Introduction
The Hh-Gli signaling pathway plays a crucial role in
embryogenesis, but it is mostly inactive in adult tissues under
normal conditions. The signaling transduction is initialized by the
ligand Hedgehog with three different isoforms in humans: the Sonic
Hedgehog (Shh), the Indian Hedgehog (Ihh) and the Desert Hedgehog
(Dhh), which bind to the transmembrane receptor Patched (Ptch),
inducing their internalization and translocation of Smoothened
(Smo) to the membrane. A cytoplasmatic cascade of phosphorylation
events leads to activation of the transcription factor Gli, which
translocates to the nucleus and initializes target gene
transcription (1). The pathway
activity is associated with the formation of various tissues and
organs: neural tube, limbs, gastrointestinal system, lung, hair
follicles, cartilage and bone (2),
pancreas (3) and ganglia (4). In the adult organism it is associated
with somatic stem cells, for example, breast (5), neural (6), lung (7), skin (8) and erythropoietic cells (9). When deregulated, it can lead to
various tumors, for example, basocellular carcinoma, melanoma,
trychoepithelioma, rhabdomyosarcoma, digestive tract tumors,
prostate tumors, small-cell lung cancer, squamous lung cancer,
pancreatic cancer, breast cancer and ovarian cancer (10).
The role of Hh-Gli signaling in ovarian development
and malignancy has been explored by several groups. It has been
shown that the pathway is involved in embryonic development and
maturation of Drosophila ovaries, while in the adult fly it
is involved in the division of stem cells giving rise to follicles
(11). In mammals, the Hh-Gli
pathway is also involved in embryonic development of the ovary, and
in the adult organism components of the pathway are expressed in
reproductive tissues (12). In the
adult ovary, the Hh-Gli signaling is active in the granulose cells
surrounding the follicle, and it can be additionally activated by
exogenous Shh protein (13).
Ovarian carcinomas express Hh-Gli pathway genes, and
in ovarian cancer cell lines cyclopamine, a known Hh-Gli pathway
inhibitor, causes G1 cell cycle arrest (14). Also, ovarian carcinomas frequently
show loss of one allele of the PTCH1 gene (15), and ovarian teratomas show increased
methylation of the PTCH1 promoter (16).
Ovarian dermoids are mature teratomas which often
contain various differentiated tissues, for example glandular
tissue, multilayered epithelium, hair follicles, bone, teeth and
cartilage. Their development is attributed to aberrant meiosis of
germinal cells within the ovary. Depending on the phase of meiosis
during which the errors occur, the teratomas are roughly divided
into 5 subtypes (17). Reports on
the malign transformation of teratomas into carcinomas mostly
describe squamous cell carcinoma, melanoma, adenocarcinoma,
neuroectodermal tumors, glioblastoma and sarcoma (18). Such events are rare; they occur in
postmenopausal women in up to 2% of all teratomas, and most
frequently progress into squamous cell carcinoma (19).
Our lab has been studying the mechanisms by which
the Ptch protein takes part in the Hh-Gli signal transduction
pathway and how aberrations in the pathway can lead to malignancy.
Our previous findings showed the role of the PTCH1 gene in
ovarian dermoids (20) and its
association with Cyclin D (21),
indicating the role of Hh-Gli signaling in the pathogenesis of this
heterogenic neoplasia.
Materials and methods
Immunohistochemical and immunofluorescent
staining
Paraffin tissue slides were routinely prepared in
the Department of Obstetrics and Gynecology, School of Medicine,
Zagreb. For immunohistochemical staining, tissues were
deparaffinized, endogenous peroxidase was blocked, the slides were
heated in epitope-retrieval solution for 5 min at 85°C to enable
correct epitope folding, and stained using the following antibodies
diluted 1:100: goat polyclonal anti-Ptch (Santa Cruz Biotechnology
Inc., Santa Cruz, CA, USA; sc-6147) (22), rabbit polyclonal anti-Smo (Santa
Cruz; sc-19943) (23), rabbit
polyclonal anti-Gli1 (Santa Cruz; sc-20687) (24) and rabbit polyclonal anti-Hh (Santa
Cruz; sc-9024) (13). Secondary
antibodies were bovine anti-goat-HRP and bovine anti-rabbit-HRP,
used at 1:100 dilution. For negative control, slides were treated
in the same way omitting the primary antibody step. For positive
control, parallel staining was performed on squamous cell carcinoma
slides which were positive for Ptch, Smo, Gli1 and Shh in our
previous study (25).
For immunofluorescence, cells were seeded on glass
coverslips and treated with 7.5 μM cyclopamine or 3
ng/μl Shh protein. After 24 h, cells were fixed using 3.5%
paraformaldehyde solution, permeabilized with methanol, heated in
epitope-retrieval solution for 5 min at 85°C to enable correct
epitope folding, and stained with the same primary antibodies used
for immunohistochemistry, using the same dilutions. Secondary
antibodies were conjugated with either Texas Red (TR) or
Fluorescein (FITC), used at 1:100 dilution: donkey anti-goat-TR
(Santa Cruz; sc-2783), mouse anti-goat-FITC (Santa Cruz; sc-2356),
donkey anti-rabbit-TR (Santa Cruz, sc-2784), donkey anti-mouse-FITC
(Santa Cruz; sc-2099) and nuclei were stained with DAPI. For
negative control, slides were treated in the same way omitting the
primary antibody step.
Mutation detection
The PTCH1 gene was divided into 24 PCR
products and analyzed using high-resolution melting analysis
(26), followed by sequencing in
both directions (27–29). For the SMO gene, only exons
9 and 10 were analyzed in the same manner (30).
Cell culture experiments
Dermoid tissue collected during surgery was stored
for a maximum of 2–3 h in serum-free MEM culture medium at 4°C. For
primary culture, tissue was washed briefly in trypsin, and then cut
into small fragments and digested in 5 ml trypsin overnight at 37°C
and 5% CO2. The following day the cells were resuspended
and plated in several 10 cm petri dishes in MEM supplemented with
20% human serum (HS). Dishes were regularly checked for cell
colonies, which usually appeared after a week, approximately.
Individual colonies were collected and grown separately, as
separate clone lines.
Ten established primary clone lines and two ovarian
cancer cell lines (COLO-704 and SkOv-3, a kind gift from Dr K.
Rajalingam) were grown in MEM supplemented with 10% HS or 0.5% HS.
Cells were treated with 7.5 μM cyclopamine (Toronto Research
Chemicals), 7.5 μM tomatidine (Sigma-Aldrich, St. Louis, MO,
USA), or 3 ng/μl Shh protein (a kind gift from Dr A. Kenney)
for 24, 48 or 72 h. RNA was extracted using TRIzol reagent
(Invitrogen, Carlsbad, CA, USA) and proteins were extracted by
standard methods. MTT assay was performed as per the manufacturer’s
instructions. For western blot analysis, the primary antibodies for
Ptch, Smo, Gli1 and Hh were the same ones that were used for
immunofluorescent staining, as well as goat anti-Actin (Santa Cruz;
sc-1616). Secondary antibodies were bovine anti-goat-HRP and bovine
anti-rabbit-HRP, and the signal was detected using SuperSignal West
Pico (Pierce Biotechnology Inc., Rockford, IL, USA; #34077).
Quantitative real-time PCR (qRT-PCR)
One microgram of total RNA was reverse transcribed
using TaqMan Reverse Transcription Reagents (Applied Biosystems).
Quantitative real-time PCR was performed with the following
primers: ARP F, 5′-GGCACCATTGAAATCCTGAGTGATGTG-3′ and
ARP R, 5′-TTGCGGACACCCTCCAGGAAGC-3′; PTCH F,
5′-TCCTCGTGTGCGCTGTCTTCCTTC-3′ and PTCH R,
5′-CGTCAGAAAGGCCAAAGCAACGTGA-3′; SMO F,
5′-CTGGTACGAGGACGTGGAGG-3′ and SMO R, 5′-AGG
GTGAAGAGCGTGCAGAG-3′; GLI1 F, 5′-GCCGTGT
AAAGCTCCAGTGAACACA-3′ and GLI1 R, 5′-TCCCACT
TTGAGAGGCCCATAGCAAG-3′; SHH F, 5′-GAAAGC AGAGAACTCGGTGG-3′
and SHH R, 5′-GGTAAGT GAGGAAGTCGCTG-3′ (25); SUFU F, 5′-AACAGCAA
ACCTGTCCTTCC-3′ and SUFU R, 5′-TCAGATGTACG CTCTCAAGC-3′
(31); β-catenin F,
5′-GCGTGGACAA TGGCTACTCA-3′ and β-catenin R, 5′-CCGCTTTTCT
GTCTGGTTCC-3′ (32). The reaction
was performed in a 10 μl reaction using IQ SYBR-Green
supermix (Bio-Rad), under the following conditions: initial
denaturation at 95°C for 3 min, and 40 cycles of 95°C for 15 sec
and 61°C for 1 min. Melting curve of each product was examined
after each real-time PCR experiment to verify their specificity,
and expression was normalized using the ARP housekeeping
gene. Relative gene expression was calculated using the
2−ΔΔCt formula, with RNA from the normal
ovary used as the reference. Normal ovary sample was a pool of 9
RNA samples from healthy ovaries, taken during surgery at the
Department of Obstetrics and Gynecology from patients operated for
reasons other than malignant transformation. All patients gave
their informed consent before the samples were obtained, and the
samples were collected with the approval of the hospital’s Ethics
Committee (No 01-600/34-1-2007) in accordance with the Health Care
Laws of the Republic of Croatia (NN121/03).
Confocal microscopy
Laser scanning confocal microscopy was performed on
the Leica TCS SP2 AOBS instrument. FITC emission was excited with
the 488 nm laser and detected in the 499–576 nm range, whereas TR
emission was excited with the 543 nm laser and detected in the
605–712 nm range. DAPI emission was excited with the 405 nm laser
and detected in the 413–466 nm range. In order to prevent
bleed-through of the FITC emission into the TR channel, images in
the two channels were recorded sequentially. Cross-excitation of
the FITC fluorophore by the green 543 nm laser was eliminated by
adjusting the imaging conditions.
Results
Hh-Gli signaling pathway proteins can be
detected in ovarian dermoids
Ovarian dermoids show a high level of
differentiation, with many structures typical for skin (epithelium,
mucous glands, serous glands), and stromal and cortical regions of
ovaries. Immunohistochemical staining of dermoid tissue sections
revealed the presence of the main components of the Hh-Gli
signaling pathway (Fig. 1). Ptch
intensively stains the epithelium and many types of glandular
tissue present in the sections, but not the connective tissue. It
is mostly localized to the cell membrane, but in many cells it is
also localized close to the nucleus (Fig. 1, insets). Smo is typically found in
the same regions as Ptch. Staining of Gli1 is weak and mostly
limited to the basal layer of the epithelium, but also within the
oocyte in the follicles. Hh protein shows mostly epithelial
staining, with the strongest intensity in the basal layer, but it
is also localized to oocytes in the follicles and to zona pellucida
in the secondary follicles.
No PTCH1 or SMO mutations are detected in
ovarian dermoids
To test whether the pathway activation is caused by
inactivating mutations in the PTCH1 gene or activating
mutations in the SMO gene, the entire coding region of
PTCH1 including its promoter, and exons 9 and 10 of
SMO were examined for mutations. No mutations were found in
the SMO hot-spot region (exons 9 and 10). In the
PTCH1 gene only three known polymorphisms were detected and
all three were in heterozygous form. Polymorphisms were detected in
exon 2 (c.318C>T), exon 12 (c.1665T>C) and intron 15
(c.2560IVS+9G>C). The two exonic polymorphisms cause no change
in the amino acid sequence, and all three polymorphisms are well
documented (Patched Mutation Database www.cybergene.se/PTCH/).
Primary cultures derived from ovarian
dermoids have a limited lifespan
Ten distinct clone lines were established from two
dermoids, 5 lines from each sample. These cultures were established
and maintained in MEM supplemented with 10% human serum (HS) to
match the conditions in vivo as closely as possible.
Developed primary lines are vimentin-positive, most likely
mesenchymal in origin, with fibroblast morphology. Primary cultures
had a limited lifespan and started to show morphology typical of
‘old’ cells at approximately the 10th passage (elongated shape,
vacuolization, slowed division), and usually died out by the 15th
passage. All experiments were performed at a passage number
<10.
Hh-Gli signaling pathway expression in
ovarian dermoid primary cultures
mRNA expression was detected for PTCH1,
SMO, GLI1, SUFU, and β-catenin in all
ten clone lines. SHH expression was not detectable after 40
cycles of quantitative real-time PCR in 5/10 clone lines. Since the
healthy ovarian tissue shows no expression of Hh-Gli pathway
proteins when examined by immunohistochemistry (14), we examined the gene expression
levels in the healthy ovary and compared them to gene expression
levels in dermoid cultures. PTCH1 and GLI1
expression, which are considered major markers of pathway activity,
were upregulated in the dermoids compared to the normal ovary.
PTCH1 levels were 40–100-fold higher in the dermoid than in
the normal ovary, while GLI1 was not detectable in the
normal ovary, but was regularly detected in the dermoids with
variations between the clone lines. β-catenin, which is
regulated by the Hh-Gli signaling (33), was 25–40-fold more strongly
expressed in the dermoid lines compared to the normal ovary
(Fig. 2). Similar expression
levels of the tested genes were also detected in the ovarian
carcinoma cell lines SkOv-3 and COLO-704, which are also shown in
Fig. 2.
Hh-Gli pathway proteins were also detected by
western blot analysis. The Ptch protein was regularly detected in
all clone lines. Smo and Gli1 expression was weaker than that of
Ptch.
The Hh antibody we used shows reactivity to all
three Hh proteins (Shh, Dhh, Ihh). In our experiments we detected
one or two bands coresponding to approximately 50 kDa. Ovarian
carcinoma cell lines showed a similar expression pattern of Hh-Gli
pathway proteins. Cells grown in 0.5% HS, mimicking starvation,
showed a far weaker signal for all pathway proteins, and Gli1 and
Smo were often undetectable under these conditions (data not
shown). Treatment with cyclopamine, tomatidine, or Shh protein did
not change protein expression levels in any clone or cell line
(Fig. 3).
Primary cultures show reactivity to
cyclopamine and Shh protein
Since all clone lines derived from two tumors showed
expression of PTCH1, SMO, GLI1 and
SUFU, one clone line from each tumor was used to determine
the effect of the Smo inhibitor cyclopamine and responsiveness to
Shh ligand. Cyclopamine-treated cells showed a significant delay in
proliferation as determined by MTT proliferation assay (Fig. 4). On the other hand, treatment with
the Shh protein caused an increase in cell proliferation compared
to non-treated cells. The ovarian carcinoma cell line shows
responsiveness to cyclopamine inhibition, but is not stimulated by
the exogenous addition of the ligand. To test whether cyclopamine
treatment affects cell cycle, as suggested by Chen et al
(14), we tested cell cycle
distribution after treatment with cyclopamine or Shh protein.
Neither treatment affects the cell cycle distribution of these
cells, suggesting a different mechanism of action from cell cycle
control (data not shown).
Hh-Gli pathway proteins show altered
localization after cyclopamine or Shh treatment
Cyclopamine and Shh treatment modulate localization
of Ptch and Gli1 proteins suggesting this as the major regulatory
step in Hh-Gli signaling in the dermoids. Their localization was
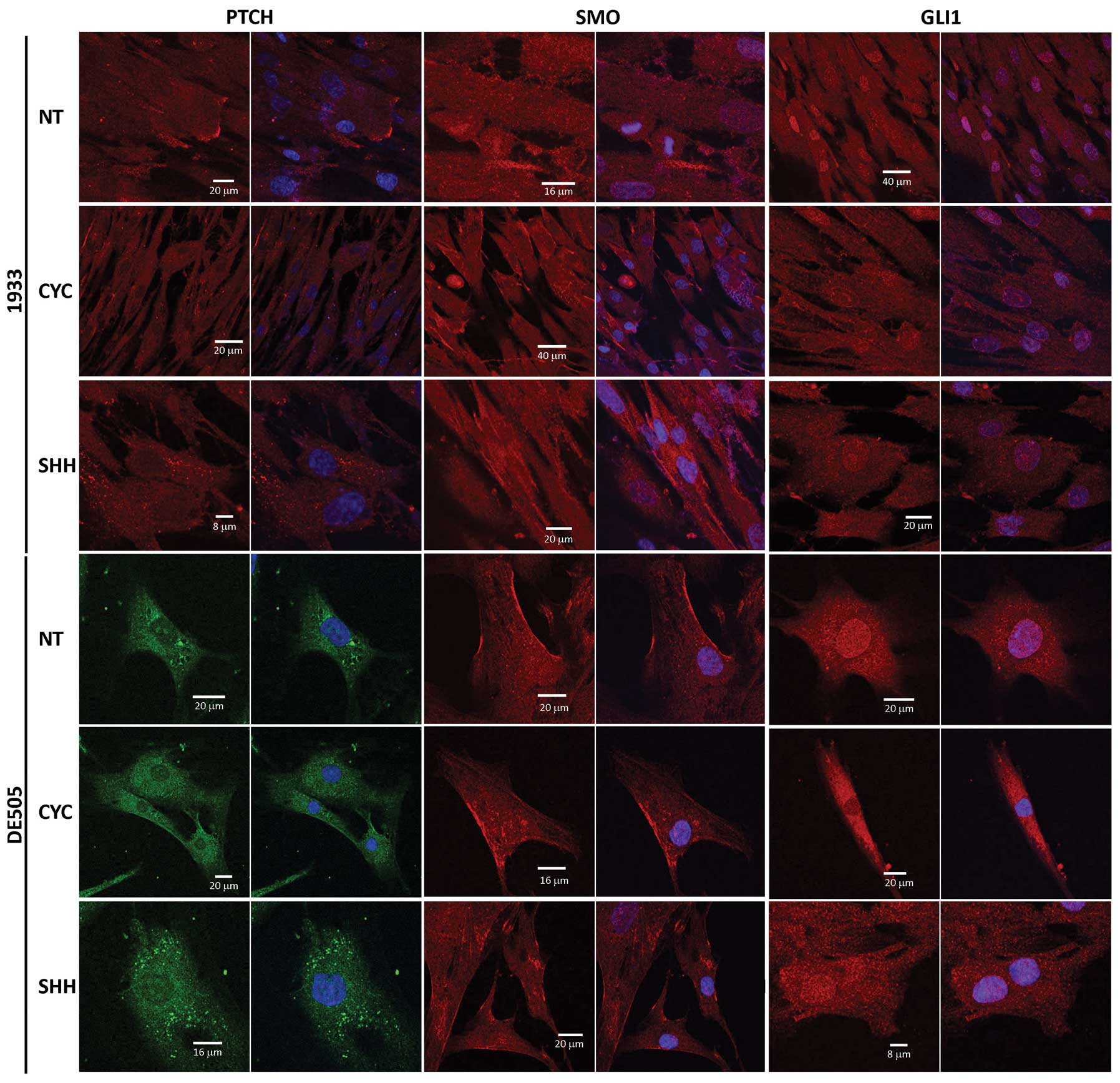
altered in all tested clone lines in the same way. Fig. 5 shows the effect of cyclopamine
(CYC) or Shh ligand (SHH) treatment on Ptch, Smo and Gli1 proteins
compared to non-treated (NT) cells.
The Ptch protein was regularly detected diffused
through the cytoplasm and in patches at the cell membrane (Fig. 5, columns 1 and 2). After
cyclopamine treatment the staining was more diffuse, while Shh
treatment induced accumulation of dot-like structures in the
cytoplasm. Smo protein was detected in the cytoplasm with a
stronger staining of the cell membrane (Fig. 5, columns 3 and 4), and remained
unchanged after either treatment. In non-treated cells the Gli1
protein was enriched in the nucleus compared to the cytoplasmic
background. After treatment with cyclopamine, staining of the
nucleus was decreased. Shh treatment had no significant effect on
Gli1, since it was already localized in the nucleus and therefore
active.
Treatment with the Shh protein had a remarkable
effect on Ptch protein localization. After 24 h small bright dots
of Ptch protein became visible in the cytoplasm, and after 48 h the
dots increased in size and intensity. These cytoplasmatic Ptch
aggregates were occasionally detected in untreated cells with
lesser intensity (Fig. 6, NT),
suggesting some baseline activity of the pathway, and were reduced
in cyclopamine-treated cells (Fig.
6, CYC). When cells were stained with Ptch and Hh antibodies,
it was clear that the aggregates contained both Ptch and Hh
protein, suggesting their joint internalization after binding
(Fig. 6, white arrows). A basal
level of pathway activity is visible in non-treated cells, is
significantly increased in Shh-treated cells and is not detectable
in cyclopamine-treated cells.
Previous studies suggest that Hh-Gli signaling in
mammals requires the primary cilia to effectively transduce the
signal (reviewed in ref. 34).
Therefore cells were stained for acetylated tubulin to check for
primary cilia formation and possible colocalization with the
pathway proteins. Despite our efforts, we were unable to find a
large number of ciliated cells regardless of growth conditions, but
occasionally cilia-like structures were detected (data not
shown).
Discussion
Involvement of Hh-Gli signaling in ovarian
carcinogenesis has been demonstrated (14,15),
but its detailed role in germ cell tumorigenesis has yet to be
thoroughly examined. We used ovarian dermoids as they show
characteristics of tumors and developmental malformations, and
Hh-Gli signaling is involved in both processes. The structures
which usually develop within dermoids belong to the tissue types
that are controlled by Hh-Gli signaling in embryonic development,
for example glandular tissue, hair follicles, bone, teeth and
cartilage. Ovarian dermoids are classified as teratomas, a type of
benign ovarian tumors, which contain differentiated elements
derived from any of the three embryonic germ layers. The term
‘teratoma’ denotes the strange manifestations of benign or
malignant tumors that possess the hallmarks of abnormal
embryogenesis (35).
Our results show different levels of gene expression
of Hh-Gli pathway genes in different clones from the same tumor
tissue sample, confirming the known observation of heterogeneity of
dermoid tissues. Developed primary lines are vimentin-positive,
most likely mesenchymal in origin, with fibroblast morphology.
However, it is unclear whether this vimentin-positive,
cytokeratine-negative phenotype is the marker of a mesenchymal
cell, or a consequence of the Hh-Gli pathway activation, which can
induce epithelial-mesenchymal transition (36,37).
Also, ovarian cysts are one of the features of the Gorlin syndrome,
which is caused by germline mutations in the PTCH1 gene.
However, we did not detect any mutations in PTCH1 or
SMO genes in the dermoid samples; only polymorphisms in
exons 2 and 12 with no effect on amino acid sequence.
Results presented in this study demonstrate that the
Hh-Gli signaling pathway is activated in the dermoid tissue, but
not in the surrounding ovarian tissue. Major members of the
pathway, namely Ptch, Smo, Gli1 and Hh, can be detected in the
dermoid tissue, although Gli1 and Hh at lower levels than Ptch and
Smo. This is not surprising since Gli1, as the transcription
factor, plays a limiting role in controlling pathway activity.
Hh-Gli signaling pathway genes PTCH1, SMO,
GLI1 and SUFU are detected in all dermoid clone
lines, with intensities similar to ovarian carcinoma cell lines.
When dermoid primary cultures are compared to the normal ovary,
PTCH1 and GLI1, major markers of pathway activity,
are strongly upregulated, as well as β-catenin, one of the
Hh-Gli signaling targets. Gli2 and Gli3 do not appear to be
relevant in Hh-Gli pathway control in ovarian dermoids, since their
gene and protein expression was extremely weak and often
undetectable. SHH gene expression was detected in only five
of the ten clone lines. When compared with western blot analysis,
one to two Hh proteins are detected in ovarian dermoid primary
cultures. Studies on mice have demonstrated that Dhh and Ihh are
expressed in granulosa cells, pre-antral and antral follicles, and
induce Hh-Gli signaling in the surrounding stroma (38). Therefore, the protein signal
detected in our cells is more likely to be one of these two
proteins.
Treatment with cyclopamine, a known inhibitor of the
Hh-Gli pathway which binds to Smo and prevents downstream
signaling, reduces cell proliferation in primary cultures and
ovarian carcinoma cell line. On the other hand, Shh protein
increases cell proliferation, and this effect has been previously
demonstrated on mammalian neuronal precursors (39).
Protein localization demonstrated by confocal
microscopy also corroborates previously published data: Gli1 is
located in the cytoplasm and in the nucleus, whereas Ptch and Smo
are located in the cytoplasm and on the cell membrane (10). Gli1 changes localization after
cyclopamine treatment, showing a reduction of the signal in the
nuclear interior.
It is well known that many membrane receptors are
internalized and then degraded or recycled to the cell membrane
(40). According to recent models,
Ptch is also internalized upon Shh binding, allowing Smo to
translocate to the cell membrane (41,42).
Indeed, our results provide evidence for this internalization
process. Addition of Shh protein induces the signal transduction,
which leads to increased Ptch internalization, and colocalization
of Ptch and Shh proteins within these granules in the cytoplasm
(Fig. 5).
It has been demonstrated that the primary cilia are
required for Hh-Gli pathway regulation in mammalian development,
where they act as a switch for turning the signaling on and off
(34). However, the cilia are not
required for the Drosophila Hh signaling, and the signaling
events involving SuFu and Gli proteins are independent of cilia
(43). Here, we detected
structures that stain for acetylated tubulin, and resemble the
primary cilia. However there was no enrichment of the Smo protein
inside the cilia. Since the primary cilia are usually found on the
surface of most growth-arrested and differentiated cells, it is not
surprising that they were not detected more often in primary
dermoid cells, since these were quite proliferative in culture. The
role of primary cilia in tumorigenesis remains unclear, and
published reports state that either the presence or the absence of
cilia can enhance tumor growth, depending on the molecular context
(44,45). It has been reported that Hh-Gli
pathway responsiveness correlates with the presence of the primary
cilia in prostate development (46). However, when prostate cancer cell
lines were examined under different growth conditions, there was no
evidence of cilia formation (46).
The cilia were also normally detected in the ovary, more
specifically in the granulosa cells of the pre-antral and antral
follicles (47). When cultured,
these cells exhibit cilia only after serum starvation and growth
arrest, while normal interphase cells show a complex cytoskeletal
network but no primary cilia (47). This, as well as our data, suggests
that the pathway may be regulated differently in normal development
compared to cancer, and further investigation of these differences
may lead to potential Hh-Gli pathway-targeting drugs.
Teglund and Toftgard (1) proposed that Hedgehog-associated
tumors may have four different mechanisms of activation: the
ligand-independent, the ligand-dependent autocrine, the
ligand-dependent paracrine, and the ligand-dependent reverse
paracrine mechanism. The dermoids express Hh ligand, and show
increased proliferation and changes in protein localization
consistent with pathway activity. A basal level of pathway activity
can be seen in all the clone lines and this is greatly increased by
addition of the Shh ligand. Based on our observations, the
mechanism of Hedgehog activation in the ovarian dermoids could be
the ligand-dependent autocrine pathway, which can also be
stimulated by paracrine signals. The basal level of autocrine
activity may keep the cells in a ‘ready’ state, prepared to react
to outside stimulus, possibly by a signal from granulosa cells or
developing follicles which are known to produce the Hh ligands.
Since dermoids are considered to develop from aberrantly activated
germinal cells within the ovary, their microenvironment which
produces Hh ligands provides a proliferation signal and enables
dermoid formation.
Acknowledgements
This study was funded by the Croatian
Ministry of Science, Education and Sports, grant no.
098-0982464-2461. The authors wish to thank all the patients who
participated in the study. We would also like to thank Dr A. Kenney
for providing the Shh protein, Dr K. Rajalingam for the SkOv-3 and
COLO-704 cells, Professor Dr B. Sarcevic for vimentin and
cytokeratin characterization of clone lines, and L. Horvat, BSc,
for her assistance with the confocal microscopy.
References
|
1
|
Teglund S and Toftgård R: Hedgehog beyond
medulloblastoma and basal cell carcinoma. Biochim Biophys Acta.
1805:181–208. 2010.PubMed/NCBI
|
|
2
|
Jia J and Jiang J: Decoding the Hedgehog
signal in animal development. Cell Mol Life Sci. 63:1249–1265.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hebrok M: Hedgehog signaling in pancreas
development. Mechanisms Dev. 120:45–57. 2003. View Article : Google Scholar
|
|
4
|
Masai I, Yamaguchi M, Tonou-Fujimori N,
Komori A and Okamoto H: The hedgehog-PKA pathway regulates two
distinct steps of the differentiation of retinal ganglion cells:
the cell-cycle exit of retinoblasts and their neuronal maturation.
Development. 132:1539–1553. 2005. View Article : Google Scholar
|
|
5
|
Kalirai H and Clarke RB: Human breast
epithelial stem cells and their regulation. J Pathol. 208:7–16.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Stecca B and Ruiz i Altaba A: Brain as a
paradigm of organ growth: Hedgehog-Gli signaling in neural stem
cells and brain tumors. J Neurobiol. 64:476–490. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Watkins DN, Berman DM, Burkholder SG, Wang
B, Beachy PA and Baylin SB: Hedgehog signaling within airway
epithelial progenitors and in small-cell lung cancer. Nature.
422:313–317. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhou YX, Jia LW, Liu WM, Miao CL, Liu S,
Cao YJ and Duan EK: Role of sonic hedgehog in maintaining a pool of
proliferating stem cells in the human fetal epidermis. Hum Reprod.
21:1698–1704. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Detmer K, Thompson AJ, Garner RE, Walker
AN, Gaffield W and Dannawi H: Hedgehog signaling and cell cycle
control in differentiating erythroid progenitors. Blood Cells Mol
Dis. 34:60–70. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Scales SJ and de Sauvage FJ: Mechanisms of
Hedgehog pathway activation in cancer and implications for therapy.
Trends Pharmacol Sci. 30:302–312. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhang Y and Kalderon D: Hedgehog acts as a
somatic stem cell factor in the Drosophila ovary. Nature.
410:599–604. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
12
|
Walterhouse DO, Lamm ML, Villavicencio E
and Iannaccone PM: Emerging roles for hedgehog-patched-Gli signal
transduction in reproduction. Biol Reprod. 69:8–14. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Russel MC, Cowan RG, Harman RM, Walker AL
and Quirk SM: The hedgehog signaling pathway in the mouse ovary.
Biol Reprod. 77:226–236. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Chen X, Horiuchi A, Kikuchi N, Osada R,
Yoshida J, Shizawa T and Konishi I: Hedgehog signal is activated in
ovarian carcinomas, correlating with cell proliferation: its
inhibition leads to growth suppression and apoptosis. Cancer Sci.
98:68–76. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Byrom J, Mudaliar V, Redman CW, Jones P,
Strange RC and Hoban PR: Loss of heterozygosity at chromosome
9q22-31 is a frequent and early event in ovarian tumors. Int J
Oncol. 24:1271–1277. 2004.PubMed/NCBI
|
|
16
|
Cretnik M, Musani V, Oreskovic S, Leovic D
and Levanat S: The Patched gene is epigenetically regulated in
ovarian dermoids and fibromas, but not in basocellular carcinomas.
Int J Mol Med. 19:875–883. 2007.PubMed/NCBI
|
|
17
|
Surti U, Hoffner L, Chakravarti A and
Ferrell RE: Genetics and biology of human ovarian teratomas. I.
Cytogenetic analysis and mechanism of origin. Am J Hum Genet.
47:635–643. 1990.PubMed/NCBI
|
|
18
|
Ulbright TM: Germ cell tumors of the
gonads: a selective review emphsizing problems in differential
diagnosis, newly appreciated, and controversial issues. Mod Pathol.
18:S61–S79. 2005. View Article : Google Scholar
|
|
19
|
Bal A, Mohan H, Singh SB and Sehgal A:
Malignant transformation in mature cystic teratoma of the ovary:
report of five cases and review of the literature. Arch Gynecol
Obstet. 275:179–182. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Levanat S, Pavelic B, Crnic I, Oreskovic S
and Manojlovic S: Involvement of PTCH gene in various
noninflammatory cysts. J Mol Med. 78:140–146. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Levanat S, Kappler R, Hemmerlein B, Doring
P, Musani V, Komar A, Orešković S, Pavelić B and Hahn H: Analysis
of alterations of the PTCH1 signaling pathway in ovarian dermoids.
Int J Mol Med. 14:793–799. 2004.PubMed/NCBI
|
|
22
|
Yoshizaki A, Nakayama T, Naito S, Wen CY
and Sekine I: Expressions of sonic hedgehog, patched, smoothened
and Gli-1 in human intestinal stromal tumors and their correlation
with prognosis. World J Gastroenterol. 12:5687–5691.
2006.PubMed/NCBI
|
|
23
|
Liao X, Siu MK, Au CW, Wong ES, Chan HY,
Ip PP, Ngan HY and Cheung AN: Aberrant activation of hedgehog
signaling pathway in ovarian cancers: effect on prognosis, cell
invasion and differentiation. Carcinogenesis. 30:131–140. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Regl G, Neill GW, Eichberger T, Kasper M,
Ikram MS, Koller J, Hintner H, Quinn AG, Frischauf AM and Aberger
F: Human GLI2 and GLI1 are part of a positive feedback mechanism in
basal cell carcinoma. Oncogene. 21:5529–5539. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Leovic D, Sabol M, Ozretic P, Musani V,
Car D, Marjanovic K, Zubcic V, Sabol I, Sikora M, Grce M, et al:
Hh-Gli signaling pathway activity in oral and oropharyngeal
squamous cell carcinoma. Head Neck. 34:104–112. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Cvok ML, Cretnik M, Musani V, Ozretic P
and Levanat S: New sequence variants in BRCA1 and BRCA2 genes
detected by high-resolution melting analysis in an elderly healthy
female population in Croatia. Clin Chem Lab Med. 46:1376–1383.
2008.PubMed/NCBI
|
|
27
|
Boutet N, Bignon YJ, Drouin-Garraud V,
Sarda P, Longy M, Lacombe D and Gorry P: Spectrum of PTCH mutations
in French patients with Gorlin syndrome. J Invest Dermatol.
121:478–481. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Hahn H, Wicking C, Zaphiropoulous PG,
Gailani MR, Shanley S, Chidambaram A, Vorechovsky I, Holmberg E,
Unden AB, Gillies S, et al: Mutations of the human homolog of
Drosophila patched in the nevoid basal cell carcinoma syndrome.
Cell. 85:841–851. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Lench NJ, Telford EA, High AS, Markham AF,
Wicking C and Wainwright BJ: Characterisation of human patched germ
line mutations in naevoid basal cell carcinoma syndrome. Hum Genet.
100:497–502. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Xie J, Murone M, Luoh SM, Ryan A, Gu Q,
Zhang C, Bonifas JM, Lam CW, Hynes M, Goddard A, et al: Activating
Smoothened mutations in sporadic basal-cell carcinoma. Nature.
391:90–92. 1998. View
Article : Google Scholar : PubMed/NCBI
|
|
31
|
Koch A, Waha A, Hartmann W, Milde U,
Goodyer CG, Sorensen N, Berthold F, Digon-Sontgerath B, Kratzschmar
J, Wiestler OD and Pietsch T: No evidence for mutations or altered
expression of the Suppressor of Fused (SUFU) in primitive
neuroectodermal tumours. Neuropath Applied Neurobiol. 30:532–539.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Wong SC, Lo SF, Cheung MT, Ng KO, Tse CW,
Lai BS, Lee KC and Lo YM: Quantification of plasma β-catenin mRNA
in colorectal cancer and adenoma patients. Clin Cancer Res.
10:1613–1617. 2004.
|
|
33
|
Mullor JL, Dahmane N, Sun T and Ruiz i
Altaba A: Wnt signals are targets and mediators of Gli function.
Curr Biol. 11:769–773. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Veland IR, Awan A, Pedersen LB, Yoder BK
and Christensen ST: Primary cilia and signaling pathways in
mammalian development, health and disease. Nephron Physiol.
111:39–53. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Solter D: From teratocarcinomas to
embryonic stem cells and beyond: a history of embryonic stem cell
research. Nature Review Genet. 7:312–327. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Huber MA, Kraut N and Beug H: Molecular
requirements for epithelial-mesenchymal transition during tumor
progression. Curr Opin Cell Biol. 17:548–558. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Savagner P: The epithelial-mesenchymal
transition (EMT) phenomenon. Ann Oncol 21. (Suppl 7): vii89–vii92.
2010.PubMed/NCBI
|
|
38
|
Wijgerde M, Ooms M, Hoogerbrugge JW and
Anton Grootegoed J: Hedgehog signaling in mouse ovary: Indian
Hedgehog and Desert Hedgehog from granulosa cells induce target
gene expression in developing theca cells. Endocrinology.
146:3558–3566. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Kenney AM and Rowitch DH: Sonic Hedgehog
promotes G(1) cyclin expression and sustained cell cycle
progression in mammalian neuronal precursors. Mol Cell Biol.
20:9055–9067. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Acconcia F, Sigismund S and Polo S:
Ubiquitin in trafficking: the network at work. Exp Cell Res.
315:1610–1618. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Rohatgi R, Milenkovic L and Scott MP:
Patched1 regulates Hedgehog signaling at the primary cilium.
Science. 317:372–376. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Lu X, Liu S and Kornberg TB: The
C-terminal tail of the Hedgehog receptor Patched regulates both
localization and turnover. Genes Dev. 20:2539–2551. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Chen MH, Wilson CW, Li YJ, Law KKL, Lu CS,
Gacayan R, Zhang X, Hui CC and Chuang PT: Cilium-independent
regulation of Gli protein function by Sufu in Hedgehog signaling is
evolutionarily conserved. Genes Dev. 23:1910–1928. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Han YG, Kim HJ, Dlugosz AA, Ellison DW,
Gilbertson RJ and Alvarez-Buylla A: Dual and opposing roles of
primary cilia in medulloblastoma development. Nature Med.
15:1062–1065. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Wong SY, Seol AD, So PL, Ermilov AE,
Bichakjian CK, Epstein EE Jr, Dlugosz AA and Reiter JF: Primary
cilia can both mediate and suppress Hedgehog pathway-dependent
tumorigenesis. Nature Med. 15:1055–1061. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Zhang J, Lipinski RJ, Gipp JJ, Shaw AK and
Bushman W: Hedgehog pathway responsiveness correlates with the
presence of primary cilia on prostate stromal cells. BMC Dev Biol.
9:502009. View Article : Google Scholar
|
|
47
|
Teilmann SC, Byskov AG, Pedersen PA,
Wheatley DN, Pazour GJ and Christensen ST: Localization of
transient receptor potential ion channels in primary and motile
cilia of the female murine reproductive organs. Mol Reprod Dev.
71:444–452. 2005. View Article : Google Scholar : PubMed/NCBI
|