Introduction
The full-length cDNA encoding human protein of
extracellular heparan sulfate 6-O-endosulfatase 1, named HSulf-1,
has been recently identified and characterized (1,2).
HSulf-1 has a unique structural feature, enzymatic activity and
signaling function which could selectively remove 6-O-sulfate from
heparan sulfate proteoglycans (HSPGs) (3,4).
Since HSPGs serve as co-receptors for numerous heparin-binding
growth factors, cytokines, chemokines and adhesion molecules, they
could act as key regulator of growth factor and cell signaling in
the extracellular matrix and on the cell surface (5,6).
HSulf-1 could change HSPG-related signaling pathways, such as
heparin-binding epidermal growth factor (HB-EGF), fibroblast growth
factor-2 (FGF-2), hepatocyte growth factor (HGF), and vascular
endothelial growth factor (VEGF). Therefore, dysregulation of
HSulf-1 may exert significant effect on cell growth, angiogenesis
and tumorigenesis (7–14). Previous studies have identified
HSulf-1 as a downregulated gene in some tumor types including
ovarian cancer, hepatocellular cancer, and head and neck squamous
cell carcinoma (7,8). Re-expression of HSulf-1 diminished
heparin-binding growth factor signaling, inhibited cell
proliferation, migration, invasion, angiogenesis and promoted
drug-induced apoptosis in vitro (7–9) and
in vivo (10–12).
Ovarian cancer is the leading cause of death from
gynecological malignancies and accounts for 4% of all cancer types
in American women (15). Despite
improved methods of surgery, chemotherapy, radiotherapy and new
biological therapies, the mortality in women with advanced,
persistent or recurrent ovarian cancer still remains at a high
level (16). Cisplatin (DDP), one
of the most important chemotherapy drugs, has been widely used for
the treatment of ovarian carcinoma. Despite the significant
efficacy at treating ovarian cancer, cisplatin still has many
problems in clinical use, such as severe side effects and
resistance to the drug which undermine the curative potential of
cisplatin. The antitumor mechanisms of DDP involve its capability
to form bifunctional DNA crosslinks, failure to repair damaged DNA,
cell cycle arrest, interference with DNA replication and the
subsequent induction of cell death through apoptosis, necrosis, or
both (17–20). The expression of HSulf-1 is
repressed in the majority (∼75%) of tumor tissues originated from
ovarian cancer patients (7). In an
effort to develop more effective and novel therapeutic approaches
to combat ovarian cancer, we sought to develop combination therapy
of HSulf-1 with DDP to treat ovarian cancer.
The application of gene therapy in cancer treatment
has now been widely recognized, but the clinical employment of gene
therapy is restricted mainly due to the lack of safe and efficient
gene delivery technologies (21–24).
At present, cationic polyethyleneimine (PEI) has been used as one
of the most effective non-viral gene transfection agents. However,
PEI is not biodegradable and its application has been compromised
mainly due to the correlation of cytotoxicity, transfection
efficiency and its chain length (25,26).
Intensive research has been carried out to couple short PEI chains
into a longer one using biodegradable linkers to overcome this
issue (27–30). In our previous study, the low
molecular weight PEI was chemically conjugated into biodegradable
cationic nanogels by heparin and HPEI could serve as a safe and
efficient non-viral gene vector (31).
In the present study, we used HPEI nanogels
delivering HSulf-1 combined with DDP to investigate the antitumor
efficiency of combination therapy on human ovarian cancer. HSulf-1
exhibited significant capability of inhibiting tumor growth by way
of reducing angiogenesis, decreasing cell proliferation and
inducing apoptosis. The antitumor efficacy of HSulf-1 was enhanced
when combined with DDP. Additionally, HPEI nanogels displayed
efficient transfection without any conspicuous toxicity.
Materials and methods
Cell culture
The human ovarian epithelial serous
cystadenocarcinoma cell line SKOV3, obtained from American Type
Culture Collection (ATCC, Manassas, VA) was cultured in Dulbecco’s
modified Eagle’s medium (DMEM) containing 10% heat-inactivated
fetal bovine serum (FBS), 2 mM L-glutamine, 0.1 mg/ml of
streptomycin and 100 U/ml of penicillin. The SKOV3 cells were
incubated in a humidified atmosphere containing 5% CO2
at 37°C and passaged every 3 days at a split ratio of 1:3 using
trypsin.
Construction and purification of
plasmid
The pVAX plasmid (Invitrogen, Carlsbad, CA)
expressing HSulf-1 (GenBank accession number NM-001128205.1
GI:189571640) named pHSulf-1, was constructed in our laboratory as
previously mentioned (32). The
pVAX plasmid without HSulf-1 cDNA was used as an empty vector
(named pEP). A broad scale preparation of plasmid DNA was purified
using an EndoFree Plasmid Giga kit (Qiagen, Chatsworth, CA). The
purified plasmids were eventually dissolved in Tris-EDTA buffer,
stored at −20°C for further use. The recombinant pHSulf-1 was
confirmed by restriction digestion and DNA sequencing.
Synthesis of HPEI nanogels
The biodegradable cationic nanogel-HPEI were
synthesized at the State Key Laboratory of Biotherapy and Cancer
Center as previously described (31). Briefly, 50 mg heparin was dissolved
in 100 ml 2-N-morpholino ethanesulfonic acid (MES) buffer solution
(0.05 M), and then, to activate the carboxylic acid groups of
heparin, 20 mg 1-ethyl-3-3-dimethylaminopropyl carbodiimide (EDC)
and 30 mg N-hydroxysuccinimide (NHS) were added into the solution.
The solution was dropped into 20 ml PEI2K solution (7.5 mg/ml)
after 2 h of this reaction at room temperature. The persistently
stirred reaction was carried out at room temperature overnight.
Then, the synthetic HPEI nanogels were dialyzed in distilled water
for 3 days. Subsequently, the HPEI nanogels were filtered by a
syringe filter. The HPEI nanogels were adjusted to a final
concentration of 1 mg/ml and stored at 4°C for future use.
Preparation of plasmid/HPEI complexes and
transfection in vitro
The SKOV3 cell transfection was carried out using
HPEI. To determine the optimal plasmid/HPEI ratio (μg/μg) for
efficient gene delivery, we used the recombinant pVAX plasmid
coding the green fluorescent protein (GFP) in a series of
experiments with different plasmid/HPEI ratios transfecting SKOV3
cells in vitro. A maximum efficiency of transfection was
obtained when 2 μg plasmid/20 μg HPEI was used (data not
shown).
The SKOV3 cells were seeded in 6-well plates at a
density of 2×105/well and incubated for 24 h to reach
80% confluence. Plasmid (pHSulf-1 or pEP, 2 μg)/HPEI complexes (20
μg) were prepared in 1 ml DMEM medium without serum. DDP was
prepared at a concentration of 5.0 μg/ml. Normal saline (NS) was
used as control. After the cells were incubated for 6 h, the medium
was replaced by 2 ml DMEM supplemented with 10% FBS and cultured
for an additional 48 h. The cells and the supernatants were
collected for further assay. All transfections were performed in
triplicate.
Detection of HSulf-1 mRNA expression by
RT-PCR
The reverse transcription polymerase chain reaction
(RT-PCR) was performed to detect the stable expression of HSulf-1
mRNA in transfected SKOV3 cells and intraperitoneal tumor tissues.
The upstream primer and downstream primer for HSulf-1 were
5′-CGCGGATCCAAGATGAAGTATTCTTGCTGTGC-3′ and
5′-CGCGATATCTTAACCTTCCCATCCATCCCATA-3′, respectively. The total-RNA
of each experimental group was extracted using TRIzol reagent
(Invitrogen). The RT-PCR reactions with the isolated total-RNA (0.5
μg) were carried out as follows: reverse transcription (50°C, 30
min); denaturation (94°C, 2 min); amplification for 35 cycles
(94°C, 0.5 min), annealing (60°C, 0.5 min) and extension (72°C for
3 min); a terminal elongation procedure (72°C, 10 min). After the
reactions were completed, each RT-PCR product of 10 μl was
electrophoresed in a 1.0% agarose gel.
Western blot analysis
To confirm whether HSulf-1 was re-expressed in the
transfected SKOV3 cells and intraperitoneal tumor tissues, the
sample of each experimental group was lysed in modified RIPA lysis
buffer containing 1 mM PMSF. We used the BioRad protein assay
(Bio-Rad, Hercules, CA) to detect the protein concentrations of
lysates. Equal amounts of protein were loaded onto the 8% SDS-PAGE
for electrophoresis and transferred to PVDF membranes (Millipore,
Billerica, MA). Then, the blots were incubated with rabbit
anti-human polyclonal antibody against HSulf-1 (diluted 1:800,
Santa Cruz Biotechnology, Santa Cruz, CA) and the horseradish
peroxidase-conjugated secondary antibody. The immunoreactive bands
were detected by chemiluminescence. GAPDH was used as the internal
standard.
Tumor model and treatment
The female athymic BALB/c nude mice (6–8 weeks old,
18–20 g each) were used to establish the intraperitoneal xenograft
tumor model of human ovarian cancer as previously described
(33). This animal experiment
procedure was approved by the Institutional Animal Care and
Treatment Committee of Sichuan University.
SKOV3 cell suspension (5×106 cells in 100
μl DMEM) was injected subcutaneously in the backs of 5 nude mice.
When the diameter of s.c. tumors was approx. 1 cm, tumors were
harvested for i.p. inoculation. Seven days after i.p. tumor
inoculation, 30 mice were randomly allocated into five groups (six
mice/per group) to receive the following i.p. administration: i)
untreated, normal saline (NS); ii) 5 μg pEP/50 μg HPEI complexes,
every two days for 12 times; iii) 5 μg pHSulf-1/50 μg HPEI
complexes, every two days 12 times; iv) DDP (3 mg/kg, Qinu Pharmacy
Corporation, China), weekly for 3 times; v) combination therapy
containing treatment of 5 μg pHSulf-1/50 μg HPEI complexes for 12
times and DDP (3 mg/kg) for 3 times (volume 100 μl). All mice were
sacrificed 3 days after the last injection and the intraperitoneal
tumors were removed and weighed. When sacrificed, each mouse was
observed and recorded in terms of ascites, and the number and
location of peritoneally disseminated macroscopic tumors.
Immunohistochemistry staining
The intraperitoneal tumor tissues were fixed in 10%
formalin (pH 7.0), embedded in paraffin, and then cut into sections
(3–5 μm). The primary antibody of Ki-67 and CD31 immunostaining for
intraperitoneal tumors is the rabbit anti-human Ki-67 antibody
(diluted 1:100; Thermo Scientific, Hudson, NH) and goat anti-mouse
CD31 (diluted 1:100; Santa Cruz Biotechnology), respectively. The
tumor sections were deparaffinized and rehydrated at first.
Heat-induced antigen retrieval was performed in 10 mM citrate
buffer (pH 6.0) at 120°C, and then endogenous peroxidase activity
was blocked by 3% H2O2. The sections were
treated with 10% normal goat or rabbit serum to reduce non-specific
staining, and then incubated with the primary antibody,
biotinconjugated secondary antibody, streptavidin-biotin complex
(SABC) successively. The immunoreaction was observed using
diaminobenzidine (DAB) peroxide solution.
To quantify microvessel density (MVD), the sections
were first scanned at ×100 magnification to identify the areas
having the highest vascular density without necrosis. The number of
microvessels was counted in this areas at high-power field (hpf).
In order to quantify the proliferation index, the percentage of
Ki-67-positive cells was observed in 10 random fields without
necrosis at a magnification, ×400.
Apoptotic analysis
Apoptosis in vivo was analysed using terminal
deoxynucleotidyl transferase-mediated dUTP nick end-labeling
(TUNEL) assay in accordance with the manufacturer’s protocol
(Promega, Madison, WI). Cell nuclei stained with dark green
fluorescence were defined as TUNEL-positive and observed by
fluorescence microscopy. The apoptosis index was analysed in 10
random fields without necrosis at ×400 magnification.
Alginate-encapsulated tumor cell
assay
SKOV3 ovarian tumor cells were resuspended in a 1.5%
alginate (Sigma) solution, and then released into a solution of 250
mM CaCl2 to form the alginate bead. Alginate beads
(2×105 cells/bead) were embedded s.c. into both dorsal
sides of the BALB/c nude mice under anesthesia (4 beads/mouse).
Mice were treated with i.p. administration of 0.9% NS, pEP/HPEI
complexes, pHSulf-1/HPEI complexes, DDP or pHSulf-1/HPEI complexes
plus DDP, respectively. Treatment was carried out on the second day
of implanting beads. The mice were injected intravenously with 100
μl FITC-dextran (Sigma) (100 mg/kg) solution after 14 days. After
FITC-dextran injection, alginate beads covering blood vessels were
photographed after being exposed surgically and then quickly
removed in 20 min. The uptake of FITC-dextran was measured as
described (34,35).
Toxicity assessment
To evaluate the underlying side effects and toxicity
of the combination therapy, the animal weight was monitored every
four days and the correlative indices such as anorexia, diarrhea,
skin ulceration and toxic death were monitored consecutively during
the whole therapeutic procedure. The blood biochemical parameters
such as ALT, AST, TBIL, ALB, BUN and CREA were detected to assess
the potential side effects of the treatment at the end point of the
experiment. Various organs (heart, liver, spleen, lung, kidney,
brain, etc.) were harvested, fixed in 10% formalin (pH 7.0) and
embedded in paraffin after sacrifice. Sections of these tissues
(3–5 μm) were stained with H&E.
Statistical analysis
Comparisons of all numerical values among the
different groups were performed using one-way analysis of variance
(ANOVA) and the unpaired Student’s t-test. A value of P<0.05 was
defined as statistically significant.
Results
Preparation and characterization of HPEI
nanogels
As shown in Fig.
1A, in presence of EDC/NHS, the reaction between heparin and
PEI2K was generated. When EDC/NHS activated heparin was dropped
into PEI2K solution, one heparin molecule reacted with several
PEI2K molecules and cross-linkage between heparin and PEI2K
appeared. Consequently, the HPEI nanogels were formed through amide
bonds.
 | Figure 1Synthesis of HPEI nanogels and
expression of HSulf-1 in vitro and in vivo. (A)
Preparative method of HPEI nanogels. Reaction between heparin and
PEI2K was generated when catalyzed by EDC/NHS. (B) Identification
of the expression of HSulf-1 in transfected SKOV3 cells in
vitro by RT-PCR and western blot analysis. Lane 1, SKOV3
ovarian cancer cells were treated with NS; lane 2, pEP/HPEI
complexes; lane 3, pHSulf-1/HPEI complexes; lane 4, DDP; or lane 5,
pHSulf-1/HPEI plus DDP, respectively. Left, PT-PCR indicated only
pHSulf-1/HPEI complexes treated cells had positive band of HSulf-1
(2,616 bp). GAPDH was used as the internal standard. Right, the
result of western blot analysis showed a positive band (∼130 kDa)
only occurred in cells transfected with pHSulf-1/HPEI complexes in
contrast to control groups. (C) Expression of HSulf-1 in
vivo. The intraperitoneal carcinomatosis model in nude mice was
established and intraperitoneally administered with: lane 1, NS;
lane 2, pEP/HPEI complexes; lane 3, pHSulf-1/HPEI complexes; lane
4, DDP; or lane 5, pHSulf-1/HPEI plus DDP, respectively (80x70 mm,
300 DPI). |
Stable expression of HSulf-1 in
vitro
To examine whether the pHSulf-1/HPEI complexes
resulted in expression of HSulf-1 in SKOV3 ovarian cancer cells by
transfection in vitro, SKOV3 cells were seeded in 6-well
plates and incubated with NS, pEP/HPEI complexes, pHSulf-1/HPEI
complexes, DDP or pHSulf-1/HPEI complexes plus DDP, respectively.
Stable expression of HSulf-1 was detected using PT-PCR and western
blot analysis after transfection for 48 h. The HPEI nanogels
efficiently transfected pHSulf-1 into SKOV3 cells in vitro
and HSulf-1 could be expressed in SKOV3 cells after transfection
with pHSulf-1/HPEI complexes, compared with control groups
(Fig. 1B).
Expression of HSulf-1 in vivo
An intraperitoneal xenograft model of human ovarian
cancer was established to detect whether the pHSulf-1/HPEI
complexes led to expression of HSulf-1 on SKOV3 cell line in
vivo. The nude mice were injected with the above five agents,
respectively. All mice were sacrificed after the last
administration of 3 days and the intraperitoneal tumors were used
for RT-PCR and western blot analysis. The pHSulf-1/HPEI complexes
resulted in expression of HSulf-1 in vivo, but no expression
of HSulf-1 could be examined in the three groups which were not
treated with pHSulf-1/HPEI complexes (Fig. 1C).
Tumor suppressor function of the
combination of HSulf-1 plus DDP
We established an intraperitoneal xenograft model of
human ovarian cancer in nude mice to further investigate the
efficacy of combination HSulf-1 with DDP in suppressing the growth
of human ovarian tumor in vivo. As shown in Fig. 2, the group treated with
pHSulf-1/HPEI or DDP alone exhibited significant inhibition of
tumor growth (mean tumor weight, 0.38±0.19 and 0.48±0.10 g,
respectively). The tumor growth of the two groups was significantly
inhibited, compared with the group treated with NS and pEP/HPEI
complexes (mean tumor weight, 1.62±0.36 and 1.51±0.30 g,
respectively, P<0.01). However, the group of combination therapy
exhibited an enhanced effect on tumor suppression, compared with
the group treated with pHSulf-1/HPEI or DDP alone (mean tumor
weight, 0.10±0.06 g, P<0.01).
In the NS and pEP/HPEI groups, each mouse developed
intraperitoneally macroscopic disseminated tumor nodules and a
portion of them emerged bloody ascites (Table I). Microscopic examination verified
that livers of three and four of the six mice developed tumor
invasion, and the hemorrhagic ascites emerged in two and three of
the six mice in pEP/HPEI and NS group, respectively. In the other
three groups, the intraperitoneal tumor nodules were limited to the
pelvis. No tissues and organs were obviously invaded by tumor and
no ascites were found in these mice. One of the six mice in the
group of combination therapy had no macroscopic tumor when
sacrificed.
 | Table ICharacterization of intraperitoneal
xenografs of human ovarian cancer in different treatment group in
nude mice (100×40 mm, 300 DPI). |
Table I
Characterization of intraperitoneal
xenografs of human ovarian cancer in different treatment group in
nude mice (100×40 mm, 300 DPI).
| Treatment
(n=6) | Number of
nodules | Mean weight (g,
mean ± SD) | Liver invaded by
tumors | Ascites |
|---|
| NS | 8±2 | 1.62±0.36 | 4 | 3 |
| pEP/HPEI | 6±2 | 1.51±0.30 | 3 | 2 |
| pHSulf-1/HPEI | 3±1 | 0.38±0.19 | 0 | 0 |
| DDP | 3±1 | 0.48±0.10 | 0 | 0 |
|
pHSulf-1/HPEI+DDP | 2±1 | 0.10±0.06 | 0 | 0 |
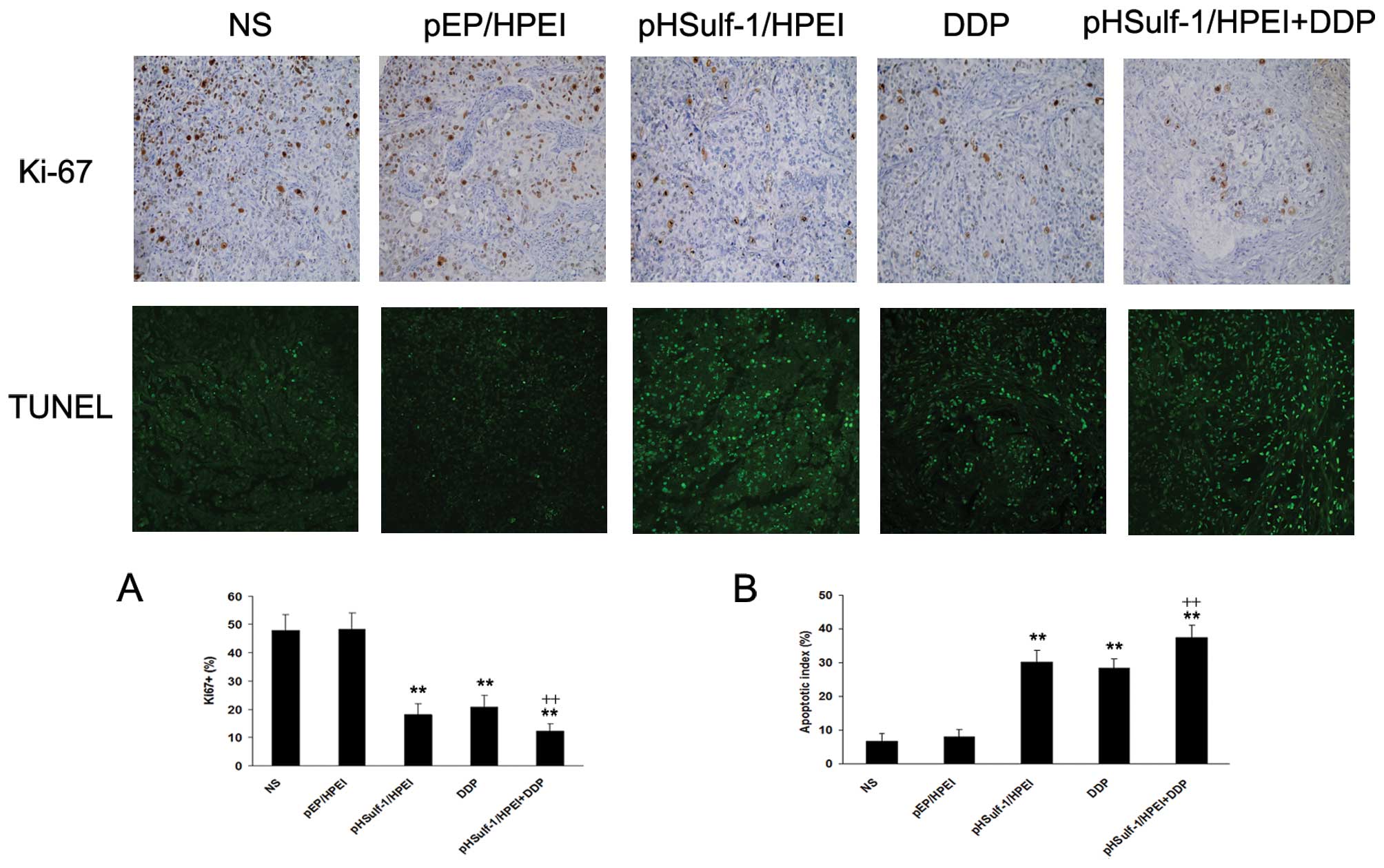
Inhibition of tumor cell proliferation in
vivo
Ki-67 immunostaining was used to evaluate the
proportion of proliferative tumor cells in each treatment group
(Fig. 3). The empty vector and NS
group demonstrated significant tumor cell proliferation in
vivo. Nuclear staining in tumor tissues of pHSulf-1/HPEI and
DDP single therapy groups showed less staining for Ki-67 compared
with the NS and pEP/HPEI groups (P<0.01). The mean percentage of
Ki-67 positive cells in the pHSulf-1/HPEI group was not
significantly different from that of DDP group (P>0.05). The
cell proliferation dramatically decreased in tumors treated with
pHSulf-1/HPEI plus DDP compared with control groups
(P<0.01).
Induction of apoptosis in vivo
The pHSulf-1/HPEI and DDP monotherapy resulted in a
significant increase of apoptotic tumor cells compared with the NS
and pEP/HPEI treated groups (P<0.01), respectively. No
significant difference was detected in the percentage of apoptotic
cells between the two groups (P>0.05). The tumor tissues of the
combination therapy group showed increased positive nuclei compared
with the pHSulf-1/HPEI and DDP monotherapy groups (P<0.01).
However, positive nuclei were rare in tumor tissues of the empty
vector and NS groups, as shown in Fig.
3.
Inhibition of angiogenesis in vivo
Angiogenesis in tumor tissues was estimated by
immunostaining using CD31 antibody which had high specific affinity
for vascular endothelial cells. A significant reduction of MVD
could be observed in pHSulf-1/HPEI and DDP treated groups compared
with that in NS and pEP/HPEI treated groups (P<0.01; Fig. 4). The MVD decreased more obviously
in tumors of combination therapy group compared with NS and
pEP/HPEI groups (P<0.01). The capability of antiangio-genesis
was also evaluated by alginate-encapsulated tumor cell assay and
the quantification of the FITC-dextran uptake (Fig. 4). New blood vessels in alginate
beads from the mice treated with pHSulf-1/HPEI and DDP were sparse,
but twisted and rich blood vessels could be observed in alginate
beads of NS and pEP/HPEI treated groups. Blood vessels were
obviously few in alginate beads of pHSulf-1/HPEI plus DDP group. In
addition, the results of FITC-dextran uptake were similar to what
we observed in alginate beads of different group.
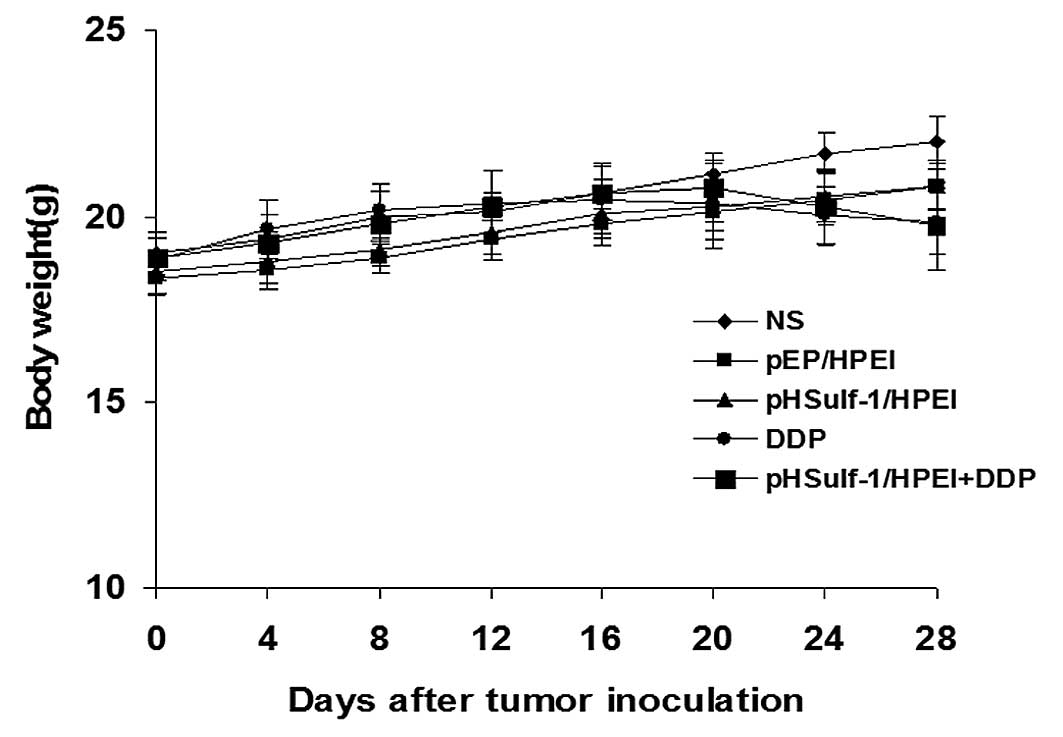
Toxicity observation
The animal weight, considered as a parameter for
evaluating anorexia, physical status or cachexia, was monitored
every four days. There was a minor decrease of the body weights in
the DDP and pHSulf-1/HPEI plus DDP groups, and no apparent
differences were found between the two groups. Meanwhile, no
significant differences in body weight were observed among the five
groups (P>0.05, Fig. 5). In
addition, no gross changes such as abnormal behavior and ruffling
of fur were detected. The results of blood biochemical parameters
and H&E histological staining indicated that there were no
obvious pathological and biochemical alterations in various
important organs.
Discussion
HSulf-1, a heparin-degrading extracellular
endosulfatase which could selectively remove 6-O-sulfate from
heparan sulfate is downregulated in the majority of examined cancer
cell lines and it is markedly diminished or undetectable in ∼75% of
ovarian cancers (7). Re-expression
of HSulf-1 diminishes signaling of various heparin-binding growth
factors, decreases cell proliferation, invasion and enhances
drug-induced apoptosis in vitro (7–9,13).
Some studies also showed that HSulf-1 could inhibit tumorigenesis
and angiogenesis, and promote drug-induced apoptosis in vivo
(10–12). The contribution of HSulf-1 to
growth factor signaling and its effects on human cancerigenesis are
under intensive investigation. In our previous study, we
demonstrated that HSulf-1 could effectively inhibit intraperitoneal
xenograft growth of human ovarian cancer (32). The findings that expression of
HSulf-1 in cancer cells could inhibit tumori-genesis and tumor
angiogenesis indicate that the modification of HSPG polysaccharide
structure might be a reasonable approach for the therapy of cancer,
in addition to, or in combination with conventional chemotherapy
and antiangiogenic therapy.
Re-expression of HSulf-1 could sensitize the cancer
cells to traditionally used chemotherapeutic agents such as
cisplatin and paclitaxel (7–9,12–14).
At present, cisplatin (DDP) has been widely used for the treatment
of ovarian carcinoma. Severe side-effects and resistance to drug
are common problems encountered in the procedure of cancer therapy,
despite the significant efficacy of DDP in treating cancers. The
therapy that combines conventional chemotherapeutic agents with
inhibition of angiogenesis could have an additive effect on
suppression of tumor growth and metastases in vivo (36). Therefore, in this study, we used
the combination therapy of HSulf-1 plus DDP to develop more
effective and novel therapeutic approach to treat ovarian cancer.
Our finding indicated that HSulf-1 could inhibit tumorigenesis and
angiogenesis in vivo, and the effect of HSulf-1 on ovarian
cancer was markedly enhanced by combination with DDP. The
antitumor, antiangiogenesis, antimetastasis, and antiapoptosis
effects of HSulf-1 plus DDP were remarkably greater than those of
HSulf-1 or DDP alone.
The mechanism of how this combination therapy exerts
its effect is not clear. Angiogenesis, the development of new blood
vessels from existing vasculature, is an essential factor for solid
tumor growth and metastasis because beyond a critical extent, the
tumor fails to expand further without neovascularization. The
angiogenesis is controlled by the balance between various
angiogenic and antiangiogenic agents (37,38).
HSulf-1 may play a potential regulatory role in this multiple
biological event, because heparan sulfate could interact with a
great deal of these agents to enhance or inhibit their activities.
HSulf-1 could modulate the function of heparan sulfate binding
VEGF165 in proliferation and angiogenesis (11). The blockade of VEGF signaling can
significantly enhance the efficacy of chemotherapeutic regimens
(39). In this study, inhibition
of angiogenesis by HSulf-1 was evaluated by CD31 and
alginate-encapsulated tumor cell assay. Apparent decrease in tumor
vessel formation was observed in the HSulf-1 treated group compared
with the empty vector or NS treated group, indicating that HSulf-1
inhibited angiogenesis in vivo. This demonstrates that the
intraperitoneal tumor might be exposed to an insufficient supply of
oxygen and nutrients, which represses tumor growth, in agreement
with the universally accepted concept that the growth of solid
tumors such as breast and ovarian cancers depend on their
capability of inducing tumor angiogenesis (40). In addition, angiogenesis in the
HSulf-1 plus DDP group was more significantly suppressed compared
with any other group. However, the exact antiangiogenic mechanism
of this combination therapy remains unclear.
DDP is highly effective in the treatment of ovarian
cancers. The biochemical mechanisms of DDP cytotoxicity involve
binding of the drug to DNA and non-DNA targets, and the subsequent
provocation of cell death through apoptosis, necrosis, or both
(17). These antitumor mechanisms
of DDP refer to interfering with DNA replication, forming lethal
intrastrand DNA crosslinks, arresting cell cycle, and inducing cell
apoptosis (18–20). In the present study, the antitumor
and antiapoptosis effects were markedly enhanced by the combination
therapy of HSulf-1 plus DDP, compared with the monotherapy of
HSulf-1 or DDP. These results were consistent with the previous
findings indicating that re-expression of HSulf-1 increased
cisplatin-mediated cytotoxicity in ovarian cancer (7,12,13).
Further studies by Lai et al (7) indicated that the typical biochemical
hallmarks of apoptosis by the combination therapy of HSulf-1 plus
DDP included cytochrome c release from mitochondria and DNA
fragmentation. HSulf-1 induced apoptosis by modulating the
sensitivity of ovarian cancer cells to other stimuli, not by
itself. Higher expression of HSulf-1 was related with somewhat
higher induction of apoptosis.
With respect to the combination therapy, tumor
growth might be influenced not only by direct cytotoxicity but also
by inhibition of new vessel formation, and HSulf-1 could enhance
the antitumor effect of DDP, as well as decreasing development of
drug resistance to DDP. On the other hand, the suppression of
angiogenesis might lead to the death of tumor cells most distal to
the established vasculature, thereby reducing tumor volume and
facilitating access of the DDP throughout the tumor tissue.
Gene therapy, as a promising strategy to treat
cancer, has achieved exciting progress in the past two decades. The
development of safe and efficient gene carriers is one of the
prerequisites for the successful application of gene therapy. The
non-viral vectors, such as cationic lipids and polymers have many
advantages over viral ones: low immunogenicity, easy to produce and
no limitation to the size of transferred DNA molecules, but the
toxicity is still a hindrance for the application of non-viral
vectors in gene therapy (21,23,24).
Polyethylenimine (PEI), one of the most successful and widely
studied gene delivery cationic polymers, is effective in gene
delivery on account of its condensation of DNA, which facilitates
endocytosis, and ‘proton sponger’ quality, which can prevent DNA
from endosomal disruption (25,31).
Because PEI is not biodegradable and has the shortcoming of an
association between transfection efficiency and cytotoxicity
(25,26), in our previous study, the low
molecular weight PEI was conjugated chemically into biodegradable
cationic nanogels by heparin, to develop a novel gene delivery
vector (31,32). HPEI nanogels were quickly degraded
into low molecular weight PEI followed by excretion through urine
in vivo and they also exhibited better blood compatibility,
lower cytotoxicity, and stability in vitro, therefore, we
used HPEI nanogels as the gene carrier in the present study.
Our data revealed that HPEI nanogels could
efficiently deliver the HSulf-1 gene into SKOV3 ovarian cancer
cells, and the expression of HSulf-1 in vitro and in
vivo was detected at the mRNA and protein level. In this study,
there were no apparent cytotoxicity and systemic toxic effects.
Even though slight weight loss was observed in the DDP and HSulf-1
plus DDP-treated groups, it might be due to the toxic reaction of
DDP. HSulf-1 delivered by HPEI nanogels exhibited an excellent
tolerance in the procedure of intraperitoneal cancer treatment.
In conclusion, HSulf-1 shows potential for an
effective anti-tumor capability against human ovarian carcinoma by
way of inhibiting angiogenesis, reducing cell proliferation and
inducing apoptosis. The antitumor activity of HSulf-1 was enhanced
when combined with DDP. HPEI nanogels could serve as an efficient
gene transfer vector, without apparent toxicity. Thus, HPEI
nanogels delivering HSulf-1 combined with DDP might become a new
and promising therapeutic strategy against human ovarian
cancer.
Acknowledgements
This study was supported by the
National Key Basic Research Program (973 Program) of China
(2010CB529905 and 2011CB910703) and NIH81071862.
References
|
1
|
Shridhar V, Sen A, Chien J, Staub J, Avula
R, Kovats S, Lee J, Lillie J and Smith DI: Identification of
underexpressed genes in early- and late-stage primary ovarian
tumors by suppression subtraction hybridization. Cancer Res.
62:262–270. 2002.PubMed/NCBI
|
|
2
|
Morimoto-Tomita M, Uchimura K, Werb Z,
Hemmerich S and Rosen SD: Cloning and characterization of two
extracellular heparin-degrading endosulfatases in mice and humans.
J Biol Chem. 277:49175–49185. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ai X, Do AT, Kusche-Gullberg M, Lindahl U,
Lu K and Emerson CP Jr: Substrate specificity and domain functions
of extracellular heparan sulfate 6-O-endosulfatases, QSulf1 and
QSulf2. J Biol Chem. 281:4969–4976. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Dhoot GK, Gustafsson MK, Ai X, Sun W,
Standiford DM and Emerson CP Jr: Regulation of Wnt signaling and
embryo patterning by an extracellular sulfatase. Science.
293:1663–1666. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Selva EM and Perrimon N: Role of heparan
sulfate proteoglycans in cell signaling and cancer. Adv Cancer Res.
83:67–80. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lin X: Functions of heparan sulfate
proteoglycans in cell signaling during development. Development.
131:6009–6021. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lai JP, Chien J, Staub J, Avula R, Greene
EL, Matthews TA, Smith DI, Kaufmann SH, Roberts LR and Shridhar V:
Loss of HSulf-1 up-regulates heparin-binding growth factor
signaling in cancer. J Biol Chem. 278:23107–23117. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lai JP, Chien J, Strome SE, Staub J,
Montoya DP, Greene EL, Smith DI, Roberts LR and Shridhar V: HSulf-1
modulates HGF-mediated tumor cell invasion and signaling in head
and neck squamous carcinoma. Oncogene. 23:1439–1447. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lai JP, Chien JR, Moser DR, Staub JK,
Aderca I, Montoya DP, Matthews TA, Nagorney DM, Cunningham JM,
Smith DI, Greene EL, Shridhar V and Roberts LR: hSulf1sulfatase
promotes apoptosis of hepatocellular cancer cells by decreasing
heparin-binding growth factor signaling. Gastroenterology.
126:231–248. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Dai Y, Yang Y, MacLeod V, Yue X, Rapraeger
AC, Shriver Z, Venkataraman G, Sasisekharan R and Sanderson RD:
HSulf-1 and HSulf-2 are potent inhibitors of myeloma tumor growth
in vivo. J Biol Chem. 280:40066–40073. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Narita K, Staub J, Chien J, Meyer K, Bauer
M, Friedl A, Ramakrishnan S and Shridhar V: HSulf-1 inhibits
angiogenesis and tumorigenesis in vivo. Cancer Res. 66:6025–6032.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Liu P, Khurana A, Rattan R, Kalloger S,
Dowdy S, Gilks B and Shridhar V: Regulation of HSulf-1 expression
by variant hepatic nuclear factor 1 in ovarian cancer. Cancer Res.
69:4843–4850. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Staub J, Chien J, Pan Y, Qian X, Narita K,
Aletti G, Roberts LR, Molina J and Shridhar V: Epigenetic silencing
of HSulf-1 in ovarian cancer: implications in chemoresistance.
Oncogene. 26:4969–4978. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lai JP, Sandhu DS, Shire AM and Roberts
LR: The tumor suppressor function of human sulfatase 1 (SULF1) in
carcinogenesis. J Gastrointest Cancer. 39:149–158. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Jemal A, Siegel R, Xu J and Ward E: Cancer
statistics, 2010. CA Cancer J Clin. 60:277–300. 2010. View Article : Google Scholar
|
|
16
|
Ozols RF: Progress in ovarian cancer: an
overview and perspective. EJC (Suppl). 1:43–55. 2003. View Article : Google Scholar
|
|
17
|
Gonzalez VM, Fuertes MA, Alonso C and
Perez JM: Is cisplatin-induced cell death always produced by
apoptosis? Mol Pharmacol. 59:657–663. 2001.PubMed/NCBI
|
|
18
|
Fuertes MA, Alonso C and Perez JM:
Biochemical modulation of cisplatin mechanisms of action:
enhancement of antitumor activity and circumvention of drug
resistance. Chem Rev. 103:645–662. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Reed E: Platinum-DNA adduct, nucleotide
excision repair and platinum based anti-cancer chemotherapy. Cancer
Treat Rev. 24:331–344. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Cohen SM and Lippard SJ: Cisplatin: from
DNA damage to cancer chemotherapy. Prog Nucleic Acid Res Mol Biol.
67:93–130. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Edelstein ML, Abedi MR, Wixon J and
Edelstein RM: Gene therapy clinical trials worldwide 1989–2004-an
overview. J Gene Med. 6:597–602. 2004.
|
|
22
|
Anderson DG, Peng W, Akinc A, Hossain N,
Kohn A, Padera R, Langer R and Sawicki JA: A polymer library
approach to suicide gene therapy for cancer. Proc Natl Acad Sci
USA. 101:16028–16033. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ferber D: Gene therapy. Safer and
virus-free? Science. 294:1638–1642. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Lv H, Zhang S, Wang B, Cui S and Yan J:
Toxicity of cationic lipids and cationic polymers in gene delivery.
J Control Release. 114:100–109. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Neu M, Fischer D and Kissel T: Recent
advances in rational gene transfer vector design based on
poly(ethyleneimine) and its derivatives. J Gene Med. 7:992–1009.
2005. View
Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kunath K, von Harpe A, Fischer D, Petersen
H, Bickel U, Voigt K and Kissel T: Low-molecular-weight
polyethyleneimine as a non-viral vector for DNA delivery:
comparison of physicochemical properties, transfection efficiency
and in vivo distribution with high-molecular-weight
polyethyleneimine. J Control Release. 89:113–125. 2003. View Article : Google Scholar
|
|
27
|
Wen Y, Pan S, Luo X, Zhang X, Zhang W and
Feng M: A biodegradable low molecular weight polyethyleneimine
derivative as low toxicity and efficient gene vector. Bioconjug
Chem. 20:322–332. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Gosselin MA, Guo W and Lee RJ: Efficient
gene transfer using reversibly cross-linked low molecular weight
polyethyleneimine. Bioconjug Chem. 12:989–994. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Forrest ML, Koerber JT and Pack DW: A
degradable polyethyleneimine derivative with low toxicity for
highly efficient gene delivery. Bioconjug Chem. 14:934–940. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Jeon O, Yang HS, Lee TJ and Kim BS:
Heparin-conjugated polyethyleneimine for gene delivery. J Control
Release. 132:236–242. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Gou ML, Men K, Zhang J, Li YH, Song J, Luo
S, Shi HS, Wen YJ, Guo G, Huang MJ, Zhao X, Qian ZY and Wei YQ:
Efficient inhibition of C-26 colon carcinoma by VSVMP gene
delivered by biodegradable cationic nanogel derived from
polyethyleneimine. ACS Nano. 4:5573–5584. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Liu P, Gou ML, Yi T, Xie C, Qi XR, Zhou
ST, Deng HX, Wei YQ and Zhao X: Efficient inhibition of an
intraperitoneal xenograft model of human ovarian cancer by HSulf-1
gene delivered by biodegradable cationic heparin-polyethyleneimine
nanogels. Oncol Rep. 27:363–370. 2012.
|
|
33
|
Lin XJ, Chen XC, Wang L, Wei YQ, Kan B,
Wen YJ, He X and Zhao X: Dynamic progression of an intraperitoneal
xenograft model of human ovarian cancer and its potential for
preclinical trials. J Exp Clin Cancer Res. 26:467–474.
2007.PubMed/NCBI
|
|
34
|
Hoffmann J, Schirner M, Menrad A and
Schneider MR: A highly sensitive model for quantification of in
vivo tumor angiogenesis induced by alginate-encapsulated tumor
cells. Cancer Res. 57:3847–3851. 1997.PubMed/NCBI
|
|
35
|
He QM, Wei YQ, Tian L, Zhao X, Su JM, Yang
L, Lu Y, Kan B, Lou YY, Huang MJ, Xiao F, Liu JY, Hu B, Luo F,
Jiang Y, Wen YJ, Deng HX, Li J, Niu T and Yang JL: Inhibition of
tumor growth with a vaccine based on xenogeneic homologous
fibroblast growth factor receptor-1 in mice. J Biol Chem.
278:21831–21836. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Hu L, Hofmann J, Zaloudek C, Ferrara N,
Hamilton T and Jaffe RB: Vascular endothelial growth factor
immunoneutralization plus paclitaxel markedly reduces tumor burden
and ascites in athymic mouse model of ovarian cancer. Am J Pathol.
161:1917–1924. 2002. View Article : Google Scholar
|
|
37
|
Folkman J: What is the evidence that
tumors are angiogenesis dependent? J Natl Cancer Inst. 82:4–6.
1990. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Carmeliet P and Jain RK: Angiogenesis in
cancer and other diseases. Nature. 407:249–257. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Klement G, Baruchel S, Rak J, Man S, Clark
K, Hicklin DJ, Bohlen P and Kerbel RS: Continuous low-dose therapy
with vinblastine and VEGF receptor-2 antibody induces sustained
tumor regression without overt toxicity. J Clin Invest.
105:R15–R24. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Neri D and Bicknell R: Tumour vascular
targeting. Nat Rev Cancer. 5:436–446. 2005. View Article : Google Scholar
|