As awareness of health and wellness grows, the
benefits of Traditional Chinese Medicine (TCM) in health care and
disease treatment are increasingly recognized. TCM compounds,
valued for their targeted therapeutic effects and minimal adverse
reactions (1-3), are gaining market traction. Guided
by TCM principles, researchers employ modern scientific and
technological methods to investigate the pharmacodynamics,
pharmacology, toxicology and clinical applications of TCM
compounds. Extensive studies have elucidated the active ingredients
and mechanisms of these compounds (2), leading to significant advancements.
For instance, Qinggan Huoxue Recipe has been shown to mitigate
alcoholic liver disease (ALD) progression by inhibiting the liver X
receptor-lysophosphatidylcholine acyl-transferase 3 signaling
pathway (4). In addition,
Danggui Buxue Decoction may improve diabetic nephropathy by
modulating insulin resistance, chronic inflammation, and lipid
accumulation (5). In Jiangzhi
Granule, Xiang et al (6)
identified that kaempferol, a monomer from lotus leaf, alleviates
liver damage in non-alcoholic steatohepatitis mice by reducing
endoplasmic reticulum stress. Ginsenosides from ginseng have been
demonstrated to exhibit anti-inflammatory, antioxidant and
anti-tumor activities (7,8),
alongside neuroprotective effects through the protein kinase B
(AKT)/cyclic adenosine monophosphate (cAMP) response
element-binding protein (CREB)/brain-derived neurotrophic factor
(BDNF) pathway (9). Such
findings support the development of innovative therapeutics.
Bupleurum, a widely used TCM herb in China and other
Asian countries for managing chronic liver inflammation and viral
hepatitis (10,11), is a key component in classic TCM
formulas such as Xiao-Chai-Hu-Tang (12), Chaihu-Shugan-San (13) and Xiaoyaosan (14). Known for its anti-inflammatory,
anti-infective and hepatoprotective effects (15-18), Bupleurum has yielded >100
triterpene saponins, including saikosaponin (SS)A, B, C and D, with
SSA and SSD as primary bioactive components (11). Studies have demonstrated SSD's
diverse pharmacological effects, including anti-inflammatory,
antioxidant, anti-apoptotic, anti-fibrotic and anti-cancer
properties (19), underscoring
its therapeutic potential across a spectrum of conditions such as
myocardial injury (20), lung
injury (21), non-alcoholic
fatty liver disease (NAFLD) (22), liver fibrosis (23), glomerulonephritis (24), diabetes (25), depression (26), Alzheimer's disease (AD) (27) and various cancers (28,29).
Elucidating the action targets and molecular
mechanisms of TCM compounds is instrumental in scientifically
validating their roles in disease prevention and treatment, as well
as in identifying novel therapeutic targets and lead compounds. Our
team has dedicated extensive research to understanding SSD's
mechanisms in liver fibrosis (30-33), while valuable studies by other
researchers on SSD in additional diseases also deserve close
examination. This review synthesizes the mechanisms of SSD across a
range of diseases from its discovery to the present, highlighting
current challenges and proposing strategic solutions in SSD
research. SSD emerges as a promising monomer with multifaceted
therapeutic potential. This article will enhance researchers'
understanding of SSD's current research status and offer strategic
guidance for future studies.
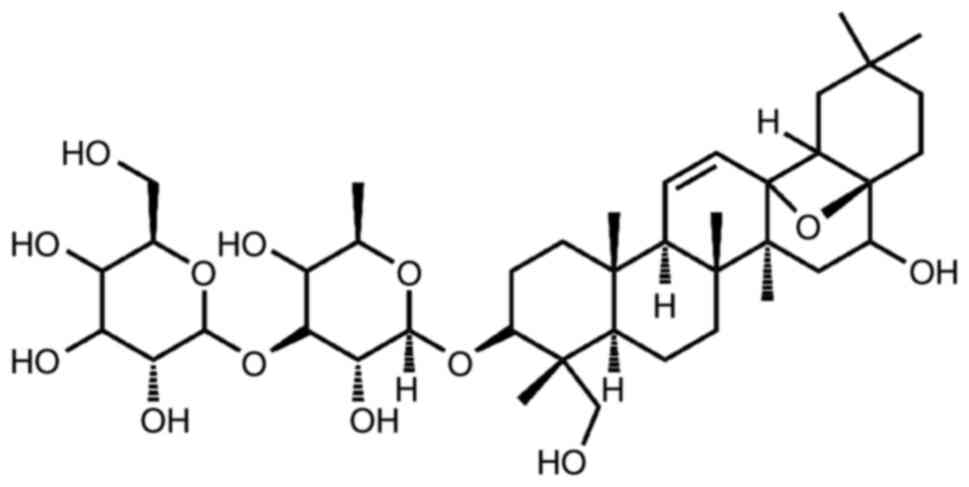
SSD, a triterpene saponin compound, dissolves well
in methanol and ethanol but has limited solubility in water.
Structurally, it resembles steroids (Fig. 1) (34,35) and possesses a chemical formula of
C42H68O13 with a molecular weight
of 780.98 (35). The extracted
SSD is a white powder, which will be considered for oral
administration in the future. The main administration methods of
SSD in existing animal experimental studies are gastric lavage and
intraperitoneal injection (24,25).
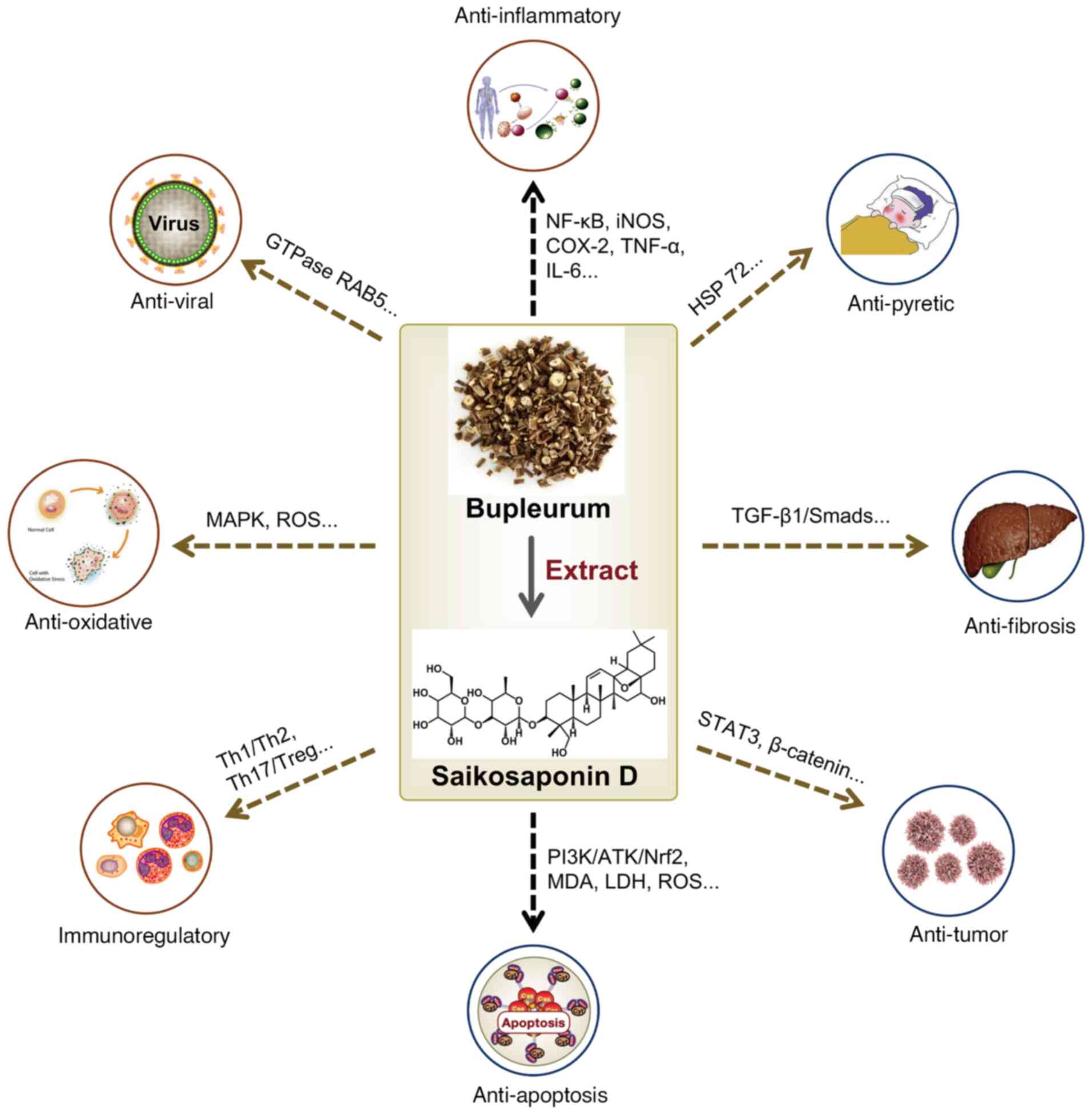
SSD possesses diverse therapeutic properties,
including anti-inflammatory, antipyretic, analgesic, antioxidant,
anti-apoptotic, antiviral, antifibrosis, immune-regulation and
anti-tumor activities (19),
with specific mechanisms supported by studies (Fig. 2).
Investigations into SSD's anti-inflammatory
mechanisms reveal its potent inhibition of lipopolysaccharide
(LPS)-induced inducible nitric oxide synthase (iNOS),
cyclooxygenase-2 (COX-2) and pro-inflammatory cytokines (TNF-α and
IL-6) in RAW264.7 cells by blocking nuclear factor-κB (NF-κB)
activation (36). Furthermore,
SSD was reported to significantly reduce cerulein-induced apoptosis
and inflammation in pancreatic AR42J cells by modulating the
mitogen-activated protein kinase (MAPK) signaling pathway (37). Heat stress, a trigger for
reactive oxygen species (ROS) production, leads to oxidative tissue
damage (38). Zhang et al
(39) reported that SSD
mitigated heat stress-induced oxidative damage in LLC-PK1 cells by
promoting antioxidant enzymes and heat shock protein 72. The
imbalance between cellular defense mechanisms and free radical
production is known as oxidative stress (OS). Research by Lin et
al (40) demonstrated that
SSD significantly reduces H2O2-induced
malondialdehyde (MDA) and lactate dehydrogenase (LDH) release,
while enhancing superoxide dismutase (SOD) activity and antioxidant
capacity, thereby protecting against PC12-cell apoptosis. The
primary mechanism involves SSD's ability to eliminate ROS and
disrupt MAPK-mediated oxidative injury, which prevents
H2O2-induced PC12-cell death (40). An additional study showed SSD's
ability to reduce OS through the phosphatidylinositol-3 kinase
(PI3K)/AKT/nuclear factor erythroid 2-related factor 2 (Nrf2)
pathway, improving muscle atrophy in chronic kidney disease (CKD)
models (41).
SSD has shown antiviral activity against enterovirus
A71 (EV-A71), which causes hand, foot and mouth disease, a virus
known to trigger autophagy (42). Li et al (43) found that SSD inhibits EV-A71
infection by effectively suppressing viral RNA replication and
subsequent viral protein synthesis, preventing EV-A71-induced cell
death.
SSD has also shown immunomodulatory benefits, as it
regulates Type 1 T-helper cell (Th1)/Th2 and Th17/T-regulatory cell
(Treg) imbalances and alleviates Hashimoto's thyroiditis (HT)
severity in mice by promoting M2 macrophage differentiation
(45).
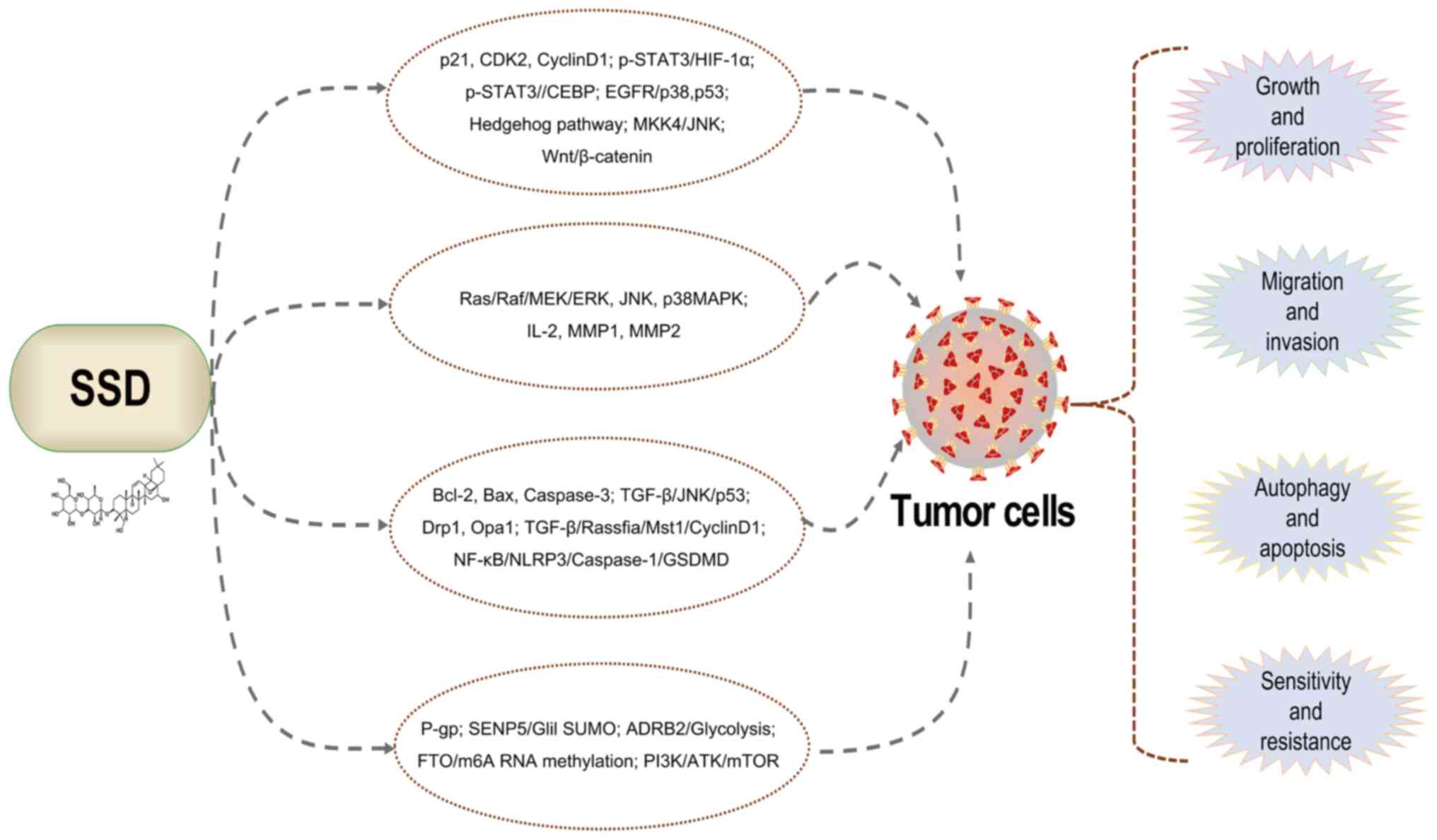
Among saikosaponins, SSD displays the strongest
anti-tumor activity, targeting various cancers through multiple
pathways (46-48). For instance, SSD inhibits
non-small cell lung cancer cell proliferation and induces apoptosis
by disrupting the signal transducer and activator of transcription
3 (STAT3) pathway (48) and also
suppresses triple-negative breast cancer cell growth through
β-catenin signaling inhibition (49).
These pharmacological insights underscore SSD's
promising potential as a therapeutic agent.
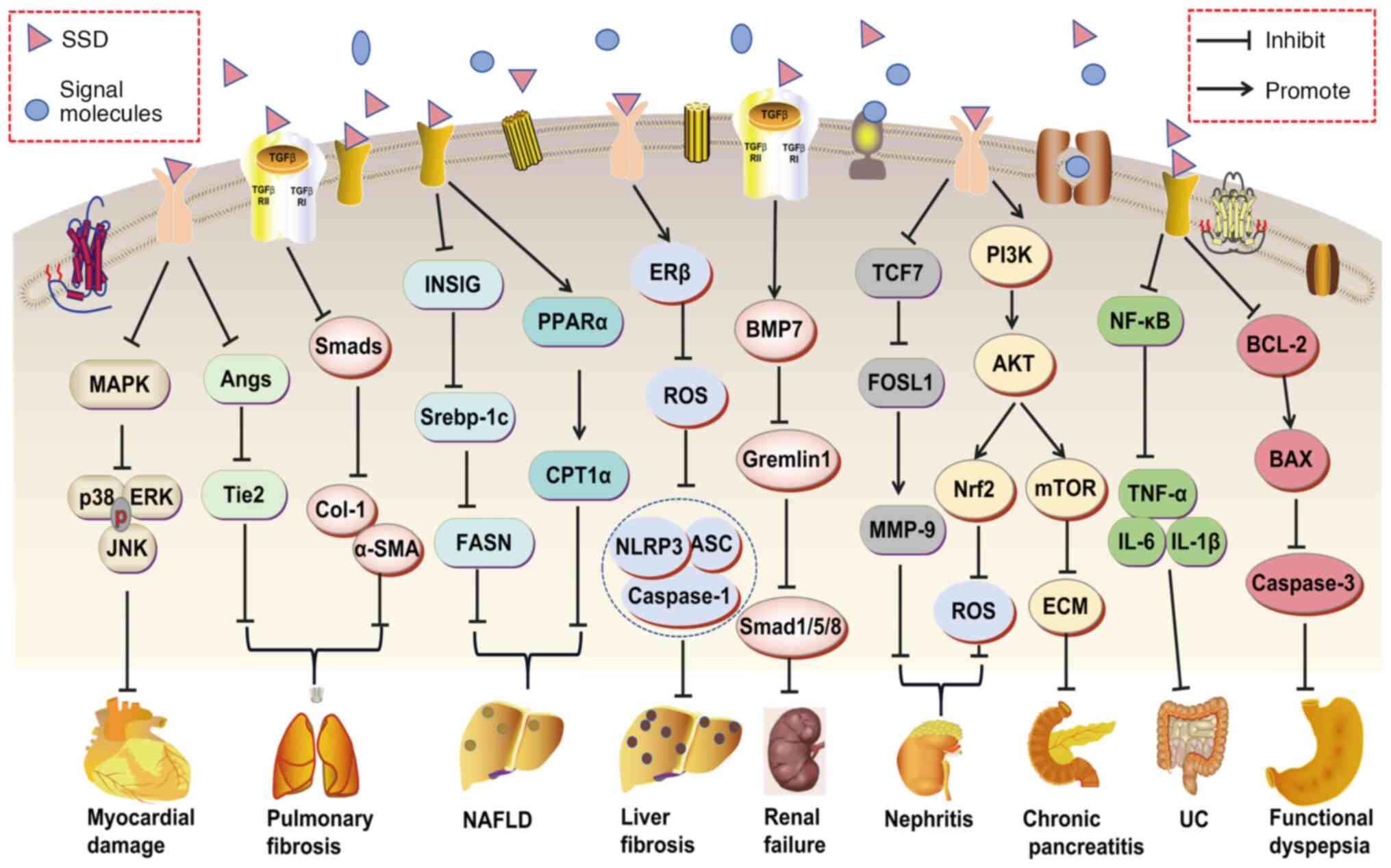
SSD demonstrates a wide range of therapeutic
properties, including anti-inflammatory, antipyretic, analgesic,
antioxidant, anti-apoptotic, antiviral, antifibrotic,
immune-regulation and anti-tumor activities. Consequently, its
pharmacological effects of inhibiting inflammation, oxidative
stress, fibrosis and so on are related to the treatment of various
diseases, such as myocardial injury (20), lung damage (50), NAFLD (22), liver fibrosis (23), glomerulonephritis (51), diabetes (25), depression (26), AD (27) and cancers (52,53) (Fig. 3). Specific molecular mechanisms
are detailed in Tables I and
II.
The oxidative damage induced by anticancer drugs
such as doxorubicin (DOX) can result in irreversible cardiotoxicity
(54). Zhang et al
(20) found that DOX
administration led to cardiac damage and dysfunction, as well as
reduced survival in mice. In H9c2 cells, DOX treatment increased
LDH leakage, cardiomyocyte apoptosis, myocardial fibrosis and
reduced cardiomyocyte volume. SSD protects cardiomyocytes from
DOX-induced cardiotoxicity by inhibiting excessive OS through the
p38-MAPK signaling pathway (20).
In a rat model of ventilator-induced lung injury
(VILI), SSD reduced pulmonary neutrophil infiltration,
myeloperoxidase levels and pro-inflammatory cytokines (MIP-2, IL-6
and TNF-α), while elevating anti-inflammatory mediators (TGF-β1 and
IL-10) (21). SSD also reduced
OS and apoptosis in lung tissue by downregulating caspases-3 and
pro-apoptotic protein Bax, while upregulating the anti-apoptotic
protein Bcl-2. These effects suggest that SSD may alleviate VILI by
suppressing inflammation, OS and apoptosis (21). Another study demonstrated that
SSD reduced LPS-induced inflammation and apoptosis in MLE-12 lung
epithelial cells and, in vivo, decreased pathological
damage, inflammation and apoptosis in cecum ligation and
perforation-induced mouse septic acute lung injury model (55).
Idiopathic pulmonary fibrosis, a group of
heterogeneous parenchymal lung disorders characterized by chronic
progressive fibrosis, represents one of the most severe
interstitial lung diseases (56). Sun et al (44) reported that SSD inhibits human
embryonic lung fibroblast proliferation and collagen production
[Collagen-1 (Col-1), α-smooth muscle actin (α-SMA)] via modulation
of the TGF-β1/Smads pathway, exerting an antifibrotic effect. A
recent study revealed that SSD reduces the expression of
angiotensin-1 (Ang-1), Ang-2 and Tie-2 (tyrosine kinase receptors
with immunoglobulin and epidermal growth factor homology domains-2)
in the angiopoietin/Tie2 pathway, which may help alleviate
pulmonary inflammation in bleomycin-induced mice (administered via
intratracheal injection) by inhibiting angiogenesis and fibrosis
(57).
Excessive acetaminophen (APAP) intake can lead to
acute liver injury, which can be fatal and necessitates effective
pharmacological intervention (58). Liu et al (59) found that SSD protected mice from
APAP-induced hepatotoxicity by primarily inhibiting NF-κB and
STAT3-mediated inflammatory signaling pathways (IL-6, C-C motif
chemokine ligand 2, IL-10). Another study indicated that SSD
mitigated carbon tetrachloride-induced acute hepatocyte damage by
suppressing OS and NLR family pyrin domain containing 3 (NLRP3)
inflammasome activation in HL-7702 cells (60). Similar protective effects were
observed in animal models (61),
where SSD administration led to reduced MDA and superoxide
production in carbon tetrachloride-treated mouse liver tissue while
enhancing SOD, glutathione peroxidase (GPx) and catalase
activities. In addition, protein levels of caspase 1, NLRP3,
apoptosis-associated speck-like protein, IL-1β and IL-18 were
diminished (61), supporting
conclusions drawn from in vitro experiments.
ALD, encompassing a range of liver damage due to
chronic excessive alcohol intake, has become a significant global
health concern (62). Hepatic
stellate cells (HSCs) play a pivotal role in ALD progression, with
activated HSCs acting as a primary source of extracellular matrix
(ECM) in the liver (63). Jiang
et al (64) demonstrated
that SSD suppressed proliferation and promoted apoptosis in
acetaldehyde-activated rat HSC-T6 cells by stimulating
autophagosome formation. SSD treatment increased caspase-3 and Bax
expression while reducing Ki67 and Bcl-2 levels. Despite its
therapeutic potential, SSD has poor bioavailability, stability and
solubility, which limits its clinical application. A promising
study aimed to enhance SSD's efficacy by formulating SSD-loaded
liposomes using a thin-film hydration method (65). Compared to the group treated with
SSD alone, mice administered SSD liposomes showed significantly
lower serum alanine aminotransferase, aspartate aminotransferase,
MDA, TNF-α, total cholesterol and triglyceride levels in liver
homogenates, while GPx and total SOD levels were significantly
elevated. These observations suggest that SSD liposomes exhibit
enhanced hepatoprotective effects in alleviating alcoholic
hepatitis in mice, likely due to their improved antioxidative and
anti-inflammatory properties (65).
Adipogenesis is the developmental process by which
preadipocytes differentiate into mature adipocytes, leading to fat
accumulation (66). Research has
shown that SSD suppresses adipogenesis in 3T3-L1 adipocytes by
promoting AMP-activated protein kinase phosphorylation and its
substrate acetyl-CoA carboxylase during the early stage of
adipogenesis, while concurrently inhibiting the phosphorylation of
the ERK1/2 and p38 MAPK pathways (67). In a study focused on SSD's
effects on NAFLD, SSD was found to significantly reduce fatty acid
biosynthesis by downregulating fatty acid synthase (FASN) and
acetyl-CoA carboxylases α (ACACA) expression and to enhance fatty
acid degradation through increased expression of COX1 and carnitine
palmitoyltransferase-1α (CPT1α) (22). Building on these outcomes, Gu
et al (68) conducted
further investigations using a high-fat diet (HFD)- and fructose
water-induced metabolic dysfunction-associated fatty liver disease
mouse model along with HepG2 cells, primary mouse hepatocytes and
adipocytes. They discovered that SSD acts as a potent peroxisome
proliferator-activated receptor α (PPARα) activator, promoting
fatty acid oxidation in hepatocytes and adipocytes while
upregulating insulin-induced genes 1 and 2 (INSIG1/2) expression,
which inhibits sterol regulatory element-binding protein 1c
(SREBP-lc) maturation and thereby reduces fatty acid production. Of
note, the lipid-regulating effects of SSD were nullified by the
PPARα inhibitor GW6471, indicating that SSD mitigates
metabolism-related fatty liver disorder via coordinated modulation
of the PPARα and INSIG/SREBP-1c pathways (68).
In a separate study on DSS-induced UC in mice, SSD
significantly decreased pro-inflammatory cytokines (TNF-α, IL-6 and
IL-1β) and elevated mRNA levels of the anti-inflammatory cytokine
IL-10 (75). The primary
mechanism involves SSD's suppression of NF-κB activation and
modulation of the gut microbiota to mitigate DSS-induced intestinal
inflammation (75).
CP is a progressive fibro-inflammatory syndrome
where acinar cell injury initiates inflammation and pancreatic
stellate cell (PSC) activation (76). Cui et al (77) found that SSD alleviates
pancreatic fibrosis by inhibiting PSC autophagy through the
PI3K/AKT/mTOR pathway. Their subsequent research showed that SSD
further reduces pancreatic injury by inhibiting acinar cell
apoptosis and inflammation via the MAPK signaling pathway (37).
Prior research has shown that SSD can prevent
aminonucleoside-induced proteinuria in rats (78). Another study indicated that SSD
protects renal tubular epithelial cells from high-glucose-induced
injury through modulation of SIRT3 (79). Li et al (51) demonstrated that SSD hinders the
progression of mesangial proliferative glomerulonephritis in rats
by downregulating TGF-β1 and reducing macrophages and
CD8+ T lymphocytes. Additionally, it has been suggested
that SSD reduces renal inflammation and apoptosis in a sepsis mouse
model by inhibiting the transcription factor-7 (TCF7)/Fos-like
antigen 1/matrix metalloproteinase (MMP)-9 axis (24), supporting its protective role in
renal inflammation.
In CKD, skeletal muscle atrophy diminishes quality
of life and increases morbidity and mortality (80). Huang et al (41) developed a muscle atrophy model
using 5/6 nephrectomized mice and dexamethasone-treated C2C12
myotubes. Their results revealed that SSD activates the
PI3K/AKT/Nrf2 pathway, reducing OS and alleviating CKD-induced
muscle atrophy (41).
Cisplatin (DDP)-induced nephrotoxicity significantly
limits the efficacy of this widely used anticancer drug (81), with inflammation and apoptosis
likely contributing to the observed renal damage. Ma et al
(82) examined the
anti-apoptotic, anti-inflammatory and antioxidant effects of SSD in
DDP-induced injury in human renal cortex and HK-2 human proximal
tubular epithelial cells. Their findings indicated that SSD
substantially increased the survival rate of DDP-treated HK-2
cells, improved nuclear morphology and reduced vesicle-3 activation
and apoptosis. In addition, SSD treatment significantly lowered
TNF-α, IL-1β and IL-6 secretion, NO generation and iNOS expression.
The underlying mechanism suggests that SSD mitigates DDP-induced
nephrotoxicity by inhibiting the MAPK and NF-κB signaling pathways
(82).
Peritoneal dialysis has the potential to enhance
life quality and prolong survival in patients with renal failure;
however, peritoneal fibrosis can frequently lead to treatment
discontinuation (83). Liu et
al (84) found that SSD may
alleviate peritoneal fibrosis in renal failure rats by modulating
the TGF-β1/BMP7/Gremlin1/Smads pathway, though further research is
needed to confirm SSD's mechanism in renal failure.
AD, a neurodegenerative disorder, manifests
primarily through dementia, memory loss and language deficits
(85). As elevated ROS levels
are implicated in OS in AD (86). Du et al (87) showed that SSD alleviates
glutamate-induced oxidative cytotoxicity in SH-SY5Y human
neuroblastoma cells by activating the Nrf2 pathway, underscoring
SSD's neuroprotective effects. However, it has been reported that
SSD may impair cognitive function in mice by inhibiting hippocampal
neurogenesis through the AKT/forkhead box G1 pathway (27). Another study found that SSD
decreases the viability of neural stem/progenitor cells by
disrupting neurotrophic factor receptor signaling, thus impairing
hippocampal neurogenesis and potentially leading to cognitive
deficits (88). These
observations suggest a complex role for SSD in AD, with both
protective and adverse effects, highlighting the need for further
research to clarify SSD's impact on neurodegeneration.
Depression is a severe, recurrent disorder
characterized by persistent low mood, anhedonia and impaired
cognitive function (89). Early
studies indicated that SSD therapy significantly increased
hippocampal neurogenesis in rats exposed to unpredictable chronic
mild stress (UCMS), as evidenced by elevated doublecortin levels.
In addition, SSD treatment boosted hippocampal neuromolecule
levels, such as p-CREB and BDNF, in UCMS rats, suggesting that SSD
may counter CMS-induced depressive behaviors partly by enhancing
hypothalamic-pituitary-adrenal axis function and supporting
hippocampal neurogenesis (90).
Further research demonstrated that SSD alleviates depressive-like
behavior in UCMS rats by reducing NF-κB and microRNA (miR)-155,
while upregulating the fibroblast growth factor 2 (91), homer protein homolog
1-metabotropic glutamate receptor 5 and mTOR signaling pathways
(92). A recent study found that
SSD alleviates depression in UCMS mice by promoting NLRP3
ubiquitination and suppressing inflammasome activation (26). SSD also reduced LPS-induced
depression-like behavior in mice by inhibiting microglial
activation and neuroinflammation, potentially through
downregulation of the mammaglobin 1/Toll-like receptor 4/NF-κB
pathway (93). These insights
suggest SSD as a promising candidate for treating depressive
disorders.
Diabetes complications include diabetic nephropathy
(DN) and diabetic peripheral neuropathy (DPN) (94). Zhao et al (79) showed that SSD protects renal
tubular epithelial cells (NRK-52E) from high glucose-induced OS by
upregulating SIRT3, elucidating its protective mechanism against
DN. Another study revealed that SSD improved body weight, lowered
blood glucose levels, alleviated mechanical and thermal
hyperalgesia, enhanced nerve conduction velocity and reduced
pro-inflammatory cytokines (TNF-α, IL-1β and IL-6) in
streptozotocin/HFD-induced DPN in rats. Of note, SSD mitigated DPN
by inhibiting the aquaporin 1/Ras homolog family member
A/Rho-associated protein kinase signaling pathway (25).
Osteoarthritis is the most prevalent joint disease
among the elderly, characterized by inflammation and autophagy
dysregulation (96). A
literature survey by Jiang et al (97) indicated that SSD may inhibit
inflammation and modulate autophagic processes by downregulating
the PI3K/AKT/mTOR signaling pathway, positioning SSD as a promising
treatment option for osteoarthritis. Wu et al (98) demonstrated in their experimental
investigation that SSD inhibited IL-1β-induced apoptosis in
differentiated ATDC 5 chondrocytes in vitro. Furthermore,
SSD effectively prevented cartilage degeneration in the knee joints
of mice and reduced the number of osteochondrocytes in the
subchondral bone. Of note, SSD activates the Nrf2/heme
oxygenase-1/ROS axis, suppresses the production of inflammatory
mediators and protects against ECM degradation, thereby delaying
the progression of osteoarthritis in destabilization of the medial
meniscus model mice (98). A
recent study also reported that SSD reduces inflammatory responses
in osteoarthritis mice and chondrocytes and mediates autophagy by
upregulating miR-199-3p, which targets TCF4 (99). These insights collectively
support the notion that SSD mitigates the development of
osteoarthritis through its anti-inflammatory and autophagic
effects.
In thyroid cancer, SSD has been shown to enhance p53
and Bax expression while reducing Bcl-2 levels, promoting apoptosis
in thyroid cancer cells. In addition, SSD notably elevates p21 and
inhibits cyclin-dependent kinase 2 and cyclin D1, leading to the
suppression of human thyroid cancer cell growth (100).
Prior research has established that SSD inhibits
growth and induces programmed cell death in non-small cell lung
cancer (NSCLC) cells (A549 and H1299), primarily by inhibiting
STAT3 phosphorylation and activating caspase 3 (52). Chen et al (46) further elucidated the anti-cancer
mechanism of SSD, revealing that it induces apoptosis in A549 cells
through two pathways: TGFα-JNK-p53 and TGFα-Rassfia-Mst1. SSD also
inhibits A549 cell proliferation by activating two pathways:
TGF-β-p53/p21/p27/p15/p16 and TGF-α/Rassfia/cyclin D1 (46). Drug resistance remains a
significant challenge in lung cancer treatment (101). In vivo and in
vitro studies by Tang et al (102) indicated that SSD reduced
p-STAT3 levels and increased Bcl-2 expression. Downregulation of
STAT3 enhanced lung cancer cell responsiveness to gefitinib,
suggesting that the combination of SSD and gefitinib may yield
improved anti-tumor effects in NSCLC cells, linked to the
inhibition of the STAT3/Bcl-2 signaling pathway. Recent
investigations have also demonstrated that SSD induces apoptosis in
HCC827 and A549 NSCLC cells by increasing ROS levels and activating
the NF-κB/NLRP3/caspase-1/gasdermin D pathway (50). These outcomes provide a strong
rationale for the use of SSD in the treatment of NSCLC.
Elevated expression of P-glycoprotein (P-gp) in
multidrug-resistant (MDR) cells poses a significant barrier to
effective cancer chemotherapy (103). Research indicates that SSD
effectively counteracts P-gp-mediated multidrug resistance in
MCF-7/adriamycin breast cancer cells (104). Another study corroborated these
findings, revealing that the combination of DOX and SSD yielded a
stronger anti-cancer effect compared to either DOX or SSD alone
(105). Furthermore, Wang et
al (49) demonstrated that
SSD significantly inhibits β-catenin and its associated downstream
target genes, leading to programmed cell death in various
triple-negative breast cancer cell lines (HCC1937, MDA-MB-468 and
MDA-MB-231 and MCF-7). Fu et al (106) expanded on the mechanisms
underlying SSD's anti-breast cancer activity, showing that it
inhibits autophagosome-lysosome fusion and induces
autophagy-independent apoptosis via caspase-3 activation in
MDA-MB-231 cells. A recent untargeted metabolomic analysis
highlighted that SSD exerts anti-breast cancer effects by
regulating basal metabolism (107), with changes in serum
metabolites observed in breast cancer mice following SSD treatment,
including alterations in sphingolipid metabolism,
glycerophospholipid metabolism and the biosynthetic pathways for
phenylalanine, tyrosine and tryptophan (107).
The integration of SSD and radiotherapy demonstrates
superior efficacy in the treatment of liver cancer compared to
their individual applications. Research indicates that SSD enhances
the radiosensitivity of SMMC-7721 liver cancer cells by regulating
the G0/G1 and G2/M cell cycle checkpoints, which correlates with
the upregulation of p53 and Bax and the downregulation Bcl-2
(116). A similar study
confirmed that SSD also increases the radiosensitivity of liver
cancer cells (SMMC-7721 and HepG2) under hypoxic conditions by
suppressing hypoxia-inducible factor-1α, thereby triggering the
upregulation of p53 and Bax and the downregulation of Bcl-2
(117). The activation of
glioma-associated oncogene (GLI) family proteins and significant
protein assimilation are characteristic of HCC cells. Hypoxia
activates the sonic hedgehog pathway, which promotes
epithelial-to-mesenchymal transition (EMT), invasion and
chemosensitivity in HCC cells, with hypoxia-dependent GLI protein
activation requiring SUMOylation (118-120). Zhang et al (121) demonstrated that SSD inhibited
liver cancer cells (Hep3B) and enhanced chemotherapy sensitivity
through sentrin-specific protease 5-dependent inhibition of Gli1
SUMOylation in hypoxic environments. Furthermore, SSD significantly
increased radiation-induced apoptosis in SMMC-7721 and MHCC97L
liver cancer cells while concurrently inhibiting cell proliferation
(122). The introduction of the
autophagy inhibitor chloroquine or the mTOR agonist MHY1485
attenuated the pharmacodynamic effects of SSD, suggesting that SSD
enhances radiation-induced apoptosis in HCC cells by promoting
autophagy through the inhibition of mTOR phosphorylation (123).
Ongoing investigations into the anti-HCC mechanisms
of SSD have included a non-targeted metabolomics study revealing
that SSD, in combination with neuropilin (NRP)-1 gene knockout,
exerts anti-liver cancer effects in vitro, primarily by
modulating lipid transport and phospholipid metabolism (124). In addition, the potential
interactions between SSD and its hypothetic target, NRP-1, are
under investigation. Li et al (125) found that SSD significantly
induced the expression and enzyme activity of cytochrome P 450
enzyme (CYP)1A2 and CYP2D6 in HepaRG cells. Another study utilizing
molecular docking indicated that SSD substantially suppressed the
expression and enzyme activity of CYP3A4 protein in HepaRG cells;
however, experimental validation is still required (126).
ICCA is a highly lethal malignant tumor, with
systemic chemotherapy using gemcitabine as the primary clinical
treatment option (127,128). Norepinephrine (NE) and
epinephrine (E) have been shown to promote ICCA cell growth and
diminish the efficacy of gemcitabine through stimulation of the β
2-adrenergic receptors (ADRB2) receptor (129). A 2023 study demonstrated that
SSD can reverse the adverse effects of gemcitabine in ICCA cells by
reducing ADRB2 levels. Furthermore, SSD inhibited drug efflux and
glycolysis in ICCA cells by modulating the expression of multidrug
resistance 1, ABC subfamily G, isoform 2 protein, hexokinase 2 and
glucose transporter 1. This suggests that SSD may counteract NE-
and E-induced gemcitabine resistance in intrahepatic
cholangiocarcinoma via downregulation of ADRB2 and glycolytic
signaling pathways (130).
GC remains a significant contributor to
cancer-related mortality, with drug resistance being a critical
factor in its poor prognosis (131). Research by Hu et al
(28) indicated that SSD
enhances the efficacy of DDP by inhibiting the growth and
invasiveness of SGC-7901 and SGC-7901/DDP-resistant cells, thereby
increasing DDP-induced apoptosis. SSD appears to enhance the
sensitivity of GC cells to DDP, potentially through inhibition of
the IKKβ/NF-κB pathway, which induces apoptosis and autophagy in GC
cells (28). A recent network
pharmacology study identified six key targets linking SSD to
gastric cancer: VEGFA, IL-2, CASP3, BCL2L1, MMP2 and MMP1.
Experimental results demonstrated that SSD induces apoptosis in
cancer cells by enhancing caspase-3 activity and elevating Bcl-2
levels, while also inhibiting migration and invasion through
upregulation of IL-2 and downregulation of MMP1 and MMP2 (132). These findings indicate that SSD
may play a role in overcoming drug resistance in GC treatment.
Pancreatic cancer remains one of the deadliest
malignancies with limited therapeutic options (133). SSD has been shown to inhibit
the growth of pancreatic cancer cells (BxPC3, PANC1 and Pan02) and
enhance the cleavage of apoptotic proteins caspase-3 and caspase-9
by activating the MAPK kinase 4-JNK signaling cascade (47). An additional in vivo and
in vitro study found SSD directly suppresses pancreatic
cancer cell apoptosis and invasion while modulating the
immunosuppressive microenvironment to reactivate local immune
responses. The primary mechanism involves reducing M2 macrophage
polarization by downregulating phosphorylated STAT6 levels and the
PI3K/AKT/mTOR signaling pathway (134). These findings suggest that SSD
holds promise as a potential treatment for pancreatic cancer.
DDP and its derivatives serve as first-line
anticancer drugs for ovarian cancer (139). Studies indicate that SSD
sensitizes drug-resistant ovarian cancer cells to DDP-induced
apoptosis by promoting mitochondrial fission and causing G2/M phase
arrest. The specific mechanism involves the enhancement of calcium
signaling, upregulating of mitochondrial fission proteins
dynamin-related protein 1 and optic atrophy 1 and inhibition of
MMPs (140).
Although human osteosarcoma has a low incidence
rate, its prognosis is often poor compared to other cancers
(142). A study assessing SSD's
therapeutic potential in osteosarcoma demonstrated that it
significantly suppresses the proliferation of 143B and MG-63 cells.
The underlying mechanism includes the upregulation of tumor protein
53 (p53) and its downstream targets (p21, p27, Bcl-2-like protein 4
and cleaved caspase-3), along with the downregulation of cyclin D1
expression levels (143).
Another study within the same year indicated that SSD, either alone
or in combination with the JNK inhibitor SP600125, inhibits
proliferation, induces apoptosis, and suppresses migration and
invasion in human osteosarcoma U2 cells. However, SP600125 alone
did not show a significant effect on U2 cells. The mechanism
involves enhancement of cytochrome release, elevation of the
Bax/Bcl-2 ratio, and activation of caspases -3, -8 and -9,
indicating that apoptosis is triggered via both mitochondrial and
death receptor pathways (144).
These findings provide a theoretical basis for the use of SSD in
osteosarcoma treatment.
An earlier study demonstrated that SSD can inhibit
apoptosis in human GBM U87 cells, primarily by significantly
suppressing the phosphorylation of AKT and ERK while promoting the
expression of phosphorylated JNK and cleaved caspase-3 (145). A subsequent investigation
revealed that SSD notably impedes the growth of RG-2, U87-MG and
U251 cells in a sugar-dependent manner, leading to a marked
increase in the proportion of apoptotic cells. Further research has
indicated that SSD induces apoptosis and autophagy by activating
endoplasmic reticulum stress in GBM cells, thereby exerting
anti-GBM effects (146).
Chemotherapy remains a critical adjunctive treatment for
glioblastoma; however, resistance to chemotherapy presents an
urgent clinical challenge for neuro-oncologists (147). Liang et al (148) discovered that SSD partially
suppressed the migration, invasion and apoptosis of LN-229 cells.
When combined with temozolomide (TMZ), SSD enhanced the
chemotherapeutic efficacy of TMZ by increasing the apoptosis rate
and LDH release in LN-229 cells, while also inhibiting the
expression of stem cell factors (octamer-binding transcription
factor 4, SRY-box transcription factor 2, myelocytomatosis viral
oncogene homolog and Kruppel-like factor 4 at both gene and protein
levels (148). These insights
lay the groundwork for the potential application of SSD in
overcoming GBM chemotherapy resistance, although further research
is required for a more comprehensive understanding.
MB is a highly malignant embryonic tumor and the
most common primary tumor of the posterior fossa in children
(149). A 2021 study
demonstrated that SSD inhibits tumor progression in MB-transplanted
mice by suppressing the Hedgehog signaling pathway (150). However, further investigations
are necessary to elucidate the mechanisms underlying SSD's anti-MB
effect.
AML remains an uncommon but potentially devastating
condition with persistently elevated mortality rates (151). The fat mass and
obesity-associated protein (FTO), an mRNA N6-methyladenosine (m6A)
demethylase, functions as an oncogene that facilitates leukemic
oncogene-driven cellular transformation and leukemogenesis
(152,153). Research by Sun et al
(154) demonstrated that SSD
significantly suppressed AML cell proliferation, induced apoptosis
and caused cell cycle arrest both in vivo and in
vitro. At the molecular level, SSD specifically targets FTO,
thereby enhancing m6A RNA methylation, which reduces the stability
of downstream gene transcripts and inhibits associated pathways.
Notably, SSD also mitigates FTO/m6A-mediated resistance to tyrosine
kinase inhibitors in leukemia (154). This investigation reveals
promising therapeutic avenues for the treatment of leukemia.
TCM monomers are single components extracted from
TCM, characterized by a clear chemical structure and established
pharmacological activity. Compared to compound formulas and
proprietary Chinese medicines, TCM monomers offer advantages such
as well-defined ingredients, clear mechanisms of action and
straightforward quality control. Consequently, they exhibit
promising applications in the treatment of various diseases
(155-158). SSD is a prominent bioactive
component of Bupleurum, demonstrating pharmacological activities
that include hepatoprotective, anti-inflammatory, antiviral and
anti-tumor effects (15,18,159). The mechanisms underlying these
pharmacological actions involve inflammation, OS, immune
modulation, autophagy and apoptosis, thereby influencing the
progression of numerous diseases (Fig. 4). An unpublished study by our
group also identified that SSD can exert anti-hepatic fibrosis
effects by modulating the expression of biological clock genes in
liver tissue, with findings set for forthcoming publication. These
investigations indicate that SSD is a potential monomer for
addressing lung diseases, kidney diseases, liver diseases and
tumors.
Natural products and botanical sources serve as
vital reservoirs for anti-cancer agents. Several natural compounds
and their derivatives, such as Matrine (160), ginsenosides (161), strychnine (162) and berbamine (163), have demonstrated anti-cancer
effects. While SSD is a natural substance with significant
anti-tumor potential, it is essential to thoroughly understand its
possible adverse effects to ensure pharmaceutical safety during its
application. Research has identified toxic effects of SSD,
including hepatotoxicity, neurotoxicity, hemolysis and
cardiotoxicity (88,164,165). Natural compounds derived from
plants are generally considered less toxic to normal cells than
chemically synthesized drugs, thus offering greater potential
safety as anti-cancer therapies (166-169).
Despite its potential, several challenges remain in
the research surrounding SSD. Firstly, pharmacokinetic studies are
insufficient. Although SSD is categorized as a triterpenoid saponin
with poor water solubility, its bioavailability may exceed current
expectations. Modern research indicates that bioavailability can be
enhanced through methods such as nanoparticle encapsulation and
liposomal formulations (65,170). In addition, investigations into
SSD metabolism reveal its conversion into specific metabolites
(e.g., prosaikogenins and saikogenins) within the body, influenced
by various transformation factors including the intestinal flora,
gastric acid and enzymes (171,172). However, a comprehensive
understanding of the pharmacokinetics of SSD after systemic
administration is currently lacking. For instance, while SSD
excretion has been documented, the specific metabolic pathways and
resulting metabolites are not fully elucidated, hindering a
complete grasp of its pharmacokinetic properties. Secondly, the
mechanistic research on SSD is limited. Current therapeutic
mechanisms attributed to SSD in treating diseases are primarily
associated with specific proteins and pathways, with insufficient
exploration of the complete upstream and downstream pathways and
the interactions between multiple pathways. Although evidence
supports SSD's significant preventive and therapeutic effects on
liver disease, the precise mechanisms of action are not fully
characterized. For instance, despite SSD's recognized therapeutic
efficacy for liver diseases, comparative analyses of its mechanisms
across various liver conditions are sparse, complicating the
differentiation and effective treatment of specific liver diseases
in clinical settings. Thirdly, the synergistic effects of SSD with
other compounds are underexplored. The treatment of tumors poses
significant challenges and the integration of traditional Chinese
and Western medicine is becoming increasingly prevalent in
oncological therapies (173,174). SSD has shown promise as an
anti-cancer monomer; however, research on its application, either
alone or in combination with other TCM monomer saponins, remains
limited. Furthermore, investigations into the synergistic effects
of SSD alongside other compounds, such as SSA and SSC, are scarce.
While studies have optimized the extraction processes for SSA and
SSD, the interactions among these components within the body and
their influence on therapeutic efficacy warrant further
examination. Lastly, there is a notable deficiency in clinical
application research. Although SSD has demonstrated significant
pharmacological activity in preclinical animal studies, there are
relatively few investigations into its clinical application. This
lack of research restricts its broader clinical utilization and
necessitates additional clinical trials to ascertain its safety and
efficacy.
In summary, future research on SSD should
prioritize pharmacokinetic studies, explore the complex
interactions of multiple proteins and pathways and foster
collaborative investigations between traditional Chinese and
Western medicine. Furthermore, in vivo and in vitro
experimental studies on SSD have shown that it is a promising drug
in the treatment of various diseases, but to the best of our
knowledge, there have been no reports of clinical trials. There
should be an increased emphasis on animal studies and clinical
trials. Integrating these research approaches will effectively
translate basic research findings into clinical practice.
Not applicable.
SG: Conceptualization, validation, writing-original
draft. YZ and CC: Software, visualization, writing-review &
editing. JL: Investigation, methodology. YaW: Visualization,
project administration. JuW and YL: Funding acquisition, project
administration, formal analysis. All authors have read the
manuscript and approved it for publication. Data authentication is
not applicable.
Not applicable.
Not applicable.
The authors declare that they have no competing
interests.
Not applicable.
This work was supported by Shanghai Natural Science Foundation
(grant no. 22ZR1459400) and Shanghai Science and Technology
Innovation Project (grant no. 22S21901100).
|
1
|
Zulkifli MH, Abdullah ZL, Mohamed Yusof
NIS and Mohd Fauzi F: In silico toxicity studies of traditional
Chinese herbal medicine: A mini review. Curr Opin Struct Biol.
80:1025882023. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Qian Z, Wang GY, Henning M and Chen Y:
Understanding health literacy from a traditional Chinese medicine
perspective. J Integr Med. 21:215–220. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ai C, Zou Y, Liu H, Yang Z and Xi J:
Traditional Chinese herbal medicine for allergic diseases: A
review. Am J Chin Med. 51:779–806. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lu Y, Shao M, Xiang H, Wang J, Ji G and Wu
T: Qinggan huoxue recipe alleviates alcoholic liver injury by
suppressing endoplasmic reticulum stress through LXR-LPCAT3. Front
Pharmacol. 13:8241852022. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Sun L, Yang Z, Zhao W, Chen Q, Bai H, Wang
S, Yang L, Bi C, Shi Y and Liu Y: Integrated lipidomics,
transcriptomics and network pharmacology analysis to reveal the
mechanisms of Danggui Buxue Decoction in the treatment of diabetic
nephropathy in type 2 diabetes mellitus. J Ethnopharmacol.
283:1146992022. View Article : Google Scholar
|
|
6
|
Xiang H, Shao M, Lu Y, Wang J, Wu T and Ji
G: Kaempferol alleviates steatosis and inf lammation during early
Non-Alcoholic steatohepatitis associated with liver X Receptor
α-Lysophosphatidylcholine acyltransferase 3 signaling pathway.
Front Pharmacol. 12:6907362021. View Article : Google Scholar
|
|
7
|
Paik S, Song GY and Jo EK: Ginsenosides
for therapeutically targeting inflammation through modulation of
oxidative stress. Int Immunopharmacol. 121:1104612023. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Li J, Li F and Jin D: Ginsenosides are
promising medicine for tumor and inflammation: A review. Am J Chin
Med. 51:883–908. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zarneshan SN, Fakhri S and Khan H:
Targeting Akt/CREB/BDNF signaling pathway by ginsenosides in
neurodegenerative diseases: A mechanistic approach. Pharmacol Res.
177:1060992022. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Teng L, Guo X, Ma Y, Xu L, Wei J and Xiao
P: A comprehensive review on traditional and modern research of the
genus Bupleurum (Bupleurum L., Apiaceae) in recent 10 years. J
Ethnopharmacol. 306:1161292023. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Sui C, Han WJ, Zhu CR and Wei JH: Recent
progress in saikosaponin biosynthesis in bupleurum. Curr Pharm
Biotechnol. 22:329–340. 2021. View Article : Google Scholar
|
|
12
|
Shao S, Jia R, Zhao L, Zhang Y, Guan Y,
Wen H, Liu J, Zhao Y, Feng Y, Zhang Z, et al: Xiao-Chai-Hu-Tang
ameliorates tumor growth in cancer comorbid depressive symptoms via
modulating gut microbiota-mediated TLR4/MyD88/NF-κB signaling
pathway. Phytomedicine. 88:1536062021. View Article : Google Scholar
|
|
13
|
Fan Q, Liu Y, Sheng L, Lv S, Yang L, Zhang
Z, Guo J, Fan Y and Hu D: Chaihu-Shugan-San inhibits
neuroinflammation in the treatment of post-stroke depression
through the JAK/STAT3-GSK3β/PTEN/Akt pathway. Biomed Pharmacother.
160:1143852023. View Article : Google Scholar
|
|
14
|
Jiao H, Fan Y, Gong A, Li T, Fu X and Yan
Z: Xiaoyaosan ameliorates CUMS-induced depressive-like and anorexia
behaviors in mice via necroptosis related cellular senescence in
hypothalamus. J Ethnopharmacol. 318:1169382024. View Article : Google Scholar
|
|
15
|
Luo K, Dai RJ, Zeng YB, Chang WJ, Deng YL
and Lv F: Triterpenoid saponins from Bupleurum marginatum and their
anti-liver fibrotic activities. J Asian Nat Prod Res. 26:858–864.
2024. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kang Y, Gao Y, Li X, Guo X, Liu Z, Li W,
Wei J and Qi Y: Bupleurum chinense exerts a mild antipyretic effect
on LPS-induced pyrexia rats involving inhibition of peripheral
TNF-α production. J Ethnopharmacol. 310:1163752023. View Article : Google Scholar
|
|
17
|
Zhou J, He X, Sun R, Yu Z, Wang C, Deng S,
Zhang B, Huang S, Han C and Li D: Lignans from Bupleurum marginatum
and their antioxidant activity. Nat Prod Res. 36:5016–5021. 2022.
View Article : Google Scholar
|
|
18
|
Sun P, Li Y, Wei S, Zhao T, Wang Y, Song
C, Xue L, Wang F, Xiao L, Wu J and Qiao M: Pharmacological effects
and chemical constituents of bupleurum. Mini Rev Med Chem.
19:34–55. 2019. View Article : Google Scholar
|
|
19
|
Li XQ, Song YN, Wang SJ, Rahman K, Zhu JY
and Zhang H: Saikosaponins: A review of pharmacological effects. J
Asian Nat Prod Res. 20:399–411. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhang YJ, Wu SS, Chen XM, Pi JK, Cheng YF,
Zhang Y, Wang XJ, Luo D, Zhou JH, Xu JY, et al: Saikosaponin D
Alleviates DOX-induced cardiac injury in vivo and in vitro. J
Cardiovasc Pharmacol. 79:558–567. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wang HW, Liu M, Zhong TD and Fang XM:
Saikosaponin-d attenuates ventilator-induced lung injury in rats.
Int J Clin Exp Med. 8:15137–15145. 2015.PubMed/NCBI
|
|
22
|
Li X, Ge J, Li Y, Cai Y, Zheng Q, Huang N,
Gu Y, Han Q, Li Y, Sun R, et al: Integrative lipidomic and
transcriptomic study unravels the therapeutic effects of
saikosaponins A and D on non-alcoholic fatty liver disease. Acta
Pharm Sin B. 11:3527–3541. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Fan J, Li X, Li P, Li N, Wang T, Shen H,
Siow Y, Choy P and Gong Y: Saikosaponin-d attenuates the
development of liver fibrosis by preventing hepatocyte injury.
Biochem Cell Biol. 85:189–195. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Yao T, Zhang L, Fu Y, Yao L, Zhou C and
Chen G: Saikosaponin-d alleviates renal inflammation and cell
apoptosis in a mouse model of sepsis via TCF7/FOSL1/matrix
metalloproteinase 9 inhibition. Mol Cell Biol. 41:e00332212021.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Xiang Q, Liu Y and Chen L: Saikosaponin d
(SSD) alleviates diabetic peripheral neuropathy by regulating the
AQP1/RhoA/ROCK signaling in streptozotocin-induced diabetic rats.
Acta Diabetol. 60:805–815. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Gao T, Wang T, Wu L, Tong Y, Tian J, Zhao
K and Wang H: Saikosaponin-d alleviates depression by promoting
NLRP3 ubiquitination and inhibiting inflammasome activation. Int
Immunopharmacol. 127:1113242024. View Article : Google Scholar
|
|
27
|
Lixing X, Zhouye J, Liting G, Ruyi Z, Rong
Q and Shiping M: Saikosaponin-d-mediated downregulation of
neurogenesis results in cognitive dysfunction by inhibiting
Akt/Foxg-1 pathway in mice. Toxicol Lett. 284:79–85. 2018.
View Article : Google Scholar
|
|
28
|
Hu J, Li P, Shi B and Tie J: Effects and
mechanisms of saikosaponin D improving the sensitivity of human
gastric cancer cells to cisplatin. ACS Omega. 6:18745–18755. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Chen LL, Xia LY, Zhang JP, Wang Y, Chen
JY, Guo C and Xu WH: Saikosaponin D alleviates cancer cachexia by
directly inhibiting STAT3. Phytother Res. 37:809–819. 2023.
View Article : Google Scholar
|
|
30
|
Wang P, Ren J, Tang J, Zhang D, Li B and
Li Y: Estrogen-like activities of saikosaponin-d in vitro: A pilot
study. Eur J Pharmacol. 626:159–165. 2010. View Article : Google Scholar
|
|
31
|
Que R, Shen Y, Ren J, Tao Z, Zhu X and Li
Y: Estrogen receptor-β-dependent effects of saikosaponin-d on the
suppression of oxidative stress-induced rat hepatic stellate cell
activation. Int J Mol Med. 41:1357–1364. 2018.
|
|
32
|
Lin L, Zhou M, Que R, Chen Y, Liu X, Zhang
K, Shi Z and Li Y: Saikosaponin-d protects against liver fibrosis
by regulating the estrogen receptor-β/NLRP3 inflammasome pathway.
Biochem Cell Biol. 99:666–674. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Zhang K, Lin L, Zhu Y, Zhang N, Zhou M and
Li Y: Saikosaponin d alleviates liver fibrosis by negatively
regulating the ROS/NLRP3 inflammasome through activating the ERβ
pathway. Front Pharmacol. 13:8949812022. View Article : Google Scholar
|
|
34
|
Kumazawa Y, Takimoto H, Nishimura C,
Kawakita T and Nomoto K: Activation of murine peritoneal
macrophages by saikosaponin a, saikosaponin d and saikogenin d. Int
J Immunopharmacol. 11:21–28. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Luo SQ, Lin LZ and Cordell GA:
Saikosaponin derivatives from Bupleurum wenchuanense.
Phytochemistry. 33:1197–1205. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Lu CN, Yuan ZG, Zhang XL, Yan R, Zhao YQ,
Liao M and Chen JX: Saikosaponin a and its epimer saikosaponin d
exhibit anti-inflammatory activity by suppressing activation of
NF-κB signaling pathway. Int Immunopharmacol. 14:121–126. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Li C, Cui L, Zhang L, Yang L, Zhuo Y, Cui
J, Cui N and Zhang S: Saikosaponin D attenuates pancreatic injury
through suppressing the apoptosis of acinar cell via modulation of
the MAPK signaling pathway. Front Pharmacol. 12:7350792021.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Kassis S, Grondin M and Averill-Bates DA:
Heat shock increases levels of reactive oxygen species, autophagy
and apoptosis. Biochim Biophys Acta Mol Cell Res. 1868:1189242021.
View Article : Google Scholar
|
|
39
|
Zhang BZ, Guo XT, Chen JW, Zhao Y, Cong X,
Jiang ZL, Cao RF, Cui K, Gao SS and Tian WR: Saikosaponin-D
attenuates heat stress-induced oxidative damage in LLC-PK1 cells by
increasing the expression of anti-oxidant enzymes and HSP72. Am J
Chin Med. 42:1261–1277. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Lin X, Wu S, Wang Q, Shi Y, Liu G, Zhi J
and Wang F: Saikosaponin-D reduces H2O2-induced PC12 cell apoptosis
by removing ROS and blocking MAPK-dependent oxidative damage. Cell
Mol Neurobiol. 36:1365–1375. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Huang M, Yan Y, Deng Z, Zhou L, She M,
Yang Y, Zhang M and Wang D: Saikosaponin A and D attenuate skeletal
muscle atrophy in chronic kidney disease by reducing oxidative
stress through activation of PI3K/AKT/Nrf2 pathway. Phytomedicine.
114:1547662023. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Su W, Huang S, Zhu H, Zhang B and Wu X:
Interaction between PHB2 and Enterovirus A71 VP1 Induces Autophagy
and Affects EV-A71 Infection. Viruses. 12:4142020. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Li C, Huang L, Sun W, Chen Y, He ML, Yue J
and Ballard H: Saikosaponin D suppresses enterovirus A71 infection
by inhibiting autophagy. Signal Transduct Target Ther. 4:42019.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Sun J, Zheng J, Shi X, Qian H, Jin C and
Xu L: Saikosaponin D inhibits proliferation and collagen production
of human embryonic lung fibroblasts by regulating TGF-β1/Smads
signaling pathway. Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi. 35:256–261.
2019.In Chinese. PubMed/NCBI
|
|
45
|
Du P, Xu J, Jiang Y, Zhao J, Gao C, Fang
Y, Yang X, Yang YP and Zhang JA: Saikosaponin-d attenuates
hashimoto's thyroiditis by regulating macrophage polarization. J
Immunol Res. 2022:74554942022. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Chen X, Liu C, Zhao R, Zhao P, Wu J, Zhou
N and Ying M: Synergetic and antagonistic molecular effects
mediated by the feedback loop of p53 and JNK between Saikosaponin D
and SP600125 on lung cancer A549 cells. Mol Pharm. 15:4974–4984.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Lai M, Ge Y, Chen M, Sun S, Chen J and
Cheng R: Saikosaponin D inhibits proliferation and promotes
apoptosis through activation of MKK4-JNK signaling pathway in
pancreatic cancer cells. Onco Targets Ther. 13:9465–9479. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Wu S, Chen W, Liu K, Ren F, Zheng D, Xu F
and Wu H: Saikosaponin D inhibits proliferation and induces
apoptosis of non-small cell lung cancer cells by inhibiting the
STAT3 pathway. J Int Med Res. 48:3000605209371632020. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Wang J, Qi H, Zhang X, Si W, Xu F, Hou T,
Zhou H, Wang A, Li G, Liu Y, et al: Saikosaponin D from Radix
Bupleuri suppresses triple-negative breast cancer cell growth by
targeting β-catenin signaling. Biomed Pharmacother. 108:724–733.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Chen M, Hu C, Yang L, Guo Q, Liang Y and
Wang W: Saikosaponin-D induces the pyroptosis of lung cancer by
increasing ROS and activating the NF-κB/NLRP3/caspase-1/GSDMD
pathway. J Biochem Mol Toxicol. 37:e234442023. View Article : Google Scholar
|
|
51
|
Li P, Gong Y, Zu N, Li Y, Wang B and
Shimizu F: Therapeutic mechanism of Saikosaponin-d in anti-Thy1 mAb
1-22-3-induced rat model of glomerulonephritis. Nephron Exp
Nephrol. 101:e111–118. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Hsu YL, Kuo PL and Lin CC: The
proliferative inhibition and apoptotic mechanism of Saikosaponin D
in human non-small cell lung cancer A549 cells. Life Sci.
75:1231–1242. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Wong VK, Zhang MM, Zhou H, Lam KY, Chan
PL, Law CK, Yue PY and Liu L: Saikosaponin-d enhances the
anticancer potency of TNF-α via overcoming its undesirable response
of activating NF-Kappa B signalling in cancer cells. Evid Based
Complement Alternat Med. 2013:7452952013. View Article : Google Scholar
|
|
54
|
Rawat PS, Jaiswal A, Khurana A, Bhatti JS
and Navik U: Doxorubicin-induced cardiotoxicity: An update on the
molecular mechanism and novel therapeutic strategies for effective
management. Biomed Pharmacother. 139:1117082021. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Song L, Lu G and Tao Y: Saikosaponin D
attenuates inflammatory response and cell apoptosis of
lipopolysaccharide-induced lung epithelial cells. Clin Respir J.
17:1017–1024. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Moss BJ, Ryter SW and Rosas IO: Pathogenic
mechanisms underlying idiopathic pulmonary fibrosis. Annu Rev
Pathol. 17:515–546. 2022. View Article : Google Scholar
|
|
57
|
Wu Y, Zhang J, Wang X, Xu Y and Zheng J:
Saikosaponin-d regulates angiogenesis in idiopathic pulmonary
fibrosis through angiopoietin/Tie-2 pathway. Acta Histochem.
125:1521002023. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Jaeschke H, Akakpo JY, Umbaugh DS and
Ramachandran A: Novel therapeutic approaches against
acetaminophen-induced liver injury and acute liver failure. Toxicol
Sci. 174:159–167. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Liu A, Tanaka N, Sun L, Guo B, Kim JH,
Krausz KW, Fang Z, Jiang C, Yang J and Gonzalez FJ: Saikosaponin d
protects against acetaminophen-induced hepatotoxicity by inhibiting
NF-κB and STAT3 signaling. Chem Biol Interact. 223:80–86. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Lin L, Que R, Shen Y, Chen Y, Yan N and Li
Y: Saikosaponin-d alleviates carbon-tetrachloride induced acute
hepatocellular injury by inhibiting oxidative stress and NLRP3
inflammasome activation in the HL-7702 cell line. Mol Med Rep.
17:7939–7946. 2018.PubMed/NCBI
|
|
61
|
Chen Y, Que R, Lin L, Shen Y, Liu J and Li
Y: Inhibition of oxidative stress and NLRP3 inflammasome by
Saikosaponin-d alleviates acute liver injury in carbon
tetrachloride-induced hepatitis in mice. Int J Immunopathol
Pharmacol. 34:20587384209505932020. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Singal AK, Bataller R, Ahn J, Kamath PS
and Shah VH: ACG Clinical Guideline: Alcoholic liver disease. Am J
Gastroenterol. 113:175–194. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Tsuchida T and Friedman SL: Mechanisms of
hepatic stellate cell activation. Nat Rev Gastroenterol Hepatol.
14:397–411. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Jiang H, Liu J, Zhang K and Zeng Q:
Saikosaponin D inhibits the proliferation and promotes the
apoptosis of rat hepatic stellate cells by inducing autophagosome
formation. Evid Based Complement Alternat Med. 2021:54517582021.
View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Yu X, Pan J, Shen N, Zhang H, Zou L, Miao
H and Xing L: Development of Saikosaponin D liposome nanocarrier
with increased hepatoprotective effect against alcoholic hepatitis
mice. J Biomed Nanotechnol. 17:627–639. 2021. View Article : Google Scholar
|
|
66
|
Byrne CD and Targher G: NAFLD: A
multisystem disease. J Hepatol. 62(1 Suppl): S47–S64. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Lim SH, Lee HS, Han HK and Choi CI:
Saikosaponin A and D inhibit adipogenesis via the AMPK and MAPK
signaling pathways in 3T3-L1 adipocytes. Int J Mol Sci.
22:114092021. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Gu Y, Duan S, Ding M, Zheng Q, Fan G, Li
X, Li Y, Liu C, Sun R and Liu R: Saikosaponin D attenuates
metabolic associated fatty liver disease by coordinately tuning
PPARα and INSIG/SREBP1c pathway. Phytomedicine. 103:1542192022.
View Article : Google Scholar
|
|
69
|
Abe H, Sakaguchi M, Odashima S and Arichi
S: Protective effect of saikosaponin-d isolated from Bupleurum
falcatum L. on CCl4-induced liver injury in the rat. Naunyn
Schmiedebergs Arch Pharmacol. 320:266–271. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Dang SS, Wang BF, Cheng YA, Song P, Liu ZG
and Li ZF: Inhibitory effects of saikosaponin-d on CCl4-induced
hepatic fibrogenesis in rats. World J Gastroenterol. 13:557–563.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Chen MF, Huang CC, Liu PS, Chen CH and
Shiu LY: Saikosaponin a and saikosaponin d inhibit proliferation
and migratory activity of rat HSC-T6 cells. J Med Food. 16:793–800.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Chen MF, Huang SJ, Huang CC, Liu PS, Lin
KI, Liu CW, Hsieh WC, Shiu LY and Chen CH: Saikosaponin d induces
cell death through caspase-3-dependent, caspase-3-independent and
mitochondrial pathways in mammalian hepatic stellate cells. BMC
Cancer. 16:5322016. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Chen Y, Que R, Zhang N, Lin L, Zhou M and
Li Y: Saikosaponin-d alleviates hepatic fibrosis through regulating
GPER1/autophagy signaling. Mol Biol Rep. 48:7853–7863. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Zeng Y, Zhou L, Wan Y, Fu T, Xu P, Zhang H
and Guan Y: Effects of Saikosaponin D on apoptosis, autophagy, and
morphological structure of intestinal cells of cajal with
functional dyspepsia. Comb Chem High Throughput Screen.
27:1513–1522. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Li P, Wu M, Xiong W, Li J, An Y, Ren J,
Xie Y, Xue H, Yan D, Li M, et al: Saikosaponin-d ameliorates
dextran sulfate sodium-induced colitis by suppressing NF-κB
activation and modulating the gut microbiota in mice. Int
Immunopharmacol. 81:1062882020. View Article : Google Scholar
|
|
76
|
Habtezion A: Inflammation in acute and
chronic pancreatitis. Curr Opin Gastroenterol. 31:395–399. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Cui LH, Li CX, Zhuo YZ, Yang L, Cui NQ and
Zhang SK: Saikosaponin d ameliorates pancreatic fibrosis by
inhibiting autophagy of pancreatic stellate cells via PI3K/Akt/mTOR
pathway. Chem Biol Interact. 300:18–26. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Abe H, Orita M, Konishi H, Arichi S and
Odashima S: Effects of saikosaponin-d on aminonucleoside nephrosis
in rats. Eur J Pharmacol. 120:171–178. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Zhao L, Zhang H, Bao J, Liu J and Ji Z:
Saikosaponin-d protects renal tubular epithelial cell against high
glucose induced injury through modulation of SIRT3. Int J Clin Exp
Med. 8:6472–6481. 2015.PubMed/NCBI
|
|
80
|
Kidney Disease: Improving Global Outcomes
(KDIGO) CKD Work Group: KDIGO 2024 clinical practice guideline for
the evaluation and management of chronic kidney disease. Kidney
Int. 105(Suppl): S117–S314. 2024. View Article : Google Scholar
|
|
81
|
Loren P, Lugones Y, Saavedra N, Saavedra
K, Páez I, Rodriguez N, Moriel P and Salazar LA: MicroRNAs involved
in intrinsic apoptotic pathway during Cisplatin-induced
nephrotoxicity: Potential use of natural products against
DDP-induced apoptosis. Biomolecules. 12:12062022. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Ma X, Dang C, Kang H, Dai Z, Lin S, Guan
H, Liu X, Wang X and Hui W: Saikosaponin-D reduces
cisplatin-induced nephrotoxicity by repressing ROS-mediated
activation of MAPK and NF-κB signalling pathways. Int
Immunopharmacol. 28:399–408. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Tomino Y: Mechanisms and interventions in
peritoneal fibrosis. Clin Exp Nephrol. 16:109–114. 2012. View Article : Google Scholar
|
|
84
|
Ruiqi L, Ming P, Qihang S, Yangyang L,
Junli C, Wei L, Chao G, Xinyue L, Kang Y and Hongtao Y:
Saikosaponin D inhibits peritoneal fibrosis in rats with renal
failure by regulation of TGFβ1/BMP7/Gremlin1/Smad pathway. Front
Pharmacol. 12:6286712021. View Article : Google Scholar
|
|
85
|
Sun Y, Zhang H, Zhang X, Wang W, Chen Y,
Cai Z, Wang Q, Wang J and Shi Y: Promotion of astrocyte-neuron
glutamate-glutamine shuttle by SCFA contributes to the alleviation
of Alzheimer's disease. Redox Biol. 62:1026902023. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Bai R, Guo J, Ye XY, Xie Y and Xie T:
Oxidative stress: The core pathogenesis and mechanism of
Alzheimer's disease. Ageing Res Rev. 77:1016192022. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Du J, Song D, Li Y, Liu J, Huang X, Li B
and Li L: Saikosaponin-D mitigates oxidation in SH-SY5Y cells
stimulated by glutamate through activation of Nrf2 pathway:
Involvement of PI3K. Neurotox Res. 40:230–240. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Qin T, Yuan Z, Yu J, Fu X, Deng X, Fu Q,
Ma Z and Ma S: Saikosaponin-d impedes hippocampal neurogenesis and
causes cognitive deficits by inhibiting the survival of neural
stem/progenitor cells via neurotrophin receptor signaling in mice.
Clin Transl Med. 10:e2432020. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Smith K: Mental health: A world of
depression. Nature. 515:1812014. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Li HY, Zhao YH, Zeng MJ, Fang F, Li M, Qin
TT, Ye LY, Li HW, Qu R and Ma SP: Saikosaponin D relieves
unpredictable chronic mild stress induced depressive-like behavior
in rats: Involvement of HPA axis and hippocampal neurogenesis.
Psychopharmacology (Berl). 234:3385–3394. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Chao B, Huang S, Pan J, Zhang Y and Wang
Y: Saikosaponin d downregulates microRNA-155 and upregulates FGF2
to improve depression-like behaviors in rats induced by
unpredictable chronic mild stress by negatively regulating NF-κB.
Brain Res Bull. 157:69–76. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Liu CY, Chen JB, Liu YY, Zhou XM, Zhang M,
Jiang YM, Ma QY, Xue Z, Zhao ZY, Li XJ, et al: Saikosaponin D
exerts antidepressant effect by regulating Homer1-mGluR5 and mTOR
signaling in a rat model of chronic unpredictable mild stress. Chin
Med. 17:602022. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Su J, Pan YW, Wang SQ, Li XZ, Huang F and
Ma SP: Saikosaponin-d attenuated lipopolysaccharide-induced
depressive-like behaviors via inhibiting microglia activation and
neuroinflammation. Int Immunopharmacol. 80:1061812020. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Papatheodorou K, Banach M, Bekiari E,
Rizzo M and Edmonds M: Complications of Diabetes 2017. J Diabetes
Res. 2018:30861672018. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Ralli M, Angeletti D, Fiore M, D'Aguanno
V, Lambiase A, Artico M, de Vincentiis M and Greco A: Hashimoto's
thyroiditis: An update on pathogenic mechanisms, diagnostic
protocols, therapeutic strategies, and potential malignant
transformation. Autoimmun Rev. 19:1026492020. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Martel-Pelletier J, Barr AJ, Cicuttini FM,
Conaghan PG, Cooper C, Goldring MB, Goldring SR, Jones G, Teichtahl
AJ and Pelletier JP: Osteoarthritis. Nat Rev Dis Primers.
2:160722016. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Jiang J, Meng Y, Hu S, Botchway BOA, Zhang
Y and Liu X: Saikosaponin D: A potential therapeutic drug for
osteoarthritis. J Tissue Eng Regen Med. 14:1175–1184. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Wu X, Zhao K, Fang X, Lu F, Cheng P, Song
X, Zhang W, Yao C, Zhu J and Chen H: Saikosaponin D inhibited IL-1β
induced ATDC 5 chondrocytes apoptosis in vitro and delayed
articular cartilage degeneration in OA model mice in vivo. Front
Pharmacol. 13:8459592022. View Article : Google Scholar
|
|
99
|
Yan M, Zhang D and Yang M: Saikosaponin D
alleviates inflammatory response of osteoarthritis and mediates
autophagy via elevating microRNA-199-3p to target transcription
Factor-4. J Orthop Surg Res. 19:1512024. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Liu RY and Li JP: Saikosaponin-d inhibits
proliferation of human undifferentiated thyroid carcinoma cells
through induction of apoptosis and cell cycle arrest. Eur Rev Med
Pharmacol Sci. 18:2435–2443. 2014.PubMed/NCBI
|
|
101
|
Passaro A, Jänne PA, Mok T and Peters S:
Overcoming therapy resistance in EGFR-mutant lung cancer. Nat
Cancer. 2:377–391. 2021. View Article : Google Scholar
|
|
102
|
Tang JC, Long F, Zhao J, Hang J, Ren YG,
Chen JY and Mu B: The Effects and mechanisms by which
Saikosaponin-D enhances the sensitivity of human non-small cell
lung cancer cells to gefitinib. J Cancer. 10:6666–6672. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Dong J, Yuan L, Hu C, Cheng X and Qin JJ:
Strategies to overcome cancer multidrug resistance (MDR) through
targeting P-glycoprotein (ABCB1): An updated review. Pharmacol
Ther. 249:1084882023. View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Li C, Guan X, Xue H, Wang P, Wang M and
Gai X: Reversal of P-glycoprotein-mediated multidrug resistance is
induced by saikosaponin D in breast cancer MCF-7/adriamycin cells.
Pathol Res Pract. 213:848–853. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Li C, Xue HG, Feng LJ, Wang ML, Wang P and
Gai XD: The effect of saikosaponin D on doxorubicin
pharmacokinetics and its MDR reversal in MCF-7/adr cell xenografts.
Eur Rev Med Pharmacol Sci. 21:4437–4445. 2017.PubMed/NCBI
|
|
106
|
Fu R, Zhang L, Li Y, Li B, Ming Y, Li Z,
Xing H and Chen J: Saikosaponin D inhibits autophagosome-lysosome
fusion and induces autophagy-independent apoptosis in MDA-MB-231
breast cancer cells. Mol Med Rep. 22:1026–1034. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Yang T, Li X, Wang X, Meng X, Zhang Z,
Zhao M and Su R: Combination of histological and metabolomic
assessments to evaluate the potential pharmacological efficacy of
saikosaponin D. J Pharm Biomed Anal. 242:1160012024. View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Wen N, Cai Y, Li F, Ye H, Tang W, Song P
and Cheng N: The clinical management of hepatocellular carcinoma
worldwide: A concise review and comparison of current guidelines:
2022 update. Biosci Trends. 16:20–30. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Jia X, Dang S, Cheng Y, Zhang X, Li M, Li
Y and Li S: Effects of saikosaponin-d on syndecan-2, matrix
metalloproteinases and tissue inhibitor of metalloproteinases-2 in
rats with hepatocellular carcinoma. J Tradit Chin Med. 32:415–422.
2012. View Article : Google Scholar
|
|
110
|
Chen Y, Chen HN, Wang K, Zhang L, Huang Z,
Liu J, Zhang Z, Luo M, Lei Y, Peng Y, et al: Ketoconazole
exacerbates mitophagy to induce apoptosis by downregulating
cyclooxygenase-2 in hepatocellular carcinoma. J Hepatol. 70:66–77.
2019. View Article : Google Scholar
|
|
111
|
Pang C, Miao H, Zuo Y, Guo N, Sun D and Li
B: C/EBPβ enhances efficacy of sorafenib in hepatoblastoma. Cell
Biol Int. 45:1897–1905. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
112
|
He SX, Luo JY, Zhao G, Xu JL, Wang YL, Fu
H and Dong L: Effect of saikosaponins-d on cyclooxygenase-2
expression of human hepatocellular carcinoma cell line SMMC-7721.
Zhonghua Gan Zang Bing Za Zhi. 14:712–714. 2006.In Chinese.
PubMed/NCBI
|
|
113
|
Lu XL, He SX, Ren MD, Wang YL, Zhang YX
and Liu EQ: Chemopreventive effect of saikosaponin-d on
diethylinitrosamine-induced hepatocarcinogenesis: Involvement of
CCAAT/enhancer binding protein β and cyclooxygenase-2. Mol Med Rep.
5:637–644. 2012.
|
|
114
|
He S, Lu G, Hou H, Zhao Z, Zhu Z, Lu X,
Chen J and Wang Z: Saikosaponin-d suppresses the expression of
cyclooxygenase-2 through the phospho-signal transducer and
activator of transcription 3/hypoxia-inducible factor-1α pathway in
hepatocellular carcinoma cells. Mol Med Rep. 10:2556–2562. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
115
|
Ren M, McGowan E, Li Y, Zhu X, Lu X, Zhu
Z, Lin Y and He S: Saikosaponin-d Suppresses COX2 Through
p-STAT3/C/EBPβ signaling pathway in liver cancer: A novel mechanism
of action. Front Pharmacol. 10:6232019. View Article : Google Scholar
|
|
116
|
Wang BF, Dai ZJ, Wang XJ, Bai MH, Lin S,
Ma HB, Wang YL, Song LQ, Ma XL, Zan Y, et al: Saikosaponin-d
increases the radiosensitivity of smmc-7721 hepatocellular
carcinoma cells by adjusting the g0/g1 and g2/m checkpoints of the
cell cycle. BMC Complement Altern Med. 13:2632013. View Article : Google Scholar : PubMed/NCBI
|
|
117
|
Wang BF, Wang XJ, Kang HF, Bai MH, Guan
HT, Wang ZW, Zan Y, Song LQ, Min WL, Lin S, et al: Saikosaponin-D
enhances radiosensitivity of hepatoma cells under hypoxic
conditions by inhibiting hypoxia-inducible factor-1α. Cell Physiol
Biochem. 33:37–51. 2014. View Article : Google Scholar
|
|
118
|
Ciepla P, Konitsiotis AD, Serwa RA,
Masumoto N, Leong WP, Dallman MJ, Magee AI and Tate EW: New
chemical probes targeting cholesterylation of Sonic Hedgehog in
human cells and zebrafish. Chem Sci. 5:4249–4259. 2014. View Article : Google Scholar
|
|
119
|
Zhang Z, Zhan X, Kim B and Wu J: A
proteomic approach identifies SAFB-like transcription modulator
(SLTM) as a bidirectional regulator of GLI family zinc finger
transcription factors. J Biol Chem. 294:5549–5561. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
120
|
Hendriks IA and Vertegaal AC: A
comprehensive compilation of SUMO proteomics. Nat Rev Mol Cell
Biol. 17:581–595. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
121
|
Zhang CY, Jiang ZM, Ma XF, Li Y, Liu XZ,
Li LL, Wu WH and Wang T: Saikosaponin-d Inhibits the hepatoma cells
and enhances chemosensitivity through SENP5-dependent inhibition of
Gli1 SUMOylation under hypoxia. Front Pharmacol. 10:10392019.
View Article : Google Scholar : PubMed/NCBI
|
|
122
|
Tian YD, Lin S, Yang PT, Bai MH, Jin YY,
Min WL, Ma HB and Wang BF: Saikosaponin-d increases the
radiosensitivity of hepatoma cells by adjusting cell autophagy. J
Cancer. 10:4947–4953. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
123
|
Wang B, Min W, Lin S, Song L, Yang P, Ma Q
and Guo J: Saikosaponin-d increases radiation-induced apoptosis of
hepatoma cells by promoting autophagy via inhibiting mTOR
phosphorylation. Int J Med Sci. 18:1465–1473. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
124
|
Lv Y, Hou X, Zhang Q, Li R, Xu L, Chen Y,
Tian Y, Sun R, Zhang Z and Xu F: Untargeted metabolomics study of
the in vitro anti-hepatoma effect of saikosaponin d in combination
with NRP-1 knockdown. Molecules. 24:14232019. View Article : Google Scholar : PubMed/NCBI
|
|
125
|
Li H, Tang Y, Wang Y, Wei W, Yin C and
Tang F: Effects of Saikosaponin D on CYP1A2 and CYP2D6 in HepaRG
cells. Drug Des Devel Ther. 14:5251–5258. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
126
|
Li H, Tang Y, Wei W, Yin C and Tang F:
Effects of saikosaponin-d on CYP3A4 in HepaRG cell and
protein-ligand docking study. Basic Clin Pharmacol Toxicol.
128:661–668. 2021. View Article : Google Scholar
|
|
127
|
Edeline J, Bridgewater J, Campillo-Gimenez
B, Neveu E, Phelip JM, Neuzillet C, Boudjema K, Rolland Y, Valle
JW, Garin E, et al: Chemotherapy with or without selective internal
radiation therapy for intrahepatic cholangiocarcinoma: Data from
clinical trials. Hepatology. 79:96–106. 2024. View Article : Google Scholar
|
|
128
|
Kelley RK, Bridgewater J, Gores GJ and Zhu
AX: Systemic therapies for intrahepatic cholangiocarcinoma. J
Hepatol. 72:353–363. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
129
|
Kanno N, Lesage G, Phinizy JL, Glaser S,
Francis H and Alpini G: Stimulation of alpha2-adrenergic receptor
inhibits cholangiocarcinoma growth through modulation of Raf-1 and
B-raf activities. Hepatology. 35:1329–1340. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
130
|
He H, Guo J, Hu Y, Zhang H, Li X, Zhang J
and Jin S: Saikosaponin D reverses epinephrine- and
norepinephrine-induced gemcitabine resistance in intrahepatic
cholangiocarcinoma by downregulating ADRB2/glycolysis signaling.
Acta Biochim Biophys Sin (Shanghai). 55:1404–1414. 2023.PubMed/NCBI
|
|
131
|
Smyth EC, Nilsson M, Grabsch HI, van
Grieken NC and Lordick F: Gastric cancer. Lancet. 396:635–648.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
132
|
Ning N, Li X, Nan Y, Chen G, Huang S, Du
Y, Gu Q, Li W and Yuan L: Molecular mechanism of Saikosaponin-d in
the treatment of gastric cancer based on network pharmacology and
in vitro experimental verification. Naunyn Schmiedebergs Arch
Pharmacol. 397:8943–8959. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
133
|
Klein AP: Pancreatic cancer epidemiology:
Understanding the role of lifestyle and inherited risk factors. Nat
Rev Gastroenterol Hepatol. 18:493–502. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
134
|
Xu X, Cui L, Zhang L, Yang L, Zhuo Y and
Li C: Saikosaponin d modulates the polarization of tumor-associated
macrophages by deactivating the PI3K/AKT/mTOR pathway in murine
models of pancreatic cancer. Int Immunopharmacol. 122:1105792023.
View Article : Google Scholar : PubMed/NCBI
|
|
135
|
Cai C, Zhang H, Ou Y, Jiang Y, Zhong D, Qi
H and Dang Q: Saikosaponin-d suppresses cell growth in renal cell
carcinoma through EGFR/p38 signaling pathway. Neoplasma.
64:518–525. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
136
|
Rizzo A, Santoni M, Mollica V, Fiorentino
M, Brandi G and Massari F: Microbiota and prostate cancer. Semin
Cancer Biol. 86:1058–1065. 2022. View Article : Google Scholar
|
|
137
|
Yao M, Yang J, Cao L, Zhang L, Qu S and
Gao H: Saikosaponin-d inhibits proliferation of DU145 human
prostate cancer cells by inducing apoptosis and arresting the cell
cycle at G0/G1 phase. Mol Med Rep. 10:365–372. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
138
|
Zhong D, Zhang HJ, Jiang YD, Wu P, Qi H,
Cai C, Zheng SB and Dang Q: Saikosaponin-d: A potential
chemotherapeutics in castration resistant prostate cancer by
suppressing cancer metastases and cancer stem cell phenotypes.
Biochem Biophys Res Commun. 474:722–729. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
139
|
Ghosh S: Cisplatin: The first metal based
anticancer drug. Bioorg Chem. 88:1029252019. View Article : Google Scholar : PubMed/NCBI
|
|
140
|
Tsuyoshi H, Wong VKW, Han Y, Orisaka M,
Yoshida Y and Tsang BK: Saikosaponin-d, a calcium mobilizing agent,
sensitizes chemoresistant ovarian cancer cells to cisplatin-induced
apoptosis by facilitating mitochondrial fission and G2/M arrest.
Oncotarget. 8:99825–99840. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
141
|
Tang TT, Jiang L, Zhong Q, Ni ZJ, Thakur
K, Khan MR and Wei ZJ: Saikosaponin D exerts cytotoxicity on human
endometrial cancer ishikawa cells by inducing apoptosis and
inhibiting metastasis through MAPK pathways. Food Chem Toxicol.
177:1138152023. View Article : Google Scholar : PubMed/NCBI
|
|
142
|
Meltzer PS and Helman LJ: New horizons in
the treatment of osteosarcoma. N Engl J Med. 385:2066–2076. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
143
|
Zhao L, Li J, Sun ZB, Sun C, Yu ZH and Guo
X: Saikosaponin D inhibits proliferation of human osteosarcoma
cells via the p53 signaling pathway. Exp Ther Med. 17:488–494.
2019.PubMed/NCBI
|
|
144
|
Gao T, Zhao P, Yu X, Cao S, Zhang B and
Dai M: Use of Saikosaponin D and JNK inhibitor SP600125, alone or
in combination, inhibits malignant properties of human osteosarcoma
U2 cells. Am J Transl Res. 11:2070–2080. 2019.PubMed/NCBI
|
|
145
|
Li Y, Cai T, Zhang W, Zhu W and Lv S:
Effects of Saikosaponin D on apoptosis in human U87 glioblastoma
cells. Mol Med Rep. 16:1459–1464. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
146
|
Liu G, Guan Y, Liu Y, Wang Y, Zhang J, Liu
Y and Liu X: Saikosaponin D inducing apoptosis and autophagy
through the activation of endoplasmic reticulum stress in
glioblastoma. Biomed Res Int. 2022:54895532022. View Article : Google Scholar : PubMed/NCBI
|
|
147
|
McKinnon C, Nandhabalan M, Murray SA and
Plaha P: Glioblastoma: Clinical presentation, diagnosis, and
management. BMJ. 374:n15602021. View Article : Google Scholar : PubMed/NCBI
|
|
148
|
Liang J, Sun J, Liu A, Chen L, Ma X, Liu X
and Zhang C: Saikosaponin D improves chemosensitivity of
glioblastoma by reducing the its stemness maintenance. Biochem
Biophys Rep. 32:1013422022.PubMed/NCBI
|
|
149
|
Northcott PA, Robinson GW, Kratz CP,
Mabbott DJ, Pomeroy SL, Clifford SC, Rutkowski S, Ellison DW,
Malkin D, Taylor MD, et al: Medulloblastoma. Nat Rev Dis Primers.
5:112019. View Article : Google Scholar : PubMed/NCBI
|
|
150
|
Luo J, Wang J, Yang J, Huang W, Liu J, Tan
W and Xin H: Saikosaponin B1 and Saikosaponin D inhibit tumor
growth in medulloblastoma allograft mice via inhibiting the
Hedgehog signaling pathway. J Nat Med. 76:584–593. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
151
|
Shimony S, Stahl M and Stone RM: Acute
myeloid leukemia: 2023 update on diagnosis, risk-stratification,
and management. Am J Hematol. 98:502–526. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
152
|
Huang Y, Su R, Sheng Y, Dong L, Dong Z, Xu
H, Ni T, Zhang ZS, Zhang T, Li C, et al: Small-molecule targeting
of oncogenic FTO demethylase in acute myeloid leukemia. Cancer
Cell. 35:677–691.e10. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
153
|
Li Z, Weng H, Su R, Weng X, Zuo Z, Li C,
Huang H, Nachtergaele S, Dong L, Hu C, et al: FTO plays an
oncogenic role in acute myeloid leukemia as a
N6-methyladenosine RNA demethylase. Cancer Cell.
31:127–141. 2017. View Article : Google Scholar
|
|
154
|
Sun K, Du Y, Hou Y, Zhao M, Li J, Du Y,
Zhang L, Chen C, Yang H, Yan F, et al: Saikosaponin D exhibits
anti-leukemic activity by targeting FTO/m6A signaling.
Theranostics. 11:5831–5846. 2021. View Article : Google Scholar :
|
|
155
|
Wang S, Long S and Wu W: Application of
traditional chinese medicines as personalized therapy in human
cancers. Am J Chin Med. 46:953–970. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
156
|
Luo Y, Wang CZ, Hesse-Fong J, Lin JG and
Yuan CS: Application of Chinese medicine in acute and critical
medical conditions. Am J Chin Med. 47:1223–1235. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
157
|
Xue J, Xu Z, Wang Q, Hou H, Wei L, Zhang
J, Zhao X, Chen L, Ding F, Ma L, et al: Clinical practice
guidelines for prevention and treatment of postoperative
gastrointestinal disorder with Integrated Traditional Chinese and
Western Medicine (2023). J Evid Based Med. 17:207–223. 2024.
View Article : Google Scholar : PubMed/NCBI
|
|
158
|
Zhang S, Liu M and Zhao L: Effects of
different Chinese traditional exercises on mental health during the
COVID-19 pandemic: A systematic review and meta-analysis. Front
Public Health. 12:14200352024. View Article : Google Scholar : PubMed/NCBI
|
|
159
|
Li X, Li X, Huang N, Liu R and Sun R: A
comprehensive review and perspectives on pharmacology and
toxicology of saikosaponins. Phytomedicine. 50:73–87. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
160
|
Chen MH, Gu YY, Zhang AL, Sze DM, Mo SL
and May BH: Biological effects and mechanisms of matrine and other
constituents of Sophora flavescens in colorectal cancer. Pharmacol
Res. 171:1057782021. View Article : Google Scholar : PubMed/NCBI
|
|
161
|
Shah MA, Abuzar SM, Ilyas K, Qadees I,
Bilal M, Yousaf R, Kassim RMT, Rasul A, Saleem U, Alves MS, et al:
Ginsenosides in cancer: Targeting cell cycle arrest and apoptosis.
Chem Biol Interact. 382:1106342023. View Article : Google Scholar : PubMed/NCBI
|
|
162
|
Alam P, Imran M, Gupta DK and Akhtar A:
Formulation of transliposomal nanocarrier gel containing strychnine
for the effective management of skin cancer. Gels. 9:8312023.
View Article : Google Scholar : PubMed/NCBI
|
|
163
|
Liu L, Yan J, Cao Y, Yan Y, Shen X, Yu B,
Tao L and Wang S: Proliferation, migration and invasion of triple
negative breast cancer cells are suppressed by berbamine via the
PI3K/Akt/MDM2/p53 and PI3K/Akt/mTOR signaling pathways. Oncol Lett.
21:702021. View Article : Google Scholar
|
|
164
|
Zhou P, Shi W, He XY, Du QY, Wang F and
Guo J: Saikosaponin D: Review on the antitumour effects, toxicity
and pharmacokinetics. Pharm Biol. 59:1480–1489. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
165
|
Zhang F, Chen L, Jin H, Shao J, Wu L, Lu Y
and Zheng S: Activation of Fas death receptor pathway and Bid in
hepatocytes is involved in saikosaponin D induction of
hepatotoxicity. Environ Toxicol Pharmacol. 41:8–13. 2016.
View Article : Google Scholar
|
|
166
|
Farhan M: Green Tea Catechins: Nature's
way of preventing and treating cancer. Int J Mol Sci. 23:107132022.
View Article : Google Scholar : PubMed/NCBI
|
|
167
|
Islam MR, Akash S, Rahman MM, Nowrin FT,
Akter T, Shohag S, Rauf A, Aljohani ASM and Simal-Gandara J: Colon
cancer and colorectal cancer: Prevention and treatment by potential
natural products. Chem Biol Interact. 368:1101702022. View Article : Google Scholar : PubMed/NCBI
|
|
168
|
Yang Z, Xiao T, Li Z, Zhang J and Chen S:
Novel Chemicals derived from tadalafil exhibit PRMT5 inhibition and
promising activities against breast cancer. Int J Mol Sci.
23:48062022. View Article : Google Scholar : PubMed/NCBI
|
|
169
|
Zhao N, Wang W, Jiang H, Qiao Z, Sun S,
Wei Y, Xie X, Li H, Bi X and Yang Z: Natural products and gastric
cancer: cellular mechanisms and effects to change cancer
progression. Anticancer Agents Med Chem. 23:1506–1518. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
170
|
Zhang GS, Hu PY, Li DX, He MZ, Rao XY, Luo
XJ, Wang YS and Wang YR: Formulations, hemolytic and
pharmacokinetic studies on saikosaponin a and saikosaponin d
compound liposomes. Molecules. 20:5889–5907. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
171
|
Shimizu K, Amagaya S and Ogihara Y:
Structural transformation of saikosaponins by gastric juice and
intestinal flora. J Pharmacobiodyn. 8:718–725. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
172
|
Liu J, Xue Y, Sun J, Fu R, Ren S, Zhang Z
and Song R: Pharmacokinetics and oral bioavailability studies of
three saikogenins in rats using a validated UFLC-MS/MS method. J
Chromatogr B Analyt Technol Biomed Life Sci. 1124:265–272. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
173
|
Ye HN, Liu XY and Qin BL: Research
progress of integrated traditional Chinese and Western medicine in
the treatment of advanced gastric cancer. World J Gastrointest
Oncol. 15:69–75. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
174
|
Ma L, Wang B, Long Y and Li H: Effect of
traditional Chinese medicine combined with Western therapy on
primary hepatic carcinoma: A systematic review with meta-analysis.
Front Med. 11:191–202. 2017. View Article : Google Scholar : PubMed/NCBI
|