Antiphospholipid syndrome (APS) is an autoimmune
disorder whose clinical manifestations are characterized by
arterial and/or venous thrombosis, pathological pregnancy and
persistent positive antiphospholipid antibodies (aPLs). A patient
can be diagnosed with APS when at least one clinical criterion and
at least one laboratory criterion are present (1). Clinical criteria include pregnancy
pathology or vascular thrombosis (2). Laboratory criteria include the
presence of titers or high titers of IgG and/or IgM in
anti-cardiolipin (aCL) and/or lupus anticoagulants on at least two
occasions, at least 6 weeks apart. The Sapporo criteria (also known
as the Sydney Criteria), revised in 2006, added IgG/IgM
anti-β2glycoprotein 1 (anti-β2GPI) antibody testing to the
laboratory criteria, and the positive detection interval was
extended from 6 weeks to 12 weeks(2). 'Non-standard' manifestations
include thrombocytopenia, APS-associated nephropathy reticulum and
cognitive impairment (3). In
addition, 'non-standard' autoantibodies such as IgA isotypes (IgA
aCL and IgA anti-β2GPI) have also been reported, but their clinical
relevance is still controversial (3,4).
To date, the pathogenesis of APS has remained
elusive. Although the role of T helper (Th) cells in the production
of aPL antibodies has long been recognized (5), there are less data on the role of T
cells in the development of APS. Studies have shown that the
activation of T lymphocytes and the production of corresponding
cytokines can be induced in patients with APS through different
ways, which can cause an imbalance among immune cells, immune
molecules and the immune system, and damage immune homeostasis
in vivo (6), which is
related to thrombosis or pathological pregnancy. It has been
reported that vitamin D (VD) has a pleiotropic effect, such as
anti-oxidation, preventing inflammatory response, reducing
immune-mediated damage and anticancer (7-10). Low serum VD levels are associated
with autoimmune diseases (11).
In vitro studies have shown that high VD levels directly
inhibit T-cell proliferation and cell-cycle progression, inhibit
the release of pro-inflammatory factors and transform the Th to the
Th2 phenotype (12). This
suggests that VD is essential for regulating T cells. In patients
with APS, decreased serum VD levels are significantly correlated
with thrombosis or obstetric complications (13-17), but their relationship with T
cells remains to be further studied. Therefore, it is required to
pay attention to the role of the relationship between VD and T
cells in APS, which provides a relevant basis for the prevention
and treatment of APS-related pathological pregnancy and thrombosis.
In the present article, the relevant literature was reviewed to
understand the potential mechanistic role of VD in APS protection.
At the same time, this review summarizes the progress of VD in the
treatment of APS and its possible adverse reactions, providing new
insights for the selection of adjuvant therapy for APS.
The estimated annual incidence of APS is 21 cases
per 100,000 individuals, with a prevalence of 50 cases per 100,000
individuals (18). The
male-to-female ratio of primary APS is ~1:3.5, and secondary APS is
mainly systemic lupus erythematosus (SLE)-related, with a ratio of
1:7 (18). Catastrophic APS
accounts for <1% of all APS cases (19). In the normal adult population,
the optimal concentration of serum 25-hydroxyVD [25(OH)D] is 30-50
ng/ml (75-125 nmol/l), while concentrations indicating insufficient
VD are 20-30 ng/ml (50-75 nmol/l). VD deficiency is defined as
levels <20 ng/ml (<50 nmol/l) (1). The reported prevalence of 25(OH)D
levels <50 nmol/l (or 20 ng/ml) is estimated to be 24% in the US
population and 37% in Canada, with an even higher prevalence in
Europe, reaching ~40% (20-23). In a survey of the normal adult
population, 67.4% of women had VD deficiency and ~87% of women were
affected by VD insufficiency, whereas ~41% of men had VD
deficiency, with prevalence rates varying across studies (24). In patients with APS, the total
prevalence of VD deficiency and insufficiency was reported to be
32.2 and 61.5%, respectively. The incidence of thrombotic events in
VD-deficient patients with APS was 62.9% and the incidence of
adverse pregnancy outcomes was 49.5% (13,25).
The biological effects of VD are mainly mediated by
the nuclear VD receptor (VDR), a member of the nuclear hormone
receptor family and a high-affinity ligand-activated transcription
factor (32). As a signaling
molecule, VDR binds to 1,25(OH)2D and the heterodimer partner, the
retinoid X receptor. This complex interacts with specific DNA
sequences in the promoter regions of target genes, known as VD
response elements, to regulate their expression (33). VDR is distributed in various
cells and tissues throughout the body, such as small intestinal
epithelial cells, endothelial cells, bone osteoblasts, the large
intestine, distal renal tubules and parathyroid glands (34-36). VDR is expressed in almost all
immune cells, including monocytes, macrophages, T and B lymphocytes
and DCs, where it regulates the differentiation and proliferation
of immune cells. However, its expression levels vary, which
indicates that VD is involved in the regulation of inflammation and
immune responses (37). T cells
are one of the target cells of VD, and VD deficiency can lead to
immune disorders, increasing the expression of procoagulant
factors, proinflammatory cytokines, adhesion molecules and tissue
factors (TF) (36).
Simultaneously, all these intermediates are associated with
thrombus formation and pathological pregnancy through mechanisms
such as increased platelet reactivity, endothelial dysfunction and
activation of the coagulation cascade and complement system
(36). Therefore, VD plays an
important role in the regulation of the immune balance and may have
therapeutic potential in the pathophysiology of APS-related
thrombosis and obstetric complications.
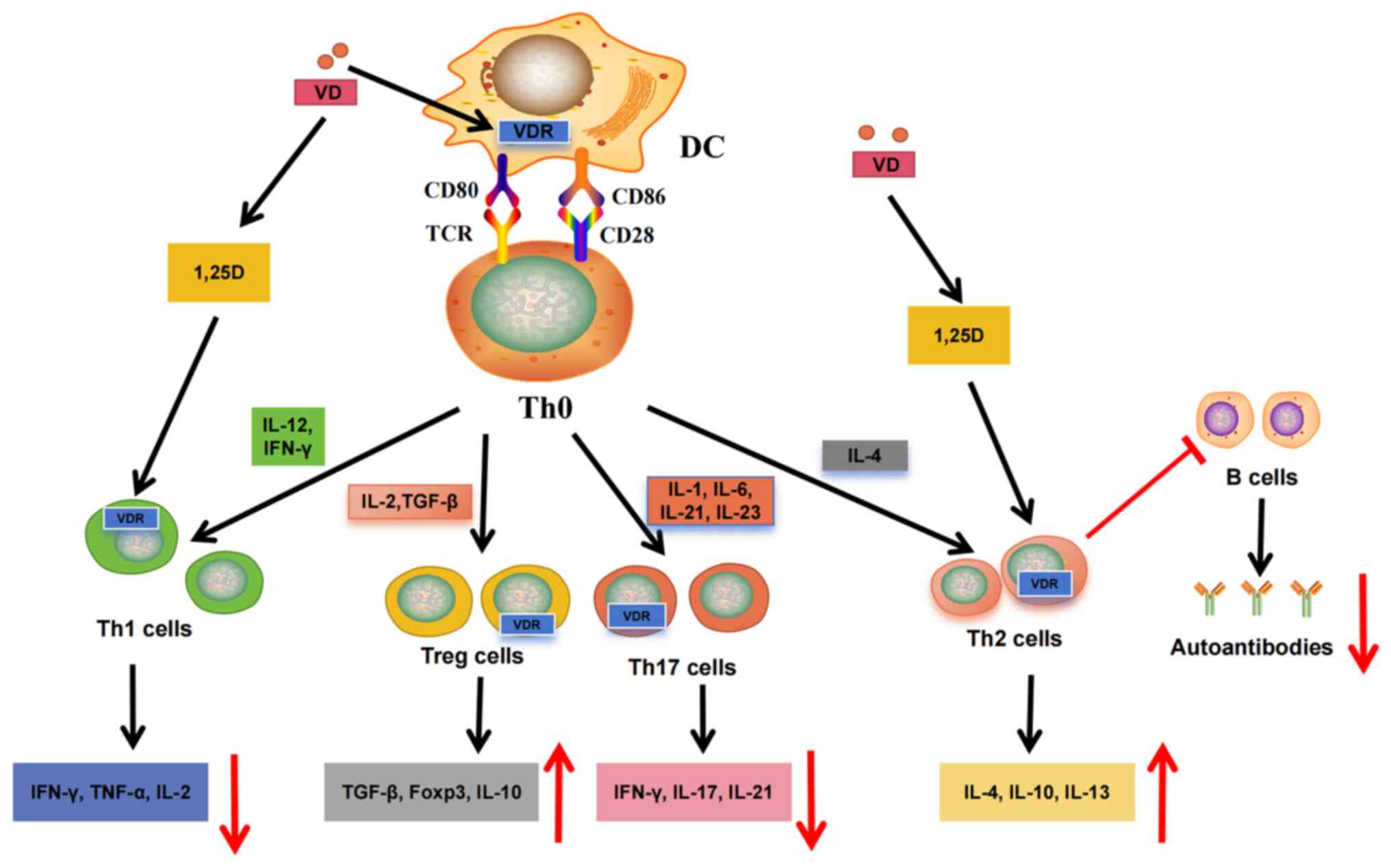
VD is known to differentially regulate innate immune
cell subsets; influence cell maturation, metabolism and antigen
presentation; and regulate cytokine production (38-40). Immature DCs usually express more
VDR, whereas mature DCs show reduced VDR expression (38). This may indicate that mature DCs
produce VD locally upon activation and that VD then acts on
immature DCs to regulate the immune response (38,41) (Fig. 1); 1,25(OH)2D can inhibit DC
maturation markers, such as CD80/CD86 and CD83 (41,42), increase the production of IL-10
and reduce proinflammatory cytokines (42,43). Thus, 1,25(OH)2D promotes an
immature, tolerant DC phenotype (44,45), thereby reducing antigen
presentation to T cells; 1,25(OH)2D primarily regulates the
proliferation of T and B cells, but studies have found that it
plays a more important role in regulating T-cell phenotypes
(46,47). After binding to autoantigens,
autoantibodies can be presented to CD4+ Th cells
together with major histocompatibility complex class II molecules
to stimulate T-cell activation and the adaptive immune response;
1,25(OH)2D reduces the cytotoxic activity of T cells by
downregulating the expression of Fas ligands (48). VD also has various effects on Th
cells, such as promoting CD4+ Th-cell differentiation,
resulting in a reduction in Th1 and Th17 cells (49). Th1 and Th17 subgroups play key
roles in different autoimmune diseases by releasing cytokines that
drive inflammatory responses. In addition, 1,25(OH)2D reduces the
production of Th1-related cytokines [IL-2, tumor necrosis factor
(TNF)-α and interferon (IFN)-γ] and Th17-related cytokines (IL-17,
IL-21, IL-22) by inhibiting Th1 and Th17 cells (50) (Fig. 1). Simultaneously, CD4+
T cells are polarized toward the Th2 phenotype by upregulating
cytokines, such as IL-4 and IL-5 (50). By enhancing the expression of
forkhead box P3, the differentiation of Treg and CD4+ T
cells involved in maintaining immune tolerance is induced, while
levels of IL-10 and transforming growth factor β1 (TGF-β1) are also
increased (50,51), thereby reducing the inflammatory
response (Fig. 1). Furthermore,
Th cells reduce the formation of autoantibodies by inhibiting the
differentiation and proliferation of B cells (Fig. 1). The aforementioned findings
indicate that VD can prevent the development of autoimmune diseases
by regulating the balance between T-cell subpopulations
(Th1/Th2/Th17/Treg) (Fig.
1).
Moderate VD levels are associated not only with
pathogen clearance but also with immune regulation in normal
pregnancy T-cell subsets. The Th1/Th2 immune response is generally
characterized by immunoinflammatory changes that occur during
embryo implantation. Th1 cells migrate into the decidua, which is
critical for regulating trophoblast invasion and contributes to
tissue remodeling and angiogenesis, thereby supporting pregnancy
(52). Th1 cells secrete various
cytokines, such as IL-2, TNF-α and IFN-γ, which, in addition to
providing immune surveillance, prevent excessive invasion of
trophoblast cells (53). Under
normal conditions, moderate levels of TNF-α protect the placental
unit and modulate the adhesion of trophoblast cells to laminin,
thereby regulating trophoblast cell invasion (53). In addition to downregulating
protease activity and extravillous trophoblastic (EVT) cell
apoptosis to prevent excessive EVT invasion, IFN-γ can induce
vascular remodeling during the implantation period to better
nourish implanted cells (54,55). Therefore, IFN-γ plays a key role
in early placental and trophoblast invasion. Toward the end of the
implantation period, Th1 immune predominance in the decidua
gradually shifts to Th2 predominance. Th2 dominance has been
demonstrated during normal pregnancy (56). After Th2 cells infiltrate the
decidua basalis, they induce local Th2 dominance through the
release of Th2 cytokines (57),
such as IL-4, IL-10 and IL-13, which inhibit the development of Th1
and Th17 immunity, promoting allograft tolerance, which favors
pregnancy (58). Th17 cells are
relatively rare (0.64-1.4%) in the peripheral blood of healthy
individuals (59). In the
decidua, IL-17+ T-cell counts parallel neutrophil
counts, indicating that neutrophil infiltration is closely related
to IL-17+ T cells (60), inducing protective immunity
against extracellular microorganisms in the uterus, which is
associated with immune protection and pregnancy. Following
implantation, sufficient VD levels in the decidua promote the
differentiation of T-cell subsets into Tregs, which maintain
maternal tolerance by inhibiting cytotoxic T and Th1 cells. Tregs
can suppress destructive immune responses and prevent autoimmune
diseases during pregnancy (61).
VD enhances the immunosuppressive function of Tregs; therefore, it
can inhibit proinflammatory Th17 cells and related cytokines (such
as IL-17), maintaining a Th17/Treg balance (62). This balance facilitates the
secretion of growth factors and extracellular matrix remodeling,
which supports trophoblastic infiltration, cytotrophoblast
development and decidual vessel remodeling (63), thus supporting placental
development.
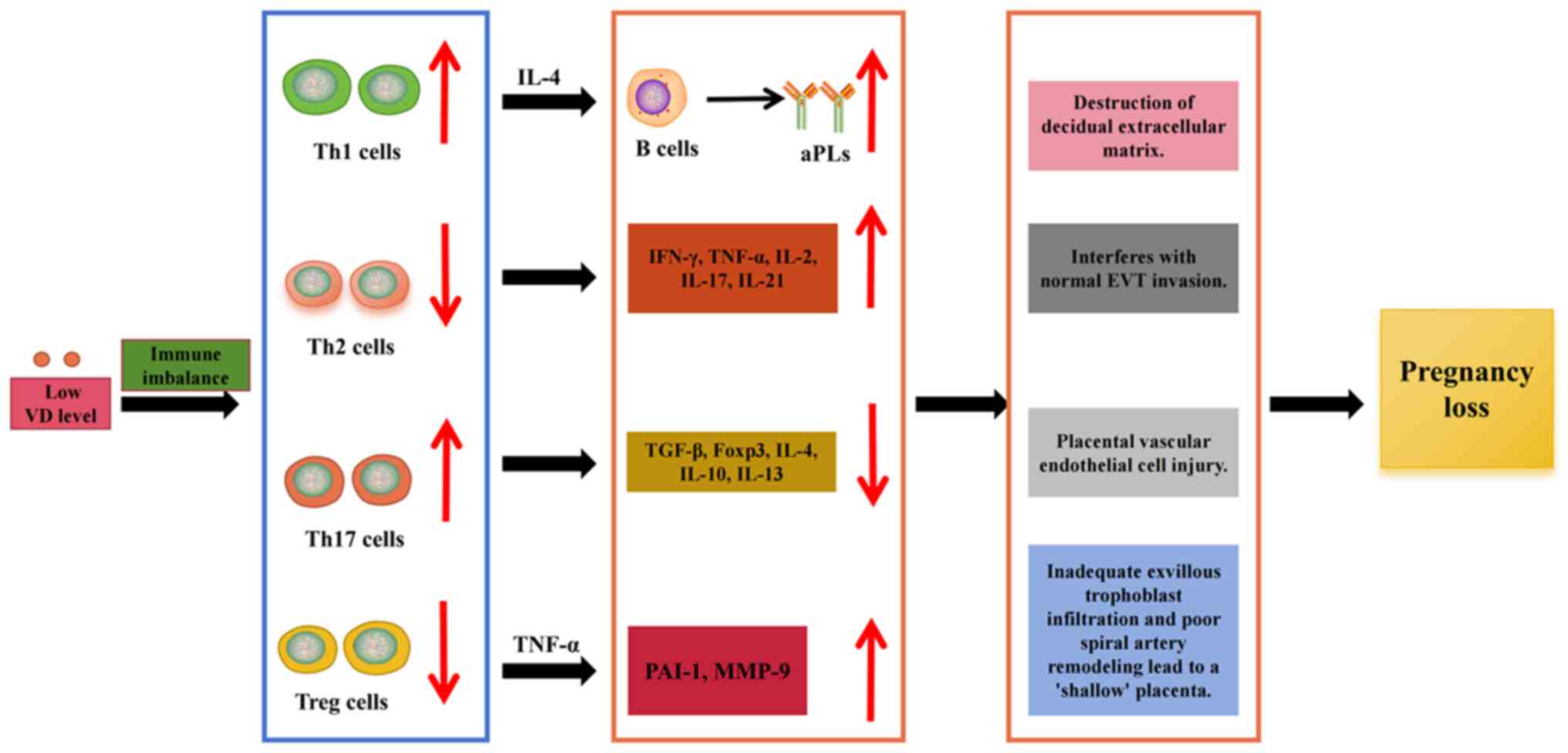
In a healthy human body, sufficient VD is important
for embryo implantation, mediating immune tolerance and promoting
embryo growth. However, T-cell dysregulation caused by VD
insufficiency or deficiency is closely linked to adverse pregnancy
outcomes in patients with APS. Insufficient or deficient VD
promotes the production of aPLs by B cells and disrupts immune
balance, leading to the overexpression of Th1 cytokines (IFN-γ and
TNF-α) and the failure of immune tolerance after implantation
(64) (Fig. 2). TNF-α increases plasminogen
activator inhibitor-1 levels derived from trophoblast cells,
reduces trophoblast cell invasion, activates endothelial cells,
induces matrix metalloproteinase-9 expression in the decidua and
degrades the decidual extracellular matrix, which interferes with
normal EVT invasion (65-67)
(Fig. 2). IFN-γ has a powerful
proinflammatory effect, stimulating chemokine secretion, activating
macrophages and increasing phagocytosis, leading to maternal
inflammation and subsequent fetal loss (Fig. 2). In addition, these cytokines
mediate cytotoxic activity against target cells, which may damage
placental vascular endothelial cells (64,68), leading to placental microthrombus
formation and subsequent pathological pregnancies (Fig. 2). The high expression of Th1
cytokines in the placentas of patients with VD insufficiency or
deficiency, particularly in preeclampsia, suggests that VD exerts a
protective effect at the fetomaternal interface (61). After the implantation phase, if
the Th1-cell immune advantage is not transferred to a Th2-cell
advantage, pathological pregnancy may ensue. However, excessive
activation of Th2 cells and the subsequent release of cytokines are
not beneficial. The Th2 cytokine IL-4 can induce the activation of
autoreactive B cells and the exacerbation of Th2 immunity during
pregnancy may worsen autoimmune diseases (69) (Fig. 2). VD deficiency can also lead to
abnormal function and reduced numbers of Treg cells in the decidua.
This condition is related to insufficient infiltration of
extravillous trophoblastic cells and poor remodeling of spiral
arteries, which, in turn, results in unstable placental development
and a 'shallow' placenta (63).
Simultaneously, an overly robust Th17-cell response can induce the
activation of decidual natural killer cells and impair the vascular
reactivity of the uterine artery, leading to embryo absorption
(70). A study showed that,
compared with the normal pregnancy group, the proportion of Th17
cells was significantly increased in aborted mice, whereas the
proportion of Treg cells and the levels of IL-10 and TGF-β1 were
significantly decreased. Following the transplantation of Treg
cells from normal pregnant mice into abortion-prone mice, the
levels of TGF-β and IL-10 significantly increased, exerting a
protective effect on the fetus (63). In addition, transplantation of
CD4+ CD25+ Treg-deficient lymphocytes into
pregnant T cell-deficient mice resulted in abortion (71). This finding suggests that the
absence or insufficiency of Tregs can lead to abortion and that VD
insufficiency or deficiency can cause an imbalance in
Th1/Th2/Treg/Th17 cells and related cytokines in patients with APS,
leading to adverse pregnancy outcomes (Fig. 2).
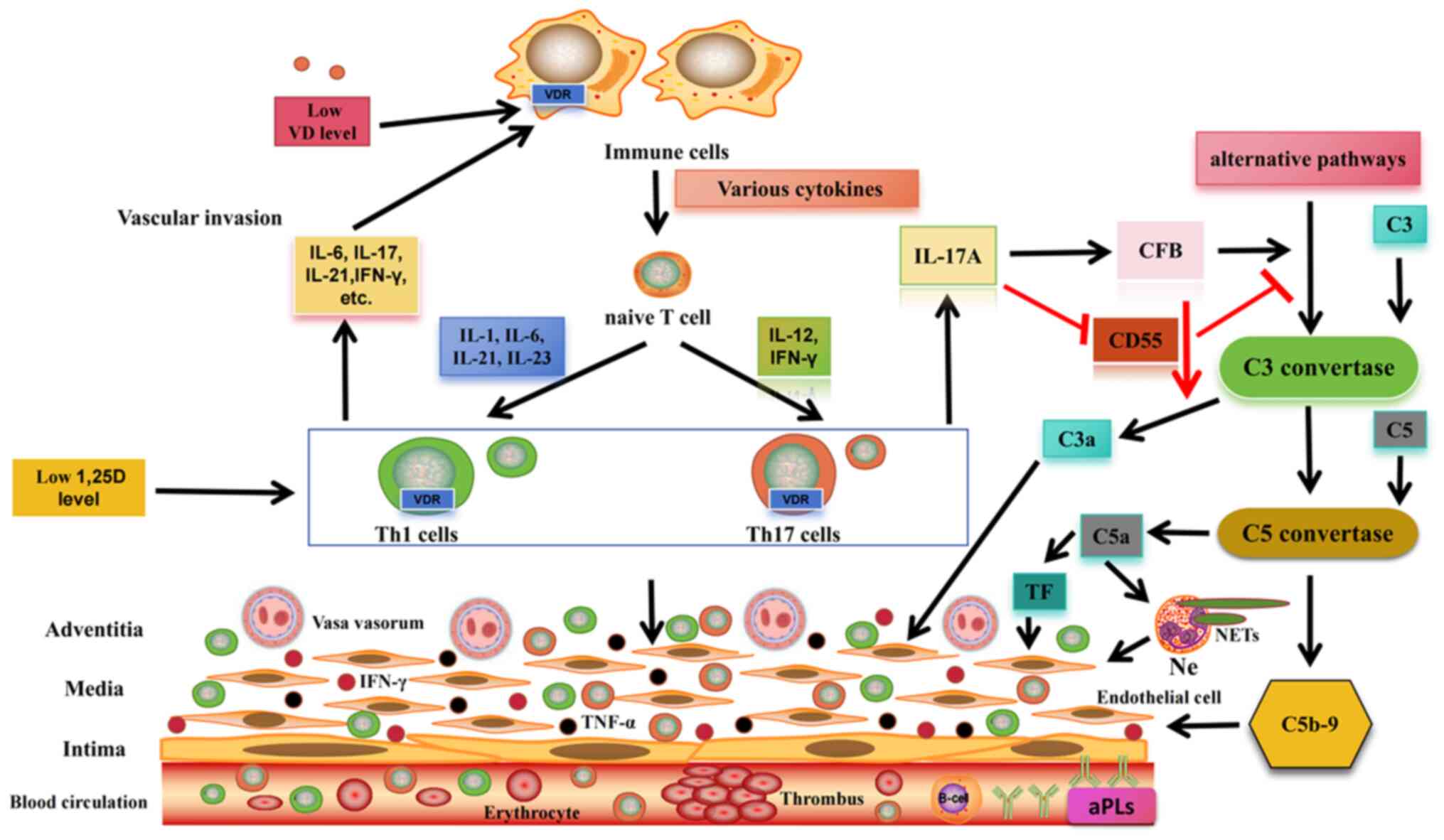
In patients with APS, VD deficiency may be closely
associated with thrombosis. Normal levels of VD can inhibit
endothelial cell angiogenic factors, modulate inflammatory
activity, reduce endothelial damage and suppress tissue factor
expression, thereby exerting antithrombotic effects. However, when
VD deficiency occurs in patients with APS, B cells may become
overactivated and differentiate into plasma cells, resulting in the
production of more aPL (Fig. 3);
aPL recognizes β2GPI and induces plaque-derived T-cell
proliferation and increased IFN-γ expression, indicating Th1 cell
activation in the artery walls of patients with APS. Local
production of IFN-γ and TNF-α following Th1-cell activation may
further stimulate these cells to present β2GPI to T cells, driving
their differentiation into Th1 effector cells (72), thereby causing endothelial cell
damage (Fig. 3). IFN-γ can also
enhance calcium flux in neutrophils and the production of reactive
oxygen species, leading to the formation of neutrophil
extracellular traps and, ultimately, thrombosis (73). In a study examining inflammatory
atherosclerotic infiltrates in patients with SLE-APS, researchers
identified β2GPI-specific T cells. Among these, 42% were polarized
Th1 cells, 38% were Th17/Th1 cells, 15% were polarized Th17 cells
and 5% were Th0 cells. Notably, no T cells were polarized into Th2
cells (72). Th2-cell deficiency
is an important risk factor for atherosclerosis. Furthermore, most
Th cells specifically producing IL-17 in patients with APS were
polarized Th17 cells. In the absence of VD, Treg cells lose their
inhibitory ability and cannot control the proliferation of
IL-17+ T cells, leading to excessive IL-17 production.
IL-17 can induce endothelial cells to secrete proinflammatory
cytokines (such as IL-6) and chemokines (including IL-8) and
increase adhesion molecule levels, particularly in combination with
TNF-α. This promotes leukocyte recruitment and endothelial-cell
invasion (73,74). In addition, IL-17A promotes
thrombosis by activating tissue factors and reducing anticoagulant
mediators, such as thrombomodulin and CD55 (73) (Fig. 3). Deficiency in complement
inhibitor CD55 disrupts the balance of the complement system,
leading to its activation. When VD levels are <40 ng/ml, CD55
expression is downregulated, whereas complement factor B expression
is significantly upregulated, indicating complement system
activation and readiness for the membrane attack complex formation
stage (75) (Fig. 3). Complement factor B, a
component of the complement alternative pathway, is responsible for
C3/C5 convertase activity. Its upregulation can lead to the
excessive production of complement fragments C3a and C5a. In
patients with APS, C5a mediates tissue factor expression via C5a
receptors, promoting procoagulant activity (76). Simultaneously, the expression of
complement component C9 is upregulated in response to reduced VD
levels, and its sustained upregulation may increase the synthesis
of C5b-9 (77). The release of
C5a and the formation of C5b-9 result in endothelial cell
activation, tissue factor expression, enhanced
neutrophil-endothelial cell interactions, neutrophil extracellular
trap formation and a lowered threshold for thrombosis. Severe cases
may present with extensive microthrombosis, possibly associated
with catastrophic APS (78,79) (Fig. 3).
The antithrombotic properties of VD have been
studied previously; aPL induces endothelial cell activation through
Toll-like receptor 4 (TLR-4)-mediated signal transduction and NF-κB
activation, which is associated with APS thrombosis (80). Normal VD levels not only inhibit
type I IFN synthesis by blocking TLR-4 signal transduction in
inflammatory cells and MYD88 innate immune signal transduction
adaptor (MyD88) signaling but also downregulate NF-κB and TF
expression, thereby inhibiting thrombosis in APS (81,82). This evidence indicates that
insufficient or deficient VD is related to APS thrombosis. In APS,
aPLs can also upregulate angiogenic factors and adhesion molecules,
such as intercellular adhesion molecule-1 (ICAM-1) and vascular
cell adhesion molecule-1 (VCAM-1, also known as ELAM-1). VD can
inhibit the expression of these molecules and reduce endothelial
cell damage (83,84). To further confirm the role of VD,
several studies have performed VDR knockouts in mice. After VDR
knockout, platelet aggregation increased significantly, the
expression of anticoagulant genes (including anticoagulant glucagon
protein, antithrombin and thrombomodulin) was downregulated and the
expression of the procoagulant TF gene was upregulated, increasing
thrombus formation (85). In
APS, the absence of VD weakens the peroxisome
proliferator-activated receptor (PPAR)γ pathway, increases
superoxide production and impairs endothelial progenitor cell
repair capacity (86).
Simultaneously, the weakened PPARγ pathway enhances angiotensin
II-induced reactive oxygen species production and activates NF-κB.
This activation induces cytokines, such as TNF-α, IL-6, ICAM-1,
VCAM-1 and E-selectin, which promote vascular injury and thrombosis
(36).
VD has immunomodulatory properties and sufficient VD
generally exerts immunosuppressive effects. Autoimmune diseases are
often triggered by external factors (including infections), leading
to immune-cell dysregulation, particularly in T cells. This
dysregulation results in an imbalance in T-cell subsets, with Th17
and Th1 cells and their associated proinflammatory cytokines
becoming significantly elevated, causing excessive immune
responses. This strong immune response can attack multiple organs
or tissues simultaneously, causing systemic damage. When VD levels
are low, autoimmune reactions may become further aggravated,
leading to disease progression and exacerbation of clinical
symptoms. In recent years, increasing attention has been paid to
the impact of low VD levels on autoimmune diseases. Studies have
demonstrated the importance of VD in diseases, such as SLE, type 1
diabetes mellitus, multiple sclerosis, autoimmune thyroid disease,
rheumatoid arthritis and psoriasis (87-118). In addition to APS, the role of
VD in these autoimmune diseases is summarized in Table I. It is worth noting that the
type and dose of VD used in the study of different autoimmune
diseases may be different (91,92,95,96,99,106,111,112,117).
Since the discovery of the VDR in cells across
various systems, the pleiotropic effects of VD have garnered much
attention. VD supplementation is important for regulating the
immune system in APS. In a study, patients were divided into two
groups: One received an intensive cholecalciferol regimen (300,000
IU at baseline, maintained at 50,000 IU/month and 850,000 IU/year),
and the other received a standard regimen (25,000 IU/month and
300,000 IU/year). The intensive regimen improved the balance of B-
and T-cell homeostasis, with an increase in Tregs and Th2 cells and
a decrease in Th17, Th1 and memory B cells (111,119). VD supplementation has been
shown to correct immune dysregulation in patients with autoimmune
diseases. In a VDR knockout mouse model, platelet aggregation was
significantly increased, the expression of anticoagulant genes
(including glucagon protein, antithrombin and thrombomodulin) was
downregulated and the TF gene was upregulated (120). After the injection of exogenous
lipopolysaccharide, VDR-knockout mice developed multi-organ
thrombosis compared to wild-type controls, suggesting VD's
potential as an antithrombotic agent (120); aPL antibodies, such as
anti-β2GPI-Abs, can trigger the clotting cascade through multiple
mechanisms, including inducing TF expression. VD may act as an
immunomodulator with antithrombotic effects. One study evaluated
179 patients with APS and found that 49.5% of patients with APS had
VD deficiency (serum levels ≤15 ng/ml), which was significantly
associated with thrombosis (58 vs. 42%; P<0.05). Neurological
and ophthalmic manifestations, pulmonary hypertension, reticular
skin changes and skin ulcers were also significantly associated
(121). In the same study,
anti-β2GPI antibodies from four patients with APS were purified and
co-cultured with VD [1,25(OH)2D, 10 nm]. VD inhibited anti-β2GPI
antibody-induced TF expression. Furthermore, VD reduced the
overexpression of adhesion molecules, such as VCAM-1 and ICAM-1, in
cultures incubated with aPL IgG antibodies (121). However, the effects of VD
supplementation in thrombotic APS have remained to be studied
prospectively. Clinical trials investigating VD supplementation for
thrombosis prevention have yielded conflicting results. For
instance, in one trial involving patients with metastatic prostate
cancer, those receiving high doses of calcitriol had significantly
fewer thrombotic events compared to placebo-treated patients, even
after adjusting for prior thrombotic history and anticoagulation
therapy (122). Conversely, in
the general population, large trials have not demonstrated any
significant benefits of VD supplementation in preventing venous
thromboembolism (123,124).
Currently, APS-related thrombosis therapy, intensive
therapy with warfarin and other vitamin K antagonists (VKA), are
standard treatments. For the first thrombotic event in APS,
switching from a direct oral anticoagulant to warfarin or another
VKA is recommended if aPL is triple-positive and direct oral
anticoagulants are already in use. The first-line treatment for
arterial and small-vessel thrombosis should be VKA (125). Low-dose aspirin and
moderate-intensity anticoagulant therapy are recommended (126). For patients with recurrent
thrombosis, heparin (including enoxaparin) at a dose of 1.5 mg/kg
administered via subcutaneous injection is recommended (127), followed by anticoagulant
therapy with warfarin, with a target international normalized ratio
of 2.0 to 3.0 (128). Heparin
not only inhibits the activation of T cells and neutrophils but
also suppresses complement activation. VD has effects similar to
those of an anticoagulant (including heparin), such as immune
modulation, inhibition of T-cell overactivation and suppression of
complement activation. In addition, supplementing VD as an
adjunctive therapy for APS-related thrombosis has additional
effects (125). The central
role of inflammation in aPL-mediated thrombosis supports VD's
potential as a treatment for APS (129). Among its many roles, VD has an
immunomodulatory effect on inflammatory activity, inhibits the
overactivation of monocytes, thereby reducing endothelial damage,
and suppresses the expression of angiogenic factors and TF in
endothelial cells (14,125). VD can also inhibit TLR-4, block
the signaling pathway of the adaptor protein MyD88 and its
downstream NF-κB in inflammatory cells, suppress inflammation and
immune responses, and upregulate thrombomodulin (14,125), thus playing an antithrombotic
role.
Regarding adverse pregnancies, the total prevalence
of aPL was significantly higher in women with recurrent pregnancy
loss (RPL) in the VD insufficiency or deficiency groups than in the
normal VD group. VD deficiency is more common in patients with RPL
having APS, and these patients are at greater risk of autoimmune
abnormalities, including APS (130). For patients with insufficient
or deficient VD combined with RPL, studies have found no
significant difference in the proportion of Th1/Th2
cytokine-expressing CD3+/CD4+ Th cells when
VD supplementation is administered compared with patients with
normal VD levels combined with RPL (129). In another study, 35 patients
with RPL and insufficient VD levels were treated with 0.5
μg/day of 1,25(OH)2D for 2 months. The proportion of
TNF-α-producing Th cells decreased significantly after treatment
compared with that before treatment (131); 1,25(OH)2D can reduce IFN-γ
production and the number of IFN-γ+ CD4+ T
cells (132). In addition,
after treatment with 3.3 million IU of intramuscular VD injections
in patients with RPL, the number of Th17 cells in the treatment
group was significantly lower than that in the control group and
the ratio of Th17/Treg cells decreased significantly more than that
in the control group (133). In
another study, patients with RPL having VD deficiency were
administered 2,000 IU of oral VD capsules per day. After 2 months
of treatment, the Treg/Th17 ratio was significantly higher than
that in women who did not receive VD supplementation (134). These findings suggest that VD
supplementation can provide immunomodulatory benefits in RPL and
may serve as an alternative therapy for APS-related thrombosis and
adverse pregnancies.
At present, there are certain limitations to the
efficacy of VD supplementation in patients with ASP. First, of the
existing articles, most of them focus on pre-clinical studies
(in vitro studies and animal experiments), and there are
also certain retrospective studies, but the changes in serum
25(OH)D levels after VD supplementation are mainly mentioned
(135,136). There may be differences between
preclinical studies and human studies, such as the impact on aPL
(121,135), and retrospective studies often
lack a lot of information, e.g. T-cell subsets were not reviewed to
analyze which T-cell subtypes were affected by VD. Therefore, it is
esteemed that there will be more large-scale prospective studies in
the future to investigate the efficacy of VD in the treatment of
patients with APS, particularly with regard to the effects of VD on
T cells (both in thrombosis and obstetric complications). In
addition, a meta-analysis was conducted on the basis of many
large-scale prospective studies, so as to better evaluate the
optimal dose range, safety and efficacy of VD in patients with APS,
particularly the effects of correcting the T-cell imbalance after
VD supplementation on thrombosis and obstetric complications,
further supporting the present conclusions and making them more
general.
Due to the increasing awareness of VD's benefits,
supplements are being used more widely. Currently, there is no
evidence that increasing the daily dose of VD to 50 μg
(2,000 IU) causes serious adverse effects in the general
population. Considering that the minimum beneficial dose for bone
health is 20 μg (800 IU), it is reasonable to recommend a
daily dose of 20 to 50 μg (800-2,000 IU). These
recommendations are supported by scholars, with evidence levels
ranging from 2 to 4 (137). In
2011, the Institute of Medicine reported the upper limits of VD
intake, highlighting the possible side effects of short-term
high-dose VD use and long-term VD supplementation. However,
toxicity may occur when serum 25(OH)D levels in patients are ≥150
ng/ml (375 nmol/l). Acute VD toxicity is usually caused by a serum
25(OH)D concentration of >150 ng/ml (375 nmol/l) following doses
>10,000 IU/day. Long-term use of doses >4,000 IU/day may lead
to chronic VD toxicity, resulting in 25(OH)D concentrations ranging
from 50 to 150 ng/ml (138).
The manifestations of VD poisoning are typically multisystemic and
are primarily due to hypercalcemia. Signs and symptoms of poisoning
are presented in Table II
(118,139-141). Generally speaking, the more
common side effects are on the gastrointestinal tract, such as
vomiting, nausea, thirst, anorexia and constipation (118,139-141).
In a study from Brazil, 21 patients who took
cholecalciferol supplementation for an average of 1 year (average
dose, 87,000 IU/day) were enrolled. Of note, 17 patients
experienced relapses, new MRI lesions or increased disabilities.
The authors also observed direct side effects of VD
supplementation, including gastrointestinal symptoms, seizures,
severe hypercalcemia, kidney failure, kidney stones and renal
calcification (142). In
another case, a patient who received high-dose cholecalciferol for
20 months (78 million IU cumulatively, 130,000 IU/day on average)
developed nausea, vomiting, muscle weakness, reversible
hypercalcemia and acute kidney injury (143). In addition, high VD doses and
specific treatment regimens may increase adverse effects. For
instance, a study of 2,256 women >70 years of age administered
500,000 IU of VD per year (~1,400 IU/day) reported a 26% increase
in fracture risk within the first 3 months after dosing (144). Similarly, annual intramuscular
injections of 300,000 IU of VD (~820 IU/day) were associated with a
49% increase in hip fracture risk (145). A case study from Brazil
reported a 19-year-old male hospitalized for anorexia, nausea and
vomiting after consuming 300 ml of an enteral formulation
containing vitamin A, D and E (totaling 5,000,000 IU of VD).
Laboratory tests revealed a serum 25(OH)D level of 150 ng/ml, total
calcium of 14.8 mg/dl (normal reference range: 8.4-11.0 mg/dl) and
serum creatinine of 2.88 mg/dl (normal reference range: 0.9-1.3
mg/dl). Hypercalcemia and acute kidney injury were treated
successfully with fluid rehydration, diuretics and zoledronic acid
(146). In a randomized
clinical trial, participants were divided into three groups: A
low-dose control group receiving 24,000 IU/month of VD3, a group
receiving 60,000 IU/month and a group receiving 24,000 IU/month
plus 300 μg calcifediol. Over 12 months, the group receiving
60,000 IU/month had a higher risk of falls compared with that of
the control group (147).
Although VD is generally safe, adverse events can occur; therefore,
clinicians should carefully monitor patients receiving
supplementation.
In addition, there are of course other causes of VD
poisoning events, e.g., an Italian study reported 3 cases of severe
hypercalcemia and renal insufficiency associated with VD poisoning.
The patients had been treated with VD preparations; the prescribed
dose was 600 IU, but the actual content was 52,800 IU, which was
related to formulation errors and long-term use. The study
highlights the need to prepare supplements such as VD according to
precise rules in order to make the amount of the potentially toxic
ingredient the correct and safe dose (148). The toxicity of VD has also been
linked to high doses of over-the-counter supplements that are taken
frequently and incorrectly prescribed by doctors as well as
incorrectly instructed by pharmacists, e.g., by providing 50,00 IU
prescriptions daily instead of once a week. For instance, a patient
with MS who initiated a self-prescribing change increased his VD3
intake from 8,000 to 88,000 IU/day over four years, resulting in an
upward trend in hypercalcaemia and creatinine rates (149).
APS is an autoimmune disease that causes
pathological pregnancy and thrombosis, significantly affecting
patients' quality of life and increasing their economic burden.
T-cell immune disorders caused by VD deficiency are critical
factors in the pathogenesis of APS thrombosis and adverse pregnancy
outcomes. VD has potent immunomodulatory effects, helping to
regulate T-cell subsets, exerting antithrombotic effects and
preventing adverse pregnancy outcomes in APS. VD supplementation is
a promising and beneficial treatment for APS. However, studies on
the correction of T-cell imbalances through VD supplementation in
patients with APS are still lacking, and the specific mechanisms at
the molecular and signaling pathway levels remain elusive.
Large-scale prospective studies are needed to confirm VD's efficacy
and mechanisms in APS treatment. Furthermore, the optimal dosage
and safety of VD supplementation should be further explored to
develop new therapeutic strategies for APS.
Not applicable.
RH, YY and CW wrote the manuscript. XH, DM, RH, YY
and YH collected the references and created the tables and figures.
JL and XH conceptualised and designed the study. All authors read
and approved the final manuscript. Data authentication is not
applicable.
Not applicable.
Not applicable.
The authors declare that they have no competing
interests.
Not applicable.
No funding was received.
|
1
|
Płudowski P, Kos-Kudła B, Walczak M, Fal
A, Zozulińska-Ziółkiewicz D, Sieroszewski P, Peregud-Pogorzelski J,
Lauterbach R, Targowski T, Lewiński A, et al: Guidelines for
preventing and treating vitamin D deficiency: A 2023 update in
Poland. Nutrients. 15:6952023. View Article : Google Scholar :
|
|
2
|
Miyakis S, Lockshin MD, Atsumi T, Branch
DW, Brey RL, Cervera R, Derksen RH, DE Groot PG, Koike T, Meroni
PL, et al: International consensus statement on an update of the
classification criteria for definite antiphospholipid syndrome
(APS). J Thromb Haemost. 4:295–306. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Sciascia S, Amigo MC, Roccatello D and
Khamashta M: Diagnosing antiphospholipid syndrome: 'extra-criteria'
manifestations and technical advances. Nat Rev Rheumatol.
13:548–560. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Sciascia S, Sanna G, Murru V, Roccatello
D, Khamashta MA and Bertolaccini ML: Anti-prothrombin (aPT) and
anti-phosphatidylserine/prothrombin (aPS/PT) antibodies and the
risk of thrombosis in the antiphospholipid syndrome. A systematic
review. Thromb Haemost. 111:354–364. 2014. View Article : Google Scholar
|
|
5
|
Kravvariti E, Konstantonis G, Tentolouris
N, Sfikakis PP and Tektonidou MG: Carotid and femoral
atherosclerosis in antiphospholipid syndrome: Equivalent risk with
diabetes mellitus in a case-control study. Semin Arthritis Rheum.
47:883–889. 2018. View Article : Google Scholar
|
|
6
|
Tektonidou MG, Vlachogiannis NI and
Sfikakis PP: T cell involvement in antiphospholipid syndrome. Clin
Immunol. 263:1102182024. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Della Nera G, Sabatino L, Gaggini M,
Gorini F and Vassalle C: Vitamin D determinants, status, and
antioxidant/anti-inflammatory-related effects in cardiovascular
risk and disease: Not the last word in the controversy.
Antioxidants (Basel). 12:9482023. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Aggeletopoulou I, Marangos M,
Assimakopoulos SF, Mouzaki A, Thomopoulos K and Triantos C: Vitamin
D and microbiome: molecular interaction in inflammatory bowel
disease pathogenesis. Am J Pathol. 193:656–668. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Muñoz A and Grant WB: Vitamin D and
cancer: An historical overview of the epidemiology and mechanisms.
Nutrients. 14:14482022. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Carlberg C and Muñoz A: An update on
vitamin D signaling and cancer. Semin Cancer Biol. 79:217–230.
2022. View Article : Google Scholar
|
|
11
|
Ao T, Kikuta J and Ishii M: The effects of
vitamin D on immune system and inflammatory diseases. Biomolecules.
11:16242021. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Boonstra A, Barrat FJ, Crain C, Heath VL,
Savelkoul HF and O'Garra A: 1alpha,25-Dihydroxyvitamin d3 has a
direct effect on naive CD4(+) T cells to enhance the development of
Th2 cells. J Immunol. 167:4974–4980. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhao H, Wei X and Yang X: A novel update
on vitamin D in recurrent pregnancy loss (Review). Mol Med Rep.
23:3822021. View Article : Google Scholar
|
|
14
|
Sayar Z, Moll R, Isenberg D and Cohen H:
Thrombotic antiphospholipid syndrome: A practical guide to
diagnosis and management. Thromb Res. 198:213–221. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Shah R, Mohammed YN, Koehler TJ, Kaur J,
Toufeili M, Pulipati P, Alqaysi A, Khan A, Khalid M, Lee Y, et al:
Antiphospholipid antibodies and vitamin D deficiency in COVID-19
infection with and without venous or arterial thrombosis: A pilot
case-control study. PLoS One. 17:e02694662022. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhang H, Ding X, Hu X, Cai YX, Chen H, Sun
C, Chen J, Li X, Zheng Z, Liao T, et al: Associations between 25
hydroxyvitamin D concentration and spontaneous abortion. BMC Public
Health. 24:18582024. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Chen C, Wang S, Zhang C, Wu X, Zhou L, Zou
X, Guan T, Zhang Z and Hao J: Association between serum vitamin D
level during pregnancy and recurrent spontaneous abortion: A
systematic review and meta-analysis. Am J Reprod Immunol.
88:e135822022. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Duarte-García A, Pham MM, Crowson CS, Amin
S, Moder KG, Pruthi RK, Warrington KJ and Matteson EL: The
epidemiology of antiphospholipid syndrome: A population-based
study. Arthritis Rheumatol. 71:1545–1552. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Petri M: Antiphospholipid syndrome. Transl
Res. 225:70–81. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Cashman KD, Dowling KG, Škrabáková Z,
Gonzalez-Gross M, Valtueña J, De Henauw S, Moreno L, Damsgaard CT,
Michaelsen KF, Mølgaard C, et al: Vitamin D deficiency in Europe:
Pandemic? Am J Clin Nutr. 103:1033–1044. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Cashman KD: Vitamin D deficiency:
defining, prevalence, causes, and strategies of addressing. Calcif
Tissue Int. 106:14–29. 2020. View Article : Google Scholar
|
|
22
|
Sarafin K, Durazo-Arvizu R, Tian L,
Phinney KW, Tai S, Camara JE, Merkel J, Green E, Sempos CT and
Brooks SP: Standardizing 25-hydroxyvitamin D values from the
Canadian health measures survey. Am J Clin Nutr. 102:1044–1050.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Amrein K, Scherkl M, Hoffmann M,
Neuwersch-Sommeregger S, Köstenberger M, Tmava Berisha A, Martucci
G, Pilz S and Malle O: Vitamin D deficiency 2.0: An update on the
current status worldwide. Eur J Clin Nutr. 74:1498–1513. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Md Isa Z, Mohd Nordin NR, Mahmud MH and
Hashim S: An Update on vitamin D deficiency status in Malaysia.
Nutrients. 14:5672022. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Islam MA, Ahmed S, Sultana S, Alam SS,
Hossan T, Gouda W, Alsaqabi F, Hassan R and Kotyla PJ: Vitamin D
status in patients with primary antiphospholipid syndrome (PAPS): A
systematic review and meta-analysis. Antibodies (Basel). 13:222024.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Fernando M, Ellery SJ, Marquina C, Lim S,
Naderpoor N and Mousa A: Vitamin D-binding protein in pregnancy and
reproductive health. Nutrients. 12:14892020. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Jäpelt RB and Jakobsen J: Vitamin D in
plants: A review of occurrence, analysis, and biosynthesis. Front
Plant Sci. 4:1362013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Tsuprykov O, Chen X, Hocher CF, Skoblo R,
Yin L and Hocher B: Why should we measure free 25(OH) vitamin D? J
Steroid Biochem Mol Biol. 180:87–104. 2018. View Article : Google Scholar
|
|
29
|
Al-Ishaq RK, Kubatka P, Brozmanova M,
Gazdikova K, Caprnda M and Büsselberg D: Health implication of
vitamin D on the cardiovascular and the renal system. Arch Physiol
Biochem. 127:195–209. 2021. View Article : Google Scholar
|
|
30
|
Keane JT, Elangovan H, Stokes RA and
Gunton JE: Vitamin D and the liver-correlation or cause? Nutrients.
10:4962018. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
de Paula FJA and Rosen CJ: Vitamin D
safety and requirements. Arch Biochem Biophys. 523:64–72. 2012.
View Article : Google Scholar :
|
|
32
|
Zmijewski MA and Carlberg C: Vitamin D
receptor(s): In the nucleus but also at membranes? Exp Dermatol.
29:876–884. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Arora J, Wang J, Weaver V, Zhang Y and
Cantorna MT: Novel insight into the role of the vitamin D receptor
in the development and function of the immune system. J Steroid
Biochem Mol Biol. 219:1060842022. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Khundmiri SJ, Murray RD and Lederer E: PTH
and vitamin D. Compr Physiol. 6:561–601. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Li YC, Chen Y and Du J: Critical roles of
intestinal epithelial vitamin D receptor signaling in controlling
gut mucosal inflammation. J Steroid Biochem Mol Biol. 148:179–183.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Mohammad S, Mishra A and Ashraf MZ:
Emerging role of vitamin D and its associated molecules in pathways
related to pathogenesis of thrombosis. Biomolecules. 9:6492019.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Battault S, Whiting SJ, Peltier SL, Sadrin
S, Gerber G and Maixent JM: Vitamin D metabolism, functions and
needs: From science to health claims. Eur J Nutr. 52:429–441. 2013.
View Article : Google Scholar
|
|
38
|
Hewison M, Freeman L, Hughes SV, Evans KN,
Bland R, Eliopoulos AG, Kilby MD, Moss PA and Chakraverty R:
Differential regulation of vitamin D receptor and its ligand in
human monocyte-derived dendritic cells. J Immunol. 170:5382–5390.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Fritsche J, Mondal K, Ehrnsperger A,
Andreesen R and Kreutz M: Regulation of 25-hydroxyvitamin D3-1
alpha-hydroxylase and production of 1 alpha,25-dihydroxyvitamin D3
by human dendritic cells. Blood. 102:3314–3316. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Harrison SR, Li D, Jeffery LE, Raza K and
Hewison M: Vitamin D, autoimmune disease and rheumatoid arthritis.
Calcif Tissue Int. 106:58–75. 2020. View Article : Google Scholar :
|
|
41
|
Hewison M, Zehnder D, Chakraverty R and
Adams JS: Vitamin D and barrier function: A novel role for
extra-renal 1 alpha-hydroxylase. Mol Cell Endocrinol. 215:31–38.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Penna G and Adorini L: 1
Alpha,25-dihydroxyvitamin D3 inhibits differentiation, maturation,
activation, and survival of dendritic cells leading to impaired
alloreactive T cell activation. J Immunol. 164:2405–2411. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Bartels LE, Jørgensen SP, Agnholt J,
Kelsen J, Hvas CL and Dahlerup JF: 1,25-dihydroxyvitamin D3 and
dexamethasone increase interleukin-10 production in CD4+ T cells
from patients with Crohn's disease. Int Immunopharmacol.
7:1755–1764. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Széles L, Keresztes G, Töröcsik D,
Balajthy Z, Krenács L, Póliska S, Steinmeyer A, Zuegel U, Pruenster
M, Rot A and Nagy L: 1,25-dihydroxyvitamin D3 is an autonomous
regulator of the transcriptional changes leading to a tolerogenic
dendritic cell phenotype. J Immunol. 182:2074–2083. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Piemonti L, Monti P, Sironi M, Fraticelli
P, Leone BE, Dal Cin E, Allavena P and Di Carlo V: Vitamin D3
affects differentiation, maturation, and function of human
monocyte-derived dendritic cells. J Immunol. 164:4443–4451. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Hewison M: Vitamin D and immune function:
An overview. Proc Nutr Soc. 71:50–61. 2012. View Article : Google Scholar
|
|
47
|
Cantorna MT, Snyder L, Lin YD and Yang L:
Vitamin D and 1,25(OH)2D regulation of T cells. Nutrients.
7:3011–3021. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Cippitelli M, Fionda C, Di Bona D, Di Rosa
F, Lupo A, Piccoli M, Frati L and Santoni A: Negative regulation of
CD95 ligand gene expression by vitamin D3 in T lymphocytes. J
Immunol. 168:1154–1166. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Xie Z, Chen J, Zheng C, Wu J, Cheng Y, Zhu
S, Lin C, Cao Q, Zhu J and Jin T: 1,25-dihydroxyvitamin D3-induced
dendritic cells suppress experimental autoimmune encephalomyelitis
by increasing proportions of the regulatory lymphocytes and
reducing T helper type 1 and type 17 cells. Immunology.
152:414–424. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Bellan M, Andreoli L, Mele C, Sainaghi PP,
Rigamonti C, Piantoni S, De Benedittis C, Aimaretti G, Pirisi M and
Marzullo P: Pathophysiological role and therapeutic implications of
vitamin D in autoimmunity: Focus on chronic autoimmune diseases.
Nutrients. 12:7892020. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Zhou Q, Qin S, Zhang J, Zhon L, Pen Z and
Xing T: 1,25(OH)2D3 induces regulatory T cell
differentiation by influencing the VDR/PLC-γ1/TGF-β1/pathway. Mol
Immunol. 91:156–164. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Germain SJ, Sacks GP, Sooranna SR, Sargent
IL and Redman CW: Systemic inflammatory priming in normal pregnancy
and preeclampsia: The role of circulating syncytiotrophoblast
microparticles. J Immunol. 178:5949–5956. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Torchinsky A, Shepshelovich J, Orenstein
H, Zaslavsky Z, Savion S, Carp H, Fain A and Toder V: TNF-alpha
protects embryos exposed to developmental toxicants. Am J Reprod
Immunol. 49:159–168. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Piccinni MP, Raghupathy R, Saito S and
Szekeres-Bartho J: Cytokines, hormones and cellular regulatory
mechanisms favoring successful reproduction. Front Immunol.
12:7178082021. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Casazza RL, Lazear HM and Miner JJ:
Protective and pathogenic effects of interferon signaling during
pregnancy. Viral Immunol. 33:3–11. 2020. View Article : Google Scholar :
|
|
56
|
Yang X, Tian Y, Zheng L, Luu T and
Kwak-Kim J: The update immune-regulatory role of pro- and
anti-inflammatory cytokines in recurrent pregnancy losses. Int J
Mol Sci. 24:1322022. View Article : Google Scholar
|
|
57
|
Michimata T, Tsuda H, Sakai M, Fujimura M,
Nagata K, Nakamura M and Saito S: Accumulation of CRTH2-positive
T-helper 2 and T-cytotoxic 2 cells at implantation sites of human
decidua in a prostaglandin D(2)-mediated manner. Mol Hum Reprod.
8:181–187. 2002. View Article : Google Scholar
|
|
58
|
Mitchell RE, Hassan M, Burton BR, Britton
G, Hill EV, Verhagen J and Wraith DC: IL-4 enhances IL-10
production in Th1 cells: Implications for Th1 and Th2 regulation.
Sci Rep. 7:113152017. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Nakashima A, Ito M, Yoneda S, Shiozaki A,
Hidaka T and Saito S: Circulating and decidual Th17 cell levels in
healthy pregnancy. Am J Reprod Immunol. 63:104–109. 2010.
View Article : Google Scholar
|
|
60
|
Cua DJ and Tato CM: Innate IL-17-producing
cells: The sentinels of the immune system. Nat Rev Immunol.
10:479–489. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Cyprian F, Lef kou E, Varoudi K and
Girardi G: Immunomodulatory effects of vitamin D in pregnancy and
beyond. Front Immunol. 10:27392019. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Minton K: Vitamin D shuts down T
cell-mediated inflammation. Nat Rev Immunol. 22:12022. View Article : Google Scholar
|
|
63
|
Zhang H, Wang S, Tuo L, Zhai Q, Cui J,
Chen D and Xu D: Relationship between maternal vitamin D levels and
adverse outcomes. Nutrients. 14:42302022. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Schröder-Heurich B, Springer CJP and von
Versen-Höynck F: Vitamin D effects on the immune system from
periconception through pregnancy. Nutrients. 12:14322020.
View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Renaud SJ, Postovit LM,
Macdonald-Goodfellow SK, McDonald GT, Caldwell JD and Graham CH:
Activated macrophages inhibit human cytotrophoblast invasiveness in
vitro. Biol Reprod. 73:237–243. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Seki H, Matuoka K, Inooku H and Takeda S:
TNF-alpha from monocyte of patients with pre-eclampsia-induced
apoptosis in human trophoblast cell line. J Obstet Gynaecol Res.
33:408–416. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Lockwood CJ, Oner C, Uz YH, Kayisli UA,
Huang SJ, Buchwalder LF, Murk W, Funai EF and Schatz F: Matrix
metalloproteinase 9 (MMP9) expression in preeclamptic decidua and
MMP9 induction by tumor necrosis factor alpha and interleukin 1
beta in human first trimester decidual cells. Biol Reprod.
78:1064–1072. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Raphael I, Nalawade S, Eagar TN and
Forsthuber TG: T cell subsets and their signature cytokines in
autoimmune and inflammatory diseases. Cytokine. 74:5–17. 2015.
View Article : Google Scholar :
|
|
69
|
Jara LJ, Medina G, Cruz-Dominguez P,
Navarro C, Vera-Lastra O and Saavedra MA: Risk factors of systemic
lupus erythematosus flares during pregnancy. Immunol Res.
60:184–192. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Travis OK, White D, Pierce WA, Ge Y,
Stubbs CY, Spradley FT, Williams JM and Cornelius DC: Chronic
infusion of interleukin-17 promotes hypertension, activation of
cytolytic natural killer cells, and vascular dysfunction in
pregnant rats. Physiol Rep. 7:e140382019. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Idali F, Rezaii-Nia S, Golshahi H, Fatemi
R, Naderi MM, Goli LB, Zarnani AH and Jeddi-Tehrani M: Adoptive
cell therapy with induced regulatory T cells normalises the
abortion rate in abortion-prone mice. Reprod Fertil Dev.
33:220–228. 2021.
|
|
72
|
Benagiano M, Borghi MO, Romagnoli J,
Mahler M, Bella CD, Grassi A, Capitani N, Emmi G, Troilo A,
Silvestri E, et al: Interleukin-17/Interleukin-21 and interferon-γ
producing T cells specific for β2 glycoprotein I in atherosclerosis
inflammation of systemic lupus erythematosus patients with
antiphospholipid syndrome. Haematologica. 104:2519–2527. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Robert M, Miossec P and Hot A: The Th17
pathway in vascular inflammation: Culprit or consort? Front
Immunol. 13:8887632022. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Cremoni M, Brglez V, Perez S, Decoupigny
F, Zorzi K, Andreani M, Gérard A, Boyer-Suavet S, Ruetsch C,
Benzaken S, et al: Th17-immune response in patients with membranous
nephropathy is associated with thrombosis and relapses. Front
Immunol. 11:5749972020. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Rawal N and Pangburn MK: Formation of high
affinity C5 convertase of the classical pathway of complement. J
Biol Chem. 278:38476–38483. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Liu X, Hu Y, Yu X, Tan Y, Yu F, Chen M and
Zhao M: Differential contributions of the C5b-9 and C5a/C5aR
pathways to microvascular and macrovascular thrombosis in
complement-mediated thrombotic microangiopathy patients. Clin
Immunol. 259:1098712024. View Article : Google Scholar
|
|
77
|
Li H, Xie X, Bai G, Qiang D, Zhang L, Liu
H, He Y, Tang Y and Li L: Vitamin D deficiency leads to the
abnormal activation of the complement system. Immunol Res.
71:29–38. 2023. View Article : Google Scholar :
|
|
78
|
Skendros P, Mitsios A, Chrysanthopoulou A,
Mastellos DC, Metallidis S, Rafailidis P, Ntinopoulou M, Sertaridou
E, Tsironidou V, Tsigalou C, et al: Complement and tissue
factor-enriched neutrophil extracellular traps are key drivers in
COVID-19 immunothrombosis. J Clin Invest. 130:6151–6157. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Chen Y, Li X, Lin X, Liang H, Liu X, Zhang
X, Zhang Q, Zhou F, Yu C, Lei L and Xiu J: Complement C5a induces
the generation of neutrophil extracellular traps by inhibiting
mitochondrial STAT3 to promote the development of arterial
thrombosis. Thromb J. 20:242022. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Borghi MO, Raschi E, Grossi C, Chighizola
CB and Meroni PL: Toll-like receptor 4 and β2 glycoprotein I
interaction on endothelial cells. Lupus. 23:1302–1304. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Matias ML, Romao-Veiga M, Ribeiro VR,
Nunes PR, Gomes VJ, Devides AC, Borges VT, Romagnoli GG, Peracoli
JC and Peracoli MT: Progesterone and vitamin D downregulate the
activation of the NLRP1/NLRP3 inflammasomes and TLR4-MyD88-NF-κB
pathway in monocytes from pregnant women with preeclampsia. J
Reprod Immunol. 144:1032862021. View Article : Google Scholar
|
|
82
|
Martinez-Moreno JM, Herencia C, Montes de
Oca A, Muñoz-Castañeda JR, Rodríguez-Ortiz ME, Díaz-Tocados JM,
Peralbo-Santaella E, Camargo A, Canalejo A, Rodriguez M, et al:
Vitamin D modulates tissue factor and protease-activated receptor 2
expression in vascular smooth muscle cells. FASEB J. 30:1367–1376.
2016. View Article : Google Scholar
|
|
83
|
Chen SF: 1 alpha, 25-Dihydroxyvitamin D3
decreased ICAM-1 and ELAM-1 expressions on pulmonary microvascular
endothelial cells and neutrophil motivation. J Steroid Biochem Mol
Biol. 52:67–70. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Jamali N, Song YS, Sorenson CM and
Sheibani N: 1,25(OH)2D3 regulates the proangiogenic activity of
pericyte through VDR-mediated modulation of VEGF production and
signaling of VEGF and PDGF receptors. FASEB Bioadv. 1:415–434.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Aihara K, Azuma H and Matsumoto T: Vitamin
D-vitamin D receptor system regulates antithrombogenicity in vivo.
Clin Calcium. 16:1173–1179. 2006.In Japanese. PubMed/NCBI
|
|
86
|
Han T, Liu M and Yang S: DJ-1 alleviates
angiotensin II-induced endothelial progenitor cell damage by
activating the PPARγ/HO-1 pathway. J Cell Biochem. 119:392–400.
2018. View Article : Google Scholar
|
|
87
|
Haseda F, Imagawa A, Murase-Mishiba Y,
Terasaki J and Hanafusa T: CD4+ CD45RA−
FoxP3high activated regulatory T cells are functionally impaired
and related to residual insulin-secreting capacity in patients with
type 1 diabetes. Clin Exp Immunol. 173:207–216. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
He LP, Song YX, Zhu T, Gu W and Liu CW:
Progress in the relationship between vitamin D deficiency and the
incidence of type 1 diabetes mellitus in children. J Diabetes Res.
2022:59535622022. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Rak K and Bronkowska M: Immunomodulatory
effect of vitamin D and its potential role in the prevention and
treatment of type 1 diabetes mellitus-A narrative review.
Molecules. 24:532018. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Roep BO: The role of T-cells in the
pathogenesis of type 1 diabetes: From cause to cure. Diabetologia.
46:305–321. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Felício KM, de Souza ACCB, Neto JFA, de
Melo FTC, Carvalho CT, Arbage TP, de Rider Brito HA, Peixoto AS, de
Oliveira AF, de Souza Resende F, et al: Glycemic variability and
insulin needs in patients with type 1 diabetes mellitus
supplemented with vitamin D: A pilot study using continuous glucose
monitoring system. Curr Diabetes Rev. 14:395–403. 2018. View Article : Google Scholar
|
|
92
|
Treiber G, Prietl B, Fröhlich-Reiterer E,
Lechner E, Ribitsch A, Fritsch M, Rami-Merhar B,
Steigleder-Schweiger C, Graninger W, Borkenstein M and Pieber TR:
Cholecalciferol supplementation improves suppressive capacity of
regulatory T-cells in young patients with new-onset type 1 diabetes
mellitus-A randomized clinical trial. Clin Immunol. 161:217–224.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Giri D, Pintus D, Burnside G, Ghatak A,
Mehta F, Paul P and Senniappan S: Treating vitamin D deficiency in
children with type I diabetes could improve their glycaemic
control. BMC Res Notes. 10:4652017. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Janyga S, Marek B, Kajdaniuk D,
Ogrodowczyk-Bobik M, Urbanek A and Bułdak Ł: CD4+ cells in
autoimmune thyroid disease. Endokrynol Pol. 72:572–583. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Chahardoli R, Saboor-Yaraghi AA, Amouzegar
A, Khalili D, Vakili AZ and Azizi F: Can supplementation with
vitamin D modify thyroid autoantibodies (Anti-TPO Ab, Anti-Tg Ab)
and thyroid profile (T3, T4, TSH) in hashimoto's thyroiditis? A
double blind, randomized clinical trial. Horm Metab Res.
51:296–301. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Penna-Martinez M, Filmann N, Bogdanou D,
Shoghi F, Huenecke S, Schubert R, Herrmann E, Koehl U, Husebye ES
and Badenhoop K: High-dose vitamin D in Addison's disease regulates
T-cells and monocytes: A pilot trial. Nutrition. 49:66–73. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Lynde CW, Poulin Y, Vender R, Bourcier M
and Khalil S: Interleukin 17A: Toward a new understanding of
psoriasis pathogenesis. J Am Acad Dermatol. 71:141–150. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Kjær TN, Thorsen K, Jessen N, Stenderup K
and Pedersen SB: Resveratrol ameliorates imiquimod-induced
psoriasis-like skin inflammation in mice. PLoS One.
10:e01265992015. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Navegantes KC, de Souza Gomes R, Pereira
PAT, Czaikoski PG, Azevedo CHM and Monteiro MC: Immune modulation
of some autoimmune diseases: The critical role of macrophages and
neutrophils in the innate and adaptive immunity. J Transl Med.
15:362017. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Balato A, Schiattarella M, Lembo S, Mattii
M, Prevete N, Balato N and Ayala F: Interleukin-1 family members
are enhanced in psoriasis and suppressed by vitamin D and retinoic
acid. Arch Dermatol Res. 305:255–262. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Prtina A, Rašeta Simović N, Milivojac T,
Vujnić M, Grabež M, Djuric D, Stojiljković MP, Soldat Stanković V,
Čolić MJ and Škrbić R: The effect of three-month vitamin D
supplementation on the levels of homocysteine metabolism markers
and inflammatory cytokines in sera of psoriatic patients.
Biomolecules. 11:18652021. View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Brożyna AA, Slominski RM, Nedoszytko B,
Zmijewski MA and Slominski AT: Vitamin D signaling in psoriasis:
Pathogenesis and therapy. Int J Mol Sci. 23:85752022. View Article : Google Scholar
|
|
103
|
Weyand CM and Goronzy JJ: The immunology
of rheumatoid arthritis. Nat Immunol. 22:10–18. 2021. View Article : Google Scholar :
|
|
104
|
van Hamburg JP, Asmawidjaja PS, Davelaar
N, Mus AMC, Cornelissen F, van Leeuwen JPTM, Hazes JM, Dolhain RJ,
Bakx PA, Colin EM and Lubberts E: TNF blockade requires 1,25(OH)2D3
to control human Th17-mediated synovial inflammation. Ann Rheum
Dis. 71:606–612. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Jeffery LE, Qureshi OS, Gardner D, Hou TZ,
Briggs Z, Soskic B, Baker J, Raza K and Sansom DM: Vitamin D
antagonises the suppressive effect of inflammatory cytokines on
CTLA-4 expression and regulatory function. PLoS One.
10:e01315392015. View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Guan Y, Hao Y, Guan Y, Bu H and Wang H:
The effect of vitamin D supplementation on rheumatoid arthritis
patients: A systematic review and meta-analysis. Front Med
(Lausanne). 7:5960072020. View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Nguyen Y, Sigaux J, Letarouilly JG,
Sanchez P, Czernichow S, Flipo RM, Soubrier M, Semerano L, Seror R,
Sellam J and Daïen C: Efficacy of oral vitamin supplementation in
inflammatory rheumatic disorders: A systematic review and
meta-analysis of randomized controlled trials. Nutrients.
13:1072020. View Article : Google Scholar
|
|
108
|
Pan L, Lu MP, Wang JH, Xu M and Yang SR:
Immunological pathogenesis and treatment of systemic lupus
erythematosus. World J Pediatr. 16:19–30. 2020. View Article : Google Scholar :
|
|
109
|
Berthelot JM, Le Goff B, Neel A, Maugars Y
and Hamidou M: NETosis: At the crossroads of rheumatoid arthritis,
lupus, and vasculitis. Joint Bone Spine. 84:255–262. 2017.
View Article : Google Scholar
|
|
110
|
Shan J, Jin H and Xu Y: T cell metabolism:
A new perspective on Th17/Treg cell imbalance in systemic lupus
erythematosus. Front Immunol. 11:10272020. View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Terrier B, Derian N, Schoindre Y, Chaara
W, Geri G, Zahr N, Mariampillai K, Rosenzwajg M, Carpentier W,
Musset L, et al: Restoration of regulatory and effector T cell
balance and B cell homeostasis in systemic lupus erythematosus
patients through vitamin D supplementation. Arthritis Res Ther.
14:R2212012. View
Article : Google Scholar : PubMed/NCBI
|
|
112
|
Petri M, Bello KJ, Fang H and Magder LS:
Vitamin D in systemic lupus erythematosus: Modest association with
disease activity and the urine protein-to-creatinine ratio.
Arthritis Rheum. 65:1865–1871. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
113
|
Ruiz-Irastorza G, Egurbide MV, Olivares N,
Martinez-Berriotxoa A and Aguirre C: Vitamin D deficiency in
systemic lupus erythematosus: Prevalence, predictors and clinical
consequences. Rheumatology (Oxford). 47:920–923. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
114
|
Lima GL, Paupitz J, Aikawa NE, Takayama L,
Bonfa E and Pereira RMR: Vitamin D supplementation in adolescents
and young adults with juvenile systemic lupus erythematosus for
improvement in disease activity and fatigue scores: A randomized,
double-blind, placebo-controlled trial. Arthritis Care Res
(Hoboken). 68:91–98. 2016. View Article : Google Scholar
|
|
115
|
Galoppin M, Kari S, Soldati S, Pal A,
Rival M, Engelhardt B, Astier A and Thouvenot E: Full spectrum of
vitamin D immunomodulation in multiple sclerosis: Mechanisms and
therapeutic implications. Brain Commun. 4:fcac1712022. View Article : Google Scholar : PubMed/NCBI
|
|
116
|
Peelen E, Muris AH, Damoiseaux J,
Knippenberg S, Broens K, Smolders J, Cohen Tervaert JW, Hupperts R
and Thewissen M: GM-CSF production by CD4+ T cells in MS patients:
Regulation by regulatory T cells and vitamin D. J Neuroimmunol.
280:36–42. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
117
|
Soilu-Hänninen M, Aivo J, Lindström BM,
Elovaara I, Sumelahti ML, Färkkilä M, Tienari P, Atula S, Sarasoja
T, Herrala L, et al: A randomised, double blind, placebo controlled
trial with vitamin D3 as an add on treatment to interferon β-1b in
patients with multiple sclerosis. J Neurol Neurosurg Psychiatry.
83:565–571. 2012. View Article : Google Scholar
|
|
118
|
Feige J, Moser T, Bieler L, Schwenker K,
Hauer L and Sellner J: Vitamin D supplementation in multiple
sclerosis: A critical analysis of potentials and threats.
Nutrients. 12:7832020. View Article : Google Scholar : PubMed/NCBI
|
|
119
|
Piantoni S, Andreoli L, Scarsi M, Zanola
A, Dall'Ara F, Pizzorni C, Cutolo M, Airò P and Tincani A:
Phenotype modifications of T-cells and their shift toward a Th2
response in patients with systemic lupus erythematosus supplemented
with different monthly regimens of vitamin D. Lupus. 24:490–498.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
120
|
Kello N and Cho YM: Natural supplements in
antiphospholipid syndrome: A case for further study. Clin Immunol.
258:1098482024. View Article : Google Scholar
|
|
121
|
Agmon-Levin N, Blank M, Zandman-Goddard G,
Orbach H, Meroni PL, Tincani A, Doria A, Cervera R, Miesbach W,
Stojanovich L, et al: Vitamin D: An instrumental factor in the
anti-phospholipid syndrome by inhibition of tissue factor
expression. Ann Rheum Dis. 70:145–150. 2011. View Article : Google Scholar
|
|
122
|
Beer TM, Venner PM, Ryan CW, Petrylak DP,
Chatta G, Dean Ruether J, Chi KN, Curd JG and DeLoughery TG: High
dose calcitriol may reduce thrombosis in cancer patients. Br J
Haematol. 135:392–394. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
123
|
Keaney JF Jr and Rosen CJ: VITAL signs for
dietary supplementation to prevent cancer and heart disease. N Engl
J Med. 380:91–93. 2019. View Article : Google Scholar
|
|
124
|
Cannegieter SC, Doggen CJ, van Houwelingen
HC and Rosendaal FR: Travel-related venous thrombosis: Results from
a large population-based case control study (MEGA study). PLoS Med.
3:e3072006. View Article : Google Scholar : PubMed/NCBI
|
|
125
|
Cohen H, Cuadrado MJ, Erkan D,
Duarte-Garcia A, Isenberg DA, Knight JS, Ortel TL, Rahman A, Salmon
JE, Tektonidou MG, et al: 16th International congress on
antiphospholipid antibodies task force report on antiphospholipid
syndrome treatment trends. Lupus. 29:1571–1593. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
126
|
Ruiz-Irastorza G, Crowther M, Branch W and
Khamashta MA: Antiphospholipid syndrome. Lancet. 376:1498–1509.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
127
|
Chighizola CB, Ubiali T and Meroni PL:
Treatment of thrombotic antiphospholipid syndrome: The rationale of
current management-an insight into future approaches. J Immunol
Res. 2015:9514242015. View Article : Google Scholar : PubMed/NCBI
|
|
128
|
Uludag G, Onghanseng N, Tran ANT, Hassan
M, Halim MS, Sepah YJ, Do DV and Nguyen QD: Current concepts in the
diagnosis and management of antiphospholipid syndrome and ocular
manifestations. J Ophthalmic Inflamm Infect. 11:112021. View Article : Google Scholar : PubMed/NCBI
|
|
129
|
García-Carrasco M, Jiménez-Herrera EA,
Gálvez-Romero JL, Mendoza-Pinto C, Méndez-Martínez S,
Etchegaray-Morales I, Munguía-Realpozo P, Vázquez de Lara-Cisneros
L, Santa Cruz FJ and Cervera R: The anti-thrombotic effects of
vitamin D and their possible relationship with antiphospholipid
syndrome. Lupus. 27:2181–2189. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
130
|
Ota K, Dambaeva S, Han AR, Beaman K,
Gilman-Sachs A and Kwak-Kim J: Vitamin D deficiency may be a risk
factor for recurrent pregnancy losses by increasing cellular
immunity and autoimmunity. Hum Reprod. 29:208–219. 2014. View Article : Google Scholar
|
|
131
|
Chen X, Yin B, Lian RC, Zhang T, Zhang HZ,
Diao LH, Li YY, Huang CY, Liang DS and Zeng Y: Modulatory effects
of vitamin D on peripheral cellular immunity in patients with
recurrent miscarriage. Am J Reprod Immunol. 76:432–438. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
132
|
Jeffery LE, Burke F, Mura M, Zheng Y,
Qureshi OS, Hewison M, Walker LS, Lammas DA, Raza K and Sansom DM:
1,25-Dihydroxyvitamin D3 and IL-2 combine to inhibit T cell
production of inflammatory cytokines and promote development of
regulatory T cells expressing CTLA-4 and FoxP3. J Immunol.
183:5458–5467. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
133
|
Rafiee M, Gharagozloo M, Ghahiri A,
Mehrabian F, Maracy MR, Kouhpayeh S, Pieper IL and Rezaei A:
Altered Th17/Treg ratio in recurrent miscarriage after treatment
with paternal lymphocytes and vitamin D3: A double-blind
placebo-controlled study. Iran J Immunol. 12:252–262.
2015.PubMed/NCBI
|
|
134
|
Ji J, Zhai H, Zhou H, Song S, Mor G and
Liao A: The role and mechanism of vitamin D-mediated regulation of
Treg/Th17 balance in recurrent pregnancy loss. Am J Reprod Immunol.
81:e131122019. View Article : Google Scholar : PubMed/NCBI
|
|
135
|
Piantoni S, Andreoli L, Allegri F, Meroni
PL and Tincani A: Low levels of vitamin D are common in primary
antiphospholipid syndrome with thrombotic disease. Reumatismo.
64:307–313. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
136
|
Bećarević M, Sarić M, Stojanovich L,
Mirković D, Dopsaj V and Ignjatović S: Anti-annexin A5 antibodies
and 25-hydroxycholecalciferol in female patients with primary
antiphospholipid syndrome. Clin Rheumatol. 37:3359–3364. 2018.
View Article : Google Scholar
|
|
137
|
Hanley DA, Cranney A, Jones G, Whiting SJ,
Leslie WD, Cole DE, Atkinson SA, Josse RG, Feldman S, Kline GA, et
al: Vitamin D in adult health and disease: A review and guideline
statement from Osteoporosis Canada. CMAJ. 182:E610–E618. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
138
|
Ross AC, Manson JE, Abrams SA, Aloia JF,
Brannon PM, Clinton SK, Durazo-Arvizu RA, Gallagher JC, Gallo RL,
Jones G, et al: The 2011 report on dietary reference intakes for
calcium and vitamin D from the Institute of Medicine: What
clinicians need to know. J Clin Endocrinol Metab. 96:53–58. 2011.
View Article : Google Scholar :
|
|
139
|
Marcinowska-Suchowierska E,
Kupisz-Urbańska M, Łukaszkiewicz J, Płudowski P and Jones G:
Vitamin D toxicity-A clinical perspective. Front Endocrinol
(Lausanne). 9:5502018. View Article : Google Scholar : PubMed/NCBI
|
|
140
|
Rolf L, Muris AH, Bol Y, Damoiseaux J,
Smolders J and Hupperts R: Vitamin D3 supplementation in
multiple sclerosis: Symptoms and biomarkers of depression. J Neurol
Sci. 378:30–35. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
141
|
Alkundi A, Momoh R, Musa A and Nwafor N:
Vitamin D intoxication and severe hypercalcaemia complicating
nutritional supplements misuse. BMJ Case Rep. 15:e2505532022.
View Article : Google Scholar : PubMed/NCBI
|
|
142
|
Fragoso YD, Adoni T, Damasceno A, de
Albuquerque Damasceno CA, Ferreira ML, Finkelzstejn A, Gomes S,
Goncalves MV, Grzesiuk AK, Lins S, et al: Unfavorable outcomes
during treatment of multiple sclerosis with high doses of vitamin
D. J Neurol Sci. 346:341–342. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
143
|
De Vincentis S, Russo A, Milazzo M,
Lonardo A, De Santis MC, Rochira V, Simoni M and Madeo B: How much
vitamin D is too much? A case report and review of the literature.
Endocr Metab Immune Disord Drug Targets. 21:1653–1659. 2021.
View Article : Google Scholar
|
|
144
|
Sanders KM, Stuart AL, Williamson EJ,
Simpson JA, Kotowicz MA, Young D and Nicholson GC: Annual high-dose
oral vitamin D and falls and fractures in older women: A randomized
controlled trial. JAMA. 303:1815–1822. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
145
|
Smith H, Anderson F, Raphael H, Maslin P,
Crozier S and Cooper C: Effect of annual intramuscular vitamin D on
fracture risk in elderly men and women-a population-based,
randomized, double-blind, placebo-controlled trial. Rheumatology
(Oxford). 46:1852–1857. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
146
|
Rocha PN, Santos CS, Avila MO, Neves CL
and Bahiense-Oliveira M: Hypercalcemia and acute kidney injury
caused by abuse of a parenteral veterinary compound containing
vitamins A, D, and E. J Bras Nefrol. 33:467–471. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
147
|
Bischoff-Ferrari HA, Dawson-Hughes B, Orav
EJ, Staehelin HB, Meyer OW, Theiler R, Dick W, Willett WC and Egli
A: Monthly high-dose vitamin D treatment for the prevention of
functional decline: A randomized clinical trial. JAMA Intern Med.
176:175–183. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
148
|
Benemei S, Gallo E, Giocaliere E,
Bartolucci G, Menniti-Ippolito F, Firenzuoli F, Mugelli A and
Vannacci A: It's time for new rules on vitamin D food supplements.
Br J Clin Pharmacol. 76:825–826. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
149
|
Kimball S and Vieth R: Self-prescribed
high-dose vitamin D3: Effects on biochemical parameters in two men.
Ann Clin Biochem. 45:106–110. 2008. View Article : Google Scholar : PubMed/NCBI
|