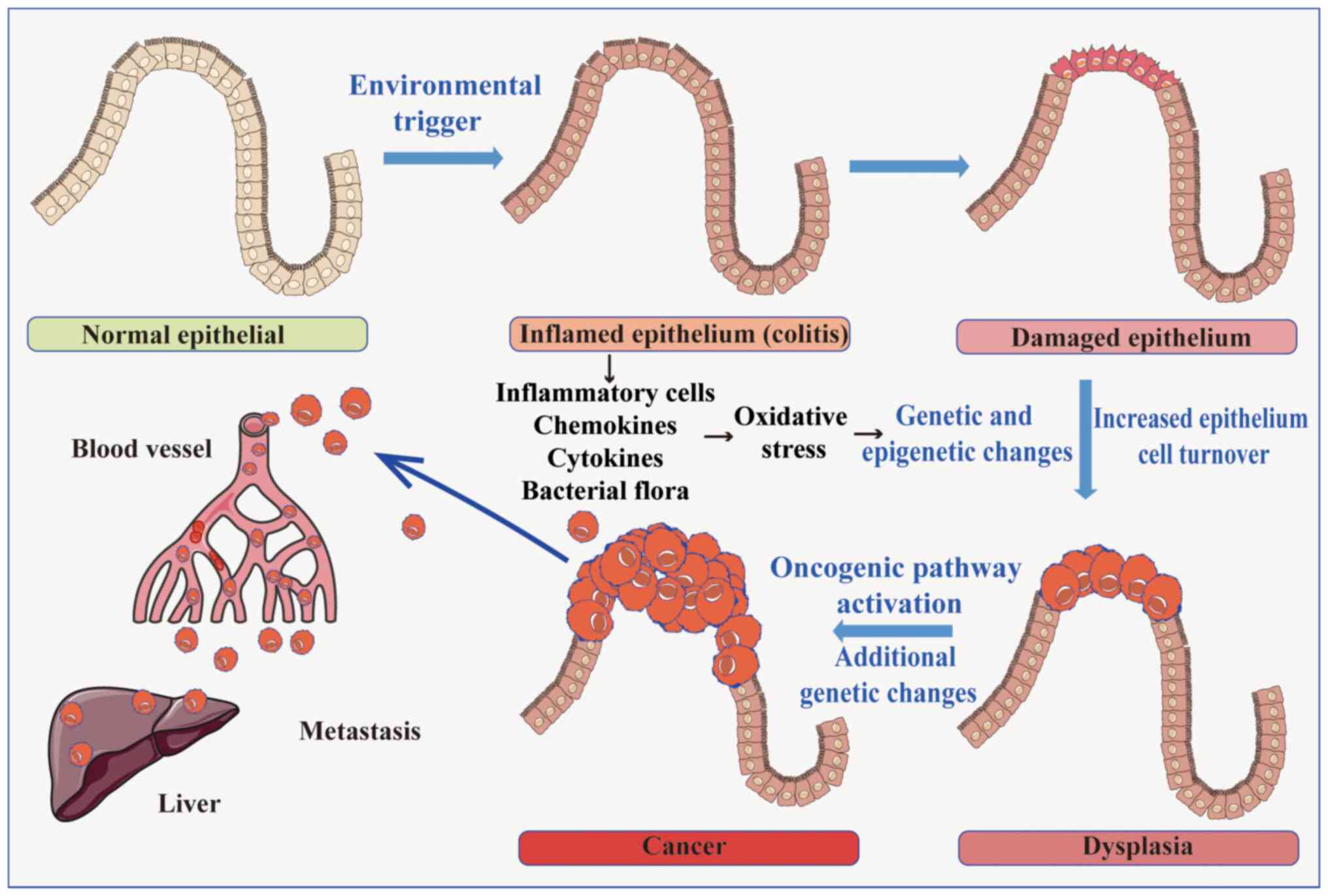
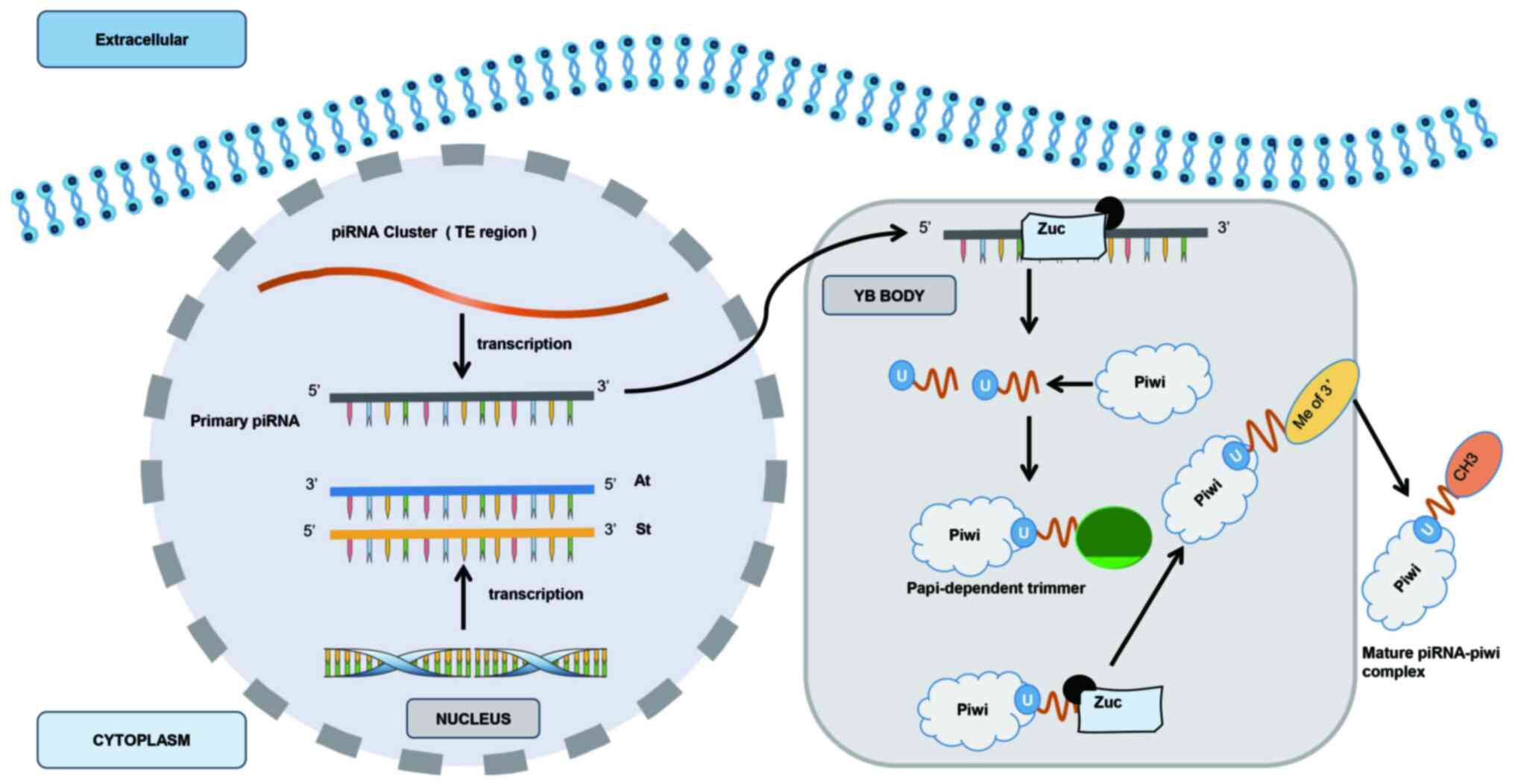
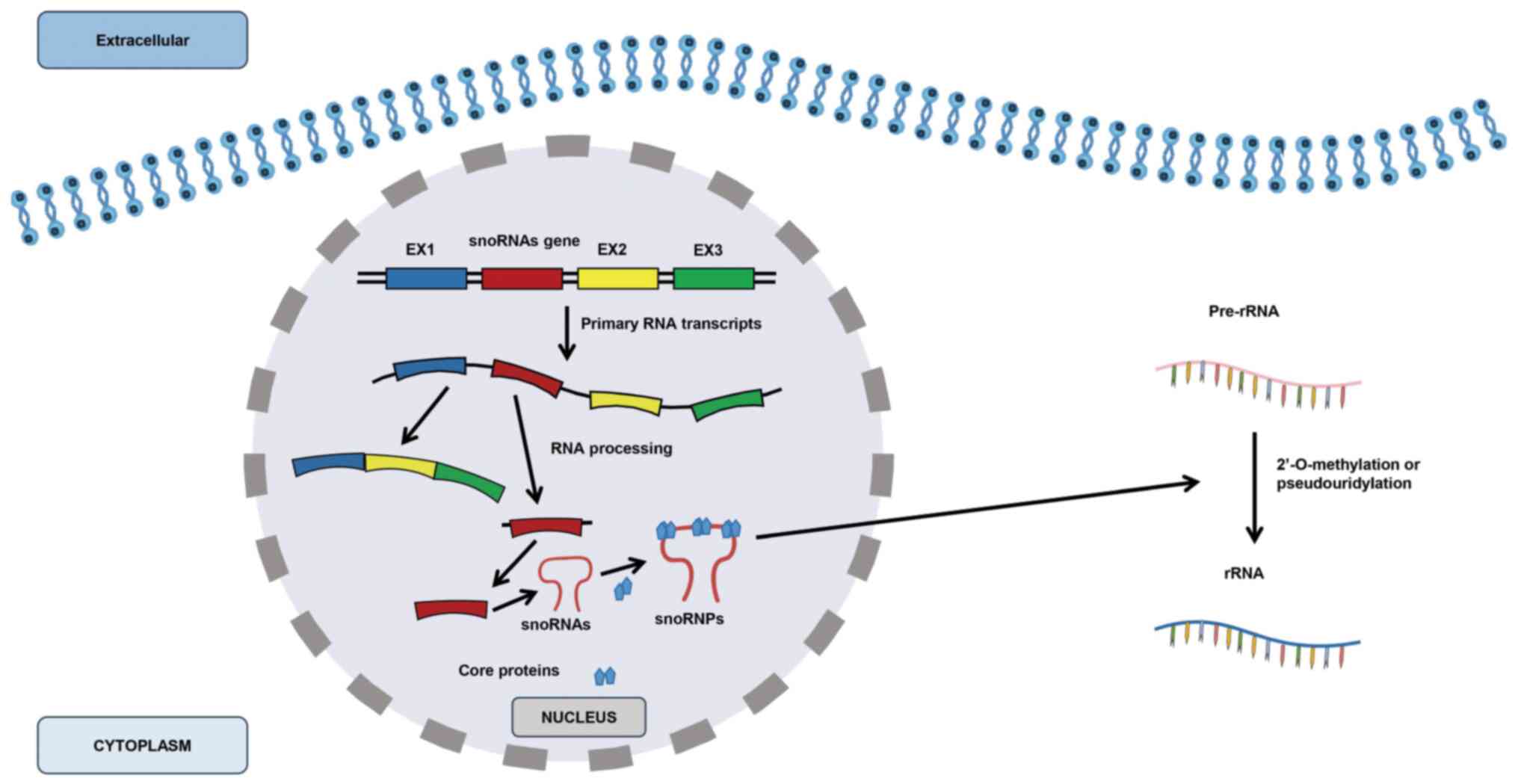
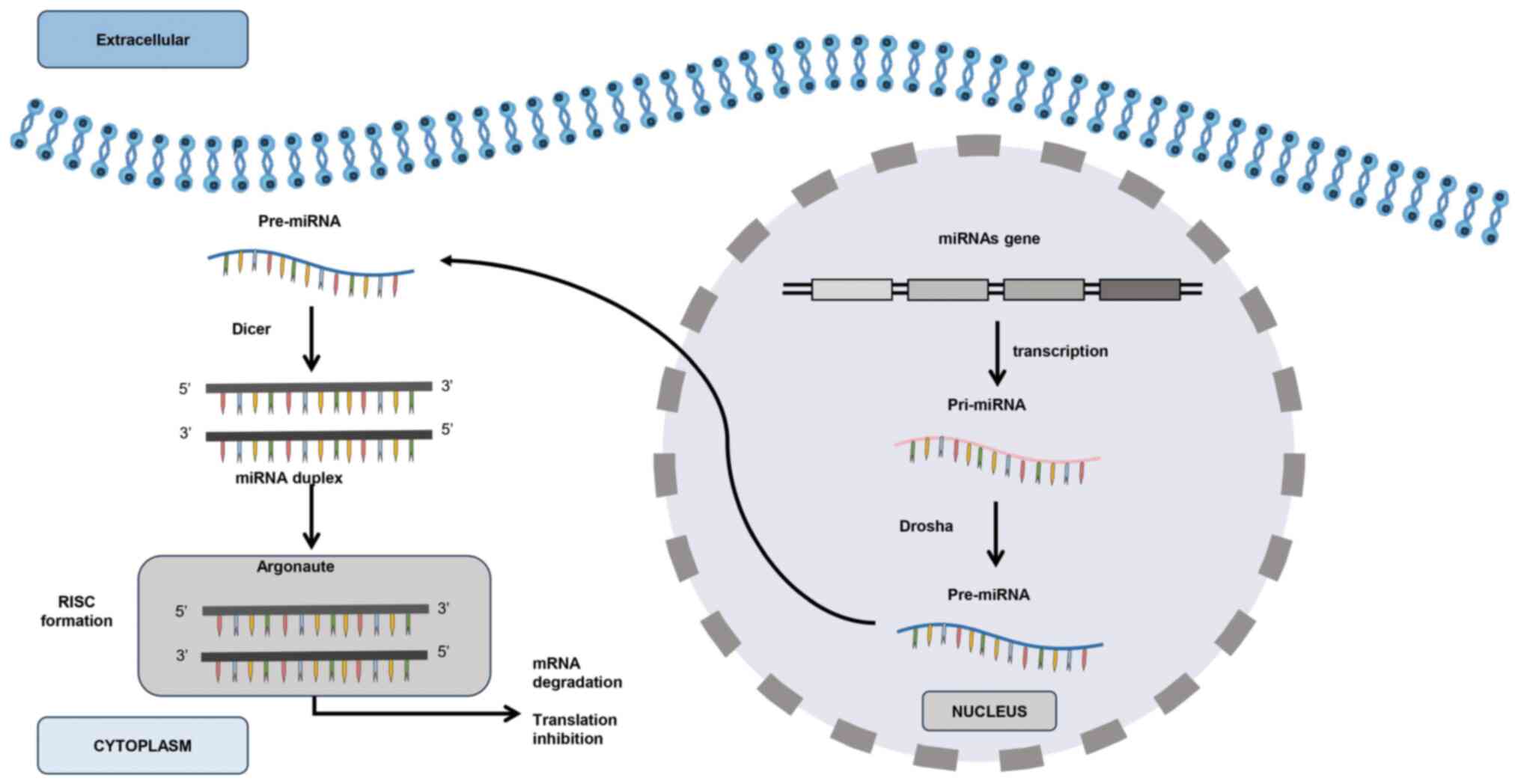
|
1
|
Yang L, Wu G, Wu Q, Peng L and Yuan L:
METTL3 overexpression aggravates LPS-induced cellular inflammation
in mouse intestinal epithelial cells and DSS-induced IBD in mice.
Cell Death Discov. 8:622022.
|
|
2
|
Patankar JV, Müller TM, Kantham S, Acera
MG, Mascia F, Scheibe K, Mahapatro M, Heichler C, Yu Y, Li W, et
al: E-type prostanoid receptor 4 drives resolution of intestinal
inflammation by blocking epithelial necroptosis. Nat Cell Biol.
23:796–807. 2021.
|
|
3
|
Krause JL, Engelmann B, Schaepe SS,
Rolle-Kampczyk U, Jehmlich N, Chang HD, Slanina U, Hoffman M,
Lehmann J, Zenclussen AC, et al: DSS treatment does not affect
murine colonic microbiota in absence of the host. Gut Microbes.
16:22978312024.
|
|
4
|
Steinwurz F, Machado MB, Veitia G De Paula
JA, Bautista Martinez S, Vergara BI, Capdevielle B, Martinez Silva
FA and Ramirez AL: Latin America consensus statement inflammatory
bowel disease: Importance of timely access to diagnosis and
treatment. Therap Adv Gastroenterol. 16:175628482312073122023.
|
|
5
|
Wang S, Dong Z and Wan X: Global,
regional, and national burden of inflammatory bowel disease and its
associated anemia, 1990 to 2019 and predictions to 2050: An
analysis of the global burden of disease study 2019. Autoimmun Rev.
23:1034982023.
|
|
6
|
Shah SC and Itzkowitz SH: Colorectal
cancer in inflammatory bowel disease: Mechanisms and management.
Gastroenterology. 162:715–730.e3. 2022.
|
|
7
|
Liu Y, Zhao C, Sun J, Wang G, Ju S, Qian C
and Wang X: Overexpression of small nucleolar RNA SNORD1C is
associated with unfavorable outcome in colorectal cancer.
Bioengineered. 12:8943–8952. 2021.
|
|
8
|
Kishore C and Karunagaran D: Non-coding
RNAs as emerging regulators and biomarkers in colorectal cancer.
Mol Cell Biochem. 477:1817–1828. 2022.
|
|
9
|
Bastet L, Korepanov AP, Jagodnik J,
Grondin JP, Lamontagne AM, Guillier M and Lafontaine DA: Riboswitch
and small RNAs modulate btuB translation initiation in Escherichia
coli and trigger distinct mRNA regulatory mechanisms. Nucleic Acids
Res. 52:5852–5865. 2024.
|
|
10
|
Xiong Q and Zhang Y: Small RNA
modifications: Regulatory molecules and potential applications. J
Hematol Oncol. 16:642023.
|
|
11
|
Kharaz YA, Zamboulis DE, Fang Y, Welting
TJM, Peffers MJ and Comerford EJ: Small RNA signatures of the
anterior cruciate ligament from patients with knee joint
osteoarthritis. Front Mol Biosci. 10:12660882023.
|
|
12
|
Soroosh A, Koutsioumpa M, Pothoulakis C
and Iliopoulos D: Functional role and therapeutic targeting of
microRNAs in inflammatory bowel disease. Am J Physiol Gastrointest
Liver Physiol. 314:G256–G262. 2018.
|
|
13
|
Huang X, Zhu X, Yu Y, Zhu W, Jin L, Zhang
X, Li S, Zou P, Xie C and Cui R: Dissecting miRNA signature in
colorectal cancer progression and metastasis. Cancer Lett.
501:66–82. 2021.
|
|
14
|
Yang X, Li Y, Li L, Liu J, Wu M and Ye M:
SnoRNAs are involved in the progression of ulcerative colitis and
colorectal cancer. Dig Liver Dis. 49:545–551. 2017.
|
|
15
|
Lan YZ, Wu Z, Chen WJ, Fang ZX, Yu XN, Wu
HT and Liu J: Small nucleolar RNA and its potential role in the
oncogenesis and development of colorectal cancer. World J
Gastroenterol. 30:115–127. 2024.
|
|
16
|
Iyer DN, Wan TM, Man JH, Sin RW, Li X, Lo
OS, Foo DC, Pang RW, Law WL and Ng L: Small RNA profiling of piRNAs
in colorectal cancer identifies consistent overexpression of
piR-24000 that correlates clinically with an aggressive disease
phenotype. Cancers (Basel). 12:1882020.
|
|
17
|
Li Y, Zhang Y, Li X, Li X, Gu X and Ju S:
Serum tRF-27-FDXX-E6XRK45 as a promising biomarker for the clinical
diagnosis in gastric cancer. Int J Med Sci. 20:1189–1201. 2023.
|
|
18
|
Dowdy SF: Endosomal escape of RNA
therapeutics: How do we solve this rate-limiting problem? RNA.
29:396–401. 2023.
|
|
19
|
Zhang Y, Belaid M, Luo X, Daci A, Limani
R, Mantaj J, Zilbauer M, Nayak K and Vllasaliu D: Probing milk
extracellular vesicles for intestinal delivery of RNA therapies. J
Nanobiotechnology. 21:4062023.
|
|
20
|
Zhang M, Xu C, Liu D, Han MK, Wang L and
Merlin D: Oral delivery of nanoparticles loaded with ginger active
compound, 6-shogaol, attenuates ulcerative colitis and promotes
wound healing in a murine model of ulcerative colitis. J Crohns
Colitis. 12:217–229. 2018.
|
|
21
|
Vaghari-Tabari M, Targhazeh N, Moein S,
Qujeq D, Alemi F, Majidina M, Younesi S, Asemi Z and Yousefi B:
From inflammatory bowel disease to colorectal cancer: What's the
role of miRNAs? Cancer Cell Int. 22:1462022.
|
|
22
|
Porter RJ, Arends MJ, Churchhouse AMD and
Din S: Inflammatory bowel disease-associated colorectal cancer:
Translational risks from mechanisms to medicines. J Crohns Colitis.
15:2131–2141. 2021.
|
|
23
|
Alotaibi AG, Li JV and Gooderham NJ:
Tumour necrosis Factor-alpha (TNF-α)-induced metastatic phenotype
in colorectal cancer epithelial cells: Mechanistic support for the
role of MicroRNA-21. Cancers (Basel). 15:6272023.
|
|
24
|
Fan W, Maoqing W, Wangyang C, Fulan H,
Dandan L, Jiaojiao R, Xinshu D, Binbin C and Yashuang Z:
Relationship between the polymorphism of tumor necrosis
factor-α-308 G>A and susceptibility to inflammatory bowel
diseases and colorectal cancer: A meta-analysis. Eur J Hum Genet.
19:432–437. 2011.
|
|
25
|
Al Obeed OA, Alkhayal KA, Al Sheikh A,
Zubaidi AM, Vaali-Mohammed MA, Boushey R, Mckerrow JH and Abdulla
MH: Increased expression of tumor necrosis factor-α is associated
with advanced colorectal cancer stages. World J Gastroenterol.
20:18390–18396. 2014.
|
|
26
|
Verna G, Liso M, Cavalcanti E, Armentano
R, Miraglia A, Monsurrò V, Chieppa M and De Santis S: Deletion of
TNF in Winnie-APCMin/+ Mice reveals its dual role in the onset and
progression of colitis-associated colorectal cancer. Int J Mol Sci.
23:151452022.
|
|
27
|
Wang S, Liu Z, Wang L and Zhang X:
NF-kappaB signaling pathway, inflammation and colorectal cancer.
Cell Mol Immunol. 6:327–334. 2009.
|
|
28
|
Gregório H, Raposo TP, Queiroga FL, Prada
J and Pires I: Investigating associations of cyclooxygenase-2
expression with angiogenesis, proliferation, macrophage and
T-lymphocyte infiltration in canine melanocytic tumours. Melanoma
Res. 26:338–347. 2016.
|
|
29
|
Agoff SN, Brentnall TA, Crispin DA, Taylor
SL, Raaka S, Haggitt RC, Reed MW, Afonina IA, Rabinovitch PS,
Stevens AC, et al: The role of cyclooxygenase 2 in ulcerative
colitis-associated neoplasia. Am J Pathol. 157:737–745. 2000.
|
|
30
|
Di Mari JF, Saada JI, Mifflin RC,
Valentich JD and Powell DW: HETEs enhance IL-1-mediated COX-2
expression via augmentation of message stability in human colonic
myofibroblasts. Am J Physiol Gastrointest Liver Physiol.
293:G719–G728. 2007.
|
|
31
|
Liu M, Xie W, Wan X and Deng T:
Clostridium butyricum modulates gut microbiota and reduces
colitis associated colon cancer in mice. Int Immunopharmacol.
88:1068622020.
|
|
32
|
Widjaja AA and Cook SA: Nonspecific
inhibition of IL6 family cytokine signalling by soluble gp130. Int
J Mol Sci. 25:13632024.
|
|
33
|
Rose-John S, Winthrop K and Calabrese L:
The role of IL-6 in host defence against infections: Immunobiology
and clinical implications. Nat Rev Rheumatol. 13:399–409. 2017.
|
|
34
|
Kishimoto T: IL-6: From its discovery to
clinical applications. Int Immunol. 22:347–352. 2010.
|
|
35
|
Wu MY, Luo YX, Jia WX, Wang DD, Sun DL,
Song J, Wang J, Niu WW and Zhang XL: miRNA-320 inhibits
colitis-associated colorectal cancer by regulating the IL-6R/STAT3
pathway in mice. J Gastrointest Oncol. 13:695–709. 2022.
|
|
36
|
Li Y, de Haar C, Chen M, Deuring J,
Gerrits MM, Smits R, Xia B, Kuipers EJ and van der Woude CJ:
Disease-related expression of the IL6/STAT3/SOCS3 signalling
pathway in ulcerative colitis and ulcerative colitis-related
carcinogenesis. Gut. 59:227–235. 2010.
|
|
37
|
Tian X, Wang S, Zhang C, Prakash YS and
Vassallo R: Blocking IL-23 signaling mitigates cigarette
smoke-induced murine emphysema. Environ Toxicol. 39:5334–5346.
2024.
|
|
38
|
Blauvelt A, Chen Y, Branigan PJ, Liu X,
DePrimo S, Keyes BE, Leung M, Fakharzadeh S, Yang YW, Muñoz-Elías
EJ, et al: Differential pharmacodynamic effects on psoriatic
biomarkers by guselkumab versus secukinumab correlate with
Long-Term efficacy: An ECLIPSE substudy. JID Innov.
4:1002972024.
|
|
39
|
Miyake Y, Tanaka K, Nagata C, Furukawa S,
Andoh A, Yokoyama T, Yoshimura N, Mori K, Ninomiya T, Yamamoto Y,
et al: Case-control study of IL23R rs76418789 polymorphism,
smoking, and ulcerative colitis in Japan. Cytokine.
183:1567432024.
|
|
40
|
Jacobse J, Pilat JM, Li J, Brown RE, Kwag
A, Buendia MA, Choksi YA, Washington MK, Williams CS, Markham NO,
et al: Distinct roles for interleukin-23 receptor signaling in
regulatory T cells in sporadic and inflammation-associated
carcinogenesis. Front Oncol. 13:12767432023.
|
|
41
|
Ljujic B, Radosavljevic G, Jovanovic I,
Pavlovic S, Zdravkovic N, Milovanovic M, Acimovic L, Knezevic M,
Bankovic D, Zdravkovic D and Arsenijevic N: Elevated serum level of
IL-23 correlates with expression of VEGF in human colorectal
carcinoma. Arch Med Res. 41:182–189. 2010.
|
|
42
|
Wu H, Wu Z, Qiu Y, Zhao F, Liao M, Zhong
Z, Chen J, Zeng Y and Liu R: Supplementing a specific synbiotic
suppressed the incidence of AOM/DSS-induced colorectal cancer in
mice. iScience. 26:1069792023.
|
|
43
|
Hughes CE and Nibbs RJB: A guide to
chemokines and their receptors. FEBSJ. 285:2944–2971. 2018.
|
|
44
|
Kłósek M, Kurek-Górecka A, Balwierz R,
Krawczyk-Łebek A, Kostrzewa-Susłow E, Bronikowska J, Jaworska D and
Czuba ZP: The effect of Methyl-derivatives of flavanone on MCP-1,
MIP-1β, RANTES, and eotaxin release by activated RAW264.7
macrophages. Molecules. 29:22392024.
|
|
45
|
Mrowicki J, Przybylowska-Sygut K, Dziki L,
Dziki L, Sygut A, Chojnacki J, Dziki A and Majsterek I: The role of
polymorphisms of genes CXCL12/CXCR4 and MIF in the risk development
IBD the Polish population. Mol Biol Rep. 41:4639–4652. 2014.
|
|
46
|
Gaines T, Garcia F, Virani S, Liang Z,
Yoon Y, Oum YH, Shim H and Mooring SR: Synthesis and evaluation of
2,5-furan, 2,5-thiophene and 3,4-thiophene-based derivatives as
CXCR4 inhibitors. Eur J Med Chem. 181:1115622019.
|
|
47
|
Ottaiano A, Franco R, Aiello Talamanca A,
Liguori G, Tatangelo F, Delrio P, Nasti G, Barletta E, Facchini G,
Daniele B, et al: Overexpression of both CXC chemokine receptor 4
and vascular endothelial growth factor proteins predicts early
distant relapse in stage II-III colorectal cancer patients. Clin
Cancer Res. 12:2795–2803. 2006.
|
|
48
|
Fellhofer-Hofer J, Franz C, Vey JA,
Kahlert C, Kalkum E, Mehrabi A, Halama N, Probst P and Klupp F:
Chemokines as prognostic factor in colorectal cancer patients: A
systematic review and meta-analysis. Int J Mol Sci.
25:53742024.
|
|
49
|
Cheng Y, Yang X, Liang L, Xin H, Dong X,
Li W, Li J, Guo X, Li Y, He J, et al: Elevated expression of CXCL3
in colon cancer promotes malignant behaviors of tumor cells in an
ERK-dependent manner. BMC Cancer. 23:11622023.
|
|
50
|
Zhang H, Shi Y, Lin C, He C, Wang S, Li Q,
Sun Y and Li M: Overcoming cancer risk in inflammatory bowel
disease: New insights into preventive strategies and pathogenesis
mechanisms including interactions of immune cells, cancer signaling
pathways, and gut microbiota. Front Immunol. 14:13389182023.
|
|
51
|
Wunderlich CM, Acker mann PJ, Oster mann
AL, Adams-Quack P, Vogt MC, Tran ML, Nikolajev A, Waisman A,
Garbers C, Theurich S, et al: Obesity exacerbates
colitis-associated cancer via IL-6-regulated macrophage
polarisation and CCL-20/CCR-6-mediated lymphocyte recruitment. Nat
Commun. 9:16462018.
|
|
52
|
Wang Y, Liu H, Zhang Z, Bian D, Shao K,
Wang S and Ding Y: G-MDSC-derived exosomes mediate the
differentiation of M-MDSC into M2 macrophages promoting
colitis-to-cancer transition. J Immunother Cancer.
11:e0061662023.
|
|
53
|
Zhang C, Zhang J, Zhang Y, Song Z, Bian J,
Yi H and Ma Z: Identifying neutrophil-associated subtypes in
ulcerative colitis and confirming neutrophils promote
colitis-associated colorectal cancer. Front Immunol.
14:10950982023.
|
|
54
|
Burrello C, Pellegrino G, Giuffrè MR,
Lovati G, Magagna I, Bertocchi A, Cribiù FM, Boggio F, Botti F,
Trombetta E, et al: Mucosa-associated microbiota drives pathogenic
functions in IBD-derived intestinal iNKT cells. Life Sci Alliance.
2:e2018002292019.
|
|
55
|
Díaz-Basabe A, Lattanzi G, Perillo F,
Amoroso C, Baeri A, Farini A, Torrente Y, Penna G, Rescigno M,
Ghidini M, et al: Porphyromonas gingivalis fuels colorectal cancer
through CHI3L1-mediated iNKT cell-driven immune evasion. Gut
Microbes. 16:23888012024.
|
|
56
|
Peng Q, Pan T, He R, Yi M, Feng L, Cui Z,
Gao R, Wang H, Feng X, Li H, et al: BTNL2 promotes
colitis-associated tumorigenesis in mice by regulating IL-22
production. EMBO Rep. 24:e560342023.
|
|
57
|
Li Y, Shi J, Liu Z, Lin Y, Xie A, Sun W,
Liu J and Liang J: Regulation of the migration of colorectal cancer
stem cells via the TLR4/MyD88 signaling pathway by the novel
surface marker CD14 following LPS stimulation. Oncol Lett.
27:602024.
|
|
58
|
Quandt J, Arnovitz S, Haghi L, Woehlk J,
Mohsin A, Okoreeh M, Mathur PS, Emmanuel AO, Osman A, Krishnan M,
et al: Wnt-β-catenin activation epigenetically reprograms T(reg)
cells in inflammatory bowel disease and dysplastic progression. Nat
Immunol. 22:471–484. 2021.
|
|
59
|
Lo Presti E, Mocciaro F, Mitri RD, Corsale
AM, Di Simone M, Vieni S, Scibetta N, Unti E, Dieli F and
Meraviglia S: Analysis of colon-infiltrating γδ T cells in chronic
inflammatory bowel disease and in colitis-associated cancer. J
Leukoc Biol. 108:749–760. 2020.
|
|
60
|
D'Este F, Della Pietra E, Badillo Pazmay
GV, Xodo LE and Rapozzi V: Role of nitric oxide in the response to
photooxidative stress in prostate cancer cells. Biochem Pharmacol.
182:1142052020.
|
|
61
|
Aslan M, Nazligul Y, Bolukbas C, Bolukbas
FF, Horoz M, Dulger AC, Erdur FM, Celik H and Kocyigit A:
Peripheral lymphocyte DNA damage and oxidative stress in patients
with ulcerative colitis. Pol Arch Med Wewn. 121:223–229. 2011.
|
|
62
|
Dincer Y, Erzin Y, Himmetoglu S, Gunes KN,
Bal K and Akcay T: Oxidative DNA damage and antioxidant activity in
patients with inflammatory bowel disease. Dig Dis Sci.
52:1636–1641. 2007.
|
|
63
|
Wang H, Wang L, Xie Z, Zhou S, Li Y, Zhou
Y and Sun M: Nitric oxide (NO) and no synthases (NOS)-Based
targeted therapy for colon cancer. Cancers (Basel).
12:18812020.
|
|
64
|
Lechner M, Lirk P and Rieder J: Inducible
nitric oxide synthase (iNOS) in tumor biology: The two sides of the
same coin. Semin Cancer Biol. 15:277–289. 2005.
|
|
65
|
Wang C, Gong G, Sheh A, Muthupalani S,
Bryant EM, Puglisi DA, Holcombe H, Conaway EA, Parry NAP,
Bakthavatchalu V, et al: Interleukin-22 drives nitric
oxide-dependent DNA damage and dysplasia in a murine model of
colitis-associated cancer. Mucosal Immunol. 10:1504–1517. 2017.
|
|
66
|
Erdman SE, Rao VP, Poutahidis T, Rogers
AB, Taylor CL, Jackson EA, Ge Z, Lee CW, Schauer DB, Wogan GN, et
al: Nitric oxide and TNF-alpha trigger colonic inflammation and
carcinogenesis in Helicobacter hepaticus-infected,
Rag2-deficient mice. Proc Natl Acad Sci USA. 106:1027–1032.
2009.
|
|
67
|
Huang Y, Jiao Z, Fu Y, Hou Y, Sun J, Hu F,
Yu S, Gong K, Liu Y and Zhao G: An overview of the functions of p53
and drugs acting either on wild- or mutant-type p53. Eur J Med
Chem. 265:1161212024.
|
|
68
|
Perri F, Pisconti S and Della Vittoria
Scarpati G: P53 mutations and cancer: A tight linkage. Ann Transl
Med. 4:5222016.
|
|
69
|
Du L, Kim JJ, Shen J, Chen B and Dai N:
KRAS and TP53 mutations in inflammatory bowel disease-associated
colorectal cancer: A meta-analysis. Oncotarget. 8:22175–22186.
2017.
|
|
70
|
Sangodkar J, Shi J, DiFeo A, Schwartz R,
Bromberg R, Choudhri A, McClinch K, Hatami R, Scheer E, Kremer-Tal
S, et al: Functional role of the KLF6 tumour suppressor gene in
gastric cancer. Eur J Cancer. 45:666–676. 2009.
|
|
71
|
Reeves HL, Narla G, Ogunbiyi O, Haq AI,
Katz A, Benzeno S, Hod E, Harpaz N, Goldberg S, Tal-Kremer S, et
al: Kruppel-like factor 6 (KLF6) is a tumor-suppressor gene
frequently inactivated in colorectal cancer. Gastroenterology.
126:1090–1103. 2004.
|
|
72
|
Cooke J, Zhang H, Greger L, Silva AL,
Massey D, Dawson C, Metz A, Ibrahim A and Parkes M: Mucosal
genome-wide methylation changes in inflammatory bowel disease.
Inflamm Bowel Dis. 18:2128–2137. 2012.
|
|
73
|
Yoshimi K, Tanaka T, Serikawa T and
Kuramoto T: Tumor suppressor APC protein is essential in mucosal
repair from colonic inflammation through angiogenesis. Am J Pathol.
182:1263–1274. 2013.
|
|
74
|
Büki G, Antal G and Bene J: Rare germline
variants in the adenomatous polyposis coli gene associated with
dental and osseous anomalies. Int J Mol Sci. 25:81892024.
|
|
75
|
Dhir M, Montgomery EA, Glöckner SC,
Schuebel KE, Hooker CM, Herman JG, Baylin SB, Gearhart SL and Ahuja
N: Epigenetic regulation of WNT signaling pathway genes in
inflammatory bowel disease (IBD) associated neoplasia. J
Gastrointest Surg. 12:1745–1753. 2008.
|
|
76
|
Papadia C, Louwagie J, Del Rio P,
Grooteclaes M, Coruzzi A, Montana C, Novelli M, Bordi C, de'
Angelis GL, Bassett P, et al: FOXE1 and SYNE1 genes
hypermethylation panel as promising biomarker in colitis-associated
colorectal neoplasia. Inflamm Bowel Dis. 20:271–277. 2014.
|
|
77
|
Kim TO, Park DI, Han YK, Kang K, Park SG,
Park HR and Yi JM: Genome-wide analysis of the DNA methylation
profile identifies the fragile histidine triad (FHIT) gene as a new
promising biomarker of Crohn's disease. J Clin Med. 9:13382020.
|
|
78
|
Bae JH, Park J, Yang KM, Kim TO and Yi JM:
Detection of DNA hypermethylation in sera of patients with Crohn's
disease. Mol Med Rep. 9:725–729. 2014.
|
|
79
|
Yi JM, Dhir M, Guzzetta AA,
Iacobuzio-Donahue CA, Heo K, Yang KM, Suzuki H, Toyota M, Kim HM
and Ahuja N: DNA methylation biomarker candidates for early
detection of colon cancer. Tumour Biol. 33:363–372. 2012.
|
|
80
|
Gasaly N, de Vos P and Hermoso MA: Impact
of bacterial metabolites on gut barrier function and host immunity:
A focus on bacterial metabolism and its relevance for intestinal
inflammation. Front Immunol. 12:6583542021.
|
|
81
|
Wang M, Ma Y, Yu G, Zeng B, Yang W, Huang
C, Dong Y, Tang B and Wu Z: Integration of microbiome, metabolomics
and transcriptome for in-depth understanding of berberine
attenuates AOM/DSS-induced colitis-associated colorectal cancer.
Biomed Pharmacother. 179:1172922024.
|
|
82
|
Uronis JM, Mühlbauer M, Herfarth HH,
Rubinas TC, Jones GS and Jobin C: Modulation of the intestinal
microbiota alters colitis-associated colorectal cancer
susceptibility. PLoS One. 4:e60262009.
|
|
83
|
Burgueño JF, Fritsch J, González EE,
Landau KS, Santander AM, Fernández I, Hazime H, Davies JM,
Santaolalla R, Phillips MC, et al: Epithelial TLR4 Signaling
Activates DUOX2 to Induce Microbiota-Driven Tumorigenesis.
Gastroenterology. 160:797–808.e6. 2021.
|
|
84
|
Fukata M, Chen A, Vamadevan AS, Cohen J,
Breglio K, Krishnareddy S, Hsu D, Xu R, Harpaz N, Dannenberg AJ, et
al: Toll-like receptor-4 promotes the development of
colitis-associated colorectal tumors. Gastroenterology.
133:1869–1881. 2007.
|
|
85
|
Viennois E, Chen F and Merlin D: NF-κB
pathway in colitis-associated cancers. Transl Gastrointest Cancer.
2:21–29. 2013.
|
|
86
|
Soleimani A, Rahmani F, Ferns GA, Ryzhikov
M, Avan A and Hassanian SM: Role of the NF-κB signaling pathway in
the pathogenesis of colorectal cancer. Gene. 726:1441322020.
|
|
87
|
Lu Y, Huang R, Ying J, Li X, Jiao T, Guo
L, Zhou H, Wang H, Tuersuntuoheti A, Liu J, et al: RING finger 138
deregulation distorts NF-кB signaling and facilities colitis Switch
to aggressive malignancy. Signal Transduct Target Ther.
7:1852022.
|
|
88
|
Li L, Liu H, Yu J, Sun Z, Jiang M, Yu H
and Wang C: Intestinal microbiota and metabolomics reveal the role
of auricularia delicate in regulating Colitis-associated colorectal
cancer. Nutrients. 15:50112023.
|
|
89
|
Kojima M, Morisaki T, Sasaki N, Nakano K,
Mibu R, Tanaka M and Katano M: Increased nuclear factor-kB
activation in human colorectal carcinoma and its correlation with
tumor progression. Anticancer Res. 24:675–681. 2004.
|
|
90
|
Onizawa M, Nagaishi T, Kanai T, Nagano K,
Oshima S, Nemoto Y, Yoshioka A, Totsuka T, Okamoto R, Nakamura T,
et al: Signaling pathway via TNF-alpha/NF-kappaB in intestinal
epithelial cells may be directly involved in colitis-associated
carcinogenesis. Am J Physiol Gastrointest Liver Physiol.
296:G850–G859. 2009.
|
|
91
|
Massacesi C, Di Tomaso E, Urban P, Germa
C, Quadt C, Trandafir L, Aimone P, Fretault N, Dharan B, Tavorath R
and Hirawat S: PI3K inhibitors as new cancer therapeutics:
Implications for clinical trial design. Onco Targets Ther.
9:203–210. 2016.
|
|
92
|
Alzahrani AS: PI3K/Akt/mTOR inhibitors in
cancer: At the bench and bedside. Semin Cancer Biol. 59:125–132.
2019.
|
|
93
|
Wang D, Zhu L, Liu H, Feng X, Zhang C, Li
T, Liu B, Liu L, Sun J, Chang H, et al: Huangqin tang alleviates
colitis-associated colorectal cancer via amino acids homeostasisand
PI3K/AKT/mtor pathway modulation. J Ethnopharmacol.
334:1185972024.
|
|
94
|
Lu ZH, Ding Y, Wang YJ, Chen C, Yao XR,
Yuan XM, Bu F, Bao H, Dong YW, Zhou Q, et al: Early administration
of Wumei wan inhibit myeloid-derived suppressor cells via PI3K/Akt
pathway and amino acids metabolism to prevent colitis-associated
colorectal cancer. J Ethnopharmacol. 333:1182602024.
|
|
95
|
Aigner P, Just V and Stoiber D: STAT3
isoforms: Alternative fates in cancer? Cytokine. 118:27–34.
2019.
|
|
96
|
Liu LQ, Nie SP, Shen MY, Hu JL, Yu Q, Gong
D and Xie MY: Tea polysaccharides inhibit colitis-associated
colorectal cancer via interleukin-6/STAT3 pathway. J Agric Food
Chem. 66:4384–4393. 2018.
|
|
97
|
Saadatdoust Z, Pandurangan AK, Ananda
Sadagopan SK, Mohd Esa N, Ismail A and Mustafa MR: Dietary cocoa
inhibits colitis associated cancer: A crucial involvement of the
IL-6/STAT3 pathway. J Nutr Biochem. 26:1547–1558. 2015.
|
|
98
|
Andersen PR, Tirian L, Vunjak M and
Brennecke J: A heterochromatin-dependent transcription machinery
drives piRNA expression. Nature. 549:54–59. 2017.
|
|
99
|
Ipsaro JJ, Haase AD, Knott SR, Joshua-Tor
L and Hannon GJ: The structural biochemistry of Zucchini implicates
it as a nuclease in piRNA biogenesis. Nature. 491:279–283.
2012.
|
|
100
|
Mohn F, Handler D and Brennecke J:
Noncoding RNA. piRNA-guided slicing specifies transcripts for
Zucchini-dependent, phased piRNA biogenesis. Science. 348:812–817.
2015.
|
|
101
|
Hayashi R, Schnabl J, Handler D, Mohn F,
Ameres SL and Brennecke J: Genetic and mechanistic diversity of
piRNA 3′-end formation. Nature. 539:588–592. 2016.
|
|
102
|
Vourekas A, Zheng K, Fu Q, Maragkakis M,
Alexiou P, Ma J, Pillai RS, Mourelatos Z and Wang PJ: The RNA
helicase MOV10L1 binds piRNA precursors to initiate piRNA
processing. Genes Dev. 29:617–629. 2015.
|
|
103
|
Kawaoka S, Hayashi N, Suzuki Y, Abe H,
Sugano S, Tomari Y, Shimada T and Katsuma S: The Bombyx
ovary-derived cell line endogenously expresses
PIWI/PIWI-interacting RNA complexes. RNA. 15:1258–1264. 2009.
|
|
104
|
Murota Y, Ishizu H, Nakagawa S, Iwasaki
YW, Shibata S, Kamatani MK, Saito K, Okano H, Siomi H and Siomi MC:
Yb integrates piRNA intermediates and processing factors into
perinuclear bodies to enhance piRISC assembly. Cell Rep. 8:103–113.
2014.
|
|
105
|
Izumi N, Shoji K, Suzuki Y, Katsuma S and
Tomari Y: Zucchini consensus motifs determine the mechanism of
pre-piRNA production. Nature. 578:311–316. 2020.
|
|
106
|
Izumi N, Shoji K, Sakaguchi Y, Honda S,
Kirino Y, Suzuki T, Katsuma S and Tomari Y: Identification and
functional analysis of the Pre-piRNA 3′trimmer in silkworms. Cell.
164:962–973. 2016.
|
|
107
|
Kamminga LM, Luteijn MJ, den Broeder MJ,
Redl S, Kaaij LJ, Roovers EF, Ladurner P, Berezikov E and Ketting
RF: Hen1 is required for oocyte development and piRNA stability in
zebrafish. EMBO J. 29:3688–3700. 2010.
|
|
108
|
Brown JW, Clark GP, Leader DJ, Simpson CG
and Lowe T: Multiple snoRNA gene clusters from Arabidopsis. RNA.
7:1817–1832. 2001.
|
|
109
|
Tycowski KT and Steitz JA: Non-coding
snoRNA host genes in drosophila: Expression strategies for
modification guide snoRNAs. Eur J Cell Biol. 80:119–125. 2001.
|
|
110
|
Deryusheva S and Gall JG: scaRNAs and
snoRNAs: Are they limited to specific classes of substrate RNAs?
RNA. 25:17–22. 2019.
|
|
111
|
Stepanov GA, Filippova JA, Komissarov AB,
Kuligina EV, Richter VA and Semenov DV: Regulatory role of small
nucleolar RNAs in human diseases. Biomed Res Int.
2015:2068492015.
|
|
112
|
O'Brien J, Hayder H, Zayed Y and Peng C:
Overview of MicroRNA biogenesis, mechanisms of actions, and
circulation. Front Endocrinol (Lausanne). 9:4022018.
|
|
113
|
Denli AM, Tops BB, Plasterk RH, Ketting RF
and Hannon GJ: Processing of primary microRNAs by the
Microprocessor complex. Nature. 432:231–235. 2004.
|
|
114
|
Han J, Lee Y, Yeom KH, Kim YK, Jin H and
Kim VN: The Drosha-DGCR8 complex in primary microRNA processing.
Genes Dev. 18:3016–3027. 2004.
|
|
115
|
Medley JC, Panzade G and Zinovyeva AY:
microRNA strand selection: Unwinding the rules. Wiley Interdiscip
Rev RNA. 12:e16272021.
|
|
116
|
Yoda M, Kawamata T, Paroo Z, Ye X, Iwasaki
S, Liu Q and Tomari Y: ATP-dependent human RISC assembly pathways.
Nat Struct Mol Biol. 17:17–23. 2010.
|
|
117
|
Frédérick PM and Simard MJ: Regulation and
different functions of the animal microRNA-induced silencing
complex. Wiley Interdiscip Rev RNA. 13:e17012022.
|
|
118
|
Tu M, Zuo Z, Chen C, Zhang X, Wang S, Chen
C and Sun Y: Transfer RNA-derived small RNAs (tsRNAs) sequencing
revealed a differential expression landscape of tsRNAs between
glioblastoma and low-grade glioma. Gene. 855:1471142023.
|
|
119
|
Chu X, He C, Sang B, Yang C, Yin C, Ji M,
Qian A and Tian Y: Transfer RNAs-derived small RNAs and their
application potential in multiple diseases. Front Cell Dev Biol.
10:9544312022.
|
|
120
|
Shen Y, Yu X, Zhu L, Li T, Yan Z and Guo
J: Transfer RNA-derived fragments and tRNA halves: Biogenesis,
biological functions and their roles in diseases. J Mol Med (Berl).
96:1167–1176. 2018.
|
|
121
|
Jiang X, Liu B, Nie Z, Duan L, Xiong Q,
Jin Z, Yang C and Chen Y: The role of m6A modification in the
biological functions and diseases. Signal Transduct Target Ther.
6:742021.
|
|
122
|
Alarcón CR, Lee H, Goodarzi H, Halberg N
and Tavazoie SF: N6-methyladenosine marks primary microRNAs for
processing. Nature. 519:482–485. 2015.
|
|
123
|
Liu J, Yue Y, Han D, Wang X, Fu Y, Zhang
L, Jia G, Yu M, Lu Z, Deng X, et al: A METTL3-METTL14 complex
mediates mammalian nuclear RNA N6-adenosine methylation. Nat Chem
Biol. 10:93–95. 2014.
|
|
124
|
Schöller E, Weichmann F, Treiber T, Ringle
S, Treiber N, Flatley A, Feederle R, Bruckmann A and Meister G:
Interactions, localization, and phosphorylation of the
m6A generating METTL3-METTL14-WTAP complex. RNA.
24:499–512. 2018.
|
|
125
|
Patil DP, Chen CK, Pickering BF, Chow A,
Jackson C, Guttman M and Jaffrey SR: m(6)A RNA methylation promotes
XIST-mediated transcriptional repression. Nature. 537:369–373.
2016.
|
|
126
|
Su R, Dong L, Li Y, Gao M, He PC, Liu W,
Wei J, Zhao Z, Gao L, Han L, et al: METTL16 exerts an
m6A-independent function to facilitate translation and
tumorigenesis. Nat Cell Biol. 24:205–216. 2022.
|
|
127
|
Knuckles P, Lence T, Haussmann IU, Jacob
D, Kreim N, Carl SH, Masiello I, Hares T, Villaseñor R, Hess D, et
al: Zc3h13/Flacc is required for adenosine methylation by bridging
the mRNA-binding factor Rbm15/Spenito to the m6A
machinery component Wtap/Fl(2)d. Genes Dev. 32:415–429. 2018.
|
|
128
|
Zhang X, Li MJ, Xia L and Zhang H: The
biological function of m6A methyltransferase KIAA1429 and its role
in human disease. PeerJ. 10:e143342022.
|
|
129
|
Bawankar P, Lence T, Paolantoni C,
Haussmann IU, Kazlauskiene M, Jacob D, Heidelberger JB, Richter FM,
Nallasivan MP, Morin V, et al: Hakai is required for stabilization
of core components of the m6A mRNA methylation machinery. Nat
Commun. 12:37782021.
|
|
130
|
Pinto R, Vågbø CB, Jakobsson ME, Kim Y,
Baltissen MP, O'Donohue MF, Guzmán UH, Małecki JM, Wu J, Kirpekar
F, et al: The human methyltransferase ZCCHC4 catalyses
N6-methyladenosine modification of 28S ribosomal RNA. Nucleic Acids
Res. 48:830–846. 2020.
|
|
131
|
Shen D, Wang B, Gao Y, Zhao L, Bi Y, Zhang
J, Wang N, Kang H, Pang J, Liu Y, et al: Detailed resume of RNA
m6A demethylases. Acta Pharm Sin B. 12:2193–2205.
2022.
|
|
132
|
Xu C, Liu K, Tempel W, Demetriades M, Aik
W, Schofield CJ and Min J: Structures of human ALKBH5 demethylase
reveal a unique binding mode for specific single-stranded
N6-methyladenosine RNA demethylation. J Biol Chem. 289:17299–17311.
2014.
|
|
133
|
Shishodia S, Demetriades M, Zhang D, Tam
NY, Maheswaran P, Clunie-O'Connor C, Tumber A, Leung IKH, Ng YM,
Leissing TM, et al: Structure-based design of selective fat mass
and obesity associated protein (FTO) inhibitors. J Med Chem.
64:16609–16625. 2021.
|
|
134
|
Zhou L, Zhang L, Lv Y, Qian J, Huang L and
Qu C: YTHDC1 inhibits autophagy-dependent NF-κB signaling by
stabilizing Beclin1 mRNA in macrophages. J Inflamm (Lond).
21:222024.
|
|
135
|
Alarcón CR, Goodarzi H, Lee H, Liu X,
Tavazoie S and Tavazoie SF: HNRNPA2B1 Is a mediator of
m(6)A-dependent nuclear RNA processing events. Cell. 162:1299–1308.
2015.
|
|
136
|
Zaccara S and Jaffrey SR: A Unified model
for the function of YTHDF proteins in regulating m6A-Modified mRNA.
Cell. 181:1582–1595.e18. 2020.
|
|
137
|
Elcheva IA, Gowda CP, Bogush D,
Gornostaeva S, Fakhardo A, Sheth N, Kokolus KM, Sharma A, Dovat S,
Uzun, et al: IGF2BP family of RNA-binding proteins regulate innate
and adaptive immune responses in cancer cells and tumor
microenvironment. Front Immunol. 14:12245162023.
|
|
138
|
Zhang Y, Wan X, Yang X, Liu X, Huang Q,
Zhou L, Zhang S, Liu S, Xiong Q, Wei M, et al: eIF3i promotes
colorectal cancer cell survival via augmenting PHGDH translation. J
Biol Chem. 299:1051772023.
|
|
139
|
Li Y, Yi Y, Gao X, Wang X, Zhao D, Wang R,
Zhang LS, Gao B, Zhang Y, Zhang L, et al: 2′-O-methylation at
internal sites on mRNA promotes mRNA stability. Mol Cell.
84:2320–2336.e26. 2024.
|
|
140
|
Zhou KI, Pecot CV and Holley CL:
2′-O-methylation (Nm) in RNA: Progress, challenges, and future
directions. RNA. 30:570–582. 2024.
|
|
141
|
Khoshnevis S, Dreggors-Walker RE, Marchand
V, Motorin Y and Ghalei H: Ribosomal RNA 2′-O-methylations regulate
translation by impacting ribosome dynamics. Proc Natl Acad Sci USA.
119:e21173341192022.
|
|
142
|
Li J, Yang Z, Yu B, Liu J and Chen X:
Methylation protects miRNAs and siRNAs from a 3′-end uridylation
activity in Arabidopsis. Curr Biol. 15:1501–1507. 2005.
|
|
143
|
Hajieghrari B and Niazi A: Phylogenetic
and evolutionary analysis of plant small RNA 2′-O-Methyltransferase
(HEN1) protein family. J Mol Evol. 91:424–440. 2023.
|
|
144
|
Saito K, Sakaguchi Y, Suzuki T, Suzuki T,
Siomi H and Siomi MC: Pimet, the Drosophila homolog of HEN1,
mediates 2′-O-methylation of Piwi-interacting RNAs at their 3′ends.
Genes Dev. 21:1603–1608. 2007.
|
|
145
|
Sklias A, Cruciani S, Marchand V,
Spagnuolo M, Lavergne G, Bourguignon V, Brambilla A, Dreos R,
Marygold SJ, Novoa EM, et al: Comprehensive map of ribosomal
2′-O-methylation and C/D box snoRNAs in Drosophila melanogaster.
Nucleic Acids Res. 52:2848–2864. 2024.
|
|
146
|
Kassab MA, Chen Y, Wang X, He B, Brown EJ
and Yu X: RNA 2′-O-methylation promotes persistent R-loop formation
and AID-mediated IgH class switch recombination. BMC Biol.
22:1512024.
|
|
147
|
Zhang T, Zhao F, Li J, Sun X, Zhang X,
Wang H, Fan P, Lai L, Li Z and Sui T: Programmable RNA
5-methylcytosine (m5C) modification of cellular RNAs by dCasRx
conjugated methyltransferase and demethylase. Nucleic Acids Res.
52:2776–2791. 2024.
|
|
148
|
Zhao Y, Xing C and Peng H: ALYREF (Aly/REF
export factor): A potential biomarker for predicting cancer
occurrence and therapeutic efficacy. Life Sci. 338:1223722024.
|
|
149
|
Wu P, Gao J, Lan G and Wang Y: The role of
RNA m5C modification in central nervous system diseases. Discov
Med. 36:1555–1571. 2024.
|
|
150
|
Van Haute L, Lee SY, McCann BJ, Powell CA,
Bansal D, Vasiliauskaitė L, Garone C, Shin S, Kim JS, Frye M, et
al: NSUN2 introduces 5-methylcytosines in mammalian mitochondrial
tRNAs. Nucleic Acids Res. 47:8720–8733. 2019.
|
|
151
|
Yang X, Yang Y, Sun BF, Chen YS, Xu JW,
Lai WY, Li A, Wang X, Bhattarai DP, Xiao W, et al: 5-methylcytosine
promotes mRNA export-NSUN2 as the methyltransferase and ALYREF as
an m5C reader. Cell Res. 27:606–625. 2017.
|
|
152
|
Deng M, Chen B, Liu Z, Wan Y, Li D, Yang Y
and Wang F: YBX1 mediates alternative splicing and maternal mRNA
decay during pre-implantation development. Cell Biosci.
12:122022.
|
|
153
|
He C, Bozler J, Janssen KA, Wilusz JE,
Garcia BA, Schorn AJ and Bonasio R: TET2 chemically modifies tRNAs
and regulates tRNA fragment levels. Nat Struct Mol Biol. 28:62–70.
2021.
|
|
154
|
Mao XL, Li ZH, Huang MH, Wang JT, Zhou JB,
Li QR, Xu H, Wang XJ and Zhou XL: Mutually exclusive substrate
selection strategy by human m3C RNA transferases METTL2A and
METTL6. Nucleic Acids Res. 49:8309–8323. 2021.
|
|
155
|
Li S, Zhou H, Liao S, Wang X, Zhu Z, Zhang
J and Xu C: Structural basis for METTL6-mediated m3C RNA
methylation. Biochem Biophys Res Commun. 589:159–164. 2022.
|
|
156
|
Ignatova VV, Kaiser S, Ho JSY, Bing X,
Stolz P, Tan YX, Lee CL, Gay FPH, Lastres PR, Gerlini R, et al:
METTL6 is a tRNA m3C methyltransferase that regulates
pluripotency and tumor cell growth. Sci Adv. 6:eaaz45512020.
|
|
157
|
Xu L, Liu X, Sheng N, Oo KS, Liang J,
Chionh YH, Xu J, Ye F, Gao YG, Dedon PC and Fu XY: Three distinct
3-methylcytidine (m3C) methyltransferases modify tRNA
and mRNA in mice and humans. J Biol Chem. 292:14695–14703.
2017.
|
|
158
|
Su A, Song R and Wong JJ: Pan-Cancer
analysis links altered RNA m7G methyltransferase
expression to oncogenic pathways, immune cell infiltrations and
overall survival. Cancer Rep (Hoboken). 7:e21382024.
|
|
159
|
Han M, Huang Q, Li X, Chen X, Zhu H, Pan Y
and Zhang B: M7G-related tumor immunity: novel insights of RNA
modification and potential therapeutic targets. Int J Biol Sci.
20:1238–1255. 2024.
|
|
160
|
Orellana EA, Liu Q, Yankova E, Pirouz M,
De Braekeleer E, Zhang W, Lim J, Aspris D, Sendinc E, Garyfallos
DA, et al: METTL1-mediated m7G modification of Arg-TCT
tRNA drives oncogenic transformation. Mol Cell. 81:3323–3338.e14.
2021.
|
|
161
|
Zhang X, Zhu WY, Shen SY, Shen JH and Chen
XD: Biological roles of RNA m7G modification and its implications
in cancer. Biol Direct. 18:582023.
|
|
162
|
Kataoka N: The nuclear Cap-binding
complex, a multi-tasking binding partner of RNA polymerase II
transcripts. J Biochem. 175:9–15. 2023.
|
|
163
|
Fu Y, Jiang F, Zhang X, Pan Y, Xu R, Liang
X, Wu X, Li X, Lin K, Shi R, et al: Perturbation of METTL1-mediated
tRNA N7-methylguanosine modification induces senescence
and aging. Nat Commun. 15:57132024.
|
|
164
|
Xiong W, Zhao Y, Wei Z, Zhao R, Ge J and
Shi B: N1-methyladenosine formation, gene regulation, biological
functions, and clinical relevance. Mol Ther. 31:308–330. 2023.
|
|
165
|
Li J, Zhang H and Wang H:
N1-methyladenosine modification in cancer biology: Current status
and future perspectives. Comput Struct Biotechnol J. 20:6578–6585.
2022.
|
|
166
|
Fukuda H, Chujo T, Wei FY, Shi SL,
Hirayama M, Kaitsuka T, Yamamoto T, Oshiumi H and Tomizawa K:
Cooperative methylation of human tRNA3Lys at positions A58 and U54
drives the early and late steps of HIV-1 replication. Nucleic Acids
Res. 49:11855–11867. 2021.
|
|
167
|
Dai X, Wang T, Gonzalez G and Wang Y:
Identification of YTH Domain-containing proteins as the readers for
N1-Methyladenosine in RNA. Anal Chem. 90:6380–6384. 2018.
|
|
168
|
Seo KW and Kleiner RE: YTHDF2 recognition
of N1-Methyladenosine (m1A)-modified RNA is
associated with transcript destabilization. ACS Chem Biol.
15:132–139. 2020.
|
|
169
|
Schaening-Burgos C, LeBlanc H, Fagre C, Li
GW and Gilbert WV: RluA is the major mRNA pseudouridine synthase in
Escherichia coli. PLoS Genet. 20:e10111002024.
|
|
170
|
Zhao Y, Ma X, Ye C, Li W, Pajdzik K, Dai
Q, Sun HL and He C: Pseudouridine detection and quantification
using bisulfite incorporation hindered ligation. ACS Chem Biol.
19:1813–1819. 2024.
|
|
171
|
Pichot F, Marchand V, Helm M and Motorin
Y: Data analysis pipeline for detection and quantification of
pseudouridine (ψ) in RNA by HydraPsiSeq. Methods Mol Biol.
2624:207–223. 2023.
|
|
172
|
Li Y, Wu S and Ye K: Landscape of RNA
pseudouridylation in archaeon Sulfolobus islandicus. Nucleic Acids
Res. 52:4644–4658. 2024.
|
|
173
|
Chang Y, Jin H, Cui Y, Yang F, Chen K,
Kuang W, Huo C, Xu Z, Li Y, Lin A, et al: PUS7-dependent
pseudouridylation of ALKBH3 mRNA inhibits gastric cancer
progression. Clin Transl Med. 14:e18112024.
|
|
174
|
Guzzi N, Cieśla M, Ngoc PCT, Lang S, Arora
S, Dimitriou M, Pimková K, Sommarin MNE, Munita R, Lubas M, et al:
Pseudouridylation of tRNA-derived fragments steers translational
control in stem cells. Cell. 173:1204–1216.e26. 2018.
|
|
175
|
Ding J, Bansal M, Cao Y, Ye B, Mao R,
Gupta A, Sudarshan S and Ding HF: MYC Drives mRNA pseudouridylation
to mitigate proliferation-induced cellular stress during cancer
development. Cancer Res. 84:4031–4048. 2024.
|
|
176
|
Rayford KJ, Cooley A, Rumph JT, Arun A,
Rachakonda G, Villalta F, Lima MF, Pratap S, Misra S and Nde PN:
piRNAs as modulators of disease pathogenesis. Int J Mol Sci.
22:23732021.
|
|
177
|
Yao Y, Li Y, Zhu X, Zhao C, Yang L, Huang
X and Wang L: The emerging role of the piRNA/PIWI complex in
respiratory tract diseases. Respir Res. 24:762023.
|
|
178
|
Nandi S, Chandramohan D, Fioriti L,
Melnick AM, Hébert JM, Mason CE, Rajasethupathy P and Kandel ER:
Roles for small noncoding RNAs in silencing of retrotransposons in
the mammalian brain. Proc Natl Acad Sci USA. 113:12697–12702.
2016.
|
|
179
|
Wu D, Fu H, Zhou H, Su J, Zhang F and Shen
J: Effects of novel ncRNA molecules, p15-piRNAs, on the methylation
of DNA and histone H3 of the CDKN2B promoter region in U937 Cells.
J Cell Biochem. 116:2744–2754. 2015.
|
|
180
|
Ding X, Li Y, Lü J, Zhao Q, Guo Y, Lu Z,
Ma W, Liu P, Pestell RG, Liang C and Yu Z: piRNA-823 is involved in
cancer stem cell regulation through altering DNA methylation in
association with luminal breast cancer. Front Cell Dev Biol.
9:6410522021.
|
|
181
|
Lee SA, Liu F, Yuwono C, Phan M, Chong S,
Biazik J, Tay ACY, Janitz M, Riordan SM, Lan R, et al: Emerging
Aeromonas enteric infections: Their association with inflammatory
bowel disease and novel pathogenic mechanisms. Microbiol Spectr.
11:e01088232023.
|
|
182
|
Huang X, Wang C, Zhang T, Li R, Chen L,
Leung KL, Lakso M, Zhou Q, Zhang H and Wong G: PIWI-interacting RNA
expression regulates pathogenesis in a Caenorhabditis elegans model
of Lewy body disease. Nat Commun. 14:61372023.
|
|
183
|
Xie L, Zhao Z, Xia H, Su S, He L, Huang Z,
Li Y, Gao M, Chen J, Peng J and Ruan Y: A novel tsRNA-5008a
promotes ferroptosis in cardiomyocytes that causes atrial
structural remodeling predisposed to atrial fibrillation. Exp Cell
Res. 435:1139232024.
|
|
184
|
Chen X, Li QH, Xie BM, Ji YM, Han Y and
Zhao Y: SNORA73B promotes endometrial cancer progression through
targeting MIB1 and regulating host gene RCC1 alternative splicing.
J Cell Mol Med. 27:2890–2905. 2023.
|
|
185
|
Li J, Niu C, Ai H, Li X, Zhang L, Lang Y,
Wang S, Gao F, Mei X, Yu C, et al: TSP50 attenuates DSS-induced
colitis by regulating TGF-β signaling mediated maintenance of
intestinal mucosal barrier integrity. Adv Sci (Weinh).
11:e23058932024.
|
|
186
|
Chelakkot C, Ghim J and Ryu SH: Mechanisms
regulating intestinal barrier integrity and its pathological
implications. Exp Mol Med. 50:1–9. 2018.
|
|
187
|
Zhao Y, Ma T, Chen W, Chen Y, Li M, Ren L,
Chen J, Cao R, Feng Y, Zhang H and Shi R: MicroRNA-124 promotes
intestinal inflammation by targeting aryl hydrocarbon receptor in
Crohn's disease. J Crohns Colitis. 10:703–712. 2016.
|
|
188
|
Guz M, Dworzański T, Jeleniewicz W,
Cybulski M, Kozicka J, Stepulak A and Celiński K: Elevated miRNA
inversely correlates with E-cadherin gene expression in tissue
biopsies from crohn disease patients in contrast to ulcerative
colitis patients. Biomed Res Int. 2020:42503292020.
|
|
189
|
Daulagala AC, Bridges MC and Kourtidis A:
E-cadherin beyond structure: A signaling hub in colon homeostasis
and disease. Int J Mol Sci. 20:27562019.
|
|
190
|
He C, Yu T, Shi Y, Ma C, Yang W, Fang L,
Sun M, Wu W, Xiao F, Guo F, et al: MicroRNA 301A promotes
intestinal inflammation and Colitis-Associated cancer development
by inhibiting BTG1. Gastroenterology. 152:1434–1448.e15. 2017.
|
|
191
|
Zou T, Jaladanki SK, Liu L, Xiao L, Chung
HK and Wang JY, Xu Y, Gorospe M and Wang JY: H19 long noncoding RNA
regulates intestinal epithelial barrier function via MicroRNA 675
by interacting with RNA-Binding protein HuR. Mol Cell Biol.
36:1332–1341. 2016.
|
|
192
|
Zhao X, Cui DJ, Yang LC, Yuan WQ and Yan
F: Long Noncoding RNA FBXL19-AS1-mediated ulcerative
colitis-associated intestinal epithelial barrier defect. Tissue Eng
Regen Med. 19:1077–1088. 2022.
|
|
193
|
Ma D, Cao Y, Wang Z, He J, Chen H, Xiong
H, Ren L, Shen C, Zhang X, Yan Y, et al: CCAT1 lncRNA promotes
inflammatory bowel disease malignancy by destroying intestinal
barrier via downregulating miR-185-3p. Inflamm Bowel Dis.
25:862–874. 2019.
|
|
194
|
Shen Y, Zhou M, Yan J, Gong Z, Xiao Y,
Zhang C, Du P and Chen Y: miR-200b inhibits TNF-α-induced IL-8
secretion and tight junction disruption of intestinal epithelial
cells in vitro. Am J Physiol Gastrointest Liver Physiol.
312:G123–G132. 2017.
|
|
195
|
Tian Y, Xu J, Li Y, Zhao R, Du S, Lv C, Wu
W, Liu R, Sheng X, Song Y, et al: MicroRNA-31 reduces inflammatory
signaling and promotes regeneration in colon epithelium, and
delivery of mimics in microspheres reduces colitis in mice.
Gastroenterology. 156:2281–2296.e6. 2019.
|
|
196
|
Chu XQ, Wang J, Chen GX, Zhang GQ, Zhang
DY and Cai YY: Overexpression of microRNA-495 improves the
intestinal mucosal barrier function by targeting STAT3 via
inhibition of the JAK/STAT3 signaling pathway in a mouse model of
ulcerative colitis. Pathol Res Pract. 214:151–162. 2018.
|
|
197
|
Chen T, Xue H, Lin R and Huang Z: MiR-34c
and PlncRNA1 mediated the function of intestinal epithelial barrier
by regulating tight junction proteins in inflammatory bowel
disease. Biochem Biophys Res Commun. 486:6–13. 2017.
|
|
198
|
Zhao L, Wang P, Liu Y, Ma J and Xue Y:
miR-34c regulates the permeability of blood-tumor barrier via
MAZ-mediated expression changes of ZO-1, occludin, and claudin-5. J
Cell Physiol. 230:716–731. 2015.
|
|
199
|
Aggeletopoulou I, Mouzaki A, Thomopoulos K
and Triantos C: miRNA Molecules-late breaking treatment for
inflammatory bowel diseases? Int J Mol Sci. 24:22332023.
|
|
200
|
Liu C, Yu C, Song G, Fan X, Peng S, Zhang
S, Zhou X, Zhang C, Geng X, Wang T, et al: Comprehensive analysis
of miRNA-mRNA regulatory pairs associated with colorectal cancer
and the role in tumor immunity. BMC Genomics. 24:7242023.
|
|
201
|
Raisch J, Darfeuille-Michaud A and Nguyen
HT: Role of microRNAs in the immune system, inflammation and
cancer. World J Gastroenterol. 19:2985–2996. 2013.
|
|
202
|
Kim HY, Kwon HY, Ha Thi HT, Lee HJ, Kim
GI, Hahm KB and Hong S: MicroRNA-132 and microRNA-223 control
positive feedback circuit by regulating FOXO3a in inflammatory
bowel disease. J Gastroenterol Hepatol. 31:1727–1735. 2016.
|
|
203
|
Zhao X, Li J, Ma J, Jiao C, Qiu X, Cui X,
Wang D and Zhang H: MiR-124a mediates the impairment of intestinal
epithelial integrity by targeting aryl hydrocarbon receptor in
Crohn's disease. Inflammation. 43:1862–1875. 2020.
|
|
204
|
Huang Z, Shi T, Zhou Q, Shi S, Zhao R, Shi
H, Dong L, Zhang C, Zeng K, Chen J and Zhang J: miR-141 Regulates
colonic leukocytic trafficking by targeting CXCL12β during murine
colitis and human Crohn's disease. Gut. 63:1247–1257. 2014.
|
|
205
|
Peng Y, Wang Q, Yang W, Yang Q, Pei Y and
Zhang W: MiR-98-5p expression inhibits polarization of macrophages
to an M2 phenotype by targeting Trib1 in inflammatory bowel
disease. Acta Biochim Pol. 67:157–163. 2020.
|
|
206
|
Qiao C, Yang L, Wan J, Liu X, Pang C, You
W and Zhao G: Long noncoding RNA ANRIL contributes to the
development of ulcerative colitis by miR-323b-5p/TLR4/MyD88/NF-κB
pathway. Biochem Biophys Res Commun. 508:217–224. 2019.
|
|
207
|
Liu T, Zhang L, Joo D and Sun SC: NF-κB
signaling in inflammation. Signal Transduct Target Ther.
2:170232017.
|
|
208
|
Wu W, He C, Liu C, Cao AT, Xue X,
Evans-Marin HL, Sun M, Fang L, Yao S, Pinchuk IV, et al: miR-10a
inhibits dendritic cell activation and Th1/Th17 cell immune
responses in IBD. Gut. 64:1755–1764. 2015.
|
|
209
|
Lin Z, Xie X, Gu M, Chen Q, Lu G, Jia X,
Xiao W, Zhang J, Yu D and Gong W: microRNA-144/451 decreases
dendritic cell bioactivity via targeting interferon-regulatory
factor 5 to limit DSS-induced colitis. Front Immunol.
13:9285932022.
|
|
210
|
Cheng X, Zhang X, Su J, Zhang Y, Zhou W,
Zhou J, Wang C, Liang H, Chen X, Shi R, et al: miR-19b
downregulates intestinal SOCS3 to reduce intestinal inflammation in
Crohn's disease. Sci Rep. 5:103972015.
|
|
211
|
Fukata T, Mizushima T, Nishimura J,
Okuzaki D, Wu X, Hirose H, Yokoyama Y, Kubota Y, Nagata K,
Tsujimura N, et al: The supercarbonate apatite-microRNA complex
inhibits dextran sodium sulfate-induced colitis. Mol Ther Nucleic
Acids. 12:658–671. 2018.
|
|
212
|
Wang Y, Wang N, Cui L, Li Y, Cao Z, Wu X,
Wang Q, Zhang B, Ma C and Cheng Y: Long Non-coding RNA MEG3
alleviated ulcerative colitis through upregulating
miR-98-5p-sponged IL-10. Inflammation. 44:1049–1059. 2021.
|
|
213
|
Hu D, Wang L, Chen X, Lin Y, Zhang S, Fan
Z and Peng F: Impact of PIWIL1 single nucleotide polymorphisms on
gastric cancer risk in a Chinese population. Genet Test Mol
Biomarkers. 27:185–192. 2023.
|
|
214
|
Lin X, Xia Y, Hu D, Mao Q, Yu Z, Zhang H,
Li C, Chen G, Liu F, Zhu W, et al: Transcriptome-wide piRNA
profiling in human gastric cancer. Oncol Reps. 41:3089–3099.
2019.
|
|
215
|
Sadoughi F, Mirhashemi SM and Asemi Z:
Epigenetic roles of PIWI proteins and piRNAs in colorectal cancer.
Cancer Cell Int. 21:3282021.
|
|
216
|
Liu Q, Chen Q, Zhou Z, Tian Z, Zheng X and
Wang K: piRNA-18 inhibition cell proliferation, migration and
invasion in colorectal cancer. Biochem Genet. 61:1881–1897.
2023.
|
|
217
|
Ray SK and Mukherjee S: Piwi-interacting
RNAs (piRNAs) and colorectal carcinoma: Emerging non-invasive
diagnostic biomarkers with potential therapeutic target based
clinical implications. Curr Mol Med. 23:300–311. 2023.
|
|
218
|
Tong Y, Guan B, Sun Z, Dong X, Chen Y, Li
Y, Jiang Y and Li J: Ratiometric fluorescent detection of exosomal
piRNA-823 based on Au NCs/UiO-66-NH2 and
target-triggered rolling circle amplification. Talanta.
257:1243072023.
|
|
219
|
Yin J, Jiang XY, Qi W, Ji CG, Xie XL,
Zhang DX, Cui ZJ, Wang CK, Bai Y, Wang J and Jiang HQ: piR-823
contributes to colorectal tumorigenesis by enhancing the
transcriptional activity of HSF1. Cancer Sci. 108:1746–1756.
2017.
|
|
220
|
Cheng J, Deng H, Xiao B, Zhou H, Zhou F,
Shen Z and Guo J: piR-823, a novel non-coding small RNA,
demonstrates in vitro and in vivo tumor suppressive activity in
human gastric cancer cells. Cancer Lett. 315:12–17. 2012.
|
|
221
|
Mai D, Ding P, Tan L, Zhang J, Pan Z, Bai
R, Li C, Li M, Zhou Y, Tan W, et al: PIWI-interacting RNA-54265 is
oncogenic and a potential therapeutic target in colorectal
adenocarcinoma. Theranostics. 8:5213–5230. 2018.
|
|
222
|
Shen L, Lin C, Lu W, He J, Wang Q, Huang
Y, Zheng X and Wang Z: Involvement of the oncogenic small nucleolar
RNA SNORA24 in regulation of p53 stability in colorectal cancer.
Cell Biol Toxicol. 39:1377–1394. 2023.
|
|
223
|
Zhang Z, Tao Y, Hua Q, Cai J, Ye X and Li
H: SNORA71A promotes colorectal cancer cell proliferation,
migration, and invasion. Biomed Res Int. 2020:82845762020.
|
|
224
|
Li X, Zhang Y, Li Y, Gu X and Ju S: A
comprehensive evaluation of serum tRF-29-R9J8909NF5JP as a novel
diagnostic and prognostic biomarker for gastric cancer. Mol
Carcinog. 62:1504–1517. 2023.
|
|
225
|
Lu S, Wei X, Tao L, Dong D, Hu W, Zhang Q,
Tao Y, Yu C, Sun D and Cheng H: A novel tRNA-derived fragment
tRF-3022b modulates cell apoptosis and M2 macrophage polarization
via binding to cytokines in colorectal cancer. J Hematol Oncol.
15:1762022.
|
|
226
|
Huang T, Chen C, Du J, Zheng Z, Ye S, Fang
S and Liu K: A tRF-5a fragment that regulates radiation resistance
of colorectal cancer cells by targeting MKNK1. J Cell Mol Med.
27:4021–4033. 2023.
|
|
227
|
Chen H, Xu Z, Cai H, Peng Y, Yang L and
Wang Z: Identifying differentially expressed tRNA-Derived small
fragments as a biomarker for the progression and metastasis of
colorectal cancer. Dis Markers. 2022:26461732022.
|
|
228
|
Tao EW, Wang HL, Cheng WY, Liu QQ, Chen YX
and Gao QY: A specific tRNA half, 5′tiRNA-His-GTG, responds to
hypoxia via the HIF1α/ANG axis and promotes colorectal cancer
progression by regulating LATS2. J Exp Clin Cancer Res.
40:672021.
|
|
229
|
Umezu T, Tanaka S, Kubo S, Enomoto M,
Tamori A, Ochiya T, Taguchi YH, Kuroda M and Murakami Y:
Characterization of circulating miRNAs in the treatment of primary
liver tumors. Cancer Rep (Hoboken). 7:e19642024.
|
|
230
|
Bi L, Zhou Y, Zhang Y and Zhang X:
MiR-27a-3p exacerbates cell migration and invasion in
right-sided/left-sided colorectal cancer by targeting
TGFBR2/TCF7L2. Cell Mol Biol (Noisy-le-Grand). 70:148–154.
2024.
|
|
231
|
Lai PS, Chang WM, Chen YY, Lin YF, Liao HF
and Chen CY: Circulating microRNA-762 upregulation in colorectal
cancer may be accompanied by Wnt-1/β-catenin signaling. Cancer
Biomark. 32:111–122. 2021.
|
|
232
|
Li T, Lai Q, Wang S, Cai J, Xiao Z, Deng
D, He L, Jiao H, Ye Y, Liang L, et al: MicroRNA-224 sustains
Wnt/β-catenin signaling and promotes aggressive phenotype of
colorectal cancer. J Exp Clin Cancer Res. 35:212016.
|
|
233
|
Yu FB, Sheng J, Yu JM, Liu JH, Qin XX and
Mou B: MiR-19a-3p regulates the Forkhead box F2-mediated
Wnt/β-catenin signaling pathway and affects the biological
functions of colorectal cancer cells. World J Gastroenterol.
26:627–644. 2020.
|
|
234
|
Avsar R, Gurer T and Aytekin A:
Bioinformatics and expression analyses of miR-639, miR-641,
miR-1915-3p and miR-3613-3p in colorectal cancer pathogenesis. J
Cancer. 14:2399–2409. 2023.
|
|
235
|
Raonić J, Ždralević M, Vučković L,
Šunjević M, Todorović V, Vukmirović F, Marzano F, Tullo A,
Giannattasio S and Radunović M: miR-29a expression negatively
correlates with Bcl-2 levels in colorectal cancer and is correlated
with better prognosis. Pathol Res Pract. 262:1554912024.
|
|
236
|
Tang W, Zhu Y, Gao J, Fu J, Liu C, Liu Y,
Song C, Zhu S, Leng Y, Wang G, et al: MicroRNA-29a promotes
colorectal cancer metastasis by regulating matrix metalloproteinase
2 and E-cadherin via KLF4. Br J Cancer. 110:450–458. 2014.
|
|
237
|
Ciesla M, Skrzypek K, Kozakowska M, Loboda
A, Jozkowicz A and Dulak J: MicroRNAs as biomarkers of disease
onset. Anal Bioanal Chem. 401:2051–2061. 2011.
|
|
238
|
Elamir A, Shaker O, Kamal M, Khalefa A,
Abdelwahed M, Abd El Reheem F, Ahmed T, Hassan E and Ayoub S:
Expression profile of serum LncRNA THRIL and MiR-125b in
inflammatory bowel disease. PLoS One. 17:e02752672022.
|
|
239
|
Abdelazim SA, Shaker OG, Ali O, El-Tawil M
and Senousy MA: Differential expression of serum miR-486 and miR-25
in ulcerative colitis and Crohn's disease: Correlations with
disease activity, extent, and location. Pathol Res Pract.
252:1549102023.
|
|
240
|
Valmiki S, Ahuja V and Paul J: MicroRNA
exhibit altered expression in the inflamed colonic mucosa of
ulcerative colitis patients. World J Gastroenterol. 23:5324–5332.
2017.
|
|
241
|
Zhu L, Li J, Gong Y, Wu Q, Tan S, Sun D,
Xu X, Zuo Y, Zhao Y, Wei YQ, et al: Exosomal tRNA-derived small RNA
as a promising biomarker for cancer diagnosis. Mol Cancer.
18:742019.
|
|
242
|
Xue M, Shi M, Xie J, Zhang J, Jiang L,
Deng X, Peng C, Shen B, Xu H and Chen H: Serum tRNA-derived small
RNAs as potential novel diagnostic biomarkers for pancreatic ductal
adenocarcinoma. Am J Cancer Res. 11:837–848. 2021.
|
|
243
|
Wang X and Hu Z: tRNA derived fragment
tsRNA-14783 promotes M2 polarization of macrophages in keloid.
Biochem Biophys Res Commun. 636:119–127. 2022.
|
|
244
|
Cai A, Hu Y, Zhou Z, Qi Q, Wu Y, Dong P,
Chen L and Wang F: PIWI-interacting RNAs (piRNAs): promising
applications as emerging biomarkers for digestive system cancer.
Front Mol Biosci. 9:8481052022.
|
|
245
|
Sabbah NA, Abdalla WM, Mawla WA,
AbdAlMonem N, Gharib AF, Abdul-Saboor A, Abdelazem AS and Raafat N:
piRNA-823 is a unique potential diagnostic Non-invasive biomarker
in colorectal cancer patients. Genes (Basel). 12:5982021.
|
|
246
|
Wang Z, Yang H, Ma D, Mu Y, Tan X, Hao Q,
Feng L, Liang J, Xin W, Chen Y, et al: Serum PIWI-Interacting RNAs
piR-020619 and piR-020450 are promising novel biomarkers for early
detection of colorectal cancer. Cancer Epidemiol Biomarkers Prev.
29:990–998. 2020.
|
|
247
|
Yin J, Qi W, Ji CG, Zhang DX, Xie XL, Ding
Q, Jiang XY, Han J and Jiang HQ: Small RNA sequencing revealed
aberrant piRNA expression profiles in colorectal cancer. Oncol Rep.
42:263–272. 2019.
|
|
248
|
Mai D, Zheng Y, Guo H, Ding P, Bai R, Li
M, Ye Y, Zhang J, Huang X, Liu D, et al: Serum piRNA-54265 is a New
Biomarker for early detection and clinical surveillance of Human
Colorectal Cancer. Theranostics. 10:8468–8478. 2020.
|
|
249
|
Weng W, Liu N, Toiyama Y, Kusunoki M,
Nagasaka T, Fujiwara T, Wei Q, Qin H, Lin H, Ma Y and Goel A: Novel
evidence for a PIWI-interacting RNA (piRNA) as an oncogenic
mediator of disease progression, and a potential prognostic
biomarker in colorectal cancer. Mol Cancer. 17:162018.
|
|
250
|
Feng J, Yang M, Wei Q, Song F, Zhang Y,
Wang X, Liu B and Li J: Novel evidence for oncogenic piRNA-823 as a
promising prognostic biomarker and a potential therapeutic target
in colorectal cancer. J Cell Mol Med. 24:9028–9040. 2020.
|
|
251
|
Sahami-Fard MH, Kheirandish S and Sheikhha
MH: Expression levels of miR-143-3p and -424-5p in colorectal
cancer and their clinical significance. Cancer Biomark. 24:291–297.
2019.
|
|
252
|
Touchaei AZ, Vahidi S and Samadani AA:
Decoding the interaction between miR-19a and CBX7 focusing on the
implications for tumor suppression in cancer therapy. Med Oncol.
41:212023.
|
|
253
|
Gil-Kulik P, Petniak A, Kluz N, Wallner G,
Skoczylas T, Ciechański A and Kocki J: Influence of clinical
factors on miR-3613-3p expression in colorectal cancer. Int J Mol
Sci. 24:140232023.
|
|
254
|
Zhang H, Zhu M, Shan X, Zhou X, Wang T,
Zhang J, Tao J, Cheng W, Chen G, Li J, et al: A panel of
seven-miRNA signature in plasma as potential biomarker for
colorectal cancer diagnosis. Gene. 687:246–254. 2019.
|
|
255
|
Koopaie M, Manifar S, Talebi MM, Kolahdooz
S, Razavi AE, Davoudi M and Pourshahidi S: Assessment of salivary
miRNA, clinical, and demographic characterization in colorectal
cancer diagnosis. Transl Oncol. 41:1018802024.
|
|
256
|
Xu Q, Lu X, Li J, Feng Y, Tang J, Zhang T,
Mao Y, Lan Y, Luo H, Zeng L, et al: Fusobacterium nucleatum
induces excess methyltransferase-like 3-mediated microRNA-4717-3p
maturation to promote colorectal cancer cell proliferation. Cancer
Sci. 113:3787–3800. 2022.
|
|
257
|
Toiyama Y, Okugawa Y, Tanaka K, Araki T,
Uchida K, Hishida A, Uchino M, Ikeuchi H, Hirota S, Kusunoki M, et
al: A panel of methylated MicroRNA biomarkers for identifying
High-Risk patients with ulcerative Colitis-associated colorectal
cancer. Gastroenterology. 153:1634–1646.e8. 2017.
|
|
258
|
Koike Y, Yin C, Sato Y, Nagano Y, Yamamoto
A, Kitajima T, Shimura T, Kawamura M, Matsushita K, Okugawa Y, et
al: Promoter methylation levels of microRNA-124 in non-neoplastic
rectal mucosa as a potential biomarker for ulcerative
colitis-associated colorectal cancer in pediatric-onset patients.
Surg Today. 54:347–355. 2023.
|
|
259
|
Bian Z, Xu C, Xie Y, Wang X, Chen Y, Mao
S, Wu Q, Zhu J, Huang N, Zhang Y, et al: SNORD11B-mediated
2′-O-methylation of primary let-7a in colorectal carcinogenesis.
Oncogene. 42:3035–3046. 2023.
|
|
260
|
Okugawa Y, Toiyama Y, Toden S, Mitoma H,
Nagasaka T, Tanaka K, Inoue Y, Kusunoki M, Boland CR and Goel A:
Clinical significance of SNORA42 as an oncogene and a prognostic
biomarker in colorectal cancer. Gut. 66:107–117. 2017.
|
|
261
|
Liu Y, Zhao C, Wang G, Chen J, Ju S, Huang
J and Wang X: SNORD1C maintains stemness and 5-FU resistance by
activation of Wnt signaling pathway in colorectal cancer. Cell
Death Discov. 8:2002022.
|
|
262
|
Shen L, Lu W, Huang Y, He J, Wang Q, Zheng
X and Wang Z: SNORD15B and SNORA5C: Novel diagnostic and prognostic
biomarkers for colorectal cancer. Biomed Res Int.
2022:82608002022.
|
|
263
|
Xu C, Bian Z, Wang X, Niu N, Liu L, Xiao
Y, Zhu J, Huang N, Zhang Y, Chen Y, et al: SNORA56-mediated
pseudouridylation of 28 S rRNA inhibits ferroptosis and promotes
colorectal cancer proliferation by enhancing GCLC translation. J
Exp Clin Cancer Res. 42:3312023.
|
|
264
|
Chen Q, Li D, Jiang L, Wu Y, Yuan H, Shi
G, Liu F, Wu P and Jiang K: Biological functions and clinical
significance of tRNA-derived small fragment (tsRNA) in tumors:
Current state and future perspectives. Cancer Lett.
587:2167012024.
|
|
265
|
Lee S, Kim J, Valdmanis PN and Kim HK:
Emerging roles of tRNA-derived small RNAs in cancer biology. Exp
Mol Med. 55:1293–1304. 2023.
|
|
266
|
Wang XY, Zhou YJ, Chen HY, Chen JN, Chen
SS, Chen HM and Li XB: 5′tiRNA-Pro-TGG, a novel tRNA halve,
promotes oncogenesis in sessile serrated lesions and serrated
pathway of colorectal cancer. World J Gastrointest Oncol.
15:1005–1018. 2023.
|
|
267
|
Tsiakanikas P, Adamopoulos PG, Tsirba D,
Artemaki PI, Papadopoulos IN, Kontos CK and Scorilas A: High
expression of a tRNA(Pro) derivative associates with poor survival
and independently predicts colorectal cancer recurrence.
Biomedicines. 10:11202022.
|
|
268
|
Xie Y, Zhang S, Yu X, Ye G and Guo J:
Transfer RNA-derived fragments as novel biomarkers of the onset and
progression of gastric cancer. Exp Biol Med (Maywood).
248:1095–1102. 2023.
|
|
269
|
Jin F, Yang L, Wang W, Yuan N, Zhan S,
Yang P, Chen X, Ma T and Wang Y: A novel class of tsRNA signatures
as biomarkers for diagnosis and prognosis of pancreatic cancer. Mol
Cancer. 20:952021.
|
|
270
|
Liu CX, Qiao XJ, Xing ZW and Hou MX: The
SNORA21 expression is upregulated and acts as a novel independent
indicator in human gastric cancer prognosis. Eur Rev Med Pharmacol
Sci. 22:5519–5524. 2018.
|
|
271
|
Ding Y, Sun Z, Zhang S, Zhou L, Xu Q, Zhou
D, Li Y, Han X, Xu H, Bai Y, et al: Identification of snoRNA
SNORA71A as a novel biomarker in prognosis of hepatocellular
carcinoma. Dis Markers. 2020:88799442020.
|
|
272
|
Kitagawa T, Taniuchi K, Tsuboi M,
Sakaguchi M, Kohsaki T, Okabayashi T and Saibara T: Circulating
pancreatic cancer exosomal RNAs for detection of pancreatic cancer.
Mol Oncol. 13:212–227. 2019.
|
|
273
|
Kumata Y, Iinuma H, Suzuki Y, Tsukahara D,
Midorikawa H, Igarashi Y, Soeda N, Kiyokawa T, Horikawa M and
Fukushima R: Exosome-encapsulated microRNA-23b as a minimally
invasive liquid biomarker for the prediction of recurrence and
prognosis of gastric cancer patients in each tumor stage. Oncol
Rep. 40:319–330. 2018.
|
|
274
|
Moshiri F, Salvi A, Gramantieri L,
Sangiovanni A, Guerriero P, De Petro G, Bassi C, Lupini L, Sattari
A, Cheung D, et al: Circulating miR-106b-3p, miR-101-3p and
miR-1246 as diagnostic biomarkers of hepatocellular carcinoma.
Oncotarget. 9:15350–15364. 2018.
|
|
275
|
Huang D, Chu Y, Qiu J, Chen X, Zhao J,
Zhang Y, Li S, Cheng Y, Shi H, Han L and Wang J: A novel diagnostic
signature of circulating tsRNAs and miRNAs in esophageal squamous
cell carcinoma detected with a microfluidic platform. Anal Chim
Acta. 1272:3415202023.
|
|
276
|
Zhou X, Liu J, Meng A, Zhang L, Wang M,
Fan H, Peng W and Lu J: Gastric juice piR-1245: A promising
prognostic biomarker for gastric cancer. J Clin Lab Anal.
34:e231312020.
|
|
277
|
Rui T, Wang K, Xiang A, Guo J, Tang N, Jin
X, Lin Y, Liu J and Zhang X: Serum Exosome-derived piRNAs could be
promising biomarkers for HCC diagnosis. Int J Nanomedicine.
18:1989–2001. 2023.
|
|
278
|
Raimondo TM, Reed K, Shi D, Langer R and
Anderson DG: Delivering the next generation of cancer
immunotherapies with RNA. Cell. 186:1535–1540. 2023.
|
|
279
|
Zabeti Touchaei A and Vahidi S: MicroRNAs
as regulators of immune checkpoints in cancer immunotherapy:
Targeting PD-1/PD-L1 and CTLA-4 pathways. Cancer Cell Int.
24:1022024.
|
|
280
|
He X and Xu C: Immune checkpoint signaling
and cancer immunotherapy. Cell Res. 30:660–669. 2020.
|
|
281
|
Shiravand Y, Khodadadi F, Kashani SMA,
Hosseini-Fard SR, Hosseini S, Sadeghirad H, Ladwa R, O'Byrne K and
Kulasinghe A: Immune checkpoint inhibitors in cancer therapy. Curr
Oncol. 29:3044–3060. 2022.
|
|
282
|
Liu Q, Guan Y and Li S: Programmed death
receptor (PD-)1/PD-ligand (L)1 in urological cancers: The
'all-around warrior' in immunotherapy. Mol Cancer. 23:1832024.
|
|
283
|
Roshani Asl E, Rasmi Y and Baradaran B:
MicroRNA-124-3p suppresses PD-L1 expression and inhibits
tumorigenesis of colorectal cancer cells via modulating STAT3
signaling. J Cell Physiol. 236:7071–7087. 2021.
|
|
284
|
Gao T, Lin YQ, Ye HY and Lin WM: miR-124
delivered by BM-MSCs-derived exosomes targets MCT1 of
tumor-infiltrating treg cells and improves ovarian cancer
immunotherapy. Neoplasma. 70:713–721. 2023.
|
|
285
|
Jin Y, Zhan X, Zhang B, Chen Y, Liu C and
Yu L: Polydatin exerts an antitumor effect through regulating the
miR-382/PD-L1 axis in colorectal cancer. Cancer Biother Radiopharm.
35:83–91. 2020.
|
|
286
|
Jiang W, Li T, Wang J, Jiao R, Shi X,
Huang X and Ji G: miR-140-3p suppresses cell growth and induces
apoptosis in colorectal cancer by targeting PD-L1. Onco Targets
Ther. 12:10275–10285. 2019.
|
|
287
|
Chen X, Hu J, Lai J, Zhang Z and Tang Z:
Dexmedetomidine attenuates LPS-Stimulated alveolar type II cells'
Injury through upregulation of miR-140-3p and partial suppression
of PD-L1 involving inactivating JNK-Bnip3 pathway. Can Respir J.
2022:84339602022.
|
|
288
|
Chen YL, Wang GX, Lin BA and Huang JS:
MicroRNA-93-5p expression in tumor tissue and its tumor suppressor
function via targeting programmed death ligand-1 in colorectal
cancer. Cell Biol Int. 44:1224–1236. 2020.
|
|
289
|
Yang M, Xiao R, Wang X, Xiong Y, Duan Z,
Li D and Kan Q: MiR-93-5p regulates tumorigenesis and tumor
immunity by targeting PD-L1/CCND1 in breast cancer. Ann Transl Med.
10:2032022.
|
|
290
|
Liu C, Liu R, Wang B, Lian J, Yao Y, Sun
H, Zhang C, Fang L, Guan X, Shi J, et al: Blocking IL-17A enhances
tumor response to anti-PD-1 immunotherapy in microsatellite stable
colorectal cancer. J Immunother Cancer. 9:e0018952021.
|
|
291
|
Ashizawa M, Okayama H, Ishigame T, Thar
Min AK, Saito K, Ujiie D, Murakami Y, Kikuchi T, Nakayama Y, Noda
M, et al: miRNA-148a-3p regulates immunosuppression in DNA mismatch
Repair-deficient colorectal cancer by targeting PD-L1. Mol Cancer
Res. 17:1403–1413. 2019.
|
|
292
|
Zhao L, Yu H, Yi S, Peng X, Su P, Xiao Z,
Liu R, Tang A, Li X, Liu F and Shen S: The tumor suppressor
miR-138-5p targets PD-L1 in colorectal cancer. Oncotarget.
7:45370–45384. 2016.
|
|
293
|
Luo Q, Shen F, Zhao S, Dong L, Wei J, Hu
H, Huang Q, Wang Q, Yang P, Liang W, et al:
LINC00460/miR-186-3p/MYC feedback loop facilitates colorectal
cancer immune escape by enhancing CD47 and PD-L1 expressions. J Exp
Clin Cancer Res. 43:2252024.
|
|
294
|
Zhang DJ, Fu ZM, Guo YY, Guo F, Wan YN and
Guan GF: Circ_0000052/miR-382-3p axis induces PD-L1 expression and
regulates cell proliferation and immune evasion in head and neck
squamous cell carcinoma. J Cell Mol Med. 27:113–126. 2023.
|
|
295
|
Cheung VTF, Gupta T, Olsson-Brown A,
Subramanian S, Sasson SC, Heseltine J, Fryer E, Collantes E, Sacco
JJ, Pirmohamed M, et al: Immune checkpoint inhibitor-related
colitis assessment and prognosis: Can IBD scoring point the way? Br
J Cancer. 123:207–215. 2020.
|
|
296
|
Ye R, Zheng H, Yang D, Lin J, Li L, Li Y,
Pan H, Dai H, Zhao L, Zhou Y, et al: irAE-colitis induced by CTLA-4
and PD-1 blocking were ameliorated by TNF blocking and modulation
of gut microbial. Biomed Pharmacother. 177:1169992024.
|
|
297
|
Grover S, Ruan AB, Srivoleti P,
Giobbie-Hurder A, Braschi-Amirfarzan M, Srivastava A, Buchbinder
EI, Ott PA, Kehl KL, Awad MM, et al: Safety of immune checkpoint
inhibitors in patients with pre-existing inflammatory bowel disease
and microscopic colitis. JCO Oncol Pract. 16:e933–e942. 2020.
|
|
298
|
Abu-Sbeih H, Faleck DM, Ricciuti B,
Mendelsohn RB, Naqash AR, Cohen JV, Sellers MC, Balaji A,
Ben-Betzalel G, Hajir I, et al: Immune checkpoint inhibitor therapy
in patients with preexisting inflammatory bowel disease. J Clin
Oncol. 38:576–583. 2020.
|
|
299
|
Perez-Ruiz E, Minute L, Otano I, Alvarez
M, Ochoa MC, Belsue V, de Andrea C, Rodriguez-Ruiz ME, Perez-Gracia
JL, Marquez-Rodas I, et al: Prophylactic TNF blockade uncouples
efficacy and toxicity in dual CTLA-4 and PD-1 immunotherapy.
Nature. 569:428–432. 2019.
|
|
300
|
Perez-Sanchez C, Barbera Betancourt A,
Lyons PA, Zhang Z, Suo C, Lee JC, McKinney EF, Modis LK, Ellson C
and Smith KGC: miR-374a-5p regulates inflammatory genes and
monocyte function in patients with inflammatory bowel disease. J
Exp Med. 219:e202113662022.
|
|
301
|
Zu M, Ma Y, Cannup B, Xie D, Jung Y, Zhang
J, Yang C, Gao F, Merlin D and Xiao B: Oral delivery of natural
active small molecules by polymeric nanoparticles for the treatment
of inflammatory bowel diseases. Adv Drug Deliv Rev.
176:1138872021.
|
|
302
|
Kumar S, Dilbaghi N, Saharan R and
Bhanjana G: Nanotechnology as emerging tool for enhancing
solubility of poorly water-soluble drugs. Bionanoscience.
2:227–250. 2012.
|
|
303
|
Gao M, Yang C, Wu C, Chen Y, Zhuang H,
Wang J and Cao Z: Hydrogel-metal-organic-framework hybrids mediated
efficient oral delivery of siRNA for the treatment of ulcerative
colitis. J Nanobiotechnol. 20:4042022.
|
|
304
|
Wei Y, Li X, Lin J, Zhou Y, Yang J, Hou M,
Wu F, Yan J, Ge C, Hu D and Yin L: Oral delivery of siRNA using
fluorinated, small-sized nanocapsules toward anti-inflammation
treatment. Adv Mater. 35:e22068212023.
|
|
305
|
Xu F, Ye ML, Zhang YP, Li WJ, Li MT, Wang
HZ, Qiu X, Xu Y, Yin JW, Hu Q, et al: MicroRNA-375-3p enhances
chemosensitivity to 5-fluorouracil by targeting thymidylate
synthase in colorectal cancer. Cancer Sci. 111:1528–1541. 2020.
|
|
306
|
Huang CZ, Zhou Y, Tong QS, Duan QJ, Zhang
Q, Du JZ and Yao XQ: Precision medicine-guided co-delivery of ASPN
siRNA and oxaliplatin by nanoparticles to overcome chemoresistance
of colorectal cancer. Biomaterials. 290:1218272022.
|
|
307
|
Ball RL, Bajaj P and Whitehead KA: Oral
delivery of siRNA lipid nanoparticles: Fate in the GI tract. Sci
Rep. 8:21782018.
|
|
308
|
Wang G, Yuan J, Cai X, Xu Z, Wang J,
Ocansey DKW, Yan Y, Qian H, Zhang X, Xu W and Mao F:
HucMSC-exosomes carrying miR-326 inhibit neddylation to relieve
inflammatory bowel disease in mice. Clin Transl Med.
10:e1132020.
|
|
309
|
Keller S, Ridinger J, Rupp AK, Janssen JW
and Altevogt P: Body fluid derived exosomes as a novel template for
clinical diagnostics. J Transl Med. 9:862011.
|
|
310
|
He C, Zheng S, Luo Y and Wang B: Exosome
theranostics: Biology and translational medicine. Theranostics.
8:237–255. 2018.
|
|
311
|
Wang D, Xue H, Tan J, Liu P, Qiao C, Pang
C and Zhang L: Bone marrow mesenchymal stem cells-derived exosomes
containing miR-539-5p inhibit pyroptosis through NLRP3/caspase-1
signalling to alleviate inflammatory bowel disease. Inflamm Res.
71:833–846. 2022.
|
|
312
|
Deng F, Yan J, Lu J, Luo M, Xia P, Liu S,
Wang X, Zhi F and Liu D: M2 macrophage-derived exosomal miR-590-3p
attenuates DSS-induced mucosal damage and promotes epithelial
repair via the LATS1/YAP/β-catenin signalling axis. J Crohns
Colitis. 15:665–677. 2021.
|
|
313
|
Lampropoulou DI, Pliakou E, Aravantinos G,
Filippou D and Gazouli M: The role of exosomal non-coding RNAs in
colorectal cancer drug resistance. Int J Mol Sci. 23:14732022.
|
|
314
|
Liang G, Zhu Y, Ali DJ, Tian T, Xu H, Si
K, Sun B, Chen B and Xiao Z: Engineered exosomes for targeted
co-delivery of miR-21 inhibitor and chemotherapeutics to reverse
drug resistance in colon cancer. J Nanobiotechnol. 18:102020.
|
|
315
|
Dasgupta I and Chatterjee A: Recent
advances in miRNA delivery systems. Methods Protoc. 4:102021.
|
|
316
|
Guo J, Jiang X and Gui S: RNA
interference-based nanosystems for inflammatory bowel disease
therapy. Int J Nanomedicine. 11:5287–5310. 2016.
|
|
317
|
Maliborska S, Holotiuk V, Partykevych Y
and Rossylna O: Prognostic significance of microRNA-100, -125b, and
-200b in patients with colorectal cancer. Exp Oncol. 45:443–450.
2024.
|
|
318
|
Jones BL and Wilcox MH: Aeromonas
infections and their treatment. J Antimicrob Chemother. 35:453–461.
1995.
|
|
319
|
Luo S, Yue M, Wang D, Lu Y, Wu Q and Jiang
J: Breaking the barrier: Epigenetic strategies to combat platinum
resistance in colorectal cancer. Drug Resist Updat.
77:1011522024.
|
|
320
|
Dong W, Wang F, Liu Q, Wang T, Yang Y, Guo
P, Li X and Wei B: Downregulation of miRNA-14669 reverses
vincristine resistance in colorectal cancer cells through PI3K/AKT
signaling pathway. Recent Pat Anticancer Drug Discov. 17:178–186.
2022.
|
|
321
|
Guo X, Li Q, Wang YF, Wang TY, Chen SJ and
Tian ZW: Reduced lipocalin 2 expression contributes to vincristine
resistance in human colon cancer cells. Recent Pat Anticancer Drug
Discov. 13:248–254. 2018.
|
|
322
|
Saurav S, Karfa S, Vu T, Liu Z, Datta A,
Manne U, Samuel T and Datta PK: Overcoming irinotecan resistance by
targeting its downstream signaling pathways in colon cancer.
Cancers (Basel). 16:34912024.
|
|
323
|
Yuan M, Chen T, Jin L, Zhang P, Xie L,
Zhou S, Fan L, Wang L, Zhang C, Tang N, et al: A carrier-free
supramolecular nano-twin-drug for overcoming irinotecan-resistance
and enhancing efficacy against colorectal cancer. J Nanobiotechnol.
21:3932023.
|
|
324
|
Chen M, Wang L, Wang F, Li F, Xia W, Gu H
and Chen Y: Quick synthesis of a novel combinatorial delivery
system of siRNA and doxorubicin for a synergistic anticancer
effect. Int J Nanomedicine. 14:3557–3569. 2019.
|
|
325
|
Liu S, Wang W, Ning Y, Zheng H, Zhan Y,
Wang H, Yang Y, Luo J, Wen Q, Zang H, et al: Exosome-mediated
miR-7-5p delivery enhances the anticancer effect of Everolimus via
blocking MNK/eIF4E axis in non-small cell lung cancer. Cell Death
Dis. 13:1292022.
|