Introduction
Colorectal cancer is the fourth most common type of
cancer in the United States and the second leading cause of
cancer-related mortality in the Western world (1). Although early diagnosis and rigorous
screenings have reduced its incidence in recent years, the
prognosis associated with metastatic disease remains bleak. Current
treatment for colorectal cancer is surgical resection combined with
radiation and chemotherapy. A number of chemotherapeutic agents
have been developed from botanical sources (2,3).
Currently, the identification of non-toxic
anticancer constituents from botanicals is essential for advancing
the treatment of colorectal cancer. Since the composition of herbal
extracts is complex, different constituents may have opposing
activities (4), which may reduce
the beneficial effects of an herb (5). Therefore, the isolation of an active
fraction from an herbal extract is essential for the effective use
of herbal medicines.
The root of Scutellaria baicalensis (S.
baicalensis) Georgi (Labiatae) is a widely used herb in the
traditional medical systems of China and Japan. The representative
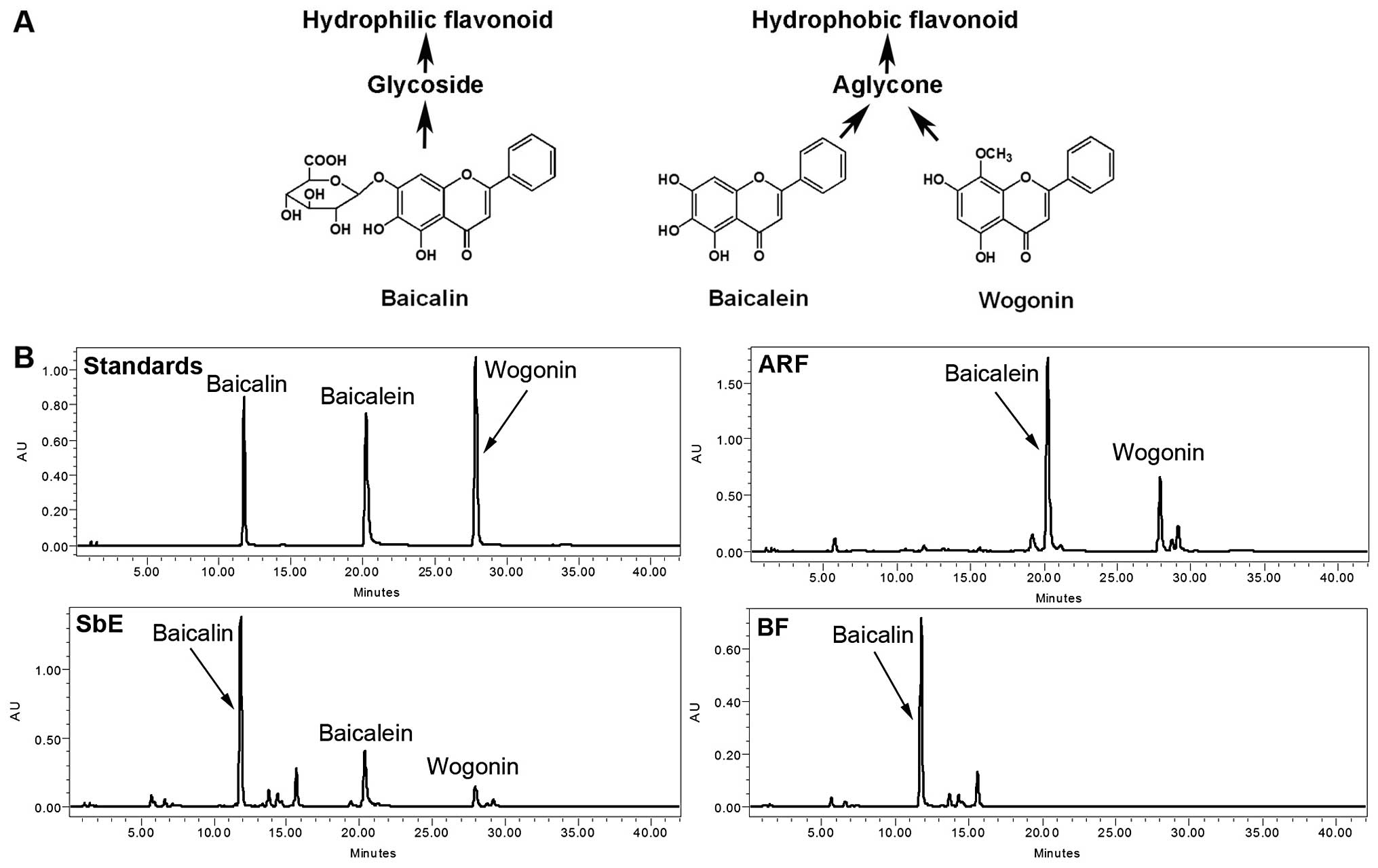
constituents of S. baicalensis are a group of flavonoids
that include baicalin, baicalein, and wogonin (Fig. 1A) (6). S. baicalensis extract (SbE)
has been used with positive results for inflammatory diseases,
allergies, hyperlipidemia and arteriosclerosis (7,8). SbE
has also been shown to exert chemopreventive effects on a variety
of cancers (9–11). Chemoprevention involves the use of
medicines, vitamins, or herbs to delay or prevent the development
of cancer.
The effect of S. baicalensis on human
colorectal cancer remains uncertain; a limited anti-proliferative
effect of SbE on human colorectal cancer cells has been reported.
Compared to its effect on liver and prostate cancer lines (9,10),
the anti-proliferative activity of SbE on human colorectal cancer
cells is limited (11,12). Although baicalein inhibits the
growth of colon cancer cells (13,14),
no such results have been obtained with baicalin, the major
constituent of SbE. Therefore, we hypothesized that since baicalin
does not significantly inhibit the growth of colorectal cancer
cells, the anti-proliferative effect of SbE may be reduced by
baicalin, the major hydrophilic flavonoid.
In this study, we prepared an aglycone-rich fraction
(ARF), which contains hydrophilic flavonoids, and a baicalin
fraction (BF) of SbE. The representative flavonoids in ARF, BF and
SbE were determined by high-performance liquid chromatography
(HPLC). We then examined the effects of ARF on the growth of human
colorectal cancer cells, the cell cycle, apoptosis and apoptotic
gene expression. The results of gene expression profiling were
validated by a cellular functional assay.
Materials and methods
Chemicals
The flavonoid standards, baicalin and baicalein,
were obtained from Sigma (St. Louis, MO) and wogonin was obtained
from the National Institute for the Control of Pharmaceutical and
Biological Products (Beijing, China). All standards were of
biochemical-reagent grade and at least 95% pure as confirmed by
HPLC. HPLC grade methanol, ethanol, n-butanol and acetonitrile were
obtained from Fisher Scientific (Pittsburgh, PA). Milli-Q water was
supplied by a water purification system (US Filter, Palm Desert,
CA). Trypsin, Dulbecco’s modified Eagle’s medium (DMEM), fetal
bovine serum (FBS), and penicillin/streptomycin solution (200X)
were obtained from Mediatech, Inc. (Herndon, VA). A CellTiter 96
Aqueous One Solution Cell Proliferation Assay kit was obtained from
Promega (Madison, WI). An Annexin V-FITC Apoptosis Detection kit,
7-amino-actinomycin D (7-AAD), FITC-conjugated cyclin A and
PE-conjugated cyclin B1 were obtained from BD Biosciences (San
Diego, CA).
Phytochemical isolation and HPLC
analysis
The roots of S. baicalensis were obtained
from Chengde (Hebei, China). The voucher samples were deposited at
the Tang Center for Herbal Medicine Research at the University of
Chicago. Dried S. baicalensis root was ground to a powder
and passed through a 40 mesh screen, then extracted with 70%
ethanol to obtain SbE. SbE was placed in water and then extracted
with ethyl acetate to obtain the ARF. After washing with n-butanol,
the water layer was dried to produce the BF. Flavonoid analysis was
performed on a Waters HPLC system consisting of a 2960 separations
module, a 996 Photodiode Array Detector (Waters, Milford, MA), and
Waters Millennium 32 software for peak identification and
integration. The separation was carried out on a Phenomenex Prodigy
ODS(2) column (150×3.2 mm, 5
μm). Acetonitrile (solvent A) and 0.03% (v/v) phosphoric
acid in water (solvent B) were used. Gradient elution started with
15% solvent A and 85% solvent B; it was changed to 28% solvent A
for 12 min, to 35% solvent A for 9 min, to 50% solvent A for 7 min,
to 95% solvent A for 2 min and then held for 2 min. Finally it was
changed to 15% solvent A for 3 min and held for 5 min. The flow
rate was 0.8 ml/min and the detection wavelength was set to 280
nm.
Cell line and cell culture
The human colorectal cancer cell lines, HCT-116 and
HT-29, were obtained from the American Type Culture Collection
(Manassas, VA) and grown in McCoy’s 5A medium supplemented with 10%
FBS and 50 IU penicillin/streptomycin in a humidified atmosphere of
5% CO2 at 37°C.
Cell proliferation analysis
Cells were seeded in 96-well plates
(1×104 cells/well). On the second day, various
concentrations of ARF, BF, SbE or flavonoids were added to the
wells. Cell proliferation was evaluated using an MTS assay
according to the manufacturer’s instructions. Briefly, after the
cells were treated with drugs for 48 h, the medium was replaced
with 100 μl of fresh medium and 20 μl of MTS reagent
(CellTiter 96® Aqueous Solution) in each well, and the
plate was returned to the incubator for 1–2 h. A 60-μl
aliquot of medium from each well was transferred to an ELISA
96-well plate and its absorbance at 490 nm was recorded. Results
were expressed as a percentage of the control (solvent vehicles set
at 100%).
Cell cycle, cyclins A and B1
analysis
Cells were seeded in 24-well tissue culture plates
(2×105 cells/well). On the second day, the medium was
changed, and the cells were treated with ARF, BF or SbE. Cells were
incubated for 48 h before harvesting. The cells were fixed gently
by adding 80% ethanol before they were placed in a freezer for 2 h.
The cells were then treated with 0.25% Triton X-100 for 5 min in an
ice bath. The cells were resuspended in 130 μl of PBS. Then,
5 μl of 7-AAD staining solution, 10 μl of cyclin
A-FITC and 10 μl of cyclin B1-PE were added to the cell
suspension. Cells were incubated in a dark room for 10 min at room
temperature and analyzed using a FACScan flow cytometer
(Becton-Dickinson, Mountain View, CA) and FlowJo 7.1.0 software
(Tree Star, Ashland, OR). For each measurement, at least 10,000
cells were counted.
Apoptotic analysis
Cells were seeded in 24-well tissue culture plates
(2×105 cells/well). On the second day, the medium was
changed and ARF, BF or SbE was added. After treatment for 48 h,
cells floating in the medium were collected. The adherent cells
were detached with 0.05% trypsin. The culture medium containing 10%
FBS (and floating cells) was then added to inactivate trypsin.
After being pipetted gently, the cells were centrifuged for 5 min
at 1,500 × g. The supernatant was removed and cells were stained
with Annexin V-FITC and propidium iodide (PI) according to the
manufacturer’s instructions. Untreated cells were used as the
control for double staining. The cells were analyzed immediately
after staining using a FACScan flow cytometer. For each
measurement, at least 20,000 cells were counted.
Reverse transcription and quantitative
real-time polymerase chain reaction (PCR)
Cells were treated with 20 μg/ml of ARF or BF
for 24 or 48 h. Total RNA was isolated using the RNAeasy kit
(Qiagen, Hilden, Germany). First strand cDNA was synthesized from 2
μg total RNA using a SuperScript II First-Strand Synthesis
System (Invitrogen, Carlsbad, CA). Quantitative real-time PCR was
performed in a reaction volume of 25 μl including 1
μl cDNA. PCR conditions were as follows: 95°C for 15 min
followed by 40 cycles at 95°C for 30 sec, 55°C for 30 sec, and 72°C
for 30 sec. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was
used as an internal reference gene to normalize the expression of
apoptotic genes. Relative quantification of apoptosis-related genes
was analyzed by the comparative threshold cycle (Ct) method. For
each sample, the Ct value of the apoptotic gene was normalized
using the formula: ΔCt = Ct (apoptotic genes) - Ct (GAPDH). To
determine relative expression levels, the following formula was
used: ΔΔCt = ΔCt (treated) - ΔCt (control). The value was used to
plot the expression of apoptotic genes using the formula
2−ΔΔCt.
Mitochondrial membrane potential (Δψm)
analysis
Δψm was estimated after staining with JC-1
(Molecular Probes, Eugene, OR). HCT-116 cells were treated for 24 h
with ARF or BF at 50 μg/ml. The control cells were grown in
medium containing the same amount of ethanol as the treated cells.
The adherent cells were then incubated in 0.5 ml of medium
containing JC-1 (2.5 μg/ml) for 20 min at 37°C, and images
were taken with a Nikon Eclipse E800 microscope (Nikon Corp.,
Champignysur-Marne, France).
Statistical analysis
Data are presented as the means ± standard error
(SE) (n=3). A one-way ANOVA determined whether the results had
statistical significance. In some cases, the Student’s t-test was
used for comparing two groups. The level of statistical
significance was set at P<0.05. For the effects of components on
HCT-116 or HT-29 cell anti-proliferation, cell cycle, cyclins and
apoptotic induction, the significance of the treatment groups vs.
the control group was assessed by the Student’s t-test.
Results
HPLC analysis of flavonoids in ARF, BF
and SbE
As shown in Fig.
1B, the representative constituents of SbE are baicalin,
baicalein and wogonin. ARF contains two hydrophobic flavonoids,
baicalein and wogonin; the major constituent of BF is baicalin. The
concentrations of these flavonoids were calculated using their
standard curves. In SbE, the concentrations of baicalin, baicalein
and wogonin were 156.7, 51.9 and 14.5 mg/g, respectively. In BF,
the concentration of baicalin was 166.8 mg/g, and baicalein and
wogonin were not detected. The concentrations of baicalein and
wogonin in ARF were 405.4 and 123.5 mg/g, respectively; baicalin
was detected only in trace amounts (5.1 mg/g). Although baicalin
accounted for 70.2% of the total flavonoids detected in SbE, in
ARF, the proportion was <1% (0.96%), suggesting that the quality
of the ARF was acceptable.
Effects of ARF, BF, SbE and flavonoids on
the proliferation of colorectal cancer cells
We used the human colorectal cancer cell lines,
HCT-116 and HT-29, to evaluate the effects of ARF, BF and SbE on
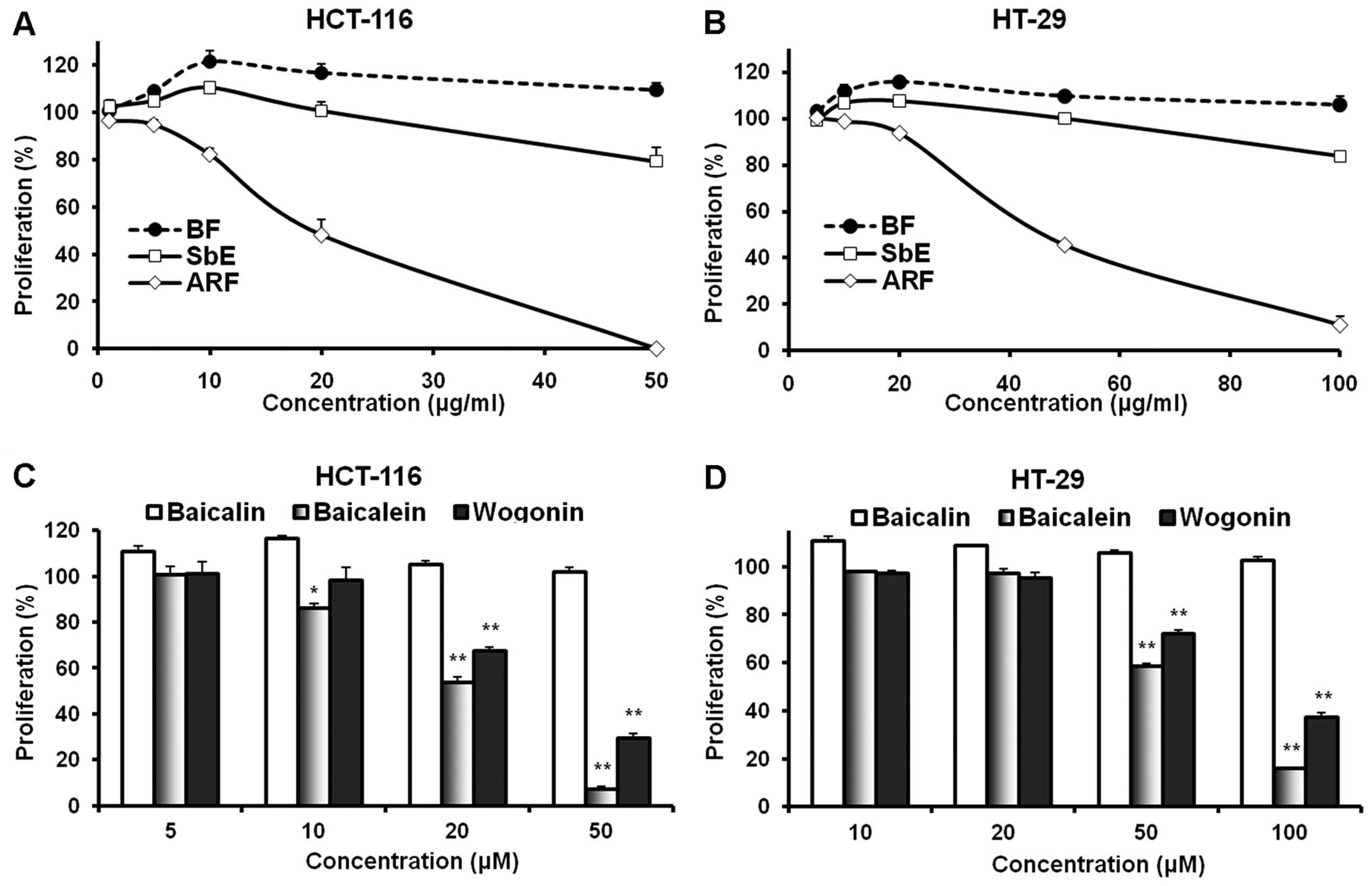
cell proliferation. As shown in Fig.
2, the complete extract, SbE, did not inhibit cell growth at
concentrations of 1–20 μg/ml for HCT-116 cells and 5-50
μg/ml for HT-29 cells. Of note, at certain concentrations,
SbE actually increased cell growth. We then observed the effects of
ARF and BF on the proliferation of cancer cells. BF, which contains
only baicalin, increased cell growth. In HCT-116 cells at 10 and 20
μg/ml, BF increased cell growth by 21.4±4.5 and 16.6±3.9%,
respectively, compared to the control (both P<0.05). By
contrast, ARF showed a potent anti-proliferative effect. At 10 and
20 μg/ml, ARF inhibited HCT-116 cell growth by 17.7±2.3%
(P<0.05 vs. control) and 51.7±6.2% (P<0.01), respectively. In
addition, treatment with 50 μg/ml of ARF inhibited cell
growth by 99.7±0.2% (P<0.01) (Fig.
2A). Similar results were observed in HT-29 cells (Fig. 2B). These observations suggest that
the effect of SbE is reduced by the BF and that ARF is the fraction
of SbE with anti-proliferative activity.
To evaluate the contributions of individual
flavonoids on the observed effects of different fractions, the
anti-proliferative activities of three representative flavonoids
were examined using HCT-116 and HT-29 cells. Among them, two are
aglycones (baicalein and wogonin) and one is a glycoside
(baicalin). As shown in Fig. 2C,
the treatment of HCT-116 cells with 5–20 μM of baicalin for
48 h did not result in a significant anti-proliferative effect; on
the contrary, at 5 and 10 μM, baicalin increased cancer cell
growth. The two aglycones, baicalein and wogonin, which are
hydrophobic flavonoids, showed significant anti-proliferative
effects at concentrations of 20–50 μM. The activity of
baicalein was more potent than that of wogonin. Similar results
were observed in the HT-29 cells (Fig.
2D). Since HT-29 is a multi-drug resistant cell line, the
active concentration of fractions and flavonoids in this cell line
is higher than the concentration in HCT-116 cells. These results
suggest that the anti-proliferative activities of flavonoid
aglycones (such as baicalein and wogonin) are significantly higher
than those of flavonoid glycosides (such as baicalin).
Effects of ARF on the cell cycle and
expression of cyclins A and B1
After treatment with 20 μg/ml ARF, BF or SbE
for 48 h, the cell cycle profile was determined. As shown in
Fig. 3, treatment with ARF
decreased the number of cells in the G1 phase, and increased the
number of cells in DNA synthesis (S) and G2/M phases significantly;
SbE and BF showed no effect on the cell cycle.
Cell cycle progression is regulated by the activity
of cyclins and cyclin-dependent kinases. Cyclin A is a key
regulator of DNA replication and mitosis in the S phase and for
passage through the G2/M phase (15). Cyclin B1 plays an important role in
the control of the G2-M transition of the cell cycle (16). To elucidate the molecular
mechanisms involved in the observed arrest of the cell cycle in the
S and G2/M phases, the expression of cyclins A and B1 was
determined (Fig. 3). After
treatment with 20 μg/ml of ARF, the expression of cyclin A
was increased to 64.1±2.3%, compared to 24.5±2.0% in the untreated
cells (P<0.01). Compared to the control (27.6±1.8%), the
expression of cyclin B1 also increased to 68.0±2.1% (P<0.01).
Cyclin A and B1 expression was not increased in the cells treated
with SbE or BF. The accumulation of cyclins A and B1 induced by ARF
was critical in promoting cell cycle arrest in the S and G2/M
phases.
Effects of ARF on induction of
apoptosis
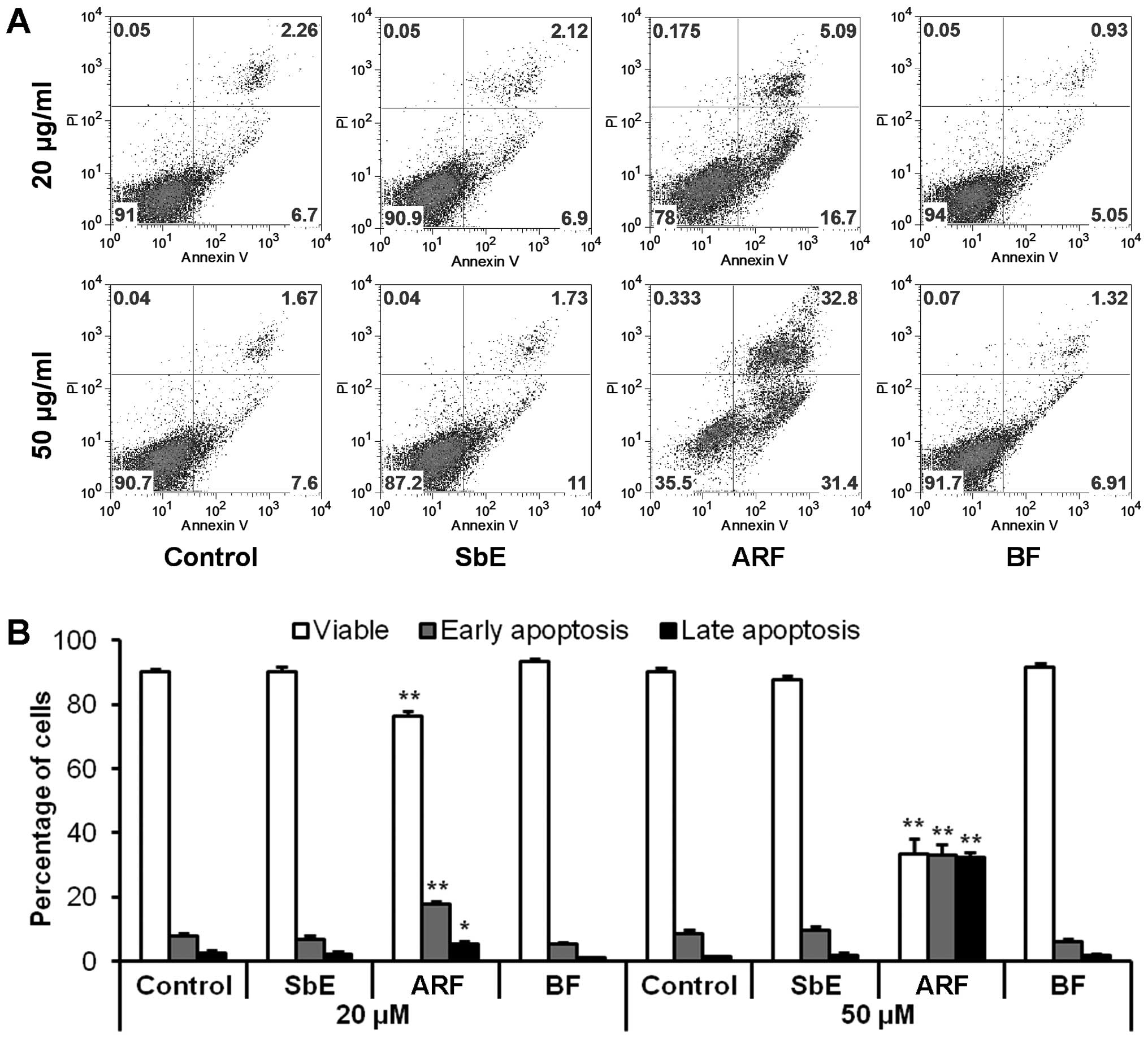
To determine whether the decrease in cell number
observed after treatment with ARF was the result of apoptosis,
cells were stained with Annexin V/PI. Annexin V can be detected in
both the early and late stages of apoptosis. PI enters the cells in
late apoptosis or necrosis. After treatment for 48 h with 20 and 50
μg/ml of ARF, compared to the control (7.8±1.1 and
8.4±1.2%), the percentage of early apoptotic cells was 17.7±0.9 and
33.1±3.3%, respectively (both P<0.01 vs. control) (Fig. 4). ARF clearly induced significant
apoptosis in the HCT-116 cells. Treatment with the same
concentration of BF or SbE did not induce apoptosis. These results
suggest that the anti-proliferative effect of ARF is caused by
apoptosis.
Effects of ARF on apoptotic-related gene
expression
We also evaluated the effect of ARF and BF on
selected pro-apoptotic genes. Among the selected targets were tumor
protein p53 (TP53), tumor protein p53 binding protein 2 (TP53BP2),
and four tumor necrosis factor (TNF) family genes: TNF super-family
member 2, CD70 molecule (CD70), tumor necrosis factor receptor
superfamily member 10a (TNFRSF10A), and tumor necrosis factor
receptor superfamily member 10b (TNFRSF10B). As shown in Table I, after treatment with ARF, the
expression of TP53 and TP53BP2 was markedly increased, suggesting
that ARF induces apoptosis partly through the upregulation of p53.
In addition, p53-wild-type cells (HCT-116) were more sensitive to
ARF than p53-mutated (HT-29) cells (Fig. 2A and B). Others studies have also
demonstrated that TP53BP2 induces apoptosis through the
mitochondrial death pathway (17,18).
 | Table IExpression of apoptosis-related genes
regulated by aglycone-rich fraction (ARF) and baicalin fraction
(BF). |
Table I
Expression of apoptosis-related genes
regulated by aglycone-rich fraction (ARF) and baicalin fraction
(BF).
| Gene symbol | Quantitative
real-time PCR primer sequences | Fold change vs.
control
|
|---|
| ARF 24 h | ARF 48 h | BF 24 h | BF 48 h |
|---|
| Pro-apoptotic
genes | | | | | |
| TP53 |
TGGCATTTGCACCTACCTCAC | 0.99 | 1.25 | −0.01 | −0.13 |
|
AACTCCCTCTACCTAACCAGC |
| TP53BP2 |
ATTAGAGGACATTTAGCGTGATG | 0.60 | 1.06 | 0.25 | 0.17 |
|
AACACTCAACAGGACAGAGAGC |
| TNF |
AGTTGTGTCTGTAATCGCCCTAC | 1.41 | 2.58 | 0.47 | 0.15 |
|
CTAAGCAAACTTTATTTCTCGCCAC |
| CD70 |
GCGTCTCAGCTTCCACCAAG | 1.39 | 3.50 | −0.39 | 0.21 |
|
TGCACTCCAAAGAAGGTCTCATC |
| TNFRSF10A |
CCACCAGCTAGAGTACATCT | −0.02 | 0.14 | −0.13 | −0.01 |
|
TGCTGTCCCATGGAGGTAG |
| TNFRSF10B |
ATCTGTCTCCCACGTCTGC | −1.12 | −2.11 | 0.10 | −1.02 |
|
CCAAGGTCCTCAAGTAGGCAATC |
| Anti-apoptotic
genes | | | | | |
| IGR1R |
ATTCCTGTTATTGCGATATACTCTG | −0.97 | −0.33 | 0.61 | 1.50 |
|
ACGTTGCCTTAGCTTCAGC |
| BCL2 |
GGCCTTCTTTGAGTTCGGTG | −3.97 | −2.07 | 1.55 | 0.28 |
|
ACAGGGCGATGTTGTCCAC |
| BCL2L1 |
GCTCCTCATGGTGGGTTCAG | −1.57 | −0.59 | 0.04 | 0.04 |
|
GCTCCCATAGCTGTTCCTG |
Another pathway to apoptosis is the death
receptor-mediated pathway. The best characterized death receptors
and ligands are those of the TNF superfamily (19,20).
We determined the effects of ARF on the expression of genes in the
TNF ligand (TNF and CD70) and receptor (TNFRSF10A and TNFRSF10B)
family. ARF upregulated TNF and CD70 expression, although the
expression of TNFRSF10A was not affected. ARF, however,
significantly downregulated TNFRSF10B expression (Table I). Based on these results, we
cannot confirm the contribution of the death receptor-mediated
pathway in ARF-induced apoptosis.
As illustrated in Fig.
2, BF increased colorectal cancer cell growth. We sought to
determine whether the expression of selected anti-apoptotic genes
is regulated by BF. Since at 20 μM, both the apoptotic
induction activity of ARF and cell proli feration exciting activity
of BF were observed, we selected this concentration to determine
both the pro-apoptotic and anti-apoptotic effects of ARF or BF. As
shown in Table I, at 24 h, IGF1R
and BCL2 were upregulated by BF; at 48 h, only IGF1R was markedly
upregulated. This observation suggests that BF enhances the
expression of certain anti-apoptotic genes. By contrast, ARF
downregulated IGF1R, BCL2 and BCL2L1 at 24 and 48 h. The Bcl-2
family consists of pro-apoptotic and anti-apoptotic genes, and the
balance in expression between these genes helps determine the death
or survival of a cell. BCL2 (Bcl-2) and BCL2L1 (Bcl-xL) are two
anti-apoptotic genes in the Bcl-2 family (21). Forced downregulation of the
anti-apoptotic Bcl-2 family genes results in mitochondrial
dysfunction and apoptosis (22).
BCL2 and BCL2L1 expression was depressed by ARF, demonstrating that
apoptosis is induced at least partly through a mitochondrial
mechanism.
Effects of ARF on Δψm
To validate the quantitative real-time PCR data, we
performed a cellular functional assay. The spatial variation in Δψm
was estimated using the JC-1 probe. This probe accumulates
specifically in the mitochondria in varying amounts according to
membrane potentials. Organelles with low Δψm accumulate a low
number of JC-1 molecules and produce a green fluorescence (485
excitation/535 emission). At high concentrations (high Δψm), the
probe aggregates exhibit a red fluorescence (535 excitation/590
emission). The loss of membrane potential is followed by a red to
green shift. As illustrated in Fig.
5, untreated HCT-116 cells exhibited red fluorescence. After
treatment with 50 μg/ml of ARF, fluorescence shifted from
red to green, indicating the loss of mitochondrial function.
Compared to the control, treatment with 50 μg/ml of BF did
not alter the Δψm. These results suggest that ARF induces apoptosis
through a mitochondrial-dependent mechanism.
Discussion
The clinical management of cancer invariably
involves diverse conventional modalities, including surgery,
chemotherapy and radiation. The complex characteristics of human
cancer may also require alternative management to improve the
therapeutic efficacy of conventional treatment and/or the quality
of life for cancer patients. Complementary and alternative medicine
(CAM) has recently gained attention for cancer management (23–25),
since CAM covers a wide spectrum of ancient to modern approaches
that expand options for preventing and treating diseases (26,27).
Botanicals have been a valuable source of
therapeutic candidates for new compounds since tremendous chemical
diversity is found across the millions of species of plants. Since
1961, nine plant-derived compounds have been approved in the US for
the treatment of cancer. Several plant-derived anticancer agents,
such as flavopiridol, acronycine, bruceantin, and thalicarpin, have
been evaluated in clinical trials in the US (28,29).
In addition, another 11 anticancer agents are being used in China
(28–30). Thus, botanicals have contributed
significantly to cancer therapy for the past 30 years, and it is
likely that this class of medication will continue to be important
in cancer therapeutics.
Over the years, the anticancer activities of SbE and
its constituents have been evaluated. Published studies have
focused on prostate and liver cancer (9,10,31).
The anti-proliferative effect of baicalin was studied in human
prostate cancer and human hepatoma cells. The results showed that
the threshold for the inhibition of cell growth by 50%
(IC50) in LNCaP prostate cancer cells was 60.8
μM, and in SK-Hep1 hepatoma cells was 25 μM (32,33).
Another major flavonoid in this herb, baicalein, possesses a
stronger anti-proliferative effect than baicalin. The
IC50 of baicalein was 29.8 μM for LNCaP cells and
9.1 μM for SK-Hep1 cells (32,33).
The two components differ in chemical structure: in baicalin, the
7-hydroxy group of baicalein is replaced by a glucuronyloxy group.
This structural difference between baicalin and baicalein may
contribute to the difference in their pharmacological activities
(34).
To evaluate the anti-colorectal cancer potential of
SbE and its fractions, we determined their anti-proliferative
activities. There are several human colorectal cancer cell lines,
of which HCT-116 is widely used in laboratory cancer research, and
has been a model for cellular pathway studies of chemotherapy on
cancer cells (35). HT-29 cells
maintain the capacity to conduct phase II metabolism and may
conjugate chemotherapeutic agents in vitro, whereas HCT-116
cells do not have this ability. Due to phase II metabolism, HT-29
cells exhibit a resistance to many drugs. Thus, we selected these
two cell lines (36,37).
The whole extract, SbE, did not exhibit significant
anti-proliferative activity. To our surprise, at certain
concentrations, SbE actually increased HCT-116 and HT-29 cell
growth. Previous reports have demonstrated that the
anti-proliferative effect of baicalein is significantly higher than
that of baicalin (32,33). In other studies, we observed that
sugar moieties on ginsenosides significantly impact their
anticancer activity. In general, the anticancer activity is
inversely correlated with the number of sugar moieties (38). Therefore, we removed the glycoside,
baicalin, from SbE to prepare an ARF and evaluated its potential
chemopreventive effect on colorectal cancer.
Of note, ARF exerted a potent anti-proliferative
effect on both colorectal cancer cell lines. The BF did not exhibit
anti-proliferative activity, and in fact, significantly increased
the growth of HCT-116 and HT-29 cells at certain concentrations. We
also evaluated the effect of three representative flavonoids on
cancer cell growth. Two aglycones, baicalein and wogonin, had
significant anti-proliferative activity; the glycoside baicalin did
not. At certain concentrations, baicalin even enhanced cancer cell
growth. The anti-proliferative effect of SbE was decreased by the
presence of baicalin. We may therefore conclude that to enhance
anticancer activity, it is necessary to remove baicalin from
SbE.
The effect of the herb on cell cycle arrest and the
induction of apoptosis in colorectal cancer cells was also
evaluated. Potent activity was observed after treatment with ARF,
as HCT-116 cells were arrested in the S and G2/M phases, both
cyclin A and cyclin B1 were upregulated, and the percentage of
apoptotic cells was significantly increased. Since apoptosis is
considered an important mechanism in the inhibition of cancer, to
further explore the mechanism of ARF-induced apoptosis, we
performed expression profiling analysis using quantitative
real-time PCR. The results showed that ARF upregulated the
expression of TP53, TP53BP2, TNF and CD70 and down-regulated the
expression of BCL2, BCL2L1 and TNFRSF10B. Many of these genes are
related to the mitochondrial apoptotic pathway. These results were
further confirmed by a cellular function assay of the Δψm.
The mitochondrial cell death pathway is mediated by
the Bcl-2 family of proteins, a group of anti-apoptotic and
proapoptotic proteins that regulate the passage of small molecules
through the mitochondrial transition pore. These molecules, such as
cytochrome c, Smac/Diablo, and apoptosis-inducing factor,
activate caspase cascades. ARF treatment led to a loss of Δψm,
suggesting that ARF induces apoptosis, at least in part, through
the mitochondrial pathway. Of note, BF did not influence the
expression of pro-apoptotic genes, instead increasing the
expression of anti-apoptotic genes such as BCL2 and BCL2L1.
To explore the possible structure-function
correlation, after comparing the effects of baicalin and baicalein
on anti-proliferation, we observed whether sugar residues on
aglycons decrease or abolish the anti-proliferative activities of
the constituent. Similar results were also observed in saponins or
ginsenosides of ginseng, another commonly used herbal medicine. The
major bioactive constituents in ginseng are ginsenosides and sugar
molecules within a ginsenoside have a high impact on tumor cells.
Anticancer activities increase with the decrease in the number of
sugar moieties. Ginsenosides with four or more sugar molecules
(e.g., Rb1 and Rc) show no significant anti-proliferative effects.
Rd with three sugar molecules weakly inhibits the growth of cancer
cells. Ginsenosides Rg3 (two sugar residues), Rh2 (one sugar
residue), IH-901 (one sugar residue), PPT (no sugar residues) and
PPD (no sugar residues) showed potent anti-proliferative effects on
different types of cancer cells (38). The data from this study suggest
that the sugar moiety in baicalin may influence the
anti-proliferative activity of baicalein.
In conclusion, to safely and effectively use the
botanical components of SbE, we prepared ARF and evaluated its
chemo-preventive effects on human colorectal cells. An apoptotic
assay and expression profiling of genes in selected pathways
revealed that ARF regulated various apoptosis-related genes. Data
from our gene expression and cellular functional analyses indicated
that the mitochondrial apoptotic pathway is responsible for the
anticancer effects of ARF. In addition, since the constituents of
SbE are complex, the development of a novel preparation protocol to
yield a high content of hydrophobic flavonoids with strong
anti-proliferative effects and removal of counteractive or inactive
constituents such as baicalin may lead to a significant improvement
in the anticancer activity of S. baicalensis.
Acknowledgements
This study was supported in part by
the NIH/NCCAM grants P01 AT004418, K01 AT005362, and the University
of Chicago Digestive Disease Research Core Center
(5P30DK042086).
References
|
1
|
Jemal A, Siegel R, Xu J, et al: Cancer
statistics, 2010. CA Cancer J Clin. 60:277–300. 2010. View Article : Google Scholar
|
|
2
|
Newman DJ and Cragg GM: Natural products
as sources of new drugs over the last 25 years. J Nat Prod.
70:461–477. 2007.PubMed/NCBI
|
|
3
|
Cassileth B, Yeung KS and Gubili J: Herbs
and other botanicals in cancer patient care. Curr Treat Options
Oncol. 9:109–116. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Sengupta S, Toh SA, Sellers LA, et al:
Modulating angiogenesis: the yin and the yang in ginseng.
Circulation. 110:1219–1225. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Matthias A, Banbury L, Bone KM, et al:
Echinacea alkylamides modulate induced immune responses in
T-cells. Fitoterapia. 79:53–58. 2008. View Article : Google Scholar
|
|
6
|
Boyle SP, Doolan PJ, Andrews CE, et al:
Evaluation of quality control strategies in Scutellaria
herbal medicines. J Pharm Biomed Anal. 54:951–957. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wang CZ, Mehendale SR, Calway T, et al:
Botanical flavonoids on coronary heart disease. Am J Chin Med.
39:661–671. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhen Z, Chang B, Li M, et al:
Anti-diabetic effects of a Coptis chinensis containing new
traditional Chinese medicine formula in type 2 diabetic rats. Am J
Chin Med. 39:53–63. 2011.
|
|
9
|
Park HJ, Lee YW, Park HH, et al: Induction
of quinone reductase by a methanol extract of Scutellaria
baicalensis and its flavonoids in murine Hepa 1c1c7 cells. Eur
J Cancer Prev. 7:465–471. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Bonham M, Posakony J, Coleman I, et al:
Characterization of chemical constituents in Scutellaria
baicalensis with antiandrogenic and growth-inhibitory
activities toward prostate carcinoma. Clin Cancer Res.
11:3905–3914. 2005.PubMed/NCBI
|
|
11
|
Ye F, Xui L, Yi J, et al: Anticancer
activity of Scutellaria baicalensis and its potential
mechanism. J Altern Complement Med. 8:567–572. 2002.
|
|
12
|
Goh D, Lee YH and Ong ES: Inhibitory
effects of a chemically standardized extract from Scutellaria
barbata in human colon cancer cell lines, LoVo. J Agric Food
Chem. 53:8197–8204. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kuntz S, Wenzel U and Daniel H:
Comparative analysis of the effects of flavonoids on proliferation,
cytotoxicity, and apoptosis in human colon cancer cell lines. Eur J
Nutr. 38:133–142. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Po LS, Chen ZY, Tsang DS, et al: Baicalein
and genistein display differential actions on estrogen receptor
(ER) transactivation and apoptosis in MCF-7 cells. Cancer Lett.
187:33–40. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yam CH, Fung TK and Poon RY: Cyclin A in
cell cycle control and cancer. Cell Mol Life Sci. 59:1317–1326.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Egloff AM, Vella LA and Finn OJ: Cyclin B1
and other cyclins as tumor antigens in immunosurveillance and
immunotherapy of cancer. Cancer Res. 66:6–9. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kobayashi S, Kajino S, Takahashi N, et al:
53BP2 induces apoptosis through the mitochondrial death pathway.
Genes Cells. 10:253–260. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Takahashi N, Kobayashi S, Kajino S, et al:
Inhibition of the 53BP2S-mediated apoptosis by nuclear factor
kappaB and Bcl-2 family proteins. Genes Cells. 10:803–811. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Fiorucci G, Vannucchi S, Chiantore MV, et
al: TNF-related apoptosis-inducing ligand (TRAIL) as a
pro-apoptotic signal transducer with cancer therapeutic potential.
Curr Pharm Des. 11:933–944. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Cretney E, Shanker A, Yagita H, et al:
TNF-related apoptosis-inducing ligand as a therapeutic agent in
autoimmunity and cancer. Immunol Cell Biol. 84:87–98. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Majors BS, Betenbaugh MJ and Chiang GG:
Links between metabolism and apoptosis in mammalian cells:
applications for anti-apoptosis engineering. Metab Eng. 9:317–326.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Willis SN and Adams JM: Life in the
balance: how BH3-only proteins induce apoptosis. Curr Opin Cell
Biol. 17:617–625. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
DiGianni LM, Garber JE and Winer EP:
Complementary and alternative medicine use among women with breast
cancer. J Clin Oncol. 20:S34–S38. 2002.PubMed/NCBI
|
|
24
|
Tian JH, Liu LS, Shi ZM, et al: A
randomized controlled pilot trial of ‘Feiji Recipe’ on quality of
life of non-small cell lung cancer patients. Am J Chin Med.
38:15–25. 2010.
|
|
25
|
Randhawa MA and Alghamdi MS: Anticancer
activity of Nigella sativa (black seed) - a review. Am J
Chin Med. 39:1075–1091. 2011.PubMed/NCBI
|
|
26
|
Richardson MA and Straus SE: Complementary
and alternative medicine: opportunities and challenges for cancer
management and research. Semin Oncol. 29:531–545. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Olaku O and White JD: Herbal therapy use
by cancer patients: a literature review on case reports. Eur J
Cancer. 47:508–514. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Da Rocha AB, Lopes RM and Schwartsmann G:
Natural products in anticancer therapy. Curr Opin Pharmacol.
1:364–369. 2001.PubMed/NCBI
|
|
29
|
Mann J: Natural products in cancer
chemotherapy: past, present and future. Nat Rev Cancer. 2:143–148.
2002. View
Article : Google Scholar : PubMed/NCBI
|
|
30
|
Lee KH: Novel antitumor agents from higher
plants. Med Res Rev. 19:569–596. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ye F, Jiang S, Volshonok H, et al:
Molecular mechanism of anti-prostate cancer activity of
Scutellaria baicalensis extract. Nutr Cancer. 57:100–110.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Chen S, Ruan Q, Bedner E, et al: Effects
of the flavonoid baicalin and its metabolite baicalein on androgen
receptor expression, cell cycle progression and apoptosis of
prostate cancer cell lines. Cell Prolif. 34:293–304. 2001.
View Article : Google Scholar
|
|
33
|
Chang WH, Chen CH and Lu FJ: Different
effects of baicalein, baicalin and wogonin on mitochondrial
function, glutathione content and cell cycle progression in human
hepatoma cell lines. Planta Med. 68:128–132. 2002. View Article : Google Scholar
|
|
34
|
Hong T, Jin GB, Cho S, et al: Evaluation
of the anti-inflammatory effect of baicalein on dextran sulfate
sodium-induced colitis in mice. Planta Med. 68:268–271. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Christopher R, Dhiman A, Fox J, et al:
Data-driven computer simulation of human cancer cell. Ann NY Acad
Sci. 1020:132–153. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Tomida A, Yun J and Tsuruo T:
Glucose-regulated stresses induce resistance to camptothecin in
human cancer cells. Int J Cancer. 68:391–396. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Akhdar H, Loyer P, Rauch C, et al:
Involvement of Nrf2 activation in resistance to 5-fluorouracil in
human colon cancer HT-29 cells. Eur J Cancer. 45:2219–2227. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Qi LW, Wang CZ and Yuan CS: American
ginseng: potential structure-function relationship in cancer
chemoprevention. Biochem Pharmacol. 80:947–954. 2010. View Article : Google Scholar : PubMed/NCBI
|