Introduction
Pancreatic cancer is a highly lethal cancer,
accounting for nearly 5% of cancer-related deaths worldwide
(1). Numerous patients with
pancreatic cancer are diagnosed in the late stages of the disease
because there are no apparent symptoms, resulting in missed
opportunities for surgical treatment. The main treatments for
pancreatic cancer are pancreatectomy and drug chemotherapy, but the
prognosis for patients is poor. Despite advances in combination
chemotherapy, the median survival of patients with pancreatic
cancer is only 10-12 months (2,3).
Due to the limited efficacy of current treatment options, targeted
therapies are receiving attention for addressing this unmet
clinical need (4). Of
significant note, antibody-drug conjugates (ADCs) have become a
popular research topic in antitumor drug development, combining the
advantages of precise targeting and efficient elimination to
achieve exact and efficient elimination of cancer cells. Since the
U.S. FDA first approved the ADC drug Mylotarg in 2000, 13 ADCs have
reached the market. However, no ADCs have yet been authorized by
the FDA for the treatment of pancreatic cancer (5). ADC treatment for pancreatic cancer
has shown promise in preclinical studies. Presently, clinical
trials of ADC drugs targeting pancreatic cancer are also underway,
including ADCs targeting claudin18.2: SHR-A1904 (NCT04928625),
TROP2: IMMU-132 (NCT01631552) and c-MET: RC108 (NCT05628857).
Despite the advances in the efficacy of these ADCs in the treatment
of pancreatic cancer, there are few studies investigating the
specific mechanisms of ADCs in pancreatic cancer, and no studies of
Nectin-4-targeted ADCs in pancreatic cancer have been reported to
the best of the authors' knowledge.
Nectin-4, a type I transmembrane cell adhesion
protein, is expressed at low levels in adults under physiological
conditions and overexpressed in various tumor tissues, and
therefore is an attractive antigen target for ADC (6). The first FDA-approved
Nectin-4-targeted ADC, enfuzumab vedotin, is a treatment for
urothelial cancer (7). According
to the literature, the overall positivity rate of pancreatic cancer
for Nectin-4 was reported to be ~71%, and the expression of
Nectin-4 in pancreatic cancer tissue was markedly higher than that
in normal pancreatic tissue (8).
Another study found that Nectin-4 may be closely associated with
Capan-2 and BxPC-3 cell proliferation. Nectin-4 may contribute to
tumor cell proliferation in human pancreatic cancer, according to
immunohistochemistry experiments (9). These findings suggest that Nectin-4
may be a potential target in pancreatic cancer and that
Nectin-4-targeted ADCs may be a potential therapeutic agent to
support further clinical studies and treatment. In the present
study, experiments were performed using Nectin-4-MMAE, 9MW2821,
which consists of an anti-Nectin-4 antibody (MW282 mAb), the
IDconnect linker and the cytotoxic molecule MMAE.
Autophagy is an important regulator of cancer cell
metabolism, and its role in various solid tumors is dynamic and
dependent on the environment (10,11). On the one hand, autophagy is a
cytoprotective mechanism that degrades damaged, degenerated and
senescent cells to provide raw materials for cell regeneration and
repair (12). On the other hand,
excessive autophagy leads to metabolic stress and cell death
(13,14). Tumor cells induce autophagy under
hypoxic and starvation conditions; inhibiting autophagy
significantly increases tumor death and suppresses tumor cell
proliferation (15). Wang et
al (16) reported that a
combination of Nectin-4-MMAE and autophagy inhibitors exhibited
synergistic antitumor effects in bladder cancer. The mechanism of
autophagy in Nectin-4-MMAE treatment of pancreatic cancer also
merits further exploration.
The present study aimed to investigate the possible
antitumor effects of Nectin-4-MMAE on pancreatic cancer both in
vivo and in vitro. Moreover, the current study delved
into the significant roles of autophagy and apoptosis in
Nectin-4-MMAE-induced pancreatic cancer cell death. Autophagy
inhibitors were combined with Nectin-4-MMAE to evaluate the
possibility of enhancing its antitumor effects and to explore the
feasibility of this combination therapy for treating pancreatic
cancer.
Materials and methods
Reagents and antibodies
9MW2821 was provided by Mabwell (Shanghai)
Biotechnology Co., Ltd. LY294002 was purchased from Selleck
Chemicals (cat. no. S1105). Antibodies from Cell Signaling
Technology, Inc. used in the present study were as follows:
anti-PARP antibody (cat. no. 9542), anti-cleaved caspase-9 antibody
(cat. no. 9502), anti-cleaved caspase-3 antibody (cat. no. 9662),
anti-phospho-AKT antibody (Ser473; cat. no. 4060),
anti-phospho-mTOR antibody (Ser2448; cat. no. 2971),
anti-phospho-p70s6k antibody (Ser371; cat. no. 9208),
anti-phospho-4EBP1 antibody (Thr45; cat. no. 2855), anti-LC3
antibody (cat. no. 3868) and anti-SQSTM1 antibody (cat. no. 8025).
All of the primary antibodies were derived from rabbits and the
dilution ratio was 1:1,000. HRP-conjugated anti-rabbit IgG
secondary antibody (1:3,000; cat. no. 7074; Cell Signaling
Technology, Inc.) and anti-β-actin antibody (rabbit; 1:5,000; cat.
no. GB15003-100; Wuhan Servicebio Technology Co., Ltd.) were also
used.
Cell culture
The human pancreatic cancer cell lines BxPC-3, YAPC
and PANC-1 were purchased from the Shanghai Typical Cultures
Depository of the Chinese Academy of Sciences. PANC-1 cells were
cultivated in DMEM media (cat. no. MA 0212; Dalian Meilun Biology
Technology Co., Ltd.), while BxPC-3 and YAPC cells were cultured in
RPMI-1640 media (cat. no. MA 0215; Dalian Meilun Biology Technology
Co., Ltd.). Cell lines were maintained in an environment with 5%
carbon dioxide, suitable humidity, and a temperature of 37°C. It
was confirmed that all cell lines are mycoplasma-free through
routine mycoplasma testing.
Cellular Nectin-4 expression assay
Cells were resuspended in ice-cold PBS. Then 100
µl (1×106 cells/ml) of the cell suspension and 2
µg/ml of anti-Nectin-4 antibody were added to a centrifuge
tube. The suspension was incubated for 30 min at 4°C while being
protected from light. The cells were washed three times and
fluorescent-conjugated secondary antibody (1:1,000; cat. no.
398004; BioLegend, Inc) was added to the appropriate tubes. The
suspension was incubated at 4°C in the dark for 30 min. After
washing the cells three times, they were resuspended in PBS for
subsequent analysis. Cells were analyzed by flow cytometry
(CytoFLEX; Beckman Coulter, Inc.) using the software CytExpert 2.4
(Beckman Coulter, Inc.).
Cell viability assay
After the indicated co-incubation time, 100
µl of MTT solution (cat. no. MB4698; Dalian Meilun Biology
Technology Co., Ltd.) was added to the cells and incubated at 37°C
for 4 h. The methylated product was dissolved with DMSO, and the
plates were gently shaken at room temperature for 5 min. An enzyme
marker was used to detect the absorbance at 490 nm.
Confocal microscopy
The internalization of ADCs and the subsequent
autophagic flow were investigated using confocal fluorescence
imaging. Using an Alexa Fluor® 488 Protein Labelling Kit
(cat. no. A10235; Thermo Fisher Scientific, Inc.), the ADCs were
labeled with Alexa Fluor® 488 and incubated for the
specified amount of time. ADCs were then applied to YAPC and BxPC-3
cells for specified durations. Autophagosomes and lysosomes were
identified using a Cyto-ID Autophagy Detection Kit (cat. no.
ENZO-51031K200; Enzo Life Sciences, Inc.). The cells were observed
using a Carl Zeiss LSM710 (Carl Zeiss AG) confocal microscope.
Western blot analysis
The cells were lysed using RIPA buffer (cat. no.
P0013D; Beyotime Institute of Biotechnology). The total protein
concentration was measured after the supernatant was collected
using the BCA Protein Assay Kit (cat. no. P0012; Beyotime
Biotechnology). Loading buffer was added and the proteins were
heated for 8 min at 100°C to denature the proteins. SDS-PAGE gels
(8-15%) were loaded with equal volumes of proteins (20 µg
per lane) and then underwent electrophoretic separation. The
proteins were subsequently transferred to a PVDF membrane. Next,
the membrane was blocked with 5% BSA (cat. no. MB4219; Dalian
Meilun Biology Technology Co., Ltd.) for 2 h at room temperature,
and incubated overnight with the primary antibody at 4°C, followed
by the secondary antibody for 2 h at room temperature. Using an ECL
kit (cat. no. MA0186; Dalian Meilun Biology Technology Co., Ltd.),
the membrane protein bands were observed. Imaging was performed
using a gel imager from Bio-Rad Laboratories, Inc. Densitometric
analysis was carried out using Image LAB 5.2 software, and the
grayscale values of the bands were calculated using ImageJ
(National Institutes of Health).
Apoptosis analysis
Apoptosis was identified by using the Annexin
V-FITC/PI Apoptosis Detection Kit (cat. no. MA0220; Dalian Meilun
Biology Technology Co., Ltd.). Cells were processed and stained
according to the manufacturer's protocol. After 1×105
cells were centrifuged, the supernatant was discarded. 195
µl of Annexin V-FITC binding buffer was added and the cells
were gently resuspended. Then, 5 µl of Annexin V-FITC and 10
µl of propidium iodide staining solution were added and
gently mixed. Incubate the samples in the dark for 15 min before
performing the detection on the instrument. Using a flow cytometer,
cells were found and examined (CytoFLEX; Beckman Coulter, Inc.)
using the software CytExpert 2.4 (Beckman Coulter, Inc.).
Transmission electron microscopy
The autophagic structure of BxPC-3 cells was
determined using transmission electron microscopy. When the cell
density was ~70%, the cells were digested with EDTA-free digestive
enzymes. After centrifugation (300 × g, 3 min, 20°C), the
supernatant was discarded and the cells were fixed using 2.5%
glutaraldehyde fixative. The samples were kept at 4°C. The
embedding, sectioning and subsequent image acquisition work were
entrusted to Wuhan Servicebio Technology Co., Ltd. The samples were
recorded using a transmission electron microscope at magnifications
of ×1,500 and ×6,000.
Tumor xenograft model
BALB/c nude male mice (n=25, 18 g, 6-weeks old) were
purchased from the Shanghai Model Organisms Center. Male mice based
the stable hormone level making it easier to determine the
antitumor efficacy of ADCs. All animals were kept in the
Experimental Animal Center of Fudan University under a 12/12 h
light/dark cycle (17). The
temperature in the animal room was controlled between 20-26°C. The
mice were given full access to regular food and water during the
study. BxPC3 cells suspended in PBS were subcutaneously injected at
a density of 1×107 cells per mouse to establish mouse
xenograft tumor model. A total of 5 groups of mice were randomly
selected, with 5 mice in each group. The treatments were as
follows: Intraperitoneal injection of PBS; single dose intravenous
injection of Nectin-4-MMAE (3 mg/kg); intraperitoneal injection of
chloroquine (50 mg/kg; cat. no. C6628; MilliporeSigma) once a day;
combination of Nectin-4-MMAE and CQ; and twice a week intravenous
injection of the positive control drug gemcitabine (50 mg/kg).
Tumor volume in mice was calculated by measuring length and width
(volume=0.5 × length × width2). After 21 days of drug
administration, the experimental animals were euthanized by
cervical dislocation operation. The researchers observed the mice
directly for undulating movements of the chest to determine
respiratory arrest. At the same time, the left side of the mice's
chest was gently touched with a finger to determine whether the
heartbeat had stopped by touch. When the mice's vital signs
completely stopped and there was no sign of recovery after
continuous observation, the mice's death status was formally
confirmed, and then the subsequent experimental process steps were
carried out. Sections of excised tumor tissue were prepared for
H&E staining.
Hematoxylin and eosin (H&E)
staining
Sectioning and H&E staining were both entrusted
to Wuhan Servicebio Technology Co., Ltd. The tumor tissue was
collected and fixed in a 4% paraformaldehyde solution for 24 h.
Subsequently, paraffin embedding was carried out, and the tissue
was sectioned into slices with a thickness of 3-4 µm. The
slices were then deparaffinized in xylene and hydrated through a
series of alcohol solutions with decreasing concentrations. Next,
the slices were stained with H&E. After staining, the slices
were dehydrated with anhydrous ethanol and made transparent with
xylene. Finally, the treated slices were mounted and observed under
the Whole Slide Scanning System (Olympus VS200).
Statistical analysis
Adobe Photoshop and Illustrator were used to create
the graphs. GraphPad Prism 8.3 (Dotmatics) and Excel (version 2019;
Microsoft Corporation) were used to analyze the data, and the
results of three independent replicate experiments are shown as the
mean ± SD. Groups were compared using a one-way ANOVA followed by
Dunnett's or Tukey's post hoc tests and the unpaired Student's
t-test. P<0.05 was considered to indicate a statistically
significant difference.
Results
ADCs targeting Nectin-4 induce apoptosis
in pancreatic cancer cell lines
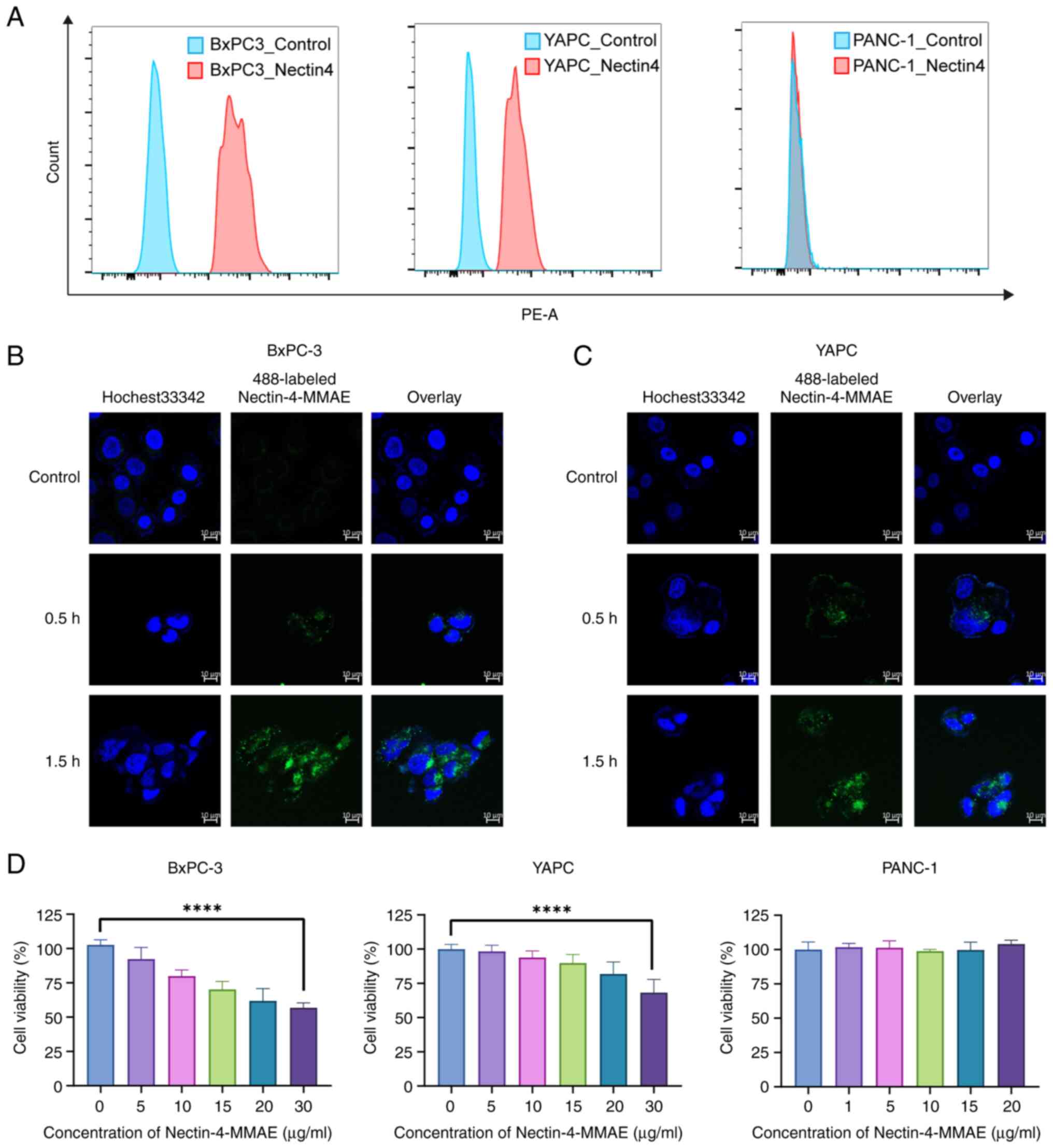
Flow cytometry screening was performed on two
Nectin-4 positive pancreatic cancer cell lines, BxPC-3 and YAPC,
and 1 Nectin-4 negative cell line, PANC-1, for subsequent
experiments (Fig. 1A). YAPC and
BxPC-3 cells were cultured with 10 µl (2 µg/ml) of
Nectin-4-MMAE labeled with Alexa Fluor® 488. After 30-90
min of treatment, intracellular green fluorescence was observed
using laser confocal microscopy, indicating that Alexa
Fluor® 488-labeled Nectin-4-MMAE bound to the cell
surface receptors and enter the cell for internalization (Fig. 1B and C). Using an MTT assay, it
was found that the cytotoxicity of BxPC-3 and YAPC cells increased
significantly as the concentration of Nectin-4-MMAE increased after
72 h, while PANC-1 cell viability was not significantly affected
(Fig. 1D). The cytotoxicity of
ADCs is not potent enough, which might depend on the targets, the
cell lines and the properties of ADCs.
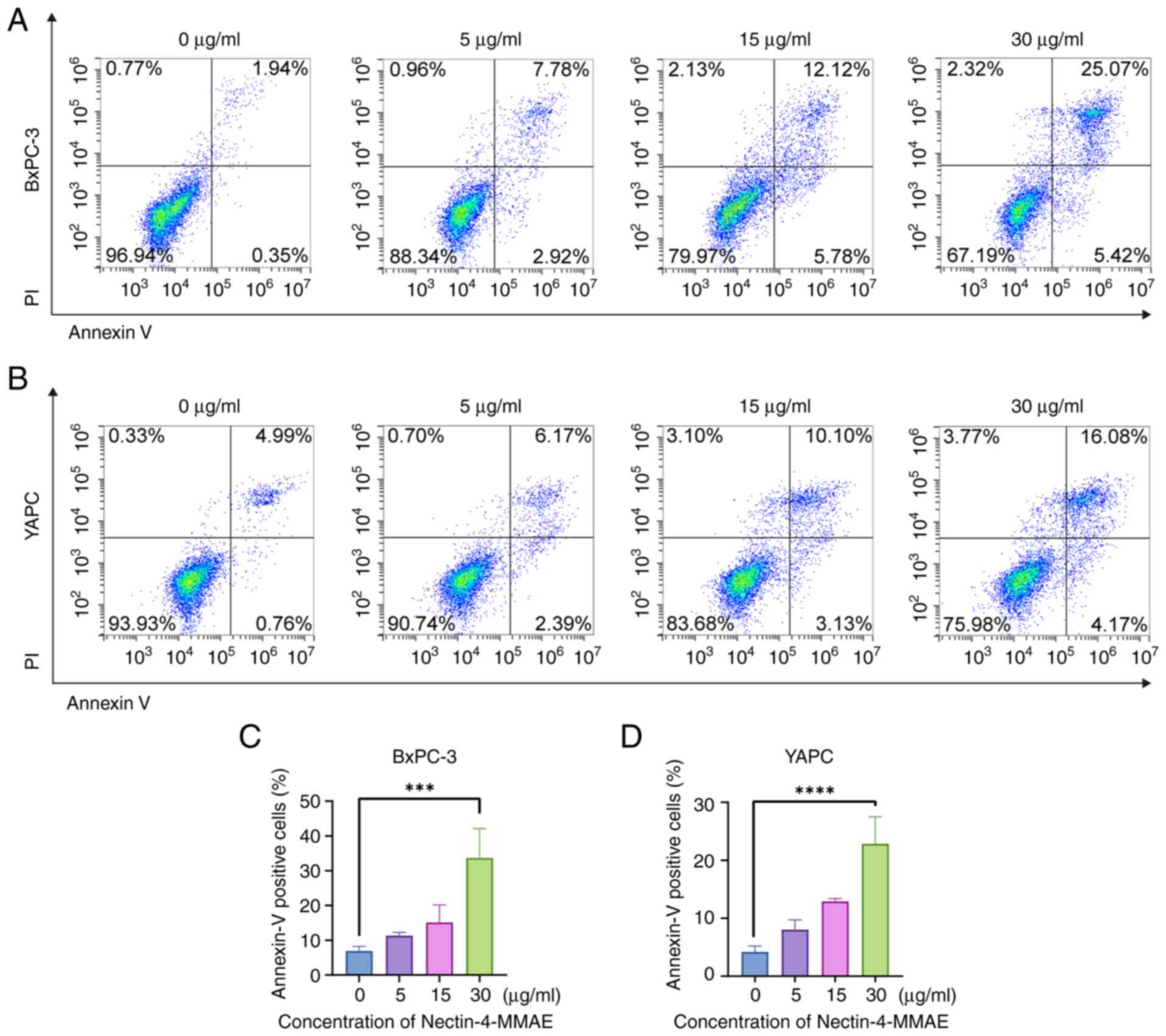
To explore the mechanism of cell elimination, cells
were analyzed for apoptosis by staining with Annexin V/PI after
BxPC-3 and YAPC cells were treated with different concentrations of
Nectin-4-MMAE for 72 h. As the concentration of Nectin-4-MMAE
within BxPC-3 and YAPC cells rises, the overall number of both
early apoptotic and late apoptotic cells similarly increased
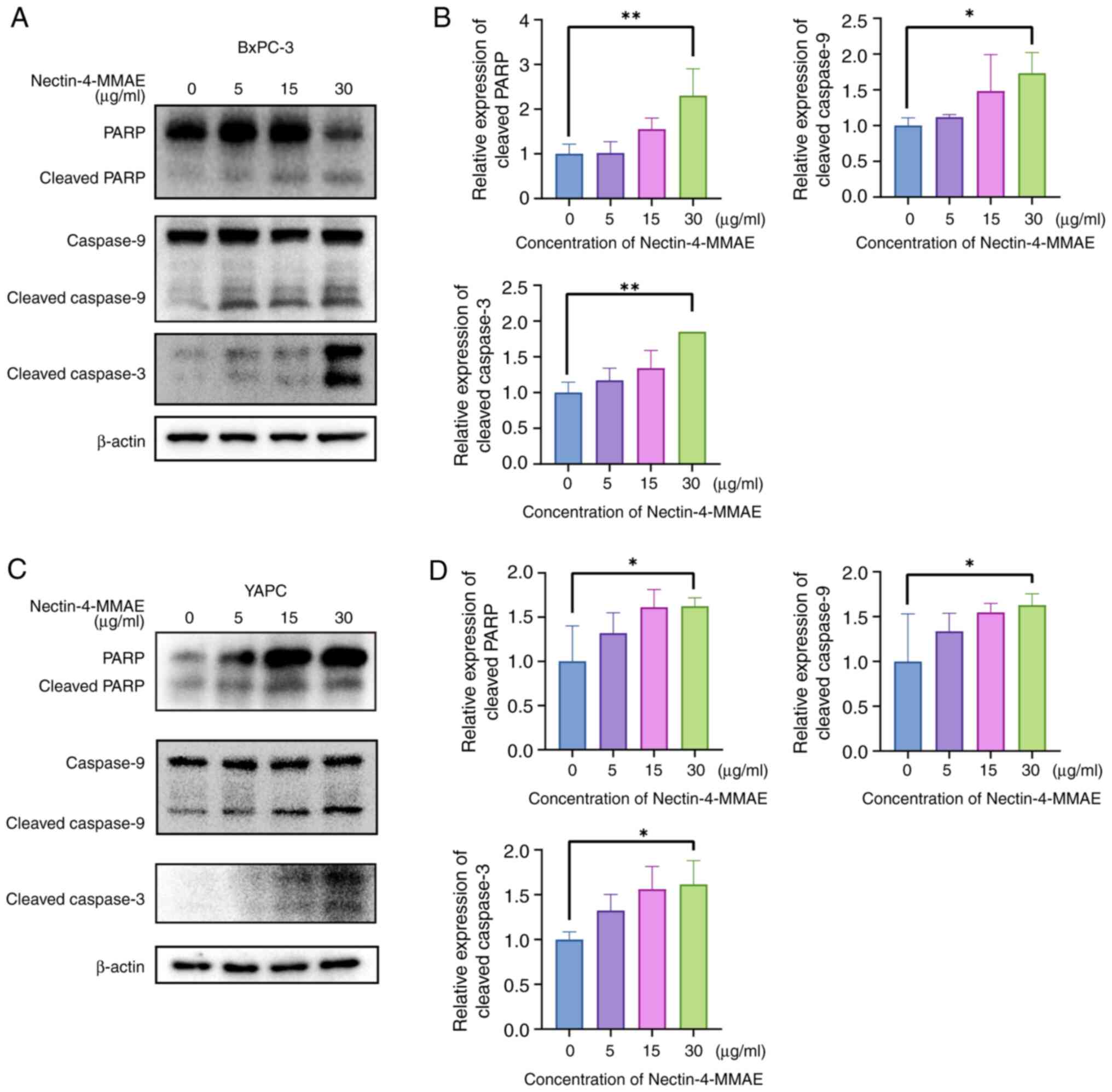
(Fig. 2A-D). The expression of
apoptosis-related proteins was analyzed in YAPC and BxPC-3 cells by
western blotting (Fig. 3A and
B). As the concentration of Nectin-4-MMAE increased, there was
a significant rise in apoptosis-related protein levels. This
finding suggested that Nectin-4-MMAE caused cell death by cleaving
PARP downstream and through apoptosis by activating the caspase
cascade. The results suggested that Nectin-4-MMAE induced apoptosis
and cytotoxicity in BxPC-3 and YAPC cells.
Nectin-4-directed ADC induces autophagy
in BxPC-3 and YAPC cells
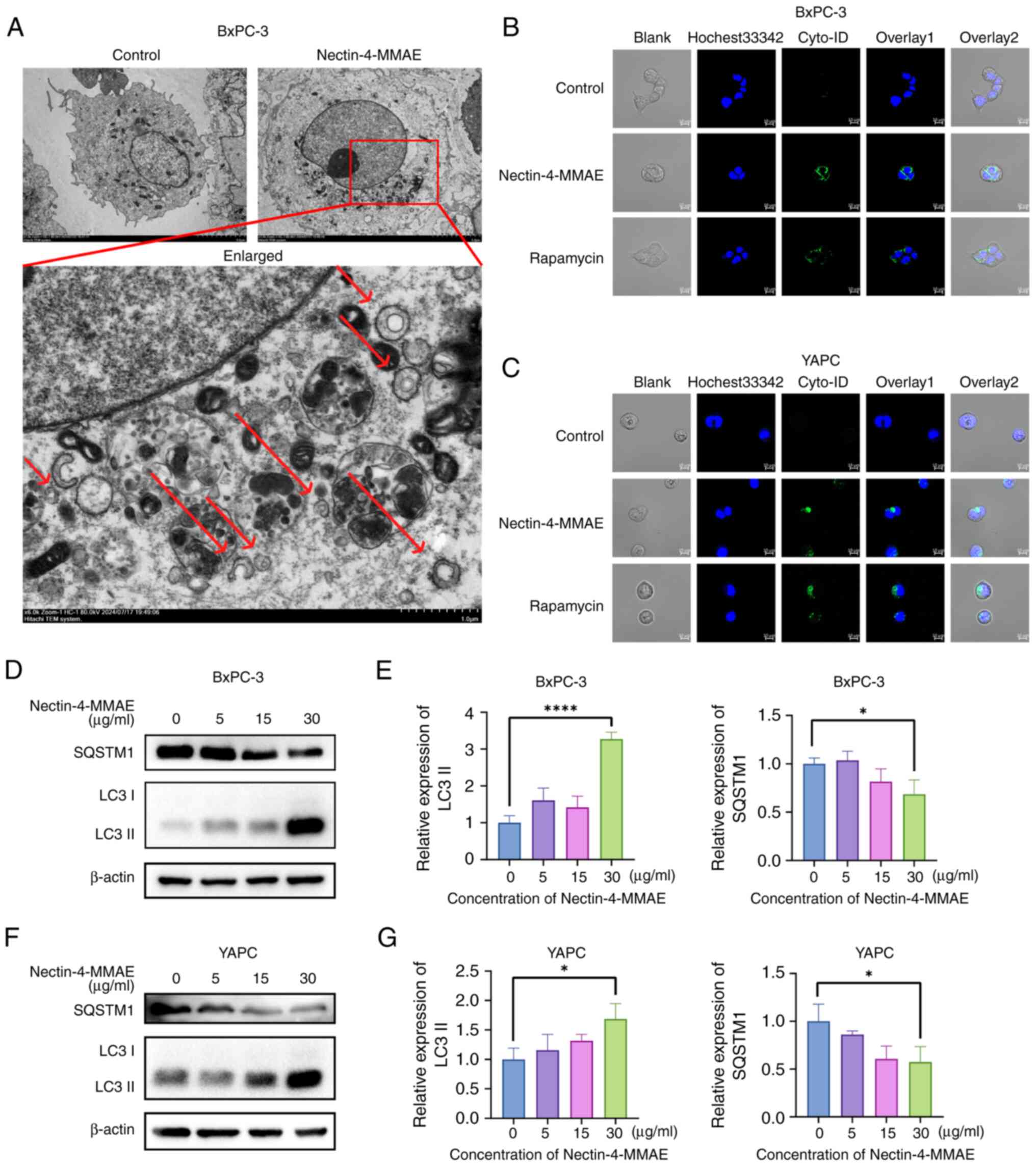
Autophagosomes in BxPC-3 cells after Nectin-4-MMAE
treatment were observed using transmission electron microscopy
(Fig. 4A). The autophagosomes
had a double-membrane structure (marked by red arrows). This result
suggested that Nectin-4-MMAE resulted in the production of
intracellular autophagic vesicles. To further confirm the
relationship between autophagy and ADCs, the cells were divided
into three groups: The negative control group, the Nectin-4-MMAE
group (15 µg/ml), and the positive control rapamycin group.
BxPC-3 and YAPC cells were plated and incubated with the
corresponding drugs for 12 h. The cells were stained with the
autophagosome dye Cyto-ID and observed by confocal microscopy. It
was found that the intensity of green fluorescence significantly
increased in the Nectin-4-MMAE groups, which was consistent with
the results of the positive control rapamycin group (Fig. 4B and C). In addition, the
expression of the autophagy marker protein SQSTM1 decreased as the
Nectin-4-MMAE concentration increased, and LC3 II expression
increased as the Nectin-4-MMAE concentration increased, suggesting
an increase in autophagy (Fig.
4D-F).
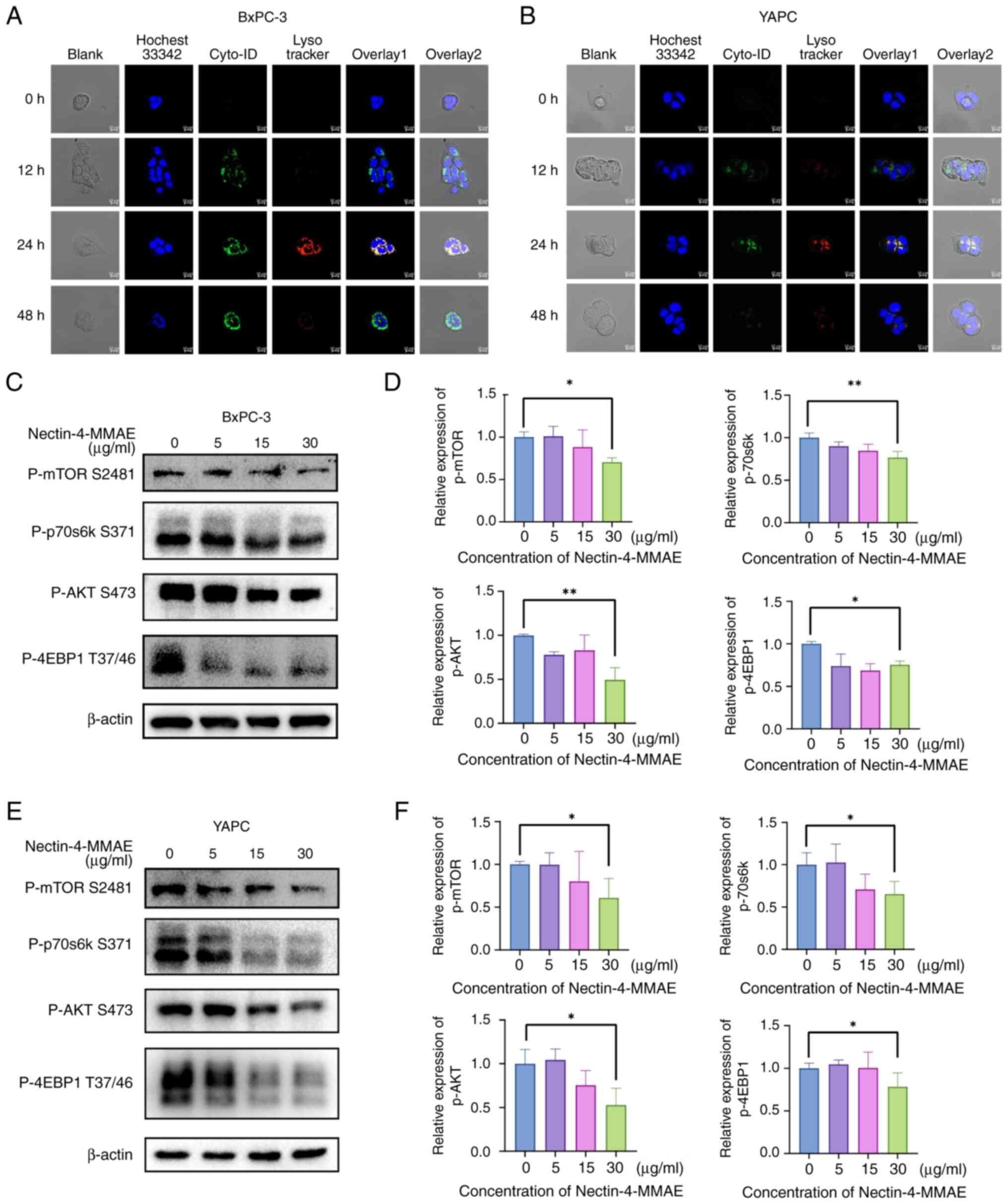
Nectin-4-directed ADCs induce autophagy
flux in BxPC-3 and YAPC cells
BxPC-3 and YAPC cells were treated with 15
µg/ml Nectin-4-MMAE for different time intervals.
Autophagosome formation was detected at 12 h and peaked at 24 h.
Lysosome formation was detected in BxPC-3 and YAPC cells after 24
h. After 48 h, lysosomes were observed in BxPC-3 cells, while
lysosomes disappeared in YAPC cells. Yellow fluorescence was
observed in the merged image at 24 h and disappeared at 48 h,
indicating that autophagosomes become autophagic lysosomes and were
subsequently degraded. This result demonstrated the entire process
of Nectin-4-MMAE-induced autophagic flux (Fig. 5A and B).
To improve understanding of how Nectin-4-MMAE
induces autophagy, the phosphorylation levels of the upstream
regulators of the classical Akt/mTOR autophagy pathway were further
examined through western blotting. It was identified that as the
quantity of Nectin-4-MMAE increased, phosphorylated (p)-Akt,
p-mTOR, p-p70S6k and p-4EBP1 protein levels dramatically decreased
(Fig. 5C-F). These results
revealed that the Akt/mTOR signaling pathway was inactivated with
increasing concentrations of Nectin-4-MMAE. Combining these results
showed that autophagy was induced by inactivating the classical
Akt/mTOR pathway, and Nectin-4-MMAE achieved the goal of
eliminating tumor cells through autophagy and apoptosis.
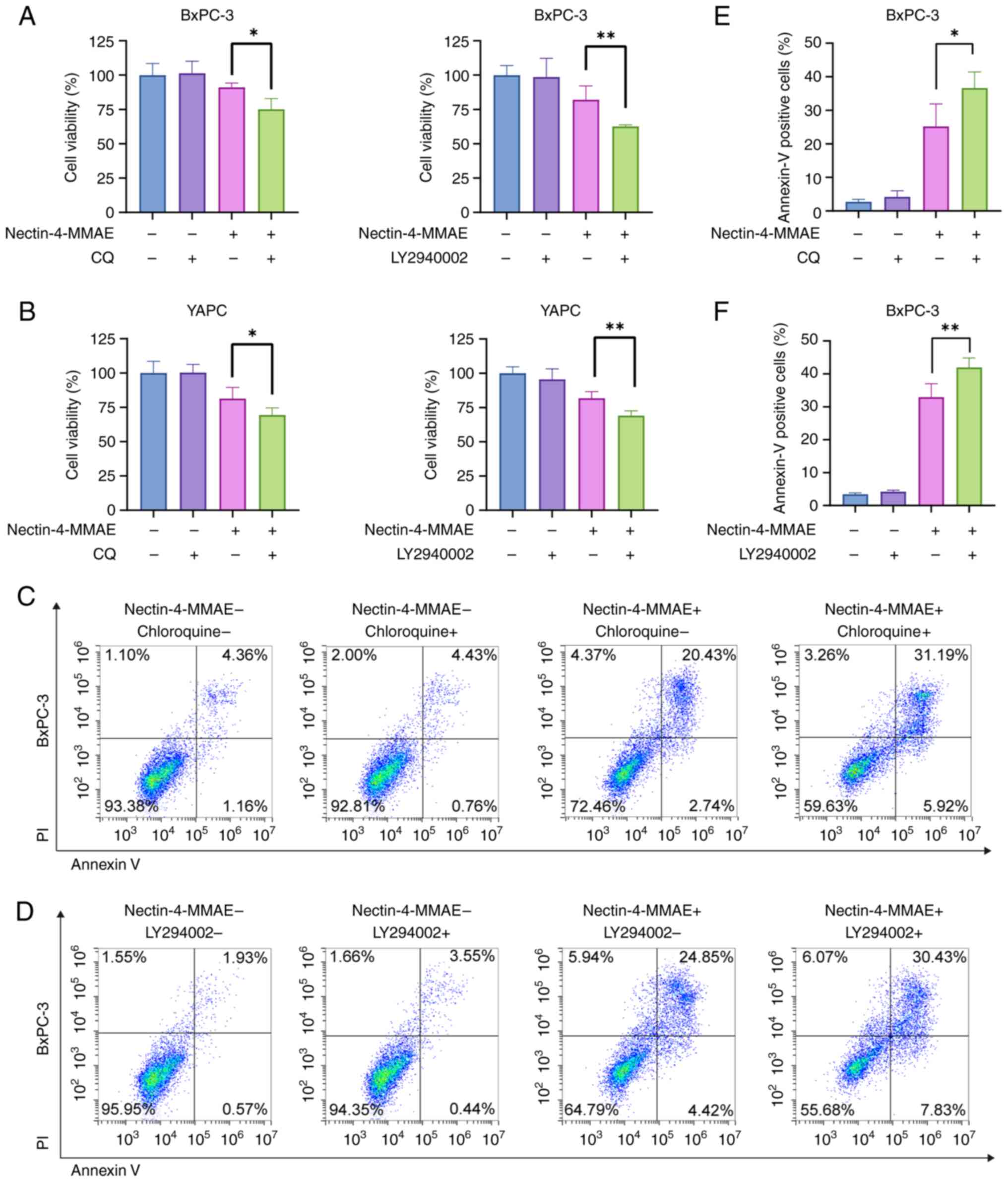
Autophagy inhibitors enhance apoptosis
induced by nectin-4-directed ADCs
Since autophagy demonstrated a crucial role in the
efficacy of Nectin-4-MMAE, it was investigated if autophagy
modulators could be combined to further improve efficacy. CQ (5
µM) and LY294002 (3 µM) autophagy inhibitors were
chosen for testing with Nectin-4-MMAE. Cell survival was
significantly lower in the combination treatment group than in the
monotreatment group (Fig. 6A and
B). In addition, flow cytometry results demonstrated a
significant increase in the proportion of Annexin-V-positive BxPC-3
cells in the combination treatment group but not in the
monotreatment group (Fig. 6C-F).
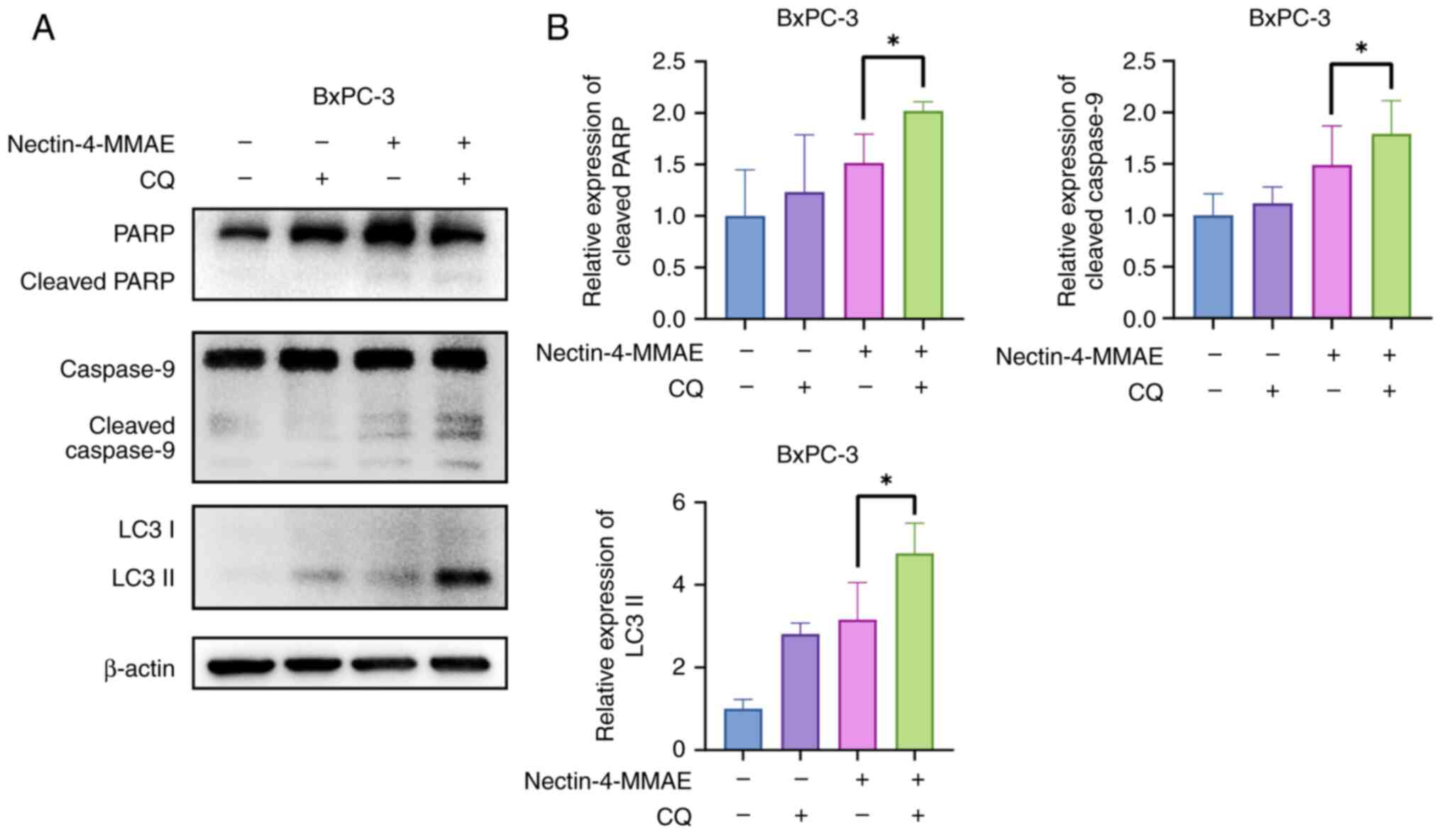
Caspase-9 and PARP were more activated in the combination treatment
group than in the monotreatment group, as evidence by cleaved
protein bands (Fig. 7A and B).
The results indicated that combining Nectin-4-directed ADCs with
autophagy inhibitors increased apoptosis and produced greater
cytotoxicity.
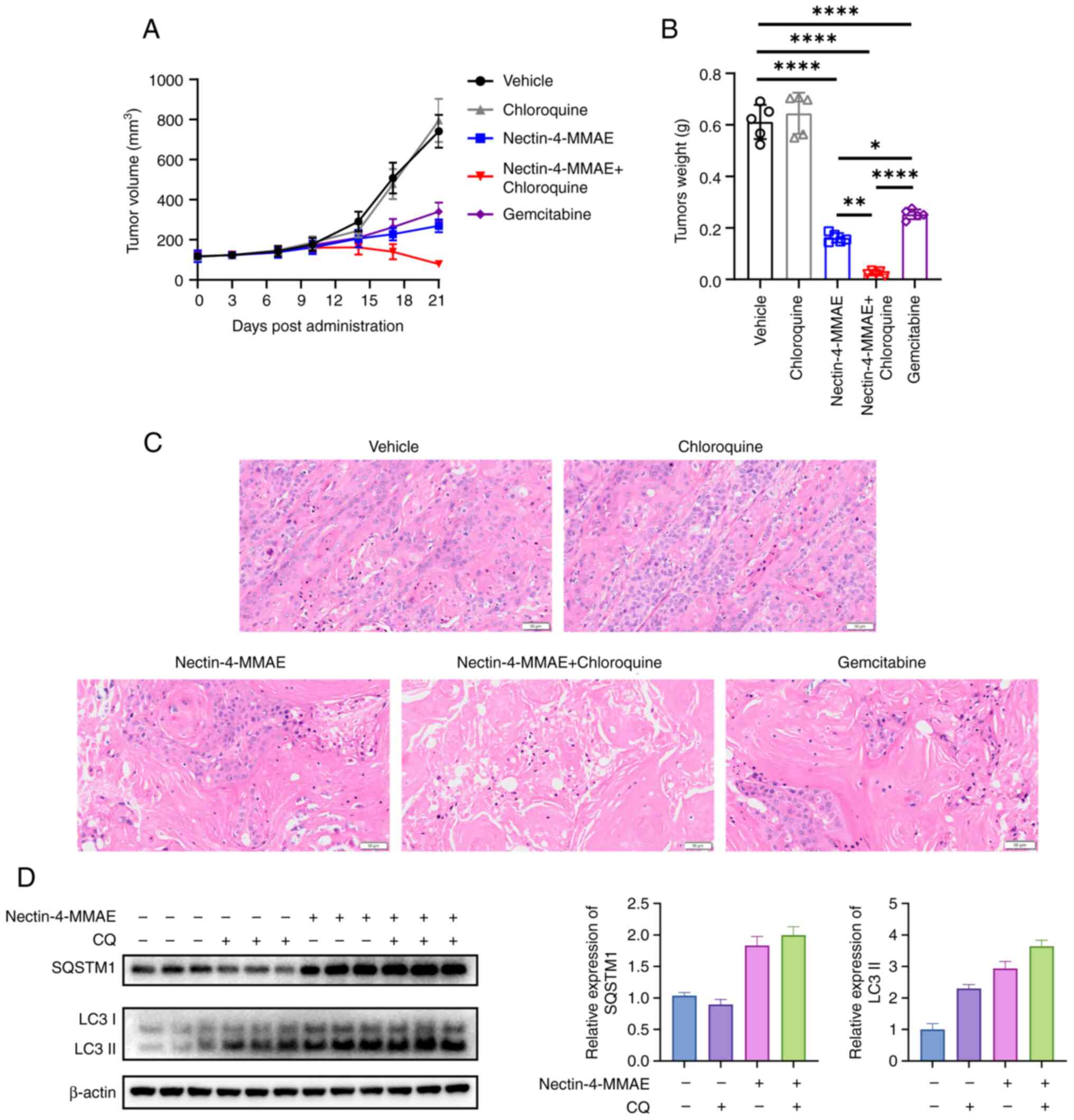
Enhancing the antitumor efficacy of
Nectin-4-directed ADC against pancreatic cancer in vivo by
inhibiting autophagy
To further confirm the in vitro results,
BxPC-3 xenograft tumor models were established to conduct in
vivo research. Mice were randomly assigned to the negative
control group, Nectin-4-MMAE group, CQ group, CQ and Nectin-4-MMAE
group, and positive control drug gemcitabine group. After 21 days
of drug administration, the maximum volume of the tumor was 904.74
mm3, maximum length was 13.84 mm and maximum width was
11.73 mm. The volume of tumors in the negative control group was
741.65±82.27 mm3, that in the gemcitabine group was
340.50±44.86 mm3, while the tumor volume in the
Nectin-4-MMAE group was 269.38±31.98 mm3, and that in
the group treated with the combination of Nectin-4-MMAE and CQ was
79.12±7.62 mm3. Mice treated with Nectin-4-MMAE showed
significantly reduced in tumor weights and volumes compared with
the negative control group. Mice treated with CQ and Nectin-4-MMAE
exhibited significantly reduced tumor weights and volumes compared
with the Nectin-4-MMAE alone group (Fig. 8A and B). Tumor tissue was
collected from mice in each group, and histological changes were
observed through H&E staining. The number of tumor cells was
notably reduced in the Nectin-4-MMAE treatment groups, and
particularly in the combined treatment group. The size of nucleoli
was reduced, nuclear divisions decreased, the density of tumor
cells was reduced, and the necrosis rate of the tumor increased
(Fig. 8C). Furthermore, the
expression levels of autophagy-related proteins in the tumor
tissues of mice were detected. CQ is an inhibitor of the late stage
of autophagy, which can inhibit the fusion of autophagosomes and
lysosomes. The degradation of LC3 II was inhibited, resulting in
its accumulation (Fig. 8D). The
results suggested that autophagy inhibitors promoted the treatment
efficacy of Nectin-4-MMAE in animal models, which was consistent
with the results of our cellular experiments.
Discussion
ADCs undergo endocytosis into target cells where
they release a cytotoxic drug that causes tumor cell death
(18). The cytotoxic drug MMAE
in Nectin-4-MMAE promotes tubulin polymerization to perturb
microtubule growth (19). The
specific protease caspases cause cell death by cleaving a variety
of protein substrates, which are also molecular markers of
apoptosis (20). The present
study found that Nectin-4-MMAE demonstrated significant
cytotoxicity and triggered caspase-dependent apoptosis in
Nectin-4-positive pancreatic cancer cells. ADC-related research is
progressing rapidly and has achieved improved therapeutic effects
in the treatment of some solid tumors. Autophagy is a double-edged
sword. Autophagy and apoptosis are closely related to each other,
as autophagy can promote cell death in concert with apoptosis or
antagonize apoptosis by promoting cell survival (21,22). The link between apoptosis and
autophagy is complex and diverse, and the signals that activate
autophagy usually derive from various stress conditions (23). The present study showed that
Nectin-4-MMAE induced the conversion of LC3-I to LC3-II and
activated autophagy in pancreatic cancer-positive cells. LC3-I is
the precursor of LC3-II. LC3-II is a docking site covalently
attached to cargo receptors on the membrane of phagosomes. The
receptor binds to the docking site, forming a core that selectively
recruits cargo. The phagosome then elongates and closes to form a
separate autophagosomal compartment (24). The proximal lysosome and
autophagosome then combine to produce an autophagic lysosome. In
the presence of lysosomal hydrolases, the cargo is degraded and
nutrients are recycled. This process was observed using electron
microscopy and confocal microscopy. Xu et al (25,26) first reported that
HuNbTROP2-HSA-MMAE significantly induced caspase-dependent
apoptosis in pancreatic cancer cells and activated cytoprotective
autophagy, which was consistent with the results of the present
study.
The AKT/mTOR pathway is crucial to tumor development
and has been implicated in the development of various malignant
tumors, including pancreatic cancer. In patients with pancreatic
cancer, 92% were found to have a KRAS mutation, which activates the
AKT/mTOR pathway, leading to alterations in cell cycle progression
and survival (27,28). mTOR consists of two multiprotein
complexes, mTORC1 and mTORC2. By modifying protein synthesis,
mTORC1 controls metabolism, whereas mTORC2 predominantly enhances
cell survival and regulates apoptosis. mTORC1 is over-activated in
a high proportion of human cancers and forms an
autophagy-regulating complex that affects autophagic vesicles. Once
activated, mTORC1 promotes cellular energy synthesis and metabolism
by phosphorylating the downstream effectors p70S6 kinase 1 and
eIF4E-binding protein (29).
mTORC1 mainly promotes energy synthesis, metabolism, autophagy
inhibition and lysosome formation. mTORC2 mainly promotes cell
survival and regulates apoptosis. After the BXPC-3 and YAPC cells
were treated with with Nectin-4-MMAE, significantly reduced levels
of p-Akt and mTOR were observed, along with the downstream proteins
4EBP1 and p70S6K. This demonstrated that Nectin-4-MMAE treatment
enhanced autophagy in pancreatic cancer cells, and tumors were
successfully suppressed.
Tumors disrupt overall homeostasis and biological
rhythms in the body and promote tumor growth, and increased
autophagy promotes cell survival in the face of environmental
stresses such as nutrient deprivation (30,31). There are two ways in which
malignant tumors affect body homeostasis: i) producing
neurohormonal modulators that act on nerve terminal receptors after
entering the circulatory system; and ii) activating circulating
immune cells to regulate the function of other organs (32). Autophagy may primarily play a
cytoprotective role, by maintaining energy homeostasis and nutrient
requirements under starvation conditions (25,33). CQ is a late-phase autophagy
inhibitor that impairs acid-dependent autophagy by increasing
intra-lysosomal pH. Nectin-4-MMAE-induced autophagy can be
inhibited by autophagy inhibitors such as CQ. When combined,
Nectin-4-MMAE and autophagy inhibitors significantly enhanced the
cytotoxic effects of Nectin-4-MMAE in the treatment of pancreatic
cancer. This finding demonstrated the protective role of autophagy
when treating pancreatic cancer with Nectin-4-MMAE.
Currently, the clinical application of ADCs in
cancer treatment still faces numerous challenges, including
off-target toxicity, drug resistance, protein aggregation and
immune response. If the antibody molecules in the ADCs have poor
selectivity and the target antigen exists in normal tissues,
cytotoxic molecules will be delivered into normal cells, thereby
causing toxic side effects. The drug resistance of human tumor
tissues to ADCs is mediated by multiple factors, including
alterations in the transport pathway of ADCs, downregulation of the
expression of target antigens, decreased lysosomal processing
capacity, overexpression of drug efflux transporters and changes in
apoptotic signaling pathways (34). Drug resistance has always been a
difficult problem in the treatment of tumors with ADCs. The
mechanisms, causes and solutions of drug resistance still urgently
need to be studied.
The present study showed that Nectin-4-MMAE exerted
potent therapeutic effects on Nectin-4 positive pancreatic cancer.
Notably, autophagy was activated and played a vital role in
Nectin-4-MMAE-mediated therapeutic effects. Nectin-4-MMAE's
therapeutic efficiency was significantly enhanced when combined
with autophagy inhibitors, indicating that autophagy inhibition may
be a novel approach to enhancing the ADCs' antitumor efficacy. In
the future, it is expected that the therapeutic effect on
pancreatic cancer will be further improved through an in-depth
exploration of the specific mechanism of action of Nectin-4 in
pancreatic cancer.
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
XZ and ZD conceived the study. ZD, JX and WZ
designed the experiments and proofread the manuscript. RF, CW and
TY performed the experiments and prepared the figures. YX and YS
analyzed the data. XZ and RF wrote the manuscript. XZ and ZD
revised the manuscript. ZD and RF confirm the authenticity of all
the raw data. All authors read and approved the final version of
the manuscript.
Ethics approval and consent to
participate
All animal experimental procedures adhered strictly
to the approved (approval no. 2025-01-SY-ZXY-02) guidelines and
protocols established by the Animal Ethical Committee of the School
of Pharmacy of Fudan University (Shanghai, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Acknowledgments
Not applicable.
Funding
No funding was received.
References
|
1
|
Bray F, Laversanne M, Sung H, Ferlay J,
Siegel RL, Soerjomataram I and Jemal A: Global cancer statistics
2022: GLOBOCAN estimates of incidence and mortality worldwide for
36 cancers in 185 countries. CA Cancer J Clin. 74:229–263. 2024.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Park W, Chawla A and O'Reilly EM:
Pancreatic Cancer: A Review. JAMA. 326:851–862. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Waters AM and Der CJ: KRAS: The critical
driver and therapeutic target for pancreatic cancer. Cold Spring
Harb Perspect Med. 8:a0314352018. View Article : Google Scholar :
|
|
4
|
Wittwer NL, Brown MP, Liapis V and
Staudacher AH: Antibody drug conjugates: Hitting the mark in
pancreatic cancer? J Exp Clin Cancer Res. 42:2802023. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Crescioli S, Kaplon H, Wang L,
Visweswaraiah J, Kapoor V and Reichert JM: Antibodies to watch in
2025. MAbs;17:24435382025.
|
|
6
|
Ungaro A, Tucci M, Audisio A, Di Prima L,
Pisano C, Turco F, Delcuratolo MD, Di Maio M, Scagliotti GV and
Buttigliero C: Antibody-drug conjugates in urothelial carcinoma: A
new therapeutic opportunity moves from bench to bedside. Cells.
11:8032022. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Khosravanian MJ, Mirzaei Y, Mer AH,
Keyhani-Khankahdani M, Abdinia FS, Misamogooe F, Amirkhani Z,
Bagheri N, Meyfour A, Jahandideh S, et al: Nectin-4-directed
antibody-drug conjugates (ADCs): Spotlight on preclinical and
clinical evidence. Life Sci. 352:1229102024. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Challita-Eid PM, Satpayev D, Yang P, An Z,
Morrison K, Shostak Y, Raitano A, Nadell R, Liu W, Lortie DR, et
al: Enfortumab vedotin antibody-drug conjugate targeting nectin-4
is a highly potent therapeutic agent in multiple preclinical cancer
models. Cancer Res. 76:3003–3013. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Nishiwada S, Sho M, Yasuda S, Shimada K,
Yamato I, Akahori T, Kinoshita S, Nagai M, Konishi N and Nakajima
Y: Nectin-4 expression contributes to tumor proliferation,
angiogenesis and patient prognosis in human pancreatic cancer. J
Exp Clin Cancer Res. 34:302015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kimmelman AC and White E: Autophagy and
tumor metabolism. Cell Metab. 25:1037–1043. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Amaravadi R, Kimmelman AC and White E:
Recent insights into the function of autophagy in cancer. Genes
Dev. 30:1913–1930. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
van der Vos KE, Eliasson P,
Proikas-Cezanne T, Vervoort SJ, van Boxtel R, Putker M, van Zutphen
IJ, Mauthe M, Zellmer S, Pals C, et al: Modulation of glutamine
metabolism by the PI(3) K-PKB-FOXO network regulates autophagy. Nat
Cell Biol. 14:829–837. 2012. View
Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lahiri V, Hawkins WD and Klionsky DJ:
Watch what you (self-) eat: Autophagic mechanisms that modulate
metabolism. Cell Metab. 29:803–826. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ktistakis NT and Tooze SA: Digesting the
expanding mechanisms of autophagy. Trends Cell Biol. 26:624–635.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Guo JY, Xia B and White E:
Autophagy-mediated tumor promotion. Cell. 155:1216–1219. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wang Y, Nan Y, Ma C, Lu X, Wang Q, Huang
X, Xue W, Fan J, Ju D, Ye D and Zhang X: A potential strategy for
bladder cancer treatment: Inhibiting autophagy to enhance antitumor
effects of Nectin-4-MMAE. Cell Death Dis. 15:2932024. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hu Q, Zhao H, Zhou K, Hua X and Zhang X:
Scarless circular mRNA-based CAR-T cell therapy elicits superior
anti-tumor efficacy. bioRxiv 2024.2008.2005.606578. 2024.
|
|
18
|
Fu Z, Li S, Han S, Shi C and Zhang Y:
Antibody drug conjugate: The 'biological missile' for targeted
cancer therapy. Signal Transduct Target Ther. 7:932022. View Article : Google Scholar
|
|
19
|
Adair JR, Howard PW, Hartley JA, Williams
DG and Chester KA: Antibody-drug conjugates-A perfect synergy.
Expert Opin Biol Ther. 12:1191–1206. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wang X: The expanding role of mitochondria
in apoptosis. Genes Dev. 15:2922–2933. 2001.PubMed/NCBI
|
|
21
|
Zang YD, Wu HJ, Chen XY, Ma ZL, Li CJ, Ma
J, Chen XG, Sheng L, Zhang S and Zhang DM: Synthesis and biological
evaluation of novel Psidium meroterpenoid derivatives against
cisplatin-induced acute kidney injury. J Med Chem. 67:14234–14255.
2024. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Qu L, Liu Y, Deng J, Ma X and Fan D:
Ginsenoside Rk3 is a novel PI3K/AKT-targeting therapeutics agent
that regulates autophagy and apoptosis in hepatocellular carcinoma.
J Pharm Anal. 13:463–482. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Li Z, Mao L, Yu B, Liu H, Zhang Q, Bian Z,
Zhang X, Liao W and Sun S: GB7 acetate, a galbulimima alkaloid from
Galbulimima belgraveana, possesses anticancer effects in colorectal
cancer cells. J Pharm Anal. 12:339–349. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Gao W, Wang X, Zhou Y, Wang X and Yu Y:
Autophagy, ferroptosis, pyroptosis, and necroptosis in tumor
immunotherapy. Signal Transduct Target Ther. 7:1962022. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Xu C, Zhu M, Wang Q, Cui J, Huang Y, Huang
X, Huang J, Gai J, Li G, Qiao P, et al: TROP2-directed
nanobody-drug conjugate elicited potent antitumor effect in
pancreatic cancer. J Nanobiotechnology. 21:4102023. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Xu C, Huang X, Hu Q, Xue W, Zhou K, Li X,
Nan Y, Ju D, Wang Z and Zhang X: Modulating autophagy to boost the
antitumor efficacy of TROP2-directed antibody-drug conjugate in
pancreatic cancer. Biomed Pharmacother. 180:1175502024. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Witkiewicz AK, McMillan EA, Balaji U, Baek
G, Lin WC, Mansour J, Mollaee M, Wagner KU, Koduru P, Yopp A, et
al: Whole-exome sequencing of pancreatic cancer defines genetic
diversity and therapeutic targets. Nat Commun. 6:67442015.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Fang P, You M, Cao Y, Feng Q, Shi L, Wang
J, Sun X, Yu D, Zhou W, Yin L, et al: Development and validation of
bioanalytical assays for the quantification of 9MW2821, a
nectin-4-targeting antibody-drug conjugate. J Pharm Biomed Anal.
248:1163182024. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Xue W, Xu C, Zhang K, Cui L, Huang X, Nan
Y, Ju D, Chang X and Zhang X: Enhancing antitumor efficacy of
CLDN18.2-directed antibody-drug conjugates through autophagy
inhibition in gastric cancer. Cell Death Discov. 10:3932024.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Slominski RM, Raman C, Chen JY and
Slominski AT: How cancer hijacks the body's homeostasis through the
neuroendocrine system. Trends Neurosci. 46:263–275. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Chen X, Zeh HJ, Kang R, Kroemer G and Tang
D: Cell death in pancreatic cancer: From pathogenesis to therapy.
Nat Rev Gastroenterol Hepatol. 18:804–823. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Mottini C, Auciello FR, Manni I, Pilarsky
C, Caputo D, Caracciolo G, Rossetta A, Di Gennaro E, Budillon A,
Blandino G, et al: The cross-talk between the macro and
micro-environment in precursor lesions of pancreatic cancer leads
to new and promising circulating biomarkers. J Exp Clin Cancer Res.
43:1982024. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Dikic I and Elazar Z: Mechanism and
medical implications of mammalian autophagy. Nat Rev Mol Cell Biol.
19:349–364. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
García-Alonso S, Ocaña A and Pandiella A:
Resistance to antibody-drug conjugates. Cancer Res. 78:2159–2165.
2018. View Article : Google Scholar : PubMed/NCBI
|