Introduction
Iron is an essential metal in the human body,
serving as a crucial component for the synthesis of hemoglobin and
myoglobin. In addition, iron plays vital roles in cellular vitality
and is involved in a wide range of biochemical and physiological
processes, including oxygen storage and transport, mitochondrial
respiration, DNA synthesis and repair, and enzymatic reactions
within cells (1). However,
excess iron can exert toxic effects on the human body due to its
active redox capacity (2),
making free iron readily accept and donate electrons, leading to
its involvement in various pathological mechanisms.
Iron plays a crucial role in cells, and its normal
accumulation and regulation are essential for the survival of cells
(3). In tumor cells, a series of
gene expressions and molecular mechanisms facilitate the
acquisition of sufficient iron to meet the high demands of growth
and metastasis (4). Various
organelles, including mitochondria (5) and lysosomes (6), probably participate in regulating
iron metabolism and redox imbalance. However, when iron excessively
accumulates, disrupting the balance of supply and demand, it can
have the opposite effect, inhibiting normal cell growth (7).
A critical analysis of current research is required
to understand the molecular regulation of iron metabolism
systematically, ranging from its physiological implications to its
therapeutic potential. For instance, the role of ferroptosis in
tumor cells has been validated using ferroptosis-inducing drugs
(8), underscoring its potential
as a novel target for anticancer therapies. Focusing on the process
of iron accumulation, regulation and ferroptosis, the following
content will primarily discuss iron metabolism and its roles in
tumor cells. Building on previous research, the potential of cancer
therapies will be further explored based on the regulation of iron
and the activation of ferroptosis.
Given the crucial role of iron in the growth and
division of various cell types, it is important not only to discuss
its impact on tumor cells themselves but also to broaden the
perspective to include the entire tumor microenvironment, with a
particular focus on its effects on immune cells. According to
existing research, iron can exert varying degrees of influence on a
wide range of immune cells (9),
thereby directly affecting the growth and development of tumor
cells. This provides a new perspective on the role of iron
metabolism in tumors, warranting systematic exploration and further
investigation. Understanding the intricate mechanisms and
regulatory pathways of iron in the tumor microenvironment,
exemplified by the process of ferroptosis, provides insight into
potential therapeutic strategies targeting ferroptosis in cancer
treatment.
This review's body is organized into four key
chapters, focusing on the multifaceted role of iron metabolism in
cancer biology, particularly ferroptosis and its therapeutic
implications: i) The Accumulation and Regulation of Iron in Tumor
Cells: This chapter delves into how tumor cells take up and
regulate iron, emphasizing the roles of various transporters and
regulatory factors. It will be analyzed how the availability of
iron influences tumor growth and survival, revealing changes in
iron homeostasis within the tumor microenvironment and their impact
on cancer cells. ii) The occurrence of ferroptosis in tumor cells:
This chapter discusses the mechanisms and biological significance
of ferroptosis, elucidating how this unique form of regulated cell
death functions in cancer. A focus was placed on the potential of
ferroptosis to inhibit tumor growth, as well as how cancer cells
respond to the challenges posed by ferroptosis. iii) Regulation of
immune cells in the tumor microenvironment by iron: This chapter
examines how iron metabolism influences the function of immune
cells within the tumor microenvironment, discussing how iron
regulates immune cell behavior and impacts immune evasion
mechanisms and the effectiveness of immunotherapy. iv) Targeting
iron metabolism for cancer treatment: Finally, this chapter
explores emerging therapeutic strategies that target iron
metabolism and ferroptosis, emphasizing the potential of
combination therapies to enhance treatment efficacy and overcome
resistance.
Accumulation and regulation of iron in tumor
cells
Impact of tumor-environment interactions:
Study of several specific tumors as examples
Cancer can strategically control body systems, such
as the neuroendocrine and immune systems, to reset homeostasis in a
way that promotes its own growth, often at the expense of the
host's well-being (10),
highlighting the intricate relationship between tumors and their
environmental influences. In general, cancer not only evades the
body's regulatory mechanisms but also acquires the ability to
influence both local and systemic homeostasis. In this section,
several typical cancers were used as examples to demonstrate the
validity of existing research that observes cancer development from
the perspectives of metabolism and the body's environment. The
present review's focus on iron metabolism arises from this very
logic.
Melanoma
Melanomagenesis is influenced by various
environmental and internal factors, including genetic
predisposition and epigenetic changes. Exposure to solar radiation
is a significant risk factor, particularly for cutaneous melanomas,
while acral melanomas, which occur in areas not directly exposed to
sunlight, primarily affect darker-skinned populations (11). The tumor microenvironment,
characterized by local neuroimmune interactions and systemic
factors, plays a crucial role in disease progression.
Hepatocellular carcinoma (HCC)
HCC is the most common type of primary liver cancer,
characterized by a multifactorial etiology that includes genetic,
environmental and behavioral influences (12). Several environmental factors,
particularly copper and iron metabolism, play important roles in
the progression and treatment resistance of HCC. For instance,
elevated ceruloplasmin levels further enhance hypoxia-inducible
factor (HIF)1α expression by decreasing iron availability, creating
a positive feedback loop that sustains the cancer cells' survival
(13). The interplay between
copper and iron homeostasis, mediated by copper metabolism MURR1
domain 10 and HIF1α, highlights a critical link between
environmental factors and the molecular mechanisms underlying HCC
progression and therapy resistance. This understanding opens up
potential therapeutic avenues aimed at disrupting these metabolic
pathways to improve treatment outcomes.
Iron uptake and accumulation
mechanisms
Cancer cells require abundant iron to sustain their
rapid proliferation and metabolic demands. Cells of tumors often
exhibit high iron metabolic activity, which supports their rapid
proliferation and survival. Iron metabolism disorders are also
common in cancers. Both iron overload and deficiency can affect
disease progression and be linked to tumorigenesis. The complexity
of iron metabolism in the tumor microenvironment lies in its
multifaceted interplay with cancer cells and surrounding tissues.
Cancer cells exhibit dynamic adaptations to fluctuations in iron
availability, finely tuning the expression of key iron metabolism
proteins to sustain their proliferation and survival. Meanwhile,
the tumor microenvironment, characterized by hypoxia and
inflammation, further modulates iron metabolism through the
activation of the secretion of iron-binding proteins. These
mechanisms highlight the sophisticated strategies employed by
cancer cells to ensure an adequate supply of iron. Cancer cells
could effectively increase their iron uptake and retention,
creating an iron-rich environment that supports their aggressive
growth and survival through comprehensive approaches, including
overexpressing transferrin (TF) receptor 1 (TfR1), upregulating
six-transmembrane epithelial antigen of the prostate (STEAP)
protein and downregulating ferroportin (FPN).
Overexpression of TfR1
Iron is primarily absorbed by the divalent metal
transporter protein 1 (DMT1) on the intestinal wall and it is
transported to various tissues with the assistance of TF, which
carries trivalent iron. And iron is mainly taken up by cells
through endocytosis. TfR1 interacts with Tf proteins to form
endocytic vesicles that facilitate the absorption of iron. Although
TfR1 is generally expressed at low levels in most normal cells, its
expression is significantly elevated in rapidly proliferating
cells, such as those found in tumors (14,15), the basal cell layer, intestinal
epithelium and certain types of immune cells (16). It is well established that TfR1
plays a crucial role in cellular iron uptake and homeostasis
(17).
TfR1 expression is precisely regulated at multiple
levels, including transcriptional and post-transcriptional
mechanisms, to meet cellular iron demands and prevent disruptions
in iron homeostasis, which can be detrimental to the cell (18). The primary regulatory mechanism
involves IRPs, specifically IRP1 and IRP2, which will be elaborated
on in the following chapters. Other regulatory factors include
oncogenes such as c-MYC, which directly binds to a conserved E box
in intron 1 of TFRC (19), and
HIF-1, which activates TFRC expression under specific conditions,
such as iron-deficient conditions, by binding to an upstream
hypoxia response element (20).
For instance, in breast cancer, the SRC oncogene (sarcoma gene)
encodes a tyrosine kinase that phosphorylates TfR1, enhancing
cancer cell survival and inhibiting apoptosis. The loss of SIRT3, a
mitochondrial deacetylase, also upregulates TfR1 by increasing
reactive oxygen species (ROS) production (21).
In cancer cells, TfR1 is significantly overexpressed
to meet the increased iron requirements necessary for their rapid
proliferation and biosynthesis. This overexpression supports
enhanced iron uptake through TfR1-mediated endocytosis, which is
crucial for DNA synthesis and cellular proliferation driven by
ribonucleotide reductase, an iron-dependent enzyme that can be
inhibited by maltose gallium (22). The phenomenon of 'iron addiction'
actually underscores the higher dependency of cancer cells on iron
compared to normal cells, making them more sensitive to iron
deprivation targeting TfR1.
Elevated intracellular iron levels protect cancer
cells from natural killer (NK) cell-mediated cytotoxicity and
apoptosis induced by tumor necrosis factor (TNF)α by inhibiting ROS
accumulation through ferritin. TfR1 can also interact with the
inhibitor of NF-κB kinase complex, enhancing NF-κB signaling and
promoting cancer cell survival. In addition, NF-κB further
upregulates TfR1 expression by regulating HIF-1α levels, creating a
feedback loop that sustains cancer cell growth (23). Moreover, TfR1 modulates
mitochondrial respiration and ROS production, both of which are
crucial for the growth and survival of malignant cells (24). For instance, in hepatocellular
carcinoma in animals, TfR1 maintains the stemness of cancer
stem-like cells and promotes malignancy by regulating iron
accumulation (25).
Due to these multifaceted roles, TfR1 is
overexpressed in various cancer types, often at levels
significantly higher than in normal cells (23-25). This overexpression is associated
with advanced stages and poor prognosis in cancers such as breast
cancer (26,27), ovarian cancer (28), esophageal squamous cell carcinoma
(29), pancreatic cancer
(30), lung cancer (31), bladder cancer (32), cholangiocarcinoma (33), cervical cancer (34), osteosarcoma (35), adrenal cortical carcinoma renal
cell carcinoma (36),
hepatocellular carcinoma (37)
and several other hematopoietic malignancies such as non-Hodgkin
lymphoma (38) and chronic
lymphocytic leukemia, as well as acute lymphoblastic leukemia
(39). Of note, in HIV-infected
patients, aggressive non-Hodgkin lymphoma shows even higher TfR1
mRNA levels compared to those in non-infected patients (40). Therefore, TfR1 overexpression is
a hallmark of cancer biology, driving iron accumulation and
supporting the aggressive and malignant behavior of cancer
cells.
Downregulation of FPN
FPN is the sole known iron export protein in
mammalian cells, responsible for transporting iron from the inside
of the cell to the extracellular space. In tumor cells, the
expression of FPN is often downregulated, leading to the
accumulation of intracellular iron. Decreased expression of FPN
results in iron retention within the cell, and may help to
contribute to an iron-rich intracellular environment that favors
cancer-cell growth and survival, and this process may be related to
the action of hepcidin (41). By
limiting the export of iron, cancer cells ensure a steady supply of
this essential metal to support their heightened metabolic and
proliferative needs. This mechanism of iron retention through FPN
downregulation is a critical factor in the dysregulated iron
homeostasis observed in numerous types of cancer.
Role of the STEAP protein family
The STEAP protein family consists of
metalloreductases that facilitate the reduction of iron and copper,
enhancing their bioavailability for cellular processes. The family
includes STEAP1-4, all of which play significant roles in iron
metabolism (42). These proteins
are primarily localized in the endosomal and plasma membranes,
where they reduce Fe(III) to Fe(II), which can then be transported
by DMT1. What is notable is that STEAP proteins are often
upregulated in cancer cells, thereby increasing the availability of
reduced iron for cellular uptake. This upregulation correlates with
enhanced cell proliferation, migration and tumor growth,
underscoring the critical role of STEAP proteins in maintaining the
iron homeostasis necessary for cancer-cell survival and
proliferation.
STEAP1 is predominantly located on the cell membrane
and is hypothesized to function as an ion channel or transporter
within tight junctions, gap junctions or cell adhesion sites,
facilitating intercellular communication. Overexpression of STEAP1
in cancer suggests its potential role in promoting cancer-cell
proliferation and invasion. Blocking STEAP1 with specific
monoclonal antibodies has been shown to increase cell death in
LNCaP cells, suggesting that STEAP1 may support cancer-cell
proliferation or inhibit apoptosis. STEAP1 also appears to
facilitate cell growth by elevating intracellular ROS levels,
indicating its involvement in both intra- and intercellular
pathways (42). STEAP2 operates
as a shuttle between the Golgi complex and the plasma membrane,
participating in both endocytic and exocytic pathways. It may
function as a receptor for endogenous and exogenous ligands or
regulate protein delivery and sorting mechanisms. STEAP2′s
colocalization with TF and TfR1 suggests a role in the endosomal TF
cycle of erythroid cells, aiding in the uptake of iron and copper
by reducing Fe3+ to Fe2+ and Cu2+
to Cu+ (43,44). It has been proved that STEAP2
increases prostate cancer-cell proliferation by regulating genes
involved in the cell cycle, causing partial cell-cycle arrest in
G0/G1 phase, and this effect is mediated through the activation of
the ERK pathway. Initially identified in studies on hypochromic
microcytic anemia in nm1054 mouse mutants, STEAP3 plays a vital
role in iron metabolism in erythroid precursors. Localization
cloning confirmed that STEAP3 is the gene causing iron deficiency
anemia in the mouse mutant nm1054; It encodes an iron and copper
reductase, which is essential for the effective transport of TF
iron (45). STEAP4 is crucial
for cellular iron and copper uptake, significantly enhancing their
absorption. It aids iron homeostasis through involvement in the TF
cycle and response to inflammatory cytokines. In cancer, STEAP4
expression may increase iron uptake, supporting cancer-cell growth
by meeting their high iron demands. Its role in inflammation could
also influence the tumor microenvironment, affecting cancer
progression (46).
In cancer, STEAP proteins are frequently
upregulated, leading to increased iron levels that support
cancer-cell proliferation, migration and tumor growth. This makes
STEAP proteins significant for cancer research, as they provide
potential therapeutic targets for disrupting iron homeostasis in
cancer cells and inhibiting tumor progression.
Iron regulatory proteins (IRPs) and
HIFs
Iron is essential for cancer cell growth and
survival, while its excessive accumulation can induce a series of
unhealthy incidents including ferroptosis, leading to oxidative
stress and cellular damage, affecting cancer-cell survival.
Therefore, understanding the surrounding molecular mechanisms
regulating iron metabolism is crucial for developing new cancer
treatment strategies. For instance, animal cells, particularly
cancer cells, regulate the expression of downstream proteins by
synthesizing certain types of regulatory proteins, thereby ensuring
an adequate supply of iron required for cellular metabolism. This
allows them to adapt to fluctuations in environmental iron levels
while avoiding the damage caused by excessive iron accumulation.
Together, IRPs and HIFs orchestrate a complex regulatory network
that ensures adequate iron supply in cancer cells. By regulating
the transcription of key genes involved in iron metabolism, IRPs
and HIFs enable cancer cells to adapt to varying iron levels and
hypoxic stress in the tumor microenvironment.
Role of IRPs in gene transcription
regulation
IRPs are critical regulators of cellular iron
homeostasis. IRPs control the expression of genes involved in iron
metabolism by binding to iron-responsive elements (IREs) located in
the untranslated regions (UTRs) of target mRNAs. There are two main
IRPs: IRP1 and IRP2. These proteins can respond to intracellular
iron levels to either stabilize or degrade mRNAs encoding key
proteins in iron metabolism (47).
When cellular iron levels are low, IRPs bind to IREs
in the 5′-UTR of mRNAs, such as those of ferritin and ferroportin,
inhibiting their translation and reducing iron storage and export.
Conversely, IRPs bind to IREs in the 3′-UTR of mRNAs like TfR1,
stabilizing the mRNA and promoting its translation, thereby
increasing iron uptake (47,48). This regulation ensures that cells
can adapt to fluctuations in iron availability, maintaining iron
homeostasis. In cancer cells, the dysregulation of IRP activity can
lead to altered expression of these iron metabolism genes,
contributing to increased iron uptake and retention that supports
tumor growth and survival.
Activation of HIF signaling pathways in
hypoxic environments and their impact on iron metabolism
HIFs are transcription factors that play a central
role in the cellular response to low oxygen levels (hypoxia), a
common characteristic of the tumor microenvironment (48,49). HIFs are composed of an
oxygen-sensitive α subunit (HIF-1α, HIF-2α) and a constitutively
expressed β subunit (HIF-1β). Under normoxic conditions, HIF-1α is
rapidly degraded via the ubiquitin-proteasome pathway. However,
under hypoxic conditions, HIF-1α is stabilized and translocates to
the nucleus, where it dimerizes with HIF-1β to activate the
transcription of various genes involved in the adaptation to
hypoxia (49,50).
HIFs significantly influence iron metabolism by
upregulating the expression of genes that enhance iron uptake and
utilization. For instance, as previously mentioned, molecules such
as HIF-1α can regulate downstream iron absorption by modulating the
expression levels of TfR1 (51).
Besides, HIFs also increase the expression of DMT1, which
facilitates the transport of iron into the cytoplasm. In addition,
HIFs can be used as a hypoxia signal, upregulating heme oxygenase-1
(HO-1), an enzyme that releases iron from heme, contributing to
intracellular iron availability (52), which highlights the unique role
of the HIF family in regulating iron metabolism.
In the context of cancer, activation of the HIF
signaling pathways supports the increased iron demands of rapidly
proliferating tumor cells. By enhancing iron uptake and
utilization, HIFs help to sustain the metabolic and proliferative
needs of cancer cells under hypoxic conditions. This adaptation not
only promotes tumor growth but also contributes to the aggressive
and treatment-resistant nature of hypoxic tumors (Table I; Fig. 1).
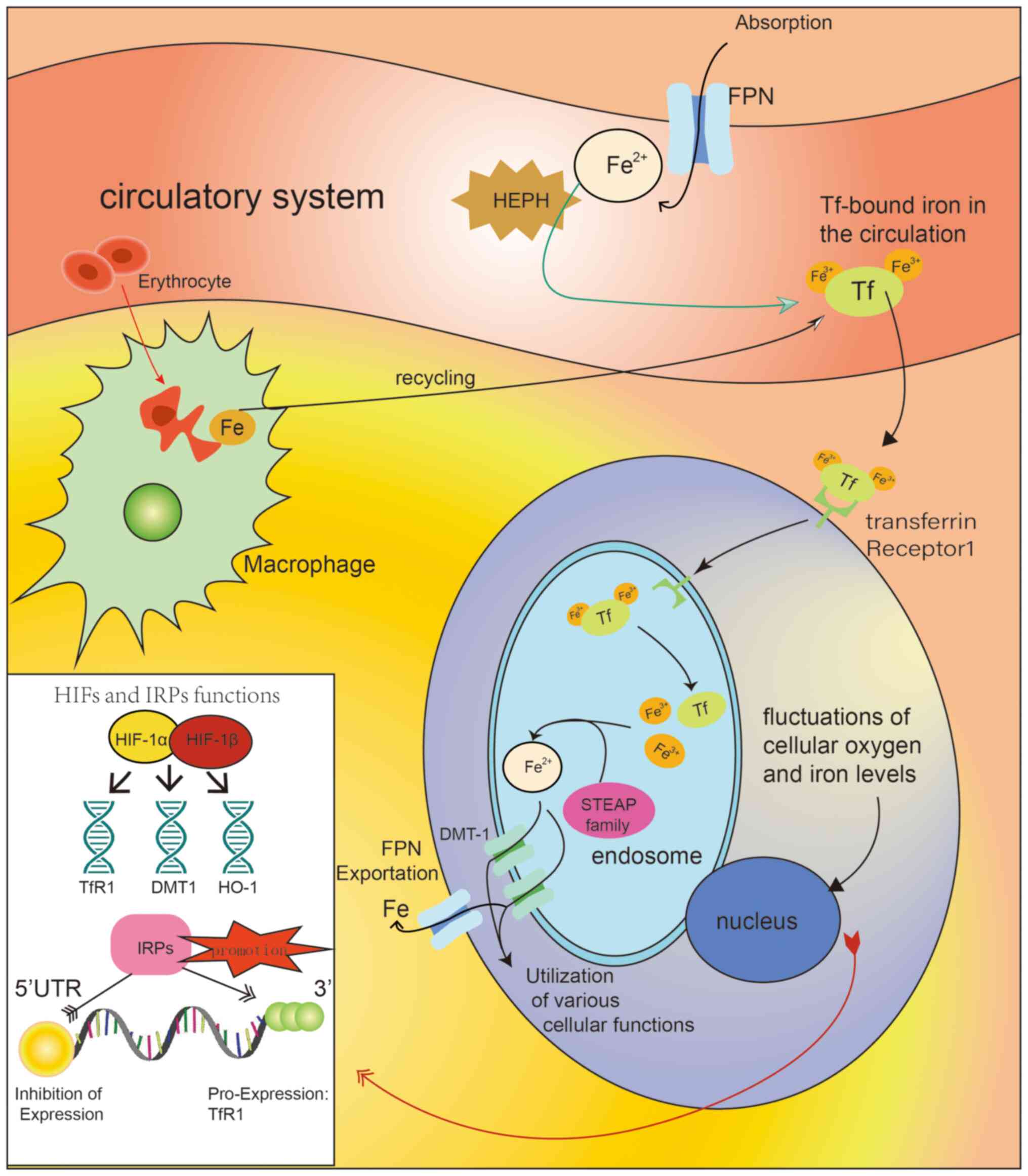 | Figure 1Diagram illustrating the mechanisms
of iron accumulation and regulation in tissue cells. The
circulatory system (e.g., blood circulation) acquires iron through
two main pathways: Absorption from external sources (mediated by
the FPN protein) and recycling. In the circulatory system, HEPH
plays its roles in interacting with iron and altering its oxidation
state. The iron is then converted to ferric iron and transported
bound to Tf. Iron transport into cells requires the interaction
between TfR1 and Tf, along with transmembrane transport by DMT-1.
Tf carries iron into endosomes by binding to its specific receptor.
After Tf releases the ferric ions (Fe3+), the
oxidation state of iron changes again and it can be transported
into the cytoplasm via DMT-1. The STEAP protein family plays a role
in converting the oxidation state of iron and promoting its
utilization in specific cell types. In cancer cells, this process
of iron accumulation is selectively enhanced. HIFs and IRPs are two
key molecules in iron homeostasis regulation, interacting with
genes and mRNA, respectively. Their different target sites result
in varying downstream effects. Typical downstream molecules include
TfR1, DMT-1 and HO-1, which influence the absorption and
utilization of iron by tissue cells through various mechanisms,
such as regulating iron uptake and affecting the release of iron
from heme. DMT-1, divalent metal transporter 1; Tf, transferrin;
TfR1, transferrin receptor 1; HIFs, hypoxia-inducible factors;
IRPs, iron regulatory proteins; HO-1, heme oxygenase 1; HEPH,
hephaestin (copper-dependent ferroxidase); FPN, ferroportin; STEAP,
six-transmembrane epithelial antigen of the prostate; 5′-UTR,
5′-untranslated region. |
 | Table IComparison of iron metabolism
pathways in neoplastic and normal cells. |
Table I
Comparison of iron metabolism
pathways in neoplastic and normal cells.
| Pathway | Neoplastic
cells | Normal cells | Canonical mechanism
(TfR1) | Non-canonical
mechanism (GPx) | (Refs.) |
|---|
| Canonical iron
uptake | Overexpression of
transferrins | Relatively low TfR1
expression | TfR1-mediated
endocytosis increased | - | (10,14,15) |
| Canonical iron
retention | Downregulated
FPN | Normal FPN
expression maintains iron homeostasis | Antagonism with
FPN | - | (41) |
| IRPs | Dysregulated IRP
activity; promotes increased iron uptake | Normal regulation
of IRPs maintains iron homeostasis | IRPs bind to IREs
in mRNAs to regulate TfR1 expression | Oxidative stress
modulating IRP function | (47) |
| HIF signaling | Upregulated HIF-1α
enhances iron uptake | HIF activation is
transient and regulated | HIF-1α stabilizes
and increases TfR1 and DMT1 expression | Oxidative stress
affecting HIF regulation | (48-50) |
| STEAP proteins | Upregulated STEAP1,
-2 and -4 enhance iron bioavailability | Normal expression,
no significant role in iron uptake | STEAP proteins
reduce Fe(III) to Fe(II) for uptake | Potential
interaction with GPx for redox balance | (43,44) |
Occurrence of ferroptosis in tumor
cells
Initially introduced in 2012, the term 'ferroptosis'
denotes iron-dependent regulated cell death triggered by excessive
lipid peroxidation and Fenton-like chemical reactions, which could
lead to irreversible membrane rupture (53). Iron accumulation and lipid
peroxidation are critical inducers of ferroptosis in various cell
models (54,55). Ferroptosis represents an
iron-dependent form of regulated cell death driven by lipid
peroxidation and oxidative stress. Ferroptosis is a cell death mode
closely related to iron metabolism. Its prerequisite is the massive
accumulation of iron, which is particularly evident in various
cancer cells. In the previous section, the conditions under which
iron accumulates in cells were discussed. Due to the fact that iron
itself is an essential component of cells, it is important to
explore the specific situations and conditions that can induce
ferroptosis, which makes the effect of iron on cells become
negative.
Specific mechanisms of ferroptosis
Iron-dependent lipid peroxidation
Ferroptosis is fundamentally driven by
iron-dependent lipid peroxidation. Iron, due to its redox-active
nature, can catalyze the formation of ROS through Fenton reactions.
These ROS, particularly hydroxyl radicals (OH·), react with
polyunsaturated fatty acids (PUFAs) in cell membranes, initiating a
chain reaction of lipid peroxidation (53). ROS-induced lipid peroxidation is
a crucial propulsive step in ferroptosis. The lipid peroxides
formed are highly reactive and can further propagate the
peroxidation chain reaction and promote more ROS production in
different parts of cells, leading to the disruption of membrane
integrity (56), DNA structure
(57) and cell death. This
process is central to ferroptosis, as it leads to the accumulation
of lipid ROS that overwhelm the cell's antioxidant defenses.
Disruption of the xc-cystine/glutamate
antiporter or glutathione peroxidase 4 (GPX4) system
Ferroptosis primarily involves balancing oxidative
damage and antioxidant defenses. In fact, the destruction and
failure of antioxidant mechanisms are crucial in the process of
ferroptosis. The glutathione (GSH)-GPX4 antioxidant system plays a
crucial role in protecting cells from iron-induced death (58). The xc-system imports cystine as a
limiting substrate for GSH synthesis in exchange for glutamate
(Glu). GPX4 utilizes GSH as a reducing cofactor to convert
phospholipid hydroperoxides into non-toxic lipid alcohols, thereby
inhibiting ferroptosis in tumor cells (58,59).
A key mechanism in ferroptosis is the disruption of
the xc-cystine/glutamate antiporter or the GPX4 system (58). The xc-antiporter imports cystine
into the cell in exchange for glutamate. Cystine is then reduced to
cysteine, which is essential for the synthesis of GSH, the major
cellular antioxidant. When the function of the xc-antiporter is
inhibited, intracellular cystine levels drop, leading to a decrease
in GSH synthesis. Without sufficient GSH, the activity of GPX4, an
enzyme that reduces lipid hydroperoxides to non-toxic lipid
alcohols, is compromised. This results in the accumulation of lipid
hydroperoxides, which can then undergo iron-catalyzed decomposition
to form toxic lipid radicals, further promoting ferroptosis.
Role of lipoxygenases (LOX) in the
endoplasmic reticulum-associated compartment
LOX play a significant role in ferroptosis. LOX
inhibitors, such as Baicalein and nordihydroguaiaretic acid,
protect acute lymphoblastic leukemia cells from ferroptosis induced
by GPX4 inhibition (60).
Similarly, 12/15-LOX inhibitors and arachidonate 15-lipoxygenase
(ALOX15) silencing reduce ferroptotic cell death in cancer cells,
while ALOX15 overexpression enhances it (61). Lysyl oxidase is another molecule
that plays a similar role to LOX, which promotes ferroptosis
through ERK-dependent 5-lipoxygenase phosphorylation, leading to
lipid ROS accumulation in neuronal cells (62). However, the role of LOX in
ferroptosis may be more complex, as certain LOX inhibitors protect
cells by acting as radical-trapping antioxidants rather than
through LOX inhibition. While LOX activity may contribute to
initiating ferroptosis, lipid autoxidation appears to drive the
cell death process (63).
Interplay with other cellular
systems
In addition to the primary mechanisms described,
ferroptosis involves complex interactions with other cellular
systems. For instance, the iron-sulfur cluster biosynthesis pathway
and mitochondrial iron regulation are intimately linked with
ferroptosis. Mitochondria, as major sites of iron metabolism and
ROS production, contribute to the iron pool and oxidative stress
that drive ferroptosis (64).
It is worth mentioning that multiple studies have
shown that certain molecules can promote or inhibit ferroptosis by
regulating iron levels. This process occurs through the action on
TfR molecules to modulate lipid peroxidation (65,66). For instance, Chen et al
(65) clarified the
characteristics of ferroptosis through the TfR1 mechanism when
studying other molecular mechanisms. The actions of these molecules
also make them key players indirectly related to the ferroptosis
process. Molecules like ubiquinone (CoQ10) may play a role in this
process. It is also universally acknowledged that ferroptosis
suppressor protein 1 (FSP1) is another molecule which acts
independently of GPX4. FSP1, when recruited to the plasma membrane
through myristoylation, reduces lipid peroxidation by regenerating
CoQ10 to its antioxidant form, ubiquinol, thus protecting against
ferroptosis (67).
Other antioxidant systems, such as
apoptosis-inducing factor mitochondria-associated 2-coenzyme Q10,
tetrahydrobiopterin and the endosomal sorting complex required for
transport III membrane repair system, counteract iron death in
solid tumors. Furthermore, autophagy, particularly ferritinophagy
(the autophagic degradation of ferritin), releases free iron within
the cell, further promoting ferroptosis. Ferroptosis was initially
considered autophagy-independent. Recent studies, however, have
shown that excessive activation of selective autophagy promotes
iron accumulation and lipid peroxidation, thereby inducing
ferroptosis. Selective autophagy mechanisms include iron protein
engulfment induced by nuclear receptor coactivator 4, molecular
chaperone protein-dependent autophagy regulated by heat shock
protein 90, lipid engulfment mediated by RAS oncogene family member
RAB7A, member RAS oncogene family, and clock autophagy associated
with sequestosome 1, selectively degrading iron proteins, GPX4 and
lipid droplets to increase intracellular iron and free fatty acid
levels, accelerating lipid peroxidation and promoting ferroptosis
(68).
Several oncogenic pathways closely relate to
ferroptosis. For instance, numerous KRAS-mutated pancreatic cancers
are sensitive to ferroptosis inducers such as Erastin (69). Furthermore, emerging evidence
suggests that as a tumor suppressor gene, p53 inhibits cystine
uptake and sensitizes cells to ferroptosis by downregulating the
expression of the Xc system (70). However, studies also indicate
that p53 can transcriptionally restrict Erastin-induced ferroptosis
by blocking dipeptidyl peptidase 4 activity in a non-dependent
manner (71).
These intricate molecular mechanisms underscore the
complexity of ferroptosis and its regulation by various cellular
pathways. Understanding these mechanisms provides a foundation for
developing targeted therapies that can modulate ferroptosis in
cancer cells, potentially leading to novel anticancer
strategies.
Protective mechanisms against
ferroptosis in cancer cells
Under high demand for iron, cancer cells have a
series of specific mechanisms to prevent ferroptosis. By listing
and exploring these mechanisms, ferroptosis may be initiated by
targeting key points in these mechanisms, providing new
opportunities for cancer treatment.
Nuclear factor erythroid 2-related
factor 2 (Nrf2) pathway
Nrf2 is a transcription factor that protects cells
from toxic and oxidative damage by regulating the expression of a
variety of genes. Under normal conditions, Nrf2 is continuously
degraded via the kelch like ECH associated protein 1
(Keap1)-mediated ubiquitin-proteasome pathway. Under conditions of
cellular stress, the protein p62 can upregulate Nrf2 expression by
inactivating Keap1, and the activation of the p62-Keap1-Nrf2
pathway helps protect cells from ferroptosis (72). Nrf2 also interacts with small Maf
proteins to activate the transcription of antioxidant genes such as
quinone oxidoreductase 1 and HO-1, contributing to iron
homeostasis. Nrf2 regulates ferroptosis through the sigma-1
receptor (SIR), which is a ligand-regulated chaperone protein. SIR
is overexpressed in certain cancer cells and helps protect against
ferroptosis by upregulating ROS accumulation via the Nrf2 pathway
(72,73). Inhibition of SIR can enhance
ferroptosis by increasing Fe2+, GSH and lipid
peroxidation. Hence, targeting the Nrf2 pathway may be a potential
strategy to induce ferroptosis in cancer cells.
Numerous studies have demonstrated that Nrf2 plays a
protective role against chemical-induced carcinogenesis. For
instance, Nrf2 knockout mice (Nrf2−/−) are more
susceptible to tumor formation in the stomach, bladder and skin
when exposed to carcinogens (74). Nrf2-null mice showed increased
gastric neoplasia after exposure to benzo(a)pyrene compared to
wild-type mice. Higher tumor incidences were also reported in the
intestines of Nrf2-deficient mice challenged with azoxymethane and
dextran sodium sulfate, as well as in the bladder following
exposure to N-nitrosobutyl (4-hydroxybutyl) amine, and in the skin
after exposure to potent carcinogens such as
dimethylbenz(a)anthracene and tetradecanoylphorbol-13-acetate. The
protective mechanism of Nrf2 is attributed to its ability to reduce
ROS and DNA damage in cells. In addition, mice with reduced Nrf2
expression due to a single-nucleotide polymorphism (SNP) in the
Nrf2 gene promoter are more vulnerable to hyperoxia-induced lung
damage (75). This protective
role is supported by human studies where individuals with a similar
SNP have lower Nrf2 mRNA levels and a higher risk of developing
non-small-cell lung cancer.
Conversely, prolonged activation of Nrf2 is
associated with the progression of various cancers, including lung,
breast, head and neck, ovarian and endometrial carcinomas (76). High levels of Nrf2 in tumors are
associated with poor patient prognosis, likely due to its role in
enhancing cancer cell proliferation, chemoresistance and
radioresistance. Elevated Nrf2 levels are observed in cancer cells
resistant to chemotherapeutic agents, such as etoposide,
carboplatin, cisplatin, 5-fluorouracil and doxorubicin. Silencing
Nrf2 in these cells has been shown to restore drug sensitivity.
Nrf2 also promotes cancer cell proliferation by activating genes
involved in the pentose phosphate pathway (77) and other metabolic pathways,
supporting glucose flux and generating purines necessary for DNA
and RNA synthesis. In addition, Nrf2 helps maintain the redox
balance and glutathione synthesis, further accelerating cancer cell
proliferation. The dual role of Nrf2 in cancer underscores the
complexity of its function (78). While transient activation of Nrf2
in normal cells is protective, constitutive activation in cancer
cells enhances their survival and progression. Therefore,
selectively targeting Nrf2 in cancer cells could potentially
improve the efficacy of chemotherapy and radiation therapy by
overcoming chemoresistance and radioresistance. Of note, the dual
role of this molecule also suggests the possibility of more complex
interconnections with iron transport-related molecules, including
TfR1. The magnitude and extent of the impact remains worth
exploring. Recent research advances have shown that Nrf2 can be
modulated or completely silenced by the action of certain
molecules, including curcumin. This process is associated with a
variety of molecules, including TfR1 (79).
Epigenetic modifications and metabolic
molecules
RNA-binding proteins (RBPs) alter RNA fate by
influencing splicing, transport, translation and degradation. For
instance, DAZ-associated protein 1 is an RBP that stabilizes solute
carrier family 7 member 11 (SLC7A11) mRNA, reducing ferroptosis in
cancer cells. Alpha-enolase 1, traditionally a glycolytic enzyme,
also acts as an RBP, promoting the degradation of IRP1 mRNA,
maintaining iron homeostasis and protecting cells from ferroptosis
(80). Circular RNAs (circRNAs)
are single-stranded noncoding RNAs that form covalently closed
loops. Circ0097009, for example, stabilizes SLC7A11 by sponging
microRNA-1261, thereby protecting cells from ferroptosis (81).
Lactate, an important metabolic intermediate, enters
cancer cells mainly through monocarboxylate transporter 1,
regulated by hydroxycarboxylic acid receptor 1. Lactate increases
the synthesis of monounsaturated fatty acids and decreases the
synthesis of PUFAs, inhibiting lipid peroxidation and ferroptosis
(82). This process involves the
upregulation of sterol regulatory element-binding protein 1 and
stearoyl-CoA desaturase-1, facilitated by enhanced ATP production
and disrupted AMPK signaling. In addition, inactivation of the AMPK
pathway upregulates branched-chain amino acid aminotransferase 2,
another ferroptosis inhibitor that increases intracellular
glutamate levels, enhancing system xc− activity and
cystine uptake.
Overall, it is clear that these metabolic processes
highlight the importance of various metabolites in protecting
against ferroptosis, thus revealing potential therapeutic targets
in cancer treatment.
Regulation of immune cells in the tumor
microenvironment by iron
In the tumor microenvironment, iron and its
metabolism not only affect tumor cells, but also have a series of
effects on immune cells. This section will comprehensively describe
the effects of iron on immune cells and attempt to provide new
possible perspectives for immune cell activation in the tumor
microenvironment.
Regulation of the tumor immune
microenvironment by iron metabolism
Iron regulation of macrophage
polarization
Macrophages play a crucial role in the tumor immune
microenvironment, significantly influencing tumor progression and
development through their polarization states. Macrophages can
polarize into three primary types: Unactivated M0 macrophages,
classically activated M1 macrophages and alternatively activated M2
macrophages. M1 macrophages exhibit pro-inflammatory functions,
secreting cytokines such as TNF-α and IL-1β, primarily functioning
in antimicrobial and antitumor activities. By contrast, M2
macrophages have anti-inflammatory roles, secreting TGF-β and
platelet-derived growth factor, contributing to tissue repair and
tumor promotion.
Regarding iron metabolism, M1 and M2 macrophages
exhibit distinct characteristics (83). M1 macrophages tend to store iron,
which helps them combat bacteria and tumor cells. They exhibit
increased expression of iron-sequestering proteins such as ferritin
and decreased expression of iron-exporting proteins such as FPN.
Conversely, M2 macrophages are inclined to release iron due to
their high expression of CD163 and CD94, as well as iron-exporting
protein FPN, and lower ferritin expression. The released iron
promotes cell proliferation, matrix remodeling and immune
regulation, aligning with the functions of M2 macrophages. Iron
overload induces M1 macrophage activation, leading to the
expression of TNF-α, IL-12p40 and CD163 (84). This suggests that iron can
promote M1 polarization under certain conditions. However, iron may
also promote M2 polarization under different circumstances,
demonstrating the heterogeneity and dynamic nature of macrophages
in various environments. Mechanistically, M1 polarization is
usually accompanied by downregulation of FPN and upregulation of
TfR1. Low FPN expression and high ferritin expression facilitate M1
polarization. Conversely, M2 polarization is typically associated
with upregulation of TF receptors and lipid carriers.
Tumor-associated macrophages (TAMs), which often
exhibit an M2-like phenotype, can release iron, further supporting
tumor growth and immune evasion. Targeting iron metabolism in
cancer therapy has emerged as a novel strategy. Modulating iron
levels in the tumor microenvironment can alter the polarization of
TAMs, potentially shifting them from a tumor-promoting M2 phenotype
to a tumor-suppressing M1 phenotype.
In summary, iron metabolism significantly influences
macrophage polarization. Macrophages are more prone to M1
polarization in iron-rich environments, while iron-deficient
conditions favor M2 polarization. Iron chelators inducing
iron-deficient environments often lead to M2 polarization, though
specific studies on M2 subtypes are limited. The promoting effect
of M2 polarization on tumors makes it a possible target for cancer
treatment. These findings suggest a need for further investigation
into the underlying mechanisms of iron metabolism's effect on
macrophage polarization.
Iron regulation of neutrophil
recruitment and inflammation
Neutrophils are critical cells in the innate immune
system, playing essential roles in defending against microbial
invasion. Iron metabolism also plays a key role in neutrophil
function. Neutrophils express iron metabolism-related proteins such
as TfR1, ferritin heavy chain and FPN (85), enabling them to absorb or release
iron when stimulated. Iron is crucial for neutrophil function; for
example, the iron-dependent metalloprotein myeloperoxidase in
neutrophils exerts antibacterial effects through its
Fe3+/Fe2+ redox state (86).
Hepcidin, a peptide that increases intracellular
iron by inducing FPN degradation, also increases neutrophil
recruitment by inducing the production of C-X-C motif chemokine
ligand 1 (87). This process is
critical in acute inflammation and the body's initial response to
infection. Conversely, iron chelators such as deferasirox can
significantly reduce neutrophil-mediated inflammation, highlighting
the potential therapeutic role of modulating iron levels to control
excessive inflammatory responses (88). Under chronic inflammatory
conditions, iron metabolism dysregulation in neutrophils can
exacerbate tissue damage and disease progression. Elevated iron
levels can enhance the production of ROS by neutrophils, leading to
increased oxidative stress and damage to surrounding tissues. This
underscores the importance of balanced iron homeostasis in
maintaining appropriate neutrophil function and preventing chronic
inflammation.
These findings highlight the significant regulatory
role of iron metabolism in neutrophil recruitment and inflammation;
however, direct evidence remains limited. Further research is
needed to elucidate the precise mechanisms by which iron influences
neutrophil behavior and to develop targeted therapies for
conditions involving neutrophil-mediated inflammation.
Iron and natural killer (NK)
cells
Iron plays a crucial role in the function and
metabolism of NK cells. NK cells increase iron absorption during
viral infection to meet their metabolic needs and antiviral
activity (89). Iron deficiency
can impair the cytotoxicity and cytokine production of NK cells,
leading to increased susceptibility to viral infections and cancer.
In obesity, iron deficiency can lead to metabolic defects in NK
cells, mitochondrial adaptation and cytokine responses.
It is clear that iron deficiency leads to the loss
of NK-cell function, indicating that iron is essential not only for
NK-cell development and proliferation but also for their activation
and function in antiviral responses. The impaired function of NK
cells under iron-deficient conditions can result in decreased
cytotoxic activity and reduced production of critical cytokines
such as IFN-γ, weakening the body's ability to combat viral
infections and tumor cells (89). Conversely, iron overload can also
negatively impact NK-cell function by promoting oxidative stress
and cellular damage. Excess iron can lead to the generation of ROS,
which can impair NK cell viability and function (90). Maintaining optimal iron levels is
crucial for effective NK cell-mediated immune responses against
pathogens and cancer cells (89,90).
Iron and adaptive immunity
Iron metabolism is also vital in the adaptive immune
system. The function and differentiation of T cells and B cells
depend on iron availability. T-cell activation and proliferation
are regulated by iron absorption mediated by TfR1 (CD71), with iron
deficiency resulting in T-cell dysfunction and delayed immune
responses. Iron is also required for the proliferation of activated
T cells, as it is necessary for DNA synthesis and cellular
respiration. Iron deficiency can lead to impaired T-cell
proliferation, reduced cytokine production and compromised immune
responses (91). In addition,
iron chelation can be used therapeutically to inhibit T-cell
proliferation in autoimmune diseases, where excessive T-cell
activity contributes to disease pathology. Iron deficiency leads to
weakened antibody responses, compromising the body's ability to
mount effective humoral immunity (89). The regulation of iron levels is
therefore critical for maintaining balanced and effective adaptive
immune responses.
Summary
Iron metabolism plays a critical regulatory role in
the tumor immune microenvironment by influencing the functions of
macrophages, neutrophils, NK cells, T cells and B cells. Future
research should further explore the specific impacts of iron
metabolism on these immune cells and their mechanisms in diseases
to provide new insight for treating conditions such as cancer and
autoimmune diseases. Understanding the intricate relationship
between iron metabolism and immune function will pave the way for
novel therapeutic approaches targeting iron homeostasis to modulate
immune responses and improve disease outcomes.
Ferroptosis and immunotherapy:
Interplay to enhance antitumor efficacy
Ferroptosis has been identified as a crucial player
in the modulation of immune responses within the tumor
microenvironment. Its role in immunotherapy, a treatment modality
that leverages the immune system to combat cancer, is becoming
increasingly evident. This section will delve into the intricate
relationship between ferroptosis and immune responses in detail,
emphasizing strategies that exploit iron metabolism to enhance the
efficacy of tumor immunotherapy.
Immunogenic cell death and
ferroptosis
Ferroptosis shares several characteristics with
other forms of immunogenic cell death, such as apoptosis and
necroptosis, which can stimulate antitumor immunity. Ferroptotic
cells release lipid mediators that serve as 'find me' signals,
attracting antigen-presenting cells (APCs) and other immune cells
to the tumor microenvironment. These lipid mediators, including
oxidized phospholipids and eicosanoids, play pivotal roles in
modulating immune responses.
For instance, inducible GPX4 depletion in cancer
cells triggers the release of eicosanoids, which are involved in
the regulation of inflammation and immune responses (92). Conversely, enhancing GPX4
activity through TNF or IL-1β stimulation can suppress the
activation of pro-inflammatory lipid mediators like leukotriene B4
(LTB4), mediated by NF-κB signaling. Given LTB4′s significant role
in carcinogenesis, understanding its modulation through ferroptosis
is crucial for designing therapeutic strategies.
In addition, lipid peroxidation products released
during ferroptosis, such as oxidized phosphatidylethanolamines,
have immunomodulatory effects (92). These oxidized lipids can promote
the activity of APCs, leading to enhanced phagocytosis and
clearance of apoptotic cells. For instance, macrophages
preferentially engulf apoptotic cells with peroxidized
phosphatidylserine on their outer membranes. Furthermore,
ferroptotic cells secrete 1-steaoryl-2-15-HpET
E-sn-glycero-3-phosphatidylethanolamine, an 'eat-me' signal that
activates macrophages for phagocytosis. These findings suggest that
the oxidized lipids from ferroptotic cells can modulate immune cell
activity, although this hypothesis requires further experimental
validation.
Immune cell resistance to
ferroptosis
Certain immunosuppressive cells, such as M2-type
macrophages, regulatory T cells (Tregs) and myeloid-derived
suppressor cells (MDSCs), exhibit resistance to ferroptosis by
expressing high levels of GPX4 or other protective components.
Inducing ferroptosis in these cells can lead to their death and
reverse their tumor-promoting functions. Of note, M1 macrophages
are more resistant to ferroptosis than M2 macrophages, even in the
absence of GPX4. This resistance is attributed to the high
expression of inducible nitric oxide synthase and the production of
nitric oxide radicals (NO·), which can inhibit lipid peroxidation
reactions. Thus, ferroptosis inducers can trigger ferroptosis in M2
macrophages or repolarize them to the M1 phenotype, thereby
enhancing antitumor effects.
Similarly, Tregs activated by T-cell receptor/CD28
co-stimulation upregulate GPX4 expression, reducing ferroptosis
occurrence. Deletion of the GPX4 gene in Tregs leads to excessive
lipid peroxide accumulation and ferroptosis, promoting an antitumor
immune response through IL-1β production and T helper 17 cell
activation. MDSCs, on the other hand, rely on lipid transport and
metabolism for their function. Polymorphonuclear MDSCs undergo
lipid peroxidation via myeloperoxidase and transfer lipids to
dendritic cells, inhibiting cross-presentation and promoting tumor
growth. MDSCs also accumulate specific lipid species, such as
arachidonic acid esterified triglycerides and prostaglandin E2,
which confer resistance to ferroptosis (93). Furthermore, MDSCs deplete cystine
and cysteine from the extracellular environment, depriving T cells
of these essential amino acids needed for activation (94). Unlike antigen-presenting cells,
MDSCs do not export cysteine, further limiting its availability to
T cells (94). In addition,
MDSCs downregulate L-selectin on T cells, disrupting their
trafficking patterns and inhibiting activation (95). The p53 pathway also regulates
ferroptosis in MDSCs, where increased p53 stability inhibits ROS
production and ferroptosis. Due to the special role of immune
suppressive cells, represented by MDSCs, in negatively regulating
immune responses in cancer and other diseases, it is important to
focus on promoting ferroptosis as a starting point to reduce immune
escape of tumor cells.
Enhancing tumor immunotherapy through
ferroptosis
Recent studies have revealed a novel mechanism by
which immune cells exert antitumor effects through promoting
ferroptosis in cancer cells. Interferon-gamma (IFNγ) released by
CD8+ T cells has a crucial role in this process by downregulating
the expression of SLC3A2 and SLC7A11, two subunits of the
glutamate-cystine antiporter system xc- (96). This impairs cystine uptake by
tumor cells, leading to increased lipid peroxidation and
ferroptosis (97). In addition,
transforming growth factor (TGF)-β1 has been shown to sensitize
cancer cells to IFNγ-induced ferroptosis by further decreasing
system xc-expression (97). The
induction of ferroptosis in cancer cells enhances tumor
immunogenicity and promotes the activation of immune cells,
creating a positive feedback loop (98). These findings suggest that
targeting the ferroptosis pathway in combination with immunotherapy
could be a promising approach for cancer treatment.
These interactions suggest potential therapeutic
strategies to enhance immunotherapy efficacy by modulating
ferroptosis. For instance, targeting GPX4 in immunosuppressive
cells like Tregs and MDSCs can induce ferroptosis, reversing their
tumor-promoting functions and enhancing the immune response. In
addition, it is worth noting that leveraging cytokines such as IFNγ
and TGF-β1 to induce ferroptosis in tumor cells can synergize with
existing immunotherapies, leading to improved antitumor outcomes.
By combining drugs that promote ferroptosis with traditional immune
checkpoint inhibitors, the killing efficiency of immune cells
against tumor cells can be improved. In addition, given the
importance of ferroptosis in the tumor microenvironment, further
research on how these signaling pathways specifically function and
their differences in different types of cancer will help develop
more precise treatment methods. Overall, ferroptosis not only
serves as a mechanism of cell death, but also as a strategy for
regulating immune responses, demonstrating its broad potential in
cancer treatment (Fig. 2).
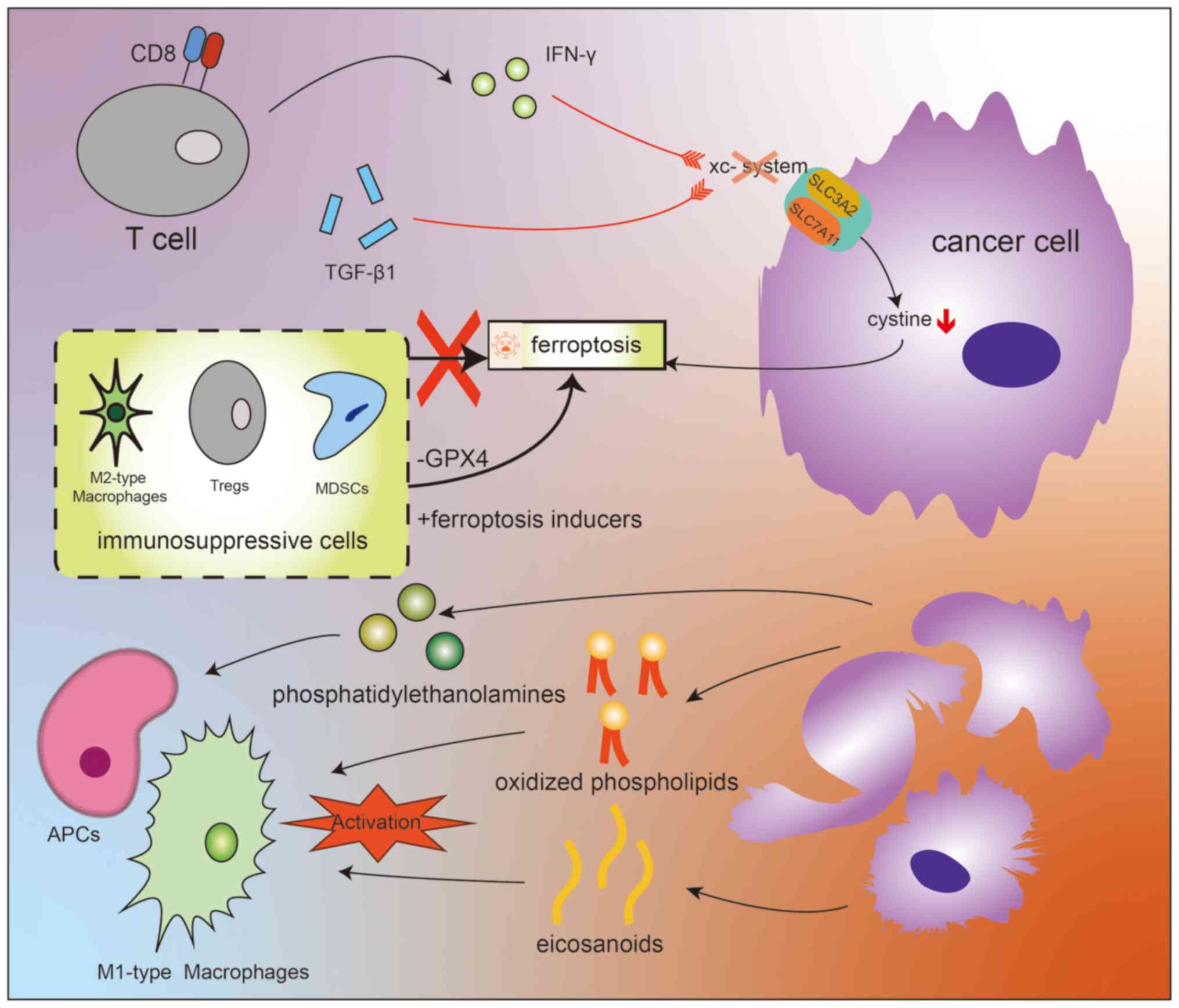 | Figure 2Diagram illustrating the integrated
effects of ferroptosis and the tumor microenvironment. CD8+ T cells
indirectly induce ferroptosis in tumor cells by targeting the
xc-system with small molecules. This process is primarily related
to the actions of the aforementioned molecules on the two core
components, SLC3A2 and SLC7A11, which together directly determine
the ability of the xc-system to uptake cystine. Cystine is involved
in the crucial lipid peroxidation process associated with
ferroptosis. Some immunosuppressive cells' (including M2-type
macrophages, Tregs and MDSCs) resistance to ferroptosis can be
overcome by certain methods such as reducing GPX4 levels or using
ferroptosis inducers. Oxidative lipids resulting from ferroptosis
activate APCs, triggering a series of physiological processes,
including the activation of macrophage phagocytosis. Among them,
various lipid molecules, including oxidized phospholipids and
eicosanoids, play a key role in the activation of M1-type
macrophages. The fundamental pathways through which they exert
their effects are illustrated in this diagram. APCs,
antigen-presenting cells; CD8+ T cells, CD8 positive T cells;
MDSCs, myeloid-derived suppressor cells; GPX4, glutathione
peroxidase 4; Tregs, regulatory T cells; xc− system,
cystine/glutamate antiporter system; SLC3A2, solute carrier family
3 member 2; SLC7A11, solute carrier family 7 member 11; IFN,
interferon; TGF, transforming growth factor. |
Targeting iron metabolism for cancer
treatment
Targeting ferroptosis in cancer
therapy
Recent advances in cancer research have highlighted
the therapeutic potential of ferroptosis-inducing agents (FINs)
(8). These agents, along with
various nanomaterials designed to locally induce ferroptosis or
enhance the activity of FINs, have shown promise in treating
cancer. Ferroptosis has also been recognized to be implicated in
the tumor-suppressive effects of conventional cancer therapies such
as radiotherapy, chemotherapy, targeted therapy and immunotherapy.
By inducing ferroptosis, FINs could enhance the efficacy of these
treatments, particularly in cancers with specific characteristics.
Ferroptosis represents a significant vulnerability in certain
cancer types, making it an attractive target for therapy (8,99). For instance, lung and breast
cancer cells are more sensitive to ferroptosis compared to their
normal epithelial counterparts, suggesting a therapeutic window for
selective ferroptosis induction in tumors while sparing normal
tissues. As mentioned earlier, overcoming ferroptosis resistance,
which is mediated by various genetic and molecular mechanisms, is
another potential strategy. Disrupting these resistance mechanisms
may re-sensitize cancer cells to ferroptosis and enhance the
effectiveness of FINs (8).
Combining FINs with conventional therapies is
another promising approach. Conventional treatments can trigger
ferroptosis, and enhancing this effect with FINs can potentiate
their therapeutic efficacy. For instance, radiotherapy induces
ferroptosis through multiple mechanisms and combining it with FINs
targeting SLC7A11 or GPX4 can radiosensitize cancer cells.
Similarly, chemotherapeutic agents like gemcitabine and cisplatin
induce GPX4 expression, and inhibiting GPX4 can increase the
sensitivity of cancer cells to these drugs. Immunotherapy, when
combined with FINs, can also boost ferroptosis induction and
overcome resistance to immune checkpoint inhibitors (100).
The integration of ferroptosis induction with
conventional therapies offers a comprehensive strategy to combat
cancer, particularly in resistant or aggressive tumors. As research
progresses, refining these approaches to enhance specificity,
efficacy and safety will be crucial in transforming the cancer
treatment landscape.
Potential future therapeutic
strategies
In the realm of cancer therapy, targeting iron
metabolism holds considerable promise due to the essential role of
iron in tumor growth and survival. Several strategies are being
explored, focusing on disrupting iron homeostasis in cancer cells,
as well as immunosuppressive cells.
It is the significant role of iron in cancer biology
that has prompted the exploration of new therapeutic strategies
targeting iron metabolism. This approach aims to exploit the
dependency of cancer cells on iron for proliferation and survival,
thereby offering promising new avenues for cancer treatment. While
challenges exist, such as adaptive resistance and potential side
effects, ongoing research and combination approaches may enhance
the efficacy and specificity of these treatments, paving the way
for more effective cancer therapies. The tumor immunotherapies
targeting iron metabolism that have been initiated or are currently
under research were elaborated on in this chapter. Although these
therapies only provide certain possible ideas and there are still
unresolved issues, they fully demonstrate the great application
value and broad prospects of iron metabolism-centered treatment
(Table II).
 | Table IITumor immunotherapies targeting iron
metabolism that have been initiated or are currently being
researched. |
Table II
Tumor immunotherapies targeting iron
metabolism that have been initiated or are currently being
researched.
| Potential
therapeutic strategy | Mechanism | Examples | Challenges |
|---|
| Iron chelation | Sequestering iron
to deprive cancer cells | DFO, DFP, DFX,
Dp44mT, Super-polyphenols | Adaptive
resistance, side effects in non-cancerous tissues |
| Targeting TfR1 | Reducing iron
uptake by cancer cells | Monoclonal
antibodies, siRNA | Variability in TfR1
expression in normal tissues |
| Inhibition of
HIFs | Disrupting hypoxia
response and iron retention | YC-1 | Optimizing
therapeutic potential, understanding systemic impacts |
| Modulation of
hepcidin-ferroportin | Regulating iron
export to reduce intracellular iron in cancer cells | Angelica
sinensis polysaccharide, anti-hemojuvelin antibodies | Long-term efficacy,
systemic effects |
| Inducing
ferroptosis | Promoting
iron-dependent cell death via lipid peroxidation | System
xc− inhibitors, GPX4 inhibitors | Identifying
specific vulnerabilities, combination with other treatments |
Limitations of therapies targeting iron
metabolism mechanisms
Despite its significant potential for application,
research related to iron metabolism and ferroptosis currently shows
limitations that impact the translation of iron metabolism theories
into therapies. The mechanisms underlying ferroptosis remain to be
fully elucidated and various triggering factors still need further
identification, which affects the safety of selectively controlling
ferroptosis. For instance, systemic targeting of GPX4 may lead to
toxicity, including kidney damage, neurodegeneration and injury to
other organs (101). In
addition, the diverse and complex effects of iron metabolism on
immune cells make clinical outcomes difficult to control. In the
future, these issues may be further addressed with the development
of more precisely targeted drugs.
Conclusion and future perspectives
The complex relationship between iron metabolism and
tumor biology is a complex network that significantly impacts tumor
progression and the tumor microenvironment. Tumor cells and their
associated stromal cells orchestrate the finely tuned regulation of
iron uptake, accumulation and homeostasis, which is crucial for
their survival and proliferation. Ferroptosis, a form of regulated
cell death driven by iron-dependent lipid peroxidation, is a potent
tumor-suppressive mechanism. However, cancer cells have developed
sophisticated strategies to evade ferroptosis, which poses a
challenge to their therapeutic exploitation. The role of iron
metabolism in modulating the tumor immune microenvironment adds
another layer of intricacy. Iron availability and regulation
influence the function of tumor-associated immune cells, affecting
immune surveillance and evasion. The relationship between
ferroptosis and immune responses creates new avenues for enhancing
cancer immunotherapy. By targeting iron metabolism, it is possible
to disrupt the protective barriers of tumors and sensitize them to
immune-mediated destruction.
Current therapeutic approaches leveraging
ferroptosis show promise, yet the full potential of iron
metabolism-based strategies remains underexplored. Future research
should prioritize several areas, including targeting cancer cells
to iron-dependent proteins to ensure their iron supply and
conducting further practical research on the iron-mediated death of
immunosuppressive proteins. The future of cancer therapy lies in a
comprehensive approach that integrates our understanding of iron
metabolism and ferroptosis with advanced therapeutic strategies. By
unravelling the complexities of iron regulation in tumors and
developing innovative treatments, the way may be paved for more
effective and personalized cancer therapies. Ongoing research and
future discoveries hold the promise of transforming the landscape
of cancer treatment, offering hope for improved patient outcomes
and long-term survival.
Availability of data and materials
Not applicable.
Authors' contributions
LFW designed the study, XRB collected the related
papers and drafted the manuscript, and LFW and XRB generated the
figures and critically reviewed the manuscript. All authors have
read and approved the final manuscript. Data authentication is not
applicable.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Acknowledgements
Not applicable.
Funding
No funding was received.
References
|
1
|
Dixon SJ and Stockwell BR: The role of
iron and reactive oxygen species in cell death. Nat Chem Biol.
10:9–17. 2014. View Article : Google Scholar
|
|
2
|
Schümann K, Ettle T, Szegner B, Elsenhans
B and Solomons NW: On risks and benefits of iron supplementation
recommendations for iron intake revisited. J Trace Elem Med Biol.
21:147–168. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Muckenthaler MU, Rivella S, Hentze MW and
Galy B: A red carpet for iron metabolism. Cell. 168:344–361. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Rodriguez R, Schreiber SL and Conrad M:
Persister cancer cells: Iron addiction and vulnerability to
ferroptosis. Mol Cell. 82:728–740. 2022. View Article : Google Scholar :
|
|
5
|
Gao M, Yi J, Zhu J, Minikes AM, Monian P,
Thompson CB and Jiang X: Role of mitochondria in ferroptosis. Mol
Cell. 73:354–363.e3. 2019. View Article : Google Scholar :
|
|
6
|
Kurz T, Eaton JW and Brunk UT: The role of
lysosomes in iron metabolism and recycling. Int J Biochem Cell
Biol. 43:1686–1697. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Silva B and Faustino P: An overview of
molecular basis of iron metabolism regulation and the associated
pathologies. Biochim Biophys Acta. 1852:1347–1359. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Liang C, Zhang X, Yang M and Dong X:
Recent progress in ferroptosis inducers for cancer therapy. Adv
Mater. 31:e19041972019. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Mu Q, Chen L, Gao X, Shen S, Sheng W, Min
J and Wang F: The role of iron homeostasis in remodeling immune
function and regulating inflammatory disease. Sci Bull (Beijing).
66:1806–1816. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Slominski RM, Raman C, Chen JY and
Slominski AT: How cancer hijacks the body's homeostasis through the
neuroendocrine system. Trends Neurosci. 46:263–275. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Slominski RM, Kim TK, Janjetovic Z,
Brożyna AA, Podgorska E, Dixon KM, Mason RS, Tuckey RC, Sharma R,
Crossman DK, et al: Malignant melanoma: An overview, new
perspectives, and vitamin D signaling. Cancers (Basel).
16:22622024. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ajoolabady A, Tang D, Kroemer G and Ren J:
Ferroptosis in hepatocellular carcinoma: Mechanisms and targeted
therapy. Br J Cancer. 128:190–205. 2023. View Article : Google Scholar :
|
|
13
|
Yang M, Wu X, Hu J, Wang Y, Wang Y, Zhang
L, Huang W, Wang X, Li N, Liao L, et al: COMMD10 inhibits HIF1α/CP
loop to enhance ferroptosis and radiosensitivity by disrupting
Cu-Fe balance in hepatocellular carcinoma. J Hepatol. 76:1138–1150.
2022. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Chen F, Fan Y, Hou J, Liu B, Zhang B,
Shang Y, Chang Y, Cao P and Tan K: Integrated analysis identifies
TfR1 as a prognostic biomarker which correlates with immune
infiltration in breast cancer. Aging (Albany NY). 13:21671–21699.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Candelaria PV, Leoh LS, Penichet ML and
Daniels-Wells TR: Antibodies targeting the transferrin receptor 1
(TfR1) as direct anti-cancer agents. Front Immunol. 12:6076922021.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Soyer HP, Smolle J, Torne R and Kerl H:
Transferrin receptor expression in normal skin and in various
cutaneous tumors. J Cutan Pathol. 14:1–5. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Gammella E, Buratti P, Cairo G and
Recalcati S: The transferrin receptor: The cellular iron gate.
Metallomics. 9:1367–1375. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kawabata H: Transferrin and transferrin
receptors update. Free Radic Biol Med. 133:46–54. 2019. View Article : Google Scholar
|
|
19
|
Thompson EB: The many roles of c-Myc in
apoptosis. Annu Rev Physiol. 60:575–600. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Chen BY, Pathak JL, Lin HY, Guo WQ, Chen
WJ, Luo G, Wang LJ, Sun XF, Ding Y, Li J, et al: Inflammation
triggers chondrocyte ferroptosis in TMJOA via HIF-1α/TFRC. J Dent
Res. 103:712–722. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Finley LWS, Carracedo A, Lee J, Souza A,
Egia A, Zhang J, Teruya-Feldstein J, Moreira PI, Cardoso SM, Clish
CB, et al: SIRT3 opposes reprogramming of cancer cell metabolism
through HIF1α destabilization. Cancer Cell. 19:416–428. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Chitambar CR, Al-Gizawiy MM, Alhajala HS,
Pechman KR, Wereley JP, Wujek R, Clark PA, Kuo JS, Antholine WE and
Schmainda KM: Gallium maltolate disrupts tumor iron metabolism and
retards the growth of glioblastoma by inhibiting mitochondrial
function and ribonucleotide reductase. Mol Cancer Ther.
17:1240–1250. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kenneth NS, Mudie S, Naron S and Rocha S:
TfR1 interacts with the IKK complex and is involved in IKK-NF-κB
signalling. Biochem J. 449:275–284. 2013. View Article : Google Scholar
|
|
24
|
Jeong SM, Hwang S and Seong RH:
Transferrin receptor regulates pancreatic cancer growth by
modulating mitochondrial respiration and ROS generation. Biochem
Biophys Res Commun. 471:373–379. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Polak KZ, Schaffer P, Donaghy D, Zenk MC
and Olver CS: Iron, hepcidin, and microcytosis in canine
hepatocellular carcinoma. Vet Clin Pathol. 51:208–215. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Habashy HO, Powe DG, Staka CM, Rakha EA,
Ball G, Green AR, Aleskandarany M, Paish EC, Douglas Macmillan R,
Nicholson RI, et al: Transferrin receptor (CD71) is a marker of
poor prognosis in breast cancer and can predict response to
tamoxifen. Breast Cancer Res Treat. 119:283–293. 2010. View Article : Google Scholar
|
|
27
|
Yang DC, Wang F, Elliott RL and Head JF:
Expression of transferrin receptor and ferritin H-chain mRNA are
associated with clinical and histopathological prognostic
indicators in breast cancer. Anticancer Res. 21:541–549.
2001.PubMed/NCBI
|
|
28
|
Basuli D, Tesfay L, Deng Z, Paul B,
Yamamoto Y, Ning G, Xian W, McKeon F, Lynch M, Crum CP, et al: Iron
addiction: A novel therapeutic target in ovarian cancer. Oncogene.
36:4089–4099. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Chan KT, Choi MY, Lai KKY, Tan W, Tung LN,
Lam HY, Tong DK, Lee NP and Law S: Overexpression of transferrin
receptor CD71 and its tumorigenic properties in esophageal squamous
cell carcinoma. Oncol Rep. 31:1296–1304. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ryschich E, Huszty G, Knaebel HP, Hartel
M, Büchler MW and Schmidt J: Transferrin receptor is a marker of
malignant phenotype in human pancreatic cancer and in
neuroendocrine carcinoma of the pancreas. Eur J Cancer.
40:1418–1422. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Kondo K, Noguchi M, Mukai K, Matsuno Y,
Sato Y, Shimosato Y and Monden Y: Transferrin receptor expression
in adenocarcinoma of the lung as a histopathologic indicator of
prognosis. Chest. 97:1367–1371. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Smith NW, Strutton GM, Walsh MD, Wright
GR, Seymour GJ, Lavin MF and Gardiner RA: Transferrin receptor
expression in primary superficial human bladder tumours identifies
patients who develop recurrences. Br J Urol. 65:339–344. 1990.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Jamnongkan W, Thanan R, Techasen A, Namwat
N, Loilome W, Intarawichian P, Titapun A and Yongvanit P:
Upregulation of transferrin receptor-1 induces cholangiocarcinoma
progression via induction of labile iron pool. Tumour Biol.
39:10104283177176552017. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Xu X, Liu T, Wu J, Wang Y, Hong Y and Zhou
H: Transferrin receptor-involved HIF-1 signaling pathway in
cervical cancer. Cancer Gene Ther. 26:356–365. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Wu H, Zhang J, Dai R, Xu J and Feng H:
Transferrin receptor-1 and VEGF are prognostic factors for
osteosarcoma. J Orthop Surg Res. 14:2962019. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Greene CJ, Attwood K, Sharma NJ, Gross KW,
Smith GJ, Xu B and Kauffman EC: Transferrin receptor 1 upregulation
in primary tumor and downregulation in benign kidney is associated
with progression and mortality in renal cell carcinoma patients.
Oncotarget. 8:107052–107075. 2017. View Article : Google Scholar :
|
|
37
|
Adachi M, Kai K, Yamaji K, Ide T, Noshiro
H, Kawaguchi A and Aishima S: Transferrin receptor 1 overexpression
is associated with tumour de-differentiation and acts as a
potential prognostic indicator of hepatocellular carcinoma.
Histopathology. 75:63–73. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Das Gupta A, Patil J and Shah VI:
Transferrin receptor expression by blast cells in acute
lymphoblastic leukemia correlates with white cell count &
immunophenotype. Indian J Med Res. 104:226–233. 1996.PubMed/NCBI
|
|
39
|
Hagag AA, Badraia IM, Abdelmageed MM,
Hablas NM, Hazzaa SME and Nosair NA: Prognostic value of
transferrin receptor-1 (CD71) expression in acute lymphoblastic
leukemia. Endocr Metab Immune Disord Drug Targets. 18:610–617.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Maguire A, Chen X, Wisner L, Ramsower C,
Glinsmann-Gibson B and Rimsza LM: Over-expression of transferrin
receptor (TFRC/CD71) and low expression of innate and adaptive
immune cell subsets in HIV-associated, GCB-DLBCL by digital gene
expression profiling. Blood. 134(Suppl 1): S27832019. View Article : Google Scholar
|
|
41
|
Joachim JH and Mehta KJ: Hepcidin in
hepatocellular carcinoma. Br J Cancer. 127:185–192. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Li P, Wu X, Chen P and Gu Z: Prognostic
significance of iron metabolism related genes in human lung
adenocarcinoma. Cancer Manag Res. 15:203–216. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Grunewald TGP, Bach H, Cossarizza A and
Matsumoto I: The STEAP protein family: Versatile oxidoreductases
and targets for cancer immunotherapy with overlapping and distinct
cellular functions. Biol Cell. 104:641–657. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Ohgami RS, Campagna DR, McDonald A and
Fleming MD: The steap proteins are metalloreductases. Blood.
108:1388–1394. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Ohgami RS, Campagna DR, Greer EL,
Antiochos B, McDonald A, Chen J, Sharp JJ, Fujiwara Y, Barker JE
and Fleming MD: Identification of a ferrireductase required for
efficient transferrin-dependent iron uptake in erythroid cells. Nat
Genet. 37:1264–1269. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
46
|
Scarl RT, Lawrence CM, Gordon HM and
Nunemaker CS: STEAP4: Its emerging role in metabolism and
homeostasis of cellular iron and copper. J Endocrinol.
234:R123–R134. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Muckenthaler MU, Galy B and Hentze MW:
Systemic iron homeostasis and the iron-responsive
element/iron-regulatory protein (IRE/IRP) regulatory network. Annu
Rev Nutr. 28:197–213. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Galy B, Conrad M and Muckenthaler M:
Mechanisms controlling cellular and systemic iron homeostasis. Nat
Rev Mol Cell Biol. 25:133–155. 2024. View Article : Google Scholar
|
|
49
|
Ke Q and Costa M: Hypoxia-inducible
factor-1 (HIF-1). Mol Pharmacol. 70:1469–1480. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Rashid M, Zadeh LR, Baradaran B, Molavi O,
Ghesmati Z, Sabzichi M and Ramezani F: Up-down regulation of HIF-1α
in cancer progression. Gene. 798:1457962021. View Article : Google Scholar
|
|
51
|
Yang L, Liu Q, Lu Q, Xiao JJ, Fu AY, Wang
S, Ni L, Hu JW, Yu H, Wu X and Zhang BF: Scavenger receptor class B
type I deficiency induces iron overload and ferroptosis in renal
tubular epithelial cells via hypoxia-inducible
factor-1α/transferrin receptor 1 signaling pathway. Antioxid Redox
Signal. 41:56–73. 2024. View Article : Google Scholar
|
|
52
|
Clérigues V, Murphy CL, Guillén MI and
Alcaraz MJ: Haem oxygenase-1 induction reverses the actions of
interleukin-1β on hypoxia-inducible transcription factors and human
chondrocyte metabolism in hypoxia. Clin Sci (Lond). 125:99–108.
2013. View Article : Google Scholar
|
|
53
|
Dixon SJ, Lemberg KM, Lamprecht MR, Skouta
R, Zaitsev EM, Gleason CE, Patel DN, Bauer AJ, Cantley AM, Yang WS,
et al: Ferroptosis: An iron-dependent form of nonapoptotic cell
death. Cell. 149:1060–1072. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Nakamura T, Naguro I and Ichijo H: Iron
homeostasis and iron-regulated ROS in cell death, senescence and
human diseases. Biochim Biophys Acta Gen Subj. 1863:1398–1409.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Rochette L, Dogon G, Rigal E, Zeller M,
Cottin Y and Vergely C: Lipid peroxidation and iron metabolism: Two
corner stones in the homeostasis control of ferroptosis. Int J Mol
Sci. 24:4492022. View Article : Google Scholar
|
|
56
|
Bhattacharyya A, Chattopadhyay R, Mitra S
and Crowe SE: Oxidative stress: An essential factor in the
pathogenesis of gastrointestinal mucosal diseases. Physiol Rev.
94:329–354. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Kiran KR, Deepika VB, Swathy PS, Prasad K,
Kabekkodu SP, Murali TS, Satyamoorthy K and Muthusamy A:
ROS-dependent DNA damage and repair during germination of NaCl
primed seeds. J Photochem Photobiol B. 213:1120502020. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Ursini F and Maiorino M: Lipid
peroxidation and ferroptosis: The role of GSH and GPx4. Free Radic
Biol Med. 152:175–185. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Xie Y, Kang R, Klionsky DJ and Tang D:
GPX4 in cell death, autophagy, and disease. Autophagy.
19:2621–2638. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Probst L, Dächert J, Schenk B and Fulda S:
Lipoxygenase inhibitors protect acute lymphoblastic leukemia cells
from ferroptotic cell death. Biochem Pharmacol. 140:41–52. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Shintoku R, Takigawa Y, Yamada K, Kubota
C, Yoshimoto Y, Takeuchi T, Koshiishi I and Torii S:
Lipoxygenase-mediated generation of lipid peroxides enhances
ferroptosis induced by erastin and RSL3. Cancer Sci. 108:2187–2194.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Chen W, Yang A, Jia J, Popov YV, Schuppan
D and You H: Lysyl oxidase (LOX) family members: rationale and
their potential as therapeutic targets for liver fibrosis.
Hepatology. 72:729–741. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Shah R, Shchepinov MS and Pratt DA:
Resolving the role of lipoxygenases in the initiation and execution
of ferroptosis. ACS Cent Sci. 4:387–396. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Yang Y, Karakhanova S, Hartwig W, D'Haese
JG, Philippov PP, Werner J and Bazhin AV: Mitochondria and
mitochondrial ROS in cancer: Novel targets for anticancer therapy.
J Cell Physiol. 231:2570–2581. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Chen L, Ma Y, Ma X, Liu L, Jv X, Li A,
Shen Q, Jia W, Qu L, Shi L and Xie J: TFEB regulates cellular
labile iron and prevents ferroptosis in a TfR1-dependent manner.
Free Radic Biol Med. 208:445–457. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Wang D, Liang W, Huo D, Wang H, Wang Y,
Cong C, Zhang C, Yan S, Gao M, Su X, et al: SPY1 inhibits neuronal
ferroptosis in amyotrophic lateral sclerosis by reducing lipid
peroxidation through regulation of GCH1 and TFR1. Cell Death
Differ. 30:369–382. 2023. View Article : Google Scholar :
|
|
67
|
Koppula P, Lei G, Zhang Y, Yan Y, Mao C,
Kondiparthi L, Shi J, Liu X, Horbath A, Das M, et al: A targetable
CoQ-FSP1 axis drives ferroptosis- and radiation-resistance in KEAP1
inactive lung cancers. Nat Commun. 13:22062022. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Liu J, Kuang F, Kroemer G, Klionsky DJ,
Kang R and Tang D: Autophagy-dependent ferroptosis: Machinery and
regulation. Cell Chem Biol. 27:420–435. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Yang Y, Luo M, Zhang K, Zhang J, Gao T,
Connell DO, Yao F, Mu C, Cai B, Shang Y and Chen W: Nedd4
ubiquitylates VDAC2/3 to suppress erastin-induced ferroptosis in
melanoma. Nat Commun. 11:4332020. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Li Y, Cao Y, Xiao J, Shang J, Tan Q, Ping
F, Huang W, Wu F, Zhang H and Zhang X: Inhibitor of
apoptosis-stimulating protein of p53 inhibits ferroptosis and
alleviates intestinal ischemia/reperfusion-induced acute lung
injury. Cell Death Differ. 27:2635–2650. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Kang R, Kroemer G and Tang D: The tumor
suppressor protein p53 and the ferroptosis network. Free Radic Biol
Med. 133:162–168. 2019. View Article : Google Scholar
|
|
72
|
Bellezza I, Giambanco I, Minelli A and
Donato R: Nrf2-Keap1 signaling in oxidative and reductive stress.
Biochim Biophys Acta Mol Cell Res. 1865:721–733. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Dodson M, Castro-Portuguez R and Zhang DD:
NRF2 plays a critical role in mitigating lipid peroxidation and
ferroptosis. Redox Biol. 23:1011072019. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Han P, Wang X, Zhou T, Cheng J, Wang C,
Sun F and Zhao X: Inhibition of ferroptosis attenuates oligospermia
in male Nrf2 knockout mice. Free Radic Biol Med. 193:421–429. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Yamaguchi Y, Kamai T, Higashi S, Murakami
S, Arai K, Shirataki H and Yoshida KI: Nrf2 gene mutation and
single nucleotide polymorphism rs6721961 of the Nrf2 promoter
region in renal cell cancer. BMC Cancer. 19:11372019. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Rojo de la Vega M, Chapman E and Zhang DD:
Nrf2 and the hallmarks of cancer. Cancer Cell. 34:21–43. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Tang YC, Hsiao JR, Jiang SS, Chang JY, Chu
PY, Liu KJ, Fang HL, Lin LM, Chen HH, Huang YW, et al:
c-MYC-directed NRF2 drives malignant progression of head and neck
cancer via glucose-6-phosphate dehydrogenase and transketolase
activation. Theranostics. 11:5232–5247. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Menegon S, Columbano A and Giordano S: The
dual roles of NRF2 in cancer. Trends Mol Med. 22:578–593. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Zhou Y, Jia Z, Wang J, Huang S, Yang S,
Xiao S, Xia D and Zhou Y: Curcumin reverses erastin-induced
chondrocyte ferroptosis by upregulating Nrf2. Heliyon.
9:e201632023. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Zhang T, Sun L, Hao Y, Suo C, Shen S, Wei
H, Ma W, Zhang P, Wang T, Gu X, et al: ENO1 suppresses cancer cell
ferroptosis by degrading the mRNA of iron regulatory protein 1. Nat
Cancer. 3:75–89. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Arabpour J, Rezaei K, Khojini JY, Razi S,
Hayati MJ and Gheibihayat SM: The potential role and mechanism of
circRNAs in Ferroptosis: A comprehensive review. Pathol Res Pract.
255:1552032024. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Zhao Y, Li M, Yao X, Fei Y, Lin Z, Li Z,
Cai K, Zhao Y and Luo Z: HCAR1/MCT1 regulates tumor ferroptosis
through the lactate-mediated AMPK-SCD1 activity and its therapeutic
implications. Cell Rep. 33:1084872020. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Ganz T: Macrophages and iron metabolism.
Microbiol Spectr. 4:2016. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Zhu L, Zhao Q, Yang T, Ding W and Zhao Y:
Cellular metabolism and macrophage functional polarization. Int Rev
Immunol. 34:82–100. 2015. View Article : Google Scholar
|
|
85
|
Cronin SJF, Woolf CJ, Weiss G and
Penninger JM: The role of iron regulation in immunometabolism and
immune-related disease. Front Mol Biosci. 6:1162019. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Arnhold J, Furtmüller PG and Obinger C:
Redox properties of myeloperoxidase. Redox Rep. 8:179–186. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Malerba M, Louis S, Cuvellier S, Shambat
SM, Hua C, Gomart C, Fouet A, Ortonne N, Decousser JW, Zinkernagel
AS, et al: Epidermal hepcidin is required for neutrophil response
to bacterial infection. J Clin Invest. 130:329–334. 2020.
View Article : Google Scholar :
|
|
88
|
Puri S, Kumar R, Rojas IG, Salvatori O and
Edgerton M: Iron chelator deferasirox reduces candida albicans
invasion of oral epithelial cells and infection levels in murine
oropharyngeal candidiasis. Antimicrob Agents Chemother.
63:e02152–18. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Littwitz-Salomon E, Moreira D, Frost JN,
Choi C, Liou KT, Ahern DK, O'Shaughnessy S, Wagner B, Biron CA,
Drakesmith H, et al: Metabolic requirements of NK cells during the
acute response against retroviral infection. Nat Commun.
12:53762021. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Yao L, Hou J, Wu X, Lu Y, Jin Z, Yu Z, Yu
B, Li J, Yang Z, Li C, et al: Cancer-associated fibroblasts impair
the cytotoxic function of NK cells in gastric cancer by inducing
ferroptosis via iron regulation. Redox Biol. 67:1029232023.
View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Li L, Xia Y, Yuan S, Li F, Xie X, Luo Y,
Yang XP and He R: Iron deprivation restrains the differentiation
and pathogenicity of T helper 17 cell. J Leukoc Biol.
110:1057–1067. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Kagan VE, Mao G, Qu F, Angeli JP, Doll S,
Croix CS, Dar HH, Liu B, Tyurin VA, Ritov VB, et al: Oxidized
arachidonic and adrenic PEs navigate cells to ferroptosis. Nat Chem
Biol. 13:81–90. 2017. View Article : Google Scholar
|
|
93
|
Veglia F, Tyurin VA, Blasi M, De Leo A,
Kossenkov AV, Donthireddy L, To TKJ, Schug Z, Basu S, Wang F, et
al: Fatty acid transport protein 2 reprograms neutrophils in
cancer. Nature. 569:73–78. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Srivastava MK, Sinha P, Clements VK,
Rodriguez P and Ostrand-Rosenberg S: Myeloid-derived suppressor
cells inhibit T-cell activation by depleting cystine and cysteine.
Cancer Res. 70:68–77. 2010. View Article : Google Scholar
|
|
95
|
Ostrand-Rosenberg S: Myeloid-derived
suppressor cells: More mechanisms for inhibiting antitumor
immunity. Cancer Immunol Immunother. 59:1593–1600. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Wang W, Green M, Choi JE, Gijón M, Kennedy
PD, Johnson JK, Liao P, Lang X, Kryczek I, Sell A, et al:
CD8+ T cells regulate tumour ferroptosis during cancer
immunotherapy. Nature. 569:270–274. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Kong R, Wang N, Han W, Bao W and Lu J:
IFNγ-mediated repression of system xc− drives
vulnerability to induced ferroptosis in hepatocellular carcinoma
cells. J Leukoc Biol. 110:301–314. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Zhao L, Zhou X, Xie F and Zhang L, Yan H,
Huang J, Zhang C, Zhou F, Chen J and Zhang L: Ferroptosis in cancer
and cancer immunotherapy. Cancer Commun (Lond). 42:88–116. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Lei G, Zhang Y, Koppula P, Liu X, Zhang J,
Lin SH, Ajani JA, Xiao Q, Liao Z, Wang H and Gan B: The role of
ferroptosis in ionizing radiation-induced cell death and tumor
suppression. Cell Res. 30:146–162. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Zhai X, Lin Y, Zhu L, Wang Y, Zhang J, Liu
J, Li L and Lu X: Ferroptosis in cancer immunity and immunotherapy:
Multifaceted interplay and clinical implications. Cytokine Growth
Factor Rev. 75:101–109. 2024. View Article : Google Scholar
|
|
101
|
Stockwell BR: Ferroptosis turns 10:
Emerging mechanisms, physiological functions, and therapeutic
applications. Cell. 185:2401–2421. 2022. View Article : Google Scholar : PubMed/NCBI
|
















