The placenta is a transitory but key organ that
undergoes changes during pregnancy ensuring the normal fetal
development (1,2). Placental development is a process
tightly regulated during pregnancy and its alteration can lead to
complications such as preeclampsia (PE) (3), fetal growth restriction (FGR)
(4), gestational trophoblastic
disease (5), preterm delivery
(6) and gestational diabetes
mellitus (GDM) (7). In addition,
placental development is impaired by exposure to exogenous agents
such as bacteria (8), viruses
(9) and pollutants (10,11) that can alter the normal placental
function.
Preeclampsia (PE) is a hypertensive disorder of
pregnancy with incidence between 2 and 10% of pregnancies worldwide
(12). It is generally diagnosed
from the second trimester of gestation and is clinically
characterized by de novo maternal hypertension (diastolic
blood pressure of 90 mmHg and/or systolic blood pressure of 140
mmHg) and proteinuria (>300 mg/24 h) (13,14). A high body mass index, previous
preeclamptic pregnancy, advanced maternal age and nulliparity are
important risk factors of PE (15,16).
Although clinical diagnosis of PE occurs after 20
weeks of pregnancy, it is hypothesized that placental impairment
begins during early stage of pregnancy and may be due to poor
trophoblast invasion (12). Poor
endometrial invasion of the extravillous trophoblast (EVT) into the
maternal uterine wall impairs proper remodelling of endometrium
spiral arteries, altering vascular perfusion and leading to a
hypoxic environment. This causes increased oxidative stress and
inflammation that leads to trophoblast immaturity and altered
angiogenesis of placental villi (17,18).
Depending on the gestational age of occurrence of
clinical signs and symptoms, PE is divided into late-(≥34 weeks of
gestation) and early-onset PE (<34 weeks of gestation).
Late-onset PE accounts for the majority of preeclampsia cases
(~90%), while early-onset PE is less common but associated with
higher rates of neonatal mortality and a greater degree of maternal
morbidity compared with late-onset PE (19,20).
Late-onset PE is a serious condition since it can
lead to eclampsia and hemolysis, elevated liver enzyme and low
platelet syndrome (20,21). Early-onset PE is associated with
impaired remodelling of the uterine spiral arteries, which leads to
hypoxia, trophoblast immaturity, maternal systemic inflammation,
vascular dysfunction and FGR (18,22,23) while late-onset PE is associated
with maternal endothelial dysfunction (19,22). Both late- and early-onset PE show
increased oxidative stress and inflammatory response that can cause
maternal and fetal complications (19,20,24). Thus, early- and late-onset PE are
considered distinct forms of PE with different pathophysiology and
pregnancy outcomes.
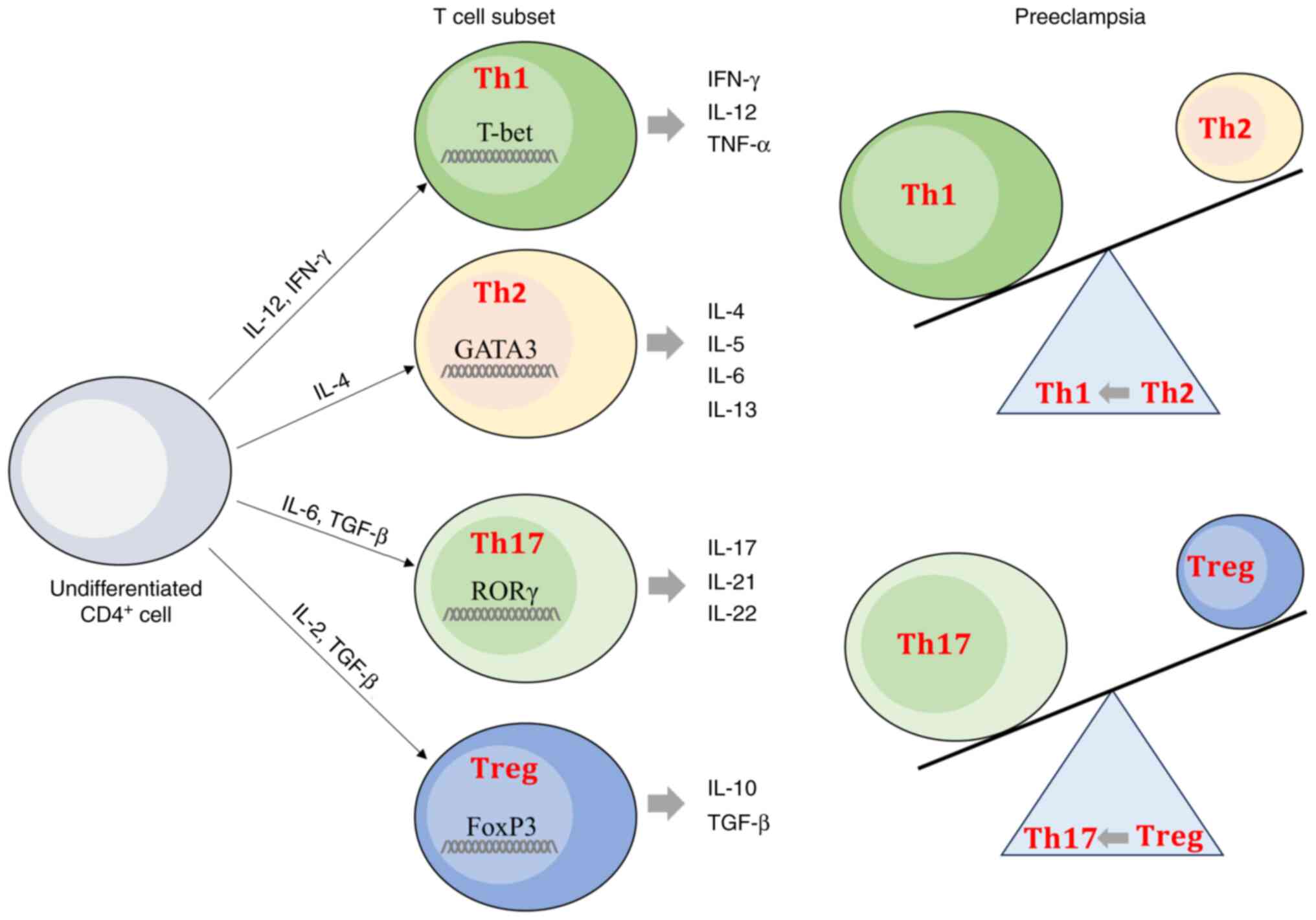
PE pregnancies show an imbalance between
inflammatory Th1/Th17/Th2 and Treg profiles (30,31). In PE pregnancies, CD4+
T cell subsets have a high expression of transcription factor T box
(T-bet) and retinoic acid receptor-related orphan receptor γ
(RORγt), which are characteristic of Th1 and Th17 profiles,
respectively, and decreased expression of GATA 3 binding protein
(GATA-3), and forkhead box P3 (FoxP3), associated with Th2 and Treg
profiles, respectively (32).
Thus, PE pregnancies have a higher percentage of Th17 cells, while
levels of Treg cells are lower, suggesting a shift from Treg toward
Th17 cells in PE (33). PE
pregnancies are also characterized by a shift from Th2 cells toward
Th1 cells (34). An imbalance of
T cell subsets can alter the immune microenvironment, leading to
pregnancy complications, including PE (30,33).
Signal transducer and activator of transcription 3
(STAT3) is a signal transducer protein activated by the binding of
cytokine or growth factors to their specific receptors. STAT3
serves a key role in regulating immune response by modulating
Th1/Th17/Th2 and Treg gene expression profiles (35).
The aim of the present review is to provide an
overview of the role of STAT3 signaling in PE pregnancy and
understand the potential therapeutic use of STAT3 modulators to
provide new therapeutic approaches in treatment and management of
PE.
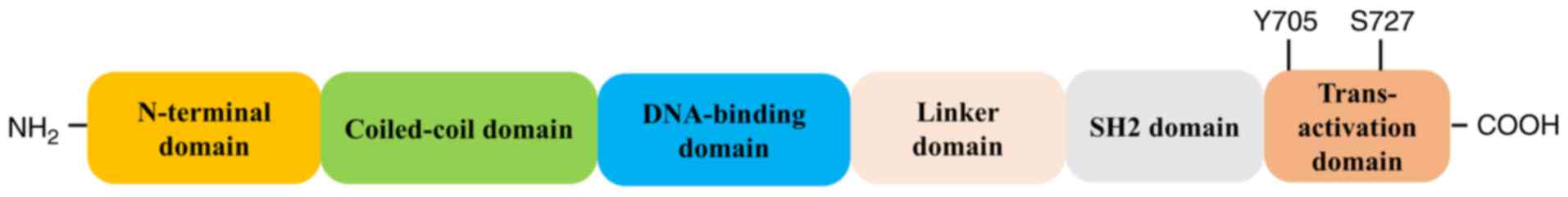
STAT family of proteins comprises seven
transcription factors: STAT1, STAT2, STAT3, STAT4, STAT5a, STAT5b
and STAT6. STAT3 has 770 amino acids and is organized in six
domains (Fig. 2). The N-terminal
domain is involved in interaction with other co-activators [such as
c-Jun and CREB binding protein (CBP) p300]; the coiled-coil domain
is necessary for the binding to the activated receptor, the
DNA-binding domain is involved in recognizing the target DNA
consensus sequence, the SH2 domain is necessary for STAT3
dimerization and recognizes the phosphorylated (p) tyrosine motifs
(Y705 residue) in the STAT3 trans-activation domain (which contains
conserved Tyr and Ser residues). STAT family members can form
heterodimers (with another member of the STAT protein family) or
homodimers (with another identical STAT protein) to initiate
transcriptional activity of STAT-dependent genes following ligand
stimulation (42,43).
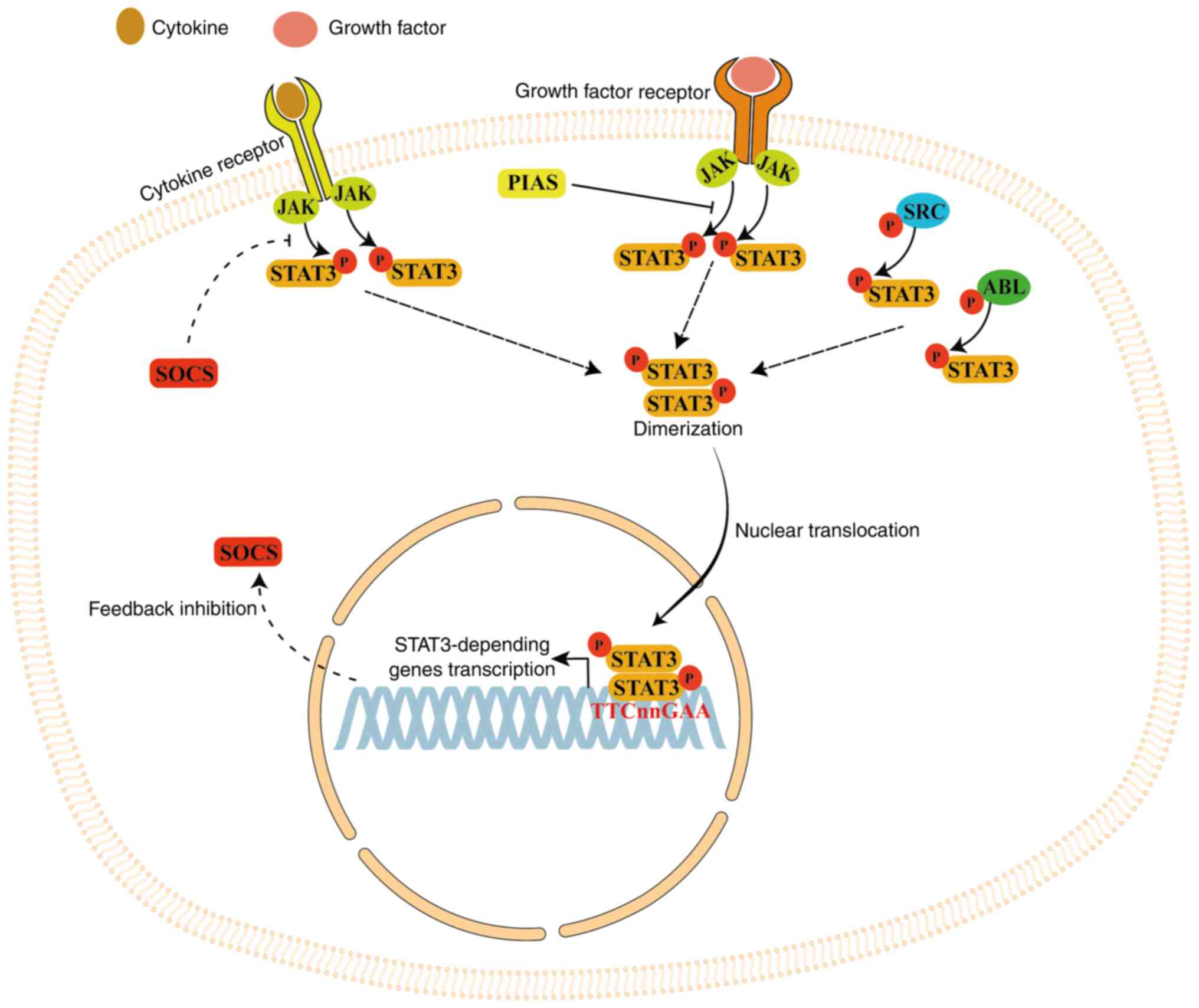
When cytokines or growth factors bind to cell
membrane receptors, STAT3 protein is activated (phosphorylated) by
Janus kinase (JAK) family proteins, which are receptor-associated
tyrosine kinases. Following phosphorylation, STAT3 forms a
homodimer that is transferred into the nucleus via the nuclear pore
complex (a GTP-dependent process) to bind the base sequence
TTCnnGAA in the promoter of STAT3-dependent genes, thereby
activating their transcription (44). STAT3 is also phosphorylated by
non-receptor tyrosine kinases such as SRC and ABL (45,46).
STAT3 long-term activation is inhibited by
suppressors of cytokine signaling (SOCS) protein, creating a
negative feedback loop (44).
STAT3 signaling is also inhibited by the protein inhibitor of
activated STAT, which blocks the DNA-binding activity of STAT3,
inhibiting its transcriptional activity (44). STAT3 signaling serves a key role
in several processes including cell proliferation, migration,
survival and differentiation (Fig.
3) (42,44,47).
STAT3 serves a key role in the transcriptional
activation of several proteins involved in the regulation of
numerous cell processes such as apoptosis, cell proliferation and
invasion (48,49). In trophoblast cells, STAT3
inhibition induces apoptosis and decreases cell proliferation and
invasion, key processes involved in normal placental development
(50-52). Thus, decreased STAT3 expression
may inhibit EVT invasion into the maternal uterine wall, causing
shallow trophoblast invasion and placental malperfusion, which are
characteristics of PE pregnancy. These effects of STAT3 on
trophoblast cells may be due to modulation of MMP activity since it
has been demonstrated that STAT3 activation inhibits tissue
inhibitor of metalloproteinase (TIMP)-1 expression (51).
STAT3 and pSTAT3 expression is significantly lower
in human PE placentas compared with normal placentas (53-60). Thus, decreased STAT3 expression
and activation in PE placental tissues serve an important role in
the pathogenesis of PE. However, in vivo models of PE show
contrasting results: To the best of our knowledge, there is only
one study that reflects results obtained in humans (61). Other studies showed an increased
pSTAT3 expression in placentas of PE models (62-65) while another study showed no
alteration in pSTAT3 expression (66). Thus, studies focused on the role
and modulation of STAT3 in in vivo models of PE must always
be assessed for pSTAT3 expression to reflect the data found in
humans.
Soluble Flt-1 (sFlt-1), also called soluble vascular
endothelial growth factor (VEGF) receptor 1, is a soluble form of
the VEGF and placental growth factor (PLGF) receptor, and plays a
key role in decreasing levels of free PLGF and VEGF, causing
endothelial dysfunction (67).
sFlt-1 levels are increased in PE and associated with PE severity
(68,69). Another important anti-angiogenic
factor is the soluble endoglin (sEng), a co-receptor for TGFβ-1 and
TGFβ-3, produced by proteolytic cleavage of endoglin and involved
in angiogenesis and endothelial cell differentiation. sEng serves a
key role in PE pathogenesis since it decreases circulating levels
of TGFβ, a key modulator of angiogenesis (70), inhibiting TGFβ pathway and
leading to endothelial dysfunction (71). Although the placenta is the
primary source of these circulating factors, it has been
demonstrated that peripheral blood mononuclear cells (PBMCs) may be
an additional source of sFlt-1 and sEng (72).
STAT3 signaling serves a key role in angiogenesis
since the inhibition of STAT3 phosphorylation suppresses this
process (76). Normally, pSTAT3
is strongly expressed in the endothelial cells of first and second
trimester placentas but its expression notably decreases in the
third trimester, when angiogenesis is completed (77). However, pSTAT3 expression is
notably increased in endothelial cells of PE placentas compared
with normotensive controls, suggesting an association between STAT3
activation and placental angiogenesis defects in PE (78).
Placental factors in maternal circulation cause
systemic endothelial dysfunction in PE pregnancy, increasing the
risk of cardiovascular disease (CVD) following delivery (79-81). Christensen et al (82) found that human umbilical vein
endothelial cell treatment with serum from patients with PE notably
decreases STAT3(Y705) phosphorylation compared with serum from
uncomplicated pregnancy. Moreover, sera from patients with previous
PE, current hypertension and carotid atherosclerotic plaques shows
significantly lower STAT3(Y705) phosphorylation capabilities
compared with healthy controls with previous uncomplicated
pregnancies 8-18 years after delivery. Thus, decreased
serum-induced endothelial STAT3(Y705) activation may play an
important role in PE-associated endothelial dysfunction and reduced
endothelial STAT3(Y705) phosphorylation and may increase
post-preeclamptic CVD risk after delivery (82).
Decreased STAT3 expression in PE placentas may favor
trophoblast apoptosis in the hypoxic environment at the beginning
of placentation (50-52). Moreover, decreased expression of
STAT3 is associated with reduced cell proliferation and invasion
due to decreased activity of MMPs (51). Decreased STAT3 expression can
inhibit EVT invasion into the maternal uterine wall, causing a
shallow trophoblast invasion, which is one of the primary causes of
PE occurrence (12). Since STAT3
expression is significantly increased in CD4+ and
CD8+ T lymphocytes isolated from patients with PE
(73), STAT3 pathway is also
involved, at least in part, in the increased inflammatory cytokines
found in serum of patients with PE (83). Therefore, investigating the
effects of STAT3 signaling modulation in PE pregnancy is necessary
to understand and treat this complication of pregnancy.
Natural compounds, also known as phytotherapeutics,
are biological substances found in several plants, bacteria, fungi
and marine organisms and are used worldwide due to
anti-inflammatory, antioxidant and anticancer effects (84-87).
Vitamin D is a pro-hormone primarily obtained from
exposure of the skin to ultraviolet B radiation and can regulate
inflammatory responses, favouring the shift from a pro-inflammatory
to a tolerogenic immune response by modulating numerous cells of
the immune system including CD4+ T cells (93-95). Vitamin D deficiency may
contribute to PE onset, altering Th1/Th17 and Th2/Treg profiles
(96). Ribeiro et al
(97) found that plasma levels
of vitamin D are lower in PE compared with normal pregnancies.
Vitamin D treatment of CD4+ T cells isolated from PE
decreases STAT1/STAT4/T-bet and STAT3/RORγt activation, while
increasing STAT6/GATA-3 and STAT5/FoxP3 activation. Treatment of
PBMCs isolated from patients with PE with vitamin D also decreases
levels of IFNγ, TNFα, IL-17, IL-22, IL-23 and IL-6, while
increasing IL-10 and TGF-β levels, suggesting an immunomodulatory
effect of vitamin D on STAT signaling that favours the shift from
Th1/Th17 to Th2/Treg profiles. Vitamin D treatment may exert a
beneficial effect in ameliorating systemic inflammation
characterising PE pregnancies (97).
Pravastatin is a statin normally used to treat CVD
but it can also reduce cholesterol content, alleviate inflammation,
decrease oxidative stress and regulate endothelial functions
(106,107). Wang et al (63) found that the expression of serum
IL-6 in PE rat model (obtained by deoxycorticosterone acetate
injection) is markedly higher than in controls and significantly
reduced in PE rats treated with pravastatin. Moreover, PE rats show
increased expression of pSTAT3 compared with controls rats.
However, pSTAT3 expression is significantly decreased in PE rats
treated with pravastatin. The proliferation of rat trophoblast
cells is significantly decreased in PE rats (due to increased
apoptosis) compared with controls but significantly increased in PE
rats treated with pravastatin (which reduced apoptosis), indicating
that pravastatin can inhibit IL-6/STAT3 signaling, decreasing the
apoptosis of trophoblast cells in PE rats (63). These data are in contrast with
another study that evaluated the role of pravastatin in a PE-like
mice model (obtained by adenoviral overexpression of sFlt-1), which
showed high blood pressure, abnormal vascular reactivity and
proteinuria similar to PE (108,109). However, pSTAT3 placental
expression is significantly higher in PE-like mice treated with
pravastatin compared with untreated and control (normal) groups,
suggesting that pravastatin can prevent PE and modulate STAT3
activation, further validating a beneficial role of statins in
preventing PE (66). The
contrasting results may be due to the different PE models.
Sulfasalazine is an anti-inflammatory drug used to
treat arthritis and inflammatory bowel disease (110,111). However, sulfasalazine decreases
placental secretion of the anti-angiogenic factor sFlt-1,
expression of which is regulated by the epidermal growth factor
receptor (EGFR) signaling pathways (112). By using primary cytotrophoblast
cells, Hastie et al (113) found that sulfasalazine
decreases sFlt-1 secretion, downregulating EGFR expression.
Additionally, sulfasalazine notably decreases protein expression of
ERK1/2 and STAT3, which are key adaptor molecules downstream of
EGFR. Thus, sulfasalazine decreases sFlt-1 secretion,
downregulating EGFR/ERK and EGFR/STAT3 signaling (113).
Nω-nitro-L-arginine methyl ester (L-NAME) is a
L-arginine analogue used as a nitric oxide synthase inhibitor to
treat hypotension. Due to these effects, it is also used to
establish the PE-like rat model (114). L-NAME administration causes a
decreased expression of STAT3 and pSTAT3 in the placenta of PE
rats, suggesting a role of STAT3 signaling in the development of PE
(61). Rats treated with L-NAME
are widely used as an in vivo model to study PE
pathophysiology (115-118).
Montelukast is a drug used in chronic asthmatic
patients planning for pregnancy and is safely used during pregnancy
(119). Montelukast is a
selective cysteinyl leukotriene receptor antagonist with
antioxidant and anti-inflammatory effects (120,121). Abdelzaher et al
(64) demonstrated that
montelukast treatment decreases oxidative stress and expression of
IL-6, TNF-α, pJAK2, and STAT3 in PE rats. Thus, montelukast exerts
anti-inflammatory effects, suppressing the IL-6/JAK2/STAT3
signaling pathway in PE rats (Table
I) (64).
ncRNAs are functional RNA molecules without
protein-coding abilities. The most studied ncRNAs include microRNA
(miRNA or miR), long ncRNA (lncRNA) and circular RNA (circRNA)
(122-124). ncRNAs regulate expression of
genes involved in several cellular processes including cell
proliferation, invasion and metabolism (122).
miRNAs are a group of endogenous small single-strand
ncRNAs that exert multifaceted functions in numerous diseases
(123-125). The miR-133 family (comprising
miR-133a and miR-133b) can affect invasion, migration and
proliferation of tumor cells (126) and plays a key role in pregnancy
complications such as recurrent spontaneous abortion (127). Placental tissue of patients
with PE shows high levels of miR-133b and decreased pJAK2 and
pSTAT3 expression. In HTR8/SVneo cells, hypoxia induces miR-133b
expression and decreases pJAK2 and pSTAT3 expression and
trophoblast migration and invasion while increasing apoptosis,
thereby proving that miR-133b may exert its functions by regulating
the JAK2/STAT3 pathway (56).
Thus, inhibiting miR-133b may improve oxidative stress injury
(induced by hypoxia) to promote the migration and invasion of
trophoblasts and suppress apoptosis by activating the JAK2/STAT3
pathway (56).
Another miRNA involved in STAT3 modulation and
altered in PE is miR-125b. This miRNA is associated with
extra-villous trophoblastic proliferation and invasion, and its
expression is notably increased in PE (128). Serum levels of miR-125b are
significantly increased in patients with PE compared with normal
pregnancies (128). Moreover,
high levels of miR-125b decrease HTR-8/SVneo cell proliferation,
invasion and migration as well as expression of STAT3, pSTAT3 and
SOCS3, demonstrating that STAT3 is a target gene of miR-125b; high
levels of miR-125b inhibit STAT3 signaling, reducing migration and
invasion of extra-villous trophoblast cells (129).
In addition to miRNAs, several studies have
demonstrated a key role of lncRNAs in pregnancy complications such
as PE (130) and GDM (131). Dendritic cells (DCs) serve a
key role as primary antigen-presenting cells at the beginning of
pregnancy and express lnc-DC, which induces DC differentiation,
maturation and STAT3 phosphorylation (132). Pregnancy complications
characterized by impaired trophoblast invasion such as PE exhibit
excessive DC maturity (133).
Moreover, it has been reported that expression of lnc-DC and pSTAT3
is increased in the decidua of patients with PE (134). Furthermore, the proportion of
Th1 cells and mature DCs is notably higher in patients with PE,
suggesting that upregulation of lnc-DC induces the over-maturation
of decidual DCs in PE, leading to an increase in Th1 cells that
lead to a persistent inflammatory response (134). Overexpression of lnc-DC in
HTR8/SVneo cells inhibits trophoblast invasion and motility by
increasing pSTAT3 levels and TIMP1 and 2 expression while
decreasing expression of MMP-9, -2 and -3 (135). Thus, lnc-DC regulates
trophoblast invasion and motility by modulating STAT3 activation
and MMP expression (135).
circRNAs are single-strand ring-like ncRNAs produced
by reverse splicing of precursor RNA following transcription and
are involved in numerous pathologies including pregnancy
complications (136).
Expression of circRNA of pregnancy-associated plasma protein A
(circPAPPA) is downregulated in PE. Knockdown of circPAPPA in
HTR8-S/Vneo cells decreases proliferation and invasion ability.
Moreover, miR-384 (which targets STAT3) is a direct target of
circPAPPA (57). STAT3
expression is decreased when circPAPPA is knocked down, suggesting
that downregulation of circPAPPA facilitates onset and development
of PE by suppressing trophoblast invasion and proliferation via
modulation of miR-384/STAT3 signaling (Table II) (57).
B7-H4 is a type I transmembrane glycoprotein
belonging to the B7 family of immune checkpoint proteins, which are
involved in regulation of immune response, preventing its excessive
activation (137). B7-H4 is
normally expressed in the placental villous but its expression
significantly decreases in decidua of patients with PE compared
with normal controls (138).
Contrarily, B7-H4 serum levels are notably increased in patients
with PE (139). B7-H4 treatment
of SGHPL-5 trophoblast cells inhibits proliferation, migration and
invasion while promoting apoptosis by decreasing pAKT, pPI3K and
pSTAT3 expression (140). Thus,
B7-H4 may serve an important role in shallow trophoblast invasion
in PE.
RAR-related orphan receptor A (RORA) is a member of
the ROR subfamily that serves as a transcription factor in the
regulatory region of ROR-responsive genes; its expression is
induced by hypoxia (141,142). It has been reported that RORA
expression is increased in PE tissues but also in HTR-8/SVneo cells
exposed to hypoxic conditions. Silencing of RORA in HTR-8/SVneo
cells increases migration and proliferation while decreasing pSTAT3
and pJAK2 expression. The inhibitory effects of RORA silencing are
reversed when cells are treated with the STAT3 activator RO8191
(143). Thus, RORA regulates
trophoblast cell proliferation and migration via the JAK2/STAT3
signaling pathway (143).
Basal cell adhesion molecule (BCAM) belongs to the
immunoglobulin superfamily and is involved in cellular processes
such as cell adhesion, migration and invasion (144). Liu et al (58) found that BCAM expression is
significantly decreased in PE placenta. Moreover, silencing BCAM in
HTR-8/SVneo and JAR cells leads to decreased trophoblast
proliferation, migration and invasion and suppresses pSTAT3(Y705)
expression through the downregulation of phosphoinositide-3-kinase
regulatory subunit 6 (PIK3R6) expression, a kinase that
phosphorylates STAT3. However, phosphorylation on S727 of STAT3 is
not altered by BCAM deficiency. In addition, adenoviruses
containing BCAM short hairpin RNA genes (Ad-shBCAM) cause BCAM
deficiency and a PE-like phenotype with elevated systolic blood
pressure, proteinuria and FGR. Accordingly, the expression of BCAM,
PIK3R6 and pSTAT3 is downregulated in Ad-shBCAM rats (58). Thus, BCAM deficiency decreases
trophoblast proliferation, migration and invasion by inhibiting
PIK3R6/STAT3 signaling (58).
NADPH oxidase 2 (Nox2) is an important source of ROS
and serves a key role in ferroptosis (145,146), a type of programmed cell death
due to iron-dependent lipid peroxidation (147). STAT3 serves as an
oxidation-responsive transcription factor and plays a key role in
regulating ferroptosis as it can bind the promoter region of
ferroptosis-associated genes such as glutathione peroxidase 4
(GPX4) (148,149). A previous study (59) found that Nox2 expression is
significantly higher in PE placentas while STAT3 and GPX4
expression is decreased. Moreover, STAT3 and GPX4 gene expression
is notably decreased by hypoxia and RAS-selective lethal compound 3
(RLS3)-induced ferroptosis while their expression is restored when
ferroptosis is inhibited with Ferrostatin-1 (Fer-1). Silencing Nox2
in HTR8/SVneo cells inhibits ferroptosis and increases STAT3 and
GPX4 expression (59). Thus,
Nox2 may trigger ferroptosis in PE via modulation of the STAT3/GPX4
pathway (59).
NOP2/Sun5 (NSUN5) is an RNA methyltransferase
involved in important cell processes such as mitochondria assembly
and cell proliferation (150).
Zhang et al (151) found
a notable association between NSUN5 polymorphism (rs77133388) and
PE. Pregnant single-base mutant mice (NSUN5 R295C at rs77133388)
exhibit PE symptoms and reduced decidualization. Additionally, the
aforementioned study found decreased IL-11Rα, cyclin D3, pJAK2 and
pSTAT3 expression in NSUN5 R295C mice, suggesting that NSUN5
mutation potentially alters decidualization through
IL-11Rα/JAK2/STAT3/cyclin D3 signaling, favouring PE occurrence
(151).
Hypoxia-inducible factors (HIFs; HIF-α and HIF-β
subunits) are hypoxia-induced transcription factors that regulate
cellular processes under hypoxic condition. HIF-1α, HIF-2α and
HIF-3α are paralogs of the HIF-α subunit; while HIF-1α and HIF-2α
are considered the two master regulators of hypoxic response,
little is known about HIF-3α (152). Qu et al (153) found that HIF-3α expression is
decreased in PE placentas compared with normal pregnancy. Moreover,
under chronic hypoxia (72 h), the expression of HIF-3α, pJAK and
pSTAT3 is significantly decreased while apoptosis notably
increases. Overexpression of HIF-3α in HTR8/SVneo cells markedly
increases phosphorylation of JAK/STAT, indicating that HIF-3α
upregulation can regulate the JAK/STAT pathway and serves as a
protective factor against hypoxia, favoring cell survival (153).
Annexin A1 (ANXA1) is a calcium-dependent
phospholipid-binding protein that can bind negatively charged
phospholipids and is involved in cell activities such as
anti-inflammatory response, differentiation and proliferation, cell
signal regulation and phagocytosis of apoptotic cells (154-156). ANXA1 is highly expressed in the
plasma of patients with PE (157,158). Feng et al (159) found that ANXA1, TNF-α, IL-1β,
IL-6 and IL-8 expression are increased in placental tissues of PE
rats. In addition, the aforementioned study found that silencing of
ANXA1 in trophoblast cells isolated from placentas of PE rat model
significantly decreases apoptosis and inflammatory response of
trophoblast cells. Furthermore, silencing of ANXA1 significantly
increases expression of Bcl-2 and pro-caspase-3, while
downregulating the expression of BAX, cleaved-caspase-3, TNF-α,
IL-1β, IL-6 and IL-8 (159).
Silencing of ANXA1 notably decreases phosphorylation of JAK2 and
STAT3 (159). These effects on
STAT3 and JAK2 can be explained by an indirect modulation by ANXA1.
Decreased phosphorylation of JAK2 and STAT3 may be due to decreased
expression of the cytokines TNF-α, IL-1β, IL-6 and IL-8, which
activate JAK/STAT3 pathway (160-163). Similar results were obtained by
Mo et al (164) studying
ANXA7, another member of the annexin family (154). Silencing of ANXA7 in
HTR-8/SVneo cells induces cell apoptosis and inhibits cell
proliferation by downregulating Bcl-2 protein expression (164). Silencing of ANXA7 decreases
pJAK and pSTAT3 expression (164). As it has been reported that
trophoblast viability is notably decreased when the levels of pJAK
and pSTAT3 are reduced (165),
the aforementioned results demonstrated that ANXA7 can regulate
trophoblast apoptosis by modulating the JAK/STAT3 pathway.
IL-27 is a member of the IL-12/IL-6 family of
cytokines produced by antigen-presenting cells and regulates T cell
differentiation and function, exerting pro- and anti-inflammatory
effects during immune response (166). Expression of IL-27 and its
receptor (IL-27 receptor α) is significantly increased in the
trophoblast of placentas from PE pregnancy (167). A previous study found that
IL-27 significantly inhibits HTR-8/SVneo cell invasion and
migration by favouring the expression of epithelial markers over
mesenchymal markers. Furthermore, IL-27 induces phosphorylation of
STAT1 and STAT3 (168).
Silencing of STAT1 attenuates the effects of IL-27, while silencing
STAT3 has no effect, demonstrating that IL-27 may inhibit
trophoblast cell migration and invasion by affecting
epithelial-mesenchymal transition via a STAT1-dominant pathway in
PE (168).
Heme oxygenase-1 (HO-1) is a key antioxidant enzyme
with anti-hypertensive effects (169) and cytoprotective and
anti-inflammatory functions under ischemic conditions (170). HO-1 expression is significantly
increased in PE placentas while STAT3 phosphorylation (Y705) is
notably decreased. Moreover, human placental choriocarcinoma JEG-3
cells exposed to hypoxia show increased HO-1 expression and STAT3
phosphorylation (Y705) compared with cells cultured under normoxic
conditions (60). HO-1
overexpression in JEG-3 cells significantly inhibits
hypoxia-promoted STAT3 phosphorylation (Y705), suggesting that the
overexpression of HO-1 in PE placentas might contribute to
decreased STAT3 phosphorylation (Y705) found in the placentas
complicated by PE (60). This is
consistent with a study by Xu et al (165), reporting an increased
expression of pJAK and pSTAT3 in primary third trimester
trophoblast cells exposed to hypoxia. To the best of our knowledge,
the aforementioned study is the only report of increased expression
of total STAT3 expression in PE placentas.
Ribosomal protein S4, Y-linked 1 (RPS4Y1) is a
member of the S4E family of ribosomal proteins ubiquitously
expressed and is involved in regulation of cell processes such as
apoptosis, cell migration and invasion (175,176). A previous study (177) found that RPS4Y1 levels are
significantly upregulated in PE placentas. Silencing of RPS4Y1 in
HTR8/SVneo cells induces cell invasion and increased STAT3
phosphorylation along with increased expression of N-cadherin and
vimentin. These effects are abolished when RPS4Y1 and STAT3 are
silenced, demonstrating that RPS4Y1 may be involved in PE,
affecting trophoblast cell migration and invasion via the STAT3
pathway (177).
EGF is a polypeptide involved in cell
proliferation, differentiation and survival (178). Lower levels of EGF are found in
plasma and urine of patients with PE, suggesting a potential role
of EGF in this pathology (179). A previous study (180) reported that treatment of
HTR-8/SVneo cells with EGF notably increases cell invasion.
Moreover, EGF treatment leads to an increase in phosphorylation of
ERK1/2, STAT1 and STAT3 (at both Y705 and S727 residues).
Inhibition of ERK1/2 phosphorylation by U0126 decreases
EGF-mediated invasion and pSTAT3 and pSTAT1 expression. Silencing
of STAT3 leads to decreased EGF-mediated invasion of HTR-8/SVneo
cells and pSTAT1 expression but does not have any effect on ERK1/2
activation. Silencing of STAT1 also leads to decreased EGF-mediated
invasion of HTR-8/SVneo cells and ERK1/2 and STAT3 (at S727
residue) phosphorylation (180). These results suggest crosstalk
between ERK1/2 and JAK/STAT pathways during EGF-mediated increase
of HTR-8/SVneo cells invasion; phosphorylation at S727 residue of
both STAT3 and STAT1 may be critical in this process (180).
Human leucocyte antigen-G (HLA-G) is the primary
immune modulator in embryo implantation and allows the interaction
between immune cells [such as natural killer (NK) cells] and
trophoblast cells, inhibiting NK cytotoxicity and cytokine
production (181). Moreover,
decreased HLA-G expression may contribute to PE onset (182). Silencing HLA-G in JEG-3 cells
decreases invasion capacity but does not alter cell proliferation
or apoptosis. Moreover, silencing of HLA-G decreases STAT3
activation, whereas the overexpression of HLA-G promotes STAT3
activation and invasion in JEG-3 cells, demonstrating that HLA-G is
able to regulate JEG-3 cell invasion by influencing STAT3
activation explaining implantation defects due to the low HLA-G
expression in PE (Table III)
(183).
Chorionic villi serve a key role in normal
placental development and function, allowing the transport of
nutrients and oxygen to the foetus (184). However, the development of
chorionic villi is impaired in pregnancy complications such as PE
and FGR (18). Chorionic villous
mesenchymal stem cells (CV-MSCs) are multipotent cells that are
detached from chorionic villi and differentiate in vitro
into neurocytes and hepatocytes (185). CV-MSCs serve a pivotal role in
regulating trophoblast function. Chu et al (186) found that treatment of JAR,
JEG-3 and HTR-8 cells under hypoxic conditions with CV-MSC
supernatant markedly enhances proliferation and invasion and
augments autophagy. In addition, pSTAT3 and pJAK2 levels increase
following CV-MSC treatment, suggesting that CV-MSC-dependent
JAK2/STAT3 signaling activation is a prerequisite for autophagy
upregulation in trophoblast cells and an important factor in
protecting cells from hypoxia (186).
In addition to CV-MSCs, STAT3 can also be modulated
by antiphospholipid antibodies (aPLs), which have been found in
patients affected by antiphospholipid syndrome (187,188). aPLs are at risk factor for
recurrent miscarriage and PE onset (174) as aPL can bind the
β2-glycoprotein I (β2-GPI) expressed by trophoblast cells,
triggering an inflammatory response and compromising the
invasiveness of trophoblast cells (190). A previous study (191) found that treatment of first
trimester trophoblast cells with anti-β2-GPI monoclonal antibodies
notably downregulates IL-6 secretion and pSTAT3 expression,
reducing trophoblast cell invasion. Thus, aPLs limit trophoblast
cell migration by downregulating trophoblast IL-6 secretion and
STAT3 activation (191).
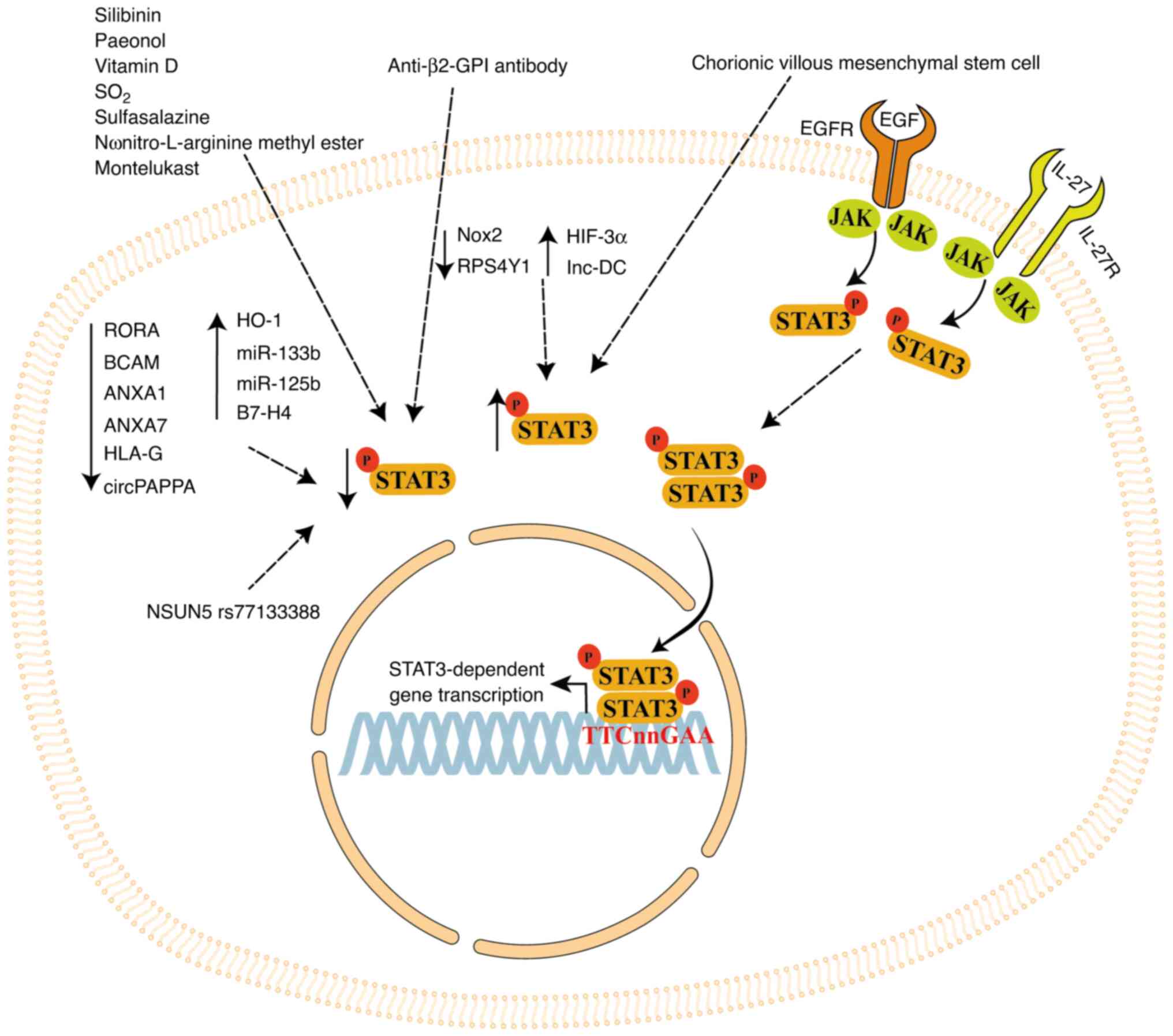
STAT3 signaling may be a promising target for
treatment of PE. The present review summarizes the role of STAT3
signaling in regulating processes in placental cell lines and in
vivo PE models. STAT3 signaling is regulated by several factors
(Fig. 4). In particular, natural
compounds such as silibinin, paeonol and vitamin D, as well as
synthetic compounds such as SO2 derivates, pravastatin,
sulfasalazine, L-NAME and montelukast, regulate STAT3 activation
and/or expression in inflammatory and trophoblast cells, regulating
inflammatory cytokine production and modulating trophoblast cell
proliferation and invasion (61,63,64,105,113).
Furthermore, STAT3 expression is modulated by
ncRNAs such as miR-133b, miR-125b, lnc-DC and circPAPPA, and
cellular modulators such as RORA, BCAM, Nox2, NSUN5, IL-27, HO-1,
RPS4Y1, EGF, HLA-G, ANXA1 and ANXA7. Thus, STAT3 signaling plays a
key role in important processes for placental development that are
impaired in PE placentas. STAT3-dependent alterations in PE may be
improved by stimulating the activation of STAT3 signaling. Therapy
focused on STAT3 regulation may improve the efficiency of the
classical treatments (e.g. magnesium sulfate, heparin) to
ameliorate PE outcomes or avoid its onset. Moreover, natural and
synthetic compounds decrease pSTAT3 expression (Fig. 4). Use of these compounds must be
tightly controlled or avoided since it can significantly worsen
STAT3-dependent cellular and molecular processes given that pSTAT3
expression is low in PE placentas (53-55).
As STAT3 expression is notably decreased in PE
placentas compared with normal placentas (53,55), its activation (especially in
pregnancy at risk of PE development) may promote processes
necessary for proper placental development (such as protecting
trophoblast cells from apoptosis in a hypoxic environment, such as
that at the beginning of placentation).
The role of STAT3 signaling in PE pregnancies has
also clinical value. As STAT3 signaling is inhibited by miR-133b,
miR-125b, B7-H4 and NSUN5 polymorphism (rs77133388), modulators
that can be detected in the blood at first trimester of pregnancy
when no PE clinical signs or symptoms are present, increased levels
of these modulators in the blood or the presence of rs77133388
could be an indicator of a pregnancy at risk of PE due to a
possible impairment of STAT3 signaling.
Not applicable.
DM reviewed and analyzed the literature and wrote
the manuscript. FP, NDS, SFG and AC reviewed and edited the
manuscript. GT conceived the study and wrote and critically revised
the manuscript. Data authentication is not applicable. All authors
have read and approved the final manuscript.
Not applicable.
Not applicable.
The authors declare that they have no competing
interests.
Not applicable.
No funding was received.
|
1
|
Tossetta G, Avellini C, Licini C,
Giannubilo SR, Castellucci M and Marzioni D: High temperature
requirement A1 and fibronectin: Two possible players in placental
tissue remodelling. Eur J Histochem. 60:27242016.
|
|
2
|
Fantone S, Giannubilo SR, Marzioni D and
Tossetta G: HTRA family proteins in pregnancy outcome. Tissue Cell.
72:1015492021. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Marzioni D, Todros T, Cardaropoli S, Rolfo
A, Lorenzi T, Ciarmela P, Romagnoli R, Paulesu L and Castellucci M:
Activating protein-1 family of transcription factors in the human
placenta complicated by preeclampsia with and without fetal growth
restriction. Placenta. 31:919–927. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Todros T, Marzioni D, Lorenzi T, Piccoli
E, Capparuccia L, Perugini V, Cardaropoli S, Romagnoli R, Gesuita
R, Rolfo A, et al: Evidence for a role of TGF-beta1 in the
expression and regulation of alpha-SMA in fetal growth restricted
placentae. Placenta. 28:1123–1132. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Marzioni D, Quaranta A, Lorenzi T, Morroni
M, Crescimanno C, De Nictolis M, Toti P, Muzzonigro G, Baldi A, De
Luca A and Castellucci M: Expression pattern alterations of the
serine protease HtrA1 in normal human placental tissues and in
gestational trophoblastic diseases. Histol Histopathol.
24:1213–1222. 2009.PubMed/NCBI
|
|
6
|
Cecati M, Sartini D, Campagna R, Biagini
A, Ciavattini A, Emanuelli M and Giannubilo SR: Molecular analysis
of endometrial inflammation in preterm birth. Cell Mol Biol
(Noisy-le-grand). 63:51–57. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Tossetta G, Fantone S, Gesuita R, Di Renzo
GC, Meyyazhagan A, Tersigni C, Scambia G, Di Simone N and Marzioni
D: HtrA1 in gestational diabetes mellitus: A possible biomarker?
Diagnostics (Basel). 12:27052022. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Licini C, Tossetta G, Avellini C, Ciarmela
P, Lorenzi T, Toti P, Gesuita R, Voltolini C, Petraglia F,
Castellucci M and Marzioni D: Analysis of cell-cell junctions in
human amnion and chorionic plate affected by chorioamnionitis.
Histol Histopathol. 31:759–767. 2016.PubMed/NCBI
|
|
9
|
Yates EF and Mulkey SB: Viral infections
in pregnancy and impact on offspring neurodevelopment: Mechanisms
and lessons learned. Pediatr Res. 96:64–72. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Fantone S, Tossetta G, Cianfruglia L,
Frontini A, Armeni T, Procopio AD, Pugnaloni A, Gualtieri AF and
Marzioni D: Mechanisms of action of mineral fibres in a placental
syncytiotrophoblast model: An in vitro toxicology study. Chem Biol
Interact. 390:1108952024. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Abrantes-Soares F, Lorigo M and Cairrao E:
Effects of BPA substitutes on the prenatal and cardiovascular
systems. Crit Rev Toxicol. 52:469–498. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Khan B, Allah Yar R, Khakwani AK, Karim S
and Arslan Ali H: Preeclampsia incidence and its maternal and
neonatal outcomes with associated risk factors. Cureus.
14:e311432022.PubMed/NCBI
|
|
13
|
No authors listed: ACOG practice bulletin
No. 202: Gestational hypertension and preeclampsia. Obstet Gynecol.
133:12019.
|
|
14
|
Magee LA, Brown MA, Hall DR, Gupte S,
Hennessy A, Karumanchi SA, Kenny LC, McCarthy F, Myers J, Poon LC,
et al: The 2021 international society for the study of hypertension
in pregnancy classification, diagnosis & management
recommendations for international practice. Pregnancy Hypertens.
27:148–169. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Chang KJ, Seow KM and Chen KH:
Preeclampsia: Recent advances in predicting, preventing, and
managing the maternal and fetal life-threatening condition. Int J
Environ Res Public Health. 20:29942023. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Gesuita R, Licini C, Picchiassi E,
Tarquini F, Coata G, Fantone S, Tossetta G, Ciavattini A,
Castellucci M, Di Renzo GC, et al: Association between first
trimester plasma htra1 level and subsequent preeclampsia: A
possible early marker? Pregnancy Hypertens. 18:58–62. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Deshpande JS, Sundrani DP, Sahay AS, Gupte
SA and Joshi SR: Unravelling the potential of angiogenic factors
for the early prediction of preeclampsia. Hypertens Res.
44:756–769. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Fantone S, Mazzucchelli R, Giannubilo SR,
Ciavattini A, Marzioni D and Tossetta G: AT-rich interactive domain
1A protein expression in normal and pathological pregnancies
complicated by preeclampsia. Histochem Cell Biol. 154:339–346.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Aneman I, Pienaar D, Suvakov S, Simic TP,
Garovic VD and McClements L: Mechanisms of key innate immune cells
in early- and late-onset preeclampsia. Front Immunol. 11:18642020.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Marin R, Chiarello DI, Abad C, Rojas D,
Toledo F and Sobrevia L: Oxidative stress and mitochondrial
dysfunction in early-onset and late-onset preeclampsia. Biochim
Biophys Acta Mol Basis Dis. 1866:1659612020. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Staff AC: The two-stage placental model of
preeclampsia: An update. J Reprod Immunol. 134-135:1–10. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Örgül G, Aydın Haklı D, Özten G, Fadiloğlu
E, Tanacan A and Beksaç MS: First trimester complete blood cell
indices in early and late onset preeclampsia. Turk J Obstet
Gynecol. 16:112–117. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Marzioni D, Lorenzi T, Altobelli E,
Giannubilo SR, Paolinelli F, Tersigni C, Crescimanno C, Monsurrò V,
Tranquilli AL, Di Simone N and Castellucci M: Alterations of
maternal plasma HTRA1 level in preeclampsia complicated by IUGR.
Placenta. 33:1036–1038. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Inversetti A, Pivato CA, Cristodoro M,
Latini AC, Condorelli G, Di Simone N and Stefanini G: Update on
long-term cardiovascular risk after pre-eclampsia: A systematic
review and meta-analysis. Eur Heart J Qual Care Clin Outcomes.
10:4–13. 2024. View Article : Google Scholar
|
|
25
|
Saito S, Shiozaki A, Nakashima A, Sakai M
and Sasaki Y: The role of the immune system in preeclampsia. Mol
Aspects Med. 28:192–209. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Hahn S, Gupta AK, Troeger C, Rusterholz C
and Holzgreve W: Disturbances in placental immunology: Ready for
therapeutic interventions? Springer Semin Immunopathol. 27:477–493.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Boulanger H, Bounan S, Mahdhi A, Drouin D,
Ahriz-Saksi S, Guimiot F and Rouas-Freiss N: Immunologic aspects of
preeclampsia. AJOG Glob Rep. 4:1003212024. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Conforti-Andreoni C, Spreafico R, Qian HL,
Riteau N, Ryffel B, Ricciardi-Castagnoli P and Mortellaro A: Uric
acid-driven Th17 differentiation requires inflammasome-derived IL-1
and IL-18. J Immunol. 187:5842–5850. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Saito S, Shiozaki A, Sasaki Y, Nakashima
A, Shima T and Ito M: Regulatory T cells and regulatory natural
killer (NK) cells play important roles in feto-maternal tolerance.
Semin Immunopathol. 29:115–122. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Saito S, Nakashima A, Shima T and Ito M:
Th1/Th2/Th17 and regulatory T-cell paradigm in pregnancy. Am J
Reprod Immunol. 63:601–610. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Figueiredo AS and Schumacher A: The T
helper type 17/regulatory T cell paradigm in pregnancy. Immunology.
148:13–21. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Ribeiro VR, Romao-Veiga M, Romagnoli GG,
Matias ML, Nunes PR, Borges VTM, Peracoli JC and Peracoli MTS:
Association between cytokine profile and transcription factors
produced by T-cell subsets in early- and late-onset pre-eclampsia.
Immunology. 152:163–173. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Eghbal-Fard S, Yousefi M, Heydarlou H,
Ahmadi M, Taghavi S, Movasaghpour A, Jadidi-Niaragh F, Yousefi B,
Dolati S, Hojjat-Farsangi M, et al: The imbalance of Th17/Treg axis
involved in the pathogenesis of preeclampsia. J Cell Physiol.
234:5106–5116. 2019. View Article : Google Scholar
|
|
34
|
Saito S and Sakai M: Th1/Th2 balance in
preeclampsia. J Reprod Immunol. 59:161–173. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Kluger MA, Luig M, Wegscheid C, Goerke B,
Paust HJ, Brix SR, Yan I, Mittrücker HW, Hagl B, Renner ED, et al:
Stat3 programs Th17-specific regulatory T cells to control GN. J Am
Soc Nephrol. 25:1291–1302. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Lee JH, Kim TH, Oh SJ, Yoo JY, Akira S, Ku
BJ, Lydon JP and Jeong JW: Signal transducer and activator of
transcription-3 (Stat3) plays a critical role in implantation via
progesterone receptor in uterus. FASEB J. 27:2553–2563. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Suman P, Malhotra SS and Gupta SK:
LIF-STAT signaling and trophoblast biology. JAKSTAT.
2:e251552013.
|
|
38
|
Fitzgerald JS, Busch S, Wengenmayer T,
Foerster K, de la Motte T, Poehlmann TG and Markert UR: Signal
transduction in trophoblast invasion. Chem Immunol Allergy.
88:181–199. 2005.PubMed/NCBI
|
|
39
|
DiFederico E, Genbacev O and Fisher SJ:
Preeclampsia is associated with widespread apoptosis of placental
cytotrophoblasts within the uterine wall. Am J Pathol. 155:293–301.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Tossetta G, Fantone S, Giannubilo SR,
Ciavattini A, Senzacqua M, Frontini A and Marzioni D: HTRA1 in
placental cell models: A possible role in preeclampsia. Curr Issues
Mol Biol. 45:3815–3828. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
He G, Xu W, Chen Y, Liu X and Xi M:
Abnormal apoptosis of trophoblastic cells is related to the
up-regulation of CYP11A gene in placenta of preeclampsia patients.
PLoS One. 8:e596092013. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Kisseleva T, Bhattacharya S, Braunstein J
and Schindler CW: Signaling through the JAK/STAT pathway, recent
advances and future challenges. Gene. 285:1–24. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Gupta S, Yan H, Wong LH, Ralph S,
Krolewski J and Schindler C: The SH2 domains of Stat1 and Stat2
mediate multiple interactions in the transduction of IFN-alpha
signals. EMBO J. 15:1075–1084. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
El-Tanani M, Al Khatib AO, Aladwan SM,
Abuelhana A, McCarron PA and Tambuwala MM: Importance of STAT3
signaling in cancer, metastasis and therapeutic interventions. Cell
Signal. 92:1102752022. View Article : Google Scholar
|
|
45
|
Blake S, Hughes TP, Mayrhofer G and Lyons
AB: The Src/ABL kinase inhibitor dasatinib (BMS-354825) inhibits
function of normal human T-lymphocytes in vitro. Clin Immunol.
127:330–339. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Cheranov SY, Karpurapu M, Wang D, Zhang B,
Venema RC and Rao GN: An essential role for SRC-activated STAT-3 in
14,15-EET-induced VEGF expression and angiogenesis. Blood.
111:5581–5591. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Murray PJ: The JAK-STAT signaling pathway:
Input and output integration. J Immunol. 178:2623–2629. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Cortés-Ballinas L, López-Pérez TV and
Rocha-Zavaleta L: STAT3 and the STAT3-regulated inhibitor of
apoptosis protein survivin as potential therapeutic targets in
colorectal cancer (Review). Biomed Rep. 21:1752024. View Article : Google Scholar
|
|
49
|
Teng Y, Ross JL and Cowell JK: The
involvement of JAK-STAT3 in cell motility, invasion, and
metastasis. JAKSTAT. 3:e280862014.PubMed/NCBI
|
|
50
|
Fang Y, Feng X, Xue N, Cao Y, Zhou P and
Wei Z: STAT3 signaling pathway is involved in the pathogenesis of
miscarriage. Placenta. 101:30–38. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Fitzgerald JS, Poehlmann TG, Schleussner E
and Markert UR: Trophoblast invasion: The role of intracellular
cytokine signaling via signal transducer and activator of
transcription 3 (STAT3). Hum Reprod Update. 14:335–344. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Borg AJ, Yong HEJ, Lappas M, Degrelle SA,
Keogh RJ, Da Silva-Costa F, Fournier T, Abumaree M, Keelan JA,
Kalionis B and Murthi P: Decreased STAT3 in human idiopathic fetal
growth restriction contributes to trophoblast dysfunction.
Reproduction. 149:523–532. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Weber M, Kuhn C, Schulz S, Schiessl B,
Schleussner E, Jeschke U, Markert UR and Fitzgerald JS: Expression
of signal transducer and activator of transcription 3 (STAT3) and
its activated forms is negatively altered in trophoblast and
decidual stroma cells derived from preeclampsia placentae.
Histopathology. 60:657–662. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Travaglino A, Raffone A, Saccone G,
Migliorini S, Maruotti GM, Esposito G, Mollo A, Martinelli P, Zullo
F and D'Armiento M: Placental morphology, apoptosis, angiogenesis
and epithelial mechanisms in early-onset preeclampsia. Eur J Obstet
Gynecol Reprod Biol. 234:200–206. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Zhang Z, Yang X, Zhang L, Duan Z, Jia L,
Wang P, Shi Y, Li Y and Gao J: Decreased expression and activation
of Stat3 in severe preeclampsia. J Mol Histol. 46:205–219. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Yang HY: MiR-133b regulates oxidative
stress injury of trophoblasts in preeclampsia by mediating the
JAK2/STAT3 signaling pathway. J Mol Histol. 52:1177–1188. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Zhou W, Wang H, Yang J, Long W, Zhang B,
Liu J and Yu B: Down-regulated circPAPPA suppresses the
proliferation and invasion of trophoblast cells via the
miR-384/STAT3 pathway. Biosci Rep. 39:BSR201919652019. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Liu M, Liao L, Gao Y, Yin Y, Wei X, Xu Q,
Gao L and Zhou R: BCAM deficiency may contribute to preeclampsia by
suppressing the PIK3R6/p-STAT3 signaling. Hypertension.
79:2830–2842. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Xu X, Zhu M, Zu Y, Wang G, Li X and Yan J:
Nox2 inhibition reduces trophoblast ferroptosis in preeclampsia via
the STAT3/GPX4 pathway. Life Sci. 343:1225552024. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Qu HM, Qu LP, Li XY and Pan XZ:
Overexpressed HO-1 is associated with reduced STAT3 activation in
preeclampsia placenta and inhibits STAT3 phosphorylation in
placental JEG-3 cells under hypoxia. Arch Med Sci. 14:597–607.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Zhang Z, Wang X, Wang J and Zhang L: The
decreased expression of Stat3 and p-Stat3 in preeclampsia-like rat
placenta. J Mol Histol. 49:175–183. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Wang H, Liu ML, Chu C, Yu SJ, Li J, Shen
HC, Meng Q and Zhang T: Paeonol alleviates placental inflammation
and apoptosis in preeclampsia by inhibiting the JAK2/STAT3
signaling pathway. Kaohsiung J Med Sci. 38:1103–1112. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Wang GJ, Yang Z, Huai J and Xiang QQ:
Pravastatin alleviates oxidative stress and decreases placental
trophoblastic cell apoptosis through IL-6/STAT3 signaling pathway
in preeclampsia rats. Eur Rev Med Pharmacol Sci. 24:12955–12962.
2020.PubMed/NCBI
|
|
64
|
Abdelzaher WY, Mostafa-Hedeab G, Bahaa HA,
Mahran A, Atef Fawzy M, Abdel Hafez SMN, Welson NN and Rofaeil RR:
Leukotriene receptor antagonist, montelukast ameliorates
L-NAME-induced pre-eclampsia in rats through suppressing the
IL-6/Jak2/STAT3 signaling pathway. Pharmaceuticals (Basel).
15:9142022. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
George EM and Arany I: Induction of heme
oxygenase-1 shifts the balance from proinjury to prosurvival in the
placentas of pregnant rats with reduced uterine perfusion pressure.
Am J Physiol Regul Integr Comp Physiol. 302:R620–R626. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Saad AF, Diken ZM, Kechichian TB, Clark
SM, Olson GL, Saade GR and Costantine MM: Pravastatin effects on
placental prosurvival molecular pathways in a mouse model of
preeclampsia. Reprod Sci. 23:1593–1599. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Lecarpentier E and Tsatsaris V: Angiogenic
balance (sFlt-1/PlGF) and preeclampsia. Ann Endocrinol (Paris).
77:97–100. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Kar M: Role of biomarkers in early
detection of preeclampsia. J Clin Diagn Res. 8:BE01–BE04.
2014.PubMed/NCBI
|
|
69
|
Levine RJ, Maynard SE, Qian C, Lim KH,
England LJ, Yu KF, Schisterman EF, Thadhani R, Sachs BP, Epstein
FH, et al: Circulating angiogenic factors and the risk of
preeclampsia. N Engl J Med. 350:672–683. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
van Meeteren LA, Goumans MJ and ten Dijke
P: TGF-β receptor signaling pathways in angiogenesis; emerging
targets for anti-angiogenesis therapy. Curr Pharm Biotechnol.
12:2108–2120. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Ives CW, Sinkey R, Rajapreyar I, Tita ATN
and Oparil S: Preeclampsia-pathophysiology and clinical
presentations: JACC state-of-the-art review. J Am Coll Cardiol.
76:1690–1702. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Rajakumar A, Michael HM, Rajakumar PA,
Shibata E, Hubel CA, Karumanchi SA, Thadhani R, Wolf M, Harger G
and Markovic N: Extra-placental expression of vascular endothelial
growth factor receptor-1, (Flt-1) and soluble Flt-1 (sFlt-1), by
peripheral blood mononuclear cells (PBMCs) in normotensive and
preeclamptic pregnant women. Placenta. 26:563–573. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Zolfaghari MA, Motavalli R,
Soltani-Zangbar MS, Parhizkar F, Danaii S, Aghebati-Maleki L, Noori
M, Dolati S, Ahmadi M, Samadi Kafil H, et al: A new approach to the
preeclampsia puzzle; MicroRNA-326 in CD4+ lymphocytes
might be as a potential suspect. J Reprod Immunol. 145:1033172021.
View Article : Google Scholar
|
|
74
|
Mathur AN, Chang HC, Zisoulis DG,
Stritesky GL, Yu Q, O'Malley JT, Kapur R, Levy DE, Kansas GS and
Kaplan MH: Stat3 and Stat4 direct development of IL-17-secreting Th
cells. J Immunol. 178:4901–4907. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Davis GK, Fehrenbach DJ and Madhur MS:
Interleukin 17A: Key player in the pathogenesis of hypertension and
a potential therapeutic target. Curr Hypertens Rep. 23:132021.
View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Bowman T, Garcia R, Turkson J and Jove R:
STATs in oncogenesis. Oncogene. 19:2474–2488. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Bushway ME, Gerber SA, Fenton BM, Miller
RK, Lord EM and Murphy SP: Morphological and phenotypic analyses of
the human placenta using whole mount immunofluorescence. Biol
Reprod. 90:1102014. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Zozzaro-Smith PE, Bushway ME, Gerber SA,
Hebert D, Pressman EK, Lord EM, Miller RK and Murphy SP: Whole
mount immunofluorescence analysis of placentas from normotensive
versus preeclamptic pregnancies. Placenta. 36:1310–1317. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Lykke JA, Langhoff-Roos J, Sibai BM, Funai
EF, Triche EW and Paidas MJ: Hypertensive pregnancy disorders and
subsequent cardiovascular morbidity and type 2 diabetes mellitus in
the mother. Hypertension. 53:944–951. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Männistö T, Mendola P, Vääräsmäki M,
Järvelin MR, Hartikainen AL, Pouta A and Suvanto E: Elevated blood
pressure in pregnancy and subsequent chronic disease risk.
Circulation. 127:681–690. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Rana S, Lemoine E, Granger JP and
Karumanchi SA: Preeclampsia: Pathophysiology, challenges, and
perspectives. Circ Res. 124:1094–1112. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Christensen M, Petersen JL, Sivanandam P,
Kronborg CS, Knudsen UB and Martensen PM: Reduction of
serum-induced endothelial STAT3(Y705) activation is associated with
preeclampsia. Pregnancy Hypertens. 25:103–109. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Harmon AC, Cornelius DC, Amaral LM,
Faulkner JL, Cunningham MW Jr, Wallace K and LaMarca B: The role of
inflammation in the pathology of preeclampsia. Clin Sci (Lond).
130:409–419. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Tossetta G, Fantone S, Licini C, Marzioni
D and Mattioli-Belmonte M: The multifaced role of HtrA1 in the
development of joint and skeletal disorders. Bone. 157:1163502022.
View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Tossetta G, Fantone S, Busilacchi EM, Di
Simone N, Giannubilo SR, Scambia G, Giordano A and Marzioni D:
Modulation of matrix metalloproteases by ciliary neurotrophic
factor in human placental development. Cell Tissue Res.
390:113–129. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Tossetta G, Fantone S, Piani F,
Crescimanno C, Ciavattini A, Giannubilo SR and Marzioni D:
Modulation of NRF2/KEAP1 signaling in preeclampsia. Cells.
12:15452023. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Tossetta G, Fantone S, Marzioni D and
Mazzucchelli R: Role of natural and synthetic compounds in
modulating NRF2/KEAP1 signaling pathway in prostate cancer. Cancers
(Basel). 15:30372023. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Ray PP, Islam MA, Islam MS, Han A, Geng P,
Aziz MA and Mamun AA: A comprehensive evaluation of the therapeutic
potential of silibinin: A ray of hope in cancer treatment. Front
Pharmacol. 15:13497452024. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Xu LN, Xu RA, Zhang D, Su SS, Xu HY, Wu Q
and Li YP: The changes of expressive levels of IL-17A, STAT3, and
RORγt in different invasive pulmonary aspergillosis mice. Infect
Drug Resist. 11:1321–1328. 2018. View Article : Google Scholar :
|
|
90
|
Ribeiro VR, Romao-Veiga M, Nunes PR, de
Oliveira LRC, Romagnoli GG, Peracoli JC and Peracoli MTS: Silibinin
downregulates the expression of the Th1 and Th17 profiles by
modulation of STATs and transcription factors in pregnant women
with preeclampsia. Int Immunopharmacol. 109:1088072022. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Zhang L, Li DC and Liu LF: Paeonol:
Pharmacological effects and mechanisms of action. Int
Immunopharmacol. 72:413–421. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Chen X, Zhang Z, Zhang X, Jia Z, Liu J,
Chen X, Xu A, Liang X and Li G: Paeonol attenuates heart failure
induced by transverse aortic constriction via ERK1/2 signalling.
Pharm Biol. 60:562–569. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Feldman D, Krishnan AV, Swami S,
Giovannucci E and Feldman BJ: The role of vitamin D in reducing
cancer risk and progression. Nat Rev Cancer. 14:342–357. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Zhang H, Shih DQ and Zhang X: Mechanisms
underlying effects of 1,25-Dihydroxyvitamin D3 on the Th17 cells.
Eur J Microbiol Immunol (Bp). 3:237–240. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Sassi F, Tamone C and D'Amelio P: Vitamin
D: Nutrient, hormone, and immunomodulator. Nutrients. 10:16562018.
View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Xu L, Lee M, Jeyabalan A and Roberts JM:
The relationship of hypovitaminosis D and IL-6 in preeclampsia. Am
J Obstet Gynecol. 210:149.e1–e7. 2014. View Article : Google Scholar
|
|
97
|
Ribeiro VR, Romao-Veiga M, Nunes PR, de
Oliveira LRC, Romagnoli GG, Peracoli JC and Peracoli MTS:
Immunomodulatory effect of vitamin D on the STATs and transcription
factors of CD4+ T cell subsets in pregnant women with
preeclampsia. Clin Immunol. 234:1089172022. View Article : Google Scholar
|
|
98
|
Bhasin D, Cisek K, Pandharkar T, Regan N,
Li C, Pandit B, Lin J and Li PK: Design, synthesis, and studies of
small molecule STAT3 inhibitors. Bioorg Med Chem Lett. 18:391–395.
2008. View Article : Google Scholar
|
|
99
|
Huang W, Dong Z, Wang F, Peng H, Liu JY
and Zhang JT: A small molecule compound targeting STAT3 DNA-binding
domain inhibits cancer cell proliferation, migration, and invasion.
ACS Chem Biol. 9:1188–1196. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Schust J, Sperl B, Hollis A, Mayer TU and
Berg T: Stattic: A small-molecule inhibitor of STAT3 activation and
dimerization. Chem Biol. 13:1235–1242. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Mendola P, Nobles C, Williams A, Sherman
S, Kanner J, Seeni I and Grantz K: Air pollution and preterm birth:
Do air pollution changes over time influence risk in consecutive
pregnancies among low-risk women? Int J Environ Res Public Health.
16:33652019. View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Yang S, Tan Y, Mei H, Wang F, Li N, Zhao
J, Zhang Y, Qian Z, Chang JJ, Syberg KM, et al: Ambient air
pollution the risk of stillbirth: A prospective birth cohort study
in Wuhan, China. Int J Hyg Environ Health. 221:502–509. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Zhang H, Dong H, Ren M, Liang Q, Shen X,
Wang Q, Yu L, Lin H, Luo Q, Chen W, et al: Ambient air pollution
exposure and gestational diabetes mellitus in Guangzhou, China: A
prospective cohort study. Sci Total Environ. 699:1343902020.
View Article : Google Scholar
|
|
104
|
Wang Q, Zhang H, Liang Q, Knibbs LD, Ren
M, Li C, Bao J, Wang S, He Y, Zhu L, et al: Effects of prenatal
exposure to air pollution on preeclampsia in Shenzhen, China.
Environ Pollut. 237:18–27. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Hu L, Huang B, Bai S, Tan J, Liu Y and
Chen H, Liu Y, Zhu L, Zhang J and Chen H: SO2
derivatives induce dysfunction in human trophoblasts via inhibiting
ROS/IL-6/STAT3 pathway. Ecotoxicol Environ Saf. 210:1118722021.
View Article : Google Scholar
|
|
106
|
Sirtori CR: The pharmacology of statins.
Pharmacol Res. 88:3–11. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Marrs CC and Costantine MM: Should we add
pravastatin to aspirin for preeclampsia prevention in high-risk
women? Clin Obstet Gynecol. 60:161–168. 2017. View Article : Google Scholar :
|
|
108
|
Lu F, Bytautiene E, Tamayo E, Gamble P,
Anderson GD, Hankins GD, Longo M and Saade GR: Gender-specific
effect of overexpression of sFlt-1 in pregnant mice on fetal
programming of blood pressure in the offspring later in life. Am J
Obstet Gynecol. 197:418.e1–e5. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Maynard SE, Min JY, Merchan J, Lim KH, Li
J, Mondal S, Libermann TA, Morgan JP, Sellke FW, Stillman IE, et
al: Excess placental soluble fms-like tyrosine kinase 1 (sFlt1) may
contribute to endothelial dysfunction, hypertension, and
proteinuria in preeclampsia. J Clin Invest. 111:649–658. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Korthals-de Bos I, Van Tulder M, Boers M,
Verhoeven AC, Adèr HJ, Bibo J, Boonen A and Van Der Linden S:
Indirect and total costs of early rheumatoid arthritis: A
randomized comparison of combined step-down prednisolone,
methotrexate, and sulfasalazine with sulfasalazine alone. J
Rheumatol. 31:1709–1716. 2004.PubMed/NCBI
|
|
111
|
Cai C, Lu J, Lai L, Song D, Shen J, Tong
J, Zheng Q, Wu K, Qian J and Ran Z: Drug therapy and monitoring for
inflammatory bowel disease: A multinational questionnaire
investigation in Asia. Intest Res. 20:213–223. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
112
|
Hastie R, Brownfoot FC, Pritchard N,
Hannan NJ, Cannon P, Nguyen V, Palmer K, Beard S, Tong S and
Kaitu'u-Lino TJ: EGFR (epidermal growth factor receptor) signaling
and the mitochondria regulate sFlt-1 (soluble FMS-like tyrosine
kinase-1) secretion. Hypertension. 73:659–670. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
113
|
Hastie R, Brownfoot FC, Cannon P, Nguyen
V, Tuohey L, Hannan NJ, Tong S and Kaitu'u-Lino TJ: Sulfasalazine
decreases soluble fms-like tyrosine kinase-1 secretion potentially
via inhibition of upstream placental epidermal growth factor
receptor signaling. Placenta. 87:53–57. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
114
|
Fantel AG, Nekahi N, Shepard TH, Cornel
LM, Unis AS and Lemire RJ: The teratogenicity of
N(omega)-nitro-L-ariginine methyl ester (L-NAME), a nitric oxide
synthase inhibitor, in rats. Reprod Toxicol. 11:709–717. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
115
|
Hao H, Li F, Wang F, Ran J, Chen Y, Yang
L, Ma H, Wang J and Yang H: Protective effect of metformin on the
NG-nitro-l-arginine methyl ester (l-NAME)-induced rat models of
preeclampsia. Biochem Biophys Res Commun. 739:1509962024.
View Article : Google Scholar : PubMed/NCBI
|
|
116
|
Liu S, Gao M, Zhang X, Wei J and Cui H:
FOXP2 overexpression upregulates LAMA4 expression and thereby
alleviates preeclampsia by regulating trophoblast behavior. Commun
Biol. 7:14272024. View Article : Google Scholar : PubMed/NCBI
|
|
117
|
Fitriana F, Soetrisno S, Sulistyowati S
and Indarto D: Evaluation of placental bed uterine in
L-NAME-induced early-onset preeclampsia (EO-PE) like the rat model.
Turk J Obstet Gynecol. 21:180–189. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
118
|
Tomruk C, Şirin Tomruk C, Denizlioğlu B,
Olukman M, Ercan G, Duman S, Köse T, Çetin Uyanıkgil EÖ, Uyanıkgil
Y and Uysal A: Effects of apelin on neonatal brain neurogenesis in
L-NAME-induced maternal preeclampsia. Sci Rep. 14:193472024.
View Article : Google Scholar : PubMed/NCBI
|
|
119
|
Palmsten K, Flores KF, Chambers CD, Weiss
LA, Sundaram R and Buck Louis GM: Most frequently reported
prescription medications and supplements in couples planning
pregnancy: The LIFE study. Reprod Sci. 25:94–101. 2018. View Article : Google Scholar :
|
|
120
|
Walia M, Lodha R and Kabra SK: Montelukast
in pediatric asthma management. Indian J Pediatr. 73:275–282. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
121
|
Yerraguravagari B, Penchikala NP, Kolusu
AS, Ganesh GS, Konduri P, Nemmani KVS and Samudrala PK: Montelukast
ameliorates scopolamine-induced Alzheimer's disease: Role on
cholinergic neurotransmission, antioxidant defence system,
neuroinflammation and expression of BDNF. CNS Neurol Disord Drug
Targets. 23:1040–1055. 2024. View Article : Google Scholar
|
|
122
|
Li P, Ma X, Huang D and Gu X: Exploring
the roles of non-coding RNAs in liver regeneration. Noncoding RNA
Res. 9:945–953. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
123
|
Hill M and Tran N: miRNA interplay:
Mechanisms and consequences in cancer. Dis Model Mech.
14:dmm0476622021. View Article : Google Scholar : PubMed/NCBI
|
|
124
|
Dong K, Hou Y, Zhang N, Duan B, Ma A and
Zhang Z: Down-regulated placental miR-21 contributes to
preeclampsia through targeting RASA1. Hypertens Pregnancy.
40:236–245. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
125
|
He H, Zhang H, Li Z, Wang R, Li N and Zhu
L: miRNA-214: Expression, therapeutic and diagnostic potential in
cancer. Tumori. 101:375–383. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
126
|
Li D, Xia L, Chen M, Lin C, Wu H, Zhang Y,
Pan S and Li X: miR-133b, a particular member of myomiRs, coming
into playing its unique pathological role in human cancer.
Oncotarget. 8:50193–50208. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
127
|
Wang X, Li B, Wang J, Lei J, Liu C, Ma Y
and Zhao H: Evidence that miR-133a causes recurrent spontaneous
abortion by reducing HLA-G expression. Reprod Biomed Online.
25:415–424. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
128
|
Licini C, Avellini C, Picchiassi E, Mensà
E, Fantone S, Ramini D, Tersigni C, Tossetta G, Castellucci C,
Tarquini F, et al: Pre-eclampsia predictive ability of maternal
miR-125b: A clinical and experimental study. Transl Res. 228:13–27.
2021. View Article : Google Scholar
|
|
129
|
Tang J, Wang D, Lu J and Zhou X: MiR-125b
participates in the occurrence of preeclampsia by regulating the
migration and invasion of extravillous trophoblastic cells through
STAT3 signaling pathway. J Recept Signal Transduct Res. 41:202–208.
2021. View Article : Google Scholar
|
|
130
|
Song X, Luo X, Gao Q, Wang Y, Gao Q and
Long W: Dysregulation of LncRNAs in placenta and pathogenesis of
preeclampsia. Curr Drug Targets. 18:1165–1170. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
131
|
Zhang TN, Wang W, Huang XM and Gao SY:
Non-coding RNAs and extracellular vehicles: Their role in the
pathogenesis of gestational diabetes mellitus. Front Endocrinol
(Lausanne). 12:6642872021. View Article : Google Scholar : PubMed/NCBI
|
|
132
|
Wang P, Xue Y, Han Y, Lin L, Wu C, Xu S,
Jiang Z, Xu J, Liu Q and Cao X: The STAT3-binding long noncoding
RNA lnc-DC controls human dendritic cell differentiation. Science.
344:310–313. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
133
|
Faas MM and De Vos P: Innate immune cells
in the placental bed in healthy pregnancy and preeclampsia.
Placenta. 69:125–133. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
134
|
Zhang W, Zhou Y and Ding Y: Lnc-DC
mediates the over-maturation of decidual dendritic cells and
induces the increase in Th1 cells in preeclampsia. Am J Reprod
Immunol. 77:e126472017. View Article : Google Scholar
|
|
135
|
Zhang W, Yang M, Yu L, Hu Y, Deng Y, Liu
Y, Xiao S and Ding Y: Long non-coding RNA lnc-DC in dendritic cells
regulates trophoblast invasion via p-STAT3-mediated TIMP/MMP
expression. Am J Reprod Immunol. 83:e132392020. View Article : Google Scholar : PubMed/NCBI
|
|
136
|
Gao Q, Wang T, Pan L, Qian C, Wang J, Xin
Q, Liu Y, Zhang Z, Xu Y, He X and Cao Y: Circular RNAs: Novel
potential regulators in embryogenesis, female infertility, and
pregnancy-related diseases. J Cell Physiol. 236:7223–7241. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
137
|
Zhou L, Duan Y, Fu K, Zhang M, Li K and
Yin R: The role of B7-H4 in ovarian cancer immunotherapy: Current
status, challenges, and perspectives. Front Immunol.
15:14260502024. View Article : Google Scholar : PubMed/NCBI
|
|
138
|
Duan L, Reisch B, Iannaccone A, Hadrovic
E, Wu Y, Vogtmann R, Winterhager E, Kimmig R, Köninger A, Mach P
and Gellhaus A: Abnormal expression of the costimulatory molecule
B7-H4 in placental chorionic villous and decidual basalis tissues
of patients with preeclampsia and HELLP syndrome. Am J Reprod
Immunol. 86:e134302021. View Article : Google Scholar : PubMed/NCBI
|
|
139
|
Mach P, Nolte-Boenigk L, Droste L, Fox L,
Frank M, Schmidt B, Herse F, Verlohren S, Wicherek L, Iannaccone A,
et al: Soluble B7-H4 blood serum levels are elevated in women at
high risk for preeclampsia in the first trimester, as well as in
patients with confirmed preeclampsia. Am J Reprod Immunol.
80:e129882018. View Article : Google Scholar : PubMed/NCBI
|
|
140
|
Ma Y, Duan L, Reisch B, Kimmig R,
Iannaccone A and Gellhaus A: Impact of the immunomodulatory factor
soluble B7-H4 in the progress of preeclampsia by inhibiting
essential functions of extravillous trophoblast cells. Cells.
13:13722024. View Article : Google Scholar : PubMed/NCBI
|
|
141
|
Li H, Zhou L and Dai J: Retinoic acid
receptor-related orphan receptor RORα regulates differentiation and
survival of keratinocytes during hypoxia. J Cell Physiol.
233:641–650. 2018. View Article : Google Scholar
|
|
142
|
Chauvet C, Bois-Joyeux B and Danan JL:
Retinoic acid receptor-related orphan receptor (ROR) alpha4 is the
predominant isoform of the nuclear receptor RORalpha in the liver
and is up-regulated by hypoxia in HepG2 human hepatoma cells.
Biochem J. 364:449–456. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
143
|
Yu Y and Zhu T: RAR-related orphan
receptor: An accelerated preeclampsia progression by activating the
JAK/STAT3 pathway. Yonsei Med J. 63:554–563. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
144
|
Kikkawa Y, Ogawa T, Sudo R, Yamada Y,
Katagiri F, Hozumi K, Nomizu M and Miner JH: The lutheran/basal
cell adhesion molecule promotes tumor cell migration by modulating
integrin-mediated cell attachment to laminin-511 protein. J Biol
Chem. 288:30990–31001. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
145
|
Yang WH, Huang Z, Wu J, Ding CC, Murphy SK
and Chi JT: A TAZ-ANGPTL4-NOX2 axis regulates ferroptotic cell
death and chemoresistance in epithelial ovarian cancer. Mol Cancer
Res. 18:79–90. 2020. View Article : Google Scholar :
|
|
146
|
Cao Y, Luo F, Peng J, Fang Z, Liu Q and
Zhou S: KMT2B-dependent RFK transcription activates the TNF-α/NOX2
pathway and enhances ferroptosis caused by myocardial
ischemia-reperfusion. J Mol Cell Cardiol. 173:75–91. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
147
|
Dixon SJ, Lemberg KM, Lamprecht MR, Skouta
R, Zaitsev EM, Gleason CE, Patel DN, Bauer AJ, Cantley AM, Yang WS,
et al: Ferroptosis: An iron-dependent form of nonapoptotic cell
death. Cell. 149:1060–1072. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
148
|
Zhao Y, Wang C, Yang T, Wang H, Zhao S,
Sun N, Chen Y, Zhang H and Fan H: Chlorogenic Acid alleviates
chronic stress-induced duodenal ferroptosis via the inhibition of
the IL-6/JAK2/STAT3 signaling pathway in rats. J Agric Food Chem.
70:4353–4361. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
149
|
Zhang W, Gong M, Zhang W, Mo J, Zhang S,
Zhu Z, Wang X, Zhang B, Qian W, Wu Z, et al: Thiostrepton induces
ferroptosis in pancreatic cancer cells through STAT3/GPX4
signaling. Cell Death Dis. 13:6302022. View Article : Google Scholar
|
|
150
|
Jiang Z, Li S, Han MJ, Hu GM and Cheng P:
High expression of NSUN5 promotes cell proliferation via cell cycle
regulation in colorectal cancer. Am J Transl Res. 12:3858–3870.
2020.PubMed/NCBI
|
|
151
|
Zhang H, Li H, Yao J, Zhao M and Zhang C:
The mutation of NSUN5 R295C promotes preeclampsia by impairing
decidualization through downregulating IL-11Rα. iScience.
27:1088992024. View Article : Google Scholar
|
|
152
|
Graham AM and Presnell JS: Hypoxia
inducible factor (HIF) transcription factor family expansion,
diversification, divergence and selection in eukaryotes. PLoS One.
12:e01795452017. View Article : Google Scholar : PubMed/NCBI
|
|
153
|
Qu H, Yu Q, Jia B, Zhou W, Zhang Y and Mu
L: HIF-3α affects preeclampsia development by regulating EVT growth
via activation of the Flt-1/JAK/STAT signaling pathway in hypoxia.
Mol Med Rep. 23:682021. View Article : Google Scholar
|
|
154
|
Gerke V and Moss SE: Annexins: From
structure to function. Physiol Rev. 82:331–371. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
155
|
Alli-Shaik A, Wee S, Lim LHK and Gunaratne
J: Phosphoproteomics reveals network rewiring to a pro-adhesion
state in annexin-1-deficient mammary epithelial cells. Breast
Cancer Res. 19:1322017. View Article : Google Scholar : PubMed/NCBI
|
|
156
|
Lee SH, Lee PH, Kim BG, Seo HJ, Baek AR,
Park JS, Lee JH, Park SW, Kim DJ, Park CS and Jang AS: Annexin A1
in plasma from patients with bronchial asthma: Its association with
lung function. BMC Pulm Med. 18:12018. View Article : Google Scholar : PubMed/NCBI
|
|
157
|
Perucci LO, Carneiro FS, Ferreira CN,
Sugimoto MA, Soriani FM, Martins GG, Lima KM, Guimarães FL,
Teixeira AL, Dusse LM, et al: Annexin A1 is increased in the plasma
of preeclamptic women. PLoS One. 10:e01384752015. View Article : Google Scholar : PubMed/NCBI
|
|
158
|
Perucci LO, Vieira ÉLM, Teixeira AL, Gomes
KB, Dusse LM and Sousa LP: Decreased plasma concentrations of
brain-derived neurotrophic factor in preeclampsia. Clin Chim Acta.
464:142–147. 2017. View Article : Google Scholar
|
|
159
|
Feng J, Wang X, Li H, Wang L and Tang Z:
Silencing of Annexin A1 suppressed the apoptosis and inflammatory
response of preeclampsia rat trophoblasts. Int J Mol Med.
42:3125–3134. 2018.PubMed/NCBI
|
|
160
|
Xue Y, Luo M, Hu X, Li X, Shen J, Zhu W,
Huang L, Hu Y, Guo Y, Liu L, et al: Macrophages regulate vascular
smooth muscle cell function during atherosclerosis progression
through IL-1β/STAT3 signaling. Commun Biol. 5:13162022. View Article : Google Scholar
|
|
161
|
Cruceriu D, Baldasici O, Balacescu O and
Berindan-Neagoe I: The dual role of tumor necrosis factor-alpha
(TNF-α) in breast cancer: Molecular insights and therapeutic
approaches. Cell Oncol (Dordr). 43:1–18. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
162
|
Sreenivasan L, Wang H, Yap SQ, Leclair P,
Tam A and Lim CJ: Autocrine IL-6/STAT3 signaling aids development
of acquired drug resistance in group 3 medulloblastoma. Cell Death
Dis. 11:10352020. View Article : Google Scholar : PubMed/NCBI
|
|
163
|
Ma JH, Qin L and Li X: Role of STAT3
signaling pathway in breast cancer. Cell Commun Signal. 18:332020.
View Article : Google Scholar : PubMed/NCBI
|
|
164
|
Mo HQ, Tian FJ, Li X, Zhang J, Ma XL, Zeng
WH, Lin Y and Zhang Y: ANXA7 regulates trophoblast proliferation
and apoptosis in preeclampsia. Am J Reprod Immunol. 82:e131832019.
View Article : Google Scholar : PubMed/NCBI
|
|
165
|
Xu C, Li X, Guo P and Wang J:
Hypoxia-induced activation of JAK/STAT3 signaling pathway promotes
trophoblast cell viability and angiogenesis in preeclampsia. Med
Sci Monit. 23:4909–4917. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
166
|
Yoshida H and Hunter CA: The immunobiology
of interleukin-27. Annu Rev Immunol. 33:417–443. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
167
|
Yin N, Zhang H, Luo X, Ding Y, Xiao X, Liu
X, Shan N, Zhang X, Deng Q, Zhuang B and Qi H: IL-27 activates
human trophoblasts to express IP-10 and IL-6: implications in the
immunopathophysiology of preeclampsia. Mediators Inflamm.
2014:9268752014. View Article : Google Scholar : PubMed/NCBI
|
|
168
|
Ge H, Yin N, Han TL, Huang D, Chen X, Xu
P, He C, Tong C and Qi H: Interleukin-27 inhibits trophoblast cell
invasion and migration by affecting the epithelial-mesenchymal
transition in preeclampsia. Reprod Sci. 26:928–938. 2019.
View Article : Google Scholar
|
|
169
|
Cao J, Inoue K, Li X, Drummond G and
Abraham NG: Physiological significance of heme oxygenase in
hypertension. Int J Biochem Cell Biol. 41:1025–1033. 2009.
View Article : Google Scholar :
|
|
170
|
Tsuchihashi S, Zhai Y, Bo Q, Busuttil RW
and Kupiec-Weglinski JW: Heme oxygenase-1 mediated cytoprotection
against liver ischemia and reperfusion injury: Inhibition of type-1
interferon signaling. Transplantation. 83:1628–1634. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
171
|
Csongradi E, Docarmo JM, Dubinion JH, Vera
T and Stec DE: Chronic HO-1 induction with cobalt protoporphyrin
(CoPP) treatment increases oxygen consumption, activity, heat
production and lowers body weight in obese melanocortin-4
receptor-deficient mice. Int J Obes (Lond). 36:244–253. 2012.
View Article : Google Scholar
|
|
172
|
Downey JM, Davis AM and Cohen MV:
Signaling pathways in ischemic preconditioning. Heart Fail Rev.
12:181–188. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
173
|
Xuan YT, Guo Y, Han H, Zhu Y and Bolli R:
An essential role of the JAK-STAT pathway in ischemic
preconditioning. Proc Natl Acad Sci USA. 98:9050–9055. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
174
|
Hilfiker-Kleiner D, Hilfiker A and Drexler
H: Many good reasons to have STAT3 in the heart. Pharmacol Ther.
107:131–137. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
175
|
Andrés O, Kellermann T, López-Giráldez F,
Rozas J, Domingo-Roura X and Bosch M: RPS4Y gene family evolution
in primates. BMC Evol Biol. 8:1422008. View Article : Google Scholar : PubMed/NCBI
|
|
176
|
Chen Y, Chen Y, Tang C, Zhao Q, Xu T, Kang
Q, Jiang B and Zhang L: RPS4Y1 promotes high glucose-induced
endothelial cell apoptosis and inflammation by activation of the
p38 MAPK signaling. Diabetes Metab Syndr Obes. 14:4523–4534. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
177
|
Chen X, Tong C, Li H, Peng W, Li R, Luo X,
Ge H, Ran Y, Li Q, Liu Y, et al: Dysregulated expression of RPS4Y1
(ribosomal protein S4, Y-linked 1) impairs STAT3 (signal transducer
and activator of transcription 3) signaling to suppress trophoblast
cell migration and invasion in preeclampsia. Hypertension.
71:481–490. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
178
|
Boonstra J, Rijken P, Humbel B, Cremers F,
Verkleij A and van Bergen en Henegouwen P: The epidermal growth
factor. Cell Biol Int. 19:413–430. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
179
|
Armant DR, Fritz R, Kilburn BA, Kim YM,
Nien JK, Maihle NJ, Romero R and Leach RE: Reduced expression of
the epidermal growth factor signaling system in preeclampsia.
Placenta. 36:270–278. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
180
|
Malik A, Pal R and Gupta SK:
Interdependence of JAK-STAT and MAPK signaling pathways during
EGF-mediated HTR-8/SVneo cell invasion. PLoS One. 12:e01782692017.
View Article : Google Scholar : PubMed/NCBI
|
|
181
|
Chen LJ, Han ZQ, Zhou H, Zou L and Zou P:
Inhibition of HLA-G expression via RNAi abolishes resistance of
extravillous trophoblast cell line TEV-1 to NK lysis. Placenta.
31:519–527. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
182
|
Cecati M, Giannubilo SR, Emanuelli M,
Tranquilli AL and Saccucci F: HLA-G and pregnancy adverse outcomes.
Med Hypotheses. 76:782–784. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
183
|
Liu X, Gu W and Li X: HLA-G regulates the
invasive properties of JEG-3 choriocarcinoma cells by controlling
STAT3 activation. Placenta. 34:1044–1052. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
184
|
Huppertz B: The anatomy of the normal
placenta. J Clin Pathol. 61:1296–1302. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
185
|
Kannaiyan J, Muthukutty P, Iqbal MDT and
Paulraj B: Villous chorion: A potential source for pluripotent-like
stromal cells. J Nat Sci Biol Med. 8:221–228. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
186
|
Chu Y, Zhu C, Yue C, Peng W, Chen W, He G,
Liu C, Lv Y, Gao G, Yao K, et al: Chorionic villus-derived
mesenchymal stem cell-mediated autophagy promotes the proliferation
and invasiveness of trophoblasts under hypoxia by activating the
JAK2/STAT3 signaling pathway. Cell Biosci. 11:1822021. View Article : Google Scholar
|
|
187
|
Valesini G and Alessandri C: New facet of
antiphospholipid antibodies. Ann N Y Acad Sci. 1051:487–497. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
188
|
Di Simone N, Caliandro D, Castellani R,
Ferrazzani S and Caruso A: Interleukin-3 and human trophoblast: In
vitro explanations for the effect of interleukin in patients with
antiphospholipid antibody syndrome. Fertil Steril. 73:1194–1200.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
189
|
Branch DW and Khamashta MA:
Antiphospholipid syndrome: Obstetric diagnosis, management, and
controversies. Obstet Gynecol. 101:1333–1344. 2003.PubMed/NCBI
|
|
190
|
Mulla MJ, Brosens JJ, Chamley LW, Giles I,
Pericleous C, Rahman A, Joyce SK, Panda B, Paidas MJ and Abrahams
VM: Antiphospholipid antibodies induce a pro-inflammatory response
in first trimester trophoblast via the TLR4/MyD88 pathway. Am J
Reprod Immunol. 62:96–111. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
191
|
Mulla MJ, Myrtolli K, Brosens JJ, Chamley
LW, Kwak-Kim JY, Paidas MJ and Abrahams VM: Antiphospholipid
antibodies limit trophoblast migration by reducing IL-6 production
and STAT3 activity. Am J Reprod Immunol. 63:339–348. 2010.
View Article : Google Scholar : PubMed/NCBI
|