Contents
Introduction
Pol and other DNA metabolic enzyme inhibition by VK
quinone derivatives in vitro
Effects of VK quinone derivatives on cultured
macrophage cells
Effects of NQ on inflammation in vivo
Discussion
Conclusion
Introduction
Vitamin K (VK) is a hydrophobic (i.e., insoluble in
water) human vitamin. It is needed for the synthesis of proteins
required for blood coagulation (1). Normally, VK is produced by bacteria
in the intestines and dietary deficiency is extremely rare unless
the intestines are badly damaged. VK is essentially involved in the
carboxylation of certain glutamate residues in proteins to form
γ-carboxyglutamate residues, which are usually involved in binding
calcium (2,3). Recently, DNA microarray was used to
identify the effect of VK status on gene expression in rat liver.
The expression of genes involved in the acute inflammation response
was enhanced in rats fed a VK-deficient diet relative to the
control and VK-supplemented diet groups (4).
VK comprises a family of structurally similar,
fat-soluble 2-methyl-1,4-naphthoquinones, including phylloquinone
(VK1), menaquinone (VK2) and menadione (VK3).
The structures are shown in Fig.
1. 1,4-Naphthoquinones (NQs) (compound 7 of Fig. 1) form a family of compounds
characterized by a naphthalene ring that contains two carbonyl
moieties at positions 1 and 4 and that, in the case of VK, is
substituted at positions 2 and 3. All members of the VK family
possess an identical NQ skeleton with various isoprenyl chains that
distinguish them. VK1 and VK2 differ only in
the prosthetic group at position 3. VK1 possesses a
phytyl group (a partially saturated poly-isoprenoid group) at
position 3, whereas VK2 possesses a repeating
unsaturated trans-poly-isoprenyl group. The IUPAC-IUB Commission on
Biochemical Nomenclature abbreviates VK2 as ‘MK-n’,
where ‘n’ signifies the number of unsaturated isoprene units that
compose the isoprenyl chain at the 3-position. The isoprenyl chain
of MK-n can vary in length from C5 (n=1) to C65 (n=13); for
example, menaquinone 4 (MK-4) could also be written as
K2(20). MK-4
(=VK2) has three isoprene units plus the first saturated
group beginning at position 3, totaling four (compound 2 of
Fig. 1). The most common form of
VK in animals is VK2 in its MK-4 structure, which is
produced by intestinal bacteria from exogenous NQs and transformed
endogenously in our own cells (5).
VK3 possesses a much simpler structure, with no
aliphatic chain prosthetic group at position 3 (compound 6 of
Fig. 1).
 | Figure 1Structures of VKs and their related
quinone derivatives. 1, Vitamin K1 (VK1); 2,
Vitamin K2 (VK2 = MK-4); 3, MK-3; 4, MK-2; 5,
MK-1; 6, Vitamin K3 (VK3 = 1-methyl-NQ); 7,
1,4-naphthoquinone (NQ); 8, 1,2-dimethyl-1,4-naphthoquinone
(1,2-dimethyl-NQ); 9, benzoquinone (BQ); 10, 9,10-anthraquinone
(AQ) and 11, 5,12-naphthacenequinone (NCQ). |
VK1 and VK2 are naturally
occurring types of VK. VK1 is synthesized by plants and
can be found in such foods as spinach, broccoli, lettuce and
soybeans. VK2 is primarily produced by bacteria in the
anterior part of the gut and the intestines. The MK-4 and MK-7
forms of VK2 are found in meat, eggs, dairy products and
natto, which is fermented food from soybeans. MK-4 is synthesized
by animal tissues; other forms of VK2 (mainly MK-7) are
synthesized by bacteria during fermentation (6). In natto 0% of VK is in the MK-4 form
and in cheese 2–7% is in this form (7). VK2 forms with 2–13
isoprene units, including MK-1, MK-2 and MK-3, have been found in
human and animal tissues (8). On
the other hand, Booth reported that though VK2
derivatives are synthesized in the intestine, intestinal MKs are
not believed to be the primary source of VK; VK1 is the
primary dietary source; MK-4 and MK-7 are relatively minor sources
in the average diet (9). Although
VK3 is considered a synthetic analogue, Billeter et
al found that VK1 can be cleaved to form
VK3 by bacteria in the intestine (10). After absorption, VK3 is
thought to become alkylated into biologically active isoprenylated
VK2(11). However,
VK3 cannot exert all of the functions of natural VK, a
finding that is ascribed to its limited transformation into the
fat-soluble vitamin forms (12,13).
VKs have quinone as the principle chemical feature.
Quinones are a class of organic compounds that are formally derived
from aromatic compounds by exchanging an even number of -CH═ groups
by -C(═O)- groups, with any necessary rearrangement of double
bonds, resulting in a fully conjugated cyclic dione structure. The
toxicological properties of quinones, which act as alkylating
agents, have been examined. For example, quinones are known to
interact with flavoproteins to generate reactive oxygen species
(ROS) that can induce biological injury (14–17).
In this study, we focus on VKs and their related
quinone derivatives and review their possible bioactivity, such as
anti-inflammatory effects based on the selective inhibition of DNA
polymerase [i.e., DNA-dependent DNA polymerase (pol), E.C. 2.7.7.7]
species.
Pol and other DNA metabolic enzyme
inhibition by VK quinone derivatives in vitro
Mammalian pol species
Pol catalyzes the polymerization of
deoxyribonucleotides alongside a DNA strand, which it ‘reads’ and
uses as a template (18). The
newly polymerized molecule is complementary to the template strand
and identical to the template’s partner strand. Pol can add free
nucleotides only to the 3′-end of the newly formed strand, meaning
that elongation of the new strand occurs in a 5′- to -3′
direction.
The human genome encodes at least 15 DNA polymerases
(pols) that conduct cellular DNA synthesis (19,20).
Eukaryotic cells contain 3 replicative pols (α, δ and ɛ), 1
mitochondrial pol (γ) and at least 11 non-replicative pols (β, ζ,
η, θ, ι, κ, λ, μ, ν, terminal deoxynucleotidyl transferase (TdT)
and REV1) (21,22). Pols have a highly conserved
structure, which means that their overall catalytic subunits show
little variance among species. Enzymes with conserved structures
usually perform important cellular functions, the maintenance of
which provides evolutionary advantages. On the basis of sequence
homology, eukaryotic pols can be divided into 4 main families,
termed A, B, X and Y (22). Family
A includes mitochondrial pol γ, as well as pols θ and ν. Family B
includes 3 replicative pols (α, δ and ɛ) and pol ζ. Family X
comprises pols β, λ and μ, as well as TdT; lastly, family Y
includes pols η, ι and κ, in addition to REV1.
An assay method to detect pol inhibitors has been
established (23,24). The substrates of the pols were
synthesized DNA, such as poly(dA)/oligo(dT)18 (A/T =
2/1) and tritium-labeled 2′-deoxythymidine 5′-triphosphate
([3H]-dTTP) as DNA template-primer substrate and
nucleotide [2′-deoxynucleotide 5′-triphosphate (dNTP)] substrate,
respectively. The [3H]-dTTP incorporated radioactive DNA
products were collected on DEAE-cellulose paper discs and
radioactivity was measured in a scintillation counter. Activity
without the inhibitor was considered 100% and the remaining
activity at each concentration of the inhibitor was determined
relative to this value. One unit of pol activity was defined as the
amount of enzyme that catalyzed the incorporation of 1 nmol dNTP
(i.e., dTTP) into synthetic DNA template-primers in 60 min at 37°C
under the normal reaction conditions for each enzyme. Pols from
mammal, fish, insect and plant, which were purified and have high
activity, were prepared according to previous reports (25).
Effect of VK quinone derivatives on
mammalian pol activity
Although many researchers found and reported
inhibitors against all eukaryotic pol species, mainly nucleotide
analogues, we have been studying selective inhibitors of each
mammalian pol derived from natural products, including food
materials and nutrients, for more than 15 years (26–28).
In fat-soluble vitamins, VK3, but not VK1 or
VK2, is a potent and specific inhibitor of human pol γ
(29–33). Therefore, in this review, VKs and
their related quinone derivatives, which are the 11 compounds in
Fig. 1, were the focus.
The inhibition of the biochemical action of four
mammalian pols, namely calf pol α, human pol γ, human pol κ and
human pol λ, induced by the administration of 50 μM of each
compound, was investigated in vitro(34–36).
Pols α, γ, κ and λ were used as representatives of the B-, A-, Y-
and X-families of pols, respectively (19–21).
As shown in Fig. 2, MK-3, MK-2 and
MK-1, which are chemically synthesized intermediates between
VK2 and VK3, inhibited the activity of
mammalian pols α, κ and λ, whereas VK2 (=MK-4) and
VK1 had no effect on pol activity. VK3
selectively inhibited pol γ among the mammalian pols tested. Among
compounds 1–6 in Fig. 1, the
inhibitory effect of these compounds on pols α, κ and λ ranked as
follows: MK-2 > MK-1 > MK-3 > VK1 =
VK2 = VK3; and the inhibitory effect of these
compounds on pol γ ranked as follows: VK3 > MK-1 >
MK-2 > MK-3 > VK1 = VK2. The 50%
inhibitory concentration (IC50) values of MK-2 against
pols α, γ, κ and λ were 27.6, 68.8, 35.3 and 24.6 μM,
respectively (34).
Of the VK3 based quinone compounds 6–11,
NQ and 1,4-benzoquinone (BQ) most strongly inhibited the activities
of pols α and λ, but other compounds did not have an influence.
VK3 (1-methyl-NQ) and 1,2-methyl-NQ selectively
suppressed pol γ activity among the mammalian pols tested and
VK3 was a stronger pol γ inhibitor than 1,2-methyl-NQ.
By contrast, 9,10-anthraquinone (AQ) and 5,12-naphthacenequinone
(NCQ) had no effect on the activities of these pols. The inhibitory
effects of the 11 compounds on the activities of pols α and λ were
ranked as follows: NQ > BQ > MK-2 > MK-1 > MK-3 >
VK1 = VK2 = VK3 = 1,2-methyl-NQ =
AQ = NCQ.
When activated DNA, via DNA digestion by bovine
deoxyribonuclease I and dNTP were used as the DNA template-primer
and nucleotide substrate, respectively, instead of
poly(dA)/oligo(dT)18 (A/T = 2/1) and dTTP, respectively,
the mode of inhibition by these compounds did not change (34–36).
Effect of NQ on pols and other DNA
metabolic enzymes
Among the VKs and their related quinone derivatives
investigated, NQ displayed the strongest inhibitory effect on
mammalian pols α and λ (Fig. 2)
and is therefore the focus of this section. As described briefly in
the previous section, ten mammalian pol species including pols α,
β, γ, δ, ɛ, ι, η, κ and λ and TdT are obtainable; however, pols ζ,
θ, μ, ν and REV1 are not yet available (Table I). Currently, eukaryotes are
thought to express at least 15 species of pols (19,20)
and we are still in an era when most pols are very difficult to
obtain in purified form in a laboratory. Table I shows the inhibitory effect
(IC50 value) of NQ against various pol species including
the ten eukaryotic pols that can be obtained (35). This compound inhibited the activity
of all of the pols from mammals and 50% inhibition of the A-, B-,
X- and Y-families of pols was observed at a dose of 73.0,
6.65–67.2, 5.57–128 and 68.2–72.0 μM, respectively. Calf pol
α and human pol λ were inhibited most strongly in the B- and
X-families, respectively. These results suggested that the strength
of the inhibitory effect of NQ on mammalian pol families can be
ranked as follows: B-family pols = X-family pols > A-family pols
= Y-family pol. NQ showed the strongest inhibition of pol λ
activity among the pols investigated, with an IC50 value
of 5.57 μM. This compound also inhibited the activity of the
animal pols from fish (cherry salmon) and insect (fruit fly) at
almost the same concentrations that inhibited the activity of
mammalian pols (35).
 | Table IIC50 values of NQ on the
activities of various pols and other DNA metabolic enzymes. |
Table I
IC50 values of NQ on the
activities of various pols and other DNA metabolic enzymes.
| Enzyme | IC50
value of NQ (7) (μM) |
|---|
| Mammalian DNA
polymerases | |
| A-Family | |
| Human DNA
polymerase γ | 73.0±3.7 |
| B-Family | |
| Calf DNA
polymerase α | 6.65±0.35 |
| Human DNA
polymerase δ | 67.2±3.5 |
| Human DNA
polymerase ɛ | 61.4±3.2 |
| X-Family | |
| Rat DNA
polymerase β | 128±6.4 |
| Human DNA
polymerase λ | 5.57±0.28 |
| Calf terminal
deoxynucleotidyl transferase | 55.6±2.88 |
| Y-Family | |
| Human DNA
polymerase η | 72.0±4.2 |
| Mouse DNA
polymerase ι | 70.2±4.0 |
| Human DNA
polymerase κ | 68.2±3.9 |
| Fish DNA
polymerases | |
| B-Family | |
| Cherry salmon
DNA polymerase δ | 68.4±3.5 |
| Insect DNA
polymerases | |
| B-Family | |
| Fruit fly DNA
polymerase α | 8.30±0.38 |
| Fruit fly DNA
polymerase δ | 69.5±3.7 |
| Fruit fly DNA
polymerase ɛ | 64.1±3.4 |
| Plant DNA
polymerases - | |
| B-Family | |
| Cauliflower DNA
polymerase α | >100 |
| Prokaryotic DNA
polymerases | |
| E. coli
DNA polymerase I | >100 |
| Taq DNA
polymerase | >100 |
| T4 DNA
polymerase | >100 |
| Other DNA metabolic
enzymes | |
| Calf primase of
DNA polymerase α | >100 |
| T7 RNA
polymerase | >100 |
| T4 polynucleotide
kinase | >100 |
| Bovine
deoxyribonuclease I | >100 |
By contrast, NQ had minimal influence on the
activity of plant (cauliflower) pol α or prokaryotic pols, such as
E. coli pol I, Taq pol, or T4 pol (Table I) (35). The three-dimensional structures of
eukaryotic pols are likely to differ greatly from those of
prokaryotic pols (18,19). NQ did not inhibit the activity of
other DNA metabolic enzymes, such as calf primase pol α, T7 RNA
polymerase, T4 polynucleotide kinase, or bovine deoxyribonuclease
I. These results suggest that NQ may be a selective inhibitor of
animal pols, especially the B- and X-families of pols containing
pol λ.
To test whether NQ-induced inhibition resulted from
the ability of this compound to bind to DNA or to the enzyme, the
thermal transition of DNA in the presence or absence of NQ was
measured (35). The interaction of
NQ with dsDNA was investigated based on the thermal transition of
dsDNA by measuring the melting temperature (Tm) of dsDNA with an
excess amount of NQ (100 μM each) using a spectrophotometer
equipped with a thermoelectric cell holder. A thermal transition of
Tm was not observed within the concentration range of the compound
used in the assay, whereas a typical intercalating compound used as
a positive control (ethidium bromide, 15 μM) produced a
clear thermal transition. It also was investigated whether an
excessive amount of nucleic acid [poly(rC)] or protein [bovine
serum albumin (BSA)] prevented the inhibitory effect of NQ to
determine whether the inhibitory effect resulted from non-specific
adhesion of these molecules to animal pols, or from selective
binding to specific sites (35).
Poly(rC) and BSA had little or no influence on the pol inhibitory
effect of NQ, suggesting that this compound selectively binds to
the target enzyme molecule. These observations indicated that NQ
does not act as a DNA intercalating agent or a DNA template-primer
substrate, but that this compound can directly bind to the enzyme
and inhibit its activity. Collectively, these results suggest that
NQ may be a potent and specific inhibitor of animal pols,
especially pol λ.
Effects of VK quinone derivatives on
cultured macrophage cells
In many inflammatory responses, activation of
nuclear factor (NF)-κB is the rate-limiting step of the
inflammatory mechanism (37). The
five members of the mammalian NF-κB family, namely p65 (RelA),
RelB, c-Rel, p50/p105 (NF-κB1) and p52/p100 (NF-κB2), exist in
unstimulated cells as homodimers or heterodimers bound to proteins
of the IκB family (38). The
binding of NF-κB to IκB prevents NF-κB from translocating to the
nucleus, thereby maintaining NF-κB in an inactive state. NF-κB
proteins are characterized by the presence of a conserved
300-amino-acid Rel homology domain located in the N-terminus of the
protein and this domain is responsible for dimerization with NF-κB,
interaction with IκB and binding to DNA (38). The translocated NF-κB proteins work
as transcription factors and regulate the expression of various
genes that encode proinflammatory cytokines such as tumor necrosis
factor (TNF)-α and interleukin (IL)-12, which have been shown to
play important roles in sustained inflammatory responses (39–41).
Effect of VK quinone derivatives on
LPS-induced TNF-α production
Next, it was investigated whether VKs and their
related quinone derivatives can inhibit the inflammatory response
in cultured mouse macrophage RAW264.7 cells treated with
lipopolysaccharide (LPS), which stimulates macrophages to release
inflammatory cytokines such as TNF-α (34–36).
The cells were placed in a 12-well plate at a concentration of
5×104 cells/well and incubated for 24 h. The cells were
pre-treated with each compound (final concentrations of 5 and 10
μM) for 30 min before the addition of 100 ng/ml of LPS.
After stimulation with LPS for 24 h, the cell culture medium was
collected to measure the amount of TNF-α secreted. The
concentration of TNF-α in the culture medium was quantified by
using a commercially available enzyme-linked immunosorbent assay
(ELISA) development system.
In RAW264.7 cells, no compound showed cytotoxicity
at 10 μM because the 50% lethal dose (LD50)
values of these eleven compounds were >10 μM (34–36).
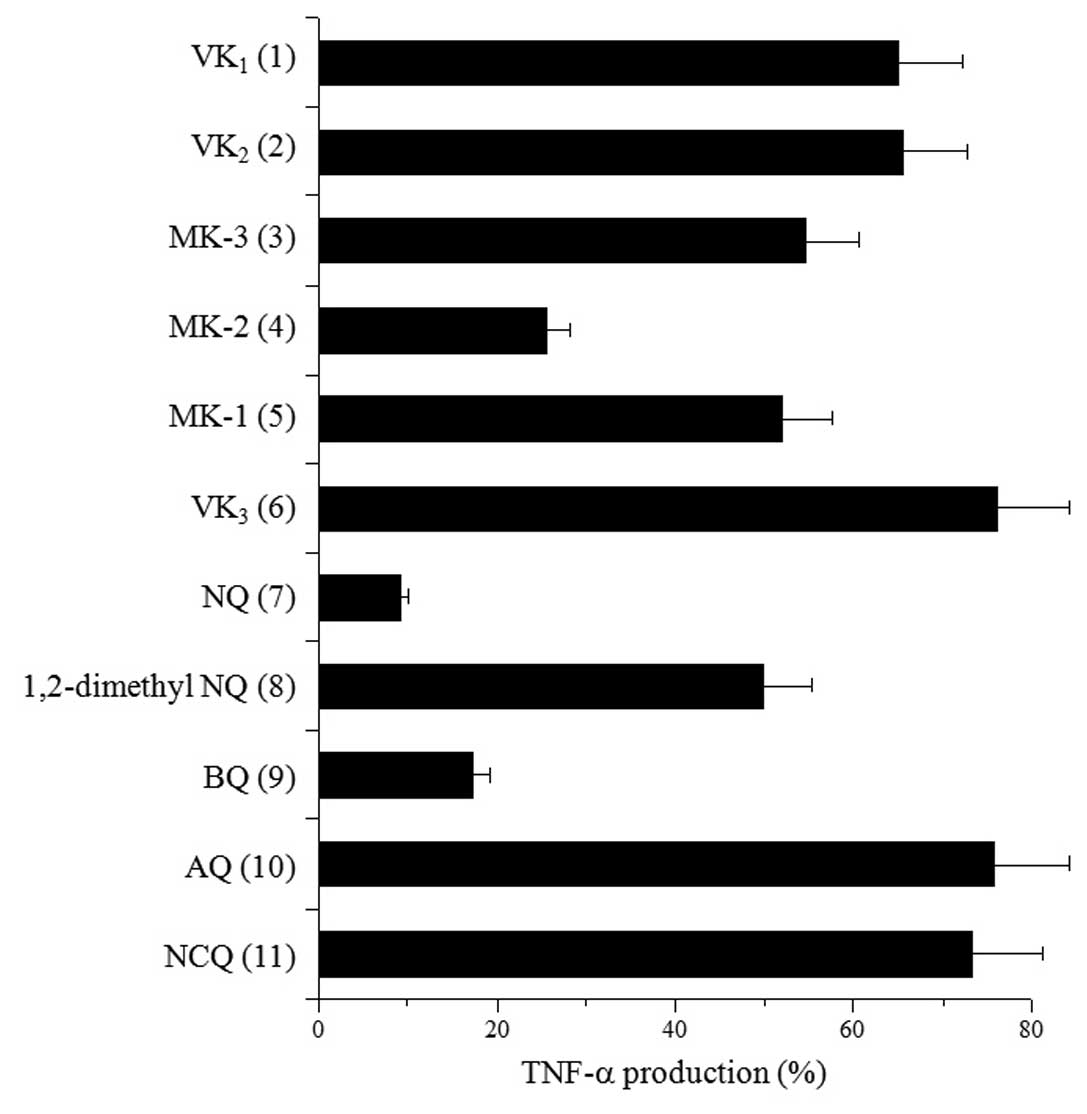
Among VKs and the VK2-VK3 intermediates
(i.e., compounds 1–6), MK-2 at 10 μM significantly
suppressed the LPS-stimulated production of TNF-α and other
compounds hardly suppressed (Fig.
3). In the VK3 related quinone compounds (i.e.,
compounds 6–11), NQ and BQ at 10 μM were potent suppressors
of TNF-α production and 1,2-dimethyl NQ moderately suppressed TNF-α
production. By contrast, VK3, AQ and NCQ displayed
hardly any effect on TNF-α production. The inhibitory effects of NQ
and BQ were, respectively, the first and second strongest among
these 11 compounds tested; the order of this effect was NQ > BQ
> MK-2 > MK-3 = MK-1 = 1,2-dimethyl-NQ > VK1 =
VK2 = VK3 = AQ = NCQ. The effect of these
compounds on the suppression of LPS-evoked TNF-α production showed
almost the same tendency as the inhibitory effect on mammalian pols
α and λ. These results suggest that VKs and their related quinone
derivatives, such as NQ, might inhibit the activities of mammalian
pols and then prevent the production of TNF-α in LPS-induced
macrophages, but not affect the cell growth.
Effect of NQ on LPS-induced pols
expression and inflammatory response
Because there was a relationship between the in
vitro pols α and λ inhibition and LPS-induced TNF-α suppression
in cultured mouse macrophage RAW264.7 cells by VK quinone
derivatives, the effect of NQ, the strongest pols α and λ inhibitor
and TNF-α suppressor, on the inflammatory response in the cells was
investigated (Mizushina et al, unpublished data). RAW264.7
cells in a 6-well plate at a concentration of 5×105
cells/well were incubated with 10 μM of NQ or DMSO as a
vehicle control for 30 min, followed by treatment with 100 ng/ml of
LPS for 30 min. After treatment, cells were harvested and the
nuclear protein extract was prepared. The protein concentration of
each extract was obtained and the amount of the β-actin band was
used as a control in western blotting.
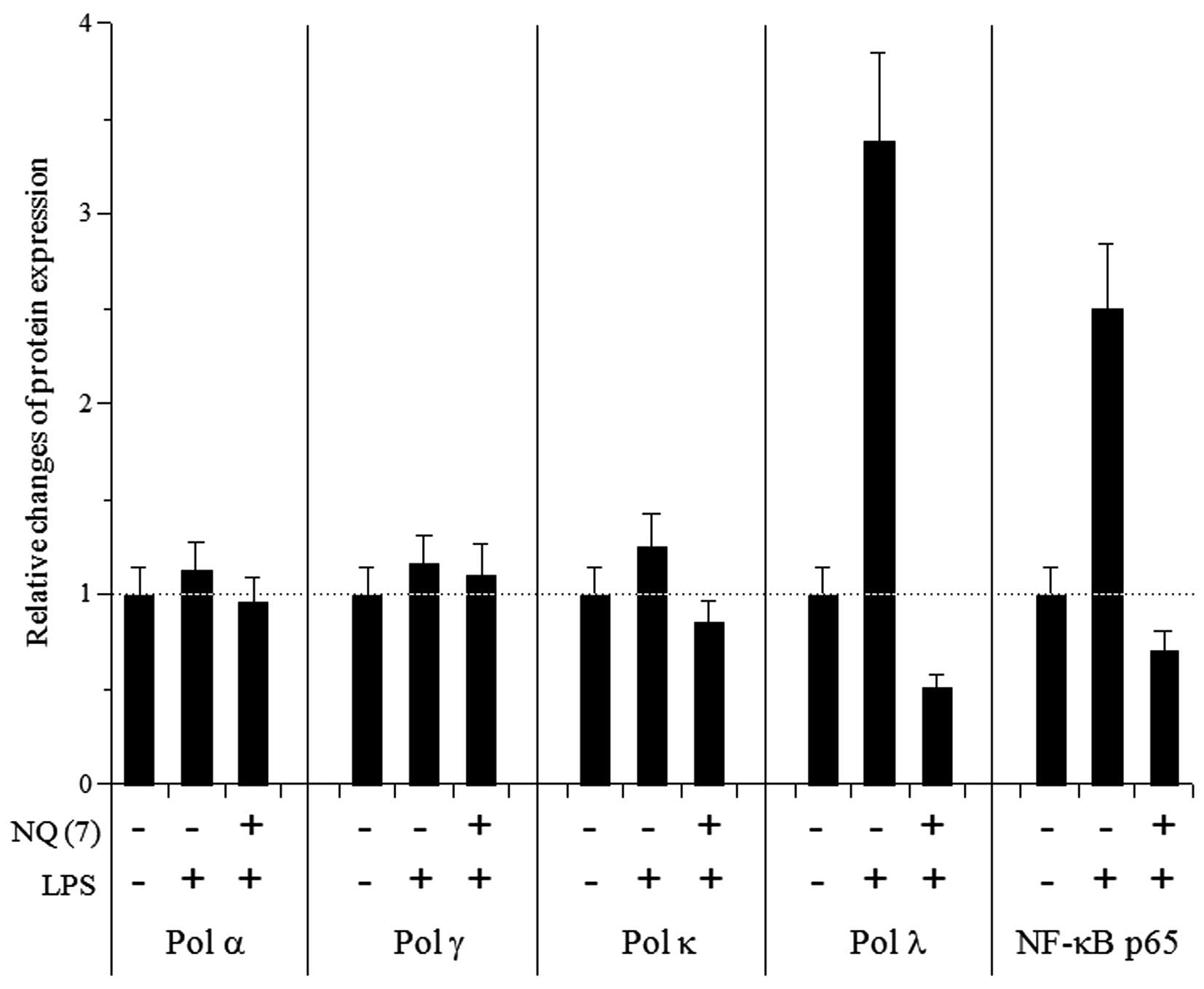
First, the effect of NQ on the protein expression
level of pols α, γ, κ and λ in LPS-treated RAW264.7 cells was
measured using western blotting. As shown in Fig. 4, these macrophages underwent a more
than 3-fold increase in the expression of pol λ after LPS
stimulation, but that this increase was significantly suppressed by
10 μM NQ. By contrast, the protein expression levels of
other mammalian pols, including pol α, were not influenced by LPS
and NQ. These results suggest that there is a positive correlation
between inflammatory induction by LPS and pol λ expression; thus,
not only the DNA polymerization activity, but also the protein
expression of DNA repair-related pol λ is likely to be important in
inflammation.
The inflammatory cytokine TNF-α activates the NF-κB
signaling pathway by binding to the TNF-α receptor (TNFR) and
thereby initiates an inflammatory response, resulting in various
inflammatory diseases (42).
Therefore, the inhibitory effect of NQ on the LPS-induced nuclear
translocation of NF-κB in RAW264.7 cells was examined. Using
western blot analysis, it was revealed that the amount of NF-κB
nuclear translocation in RAW264.7 cells was 2.5-fold greater after
LPS treatment and that 10 μM NQ was sufficient to inhibit
the LPS-stimulated nuclear translocation of NF-κB by 0.7-fold
(Fig. 4). These results
demonstrate that this compound can strongly suppress the nuclear
translocation of NF-κB by inhibiting the production of TNF-α.
Anti-oxidative activity has been reported to be
linked to anti-inflammatory activity (43); therefore, the anti-oxidative
activity of NQ against the production of ROS induced by TNF-α was
investigated according to the method of a previous report (44). In RAW264.7 cells, at 10 μM,
NQ decreased the production of ROS induced by 50 ng/ml of TNF-α to
49.2%, suggesting that this compound possesses anti-oxidative
activity (Mizushina et al, unpublished data).
Effects of NQ on inflammation in
vivo
Effect of NQ on LPS-induced mouse
inflammation
To assess the anti-inflammatory effects of NQ in
vivo, the inhibitory activity of this compound against
LPS-induced acute inflammation was investigated (35). Treatment with 250 μg/kg body
weight (BW) of LPS significantly increased the serum TNF-α level (5
ng/ml) and intraperitoneal injection of 20 mg/kg BW of NQ strongly
decreased this LPS-induced TNF-α production to 92.1%. Thus, the
in vivo data obtained in the mouse study gave the same trend
as the data obtained from cultured mouse macrophage cells.
Effect of NQ on DSS-induced colitis in
mice
To evaluate the therapeutic effects of NQ on
colitis, a dextran sulfate sodium (DSS)-induced colitis mouse model
was used (36). DSS is a sulfate
polysaccharide that has been very widely used to induce
inflammation in experimental models of inflammatory bowel disease
(45,46). The 2.5% (wt/vol) DSS-treated
C57BL/6J mice were injected orally with either NQ at 20 mg/kg BW or
corn oil as a vehicle control once daily (total 4 oral injections)
and then were sacrificed. During the experiment, the BW of the mice
was measured daily and the relative BW was calculated as the BW of
a mouse on Day 12 relative to the initial BW of the same mouse on
Day 0.
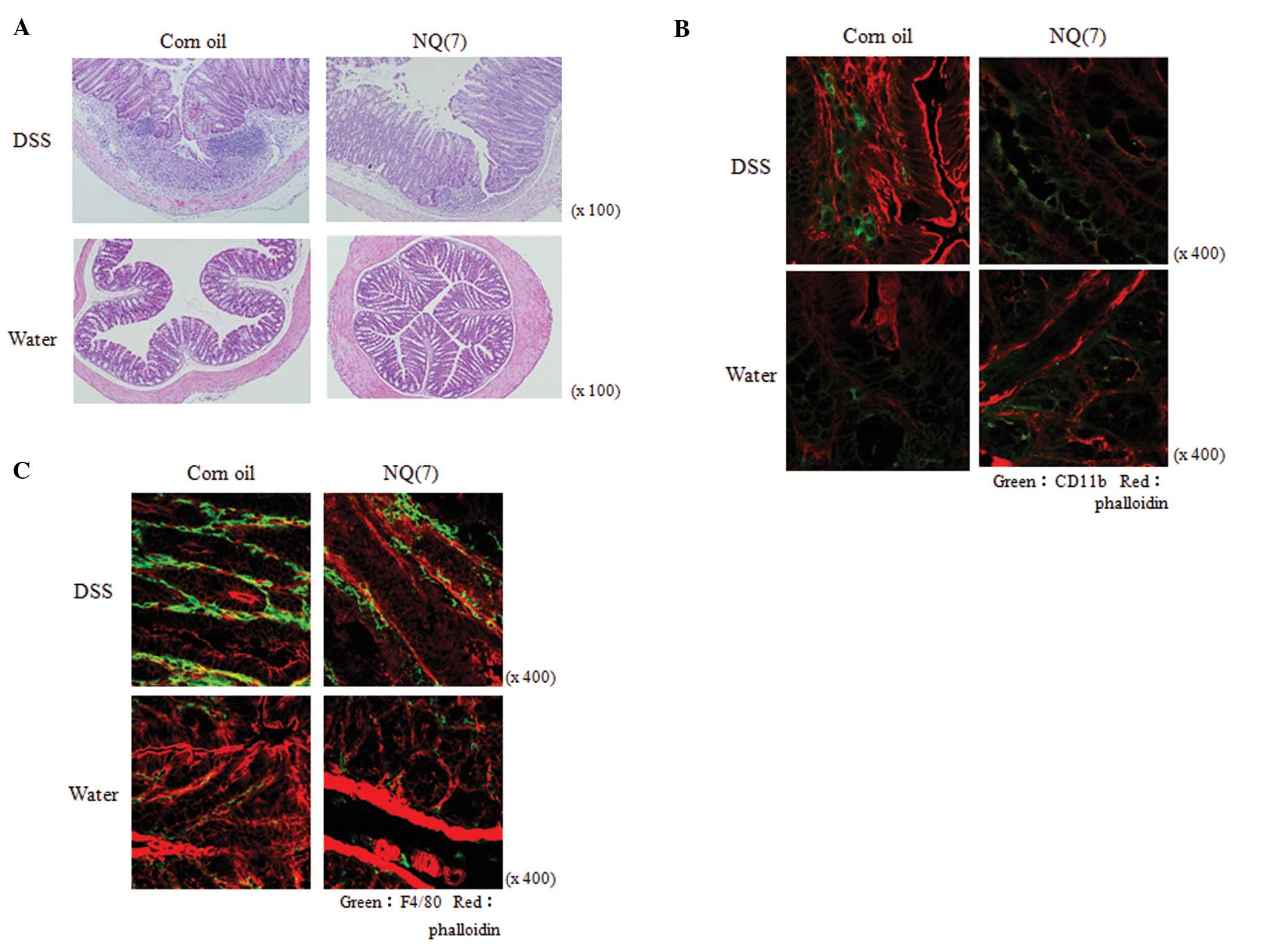
As a result, it was found that NQ significantly
improved the decreased BW of the colitis mice. This compound also
reduced the DSS-induced shortening of colon length. In a
histopathological examination with hematoxylin and eosin (H&E)
staining (Fig. 5A), NQ attenuated
the degree of tissue injury induced by DSS. RNA was isolated from
the colon epithelia of mice treated with DSS and NQ and the
expression levels of TNF-α mRNA were examined by quantitative
real-time PCR. In DSS-induced colitis mice, the expression level of
TNF-α mRNA was elevated and the administration of NQ led to a
reduction in TNF-α production in the colon epithelium. The frozen
colon sections obtained from the mice were stained with anti-CD11b
antibody to detect monocytes including macrophages (Fig. 5B) and anti-F4/80 antibody to detect
macrophages (Fig. 5C). As a
result, it was found that NQ significantly attenuated macrophage
infiltration into the large intestinal submucosa of the mice;
therefore, NQ might be useful as a therapeutic anti-inflammatory
drug.
Discussion
The relationship between the bioactivity
and the structures of VK quinone derivatives
This review described the bioactivities of VKs and
their quinone derivatives of compounds 1–11 in Fig. 1. Among the intermediates between
VK2 (MK-4) and VK3, such as compounds 2–6,
MK-2 showed the strongest effects on inhibition of mammalian pols α
and λ and LSP-induced TNF-α prevention in the inflammatory response
(Figs. 2 and 3); therefore, the length of the isoprenyl
chain of MK-n at the 3-position of VK2 is essential for
these inhibitory activities. On the other hand, NQ showed the
strongest of these effects among all 11 compounds tested. NQ,
VK3 and 1,2-dimethyl-NQ have none, one and two methyl
groups in addition to their NQ backbone, respectively. BQ, NQ, AQ
and NCQ consist of a polycyclic aromatic hydrocarbon, such as
benzene, naphthalene, anthracene, or tetracence, respectively and
these compounds are four major quinone derivatives that have two
ketone groups at positions 1 and 4. Thus, the quinone ring
structure based on NQ, which has no methyl side group, must be very
important for the bioactivity. As reported previously, the phenolic
compound curcumin, which is a known anti-inflammatory agent, is a
pol-λ-specific inhibitor (27,47,48).
Intriguingly, pol λ is also the principle molecular target of VKs
and their quinone derivatives based on NQ.
DNA repair-related pol inhibition and
anti-inflammation
Inflammatory mediators, such as LPS and DSS, quickly
stimulate ROS (46) and ROS are
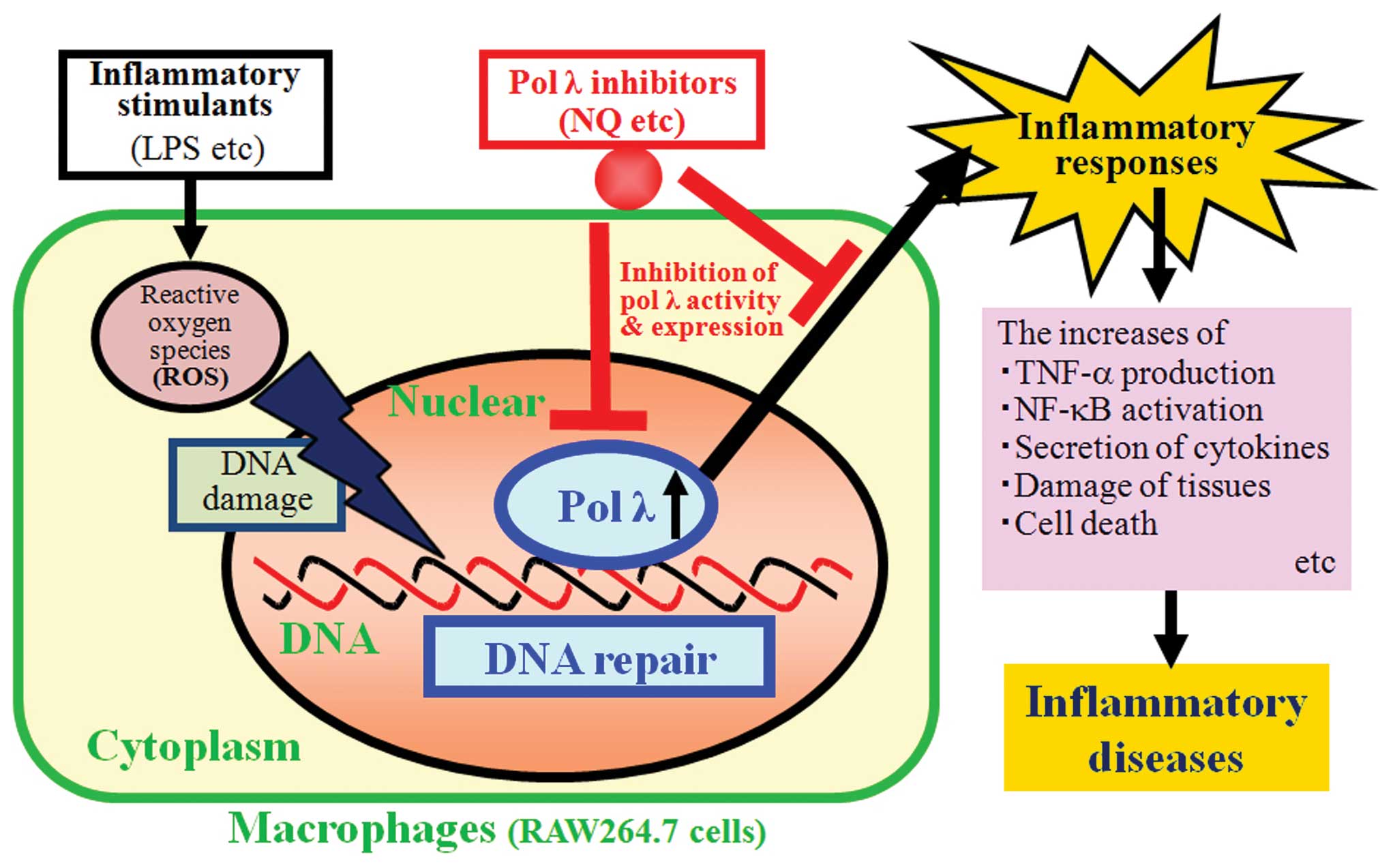
known to mediate oxidative DNA damage. As shown in Fig. 6, DNA repair pols such as pol λ
induce protein expression and increase DNA polymerization activity
to repair the damaged DNA. Furthermore, we consider that pol λ
might have a great effect on inflammatory responses, such as TNF-α
production, NF-κB activation, secretion of cytokines [e.g.
interferons and interleukins], tissue damage and cell death. The
results summarized in this review suggest that inhibition of DNA
repair by pol λ is related to anti-inflammatory pathways and that
pol λ inhibitors such as NQ might be chemotherapeutic drugs for
inflammatory diseases. The detailed molecular mechanism underlying
the correlation between DNA repair inhibition by pol λ and
anti-inflammatory responses is not yet known; therefore,
experiments with small interfering RNA (siRNA) targeting pol λ
would help in further analyses.
As mentioned above, eukaryotic cells reportedly
contain 15 pol species belonging to four families (3,4).
Among the X-family of pols, pol λ has an unclear biochemical
function, although it seems to work in a similar way to pol β
(49). Pol β is involved in the
short-patch base excision repair (BER) pathway (50–53),
as well as playing an essential role in neural development
(54). Pol λ was found to possess
5′-deoxyribose-5-phosphate (dRP) lyase activity, but not
apurinic/apyrimidinic (AP) lyase activity (55). Pol λ is able to substitute for pol
β during in vitro BER, suggesting that pol λ also
participates in BER. Northern blot analysis indicated that
transcripts of pol β are abundantly expressed in the testis, thymus
and brain in rats (56), whereas
pol λ is efficiently transcribed mostly in the testis (57). Bertocci et al reported that
mice in which pol λ expression is knocked down are not only viable
and fertile, but also display a normal hyper-mutation pattern
(58).
As well as causing inflammation, DSS influences cell
proliferation and has physiological effects on florid epithelial
cells in colitis, because it has colonic tumor promoter activity
(45). Therefore,
anti-inflammatory agents are expected to suppress DNA
replication/repair/recombination in nuclei in relation to the
action of DSS. Because pol λ is a DNA repair-related pol (49), the molecular target of the VK
quinone derivatives, such as NQ, is in good agreement with this
expected mechanism of anti-inflammatory agents. As a result, any
inhibitor of DNA repair-related pol λ might also be an inflammatory
suppressor.
Conclusion
This review summarizes data showing that a positive
correlation between mammalian pol inhibitory activity and the
anti-inflammatory response. The inflammatory mediators, such as LPS
and DSS, induce ROS and ROS mediates oxidative DNA damage. As shown
in Fig. 6, DNA repair-related
pols, such as pol λ, induce the protein expression and increase the
activity of these pols to repair the damaged DNA. Furthermore, we
consider that pol λ has effects on the inflammatory responses, such
as TNF-α production, NF-κB activation, secretion of cytokines,
damage of tissues and cell death. These phenomena suggest that the
inhibition of pol λ activity is related to the anti-inflammation
and pol λ inhibitors, some VKs and their related quinone
derivatives, such as NQ, could be chemotherapeutic drugs for
inflammatory diseases.
Abbreviations:
|
VK
|
vitamin K
|
|
pol
|
DNA-dependent DNA polymerase (E.C.
2.7.7.7)
|
|
NQ
|
1,4-naphthoquinone
|
|
ROS
|
reactive oxygen species
|
|
TdT
|
terminal deoxynucleotidyl
transferase
|
|
dTTP
|
2′-deoxythymidine-5′-triphosphate
|
|
dNTP
|
2′-deoxynucleotide-5′-triphosphate
|
|
AQ
|
9,10-anthraquinone
|
|
BQ
|
1,4-benzoquinone
|
|
NCQ
|
5,12-naphthacenequinone
|
|
IC50
|
50% inhibitory concentration
|
|
BSA
|
bovine serum albumin
|
|
NF-κB
|
nuclear factor-κB
|
|
TNF-α
|
tumor necrosis factor-α
|
|
LPS
|
lipopolysaccharide
|
|
BW
|
body weight
|
|
DSS
|
dextran sulfate sodium
|
|
H&E
|
hematoxylin and eosin
|
Acknowledgements
Y.M. acknowledges Grants-in-Aid from
Scientific Research (C) (no. 24580205) from MEXT (Ministry of
Education, Culture, Sports, Science and Technology, Japan), Takeda
Science Foundation (Japan) and the Nakashima Foundation (Japan).
This study was supported in part by grants for the MEXT-Supported
Program for the Strategic Research Foundation at Private
Universities, 2012-2016 (Y.M.), the Global COE Program ‘Global
Center of Excellence for Education and Research on Signal
Transduction Medicine in the Coming Generation’ from MEXT (T.A. and
M.Y.) and for the Young Researchers Training Program for Promoting
Innovation from MEXT through the Special Coordination Fund for
Promoting Science and Technology (S.N. and T.A.).
References
|
1
|
Furie B and Furie BC: Molecular basis of
vitamin K-dependent γ-carboxylation. Blood. 75:1753–1762. 1990.
|
|
2
|
Suttie JW: Synthesis of vitamin
K-dependent proteins. FASEB J. 7:445–452. 1993.PubMed/NCBI
|
|
3
|
Shearer MJ: Role of vitamin K and Gla
proteins in the pathophysiology of osteoporosis and vascular
calcification. Curr Opin Clin Nutr Metab Care. 3:433–438. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ohsaki Y, Shirakawa H, Hiwatashi K,
Furukawa Y, Mizutani T and Komai M: Vitamin K suppresses
lipopolysaccharide-induced inflammation in the rat. Biosci
Biotechnol Biochem. 70:926–932. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Seegers WH and Bang NU: Blood Clotting
Enzymology. Academic Press; New York, NY: 1967
|
|
6
|
Elder SJ, Haytowitz DB, Howe J, Peterson
JW and Booth SL: Vitamin K contents of meat, dairy, and fast food
in the U.S. diet. J Agric Food Chem. 54:463–467. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Tsukamoto Y, Ichise H, Kakuda H and
Yamaguchi M: Intake of fermented soybean (natto) increases
circulating vitamin K2 (menaquinone-7) and γ-carboxylated
osteocalcin concentration in normal individuals. J Bone Miner
Metab. 18:216–222. 2000.PubMed/NCBI
|
|
8
|
Suttie JW: The importance of menaquinone
in human nutrition. Annu Rev Nutr. 15:399–417. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Booth SL: Dietary vitamin K guidance: an
effective strategy for stable control of oral anticoagulation? Nutr
Rev. 68:178–181. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Billeter M, Bolliger W and Martius C:
Untersuchungen uber die umwandlung von verfutterten K-vitamin durch
austausch der seitenkette und die rolle der darmbakterien hierbei.
Biochem Z. 340:290–303. 1964.(In German).
|
|
11
|
Davidson RT, Foley AL, Engelke JA and
Suttie JW: Conversion of dietary phylloquinone to tissue
menaquinone-4 in rats is not dependent on gut bacteria. J Nutr.
128:220–223. 1998.PubMed/NCBI
|
|
12
|
Budavari S, O’Neil MJ, Smith A and
Heckelman PE: The Merck Index. Merck & Co. Inc; Rahway, NJ:
1989
|
|
13
|
Taggart WV and Matschiner JT: Metabolism
of menadione-6,7-3H in the rat. Biochemistry. 8:1141–1146. 1969.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Monks TJ, Hanzlik RP, Cohen GM, Ross D and
Graham DG: Quinone chemistry and toxicity. Toxicol Appl Pharmacol.
112:2–16. 1992. View Article : Google Scholar
|
|
15
|
O’Brien PJ: Molecular mechanisms of
quinone cytotoxicity. Chem Biol Interact. 80:1–41. 1991.
|
|
16
|
Bolton JL, Trush MA, Penning TM, Dryhurst
G and Monks TJ: Role of quinones in toxicology. Chem Res Toxicol.
13:135–160. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Cho AK, Schmitz DA, Ying Y, Rodriguez CE,
DiStefano EW, Kumagai Y, Miguel A, Eiguren A, Kobayashi T, Avol E
and Froines JR: Determination of four quinones in diesel exhaust
particles, SRM 1649a, and atmospheric PM2.5. Aerosol Sci Technol.
38:68–81. 2004. View Article : Google Scholar
|
|
18
|
Kornberg A and Baker TA: DNA replication
Chaprter. 6 2nd edition. WD Freeman and Co; New York, NY: pp.
197–225. 1992
|
|
19
|
Hubscher U, Maga G and Spadari S:
Eukaryotic DNA polymerases. Annu Rev Biochem. 71:133–163. 2002.
View Article : Google Scholar
|
|
20
|
Bebenek K and Kunkel TA: DNA repair and
replication. Adv Protein Chem. 69:137–165. 2004.
|
|
21
|
Takata K, Shimizu T, Iwai S and Wood RD:
Human DNA polymerase N (POLN) is a low fidelity enzyme capable of
error-free bypass of 5S-thymine glycol. J Biol Chem.
281:23445–23455. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Loeb LA and Monnat RJ Jr: DNA polymerases
and human disease. Nat Rev Genet. 9:594–604. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Mizushina Y, Tanaka N, Yagi H, Kurosawa T,
Onoue M, Seto H, Horie T, Aoyagi N, Yamaoka M, Matsukage A, Yoshida
S and Sakaguchi K: Fatty acids selectively inhibit eukaryotic DNA
polymerase activities in vitro. Biochim Biophys Acta. 1308:256–262.
1996. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Mizushina Y, Yoshida S, Matsukage A and
Sakaguchi K: The inhibitory action of fatty acids on DNA polymerase
β. Biochim Biophys Acta. 1336:509–521. 1997.
|
|
25
|
Mizushina Y, Motoshima H, Yamaguchi Y,
Takeuchi T, Hirano K, Sugawara F and Yoshida H: 3-O-methylfunicone,
a selective inhibitor of mammalian Y-family DNA polymerases from an
Australian sea salt fungal strain. Mar Drugs. 7:624–639. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Sakaguchi K, Sugawara F and Mizushina Y:
Inhibitors of eukaryotic DNA polymerases. Seikagaku. 74:244–251.
2002.PubMed/NCBI
|
|
27
|
Mizushina Y: Specific inhibitors of
mammalian DNA polymerase species. Biosci Biotechnol Biochem.
73:1239–1251. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Mizushina Y: Screening of novel bioactive
compounds from food components and nutrients. J Jpn Soc Nutr Food
Sci. 64:377–384. 2011. View Article : Google Scholar
|
|
29
|
Mizushina Y, Yonezawa Y and Yoshida Y:
Selective inhibition of animal DNA polymerases by fat-soluble
vitamins A, D, E and K, and their related compounds. Curr Enzyme
Inhibition. 3:61–75. 2007. View Article : Google Scholar
|
|
30
|
Sasaki R, Suzuki Y, Yonezawa Y, Ota Y,
Okamoto Y, Demizu Y, Huang P, Yoshida H, Sugimura K and Mizushina
Y: DNA polymerase γ inhibition by vitamin K3 induces
mitochondria-mediated cytotoxicity in human cancer cells. Cancer
Sci. 99:1040–1048. 2008.
|
|
31
|
Matsubara K, Kayashima T, Mori M, Yoshida
H and Mizushina Y: Inhibitory effects of vitamin K3 on
DNA polymerase and angiogenesis. Int J Mol Med. 22:381–387.
2008.PubMed/NCBI
|
|
32
|
Tanaka S, Nishiumi S, Nishida M, Mizushina
Y, Kobayashi K, Masuda A, Fujita T, Morita Y, Mizuno S, Kutsumi H,
Azuma T and Yoshida M: Vitamin K3 attenuates
lipopolysaccharide-induced acute lung injury through inhibition of
nuclear factor-κB activation. Clin Exp Immunol. 160:283–292.
2010.
|
|
33
|
Chinzei R, Masuda A, Nishiumi S, Nishida
M, Onoyama M, Sanki T, Fujita T, Moritoh S, Itoh T, Kutsumi H,
Mizuno S, Azuma T and Yoshida M: Vitamin K3 attenuates
cerulein-induced acute pancreatitis through inhibition of the
autophagic pathway. Pancreas. 40:84–94. 2011.
|
|
34
|
Mizushina Y, Maeda J, Irino Y, Nishida M,
Nishiumi S, Kondo Y, Nishio K, Kuramochi K, Tsubaki K, Kuriyama I,
Azuma T, Yoshida H and Yoshida M: Effects of intermediates between
vitamins K(2) and K(3) on mammalian DNA polymerase inhibition and
anti-inflammatory activity. Int J Mol Sci. 12:1115–1132. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Kobayashi K, Nishiumi S, Nishida M, Hirai
M, Azuma T, Yoshida H, Mizushina Y and Yoshida M: Effects of
quinone derivatives, such as 1,4-naphthoquinone, on DNA polymerase
inhibition and anti-inflammatory action. Med Chem. 7:37–44. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Aoganghua A, Nishiumi S, Kobayashi K,
Nishida M, Kuramochi K, Tsubaki K, Hirai M, Tanaka S, Azuma T,
Yoshida H, Mizushina Y and Yoshida M: Inhibitory effects of vitamin
K3 derivatives on DNA polymerase and inflammatory
activity. Int J Mol Med. 28:937–945. 2011.
|
|
37
|
Huang TT and Wuerzberger-Davis SM:
Sequential modification of NEMO/IKKÁ by SUMO-1 and ubiquitin
mediates NF-κB activation by genotoxic stress. Cell. 115:565–576.
2003.PubMed/NCBI
|
|
38
|
Hayden M and Ghosh S: Signaling to NF-κB.
Genes Dev. 18:2195–2224. 2004.
|
|
39
|
Bonizzi G and Karin M: The two NF-κB
activation pathways and their role in innate and adaptive immunity.
Trends Immunol. 25:280–288. 2004.
|
|
40
|
Wajant H, Pfizenmaier K and Scheurich P:
Tumor necrosis factor signaling. Cell Death Differ. 10:45–65. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Elson CO, Sartor RB, Tennyson GS and
Riddell RH: Experimental models of inflammatory bowel disease.
Gastroenterology. 109:1344–1367. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Aggarwal BB: Signalling pathways of the
TNF superfamily: a double-edged sword. Nat Rev Immunol. 3:745–756.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Rahman I, Biswas SK and Kirkham PA:
Regulation of inflammation and redox signaling by dietary
polyphenols. Biochem Pharmacol. 72:1439–1452. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Corda S, Laplace C, Vicaut E and Duranteau
J: Rapid reactive oxygen species production by mitochondria in
endothelial cells exposed to tumor necrosis factor-α is mediated by
ceramide. Am J Respir Cell Mol Biol. 24:762–768. 2001.PubMed/NCBI
|
|
45
|
Cooper HS, Murthy SN, Shah RS and
Sedergran DJ: Clinicopathologic study of dextran sulfate sodium
experimental murine colitis. Lab Invest. 69:238–249.
1993.PubMed/NCBI
|
|
46
|
Bhattacharyya S, Dudeja PK and Tobacman
JK: ROS, Hsp27, and IKKβ mediate dextran sodium sulfate (DSS)
activation of IκBα, NFκB, and IL-8. Inflamm Bowel Dis. 15:673–683.
2009.
|
|
47
|
Mizushina Y, Hirota M, Murakami C, Ishidoh
T, Kamisuki S, Shimazaki N, Takemura M, Perpelescu M, Suzuki M,
Yoshida H, Sugawara F, Koiwai O and Sakaguchi K: Some anti-chronic
inflammatory compounds are DNA polymerase λ-specific inhibitors.
Biochem Pharmacol. 66:1935–1944. 2003.
|
|
48
|
Mizushina Y, Takeuchi T, Kuramochi K,
Kobayashi S, Sugawara F, Sakaguchi K and Yoshida H: Study on the
molecular structure and bio-activity (DNA polymerase inhibitory
activity, anti-inflammatory activity and anti-oxidant activity)
relationship of curcumin derivatives. Curr Bioactive Compounds.
3:171–177. 2007. View Article : Google Scholar
|
|
49
|
Garcia-Diaz M, Bebenek K, Sabariegos R,
Dominguez O, Rodriguez J, Kirchhoff T, Garcia-Palomero E, Picher
AJ, Juarez R, Ruiz JF, Kunkel TA and Blanco L: DNA polymerase λ, a
novel DNA repair enzyme in human cells. J Biol Chem.
277:13184–13191. 2002.
|
|
50
|
Singhal RK and Wilson SH: Short
gap-filling synthesis by DNA polymerase β is processive. J Biol
Chem. 268:15906–15911. 1993.
|
|
51
|
Matsumoto Y and Kim K: Excision of
deoxyribose phosphate residues by DNA polymerase β during DNA
repair. Science. 269:699–702. 1995.
|
|
52
|
Sobol RW, Horton JK, Kuhn R, Gu H, Singhal
RK, Prasad R, Rajewsky K and Wilson SH: Requirement of mammalian
DNA polymerase-β in base-excision repair. Nature. 379:183–186.
1996.
|
|
53
|
Ramadan K, Shevelev IV, Maga G and
Hubscher U: DNA polymerase λ from calf thymus preferentially
replicates damaged DNA. J Biol Chem. 277:18454–18458. 2002.
|
|
54
|
Sugo N, Aratani Y, Nagashima Y, Kubota Y
and Koyama H: Neonatal lethality with abnormal neurogenesis in mice
deficient in DNA polymerase β. EMBO J. 19:1397–1404.
2000.PubMed/NCBI
|
|
55
|
Garcia-Diaz M, Bebenek K, Kunkel TA and
Blanco L: Identification of an intrinsic 5′-deoxyribose-5-phosphate
lyase activity in human DNA polymerase λ: a possible role in base
excision repair. J Biol Chem. 276:34659–34663. 2001.
|
|
56
|
Hirose F, Hotta Y, Yamaguchi M and
Matsukage A: Difference in the expression level of DNA polymerase β
among mouse tissues: high expression in the pachytene spermatocyte.
Exp Cell Res. 181:169–180. 1989.
|
|
57
|
Garcia-Diaz M, Dominguez O,
Lopez-Fernandez LA, De Lera LT, Saniger ML, Ruiz JF, Parraga M,
Garcia-Ortiz MJ, Kirchhoff T, Del Mazo J, Bernad A and Blanco L:
DNA polymerase λ, a novel DNA repair enzyme in human cells. J Mol
Biol. 301:851–867. 2000.
|
|
58
|
Bertocci B, De Smet A, Flatter E, Dahan A,
Bories JC, Landreau C, Weill JC and Reynaud CA: Cutting edge: DNA
polymerases μ and λ are dispensable for Ig gene hypermutation. J
Immunol. 168:3702–3706. 2002.
|