Contents
Telomere structure
Telomeric binding proteins
Mechanisms of telomeric lengthening in mammals
Telomeres in cell immortalization and cancer
Other diseases associated with telomerase
activity
Telomerase: structure and function
Other telomerase functions
Conclusion
Telomere structure
The ends of the eukaryotic chromosomes have special
properties when compared with chromosomal ends created by
stochastic breakdown. More than 70 years ago Muller and McClintock
established that the ends of eukaryotic chromosomes had a different
structure (1,2). Using X-rays in order to generate
chromosomical rearrangements in Drosophila, Muller could not
recover the chromosomes that were deleted in one end, in contrast
with the chromosomes that have internal deletions or
translocations. He concluded that this end should have a special
structure needed for the integrity of the chromosome and he named
it ‘telomere’. Few years after, McClintock demonstrated in maze
that broken chromosomes could stick among them to form a dicentric
chromosome highly instable by frequents breakdowns during mitosis,
resulting in the loss of a part of the chromosome. With these
experiments he concluded that one essential function of the
telomeres was to protect the fusion of the extremes of the
chromosomes.
Decades later, different molecular techniques
revealed that the extremes of the chromosomes consisted in
repetitions rich in guanidine (3).
The term telomere makes reference to a big
nucleoprotein complex found in the extremes of the chromosomes,
where their structure is different from the rest of the chromatin
(4). Functional telomeres are
stable structures not subjected to degradation, recombination or
fusion with other chromosomal ends. Furthermore, they are not
detected by the systems of damage recognition of DNA, although
technically, a chromosomal end constitutes a cut in the double
chain of DNA (5). These properties
of the eukaryotic telomeres are identified usually as the ‘capping
function’ exerted in the chromosomal ends (4). Telomeric DNA of virtually all the
eukaryotic organisms consists in short and repetitive sequences
(6). The primary sequence and the
organization of such repetitions are clearly related in many
species, since such repetitions usually contain groups of 3 or more
G, and the strand that contains them (called string G enriched)
always constitute the end 3′ of the chromosomes (6).
The number of repetitions in the telomere varies
widely among different organisms and also among different telomeres
in one organism. For all vertebrates, including humans, the
repetitive sequence is d(TTAGGG) (7). Individual telomeres may contain only
a few kb of repetitive sequence in the form of double chain, as in
some transformed cell lines or even >100 kb, as in some mouse
cells. Most of that DNA is organized in nucleosomes and only the
more distal part could be found in a special conformation as
non-nucleosomic chromatin that could be analogous to the special
structure found in telomeres of yeast or
Tetrahymena(8).
The G-rich strand of telomeric DNA is always
oriented 5′-3′ towards the terminal portion of the chromosome and
had a protrudent extreme of ∼200 nucleotides (9) as consequence of the problem of
terminal replication. The 3′ protruding G-rich strand can form
complex structures of telomeres, the G-quadruplexes.
G-quadruplexes, assume different conformations, clearly described
by Hänsel et al(11). They
are multi-stranded structures held together by square planes of
four guanines (G-quartets) interacting by forming Hoogsteen
hydrogen bonds (Fig. 1) (10,11).
Formation of such structures on telomeres can be a problem for DNA
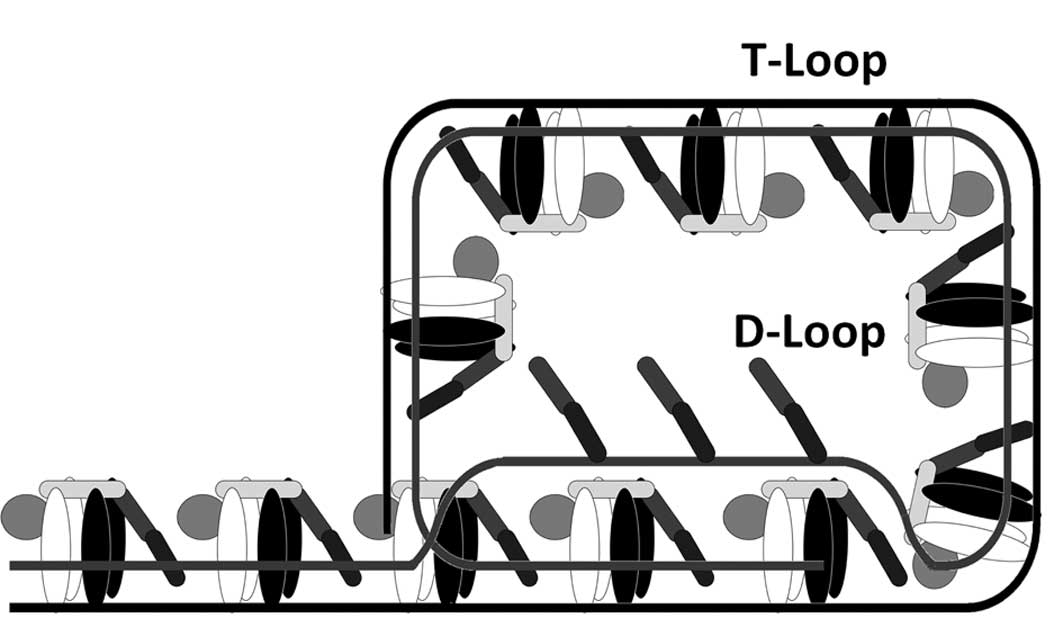
replication and telomere elongation by telomerase. Telomeres form
large loop structures called telomere loops, or T-loops which serve
to sequester the chromosome terminus (Fig. 2). Here, the single-stranded DNA
curls around in a long circle stabilized by telomere-binding
proteins. At the very end of the T-loop, the single-stranded
telomere DNA is held onto a region of double-stranded DNA by the
telomere strand disrupting the double-helical DNA and base pairing
to one of the two strands. This triple-stranded structure is called
a displacement loop or D-loop (10) (Fig.
2).
Telomeric binding proteins
Fig. 3 shows
different telomeric proteins that bind to mammal telomeres. One
general concept relates to the evolution of telomere-associated
proteins (12). Although telomere
function is conserved in different organisms, the architecture and
composition of telomere-associated proteins is remarkably varied
and seems to have changed rapidly along evolution (13). In humans, telomeres are bound by a
six-protein complex called shelterin, comprised of TRF1 and TRF2
which in turn recruits RAP1, TIN2, TPP1 and POT1 which interacts
with ss and ds telomeric DNA (14). Shelterin prevents the activation of
a DNA damage response (DDR) at chromosome ends and acts in the
regulation of telomerase activity at chromosome ends (15).
In human cells, the best known telomeric binding
proteins are TRF1 and TRF2. The first one to be identified was TRF1
(16). The C-terminal sequence of
this protein, also named teloboxes, recognize specifically a
telomeric DNA fragment, however, they require a dimerization in the
N-terminal end to bind firmly to the DNA. TRF1 is a negative
regulator of the telomeric length (17).
Another protein binding to the repeat TTAGGG is
TRF2, which is also a negative regulator of telomeric length
(18). TRF2 has unique functions
in the telomere as stabilizer of the protruding G string and is
able to prevent telomeric fusions (19). The overexpression of a dominant
negative mutant of TRF2 may cause premature senescence (20), and the activation of the apoptotic
cascade mediated by the protein kinase ATM and p53 (21). Their properties in the telomeric
length and in terminal protection could be related to its location
on the telomeric capping (22).
TRF1 and TRF2 do not allow telomerase activity,
thereby restricting or inhibiting telomere elongation (19). Also, they are involved in two
important pathways (ATM and ATR) which are potential sensors of DNA
damage. ATM is a member of the phosphoinositide 3-kinase related
protein kinase family (PIKK), along with ATR. These molecules have
serine/threonine protein kinase activity with S/TQ consensus
phosphorylation motifs on their specific substrates. ATM and ATR
are involved in inducing cell cycle arrest upon DNA damage response
pathway or through the activation of downstream kinases, Chk1 for
ATM and Chk2 for ATR. The ATM and ATR signalling kinases are
capable of inducing DNA damage response at telomeres in conditions
where shelterin activity is impaired or reduced, for example due to
telomerase shortening in senescent cells (23). ATM has a complex role at telomeres
interacting with both TRF1 and TRF2 having an effect on telomere
protection and TRF1-mediated telomere length regulation. On the
other hand, ATR pathway is triggered in response to DNA replication
stress, single stranded DNA replication stress and single stranded
DNA accumulation. The genetic analysis of the repression of the DNA
damage response in mouse cells has ascribed specific and separated
functions for TRF2 and POT1 (24).
To that effect POT1 inhibits ATR-mediated responses and TRF2
inhibits ATM.
TRF2 recruits in human telomeres the protein RAP1
whose overexpression causes telomeric elongation (25). Human Rap1 is encoded by the gene
TERF2IP, localized in chromosome 16 with pseudogenes in chromosomes
5 and 22. Rap1 is a component of the shelterin complex at mammalian
telomeres, but its in vivo role in telomere biology has
remained unknown up to date. Recently, Martinez et al have
shown that Rap1 deficiency is dispensable for telomere capping but
leads to increased telomere recombination and fragility (26). Besides its role at telomeres, RAP1
also has extratelomeric roles. Rap1 is a transcription factor that
controls the expression of glycolytic enzymes and ribosomal genes
(27). RAP1 not only binds to
telomeres but also to other non-telomeric sites preferentially
throughout the recognition of TTAGGG consensus motif (26). Extratelomeric RAP1 binding sites
are enriched at subtelomeric regions, highlighting a conserved role
for RAP1 in subtelomeric silencing. In addition, RAP1 was shown to
bind to non-coding regions in chromosomes 2, 11 and 17, which are
enriched in TTAGGG tandem repeats, raising the possibility that
RAP1 might prevent fragility and recombination at these genomic
sites. RAP1 can interact with factors other than TRF2 to help gene
transcriptional regulation, such as genes of the insulin secretion,
peroxisome proliferator-activated receptor (PPAR), signaling, and
growth hormone pathways. In another role beyond telomeres, RAP1 has
been shown to play a role as an essential modulator of the nuclear
factor-κβ (NF-κβ) mediated pathway (28).
In the case of TIN2, the gene that encodes this
protein is TINF2, formed by 9 exons in a sequence of 3032 bp,
giving origin to two transcripts, isoform 1 with 9 exons and an
alternative with 6 exons. Their functional difference is unknown.
Inhibition of PARsylation of TRF1, forms a tertiary complex with
tankyrases regulating its activity (29).
Human TPP1 is a protein encoded in humans by the ADC
gene in chromosome 16q22.1. It has 544 amino acids and a molecular
weight of 57.7 kDa. Recently Tejera et al have demonstrated
that this protein is needed for telomerase recruitment in
vivo(30).
POT1 is an acronym for ‘protection of telomeres’.
The gene of this protein localize in chromosome 7 in humans. It
contains 22 exons, giving place to 5 variants by alternative
splicing. Its higher level of expression is in testis, while the
lower is found in muscle and colon. TPP1 binds to POT1 by its
carboxyl end. This union is constant and it is needed for
localization of POT1 in telomeres and also for the regulation of
the telomeric length interacting with telomerase. In brief, POT1
regulates telomeric length acting as an activator or inhibitor of
telomerase depending the position of POT1 in the 3′ overhang. It
contributes to telomeric protection, inhibiting the formation of
anafasic bridges and avoiding the action of the DNA repair system
if it erroneously recognizes a 3′ overhang as damage (31).
Another important structural parameter governing
telomere function is that they also contain RNA, called TERRAs
(telomere repeat containing RNAs) (32). These RNAs are created from
telomeres themselves, through transcription from CpG islands with
promoter activity present in the subtelomeric regions (33). The transcription of TERRAs is
believed to be mediated by TRF1, through an interaction with Pol
II. It is unclear how TERRAs associate with telomeres.
Functionally, TERRAs are implicated in the formation of telomeric
heterochromatin, telomere protection and negative regulation of
telomerase. In addition to their roles on the telomeric chromatin,
TERRAs also exert competitive and non-competitive inhibition on
telomerase itself: they can hybridize with hTR, the telomerase RNA,
with high affinity and interact with the catalytic subunit
hTERT.
The shelterin components are quantitatively
associated with telomeres, require the TTAGGG repeats for assembly
and are present at telomeres throughout the cell cycle. However,
shelterin exerts its roles on telomere function through the
transient recruitment of accessory factors (34) and can be viewed for some aspects of
telomere function as an assembly line for them.
There are also TRF1 and TRF2 associated factors. The
main factor associated to TRF1 is tankyrase. Tankyrase is a
poly-ADP ribose onto target substrate and is particular to TRF1.
The enzyme is a molecule with well defined catalytic domain (PARP),
at the C-terminus, a His-Pro-Ser rich domain in the first
N-terminal 181 residues and, at the middle of the molecule, 24
ankyrin-repeats involved in protein-protein interactions. The
ankyrin repeats of tankyrase bind TRF1 through an interaction with
its N-terminal acidic domain and add the poly-ADP-ribose chain to
Glu residues (35). This specific
posttranslational modification leads to a significant decrease in
the affinity of TRF1 for the DNA, and results in the
ubiquitinization and proteasomal degradation of the protein.
Therefore, tankyrase is a positive regulator of telomere length
through its action of effectively removing TRF1 from telomeres.
Tankyrase has also other roles in the cytoplasm, where it
associates with the Golgi and PARsylate substrates, in a process
important for insulin-stimulated vesicle transport. In addition to
telomere length regulation and the timely events necessary for the
metaphase to anaphase transition, tankyrase 1 is also important for
the protection of telomeres. Also, tankyrase 1 has a closely
related homolog called tankyrase 2: the two molecules share a very
high degree of homology in their domains and tankyrase 2 differs
from tankyrase 1 in that it is lacking the N-terminal His-Pro-Ser
rich domain (36). Tankyrase 2 was
found to be present at telomeres, to have the capacity to PARsylate
TRF1 and to induce telomere elongation as effectively as tankyrase
1.
PINX1 is a TRF1-associated telomerase inhibitor
which associates with TRF1 through interaction with the F142 motif
present in the TRFH domain, and therefore represents one of the
activities able to bind this motif (37). The binding is highly specific as no
detectable association was found with TRF2. The domain in PINX1
responsible for the interaction with TRF1 (termed TID for
telomerase inhibitory domain) is present in the 75C-terminal
residues. The presence of the TID domain on PINX1 is needed and
sufficient for telomeric targeting through interactions with TRF1.
PINX1 is able to simultaneously interact with the telomerase
catalytic subunit providing the enzyme a physical link with TRF1
(38). PINX1 has been shown to
mediate telomere length control through inhibition of telomerase.
In cells, overexpression of PINX1 or of the TID domain leads to
telomere shortening, demonstrating that this factor is a negative
regulator of telomere length.
There are also TRF2-associated factors (Apollo, MRN
complex, PNUTS, MCPH1, WRN/FEN1 and ORC complex TERRA). Apollo
(39) is a well-characterized
5′-3′ exonuclease domain and a metallo-β-lactamese motif. Apollo is
also involved in interstrand crosslink repair, besides it roles at
telomeres (40). The recruitment
of Apollo to telomeres is completely dependent on TRF2. The
interaction between the two molecules occurs between the F120 of
TRF2 and a TBM present at the C-terminus of Apollo (37). Apollo presumably contributes to the
formation of the overhang on leading strand telomeres, and the
effective replication through the telomeric tract (41).
Another associated factor is the MRN complex,
composed of MRE11, RAD50 and NBS1, which has been implicated also
in generating the overhang on the leading telomeric strand, similar
to Apollo (42). However, a major
difference is that, unlike Apollo, mutations in the components of
the MRN complex do not show effects on the overhang on their own,
but after induction of telomere dysfunction by, for instance,
removal of TRF2. The MRN complex is implicated in the response to
certain types of DNA damage, for instance double strand breaks, and
is important in activating ATM and as an effector downstream of ATM
(43). The MRN complex could also
promote telomere elongation by downregulation of TRF1.
PNUTS, the protein phosphatase 1 nuclear-targeting
subunits, binds to the catalytic subunit of PP1 and inhibits
dephosphorylation of PP1 targets. PNUTS has a role in the general
damage response (44).
MCPH1 has three BRCT domains, two of which, at the
C-terminus, functioning as phosphopeptide binding modules on
targets involved in the DNA damage response (45). Also MCPH1 is known to have a role
in both the ATM and ATR pathways, in that it is important for
recruitment of MDC1, NBS1 and RAD1 to sites of DNA damage.
WRN and FEN1 act as a pair and both bind to TRF2.
Mutations in the WRN locus are associated with Werner syndrome, a
progeroid syndrome characterized by premature aging, high incidence
of diabetes and cardiovascular diseases, as well as a cellular
phenotype of premature senescence, high chromosomal instability and
telomere dysfunction (46). WRN is
a member of the RecQ family of helicases. Unlike the other members
of the family, WRN, in addition to DNA helicase activity has a
3′-5′ exonuclease domain found at the amino terminus. WRN has
branch migration activity and works on different substrates,
including telomeric DNA (47). WRN
was found to co-operate functionally at telomeres with shelterin
proteins TRF2 and POT1, and to be involved in telomere processing
during S phase. A physical association between WRN and TRF2 has
been documented both in vivo and in vitro(48). TRF2 and FEN1 have also been
reported to interact. Both FEN1 and WRN are recruited to telomeres
in S phase (49), consistent with
an important role with replication, perhaps in reinitiation of
stalled forks. These observations are compatible with the view that
telomeres constitute a ‘fragile site’ on the chromosome, requiring
specific activities to promote effective replication. Without the
WRN helicase activity, and FEN1 ‘gap’ exonuclease activity, both
required for efficient lagging of strand synthesis, the replication
forks experience collapse leading to incomplete replication of
telomeres (50).
The case of ORC complex-TERRA is a very interesting
one. The origin recognition complex (ORC) was discovered in yeast
as a six-subunit complex recognizing specifically the ARS
chromosomal element essential for DNA replication (51). Importantly, TRF2 recruits the ORC
complex at telomeres as well, and the ORC complex is important for
telomere function. Functional studies were performed by siRNA of
the ORC2 subunit, which resulted in chromosomal aberrations such as
increased numbers of signal-free ends and double minute
chromosomes, suggestive of strong replication defects. Telomere
loss and an increase in t-circle formation were also observed,
arguing for the ORC complex repressing recombination as well
(52). Further the stability of
the TRF-ORC1 complex was increased by TERRAs, the
telomere-transcribed RNAs, as they can associate with both proteins
and forms a ternary complex (53).
Depletion of TERRA leads to a change in chromatin structure as
detected by histone modifications (53). Thus, by inference, it appears that
TERRA is important for the maintenance of heterochromatization of
telomeres.
The complex ERCC1-XPF is a structure-specific
endonuclease involved in nucleotide excision repair (NER) pathway
and crosslink repair (54). A role
for XPF-ERCC1 is suggested in processing the overhang at normal
telomeres, affecting both strands resulting from leading or lagging
strand DNA synthesis. At present, this complex is therefore
implicated in overhang processing and, to some extent, telomere
regulation.
The Ku complex is also part of this complex
structure. Ku70/Ku80 is a heterodimer conserved along evolution. In
human cells, the Ku complex is essential for viability through its
role in repressing rapid deletions at telomeres. It is suggested
that the presence of TRF2 at telomeres and its role in T-loop
formation are important for masking the ends of chromosomes from Ku
recognition.
In addition to anchor sites located within
telomerase itself, it has been suggested that telomerase-associated
proteins facilitate the repeat addition processivity of the enzyme
in vivo. For example, the TPP1-Pot1 heterodimer stimulates
the activity and processivity of human telomerase in vitro.
TPP1 has been shown to interact with hTERT in vitro and
mutation of a conserved glycine residue in the hTERT TEN domain was
shown to suppress the stimulatory effect of TPP1-POT1 on human
telomerase processivity in vitro(55). These results are important because
they identify a physical link between the human shelterin complex
and telomerase and provide new insight into the mechanism of
processive telomere synthesis.
Mechanisms of telomeric lengthening in
mammals
A fundamental telomeric function is to act as a kind
of buffer to support the erosion of DNA chromosomic ends due to the
problem of terminal replication. Conventional DNA polymerases are
unidirectional and they cannot copy all bases in the 3′ end after
primer removal. The result is that in each replication cycle, a
given end of the chromosome cannot be synthesized completely and is
lost. If organisms could not solve this replicative problem they
could not pass they genetic charge completely from generation to
generation. As a consequence, all the species should have, at least
at the level of germinal cells, a mechanism that prevents the
incomplete replication of the genomes.
Different organisms have acquired evolutively
different methods to prevent the lost of DNA from the ends of their
chromosomes; however, most of the mammals use telomerase, a
specialized retrotranscriptase that will be described later.
Human cell lines that lack telomerase activity, are
capable of maintaining or elongating their telomeres by alternative
means, this has been called ALT (alternative lengthening of
telomeres) (56). In mammals that
exhibit ALT, sequences of DNA are copied from a telomere to the
other suggesting that ALT would involve processes of homologous
recombination (57).
In Saccaromyces cerevisiae, two ways of
homologous recombination exist involved in ALT, which depend of
RAD50 or RAD51 (58). Also, the
helicases RecQ (WRN and BLM in mammals) are required for ALT in
yeast (59). ALT mechanisms could
be increased by the elimination of mismatch repair pathways (MMR)
in yeast, and MMR machinery inhibits homologous recombination
(60).
Telomeres in cell immortalization and
cancer
Based on the initial experiments that demonstrated
that in fibroblasts, in the absence of telomerase, telomeres
shorten in direct relationship with the increasing of the number of
cell divisions, it was suggested that the loss of telomeres could
explain cell senescence after a given number of duplications in
vitro(61).
Studies of fibroblasts cultures have indicated that
the telomeric length would be better than the real age of the
donant to predict the replicative capacity of their cells (62). It has been proposed that the loss
of telomeric repeats, when reaching a critic telomeric length,
induces a signal of damage to DNA that results in the exit of the
cell cycle and replicative senescence (63). According to this model, telomeres
act as a mitotic clock that determines the replicative life of a
cell (64). The use of the
telomeric length as a marker of the cells’ replicative history has
been also described by Chang and Harley who demonstrated that
telomeric length decreases in function of the passages of
endothelial cells in culture (65). Bodnar et al gave the most
definitive proof showing that the reintroduction of the catalytic
compound of telomerase in primary human fibroblasts and in
endothelial cells that lack telomerase activity elongated the
telomeric repetitions in this cells resulting in a significant
increase in their replicative life (66).
The ‘telomeric hypothesis of cell senescence and
immortalization’ describes the potential relationship between
telomeric dynamics and immortalization (61). Telomerase is expressed in cells of
the germinal line which have long telomeres (∼10 kb) (67); in normal somatic cells, telomerase
is repressed and telomeres shorten up to a critic length whereas
the cells stop dividing (62).
The detention of the cell cycle that is imposed in
these cells is maintained by signals that activate the pathways of
the tumor suppressor genes p53 and Rb. This limit of the stage of
mortality (M1) could be altered by the transformation with viral
agents such as SV40 or the antigen T that inactivate p53 or Rb and
permit to the cells to suffer other additional cell divisions.
These cells can not yet divide indefinitely and are not considered
immortal. This proliferation is followed by ulterior telomeric
erosion, until the cells reach a second stage of mortality (M2)
whereas the telomeres are critically short (∼3 kb) (68). In this stage of crisis, the cells
could continue proliferating but showing high rates of apoptosis,
triggered by important chromosomic aberrations, where there is no
net increase in cell number. Progression beyond this point is a
very rare event which requires the alteration by mutation of
additional oncogenes and tumor suppressor genes. However, the cells
that succeed in overcoming M2 correlate strongly with the
reactivation of telomerase activity, the stabilization of telomeres
and the acquisition of an immortal phenotype. Telomerase is active
in the germ line as well as in stem cells but is inactive in most
of the somatic cells. On the other hand, telomerase is active in
most of immortalized cell lines and in 85–90% of human tumors. In a
study carried out in culture cells, representing 18 different human
tissues, it was found that 98 of the 100 studied cells, telomerase
was positive but was negative in the 22 mortal populations
analyzed. In the same way, telomerase activity was found in 90 of
the 101 biopsies that represented 12 human tumor types, but none of
the 50 normal somatic tissues was positive (69).
Clinical experience in cancer patients indicates
that some primary cancers and most metastatic lesions undergo a
period of dormancy before entering a stage of progressive growth.
Although, this may be the most crucial step in cancer progression,
the mechanisms underlying the conversion from a dormant to an
actively growing state have not been elucidated. Few studies have
focused on the role of telomerase on cancer dormancy. Gauthier
et al(70) found telomerase
activity in 73% of patients with stage IIIB and IV non-small cell
lung cancer with disseminated tumor cells, and 72% of patients with
Dukes’ stage C and D colon cancer. On the other hand, Soria et
al(71) found telomerase
activity in the peripheral blood of 21 of 25 patients with stage IV
breast cancer. In both studies, telomerase activity was not
detected in cells from healthy volunteers. Thus, detection of
telomerase activity in the blood or bone marrow appears to be
highly suggestive of disseminated cancer cells.
Pfitzenmaier et al(72) focused on telomerase activity in
disseminated tumor cells in the peripheral blood or bone marrow.
The objective of this study was to isolate homogeneous pools of
disseminated epithelial cells from bone marrow specimens of
patients with clinically localized prostate cancer obtained before
radical prostatectomy (RP). These cells were analyzed for
telomerase activity, and associations with clinical variables were
investigated. This study shows the feasibility of isolating
disseminated cancer cells for analyzing individual or pooled cells.
Compared to tissue staining, where telomerase is detected in 80–90%
of samples, they found lower rates of telomerase activity in the
disseminated tumor cells (49%). Telomerase-negative cells might
provide information on cell dormancy, as telomerase is a marker of
cell proliferation in immortal and cancer cells.
Telomerase-positive cells might predict early disease recurrence,
but a longer follow-up is needed to test this possibility.
In general, it has been accepted that telomeric
shortening is responsible of limiting the half-life of normal human
fibroblasts, as well as the expression of telomerase in the cells
is sufficient to overcome both replicative senescence and crisis
and to give them immortality. Although, the mechanisms involved in
telomerase regulation have not been completely solved, the
progressive understanding of them is creating the basis needed for
research and manipulation of telomerase activity as a potential
therapeutic target against cancer.
Other diseases associated with telomerase
activity
The first disease-associated with mutations in human
telomerase were identified in patients afflicted with a rare,
multi-system disorder called dyskeratosis congenital (73). The clinical manifestations of
dyskeratosis congenital generally appear during childhood and
include a monocutaneous triad of abnormal skin pigmentation, nail
dystrophy and oral leukoplasia. The symptoms are accompanied by a
spectrum of other somatic abnormalities such as developmental
delay, premature hair loss and organ failure. Bone marrow failure
is the principal cause of premature mortality. More recently,
telomerase mutations have been detected in the context of aplastic
anemia (74), Hoyeraal-Hreidarsson
syndrome (75) and idiopaty
pulmonary fibrosis (76). Aplastic
anemia is a hematological disorder characterized by reduced red
blood cell counts, bone marrow failure and liver and lung disease.
Hoyeraal-Hreidarsson syndrome is a multisystem disorder
characterized by bone marrow failure, immunodeficiency and severe
growth retardation. Idiopathic pulmonary fibrosis is a chronic,
progressive, and fatal disease that is defined by irreversible lung
fibrosis. The unifying molecular characteristic of these diseases
is that patients harbor telomeres that are significantly shorter
than age-matched control subjects (77).
Telomerase: structure and function
In most mammals, the maintenance of telomeric length
is carried out mainly by a specific reverse transcriptase, called
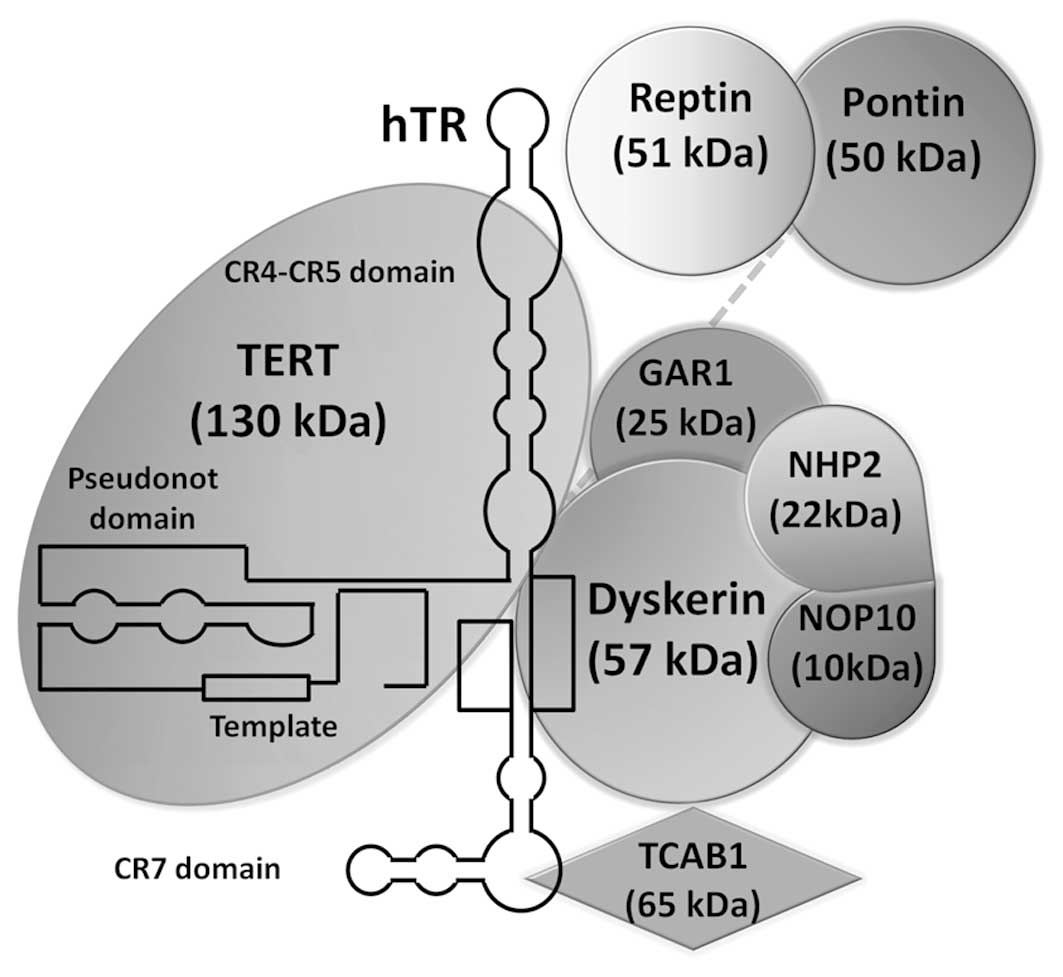
telomerase that was initially identified in ciliates (6). The human holoenzyme telomerase is a
ribonucleoprotein composed by a catalytic subunit, hTERT and an RNA
component (hRT) which acts as a template for the addition of a
short repetitive sequence dTTAGGG)n in the 3′ end of the telomeric
DNA and species-specific accessory proteins (Fig. 4) (12). These accessory proteins regulate
telomerase biogenesis, subcellular localization and function in
vivo. For instance, analysis of affinity-purified telomerase
from HeLa cells has identified integral protein components of human
telomerase: dyskerin, NHP2; NOP10, pontin/reptin, Gar1 and TCAB1
(78) (Fig. 5). Dyskerin, NHP2 and NOP10 are
required for the stability and accumulation of human telomerase RNA
(hTR) in vivo(78). The
heterotrimer of dyskerin, NOP10, and NHP2 is deposited onto each
hairpin unit of the H/ACA motif in a highly chaperoned biogenesis
process. Cotranscriptional association of the heterotrimer is
followed by an exchange of biogenesis factors for the fourth-core
subunit, GAR1, to produce a biologically functional RNP. Pontin and
reptin are two closed ATPases necessary for the stability of
dyskerin and hTR in vivo(79). The current model is that dyskerin,
pontin and reptin form a scaffold that recruits and stabilizes hTR,
and assembles the telomerase ribonucleoprotein particle. Once this
complex is formed, pontin and reptin are believed to dissociate
from the complex and release the catalytically active enzyme
(79). The subcellular location of
telomerase appears to be regulated by the recently identified TCAB1
(80). Also, while one study
claims that the human telomerase holoenzyme contains only dyskerin,
TERT, and hTR (81), other studies
establish that the human telomerase holoenzyme assembles all of the
core proteins (78).
Besides its association with telomerase, dyskerin is
a highly conserved nucleolar protein that, as part of a specialized
nucleolar RNP, catalyses the pseudouridylation of specific residues
in newly synthesized ribosomal RNAs and spliceosomal snRNA. Pontin
and reptin have multiple roles. Both ATPases are associated with
several chromating remodeling complexes and have many functions
including transcriptional regulation, DNA damage repair and
telomerase activity. They can also interact with major oncogenic
actors such as β-catenin and c-myc and regulate their oncogenic
function. Further studies are needed to elucidate the biochemical
and molecular significance of the intricate network of
protein-protein and protein-nucleic acid interactions within the
telomerase holoenzyme. Moreover, it will be important to
investigate whether the composition of the holoenzyme changes in
specific stages of the cell cycle. Normal human diploid cells that
express hTERT in transient form, acquires telomerase activity,
demonstrating that hTERT is the limiting component needed for the
restoration of the telomerase activity in these cells (64). The gen hTERT, of ∼37 kb, is present
in the human genome as a single copy sequence in the chromosome
5p15.33 (82). hTERT is a
relatively large protein (127 kDa) and the reverse transcriptase
motifs are found in its C-terminal end. Both, bioinformatics and
mutational studies have collectively established that TERT contains
three main structural elements: i) a long N-terminal extension that
contains conserved DNA and RNA-binding domains, ii) a central
catalytic RT domain and iii) a short C-terminal extension. The
N-terminal extension of most TERTs contains two conserved domains,
the TEN domains and telomerase RNA-binding domain (TRBD). The
catalytic domain of TERT is the most characterized region of the
protein and contains seven evolutionarily conserved RT motifs,
essential for enzymatic activity (83). In contrast to the N-terminus and RT
domain, the C-terminus of TERT shows only weak sequence
conservation suggesting that it may have species-specific functions
or that different amino acid sequences have evolved to fold into
similar structural domains.
The RNA molecule of telomerase has been isolated
from ciliates, yeasts, and mammals and is essential for telomerase
activity (84), but is not a
limitating factor for telomerase (85). In vertebrates, RNA of telomerase
has an extension between 382 and 559 nucleotides (86), and in humans in particular the RNA
component has an extension of 451 nucleotides of length and
contains a sequence of 11 bp (5-CUAACCCUAAC-3′) that encodes for
telomeric repeats (87). The gen
hTR is present in the human genome also as a single copy in
chromosome 3q26.
Phylogenetic comparative analysis of vertebrate TR
predicts three conserved domains: i) the pseudoknot/template core
domain, ii) the CR4/CR5 domain and iii) a box H/ACA domain
(88). The core domain is
essential for telomerase activity in vitro and in
vivo. The CR4/CR5 is required for telomerase activity but is
not essential for mTR stability in the cell and the box H/ACA
domain is essential for TR stability, processing, nuclear
localization and telomerase activity in vivo(89).
Elongation of the telomere by telomerase is a
process that happens in different stages. First, the nucleotides of
the 3′ extreme of the telomeric DNA are hybridized to the end of
the RNA template, inside the RNA domain of the telomerase complex.
The template sequence of 11 nucleotides is complementary to almost
two telomeric repeats. Secondly, the gap in the extreme of the
template is completed by synthesis, using triphosphate nucleotides
in the catalytic site of the enzyme (hTERT). In this way, a
complete hexanucleotidic repeat is assembled in the template.
Finally, the synthesized strand is translocated in 5′ direction in
order to allow the formation of a new gap and the repetition of the
cycle.
Other telomerase functions
Telomerase is essential for the long-term
proliferation potential of stem cells and cancer cells, and for
normal tissue renewal. However, other functions have been described
beyond its action at the telomeric level. Indeed, TERT can function
as a transcriptional modulator of the Wnt-β-catenin signaling
pathway (90). TERT functions as a
cofactor in a β-catenin transcriptional complex through
interactions with BRG1, which is an SWI/SNF related chromatin
remodeling protein. In addition, TERT in a complex with RMRP can
act as an RNA-dependent RNA polymerase (91). The TERT-RMRP complex acts as an
RDRP and processes RMRP into double-stranded RNA (dsRNA), which is
then processed by the endoribonuclease Dicer into small interfering
RNA (siRNA), which controls RMRP endogenous levels. Thus,
TERT-RMRP-RDRP regulates RMRP levels by a negative-feedback control
mechanism. Some evidence has been found for a role for telomerase
in the regulation of apoptosis in a telomere
maintenance-independent manner (92). TERT contains a mitochondrial
localization signal peptide at its N-terminal that targets TERT to
mitochondria where it is active (93). Furthermore, it was shown that
telomerase sensitizes mitochondrial DNA to hydrogen
peroxide-induced oxidative damage, probably through the modulation
of metal homeostasis (93). The
mitochondrial localization of telomerase also has an important role
in apoptosis (94).
Conclusions
Although pharmacological strategies for affecting
telomerase activity are beyond the scope of this review, an
excellent review by Tárkányi and Aradi (95) has been published. Although
telomerase therapeutics are not approved yet for clinical use, we
can assume that based on the promising in vitro and in
vivo results and successful clinical trials, it can be
predicted that telomerase therapeutics will be utilized soon to
combat malignancies and degenerative diseases. The active search
for modulators is justified, because telomere/telomerase system is
an extremely promising target offering possibilities to decrease
the viability of the cell for therapeutic purposes. The knowledge
of this system is vital to fulfill that aim.
Acknowledgements
This study was supported by Grants
from Quilmes National University and ANPCyT (Argentina). D.E.G.,
H.G.F., P.D.G. and D.F.A. are researchers from CONICET. Daniel
Gomez is a member of the National Cancer Institute of
Argentina.
References
|
1.
|
HJ MullerThe remaking of chromosomesThe
Collecting Net131811981938
|
|
2.
|
B McClintockThe stability of broken ends
of chromosomes in Zea maysGenetics26234282194117247004
|
|
3.
|
EH BlackburnJG GallA tandemly repeated
sequence at the termini of the extrachromosomal ribosomal RNA genes
in TetrahymenaJ Mol
Biol1202253197810.1016/0022-2836(78)90294-2
|
|
4.
|
EH BlackburnTelomeres: no end in
sightCell77621623199410.1016/0092-8674(94)90046-98205611
|
|
5.
|
LL SandellVA ZakianLoss of a yeast
telomere: arrest, recovery, and chromosome
lossCell75729739199310.1016/0092-8674(93)90493-A8242745
|
|
6.
|
CW GreiderEH BlackburnIdentification of a
specific telomere terminal transferase activity in
Tetrahymena
extractsCell43405413198510.1016/0092-8674(85)90170-93907856
|
|
7.
|
J MeyneRL RatliffRK MoyzisConservation of
the human telomere sequence (TTAGGG)n among vertebratesProc Natl
Acad Sci USA8670497053198910.1073/pnas.86.18.70492780561
|
|
8.
|
S LejnineVL MakarovJP LangmoreConserved
nucleoprotein structure at the ends of vertebrate and invertebrate
chromosomesProc Natl Acad Sci
USA9223932397199510.1073/pnas.92.6.23937892278
|
|
9.
|
WE WrightVM TesmerKE HuffmanSD LeveneJW
ShayNormal human chromosomes have long G-rich telomeric overhangs
at one endGenes Dev1128012809199710.1101/gad.11.21.28019353250
|
|
10.
|
MI ZverevaDM ShcherbakovaOA
DontsovaTelomerase: structure, functions, and activity
regulationBiochemistry7515631583201021417995
|
|
11.
|
R HänselF LöhrS Foldynová-TrantírkováE
BambergL TrantírekV DötschThe parallel G-quadruplex structure of
vertebrate telomeric repeat sequences is not the preferred folding
topology under physiological conditionsNucleic Acids
Res390576857752011
|
|
12.
|
HDM WyattSC WestTL BeattieInTERTpreting
telomerase structure and functionNucleic Acids
Res3856095612201010.1093/nar/gkq370
|
|
13.
|
BR LingerCM PriceConservation of telomere
protein complexes: shuffling though evolutionCrit Rev Biochem Mol
Biol44434446200910.3109/1040923090330732919839711
|
|
14.
|
W PalmT de LangeHow shelterin protects
mammalian telomeresAnnu Rev
Genet42301334200810.1146/annurev.genet.41.110306.13035018680434
|
|
15.
|
P MartinezMA BlascoTelomeric and
extra-telomeric roles for telomerase and the telomere-binding
proteinsNat Rev Cancer11161176201110.1038/nrc302521346783
|
|
16.
|
L ChongB van SteenselD BroccoliH
Erdjument-BromageJ HanishP TempstT de LangeA human telomeric
proteinScience27016631667199510.1126/science.270.5242.16637502076
|
|
17.
|
A BianchiT de LangeKu binds telomeric DNA
in vitroJ Biol
Chem2742122321227199910.1074/jbc.274.30.2122310409678
|
|
18.
|
A SmogorzewskaB van SteenselA BianchiS
OelmannMR ScheferG SchnappT de LangeControl of human telomere
length by TRF1 and TRF2Mol Cell
Biol2016591668200010.1128/MCB.20.5.1659-1668.200010669743
|
|
19.
|
B van SteenselT de LangeControl of
telomere length by the human telomeric protein
TRF1Nature3857407431997
|
|
20.
|
B van StenselA SmogorzewskaT de LangeTRF2
protects human telomeres from end to end
fusionsCell9240141319989476899
|
|
21.
|
J KarlsederD BroccoliY DaiS HardyT de
Langep53- and ATM-dependent apoptosis induced by telomerase lacking
TRF2Science28313211325199910.1126/science.283.5406.132110037601
|
|
22.
|
JD GriffithL ComeauS RosenfieldRM StanselA
BanchiH MossT de LangeMammalian telomeres end in a large duplex
loopCell97503514199910.1016/S0092-8674(00)80760-610338214
|
|
23.
|
U HerbigWA JoblingBP ChenDJ ChenJM
SedivyTelomere shortening triggers senescence of human cells
through a pathway involving ATM, p53 and p21 (CIP1), but not
p16(INK4a)Mol
Cell14501513200410.1016/S1097-2765(04)00256-415149599
|
|
24.
|
Y GongTA de LangeShd1-controlled POT1a
provides support for repression of ATR signaling at telomeres
through RPA exclusionMol
Cell40377387201010.1016/j.molcel.2010.10.01621070964
|
|
25.
|
CC LeeTS HuangA novel topoisomerase II
poison GL331 preferentially induces DNA cleavage at (C/G)T sites
and can cause telomere DNA damagePharm
Res18846851200110.1023/A:101104883169811474790
|
|
26.
|
P MartinezM ThanasoulaAR CarlosMammalian
Rap1 controls telomere function and gene expression through binding
to telomeric and extratelomeric sitesNat Cell
Biol8768780201010.1038/ncb208120622869
|
|
27.
|
AR BuchmanWJ KimmerlyJ RineRD KornbergTwo
DNA-binding factors recognize specific sequences at silencers,
upstream activating sequences, autonomously replicating sequences
and telomeres in Saccharomyces cerevisiaeMol Cell
Biol82102251988
|
|
28.
|
H TeoS GhoshH LueschTelomere-independent
Rap1 is an IKK adaptor and regulates NF-kappaB-dependent gene
expressionNat Cell Biol12758767201010.1038/ncb208020622870
|
|
29.
|
KK TakaiT KibeJR DonigianD FrescasT de
LangeTelomere protection by TPP1/POT1 requires tethering to TIN2Mol
Cell18647659201110.1016/j.molcel.2011.08.04322099311
|
|
30.
|
AM TejeraM Stagno d’AlcontresM
ThanasoulaTPP1 is required for TERT recruitment, telomere
elongation during nuclear reprogramming, and normal skin
development in miceDev
Cell18775789201010.1016/j.devcel.2010.03.01120493811
|
|
31.
|
P BaumannC PricePot1 and telomere
maintenanceFEBS Lett1037793784201010.1016/j.febslet.2010.05.024
|
|
32.
|
S SchoeftnerMA BlascoDevelopmentally
regulated transcription of mammalian telomeres by DNA-dependent RNA
polymerase IINat Cell Biol10228236200810.1038/ncb168518157120
|
|
33.
|
SG NergadzeBO FarnungH
WischnewskiCpG-island promoters drive transcription of human
telomeresRNA1521862194200910.1261/rna.174830919850908
|
|
34.
|
T De LangeShelterin: the protein complex
that shapes and safeguards human telomeresGenes
Dev1921002110200516166375
|
|
35.
|
SJ HsiaoS SmithTankyrase function at
telomeres, spindle poles and
beyondBiochimie908392200810.1016/j.biochi.2007.07.01217825467
|
|
36.
|
BD CookJN DynekW ChangG ShostakS SmithRole
for the related poly (ADP-Ribose) polymerases tankyrase 1 and 2 at
human telomeresMol Cell
Biol22332342200210.1128/MCB.22.1.332-342.200211739745
|
|
37.
|
Y ChenY YangM van OverbeekJR DonigianP
BaciuT de LangeM LeiA share docking motif in TRF1 and TRF2 used for
differential recruitment of telomeric
proteinsScience31910921096200810.1126/science.115180418202258
|
|
38.
|
XZ ZhouKP LuThe Pin2/TRF1-interacting
protein PinX1 is a potent telomerase
inhibitorCell107347359200110.1016/S0092-8674(01)00538-411701125
|
|
39.
|
M Van OverbeekT de LangeApollo, an
Artemis-related nuclease interacts with TRF2 and protects human
telomerase in S phaseCurr Biol1612951232200616730176
|
|
40.
|
JB BaeSS MukhopadhyayL LiuSnm1B/Apollo
mediates replication fork collapse and S phase checkpoint
activation in response to DNA interstrand
cross-linksOncogene2750455056200810.1038/onc.2008.13918469862
|
|
41.
|
JF SarthyP BaumannApollo-taking the lead
in telomere protectionMol
Cell39489491201010.1016/j.molcel.2010.08.01820797622
|
|
42.
|
Y DengX GuoDO FergusonS ChangMultiple
roles for MRE11 at uncapped
telomeresNature460914918200910.1038/nature0819619633651
|
|
43.
|
JH LeeTT PaullActivation and regulation of
ATM kinase activity in response to DNA double-strand
breaksOncogene2677417748200710.1038/sj.onc.121087218066086
|
|
44.
|
HB LandsverkF Mora-BermúdezOJ LandsverkThe
protein phosphatase 1 regulator PNUTS is a new component of the DNA
damage responseEMBO
Rep11868875201010.1038/embor.2010.13420890310
|
|
45.
|
DH MohammadMB Yaffe14-3-3 proteins, FHA
domains and BRXT domains in the DNA damage responseDNA
Repair810091017200910.1016/j.dnarep.2009.04.00419481982
|
|
46.
|
D KiplingRG FaragherProgeroid syndromes:
probing the molecular basis of aging?Mol
Pathol50234241199710.1136/mp.50.5.2349497912
|
|
47.
|
A OzgencLA LoebCurrent advances in
unraveling the function of the Werner syndrome proteinMutat
Res577237251200510.1016/j.mrfmmm.2005.03.02015946710
|
|
48.
|
PL OpreskoC Von KobbeJP LaineJ HarriganID
HicksonVA BohrTelomere binding protein TRF2 binds to and stimulates
the Werner and Bloom syndrome helicasesJ Biol
Chem2774111041119200210.1074/jbc.M20539620012181313
|
|
49.
|
A SahariaL GuittatS CrockerA LimM SteffenS
KulkarniSA StewartFlap endonuclease 1 contributes to telomere
stabilityCurr Biol18496500200810.1016/j.cub.2008.02.07118394896
|
|
50.
|
A SahariaDC TeasleyJP DuxinB DaoKB
ChiappinelliSA StewartFEN1 ensures telomere stability by
facilitating replication fork re-initiationJ Biol
Chem2852705727066201010.1074/jbc.M110.11227620551483
|
|
51.
|
JF DiffleyL LabibThe chromosome
replication cycleJ Cell Sci1158698722000
|
|
52.
|
Z DengJ DheekolluD BroccoliA DuttaPM
LiebermanThe origin recognition complex localizes to telomere
repeats and prevents telomeric-circle formationCurr
Biol1719891995200710.1016/j.cub.2007.10.05418006317
|
|
53.
|
Z DengJ NorseenA WiedmerH RietmanPM
LiebermanTERRA RNA binding to TRF2 facilitates heterochromatin
formation and ORC recruitment at telomeresMol
Cell35403413200910.1016/j.molcel.2009.06.02519716786
|
|
54.
|
A CicciaSJ ElledgeThe DNA damage response:
making it safe to play with knivesMol
Cell40179204201010.1016/j.molcel.2010.09.01920965415
|
|
55.
|
T KibeGA OsawaCE KeeganT de LangeTelomere
protection by TPP1 is mediated by POT1a and POT1bMol Cell
Biol3010591066201010.1128/MCB.01498-0919995905
|
|
56.
|
TM BryanA EnglezouL Dalla-PozzaMA DunhamRR
ReddelEvidence for an alternative mechanism for maintaining
telomere length in human tumors and tumor-derived cell linesNat
Med312711274199710.1038/nm1197-12719359704
|
|
57.
|
MA DunhamAA NeumannCL FaschingRR
ReddelTelomere maintenance by recombination in human cellsNat
Genet26447450200010.1038/8258611101843
|
|
58.
|
Q ChenA IjpmaCW GreiderTwo survivor
pathways that allow growth in the absence of telomerase are
generated by distinct telomere recombination eventsMol Cell
Biol2118191827200110.1128/MCB.21.5.1819-1827.200111238918
|
|
59.
|
P HuangFE PrydeD LesterRL MaddisonRH
BortsID HicksonEJ LouisSGS1 is required for telomere elongation in
the absence of telomeraseCurr
Biol11125129200110.1016/S0960-9822(01)00021-511231130
|
|
60.
|
A RizkiV LundblandDefects in mismatch
repair promote telomerase-independent
proliferationNature411713716200110.1038/3507964111395777
|
|
61.
|
CB HarleyAB FutcherCW GreiderTelomeres
shorten during ageing of human
fibroblastsNature345458460199110.1038/345458a02342578
|
|
62.
|
RC AllsoopCB HarleyEvidence for a critical
telomeric length in senescent human fibroblastsExp Cell
Res219130136199510.1006/excr.1995.12137628529
|
|
63.
|
J CampisiThe biology of replicative
senescenceEur J
Cancer33703709199710.1016/S0959-8049(96)00058-59282108
|
|
64.
|
H VaziriS BenchimolReconstitution of
telomerase activity in normal human cells lead to elongation of
telomeres and extended replicative life spanCurr
Biol8279282199810.1016/S0960-9822(98)70109-59501072
|
|
65.
|
E ChangCB HarleyTelomere length and
replicative aging in human vascular tissuesProc Natl Acad Sci
USA921119011194199510.1073/pnas.92.24.111907479963
|
|
66.
|
AG BodnarNW KimRB EffrosCP ChiuMechanism
of telomerase induction during T cell activationExp Cell
Res2285864199610.1006/excr.1996.02998892971
|
|
67.
|
ND HastieM DempsterMG DunlopAM ThompsonDK
GreenRC AllshireTelomere reduction in human colorectal carcinoma
and with ageingNature346866868199010.1038/346866a02392154
|
|
68.
|
CM CounterAA AvillonCE LeFeuvreNG
StewartCW GreiderCB HarleyS BacchettiTelomere shortening associated
with chromosome instability is arrested in immortal cells witch
express telomerase activityEMBO J111921192919921582420
|
|
69.
|
K DhaeeneE Van MarckR ParwareschTelomeres,
telomerase and cancer: an up-dateVirchows
Arch437116200010.1007/s004280000189
|
|
70.
|
LR GauthierC GranotierJC SoriaS FaivreV
BoigeE RaymondFD BoussinDetection of circulating carcinoma cells by
telomerase activityBr J
Cancer84631635200110.1054/bjoc.2000.166211237383
|
|
71.
|
JC SoriaLR GauthierE RaymondMolecular
detection of telomerase positive circulating epithelial cells in
metastatic breast cancer patientsClin Cancer Res59719751999
|
|
72.
|
J PfitzenmaierWJ EllisEW ArfmanS HawleyPO
McLaughlinPH LangeRL VessellaTelomerase activity in disseminated
prostate cancer cellsBJU
Int9713091313200610.1111/j.1464-410X.2006.06194.x16686730
|
|
73.
|
AJ WalneI DokalDyskeratosis Congenita: a
historical perspectiveMech Ageing
Dev1294859200810.1016/j.mad.2007.10.00618054794
|
|
74.
|
T VulliamyA MarroneI DokalPJ
MasonAssociation between aplastic anaemia and mutations in
telomerase
RNALancet2221682170200210.1016/S0140-6736(02)09087-612090986
|
|
75.
|
N NishioS KojimaRecent progress in
dyskeratosis congenitaInt J
Hematol92419424201010.1007/s12185-010-0695-520882440
|
|
76.
|
M ArmaniosTelomerase and idiopathic
pulmonary fibrosisMutat
Res7305258201210.1016/j.mrfmmm.2011.10.01322079513
|
|
77.
|
M ArmaniosSyndromes of telomere
shorteningAnnu Rev Genomics Hum
Genet104561200910.1146/annurev-genom-082908-150046
|
|
78.
|
D FuK CollinsPurification of human
telomerase complexes identifies factors involved in telomerase
biogenesis and telomere length regulationMol
Cell28773785200710.1016/j.molcel.2007.09.02318082603
|
|
79.
|
AS VeinteicherZ MengPJ MasonTD VeenstraSE
ArtandiIdentification of ATPases pontin and reptin as telomerase
components essential for holenzyme
activityCell132945957200810.1016/j.cell.2008.01.01918358808
|
|
80.
|
F ZhongSA SavageM ShkreliDisruption of
telomerase trafficking by TCAB1 mutation causes dyskeratosis
congenitaGenes Dev251116201110.1101/gad.200641121205863
|
|
81.
|
SB CohenME GrahamGO LovreczN BachePJ
RobinsonRR Red delProtein composition of catalytically active human
telomerase from immortal
cellsScience3018501853200710.1126/science.113859617395830
|
|
82.
|
LA BryceN MorrisonSF HoareS MuirWN
KeithMapping of the gene for the human telomerase reverse
transcriptase, hTERT, to chromosome 5p15.33 by fluorescence in situ
hybridizationNeoplasia2197201200010.1038/sj.neo.790009210935505
|
|
83.
|
S KyoM TakakuraT FujiwaraM
InoueUnderstanding and exploiting hTERT promoter regulation for
diagnosis and treatment of human cancersCancer
Sci9915281538200810.1111/j.1349-7006.2008.00878.x18754863
|
|
84.
|
MA BlascoW FunkB VilleponteauCW
GreiderFunctional characterization and developmental regulation of
mouse telomerase
RNAScience26912671270199610.1126/science.75444927544492
|
|
85.
|
CJ CairneyWN KeithTelomerase redefined:
integrated regulation of hTR and hTERT for telomere maintenance and
telomerase
activityBiochimie901323200810.1016/j.biochi.2007.07.02517854971
|
|
86.
|
DA TsaoCW WuYS LinMolecular cloning of
bovine telomerase
RNAGene2215158199810.1016/S0378-1119(98)00432-69852949
|
|
87.
|
GB MorinThe human telomere terminal
transferase enzyme is a ribonucleoprotein that synthesizes TTAGGG
repeatsCell59521529198910.1016/0092-8674(89)90035-42805070
|
|
88.
|
JL ChenMA BlascoCW GreiderSecondary
structure of vertebrate telomerase
RNACell100503514200010.1016/S0092-8674(00)80687-X10721988
|
|
89.
|
CA TheimerJ FeigonStructure and function
of telomerase RNACurr Opin Struct
Biol16307318200610.1016/j.sbi.2006.05.005
|
|
90.
|
JI ParkAS VenteicherJY HongTelomerase
modulates Wnt signaling by association with target gene
chromatinNature4606672200910.1038/nature0813719571879
|
|
91.
|
Y MaidaM YasukawaM FuruuchiRNA-dependent
RNA polymerase formed by TERT and the RMRP
RNANature4610230235200910.1038/nature0828319701182
|
|
92.
|
Y CongJW ShayActions of human telomerase
beyond telomeresCell Res18725732200810.1038/cr.2008.7418574498
|
|
93.
|
JH SantosJN MeyerM SkorvagaLA AnnabB Van
HoutenMitochondrial hTERT exacerbates free-radical-mediated mtDNA
damageAging
Cell6399411200410.1111/j.1474-9728.2004.00124.x15569357
|
|
94.
|
JH SantosJN MeyerB Van HoutenMitochondrial
localization of telomerase as a determinant for hydrogen
peroxide-induced mitochondrial DNA damage and apoptosisHum Mol
Genet1517571768200610.1093/hmg/ddl09816613901
|
|
95.
|
I TárkányJ AradiPharmacological
intervention strategies for affecting telomerase activity: Future
prospects to treat cancer and degenerative
diseaseBiochimie901561722008
|