Introduction
Cancer cells that survive after treatment can cause
local recurrence and distant metastases. A hierarchical model
proposes that tumor consists of proliferative cells, which have
limited life spans, and CSCs, which can self-renew, undergo
multilineage differentiation, and are suited to survive adverse
conditions in the tissue microenvironment (1). This cancer stem cell (CSC) hypothesis
may account for carcinogenesis, the functional heterogeneity
observed in tumor tissues, and tumor relapse after treatment due to
the presence of a therapy-resistant population (2).
Pancreatic ductal adenocarcinoma (PDAC) has a high
mortality rate due to its aggressive growth and high metastatic
rates (3). Multiple lines of
evidence have shown the existence of CSC fractions in PDAC
(4), which have been detected by
CSC-specific markers, including CD24 (5–7),
CD44 (5,6,8,9),
CD133 (10–13), CXCR4 (10,14),
epithelial cell adhesion molecule (EpCAM; epithelial-specific
antigen, ESA) (5), nestin
(15), and combinations of these
markers (5,10). However, definitive CSC markers for
PDAC and their clinical relevance are still controversial (16).
There is substantial evidence that PDAC does not
arise de novo, but rather progresses through a multistep
model involving non-invasive precursor lesions known as pancreatic
intraepithelial neoplasias (PanINs), and culminating in invasive
cancer (17). CXCR4 expression
begins in PanIN lesions, and the CXCR4-expressing cells appeared to
mediate pancreatic cancer metastasis (10). It has also been shown that
endogenous K-ras expression in the pancreatic nestin-positive cell
lineage induces mouse PanINs (18). These findings led to the hypothesis
that CXCR4- or nestin-positive pancreatic cells are
cancer-initiating cells of PDAC, which has been considered a
property of CSCs. However, the relationship between CSCs and PanIN
lesions remains unclear.
Most research related to CSCs has been conducted
using established cell lines or experimental animals; thus, little
is known about the localization and roles of CSCs in human tissues.
In this study, we analyzed the expression levels of CSC markers in
different grades of PanINs, using human pancreatic tissues. We also
examined the expression and roles of the CSC markers in PDAC
tissues, and compared the expression levels of these CSC markers to
those of established PDAC cell lines.
Materials and methods
Materials
The following were purchased: Histofine Simple Stain
MAX PO (R) and (M) kits from Nichirei (Tokyo, Japan); mouse
monoclonal anti-CD24 antibody from Santa Cruz Biotechnology (Santa
Cruz, CA, USA); mouse monoclonal anti-CD44, anti-nestin antibody,
mouse IgG1, and mouse IgG2A from R&D Systems Inc. (Westerville,
OH, USA); mouse polyclonal ESA antibody from Abnova (Taipei City,
Taiwan), rabbit polyclonal anti-CD133, anti-CXCR4 antibody, rabbit
IgG, and mouse IgG from Abcam plc. (Cambridge, UK); mouse
monoclonal anti-Ki 67 antibody and anti-PCNA antibody from Dako
Corp. (Santa Barbara, CA, USA); High Capacity cDNA Reverse
Transcription kit and TaqMan Gene Expression assay for CD24
(Hs02379687_s1), CD44 (Hs00153304_m1), ESA (Hs00901885_m1), CD133
(Hs01009238_m1), CXCR4 (Hs00607978_s1), nestin (Hs00707120_s1), and
18S ribosomal RNA (18S rRNA, Hs03928990_g1) from Applied Biosystems
(Foster City, CA, USA); NucleoSpin RNA II from Takara Biotechnology
(Tokyo, Japan); human serum from Lonza (Walkersville, MD, USA);
Zenon Labeling kit from Invitrogen Corp. (Carlsbad, CA, USA); and
SureLink Fluorescein (FITC) Labeling kit from KPL, Inc.
(Gaithersburg, MD, USA). All other chemicals and reagents were
purchased from Sigma Chemical Corp. (St. Louis, MO, USA).
Patients and tissues
Tissues from 105 patients with conventional PDAC
were obtained for this study (Table
I). These patients received treatment at Nippon Medical School
Hospital (Tokyo, Japan) between 1995 and 2011. None of the patients
received preoperative chemotherapy and radiotherapy. The patients
consisted of 61 males and 44 females, with a median age of 68 years
(range, 35–87 years). Clinicopathological stages were determined
according to the TNM classification system of the Union for
International Cancer Control (UICC). The mean follow-up period was
17.2 months (range, 0.4–153.1 months). Forty-six patients did not
receive any postoperative chemotherapy, whereas 59 patients
received adjuvant chemotherapy following surgery; 34 patients
received gemcitabine, 14 received uracil/tegafur, 6 received
gemcitabine and tegafur/gimeracil/oteracil potassium, 3 received
uracil/tegafur and gemcitabine and 2 received fluorouracil.
 | Table INormal, PanIN and PDAC cases. |
Table I
Normal, PanIN and PDAC cases.
| N |
|---|
| Normal pancreatic
tissue | 6 cases (53
ducts) |
| PanIN | 41 cases |
| PanIN-1 | 32 ducts |
| PanIN-2 | 50 ducts |
| PanIN-3 | 18 ducts |
| Conventional type of
PDAC | 105 cases |
| Adenosquamous
carcinoma | 7 cases |
| Anaplastic
carcinoma | 1 case |
Two PDAC subtypes, adenosquamous carcinomas (N=7)
and anaplastic carcinoma (N=1), were separately analyzed. The
normal pancreatic tissues (N=6) were obtained from patients who
underwent surgery for ectopic spleen. PanIN tissues (N=41) were
obtained from both PDAC and normal tissues. This study was carried
out in accordance with the principles embodied in the Declaration
of Helsinki, 2008; each patient gave informed consent for the use
of the pancreatic tissues.
Immunohistochemistry
Paraffin-embedded sections (3.5 μm) were subjected
to immunohistochemistry (IHC). After deparaffinization, antigen
retrieval was performed (except for nestin) at 121°C for 15 min in
a sodium citrate buffer solution (pH 6.0). Endogenous peroxidase
activity was blocked by incubation for 30 min with 0.3% hydrogen
peroxide in methanol. The tissue sections were then incubated with
each antibody in phosphate-buffered saline (PBS) containing 1%
bovine serum albumin (BSA) overnight at 4°C. The dilutions of each
primary antibody are listed in Table
II. Bound antibodies were detected with Simple Stain MAX PO (R)
or (M) reagent, using diaminobenzidine tetrahydrochloride as the
substrate. The sections were then counterstained with Mayer’s
hematoxylin. Negative control tissue sections were prepared by
omitting the primary antibody.
 | Table IIAntibodies and their application for
immunohistochemistry and flow cytometry. |
Table II
Antibodies and their application for
immunohistochemistry and flow cytometry.
| | Dilution
|
|---|
| Primary
antibodies | Isotype control | IHC | FCM |
|---|
| CD24, Santa Cruz
Biotechnology, SC-19585 | mouse IgG1 | 1:100 | 1:300 |
| CD44, R&D
Systems, BBA10 | mouse IgG2A | 1:1000 | 1:300 |
| CD133, Abcam,
ab19898 | rabbit IgG | 1:800 | 1:300 |
| CXCR4, Abcam,
ab2074 | rabbit IgG | 1:200 | 1:150 |
| ESA, Abnova,
H4072-B02P | mouse IgG | 1:100 | 1:60 |
| Nestin, R&D
Systems, MAB1259 | mouse IgG1 | 1:500 | 1:750 |
IHC staining was evaluated independently by three
investigators (S.K. and Y.M. for PanIN and Y.M and T.I. for PDAC)
who were blind to clinical and outcome data. Percentages of
positive cells (0–100%) were evaluated for each stained sample. For
PDACs, evaluation was performed within the tumor area of an
individual slide. For PanINs and normal ducts, one or more ducts
were evaluated from one slide (10) and each case or duct was subdivided
into high- and low-expression groups according to the median
percentage of positive cells for each marker.
PDAC cell lines
KLM-1, PANC-1, MIA PaCa-2, PK-1, PK-45H and PK-8
PDAC cell lines were obtained from the Cell Resource Center for
Biomedical Research, Institute of Development, Aging and Cancer,
Tohoku University (Sendai, Japan), and Capan-1 was purchased from
the American Type Culture Collection. The AsPC-1 cell line was
obtained from Dainippon Sumitomo Pharma Co. Ltd. (Osaka, Japan).
The cells were grown in RPMI-1640 medium containing 10% fetal
bovine serum (FBS) at 37°C under a humidified 5% CO2
atmosphere. Capan-1 cells were incubated in the same medium with
15% FBS.
Quantitative real-time PCR of CSC markers
in pancreatic cancer cells
All PDAC cells were seeded at 2.5×105
cells per 60-mm dish, and were incubated for 48 h. Total RNA
extraction was performed using NucleoSpin RNA II. Then, cDNA
synthesis was performed using a High Capacity cDNA Reverse
Transcription kit, following the manufacturer’s protocol.
Quantitative real-time PCR (qRT-PCR) was performed using the
StepOnePlus Real-time PCR systems (Applied Biosystems, Inc.). The
PCR reaction mixture (18 μl), containing 2 μl of template cDNA, 10
μl of TaqMan Fast Universal PCR Master Mix, and 1 μl of the TaqMan
Gene Expression assay for CD24, CD44, CD133, CXCR4, ESA, or nestin,
was placed in a 96-well reaction plate. 18S rRNA, as the internal
positive control, was amplified using the TaqMan gene expression
assay. The optimized program involved denaturation at 95°C for 20
sec, followed by 40 cycles of amplification (95°C for 1 sec and
60°C for 20 sec). Results were expressed as target/18S rRNA, as an
internal standard concentration ratio. Gene expression measurements
were performed in triplicate.
Flow cytometry
For flow cytometry (FCM), we used the same
antibodies as were used for IHC (Table
II). CD24, CD44, CD133, CXCR4, and nestin antibodies were
labeled with allophycocyanin (APC) using the Zenon mouse or rabbit
IgG Labeling kit, and ESA antibody was labeled with FITC using the
SureLink Labeling kit according to the manufacturer’s protocol.
Cells were incubated for 20 min at 4°C in PBS containing 10% human
serum. Cells (5×105 cells) were then centrifuged briefly
to remove the serum-containing medium, and incubated with antibody
for 60 min in the dark at 4°C; 1 μg of propidium iodide was added
to label dead cells. To stain nestin, cells were fixed with 4%
paraformaldehyde, and incubated with the anti-nestin antibody and
0.1% Triton-X for 60 min in the dark at room temperature. We
prepared isotype control-treated cells as a negative control
(Table II). Each marker’s
expression was determined using a BD FACSAria II flow cytometer (BD
Bioscience, Franklin Lakes, NJ, USA) and analyzed using FlowJo
version 7.6.4 (Tree Star, Inc., Ashland, OR, USA).
Statistical analysis
Results for cell proliferation are shown as mean ±
SE, and the data between different groups were compared using the
Student’s t-test. Whenever indicated, the Chi-square test and
Fisher’s exact test were used to analyze the correlation between
marker expression and clinicopathological features. Cumulative
survival rates were calculated by the Kaplan-Meier method, and the
significance of differences in survival rate was analyzed by the
log-rank test. Data between multiple groups were compared using
one-way ANOVA, then were analyzed using a post-hoc test. P<0.05
was considered significant in all analyses. Computations were
performed using the StatView J version 5.0 software package (SAS
Institute, Inc., Cary, NC, USA).
Results
Immunohistochemistry of CSCs markers in
normal, PanINs and PDAC
First, we conducted immunohistochemical analyses of
various CSC markers, in order to confirm the localizations and
differences among normal, PanIN, and PDAC tissues. Normal
pancreatic tissues were mostly negative for CD24, CD44, CXCR4, and
nestin, whereas CD133 and ESA were relatively highly expressed in
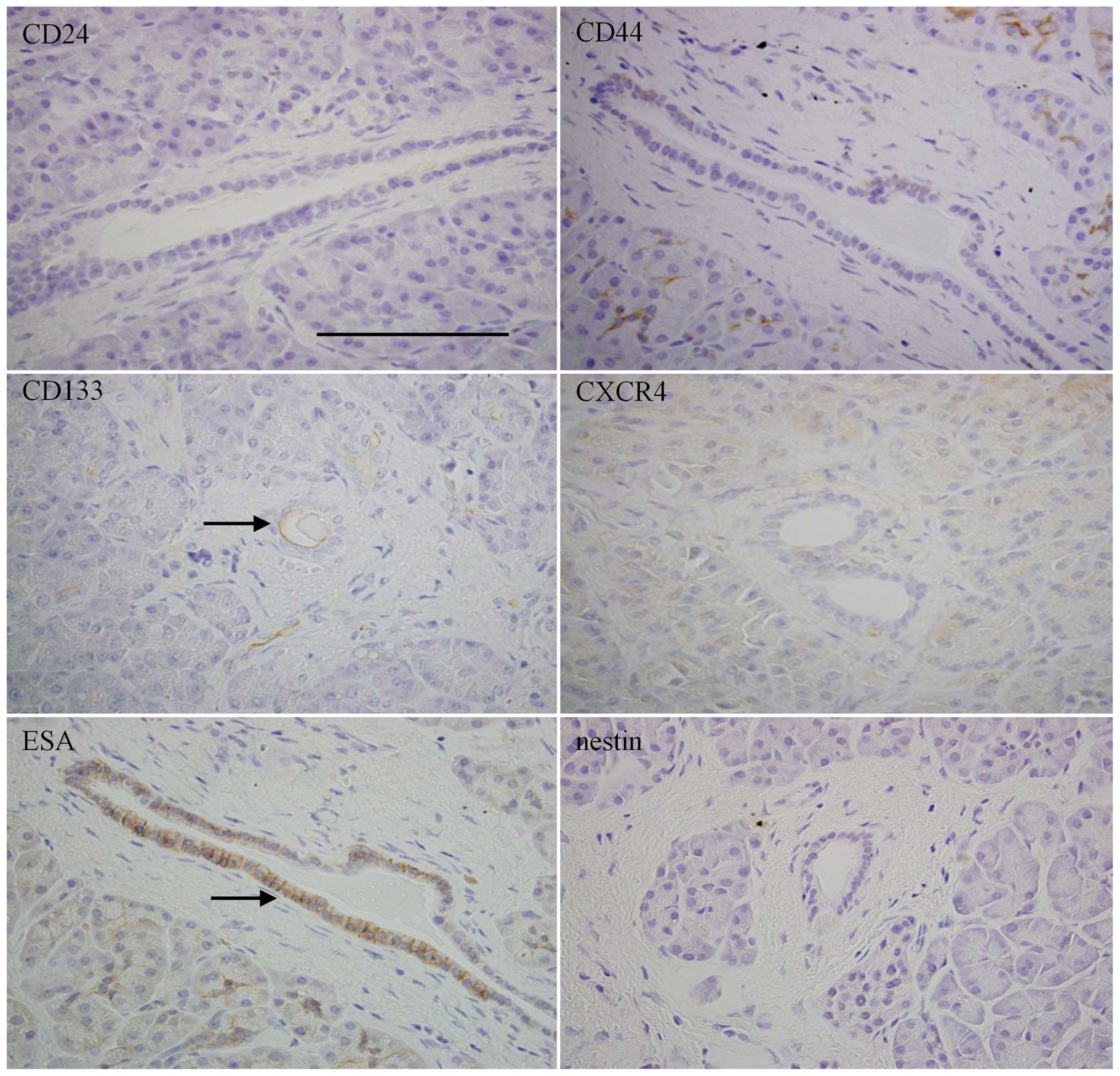
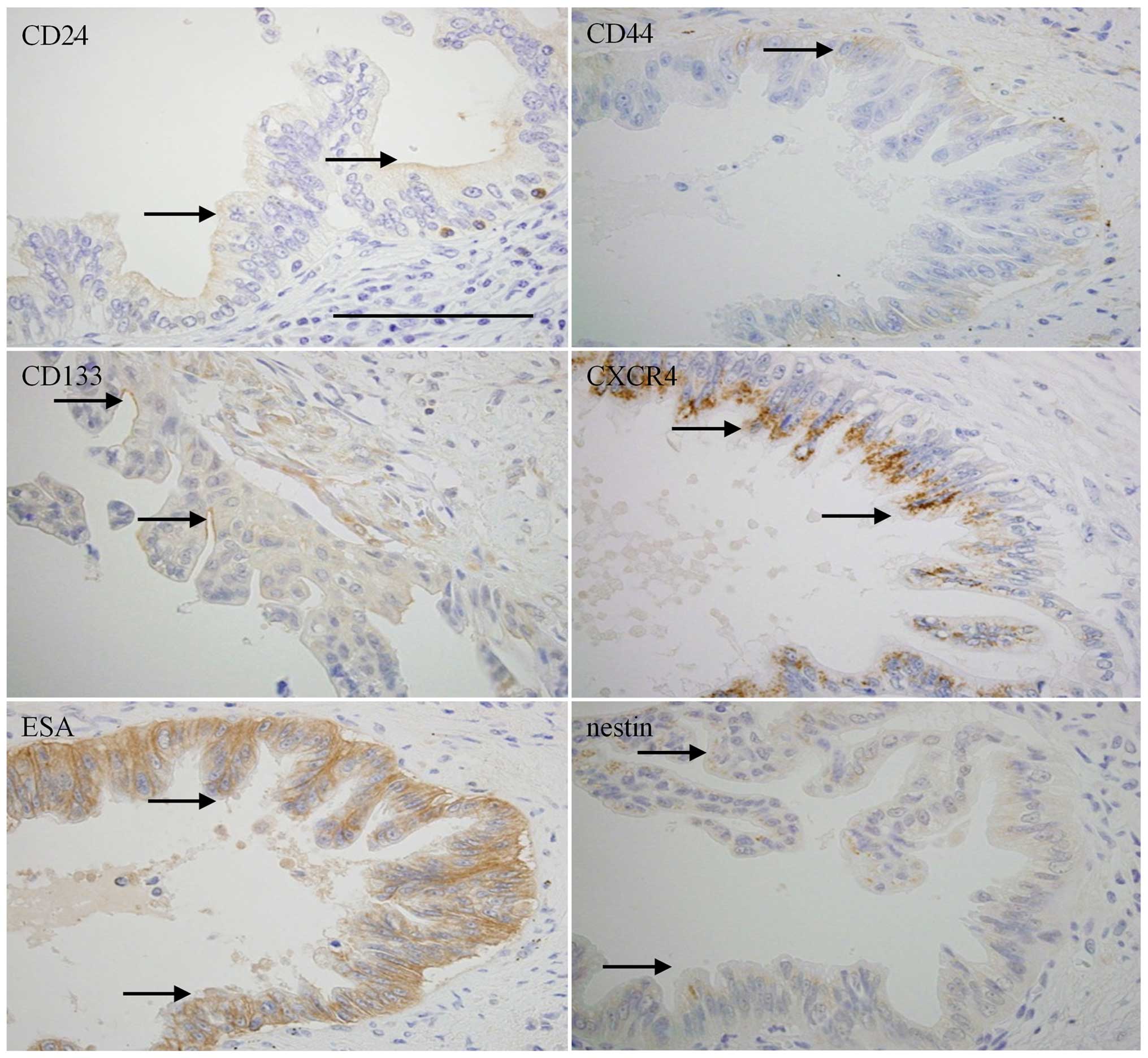
ductal cells (Fig. 1). PanIN-3
lesions showed weakly positive expressions of CD44, CD133, and
nestin in ductal cells (Fig. 2).
CD24, CXCR4 and ESA were moderately to strongly expressed in the
ductal cells of PanIN-3 lesions, with different intracellular
localization patterns: CD24 in luminal surface of cell membrane,
CXCR4 in cells with a granular staining pattern, and ESA diffusely
distributed both in cell membrane and cytoplasm (Fig. 2). PDAC showed expressions of CD24,
CD44, and CD133 in cell membranes of cancer cells; CXCR4 and nestin
were expressed in cytoplasm, and ESA was expressed in both
cytoplasm and cell membrane (Fig.
3).
Next, we compared the percentages of
immunohistochemical expression of each CSC marker in normal, PanIN,
and PDAC tissues (Table I;
Fig. 4). The expression levels of
CD24, CD44, CXCR4, ESA, and nestin showed tendencies to increase
according to the malignancy grade of PanINs, but CD133 showed an
opposite trend (Fig. 4). As
compared with normal, PanIN-1, and PanIN-2 tissues, significantly
increased CD24 expression was observed in PanIN-3 and PDAC
(Fig. 4A; **P<0.01).
Expressions of CD44 and CXCR4 were significantly increased in
PanIN-2, PanIN-3 and PDAC (Fig. 4B and
D; *P<0.05; **P<0.01). ESA staining
was relatively stronger compared to the other markers, and all
grades of PanINs and PDAC exhibited significantly higher positivity
than normal tissues (Fig. 4E;
*P<0.05; **P<0.01). PDAC showed
significantly higher positivity for nestin compared to normal,
PanIN-1 and PanIN-2 tissues (Fig.
4F; **P<0.01). In contrast to the other markers,
CD133 showed highest positivity in normal epithelial duct (Fig. 4C; **P<0.01).
To confirm that the PanIN lesions selected in the
present study possessed high proliferative ability and that they
exist as pre-cancerous lesions for PDAC, we analyzed Ki 67- and
PCNA-labeling indices. Both Ki 67- and PCNA-labeling indices
increased along with the PanIN grades, and PDAC showed
significantly higher proliferative activity than normal and PanIN
tissues (data not shown). These findings suggest that CSC markers,
including CD24, CD44, CXCR4, ESA and nestin, are involved in
proliferative activity or carcinogenesis stages of PDAC via PanIN
lesions.
CSC markers and clinicopathological
features in PDAC
Next, we examined the correlations between each CSC
marker and the clinicopathological features of PDACs. Nestin
exhibited the lowest expression, followed, in order of increasing
expression, by CD133, CD44, CD24, CXCR4 and ESA (Table III). The CSC marker expression
levels widely varied in PDAC tissues; thus, we analyzed
clinicopathological aspects for the high- and low-expression
groups, using each marker’s median as a cut-off. Correlations
between the expression levels of each CSC marker and the
clinicopathological characteristics of conventional PDAC were
analyzed. A statistically significant correlation was found between
CD133 expression levels in PDAC and pT stages (P= 0.0494), but
these data may be biased by great differences in the number of
cases for each T stage. As not expected, ESA and CXCR4 expressions
in PDAC were inversely associated with histological grade, with
well-differentiated PDAC tending to express CXCR4 and ESA (P=0.0112
and P=0.0058, respectively). Regarding metastatic features, more
severe status of venous invasion was associated with higher CD133
expression (P= 0.0056) and lower ESA expression (P=0.0243). There
were no significant differences between expression of CSC markers
and gender, age and TNM stage. These results may indicate that the
expressions of some CSC markers are associated in a different
manner with differentiation and invasiveness of PDAC.
 | Table IIIExpression of each CSC marker in IHC
and FCM. |
Table III
Expression of each CSC marker in IHC
and FCM.
| CD24 | CD44 | CD133 | CXCR4 | ESA | Nestin |
|---|
| IHC | | | | | | |
| Normal | 0.6±0.6 | 3.0±1.2 | 32.3±3.9 | 4.2±1.1 | 21.7±3.7 | 0.1±0.1 |
| PanIN-1 | 2.0±1.2 | 10.5±2.7 | 18.5±4.9 | 15.9±3.5 | 54.9±5.6 | 3.4±1.3 |
| PanIN-2 | 4.4±2.0 | 12.2±2.4 | 21.6±4.5 | 26.7±4.1 | 75.1±3.9 | 1.0±0.3 |
| PanIN-3 | 33.1±8.0 | 16.7±4.3 | 20.3±7.3 | 48.9±6.7 | 86.1±5.8 | 8.6±3.1 |
| PDAC | 26±2.1 | 15.1±1.5 | 14.5±1.6 | 35.6±2.3 | 60.6±2.6 | 13.5±1.7 |
| FCM | | | | | | |
| PDAC | 20.6±13.2 | 77.4±16.2 | 0.54±0.54 | 23.8±11.9 | 35.1±29.4 | 4.4±3.5 |
CSC markers and prognosis of PDAC
cases
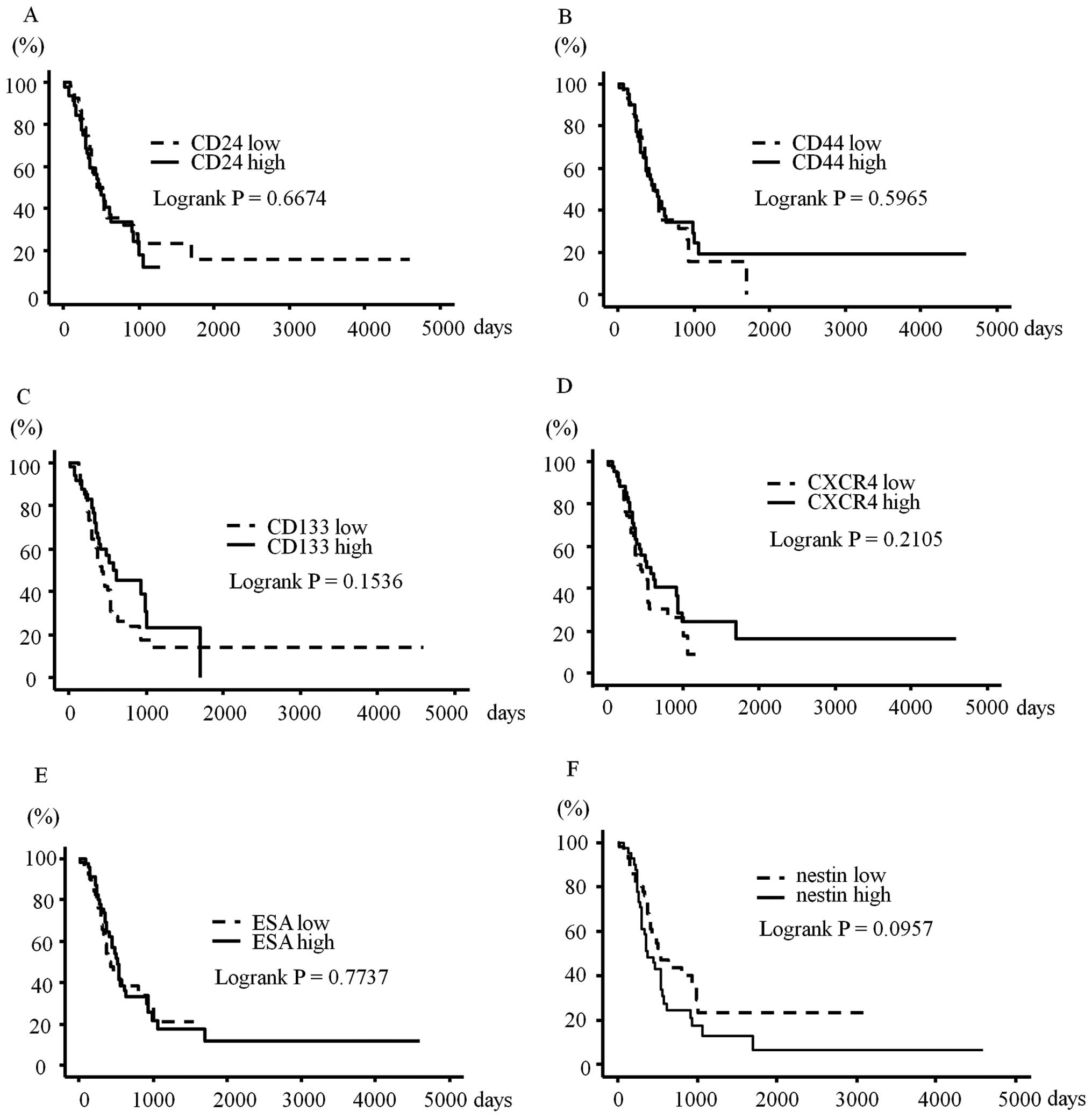
Overall survival was not significantly correlated
with any of the six CSC markers (Fig.
5). Disease-free survival was not correlated with expression of
CSC markers (data not shown). However, higher nestin expression
tended to be associated with worse overall and disease-free
survivals (P=0.0957 and P=0.0840, respectively). Previous reports
have shown that analysis of co-expressions of some CSC markers can
be more effective for detecting the CSC fraction (5,10);
therefore, we also performed survival analysis using co-expressions
of CD24, CD44, and ESA, or co-expression of CXCR4 and CD133. These
analyses did not find any significant correlations with survival
(data not shown). Furthermore, we performed the survival analyses
with the cut-off for positivity set at 10 and 30%, but still found
no statistically significant correlation (data not shown).
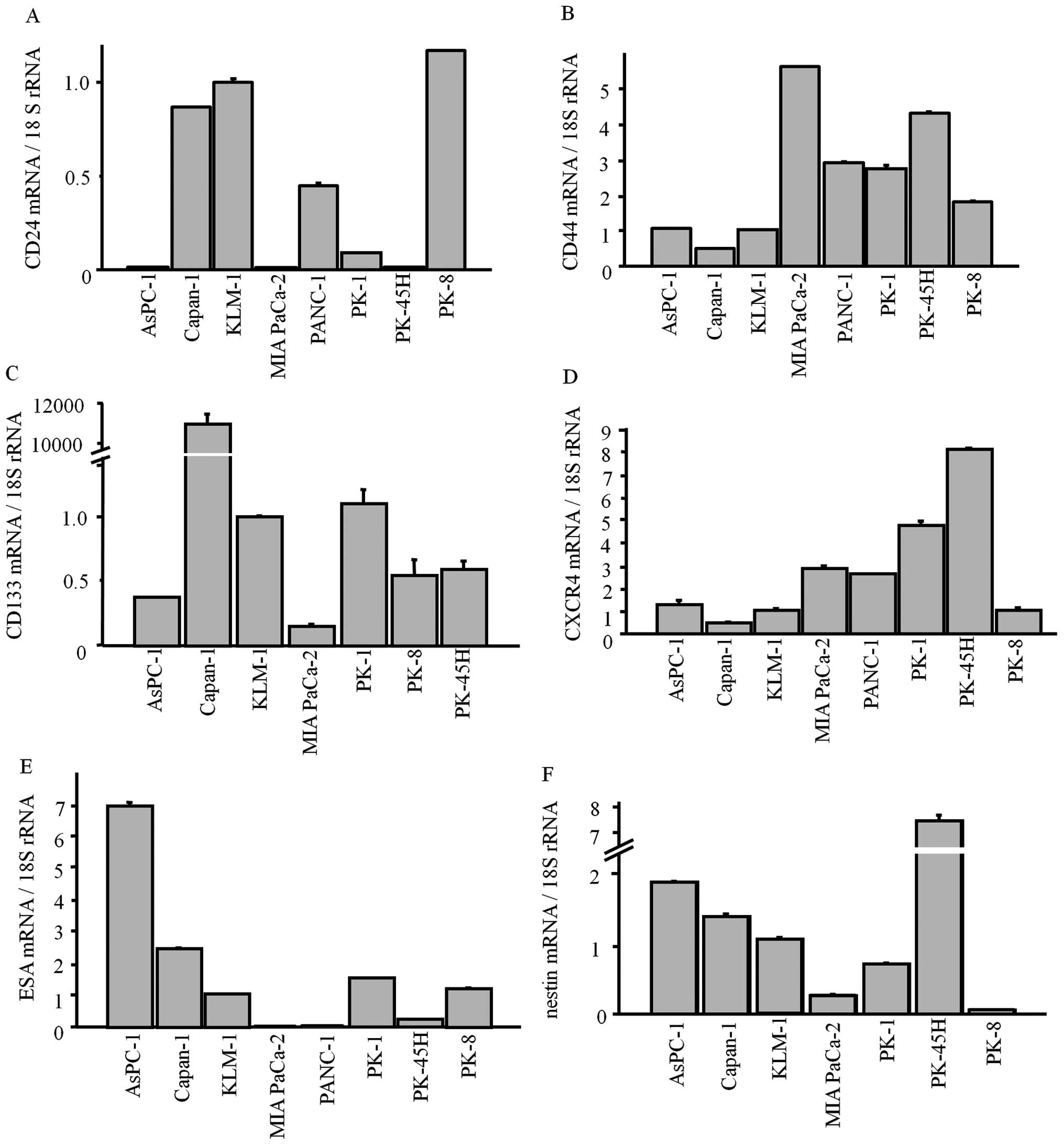
CSC markers in PDAC cell lines
Next, we examined the mRNA levels of each CSC
marker, and the corresponding protein levels in established PDAC
cell lines. All CSC markers were expressed in the PDAC cell lines
at various levels (Fig. 6). As in
the immunohistochemistry results, CD133 was weakly expressed in all
lines except for Capan-1 (Fig.
6C).
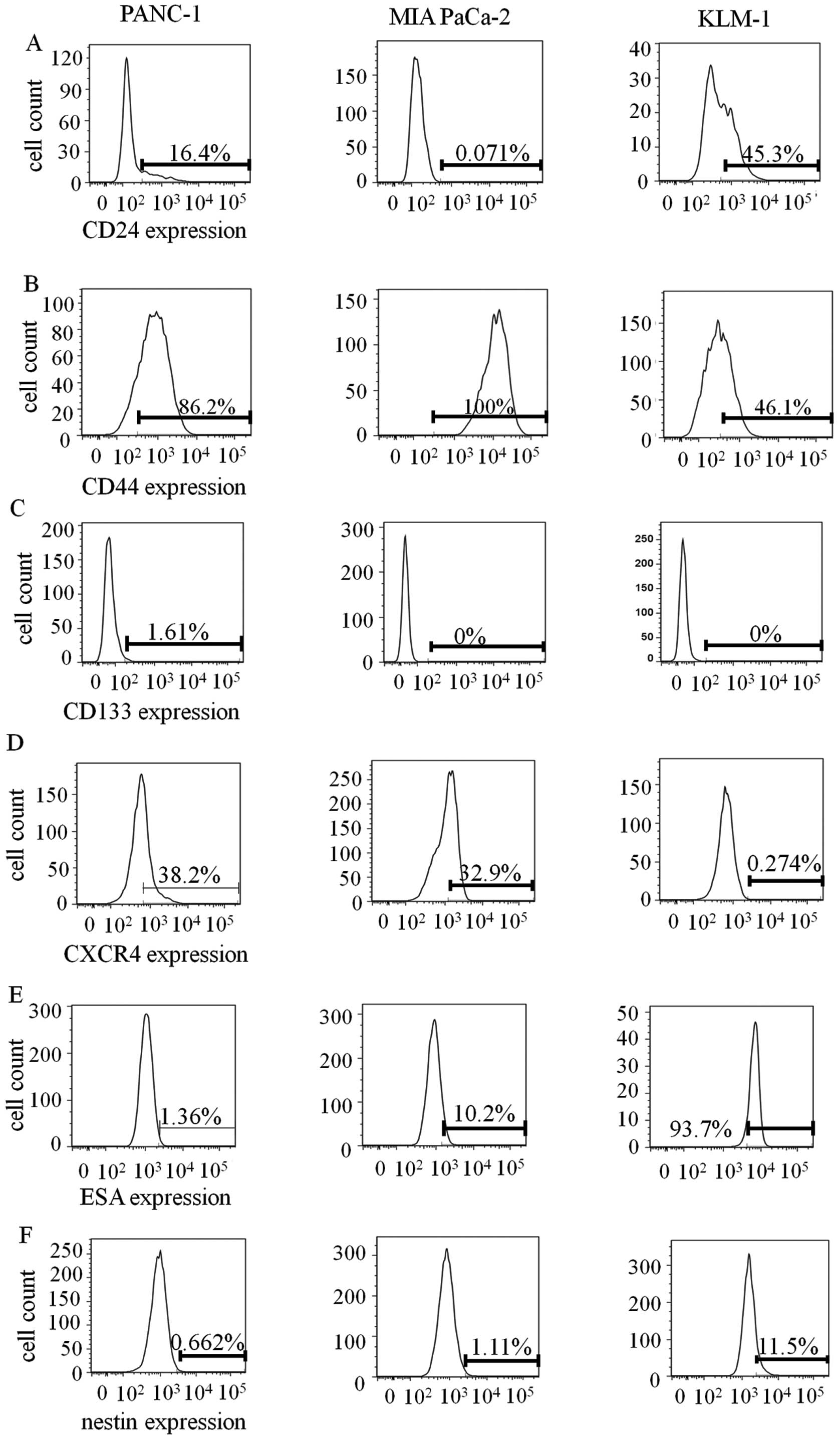
We also conducted FCM using PDAC cell lines, PANC-1,
MIA PaCa-2 and KLM-1 (Fig. 7).
Expression levels of each CSC marker varied depending on the cell
line. Protein levels of CD24, CD44, CXCR4, and nestin were measured
by FCM in PANC-1, MIA PaCa-2, and KLM-1 cells and were correlated
with their mRNA levels. Consistent with IHC and qRT-PCR analysis,
FCM showed very low CD133 expression (Fig. 7C). On the other hand, we observed
relatively high expression levels of CD44 and nestin compared to
other markers, which differed from the IHC results (Fig. 7B and F). The expression levels of
each marker were detected in the following order of increasing
percentage in PDAC cell lines: CD133 < nestin < CD24 <
CXCR4 < ESA < CD44 (Table
III). The order of increasing percentages of CSC markers in
PDAC cell lines and PDAC tissues were not identical.
Immunohistochemistry of PDAC
subtypes
Each CSC marker showed a tendency of having a
different expression pattern depending on histological types or
differentiation of PDAC cells, and expressions widely varied
between each case and cell line. These findings led us to
hypothesize that histological variety of PDACs determines the CSC
marker expression levels. Therefore, we analyzed two PDAC subtypes,
adenosquamous carcinoma (N=7) and anaplastic carcinoma (N=1), which
consist of histologically different cancer cells within an
individual sample. In adenosquamous carcinoma, CD133 and ESA were
expressed predominantly in the adenocarcinoma component, whereas
CD44 was far more strongly expressed in the squamous cell carcinoma
component (Fig. 8). CD24, CXCR4,
and nestin were expressed at similar levels in each component. ESA
showed significantly higher expression in the adenocarcinoma
component than in the squamous cell carcinoma component (Table IV, P= 0.0048). In anaplastic
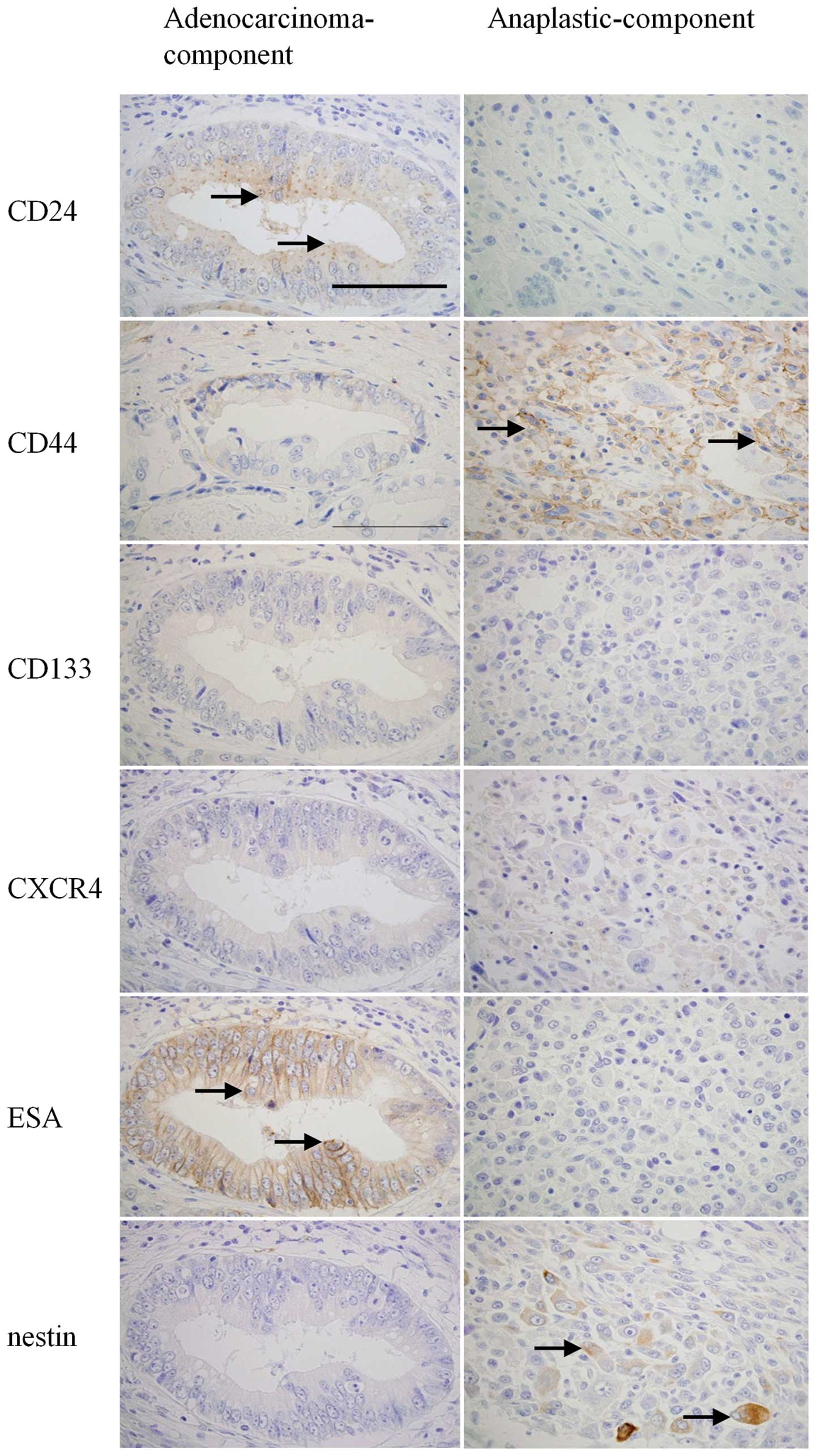
carcinoma, CD24 and ESA were predominantly expressed in the
adenocarcinoma component, while CD44 and nestin were predominantly
expressed in the anaplastic component (Fig. 9). These results indicate that
expression of each CSC marker widely varies in human tissues
depending on histological type.
 | Table IVExpression of stem cell markers in
each component of adenosquamous carcinomas. |
Table IV
Expression of stem cell markers in
each component of adenosquamous carcinomas.
| CD24 | CD44 | ESA | CD133 | CXCR4 | Nestin |
|---|
| Adenocarcinoma
component | 7.9±2.1 | 12.9±4.1 | 38.6±7.3a | 17.1±6.1 | 16.4±4.9 | 3.2±1.2 |
| Squamous cell
carcinoma component | 5.0±1.6 | 22.5±4.4 | 23.6±5.4 | 3.9±1.8 | 13.2±5.2 | 3.2±0.9 |
Discussion
Identification of pancreatic CSCs is important for
developing new therapies and elucidating the putative origin cell
of PDAC (4). The molecules that
were analyzed in the present study, have been proven to exhibit
pancreatic CSC-specific expression in previous in vitro
and/or in vivo studies. The biological roles of each CSC
marker are widely different. CD24 and CD44 function as adhesion
molecules (19,20); CD133 is related to cell polarity,
which is required for cell movement and asymmetric cell division
(21); CXCR4 functions as a
chemokine receptor (10); ESA is
considered to be a morphoregulatory molecule (22), and nestin, a class VI intermediate
filament proteins, is a marker of exocrine progenitors of normal
pancreatic tissues and involved in cell migration and cell cycle
(18,23,24).
In the present study, the expression level of each
CSC marker, except for CD133, increased with increasing progression
through the PanIN-to-PDAC sequence. ESA showed significantly higher
expression starting from PanIN-1, CD44 and CXCR4 from PanIN-2, CD24
from PanIN-3, and nestin from PDAC (not in PanINs). We also
confirmed that proliferative activity increased according to the
progression of the PanIN-to-PDAC sequence in the studied tissue
samples. These results indicate that each CSC marker is expressed
at different stages of PDAC carcinogenesis, and that the
expressions of CSC markers may relate to the proliferative activity
of PanIN and PDAC.
Some of the CSC markers were related to venous
invasion or histological grade; CD133 was positively correlated
with venous invasion, and CXCR4 and ESA were correlated with
well-differentiated PDAC. These data indicate that these markers
may be essential for metastasis or differentiation of PDACs, as
previously reported (10,25). Our analyses of adenosquamous
carcinoma and anaplastic carcinoma cases clearly showed different
expression patterns of CSC markers in each cellular component.
In conclusion, our findings show that CSC marker
expressions are related to carcinogenesis via the PanIN-to-PDAC
sequence. Furthermore, CSC markers may contribute to proliferation,
differentiation, invasiveness, or histological types of PDAC. Our
present results did not indicate any single marker as being most
important and specific for PDAC. A major CSC marker can vary
depending on various features of cancer cases; consequently,
analysis of expression level and localization of a CSC marker in
each step of PDAC progression may prove useful for developing new
detection and therapeutic modalities for PDAC.
Acknowledgements
The authors thank Ms. Y. Kawamoto, Ms.
T. Suzuki, Ms. K. Kawahara and Mr. Y. Yanagisawa (Departments of
Pathology and Integrative Oncological Pathology) for their
excellent technical assistance. We express our appreciation to Dr
Shin-ichi Tsuchiya (Division of Surgical Pathology, Nippon Medical
School Hospital) for preparing tissue blocks. This study was
supported by a Grant-in-Aid for Young Scientists (A, no. 22689038
to Y.M.), a Grant-in-Aid for Challenging Exploratory Research (No.
23650604 to Y.M.), and a Grant-in-Aid for Scientific Research (C,
no.22591531 to T.I.) from the Japan Society for the Promotion of
Science.
References
|
1
|
Bissell MJ and Labarge MA: Context, tissue
plasticity, and cancer: are tumor stem cells also regulated by the
microenvironment? Cancer Cell. 7:17–23. 2005.PubMed/NCBI
|
|
2
|
Reya T, Morrison SJ, Clarke MF and
Weissman IL: Stem cells, cancer, and cancer stem cells. Nature.
414:105–111. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
3
|
Jemal A, Siegel R, Ward E, et al: Cancer
statistics, 2008. CA Cancer J Clin. 58:71–96. 2008. View Article : Google Scholar
|
|
4
|
Hruban RH, Maitra A and Goggins M: Update
on pancreatic intraepithelial neoplasia. Int J Clin Exp Pathol.
1:306–316. 2008.PubMed/NCBI
|
|
5
|
Li C, Heidt DG, Dalerba P, et al:
Identification of pancreatic cancer stem cells. Cancer Res.
67:1030–1037. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Huang P, Wang CY, Gou SM, Wu HS, Liu T and
Xiong JX: Isolation and biological analysis of tumor stem cells
from pancreatic adenocarcinoma. World J Gastroenterol.
14:3903–3907. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ikenaga N, Ohuchida K, Mizumoto K, et al:
Characterization of CD24 expression in intraductal papillary
mucinous neoplasms and ductal carcinoma of the pancreas. Hum
Pathol. 41:1466–1474. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hong SP, Wen J, Bang S, Park S and Song
SY: CD44-positive cells are responsible for gemcitabine resistance
in pancreatic cancer cells. Int J Cancer. 125:2323–2331. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Li C, Wu JJ, Hynes M, et al: c-Met is a
marker of pancreatic cancer stem cells and therapeutic target.
Gastroenterology. 141:2218–2227.e5. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hermann PC, Huber SL, Herrler T, et al:
Distinct populations of cancer stem cells determine tumor growth
and metastatic activity in human pancreatic cancer. Cell Stem Cell.
1:313–323. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Olempska M, Eisenach PA, Ammerpohl O,
Ungefroren H, Fandrich F and Kalthoff H: Detection of tumor stem
cell markers in pancreatic carcinoma cell lines. Hepatobiliary
Pancreat Dis Int. 6:92–97. 2007.PubMed/NCBI
|
|
12
|
Kim MP, Fleming JB, Wang H, et al: ALDH
activity selectively defines an enhanced tumor-initiating cell
population relative to CD133 expression in human pancreatic
adenocarcinoma. PLoS One. 6:e206362011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Maeda S, Shinchi H, Kurahara H, et al:
CD133 expression is correlated with lymph node metastasis and
vascular endothelial growth factor-C expression in pancreatic
cancer. Br J Cancer. 98:1389–1397. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Marechal R, Demetter P, Nagy N, et al:
High expression of CXCR4 may predict poor survival in resected
pancreatic adenocarcinoma. Br J Cancer. 100:1444–1451. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kawamoto M, Ishiwata T, Cho K, et al:
Nestin expression correlates with nerve and retroperitoneal tissue
invasion in pancreatic cancer. Hum Pathol. 40:189–198. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Clevers H: The cancer stem cell: premises,
promises and challenges. Nat Med. 17:313–319. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hruban RH, Adsay NV, Albores-Saavedra J,
et al: Pancreatic intraepithelial neoplasia: a new nomenclature and
classification system for pancreatic duct lesions. Am J Surg
Pathol. 25:579–586. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Carriere C, Seeley ES, Goetze T,
Longnecker DS and Korc M: The Nestin progenitor lineage is the
compartment of origin for pancreatic intraepithelial neoplasia.
Proc Natl Acad Sci USA. 104:4437–4442. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Aigner S, Sthoeger ZM, Fogel M, et al:
CD24, a mucin-type glycoprotein, is a ligand for P-selectin on
human tumor cells. Blood. 89:3385–3395. 1997.PubMed/NCBI
|
|
20
|
Ponta H, Sherman L and Herrlich PA: CD44:
from adhesion molecules to signalling regulators. Nat Rev Mol Cell
Biol. 4:33–45. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
21
|
Immervoll H, Hoem D, Sakariassen PO,
Steffensen OJ and Molven A: Expression of the ‘stem cell marker’
CD133 in pancreas and pancreatic ductal adenocarcinomas. BMC
Cancer. 8:482008.
|
|
22
|
Raffel A, Eisenberger CF, Cupisti K, et
al: Increased EpCAM expression in malignant insulinoma: potential
clinical implications. Eur J Endocrinol. 162:391–398. 2010.
View Article : Google Scholar
|
|
23
|
Ishiwata T, Matsuda Y and Naito Z: Nestin
in gastrointestinal and other cancers: effects on cells and tumor
angiogenesis. World J Gastroenterol. 17:409–418. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Matsuda Y, Naito Z, Kawahara K, Nakazawa
N, Korc M and Ishiwata T: Nestin is a novel target for suppressing
pancreatic cancer cell migration, invasion and metastasis. Cancer
Biol Ther. 11:512–523. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Lee HJ, You DD, Choi DW, et al:
Significance of CD133 as a cancer stem cell markers focusing on the
tumorigenicity of pancreatic cancer cell lines. J Korean Surg Soc.
81:263–270. 2011. View Article : Google Scholar : PubMed/NCBI
|