Introduction
Acute lymphoblastic leukaemia (ALL) refers to a
group of malignancies arising from lymphoid progenitors that may be
of B- or T-cell lineage (B-ALL or T-ALL), resulting in
proliferation and expansion of lymphoid blasts in bone marrow,
blood and other organs. ALL has an overall incidence of 1–1.5 per
100,000 individuals and exhibits a bimodal age distribution with an
early peak between ages 2–5 years (4–5 per 100,000 individuals,
accounting for 80% of all childhood leukaemia), followed by a
second peak after the age of 50 years (1). The treatment of ALL involves complex
therapeutic programmes using intensive combination chemotherapy in
dose- and time-specific sequences resulting in survival rates
greater than 80% in children (2).
However, there is a need for novel treatment options as only 30–40%
of adults achieve long-term disease-free survival and both
childhood T-ALL and relapsed ALL are associated with poor clinical
outcome (3,4).
Microtubule targeting agents (MTAs) have been shown
to be effective against ALL cells and vincristine is a key
component of induction, consolidation and maintenance treatment
protocols (3). We have previously
described a novel MTA, pyrrolo-1,5-benzoxazepine-15 (PBOX-15),
which exhibits anti-cancer activity against a variety of human
tumour cell types, including those derived from both solid and
haematological malignancies (5–10).
Importantly, PBOX-15 and other related PBOX compounds display
minimal toxicity against normal human blood and bone marrow cells,
and are well tolerated by both tumour-bearing and healthy mice
(7,11).
MTA’s interference with tubulin dynamics during
mitotic spindle formation is well established; however, due to the
critical role of microtubules in cell motility and homeostasis, the
activity of MTAs may extend beyond inhibition of cytokinesis and
subsequent induction of apoptosis. For example, we have shown that
PBOX-15 induces apoptosis in chronic lymphocytic leukaemia (CLL)
cells independent of cell cycle arrest and this effect has been
described for other MTAs (7,12–14).
In addition, MTAs have long been known to suppress immune function
and have been used clinically in the treatment of other conditions
besides cancer, including coronary restenosis and neointimal
hyperplasia (15,16).
A crucial role for integrins in the survival and
homing of normal and neoplastic B- and T-cells cell is
well-documented (17–19). Integrins trigger intracellular
signalling pathways by forming macromolecular complexes with plasma
membrane proteins and contribute to cell adhesion, mobility, homing
and resistance to apoptosis (18,20–22).
Microtubules control integrin rearrangement and participate in the
process of focal adhesions, which are sites of interactions between
integrins and the cytoskeleton (23). We have previously shown that
PBOX-15 inhibits integrin-induced migration of T-lymphocytes via
disruption of tubulin dynamics prior to the induction of apoptosis
(24). This suggested a potential
anti-cancer activity for PBOX-15 involving disruption of leukaemic
homing and infiltration, and subsequent induction of apoptosis.
Here, we demonstrate anti-leukaemia potential of PBOX-15 by
describing multiple effects of this agent in ALL cells: a
pro-apoptotic activity, an inhibitory effect on cell adhesion and
migration, down-regulation of integrin expression and disruption of
integrin-microtubule interactions.
Materials and methods
Chemicals
PBOX-15 (pyrrolobenzoxazepine
4-acetoxy-5-(1-naphthyl)naphtha pyrrolo[1,4]-oxazepine) was
synthesised as previously described (25,26)
and prepared as a stock solution of 1 mM in ethanol. Caspase
inhibitors were obtained from Calbiochem (Darmstadt, Germany).
Unless indicated, all other reagents and chemicals were obtained
from Sigma-Aldrich Ireland Ltd. (Arklow, Ireland).
Cell culture
The T-ALL cell line CCRF-CEM and the B-ALL cell line
SD-1 were obtained from the DSMZ cell bank (Braunschweig, Germany)
for the purpose of this study. CCRF-CEM was established from the
peripheral blood of a 3-year old girl with T-ALL at relapse
(27) and SD-1 was established
from the peripheral blood of an adult female with Philadelphia
chromosome positive B-ALL (28).
Both cell lines were cultured in complete medium [RPMI-1640 medium
supplemented with 10% (v/v) foetal bovine serum (FBS), 50 U/ml
penicillin and 50 μg/ml streptomycin] under standard cell
culture conditions.
Cell viability, cell cycle and apoptosis
assays
Cell viability was determined by MTT assays (Cell
Proliferation Kit I; Roche Applied Science, West Sussex, UK)
according to the manufacturer’s protocol. Cell cycle distribution
was quantified by flow cytometric analysis of propidium iodide (PI;
Invitrogen, UK) stained cells as described previously (10). In brief, following fixation and
permeabilization with ethanol, cells were incubated with 25
μg/ml PI and 100 μg RNase A (Gentra Systems Inc.,
USA) before analysis using a FACSCalibur and CellQuest software (BD
Biosciences, Oxford, UK). Doublets were excluded and approximately
30,000 events were acquired for each sample. Apoptosis was detected
by co-staining of cells with fluorescein isothiocyanate
(FITC)-conjugated Annexin V (IQ Products, The Netherlands) and PI
(29), and quantified using a
FACSCalibur and CellQuest software. In some experiments caspase
inhibitors were added 1 h prior to PBOX-15 treatment.
Real-time impedance sensing
Real-time monitoring of adhesion and spreading of
cells was performed using xCELLigence system as per manufacturer’s
instructions (Roche Applied Science). This system measures
electrical impedance (which depends on cell number, degree of
adhesion, spreading and proliferation of the cells) across
interdigitated micro-electrodes integrated on the bottom of 96-well
E-plates. CCRF-CEM cells were seeded at a density of
1×104 cells/well in 100 μl media and allowed to
attach onto the electrode surface over time. The electrical
impedance was automatically recorded every 15 min and expressed as
an arbitrary unit called the Cell Index. Cells were treated with
PBOX-15 either prior to seeding or at 21 h post-seeding when the
cells had strongly adhered to the surface of the E-plate.
Cell adhesion assay
Adhesion of cells on anti-integrin antibody or
fibronectin coated surfaces was detected and subsequently
quantified using a cell based high content analysis (HCA) system.
The assay operates on the principle of fully automated fluorescent
microscopy described previously (30–32)
and allows multiplexing of key reporter parameters including
cell-adhesion. Untreated or PBOX-15 pre-treated cells were added to
fibronectin, anti-α4-, β1-, or β2-integrin antibody coated 24-well
or 96-well plates (Nunc, Roskilide, Denmark) at a density of
2×105 or 1×104 cells/well respectively. Cells
were allowed to adhere 2 h after which the non-adherent cells were
removed by gentle washing three times in phosphate-buffered saline
(PBS). Following fixation with 3% paraformaldehyde, adherent cells
were fluorescently stained with Rhodaminephlloidin and Hoechst to
visualize cell morphology and the nuclei, respectively. Plates were
scanned using IN Cell Analyzer 1000 automated microscope (GE
Healthcare, Buckinghamshire, UK) and images were acquired in a
stereology configuration of nine randomly selected fields per well
using a 10x objective. The quantification of cell adherence was
subsequently performed using IN Cell Investigator software.
Transwell migration assay
The transwell migration assay was performed using
standard procedure. Briefly, untreated or PBOX-15 pre-treated cells
(5×105 in 100 μl serum-free medium) were loaded
in the upper chamber of a 24-well Costar® Transwell™
inserts (Costar, Cambridge, MA) with a pore size of 5 μm.
Filters then were transferred to wells containing 600 μl
medium with or without 20% FBS. Cells were allowed to migrate for 2
h and transmigrated cells were quantified by automated microscopy
using an IN Cell Analyzer 1000. All assays were performed in
triplicate.
Integrin expression analysis by flow
cytometry
Single cell suspensions were added to individual
polystyrene tubes and Fc receptors were blocked by incubating with
FBS. Cells were washed and incubated for 30 min at 4°C with
anti-human α4-PE, β1-FITC, or β2-FITC integrin antibodies or
appropriate isotype control antibodies (BD Pharmingen, CA, USA).
Flow cytometry was performed using a FACSCalibur with CellQuest
software. PI staining was used to exclude dead cells from the
analysis. Twenty thousand events were collected for each sample,
gated to exclude debris and illustrated as histograms.
Confocal microscopy
Immunofluorescent staining and confocal microscopy
was performed as described previously (30). Briefly, cells were seeded
(2×104 cells/well) in a Lab-Tek 8-well
Permanox® slide (Nalge Nunc, NY, USA). After treatments,
as indicated in particular experiments, cells were fixed with 3%
paraformaldehyde, immunostained with anti-α4-, β1-, or β2-integrin
antibody (eBiosciences, Hatfield, UK) for 1 h, and then
permeabilized in 0.1% Triton X-100. The slides were then blocked by
the addition of 3% (v/v) goat serum in PBS, counterstained with
FITC- or TRITC-conjugated anti-α-tubulin antibody. Images were
aquired using a 63x oil emersion objective on a Zeiss 510 LSM
confocal microscope system (Carl Zeiss Jena GmbH, Jena, Germany).
At least 20 different microscopic fields were analyzed for each
sample.
Results
PBOX-15 inhibits cell growth, induces
G2/M cell cycle arrest and apoptosis in both T- and B-ALL
cells
To investigate if PBOX-15 could affect ALL cell
growth and viability, we performed a MTT-based cell proliferation
and toxicity assay. PBOX-15 caused a dose-dependent reduction in
viability of CCRF-CEM and SD-1 cells after 24 h with
IC50 values of less than 1 μM (Fig. 1A). In order to investigate the
mechanism of this PBOX-15-induced effect on ALL cells, we performed
cell cycle analysis. A G2/M-phase cell cycle arrest was observed
following 24-h treatment with 1 μM PBOX-15 in both T- and
B-ALL cells. A notable increase in the subG0 peak was evidenced in
both cell lines, indicative of increased numbers of apoptotic cells
(Fig. 1B). We have previously
shown this concentration and duration of exposure to PBOX-15 to be
minimally toxic to normal CD19+ B lymphocytes (7). In order to confirm and quantify
PBOX-15-induced apoptosis, CCRF-CEM and SD-1 cells were treated
with 1 μM PBOX-15 for 24 or 48 h, stained with Annexin V and
PI, and then analyzed by flow cytometry. A substantial increase in
both early apoptotic (lower right quadrants) and late apoptotic
(upper right quadrants) cells was detected at both 24 and 48 h
post-treatment for both cell lines (Fig. 1C). To investigate the role of
caspases in mediating PBOX-15-induced apoptosis in ALL cells,
specific pharmacologic inhibitors of caspase activity were used.
Pre-treatment of cells with the pan-caspase inhibitor, z-VAD-fmk,
significantly inhibited PBOX-15-induced apoptosis in CCRF-CEM cells
(p<0.001) and SD-1 cells (p<0.01), suggesting a requirement
for caspase activity. Pre-treatment with specific caspase-3 and 8
inhibitors significantly reduced PBOX-15-induced apoptosis in T-ALL
cells only (p<0.01) (Fig.
1D).
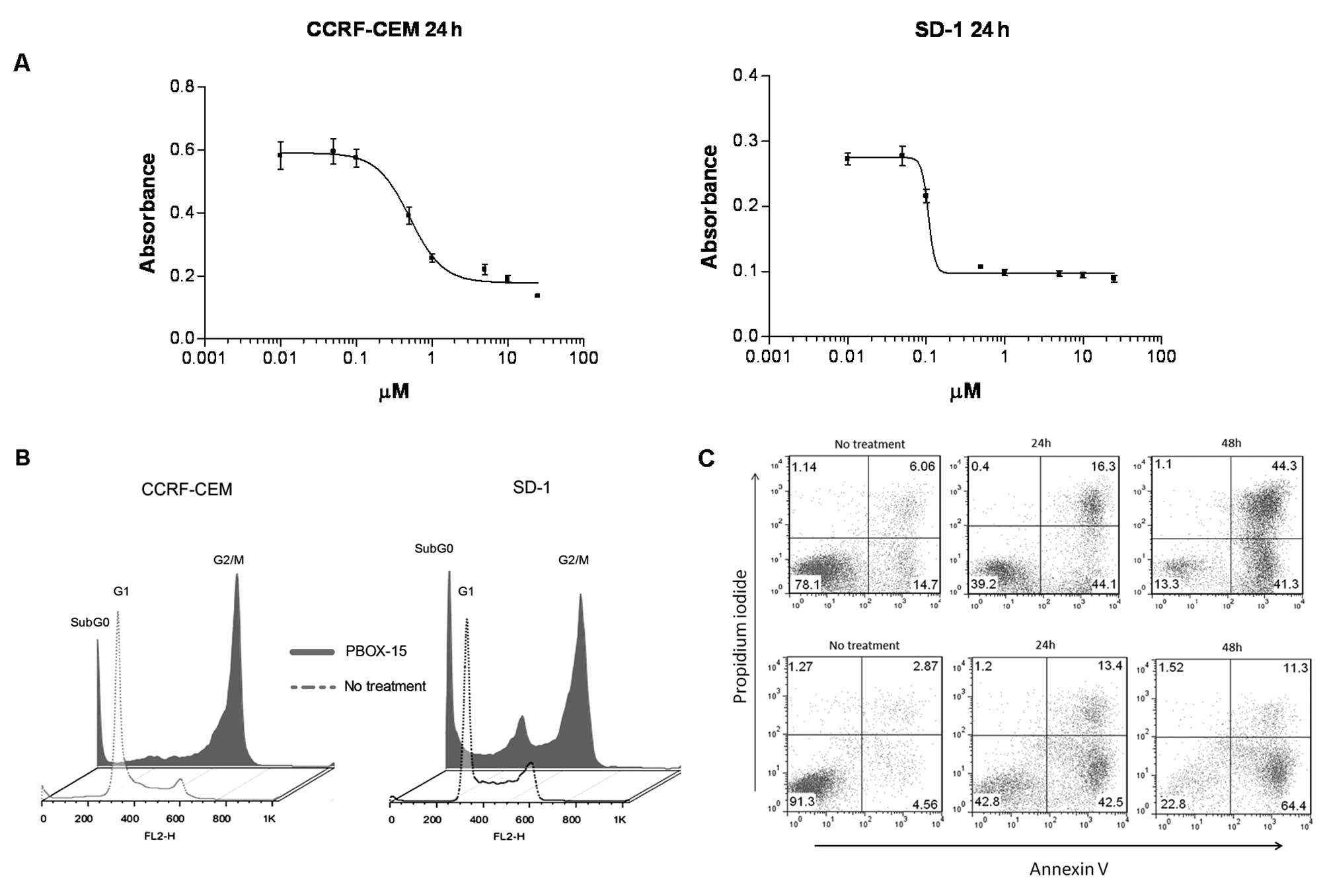 | Figure 1.PBOX-15 inhibits cell growth, induces
G2/M cell cycle arrest and apoptosis. A, Cell viability was
assessed by MTT assay following treatment of both CCRF-CEM and SD-1
cells with PBOX-15, for 24 h. Bars: SEM of triplicate samples. B,
CCRF-CEM and SD-1 cells underwent cell cycle arrest in the
G2/M-phase following 24-h treatment with 1 μM PBOX-15.
Following treatment cells were stained with PI and DNA content
(FL2-H) was analyzed by flow cytometry. C, Apoptosis induction was
assessed in CCRF-CEM cells (top panels) and SD-1 cells (bottom
panels) following no treatment (ethanol vehicle control) or 1
μM PBOX treatment at 24 or 48 h by flow cytometry analysis
of FITC-Annexin V/PI staining. Numbers represent the percentage of
total cells in each quadrant. D, Inhibition of PBOX-15 induced
apoptosis was assessed using pharmacologic inhibitors of caspases:
pan-caspase inhibitor (z-VAD-fmk, 100 μM); caspase-3
inhibitor [Z-D(OMe)QMD(OMe)-FMK, 50 μM]; caspase-8 inhibitor
[Z-IE(OMe)TD(OMe)-FMK, 50 μM]; caspase-9 inhibitor
(Ac-LEHD-CMK, 50 μM). Cells were treated directly with 1
μM PBOX-15 or pre-treated for 1 h with specific caspase
inhibitors before addition of PBOX-15. After 24 h, apoptosis was
assessed by Annexin V/PI assay. *p<0.01,
**p<0.001, n=3, by Student’s t-test compared with
PBOX-15 treated cells. Column data are illustrated as mean increase
in apoptosis compared to ethanol vehicle control treated cells ±
SEM. All data shown are representative of 3 independent
experiments. |
PBOX-15 treatment alters the adhesion and
migration of ALL cells
We next investigated the effect PBOX-15 on ALL cell
adhesion, spreading and migration prior to induction of cell cycle
arrest and subsequent apoptosis. CCRF-CEM cells grow both in
suspension and as an adherent monolayer in culture, allowing for
dynamic analysis of cell adhesion and cell spreading using the
xCELLigence electronic sensing system. CCRF-CEM cells were treated
with either 1 μM PBOX-15 or the well-characterized
microtubule targeting agent nocodozole and cellular adhesion and
spreading of the cells were monitored in real-time every 15 min for
up to 8 h. Control cells with normal cell adherence showed
continuously increasing Cell Index values, displaying a upwardly
trending curve, as the cells adhered and spread. Treatment of cells
with PBOX-15 or nocodozole inhibited their ability to adhere to the
plate surfaces and spread as observed by the curve plateaus, with
PBOX-15 treatment resulting in a more pronounced effect (Fig. 2A, top panel). In order to determine
if PBOX-15 could exert its effect on pre-adhered CCRF-CEM cells,
cells were allowed to adhere for 21 h prior to treatment (Fig. 2A, bottom panel). Following PBOX-15
treatment, cellular adherence was rapidly and substantially
decreased as evidenced by a decline in cellular electrical
impedance within 30 min. Interestingly, we observed a much more
pronounced effect on pre-adhered cells treated with PBOX-15
compared to cells treated with nocodozole.
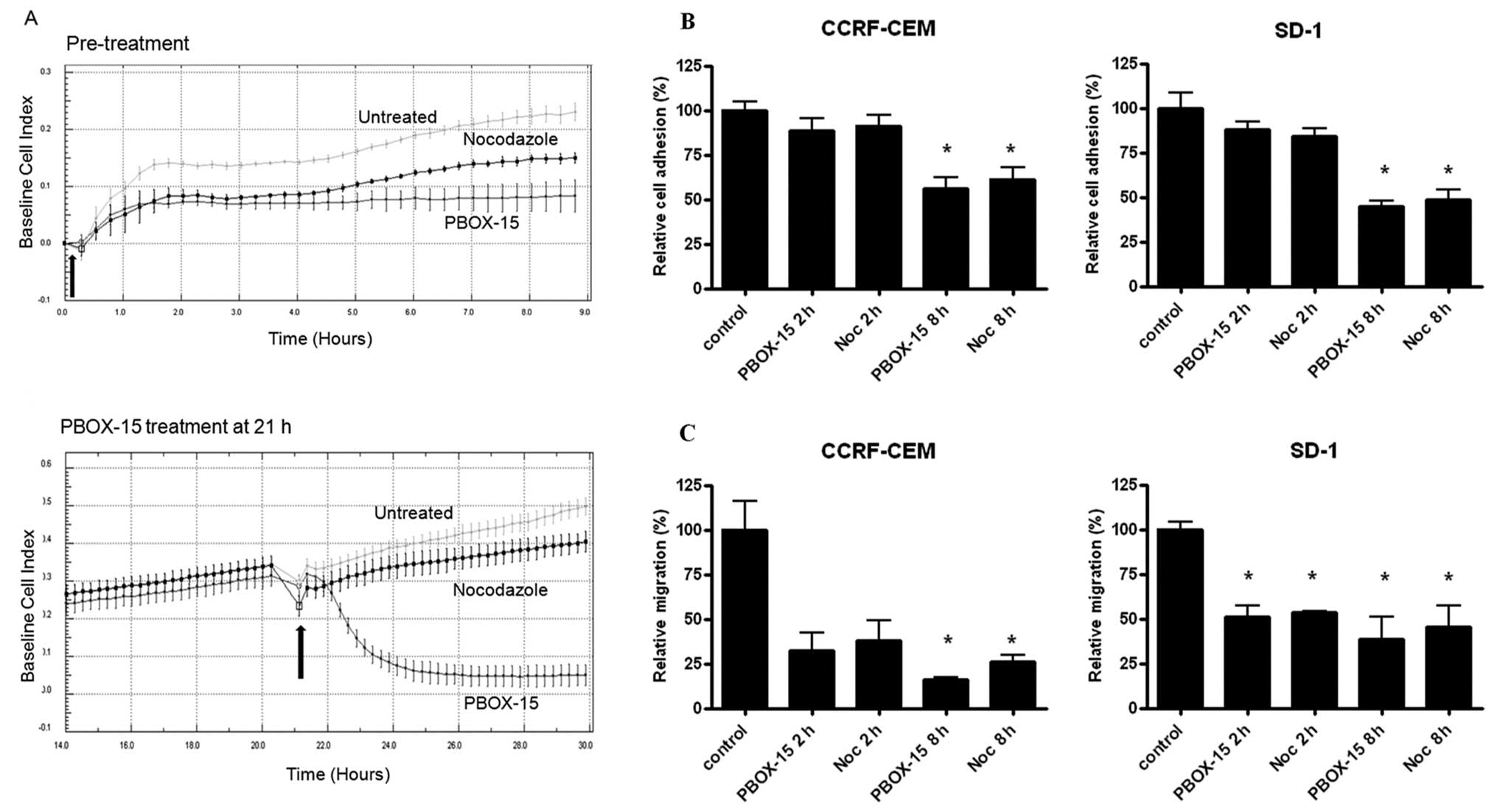 | Figure 2.PBOX-15 inhibits adhesion and
migration of T-ALL cells. A, Real-time monitoring of CCRF-CEM cell
adhesion and spreading following 1 μM PBOX-15 and 1
μM nocodozole treatment was performed using xCELLigence
electronic sensing system. This system measures electrical
impedance across interdigitated micro-electrodes integrated on the
bottom of 96-well tissue culture plates by micro-electronic sensor
technology. Cells (1×104) were seeded in the E-plate and
allowed to attach onto the electrode surface over time. The
electrical impedance of untreated (ethanol vehicle control) cells
and cells treated at time 0 h (arrow) with 1 μM PBOX-15 or 1
μM nocodozole was recorded every 15 min (top panel).
Untreated (ethanol vehicle control) cells were allowed to adhere
and spread for 21 h after which time (arrow) one group was treated
with 1 μM PBOX-15 or nocodozole and monitored for additional
9 h (bottom panel). The cell impedance, expressed as an arbitrary
unit (Cell Index), was automatically calculated on the xCELLigence
system. Data are illustrated as means ± SEM. Data shown are
representative of 3 independent experiments. B, CCRF-CEM and SD-1
cells were treated with 1 μM PBOX-15 or 1 μM
nocodozole as indicated and allowed to adhere to fibronectin coated
plates for 2 or 8 h. Plates were scanned using an IN Cell Analyzer
1000 automated microscope and cell adherence was quantified using
IN Cell Investigator software. Column data are illustrated as means
± SEM. *p<0.01, n=3, by Student’s t-test compared to
control cells. C, Following treatment with 1 μM PBOX-15 or 1
μM nocodozole for 2 and 8 h, migration potential of CCRF-CEM
and SD-1 cells were assessed by a transwell migration assay.
Significant inhibition of ALL cell migration compared to ethanol
vehicle control was observed as indicated. Column data are
illustrated as means ± SEM. *p<0.05, n=3, Student’s
t-test. |
To further investigate these early PBOX-15-induced
phenotypic changes in ALL cells, we examined the effects of PBOX-15
on ALL cell adhesion to the extracellular matrix protein
fibronectin and on cell migration. Pre-treatment of both CCRF-CEM
and SD-1 cells for 8 h with PBOX-15 significantly reduced cell
adhesion to fibronectin, with nodcodozole having a similar effect
(p<0.01; Fig. 2B). Both PBOX-15
and nocodozole potently inhibited ALL cell migration in a transwell
migration assay after 2-h exposure, with significant inhibition of
both CCRF-CEM and SD-1 cell migration after 8-h treatment
(p<0.05; Fig. 2C). To ensure
that the effects of PBOX-15 on cellular adherence, morphology and
migration were not due to the induction of cell death, PBOX-15
treated CCRF-CEM and SD-1 cells (1 μM for 2 or 8 h) were
stained with FITC-Annexin V and PI, and apoptosis was assessed by
flow cytometry. No increase in apoptotic cells was observed at up
to 8-h treatment compared with untreated cells (data not
shown).
PBOX-15 down-regulates integrin
expression and decreases integrin-mediated adhesion in ALL
cells
In order to determine if the decreased ALL cell
adhesion and migration due to PBOX-15 treatment was associated with
dysregulation of integrins involved in adhesion to fibronectin and
lymphocyte trafficking, the expression levels of α4 (CD49d), β1
(CD29) and β2 (CD18) integrins were assessed by flow cytometry
following treatment with 1 μM PBOX-15 for 8 h. A decrease in
α4- and β1-integrin expression was observed in the CCRF-CEM cells
(Fig. 3A), while a substantial
decrease in α4- and β2-integrin was observed in SD-1 cells
(Fig. 3B). To investigate if this
had a functional impact on integrin-mediated ALL cell attachment,
plates were coated with anti-α4-, anti-β1- or anti-β2-integrin and
adhesion of control or PBOX-15-treated cells (2 or 8 h) was
assessed using an HCA system. Following 8 h of PBOX-15 treatment, a
significant reduction of CCRF-CEM cell adhesion on β2 (p<0.001)
and α4 (p<0.05) integrins was observed (Fig. 3A). Similarly in the SD-1 cells, a
significant decrease in adhesion via β1 (p<0.01), β2 (p<0.05)
and α4 (p<0.01) integrins was observed (Fig. 3B).
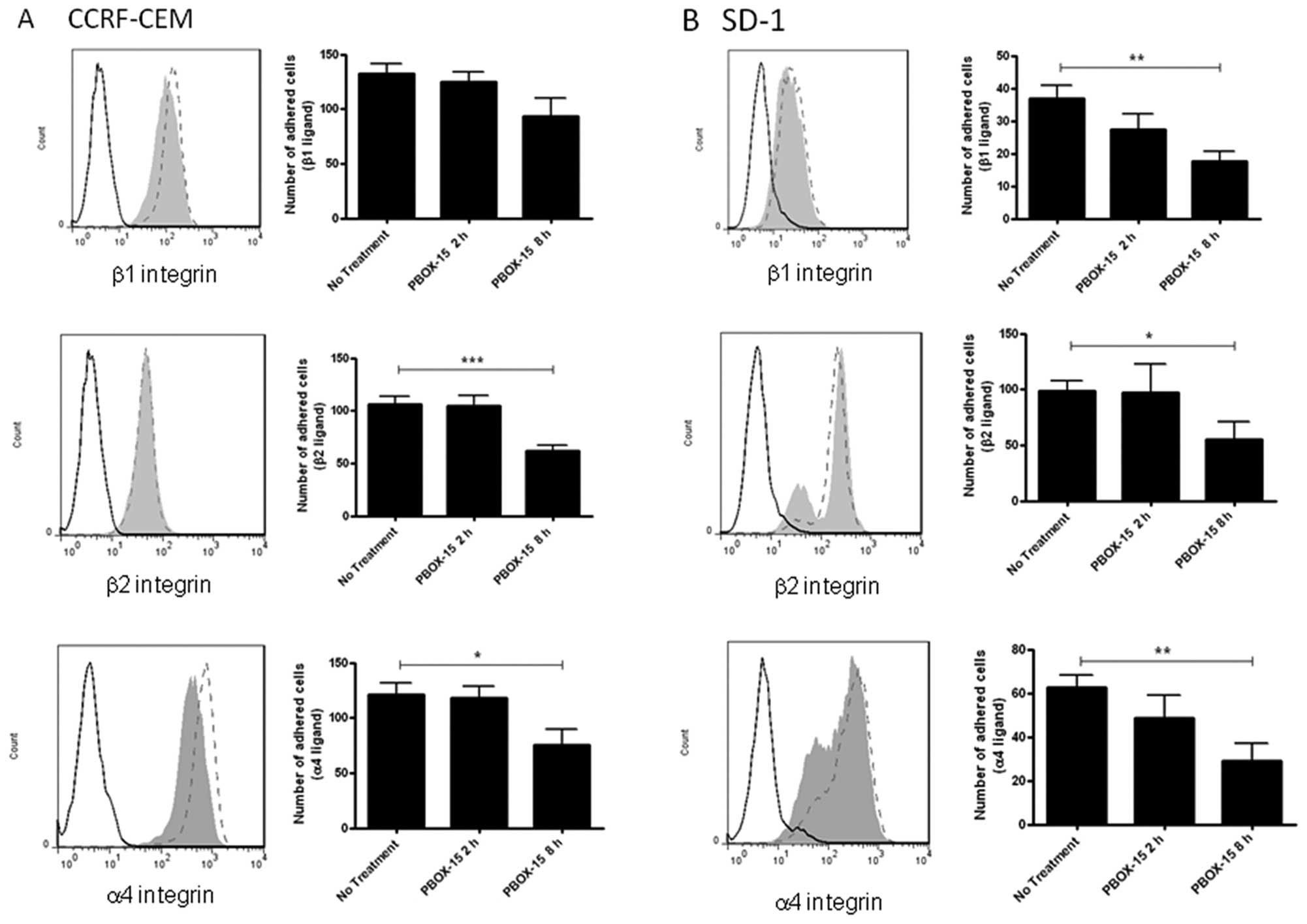 | Figure 3.PBOX-15 significantly alters
integrin-mediated adherence of ALL cells. A, CCRF-CEM and B, SD-1
cells were treated with 1 μM PBOX-15 for 8 h, stained for
α4-PE, β1-FITC or β2-FITC integrins, and analyzed by flow
cytometry. Histograms show isotype control (---), untreated
(ethanol vehicle control) cells (---), PBOX-15 treated cells (grey
area). Adhesion of cells on anti-α4, β1, or β2-integrin antibody
coated surfaces was subsequently quantified using a cell-based high
content analysis system. Adherent cells were fluorescently stained
with Rhodamine-phalloidin and Hoechst to visualize cell morphology
and the nuclei respectively. Images were acquired using an
automated microscope IN Cell Analyzer 1000 and quantified by IN
Cell Investigator software. Column data are illustrated as means ±
SEM. *p<0.05, **p<0.01,
***p<0.001, n=3, by Student’s t-test compared to ‘no
treatment’ (vehicle control). |
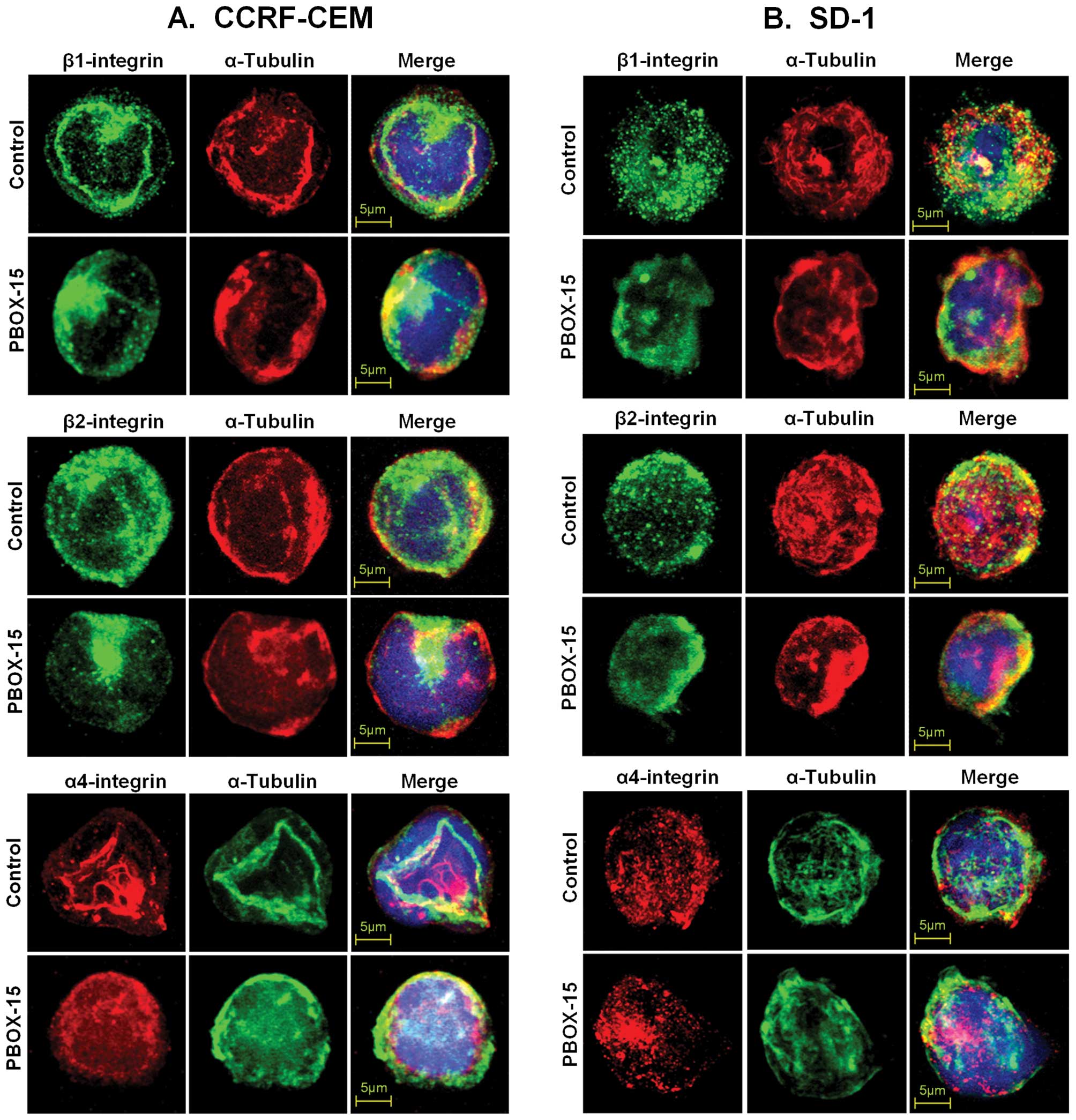
PBOX-15 interferes with lateral mobility
and clustering of integrins in ALL cells
An intact and dynamic microtubule system is required
for the cytoskeletal constraint on the lateral mobility of
integrins, and, thus, integrin-mediated adhesion. Moveover, the
microtubule cytoskeleton is known to participate in the control of
integrin avidity (23). To further
investigate if the PBOX-15-induced decrease in adhesion of ALL
cells to α4-, β1- or β2-integrin ligands was due to alteration in
lateral mobility and clustering of integrins, ALL cells were
treated with 1 μM PBOX-15 for 8 h and, subsequently, were
immunostained for α4-, β1-, or β2-integrins and α-tubulin. Confocal
microscopy revealed that the disruption of the cell membrane
distribution and clustering of integrins was concurrent with the
disruption of microtubule architecture in both CCRF-CEM (Fig. 4A) and SD-1 cells (Fig. 4B).
Discussion
The treatment outcome for ALL has improved
dramatically in recent decades with a cure rate exceeding 80% in
paediatric disease. However, a number of challenges remain which
necessitate improved therapeutic options: up to 25% of patients
relapse, resulting in relapsed ALL being the leading cause of
cancer related death in children and young adults; adult ALL
continues to have a poor prognosis; and T-ALL is associated with
unfavourable clinical features such as high white blood cell
counts, bulky adenopathy and involvement of the central nervous
system with resulting poor clinical outcomes. Here, we show
anti-cancer activity of a novel MTA compound, PBOX-15, in cell line
models representing poor prognostic subgroups of ALL: relapsed
childhood T-ALL (CCRF-CEM) and BCR-ABL positive adult B-ALL
(SD-1).
Similar to our previous studies on the effect of
PBOX-15 on other cancer cell types (5,6,10),
we showed that PBOX-15-induced G2/M cell cycle arrest and growth
inhibition was associated with the induction of apoptosis in B-ALL
and T-ALL cells. A pan-caspase pharmacological inhibitor inhibited
PBOX-15-induced apoptosis in both T-ALL and B-ALL cells, while
caspase-3 and 8 pharmacological inhibitors inhibited apoptosis in
T-ALL cells. We have recently shown caspase-8-dependent
PBOX-15-induced apoptosis in CLL and myeloma cells using a
pharmacological inhibitor and siRNA knockdown of caspase-8
expression (7,10). However, caspase inhibitors only
partially inhibited PBOX-15-induced apoptosis suggesting that
caspase-independent mechanisms are also involved in PBOX-15-induced
apoptosis in ALL cells (33).
Our previous study showing that PBOX-15 rapidly
inhibits integrin-induced T-cell migration (24) allowed us to postulate that PBOX-15
may potentially inhibit cell homing and mobility, and hence, the
metastatic potential of ALL cells. In addition, gene expression
studies on PBOX-15-treated CCRF-CEM and SD-1 cells revealed
alteration of the expression of genes involved in multiple cellular
pathways, including resistance to apoptosis and chemotaxis,
suggesting that PBOX-15 may affect multiple aspects of the ALL cell
phenotype (unpublished data). In this study, we used an
impedanciometry technique to obtain qualitative information in
real-time on the biological status of CCRF-CEM cells, including
degree of adhesion, spreading and proliferation. PBOX-15 reduced
the Cell Index of CCRF-CEM cells compared to control cells within 1
h, indicating that this effect on the phenotype of the cells was
independent of cell cycle arrest and subsequent apoptosis, and
suggesting that PBOX-15 rapidly inhibited the adhesion and
spreading of the cells (34,35).
Of note is the more pronounced and sustained effect of PBOX-15 on
the cells compared to treatment with nocodazole (Fig. 2A). This may indicate that PBOX-15
has a distinct mechanism of action compared to other tubulin
depolymerising agents and, indeed, we have shown differences in the
mechanism of action of PBOX-15 compared to nocodozole in CLL cells
(7). The effect of PBOX-15 was
more pronounced on pre-adhered cells suggesting that PBOX-15 may
have a more potent effect on cell spreading compared to nocodazole.
However, as both PBOX-15 and nocodazole had similar effects on cell
adhesion to fibronectin and cell migration, the significance of the
xCelligence data requires further investigation.
The PBOX-15-induced inhibition of ALL cell adhesion
to fibronectin and cell migration further suggests that PBOX-15 may
affect ALL homing and trafficking. Lymphocyte trafficking to
secondary organs involves tissue and lymphocyte specific multistep
adhesion cascades. Integrins are among the most dynamic adhesion
molecules and are critically important for cell migration.
Activated integrins, particularly members the α4, β1 and β2
subfamilies, mediate the firm adhesion of circulating lymphocytes
to vascular cells and are crucial for lymphocyte trafficking
(36). Very late antigen-4
(VLA-4), an α4-, β1-integrin dimer, is a receptor for fibronectin
and has been associated with B-cell survival and activation of the
phosphatidylinositol-3-kinase signalling pathway in acute leukaemia
(17,37). Recently, VLA-4 expression has been
identified as a poor prognostic marker and potential therapeutic
target in relapsed childhood B-ALL (38). We observed significantly reduced
α4-, β1- and β2-integrin mediated adhesion in PBOX-15-treated B-ALL
cells concurrently with down-regulation of the respective integrin
subunits. In comparison, down-regulation of the integrin subunits
in PBOX-15-treated T-ALL cells was less pronounced, although α4-
and β2-integrin mediated adhesion was significantly reduced.
Importantly, we show that the cell surface distribution and
clustering of α4-, β1- and β2-integrins is disrupted in both ALL
cell lines following PBOX-15 treatment. A crucial role of the
microtubule cytoskeleton in the control of integrin avidity has
been established (23). The
cytoskeleton is intimately involved in the dynamic regulation of
the adhesive state of integrins, switching from sufficiently strong
binding to maintain cell-cell contacts, to a state that allows
cells to dissociate and migrate. The microtubule system is not only
important for driving this membrane remodelling (via either an
increase or decrease of integrin avidity) but also acts as a
platform to bring together surface receptors and recruit activable
enzymes and substrates. Integrins interact with the microtubule
cytoskeleton through association of their cytoplasmic domains and
the MTA colchicine, nocodazole and taxol have been shown to
interefere with integrin clustering (23). Our data on the altered distribution
and clustering of integrins, as a result of PBOX-15-mediated
microtubule disruption, may suggest a role for integrin avidity in
signalling mechanisms and/or gene regulation in ALL cells.
Overall, this study indentifies PBOX-15 as an agent
which targets multiple aspects of the ALL cell phenotype and, as
such, warrants further investigation of its potential as a novel
therapeutic agent for the treatment of ALL.
Acknowledgements
This study was funded by the
Children’s Leukaemia Research Project and the Irish Cancer Society.
N.K.V. and equipment were part-funded by Health Research Board
(HRB) and Higher Education Authority (HEA) of Ireland.
References
|
1.
|
Jemal A, Tiwari RC, Murray T, et al:
Cancer statistics, 2004. CA Cancer J Clin. 54:8–29. 2004.
View Article : Google Scholar
|
|
2.
|
Pui CH, Robison LL and Look AT: Acute
lymphoblastic leukaemia. Lancet. 371:1030–1043. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Pui CH and Evans WE: Drug therapy -
treatment of acute lymphoblastic leukemia. N Engl J Med.
354:166–178. 2006. View Article : Google Scholar
|
|
4.
|
Faderl S, O’Brien S, Pui CH, et al: Adult
acute lymphoblastic leukemia concepts and strategies. Cancer.
116:1165–1176. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Mulligan JM, Greene LM, Cloonan S, et al:
Identification of tubulin as the molecular target of proapoptotic
pyrrolo-1,5-benzoxazepines. Mol Pharmacol. 70:60–70.
2006.PubMed/NCBI
|
|
6.
|
Greene LM, Campiani G, Lawler M, Williams
DC and Zisterer DM: BubR1 is required for a sustained mitotic
spindle checkpoint arrest in human cancer cells treated with
tubulin-targeting pyrrolo-1,5-benzoxazepines. Mol Pharmacol.
73:419–430. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
McElligott AM, Maginn EN, Greene LM, et
al: The novel tubulin-targeting agent pyrrolo-1,5-benzoxazepine-15
induces apoptosis in poor prognostic subgroups of chronic
lymphocytic leukemia. Cancer Res. 69:8366–8375. 2009. View Article : Google Scholar
|
|
8.
|
Nathwani SM, Butler S, Fayne D, et al:
Novel microtubule-targeting agents, pyrrolo-1,5-benzoxazepines,
induce apoptosis in multi-drug-resistant cancer cells. Cancer
Chemother Pharmacol. 66:585–596. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Forde JC, Maginn EN, McNamara G, et al:
Microtubule-targeting-compound PBOX-15 radiosensitizes cancer cells
in vitro. Cancer Biol Ther. 11:421–428. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Maginn EN, Browne PV, Hayden P, et al:
PBOX-15, a novel microtubule targeting agent, induces apoptosis,
upregulates death receptors, and potentiates TRAIL-mediated
apoptosis in multiple myeloma cells. Br J Cancer. 104:281–289.
2011. View Article : Google Scholar
|
|
11.
|
Bright SA, McElligott AM, O’Connell JW, et
al: Novel pyrrolo-1,5-benzoxazepine compounds display significant
activity against resistant chronic myeloid leukaemia cells in
vitro, in ex vivo patient samples and in vivo. Br J Cancer.
102:1474–1482. 2010. View Article : Google Scholar
|
|
12.
|
Vilpo JA, Koski T and Vilpo LM: Selective
toxicity of vincristine against chronic lymphocytic leukemia cells
in vitro. Eur J Haematol. 65:370–378. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Beswick RW, Ambrose HE and Wagner SD:
Nocodazole, a microtubule depolymerising agent, induces apoptosis
of chronic lymphocytic leukaemia cells associated with changes in
Bcl-2 phosphorylation and expression. Leuk Res. 30:427–436. 2006.
View Article : Google Scholar
|
|
14.
|
Moon EY and Lerner A: Benzylamide sulindac
analogues induce changes in cell shape, loss of microtubules and
G(2)-M arrest in a chronic lymphocytic leukemia (CLL) cell line and
apoptosis in primary CLL cells. Cancer Res. 62:5711–5719.
2002.PubMed/NCBI
|
|
15.
|
Aisenberg AC and Wilkes B: Studies on
suppression of immune responses by periwinkle alkaloids vincristine
+ vinblastine. J Clin Invest. 43:2394–2403. 1964.PubMed/NCBI
|
|
16.
|
Park SJ, Shim WH, Ho DS, et al: A
paclitaxel-eluting stent for the prevention of coronary restenosis.
N Engl J Med. 348:1537–1545. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Manabe A, Murti KG, Coustansmith E, et al:
Adhesion-dependent survival of normal and leukemic human-B
lymphoblasts on bone-marrow stromal cells. Blood. 83:758–766.
1994.PubMed/NCBI
|
|
18.
|
De la Fuente MT, Casanova B, Moyano JV, et
al: Engagement of alpha 4 beta 1 integrin by fibronectin induces in
vitro resistance of B chronic lymphocytic leukemia cells to
fludarabine. J Leukoc Biol. 71:495–502. 2002.PubMed/NCBI
|
|
19.
|
Scimone ML, Alfantis I, Apostolou I, von
Boehmer H and von Andrian UH: A multistep adhesion cascade for
lymphoid progenitor cell homing to the thymus. Proc Natl Acad Sci
USA. 103:7006–7011. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
20.
|
Cox D, Brennan M and Moran N: Integrins as
therapeutic targets: lessons and opportunities. Nat Rev Drug
Discov. 9:804–820. 2010. View
Article : Google Scholar : PubMed/NCBI
|
|
21.
|
Astier AL, Svoboda M, Hinds E, De Beaumont
R, Munoz O and Freedman AS: Integrins regulate survival of
pre-B-ALL cells through differential IAP and caspase-7
ubiquitination and degradation. Leukemia. 18:873–875. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
22.
|
Streuli CH and Akhtar N: Signal
co-operation between integrins and other receptor systems. Biochem
J. 418:491–506. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23.
|
Zhou XM, Li JX and Kucik DF: The
microtubule cytoskeleton participates in control of beta(2)
integrin avidity. J Biol Chem. 276:44762–44769. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
24.
|
Verma NK, Dempsey E, Conroy J, et al: A
new microtubule-targeting compound PBOX-15 inhibits T-cell
migration via post-translational modifications of tubulin. J Mol
Med. 86:457–469. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
25.
|
Campiani G, Nacci V, Fiorini I, et al:
Synthesis, biological activity, and SARs of pyrrolobenzoxazepine
derivatives, a new class of specific ‘peripheral-type’
benzodiazepine receptor ligands. J Med Chem. 39:3435–3450.
1996.PubMed/NCBI
|
|
26.
|
McGee MM, Gemma S, Butini S, et al:
Pyrrolo[1,5]benzoxa(thia) zepines as a new class of potent
apoptotic agents. Biological studies and identification of an
intracellular location of their drug target. J Med Chem.
48:4367–4377. 2005.
|
|
27.
|
Foley GE, Lazarus H, Farber S, Uzman BG,
Boone BA and McCarthy RE: Continuous culture of human lymphoblasts
from peripheral blood of a child with acute leukemia. Cancer.
18:522–529. 1965. View Article : Google Scholar : PubMed/NCBI
|
|
28.
|
Dhut S, Gibbons B, Chaplin T and Young BD:
Establishment of a lymphoblastoid cell-line, Sd-1, expressing the
p190 bcr-abl chimeric protein. Leukemia. 5:49–55. 1991.PubMed/NCBI
|
|
29.
|
Vermes I, Haanen C, Steffens-Nakken H and
Reutelingsperger C: A novel assay for apoptosis. Flow cytometric
detection of phosphatidylserine expression on early apoptotic cells
using fluorescein labelled Annexin V. J Immunol Methods. 184:39–51.
1995. View Article : Google Scholar
|
|
30.
|
Verma NK, Dourlat J, Davies AM, et al:
STAT3-stathmin interactions control microtubule dynamics in
migrating T-cells. J Biol Chem. 284:12349–12362. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
31.
|
Mohamed BM, Verma NK, Davies AM, et al:
Citrullination of proteins: a common post-translational
modification pathway induced by different nanoparticles in vitro
and in vivo. Nanomedicine. 7:1181–1195. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
32.
|
Mohamed BM, Verma NK, Prina-Mello A, et
al: Activation of stress-related signalling pathway in human cells
upon SiO2 nanoparticles exposure as an early indicator of
cytotoxicity. J Nanobiotechnology. 9:292011. View Article : Google Scholar : PubMed/NCBI
|
|
33.
|
McGrath LB, Onnis V, Campiani G, Williams
DC, Zisterer DM and McGee MM: Caspase-activated DNase
(CAD)-independent oligonucleosomal DNA fragmentation in chronic
myeloid leuka emia cells; a requirement for serine protease and
Mn2+-dependent acidic endonuclease activity. Apoptosis.
11:1473–1487. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
34.
|
Whipple RA, Matrone MA, Cho EH, et al:
Epithelial-tomesenchymal transition promotes tubulin detyrosination
and microtentacles that enhance endothelial engagement. Cancer Res.
70:8127–8137. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
35.
|
Tiruppathi C, Malik AB, Del Vecchio PJ,
Keese CR and Giaever I: Electrical method for detection of
endothelial cell shape change in real time: assessment of
endothelial barrier function. Proc Natl Acad Sci USA. 89:7919–7923.
1992. View Article : Google Scholar : PubMed/NCBI
|
|
36.
|
Von Andrian UH and Mackay CR: Advances in
immunology: T-cell function and migration - two sides of the same
coin. N Engl J Med. 343:1020–1033. 2000.
|
|
37.
|
Matsunaga T, Takemoto N, Sato T, et al:
Interaction between leukemic-cell VLA-4 and stromal fibronectin is
a decisive factor for minimal residual disease of acute myelogenous
leukemia. Nat Med. 9:1158–1165. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
38.
|
Shalapour S, Hof J, Kirschner-Schwabe R,
et al: High VLA-4 expression is associated with adverse outcome and
distinct gene expression changes in childhood B-cell precursor
acute lymphoblastic leukemia at first relapse. Haematologica.
96:1627–1635. 2011. View Article : Google Scholar : PubMed/NCBI
|