
Soltoff S, Reedquist K, Druker B, Shoelson S, Cantley L and Band H:
p120cbl is a major substrate of tyrosine phosphorylation upon B
cell antigen receptor stimulation and interacts in vivo with Fyn
and Syk tyrosine kinases, Grb2 and Shc adaptors, and the p85
subunit of phosphatidylinositol 3-kinase. J Biol Chem.
271:3187–3194. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Gold MR: Intermediary signaling effectors
coupling the B-cell receptor to the nucleus. Curr Top Microbiol
Immunol. 245:77–134. 2000.
|
|
95
|
Marshall AJ, Niiro H, Yun TJ and Clark EA:
Regulation of B-cell activation and differentiation by the
phosphatidylinositol 3-kinase and phospholipase Cgamma pathway.
Immunol Rev. 176:30–46. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Lemmon MA and Ferguson KM:
Signal-dependent membrane targeting by pleckstrin homology (PH)
domains. Biochem J. 350 Pt 1(Pt 1): 1–18. 2000.PubMed/NCBI
|
|
97
|
Scheid MP and Woodgett JR: PKB/AKT:
Functional insights from genetic models. Nat Rev Mol Cell Biol.
2:760–768. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Cross DA, Alessi DR, Cohen P, Andjelkovich
M and Hemmings BA: Inhibition of glycogen synthase kinase-3 by
insulin mediated by protein kinase B. Nature. 378:785–789. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Li J, Gao J, Zhou H, Zhou J, Deng Z, Lu Y,
Rao J, Ji G, Gu J, Yang X, et al: Inhibition of glycogen synthase
kinase 3β increases the proportion and suppressive function of
CD19+CD24hiCD27+ breg cells. Front Immunol. 11:6032882020.
View Article : Google Scholar
|
|
100
|
Kaidanovich-Beilin O and Woodgett JR:
GSK-3: Functional insights from cell biology and animal models.
Front Mol Neurosci. 4:402011. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Maurer U, Preiss F, Brauns-Schubert P,
Schlicher L and Charvet C: GSK-3 - at the crossroads of cell death
and survival. J Cell Sci. 127(Pt 7): 1369–1378. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Gold MR, Scheid MP, Santos L, Dang-Lawson
M, Roth RA, Matsuuchi L, Duronio V and Krebs DL: The B cell antigen
receptor activates the Akt (protein kinase B)/glycogen synthase
kinase-3 signaling pathway via phosphatidylinositol 3-kinase. J
Immunol. 163:1894–1905. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Cheng H, Wang L, Yang B, Li D, Wang X, Liu
X, Tian N, Huang Q, Feng R, Wang Z, et al: Cutting edge: Inhibition
of glycogen synthase kinase 3 activity induces the generation and
enhanced suppressive function of human IL-10+ FOXP3+-induced
regulatory T Cells. J Immunol. 205:1497–1502. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Mielle J, Morel J, Elhmioui J, Combe B,
Macia L, Dardalhon V, Taylor N, Audo R and Daien C: Glutamine
promotes the generation of B10+ cells via the mTOR/GSK3 pathway.
Eur J Immunol. 52:418–430. 2022. View Article : Google Scholar
|
|
105
|
Chen Z and Wang JH: How the signaling
crosstalk of B cell receptor (BCR) and Co-receptors regulates
antibody class switch recombination: A new perspective of
checkpoints of BCR signaling. Front Immunol. 12:6634432021.
View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Tsubata T, Wu J and Honjo T: B-cell
apoptosis induced by antigen receptor crosslinking is blocked by a
T-cell signal through CD40. Nature. 364:645–648. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Xu J, Foy TM, Laman JD, Elliott EA, Dunn
JJ, Waldschmidt TJ, Elsemore J, Noelle RJ and Flavell RA: Mice
deficient for the CD40 ligand. Immunity. 1:423–431. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Wang SP, Iwata S, Nakayamada S, Niiro H,
Jabbarzadeh-Tabrizi S, Kondo M, Kubo S, Yoshikawa M and Tanaka Y:
Amplification of IL-21 signalling pathway through Bruton's tyrosine
kinase in human B cell activation. Rheumatology (Oxford).
54:1488–1497. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Bishop GA and Hostager BS: Signaling by
CD40 and its mimics in B cell activation. Immunol Res. 24:97–109.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Poe JC, Smith SH, Haas KM, Yanaba K,
Tsubata T, Matsushita T and Tedder TF: Amplified B lymphocyte CD40
signaling drives regulatory B10 cell expansion in mice. PLoS One.
6:e224642011. View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Chen C, Ma J, Pi C, Huang W, Zhang T, Fu
C, Liu W and Yang YG: PPARδ inhibition blocks the induction and
function of tumor-induced IL-10+ regulatory B cells and enhances
cancer immunotherapy. Cell Discov. 9:542023. View Article : Google Scholar
|
|
112
|
Durand J, Huchet V, Merieau E, Usal C,
Chesneau M, Remy S, Heslan M, Anegon I, Cuturi MC, Brouard S and
Chiffoleau E: Regulatory B cells with a partial defect in CD40
signaling and overexpressing granzyme B transfer allograft
tolerance in rodents. J Immunol. 195:5035–5044. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
113
|
Jansen K, Cevhertas L, Ma S, Satitsuksanoa
P, Akdis M and Van De Veen W: Regulatory B cells, A to Z. Allergy.
76:2699–2715. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
114
|
Fu Q, Lee KM, Huai G, Deng K, Agarwal D,
Rickert CG, Feeney N, Matheson R, Yang H, LeGuern C, et al:
Properties of regulatory B cells regulating B cell targets. Am J
Transplant. 21:3847–3857. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
115
|
Matsumoto M, Baba A, Yokota T, Nishikawa
H, Ohkawa Y, Kayama H, Kallies A, Nutt SL, Sakaguchi S, Takeda K,
et al: Interleukin-10-producing plasmablasts exert regulatory
function in autoimmune inflammation. Immunity. 41:1040–1051. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
116
|
Baba Y, Matsumoto M and Kurosaki T:
Signals controlling the development and activity of regulatory
B-lineage cells. Int Immunol. 27:487–493. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
117
|
Bankó Z, Pozsgay J, Szili D, Tóth M, Gáti
T, Nagy G, Rojkovich B and Sármay G: Induction and differentiation
of IL-10-producing regulatory B cells from healthy blood donors and
rheumatoid arthritis patients. J Immunol. 198:1512–1520. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
118
|
Inaba A, Tuong ZK, Zhao TX, Stewart AP,
Mathews R, Truman L, Sriranjan R, Kennet J, Saeb-Parsy K, Wicker L,
et al: Low-dose IL-2 enhances the generation of IL-10-producing
immunoregulatory B cells. Nat Commun. 14:20712023. View Article : Google Scholar : PubMed/NCBI
|
|
119
|
Rosser EC, Oleinika K, Tonon S, Doyle R,
Bosma A, Carter NA, Harris KA, Jones SA, Klein N and Mauri C:
Regulatory B cells are induced by gut microbiota-driven
interleukin-1β and interleukin-6 production. Nat Med. 20:1334–1339.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
120
|
Liu X, Huang H, Gao H, Wu X, Zhang W, Yu B
and Dou X: Regulatory B cells induced by ultraviolet B through
toll-like receptor 4 signalling contribute to the suppression of
contact hypersensitivity responses in mice. Contact Dermatitis.
78:117–130. 2018. View Article : Google Scholar
|
|
121
|
Poe JC, Haas KM, Uchida J, Lee Y, Fujimoto
M and Tedder TF: Severely impaired B lymphocyte proliferation,
survival, and induction of the c-Myc:Cullin 1 ubiquitin ligase
pathway resulting from CD22 deficiency on the C57BL/6 genetic
background. J Immunol. 172:2100–2110. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
122
|
Iwata Y, Matsushita T, Horikawa M, Dilillo
DJ, Yanaba K, Venturi GM, Szabolcs PM, Bernstein SH, Magro CM,
Williams AD, et al: Characterization of a rare IL-10-competent
B-cell subset in humans that parallels mouse regulatory B10 cells.
Blood. 117:530–541. 2011. View Article : Google Scholar :
|
|
123
|
Li X, Zhong H, Bao W, Boulad N,
Evangelista J, Haider MA, Bussel J and Yazdanbakhsh K: Defective
regulatory B-cell compartment in patients with immune
thrombocytopenia. Blood. 120:3318–3325. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
124
|
Khoder A, Sarvaria A, Alsuliman A, Chew C,
Sekine T, Cooper N, Mielke S, de Lavallade H, Muftuoglu M,
Fernandez Curbelo I, et al: Regulatory B cells are enriched within
the IgM memory and transitional subsets in healthy donors but are
deficient in chronic GVHD. Blood. 124:2034–2045. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
125
|
Lee KM, Fu Q, Huai G, Deng K, Lei J,
Kojima L, Agarwal D, van Galen P, Kimura S, Tanimine N, et al:
Suppression of allograft rejection by regulatory B cells induced
via TLR signaling. JCI Insight. 7:e1522132022. View Article : Google Scholar : PubMed/NCBI
|
|
126
|
Veh J, Mangold C, Felsen A, Ludwig C,
Gerstner L, Reinhardt P, Schrezenmeier H, Fabricius D and
Jahrsdörfer B: Phorbol-12-myristate-13-acetate is a potent enhancer
of B cells with a granzyme B+ regulatory phenotype. Front Immunol.
14:11948802023. View Article : Google Scholar :
|
|
127
|
Han Y, Yu C, Yu Y and Bi L: CD25+ B cells
produced IL-35 and alleviated local inflammation during
experimental periodontitis. Oral Dis. 28:2248–2257. 2022.
View Article : Google Scholar
|
|
128
|
Zeng R, Spolski R, Casas E, Zhu W, Levy DE
and Leonard WJ: The molecular basis of IL-21-mediated
proliferation. Blood. 109:4135–4142. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
129
|
Zheremyan EA, Ustiugova AS, Uvarova AN,
Karamushka NM, Stasevich EM, Gogoleva VS, Bogolyubova AV, Mitkin
NA, Kuprash DV and Korneev KV: Differentially activated B cells
develop regulatory phenotype and show varying immunosuppressive
features: A comparative study. Front Immunol. 14:11784452023.
View Article : Google Scholar : PubMed/NCBI
|
|
130
|
Chesneau M, Mai HL, Danger R, Le Bot S,
Nguyen TV, Bernard J, Poullaouec C, Guerrif P, Conchon S, Giral M,
et al: Efficient expansion of human granzyme B-expressing B cells
with potent regulatory properties. J Immunol. 205:2391–2401. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
131
|
Thompson R and Cao X: Reassessing granzyme
B: Unveiling perforin-independent versatility in immune responses
and therapeutic potentials. Front Immunol. 15:13925352024.
View Article : Google Scholar : PubMed/NCBI
|
|
132
|
Menon M, Blair PA, Isenberg DA and Mauri
C: A regulatory feedback between plasmacytoid dendritic cells and
regulatory B cells is aberrant in systemic lupus erythematosus.
Immunity. 44:683–697. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
133
|
Mohib K, Rothstein DM and Ding Q:
Characterization and activity of TIM-1 and IL-10-reporter
expressing regulatory B cells. Methods Mol Biol. 2270:179–202.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
134
|
Carvajal Alegria G, Cornec D, Saraux A,
Devauchelle-Pensec V, Jamin C, Hillion S and Pochard P: Abatacept
promotes regulatory B cell functions, enhancing their ability to
reduce the Th1 response in rheumatoid arthritis patients through
the production of IL-10 and TGF-β. J Immunol. 207:470–482. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
135
|
Song J, Xiao L, Du G, Gao Y, Chen W, Yang
S, Fan W and Shi B: The role of regulatory B cells (Bregs) in the
Tregs-amplifying effect of Sirolimus. Int Immunopharmacol.
38:90–96. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
136
|
Wu M, Yu S, Chen Y, Meng W, Chen H, He J,
Shen J and Lin X: Acteoside promotes B cell-derived IL-10
production and ameliorates autoimmunity. J Leukoc Biol.
112:875–885. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
137
|
Mielle J, Audo R, Hahne M, Macia L, Combe
B, Morel J and Daien C: IL-10 producing B cells ability to induce
regulatory T cells is maintained in rheumatoid arthritis. Front
Immunol. 9:9612018. View Article : Google Scholar : PubMed/NCBI
|
|
138
|
Goretzki A, Lin YJ, Meier C, Dorn B,
Wolfheimer S, Jamin A, Schott M, Wangorsch A, Vieths S, Jakob T, et
al: Stimulation of naïve B cells with a fusion protein consisting
of FlaA and Bet v 1 induces regulatory B cells ex vivo. Allergy.
78:663–681. 2023. View Article : Google Scholar
|
|
139
|
Giacomini E, Rizzo F, Etna MP, Cruciani M,
Mechelli R, Buscarinu MC, Pica F, D'Agostini C, Salvetti M, Coccia
EM and Severa M: Thymosin-α1 expands deficient IL-10-producing
regulatory B cell subsets in relapsing-remitting multiple sclerosis
patients. Mult Scler. 24:127–139. 2018. View Article : Google Scholar
|
|
140
|
Li W, Wang D, Yue R, Chen X, Liu A, Xu H,
Teng P, Wang Z, Zou Y, Xu X, et al: Gut microbes enlarged the
protective effect of transplanted regulatory B cells on rejection
of cardiac allografts. J Heart Lung Transplant. 40:1502–1516. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
141
|
Liao W, Xiao H, He J, Huang L, Liao Y, Qin
J, Yang Q, Ma F and Li S: B-Cell-activating factor contributes to
elevation of the content of regulatory B cells in neonatal sepsis.
Bull Exp Biol Med. 175:72–77. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
142
|
Saulep-Easton D, Vincent FB, Quah PS, Wei
A, Ting SB, Croce CM, Tam C and Mackay F: The BAFF receptor TACI
controls IL-10 production by regulatory B cells and CLL B cells.
Leukemia. 30:163–172. 2016. View Article : Google Scholar
|
|
143
|
Hua C, Audo R, Yeremenko N, Baeten D,
Hahne M, Combe B, Morel J and Daïen C: A proliferation inducing
ligand (APRIL) promotes IL-10 production and regulatory functions
of human B cells. J Autoimmun. 73:64–72. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
144
|
Fehres CM, van Uden NO, Yeremenko NG,
Fernandez L, Franco Salinas G, van Duivenvoorde LM, Huard B, Morel
J, Spits H, Hahne M and Baeten DLP: APRIL induces a novel subset of
IgA+ regulatory B cells that suppress inflammation via expression
of IL-10 and PD-L1. Front Immunol. 10:13682019. View Article : Google Scholar :
|
|
145
|
Planelles L, Carvalho-Pinto CE, Hardenberg
G, Smaniotto S, Savino W, Gómez-Caro R, Alvarez-Mon M, de Jong J,
Eldering E, Martínez-A C, et al: APRIL promotes B-1 cell-associated
neoplasm. Cancer Cell. 6:399–408. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
146
|
Zhang Y, Li J, Zhou N, Zhang Y, Wu M, Xu
J, Shen C, An X, Shen G, Yang M, et al: The unknown aspect of BAFF:
Inducing IL-35 production by a CD5+CD1dhiFcγRIIbhi regulatory
B-cell subset in lupus. J Invest Dermatol. 137:2532–2543. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
147
|
den Hartog G, van Osch TLJ, Vos M, Meijer
B, Savelkoul HFJ, van Neerven RJJ and Brugman S: BAFF augments IgA2
and IL-10 production by TLR7/8 stimulated total peripheral blood B
cells. Eur J Immunol. 48:283–292. 2018. View Article : Google Scholar
|
|
148
|
Komlósi ZI, Kovács N, van de Veen W,
Kirsch AI, Fahrner HB, Wawrzyniak M, Rebane A, Stanic B, Palomares
O, Rückert B, et al: Human CD40 ligand-expressing type 3 innate
lymphoid cells induce IL-10-producing immature transitional
regulatory B cells. J Allergy Clin Immunol. 142:178–194.e11. 2018.
View Article : Google Scholar
|
|
149
|
Mu Y, Xu W, Liu J, Wang Y, Chen J and Zhou
Q: Mesenchymal stem cells moderate experimental autoimmune uveitis
by dynamic regulating Th17 and Breg cells response. J Tissue Eng
Regen Med. 16:26–35. 2022. View Article : Google Scholar
|
|
150
|
Franquesa M, Mensah FK, Huizinga R, Strini
T, Boon L, Lombardo E, DelaRosa O, Laman JD, Grinyó JM, Weimar W,
et al: Human adipose tissue-derived mesenchymal stem cells abrogate
plasmablast formation and induce regulatory B cells independently
of T helper cells. Stem Cells. 33:880–891. 2015. View Article : Google Scholar
|
|
151
|
Luk F, de Witte SF, Korevaar SS,
Roemeling-van Rhijn M, Franquesa M, Strini T, van den Engel S,
Gargesha M, Roy D, Dor FJ, et al: Inactivated mesenchymal stem
cells maintain immunomodulatory capacity. Stem Cells Dev.
25:1342–1354. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
152
|
Ye L, Zhang Q, Cheng Y, Chen X, Wang G,
Shi M, Zhang T, Cao Y, Pan H, Zhang L, et al: Tumor-derived
exosomal HMGB1 fosters hepatocellular carcinoma immune evasion by
promoting TIM-1+ regulatory B cell expansion. J Immunother Cancer.
6:1452018. View Article : Google Scholar :
|
|
153
|
Blache U, Tretbar S, Koehl U, Mougiakakos
D and Fricke S: CAR T cells for treating autoimmune diseases. RMD
Open. 9:e0029072023. View Article : Google Scholar : PubMed/NCBI
|
|
154
|
Cremoni M, Massa F and Sicard A:
Overcoming barriers to widespread use of CAR-Treg therapy in organ
transplant recipients. HLA. 99:565–572. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
155
|
Calderón-Gómez E and Fillatreau S:
Utilization of a lentiviral system for the generation of B cells
with regulatory properties. Methods Mol Biol. 1190:105–113. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
156
|
Alonso-Guallart P, Llore N, Lopes E,
Kofman SB, Ho SH, Stern J, Pierre G, Bruestle K, Tang Q, Sykes M
and Griesemer A: CD40L-stimulated B cells for ex-vivo expansion of
polyspecific non-human primate regulatory T cells for translational
studies. Clin Exp Immunol. 203:480–492. 2021. View Article : Google Scholar
|
|
157
|
Abreu TR, Fonseca NA, Gonçalves N and
Moreira JN: Current challenges and emerging opportunities of CAR-T
cell therapies. J Control Release. 319:246–261. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
158
|
Miliotou AN and Papadopoulou LC: CAR
T-cell Therapy: A New Era in Cancer Immunotherapy. Curr Pharm
Biotechnol. 19:5–18. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
159
|
Wilhelm A, Chambers D, Müller F, Bozec A,
Grieshaber-Bouyer R, Winkler T, Mougiakakos D, Mackensen A, Schett
G and Krönke G: Selective CAR T cell-mediated B cell depletion
suppresses IFN signature in SLE. JCI Insight. 9:e1794332024.
View Article : Google Scholar : PubMed/NCBI
|
|
160
|
Wagner JC, Ronin E, Ho P, Peng Y and Tang
Q: Anti-HLA-A2-CAR Tregs prolong vascularized mouse heterotopic
heart allograft survival. Am J Transplant. 22:2237–2245. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
161
|
Mansourabadi AH, Mohamed Khosroshahi L,
Noorbakhsh F and Amirzargar A: Cell therapy in transplantation: A
comprehensive review of the current applications of cell therapy in
transplant patients with the focus on Tregs, CAR Tregs, and
Mesenchymal stem cells. Int Immunopharmacol. 97:1076692021.
View Article : Google Scholar : PubMed/NCBI
|
|
162
|
Montgomery RA, Tatapudi VS, Leffell MS and
Zachary AA: HLA in transplantation. Nat Rev Nephrol. 14:558–570.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
163
|
Bodis G, Toth V and Schwarting A: Role of
human leukocyte antigens (HLA) in autoimmune diseases. Rheumatol
Ther. 5:5–20. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
164
|
Boardman DA, Philippeos C, Fruhwirth GO,
Ibrahim MA, Hannen RF, Cooper D, Marelli-Berg FM, Watt FM, Lechler
RI, Maher J, et al: Expression of a chimeric antigen receptor
specific for donor HLA class I enhances the potency of human
regulatory T cells in preventing human skin transplant rejection.
Am J Transplant. 17:931–943. 2017. View Article : Google Scholar
|
|
165
|
González-Galarza FF, Takeshita LY, Santos
EJ, Kempson F, Maia MH, da Silva AL, Teles e Silva AL, Ghattaoraya
GS, Alfirevic A, Jones AR and Middleton D: Allele frequency net
2015 update: new features for HLA epitopes, KIR and disease and HLA
adverse drug reaction associations. Nucleic Acids Res. 43(Database
Issue): D784–D788. 2015. View Article : Google Scholar :
|
|
166
|
Ajith A, Mamouni K, Musa A, Horuzsko DD,
Gani I, Mulloy LL and Horuzsko A: IL-10-producing memory B
regulatory cells as a novel target for HLA-G to prolong human
kidney allograft survival. Hum Immunol. 84:366–373. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
167
|
Bottomley MJ, Brook MO, Shankar S, Hester
J and Issa F: Towards regulatory cellular therapies in solid organ
transplantation. Trends Immunol. 43:8–21. 2022. View Article : Google Scholar
|
|
168
|
McNee A, Kannan A, Jull P and Shankar S:
Expanding human breg for cellular therapy in transplantation: Time
for translation. Transplantation. Oct 23–2024.Epub ahead of print.
View Article : Google Scholar : PubMed/NCBI
|
|
169
|
Geissler EK and Hutchinson JA: Cell
therapy as a strategy to minimize maintenance immunosuppression in
solid organ transplant recipients. Curr Opin Organ Transplant.
18:408–415. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
170
|
Chu Z, Zou W, Xu Y, Sun Q and Zhao Y: The
regulatory roles of B cell subsets in transplantation. Expert Rev
Clin Immunol. 14:115–125. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
171
|
Mohib K, Cherukuri A and Rothstein DM:
Regulatory B cells and transplantation: Almost prime time? Curr
Opin Organ Transplant. 23:524–532. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
172
|
Chesneau M, Michel L, Degauque N and
Brouard S: Regulatory B cells and tolerance in transplantation:
From animal models to human. Front Immunol. 4:4972013. View Article : Google Scholar
|
|
173
|
Silva HM, Takenaka MC, Moraes-Vieira PM,
Monteiro SM, Hernandez MO, Chaara W, Six A, Agena F, Sesterheim P,
Barbé-Tuana FM, et al: Preserving the B-cell compartment favors
operational tolerance in human renal transplantation. Mol Med.
18:733–743. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
174
|
Clatworthy MR, Watson CJ, Plotnek G,
Bardsley V, Chaudhry AN, Bradley JA and Smith KG: B-cell-depleting
induction therapy and acute cellular rejection. N Engl J Med.
360:2683–2685. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
175
|
Veh J, Ludwig C, Schrezenmeier H and
Jahrsdörfer B: Regulatory B cells-immunopathological and prognostic
potential in humans. Cells. 13:3572024. View Article : Google Scholar : PubMed/NCBI
|
|
176
|
Le Huu D, Matsushita T, Jin G, Hamaguchi
Y, Hasegawa M, Takehara K, Tedder TF and Fujimoto M: Donor-derived
regulatory B cells are important for suppression of murine
sclerodermatous chronic graft-versus-host disease. Blood.
121:3274–3283. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
177
|
Fang T, Koo TY, Lee JG, Jang JY, Xu Y,
Hwang JH, Park S, Yan JJ, Ryu JH, Ryu YM, et al: Anti-CD45RB
antibody therapy attenuates renal ischemia-reperfusion injury by
inducing regulatory B cells. J Am Soc Nephrol. 30:1870–1885. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
178
|
Matsushita T: Regulatory and effector B
cells: Friends or foes? J Dermatol Sci. 93:2–7. 2019. View Article : Google Scholar
|
|
179
|
Zhao H, Feng R, Peng A, Li G and Zhou L:
The expanding family of noncanonical regulatory cell subsets. J
Leukoc Biol. 106:369–383. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
180
|
Natarajan P, Singh A, McNamara JT, Secor
ER, Guernsey LA, Thrall RS and Schramm CM: Regulatory B cells from
hilar lymph nodes of tolerant mice in a murine model of allergic
airway disease are CD5+, express TGF-β, and co-localize with
CD4+Foxp3+ T cells. Mucosal Immunol. 5:691–701. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
181
|
Khalil MI, Gurski CJ, Dittel LJ, Neu SD
and Dittel BN: Discovery and Function of B-Cell IgD Low (BDL) B
Cells in Immune Tolerance. J Mol Biol. 433:1665842021. View Article : Google Scholar
|
|
182
|
Agbogan VA, Gastineau P, Tejerina E,
Karray S and Zavala F: CpG-Activated regulatory B-cell progenitors
alleviate murine graft-versus-host-disease. Front Immunol.
13:7905642022. View Article : Google Scholar : PubMed/NCBI
|
|
183
|
Lal G, Kulkarni N, Nakayama Y, Singh AK,
Sethi A, Burrell BE, Brinkman CC, Iwami D, Zhang T, Hehlgans T and
Bromberg JS: IL-10 from marginal zone precursor B cells controls
the differentiation of Th17, Tfh and Tfr cells in transplantation
tolerance. Immunol Lett. 170:52–63. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
184
|
Bao Y and Xiu DR: Adaptive transfer of B10
cells: A novel therapy for chronic rejection after solid organ
transplantation. Med Hypotheses. 81:101–103. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
185
|
Gorczynski RM, Farrokhi K, Gorczynski C,
Sadozai H, Zhu F and Khatri I: Importance of B cells to development
of regulatory T cells and prolongation of tissue allograft survival
in recipients receiving autologous bone marrow transplantation.
Immunology. 154:465–475. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
186
|
Lindner S, Dahlke K, Sontheimer K, Hagn M,
Kaltenmeier C, Barth TF, Beyer T, Reister F, Fabricius D, Lotfi R,
et al: Interleukin 21-induced granzyme B-expressing B cells
infiltrate tumors and regulate T cells. Cancer Res. 73:2468–2479.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
187
|
Horikawa M, Weimer ET, DiLillo DJ, Venturi
GM, Spolski R, Leonard WJ, Heise MT and Tedder TF: Regulatory B
cell (B10 Cell) expansion during Listeria infection governs innate
and cellular immune responses in mice. J Immunol. 190:1158–1168.
2013. View Article : Google Scholar : PubMed/NCBI
|