Introduction
Breast cancer is the most frequently diagnosed
cancer and the leading cause of cancer-related mortality among
women, accounting for 23% of all new cancer cases and 14% of cancer
deaths (1). Bone is one of the
most preferential target sites of metastasis for breast cancer and
up to 70% of women with advanced disease develop bone metastases
(2). Such lesions have devastating
effects, including pain, pathologic fractures, spinal compression
and hypercalcemia, all of which greatly compromise the quality of
life and outcome (3).
Since results from large randomized controlled
trials were published in the late 1990s, bisphosphonates have
become the standard of care for the prevention and treatment of
skeletal complications associated with bone metastases in breast
cancer (4). The third generation
nitrogen-containing bisphosphonate, zoledronic acid (ZA), is the
only bisphosphonate licensed for the treatment of bone disease
originating from a variety of solid tumors and multiple myeloma
(5). ZA reduces osteoclastic bone
resorption by inhibiting key enzymes of the mevalonate pathway
(6), including farnesyl
pyrophosphate synthase (7) and
geranylgeranyl pyrophosphate synthase (8), leading to incomplete post
translational prenylation of signaling GTPases, including Ras, Rho
and Rac (9), which ultimately
causes osteoclasts to undergo apoptosis (10).
In addition to their inhibitory effect on
osteoclasts, there is increasing preclinical evidence to suggest
that bisphosphonates exert a direct antitumor activity comprising
inhibition of tumor cell growth, induction of cancer cell apoptosis
(11–15), inhibition of tumor cell adhesion
and invasion (16–18) and anti-angiogenic activity
(19). Furthermore,
bisphosphonates used in combination with anticancer agents appear
to significantly enhance the effect of treatment. In fact, ZA has
been shown to synergistically increase breast cancer cell death
when combined with doxorubicin, paclitaxel, or cyclophosphamide
(20–22).
Several dosing schedules of ZA for the treatment of
bone metastases have been proposed; a recent study suggested that
metronomic weekly low-dose of ZA could be more effective than the
conventional ZA given every 4 weeks (23). We previously observed that the
anti-proliferative activity of ZA in breast cancer cell lines was
enhanced using a repeated treatment schedule rather than a
continuous one, and that the difference between the two schedules
was statistically relevant only in triple-negative breast cancer
lines (1). Triple-negative breast
cancer (TNBC), which accounts for approximately 15% of all breast
malignancies, is used to define tumors that lack estrogen and
progesterone receptor expression and HER-2 amplification. It is
often an aggressive disease characterized by frequent and early
relapse, a propensity for visceral involvement and shorter periods
of disease-free and overall survival with respect to other breast
cancer subgroups. The unfavorable prognosis associated with TNBC
and the lack of effective targeted therapy has made it the subject
of intensive research in recent years (24). TNBC exhibits an abundance of DNA
aberrations, suggesting that DNA repair mechanisms are defective.
Consequently, these tumors may have increased sensitivity to
agents, such as platins, which cause interstrand DNA breaks. The
sensitivity of TNBC to platinum-based chemotherapy has thus been
the focus of several recent clinical trials in neoadjuvant,
adjuvant and advanced disease settings (25).
The aim of the present study was to investigate the
activity of ZA in combination with different platinum compounds in
four breast cancer cell lines and to explore the molecular
mechanisms of action of the drugs.
Materials and methods
Cell culture
The experiments were performed on four human breast
cancer cell lines. MCF-7, SKBR3 and MDA-MB-231 were obtained from
the American Type Culture Collection, (Rockville, MD, USA), while
BRC-230 was stabilized and characterized in our laboratory
(26). Hormone receptor and HER2
status of the four cell lines are shown in Table I. Cells were cultured as a
monolayer in 75-cm2 flasks at 37°C in TF medium (45% HAM
F12 and 45% DMEM) supplemented with 10% fetal bovine serum, 1%
glutamine and 1% insulin (Mascia Brunelli S.p.a., Milan, Italy) in
a 5% CO2 atmosphere. Cells were cultured to the
exponential growth phase and then treated with ZA alone or in
combination with carboplatin or cisplatin (Cis).
 | Table IHormone receptor and HER2 status of
the four breast cancer cell lines and the primary culture. |
Table I
Hormone receptor and HER2 status of
the four breast cancer cell lines and the primary culture.
| Cell line | ER | PgR | HER2 |
|---|
| BRC-230 | − | − | − |
| MCF-7 | + | + | − |
| MDA-MB 231 | − | − | − |
| SKBR3 | − | − | + |
| Primary tumor | + | + | − |
Isolation of primary cells from a breast
cancer bone metastasis
The tumor material tissue was obtained from a
patient undergoing surgery for a bone metastasis of breast
carcinoma. The protocol was reviewed and approved by the Local
Ethics Committee and performed according to Good Clinical Practice
and the Helsinki declaration. The patient provided written informed
consent to participate in the study.
The tumor tissue was washed twice in sterile PBS 1X
supplemented with 10% penicillin/streptomycin and 5% amphotericin.
The biopsy was then disaggregated by cutting the sample with
sterile surgical blades. The obtained fragments were incubated with
collagenase type I (Millipore Corp., Billerica, MA, USA) at 37°C in
stirring conditions. The enzymatic digestion was stopped after 3–4
h by adding IMDM medium supplemented with 10% fetal bovine serum,
1% glutamine, 10% penicillin/streptomycin and 5% amphotericin and
L-glutamine. The samples were allowed to settle on the bottom of
the tube to separate tissue fragments from collagenase-released
cells. The cells were counted and seeded at a density of
10,000/cm2. Hormone receptor and HER2 status of the
primary tumor are shown in Table
I. Pan cytokeratin immunocytochemistry analysis was performed
according to the manufacturer's instructions (Epithelial Detection
kit, As-Diagnostik, Hueckeswagen, Germany) to detect the percentage
of tumor cells of the sample.
Drugs
Cis (Bristol-Myers Squibb S.p.A, Rome, Italy) was
stored at room temperature, carboplatin (Bristol-Myers Squibb
S.p.A) at 4°C, and both drugs were diluted in medium prior to use.
ZA (Zometa®), kindly provided by Novartis (East Hanover,
NJ, USA), was solubilized, stored at −20°C at a concentration of 50
mM in sterile water and diluted in medium prior to use.
Treatment schedules
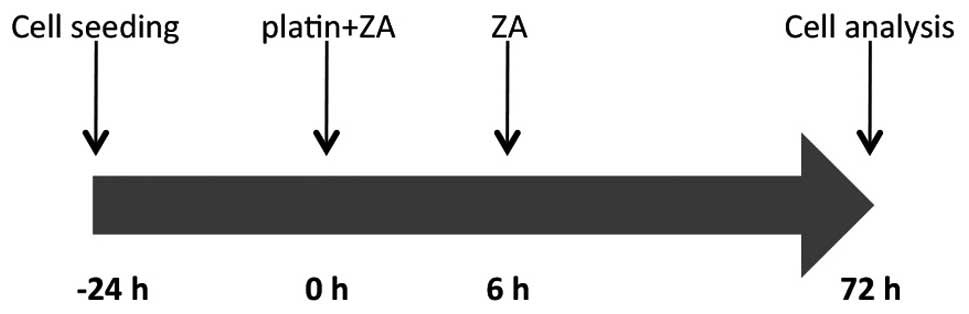
The four cell lines were exposed to ZA and either
platin, singly or in combination, for 72 h. ZA was tested at a
concentration of 50 μM for 72 h, while Cis and carboplatin
were tested at concentrations of 0.001, 0.01, 0.1, 1 and 10
μM and 1, 11 and 110 μM, respectively, for 6 h
followed by a 72-h washout. For the combination assays, cell lines
were exposed to different concentrations of Cis or carboplatin in
combination with ZA (50 μM) for 6 h, washed out and then
exposed to ZA (50 μM) for a further 72 h (Fig. 1).
Chemosensitivity assay
The sulforhodamine B (SRB) assay was used according
to the method by Skehan et al to evaluate the cytotoxic
activity of the drugs (27).
Briefly, cells were collected by trypsinization, counted and plated
at a density of 3,000 cells/well in 96-well flat-bottomed
microtiter plates. After 24 h, cells were treated with the
different schedules. The optical density (OD) of cells was
determined at a wavelength of 540 nm by a colorimetric plate
reader. Growth inhibition and cytocidal effects of the drugs were
calculated according to the formula reported by Monks et
al(28): (OD treated/OD
control) × 100% where the OD treated reflects the cell number in
treated wells and the OD control reflects the cell number in
untreated wells on the day of the assay. If the resulting
percentage ratio is above zero, a cytostatic effect has been
induced, whereas if it is below zero, cell killing has occurred.
The interaction between drugs was evaluated with the method by Kern
et al(29), subsequently
modified by Romanelli et al(30). The expected survival (defined as
the result of the observed survival for drug A alone and the
observed survival for drug B alone) and the observed survival for
the combination of drugs was used to calculate an R index (RI):
Sexp/Sobs. RI ≤0.5 indicates an antagonistic
effect between drugs, whereas ≥0.5 RI ≤1.5 indicates an additive
effect and RI ≥1.5 indicates a synergistic effect. Four biological
independent replicates of each experiment were performed.
Western blot analysis
Proteins were isolated by cell lysis with a lysis
buffer composed of 50 mM Tris-HCl (pH 8.0), 150 mM NaCl, 1% Triton
X-100 and 0.1% SDS, supplemented with 1 mM phenylmethylsulfonyl
fluoride and 1:100 protease inhibitors (Sigma-Aldrich). The protein
content was quantified using the BCA protein assay kit (Thermo
Fisher Scientific, Waltman, MA, USA). An equal amount of protein
from each sample was separated on Criterion™ Precast Gel Tris-HCl
(Bio-Rad, Hercules, CA, USA) and transferred to polyvinylidene
fluoride membranes (Millipore). The membranes were blocked for 2 h
in 5% non-fat dry milk PBS with 0.1% Tween-20 (Sigma-Aldrich,
Steinheim, Germany) at room temperature and incubated overnight at
4°C with primary antibody. After washing, the membranes were
incubated for 1 h at room temperature with horseradish
peroxidase-conjugated secondary antibody. The following primary
antibodies were used: anti-RAS (polyclonal, 1:1000) (Stressgen,
Brussels, Belgium), anti-p-MAPK (polyclonal, 1:1000), anti pM-TOR
(1:1000) (Cell Signaling Technology, Inc., Beverly, MA, USA),
anti-caspase-3 (polyclonal, 1:500), anti-caspase-8 (monoclonal,
1:500) (Alexis Biochemicals, Farmingdale, NY, USA), anti-caspase-9
(polyclonal, 1:500), anti-Mcl-1 (monoclonal 1:100) (BD Pharmingen,
San Diego, CA, USA), anti-bcl-2 (monoclonal, 1:100) (Dako Corp.,
Glostrup, Denmark), anti-p21 (monoclonal, 1:100) (BioOptica, Milan,
Italy), anti-Rho (monoclonal 1:1000) (Millipore), and anti-actin
(polyclonal, 1:5000) (Sigma-Aldrich).
TUNEL assay
Fragmented DNA generated in response to apoptotic
signals was detected by the terminal deoxynucleotidyl transferase
(TdT) nick-end labeling (TUNEL) assay. After each treatment
schedule, 106 cells were washed twice with PBS, fixed by
incubation in 1% formaldehyde on ice for 15 min, resuspended in 70%
ice cold ethanol and stored overnight. Cells were then washed twice
in PBS and resuspended in PBS containing 0.1% Triton X-100 for 5
min at 48°C. Thereafter, samples were incubated in 50 ml of
solution containing TdT and FITC conjugated dUTP deoxynucleotides
1:1 (Roche Diagnostics GmbH, Mannheim, Germany) in a humidified
atmosphere for 90 min at 37°C in the dark, washed in PBS,
counterstained with propidium iodide (2.5 mg/ml, MP Biomedicals,
Verona, Italy) and RNAse (10 kU/ml, Sigma-Aldrich) for 30 min at
48°C in the dark and analyzed by flow cytometry.
Cell cycle analysis
After all the treatment schedules, cells were fixed
in ethanol (70%), stained in a solution containing propidium iodide
(10 mg/ml, MP Biomedicals), RNAse (10 kU/ml, Sigma-Aldrich) and
NP40 (0.01%, Sigma-Aldrich) overnight at 48°C in dark conditions
and analyzed by flow cytometry. Data were expressed as fractions of
cells in the different cell cycle phases.
Scratch wound assay
We used a scratch wound assay to evaluate the
migration ability of the four cell lines after treatment. Cells
were cultured in 75-cm2 flasks, as previously described,
and were exposed to the different treatment schedules. Twenty-four
hours before the end of treatment, a uniform cell-free area was
created by scratching a confluent monolayer with a scraper. The
migration rate of the cell lines was determined by observing the
wound closure at the end of the experiments (31).
Statistical analysis
Differences between treatments in terms of
dose-response, apoptosis and cell cycle block were determined using
the Student's t-test for unpaired observations. P<0.05 was
considered to indicate statistically significant differences. In
each experiment the standard deviation did not exceed 10%.
Results
Drug sensitivity
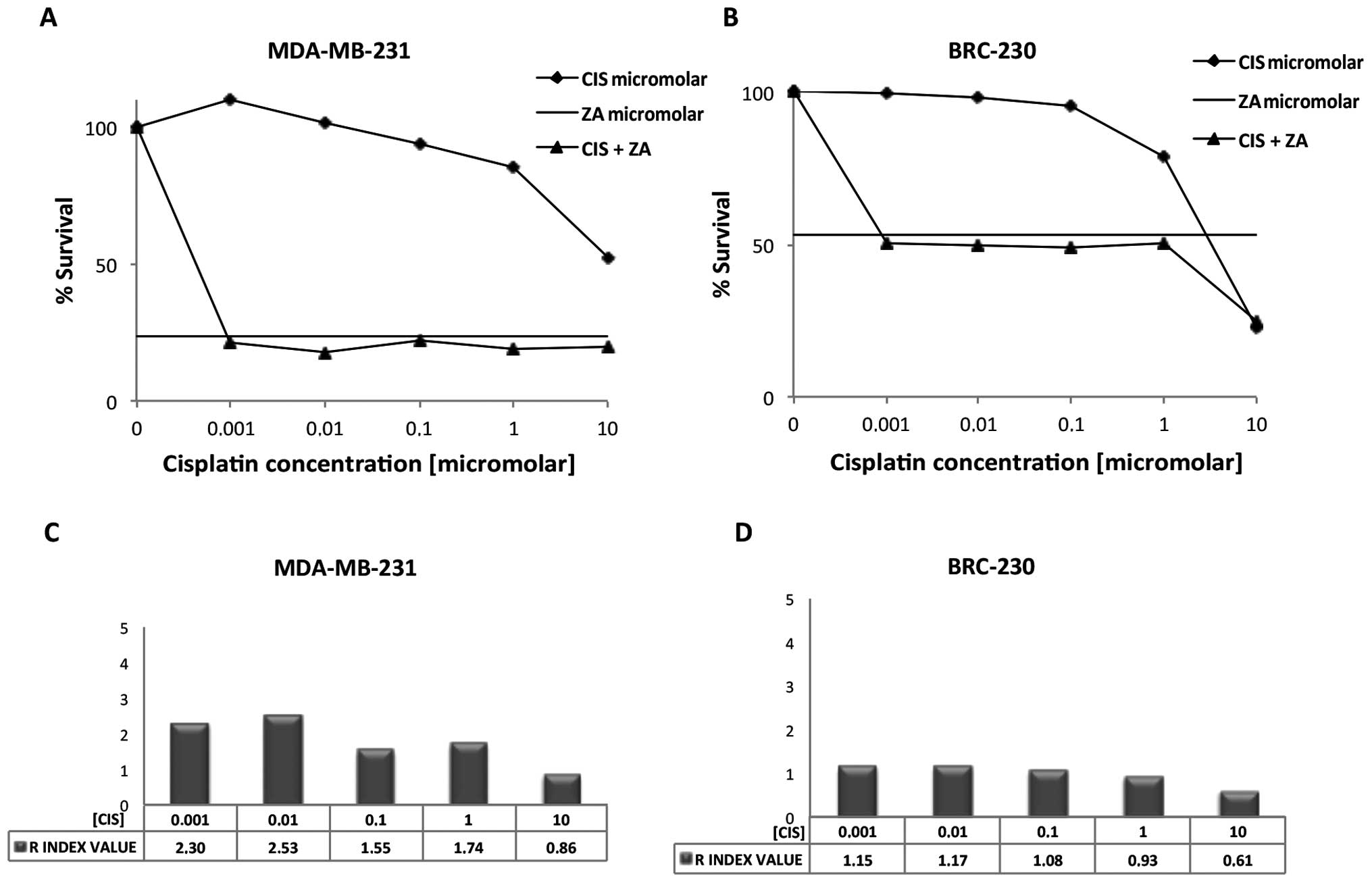
Drug combination experiments were performed using
one dose of ZA (50 μM) for 72 h and five doses of Cis
(0.001, 0.01, 0.1, 1 and 10 μM) or three doses of
carboplatin (1, 11 and 110 μM). The hormone
receptor-positive line MCF-7 and HER-2 expressing line SKBR3 showed
very low sensitivity to all the drugs tested, whether alone or in
combination (data not shown). Conversely, the Cis and ZA
combination showed a high anti-proliferative effect in the
triple-negative cell lines BRC-230 and MDA-MB-231, the latter
proving the most sensitive to treatment. IG50 was
reached at <0.001 μM with the combination, whereas it was
not reached with Cis alone, even at the highest concentration
(Fig. 2A). BRC-230 cells were more
sensitive than MDA-MB-231 to Cis alone, with an IG50 of
4.6 μM. However, the increase in growth inhibition obtained
with the drug combination was lower than that observed for
MDA-MB-231, with an IG50 of 0.005 μM (Fig. 2B). Both triple-negative lines
proved insensitive to carboplatin treatment, alone or in
combination with ZA (data not shown). No synergistic or additive
effects were observed when carboplatin or Cis were combined with ZA
in MCF-7 or SKBR3. In MDA-MB-231, the combination of ZA and Cis
produced an important synergistic effect which yielded an R index
>1.5 for all but the 10-μM Cis concentration. The
synergism was particularly evident at lower concentrations of the
platin (0.001 and 0.01 μM) (Fig. 2C). An additive effect was reached
when combining Cis and ZA in BRC-230 for all Cis concentrations,
and the interaction was once again higher at lower concentrations
of the drug (Fig. D). Conversely,
the combination of carboplatin and ZA did not produce either
additive or synergistic effects and the increase in growth
inhibition obtained with the drug combination was similar to that
obtained with ZA alone (data not shown). Based on these results, we
performed subsequent experiments using the Cis and ZA combination
in the triple-negative cell lines BRC-230 and MDA-MB-231.
Isolation of primary cancer cells from
breast bone metastasis biopsy
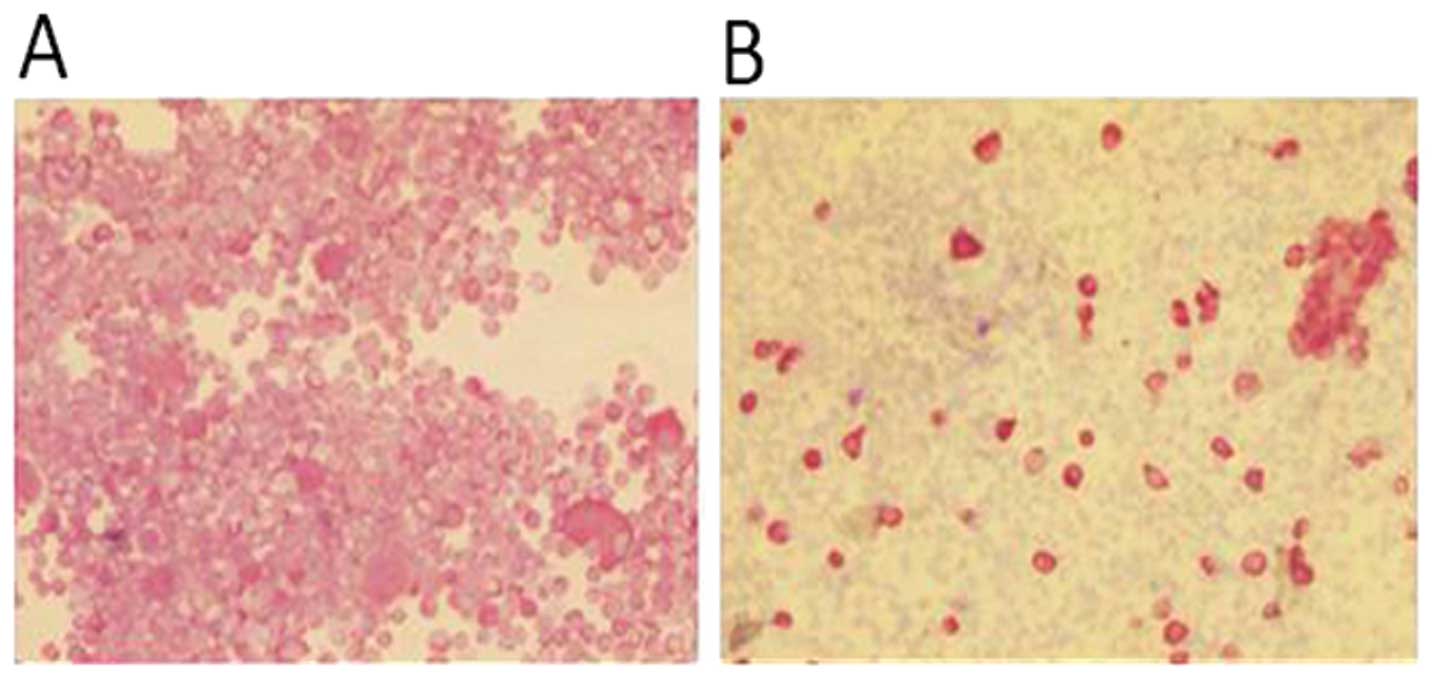
The primary culture obtained from the surgical
material was stable for 4–5 subcultures. In order to verify the
presence of tumor cells in the surgical material, the cytospin
sections were stained for pan cytokeratin immunocytochemistry
assay. The percentage of cells expressing an epithelial phenotype
was ∼20% of the whole culture (Fig.
3).
Drug sensitivity of primary culture
The drug sensitivity data of Cis and ZA were
compared with the values obtained for the cells isolated from the
bone metastasis biopsy. The IG50 obtained for the
primary culture was similar to the one obtained for MCF-7, the cell
line that presents the same HER-2 and hormone receptor pattern. The
primary culture proved to be more sensitive to Cis alone with
respect to MCF-7, IG50 of 8.0 μM for the primary
culture whereas not reached for MCF-7. However, the two cultures
showed similar sensitivity for ZA, IG50 not reached for
both cell lines, and for the combination of Cis and ZA with an
IG50 of 6.6 μM for MCF-7 and 6.9 μM for
the primary culture.
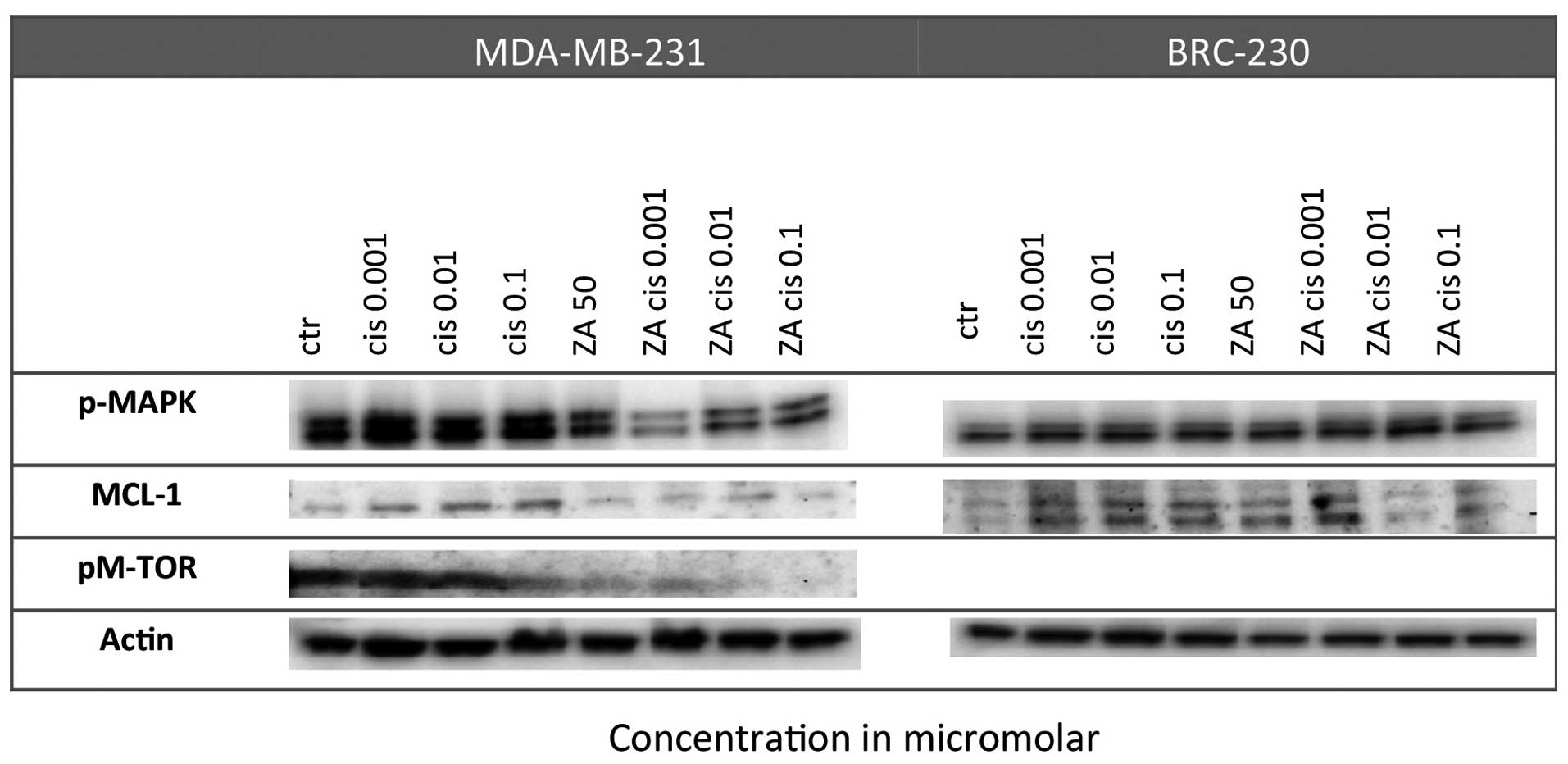
Effect on proliferation pathways
A strong reduction in pMAPK levels was observed in
BRC-230 after the Cis and ZA combination with respect to control
cells, especially at the lowest Cis dose (0.001 μm). Such a
reduction did not occur in single treatments. Furthermore, MCL-1
expression was down-regulated in the MDA-MB-231 cell line after the
combined treatment but not after single drug exposure. Finally,
pM-TOR was markedly downregulated in the MDA-MB-231 cell line
following exposure to ZA alone and especially after combined
treatment with any of the Cis concentrations (Fig. 4).
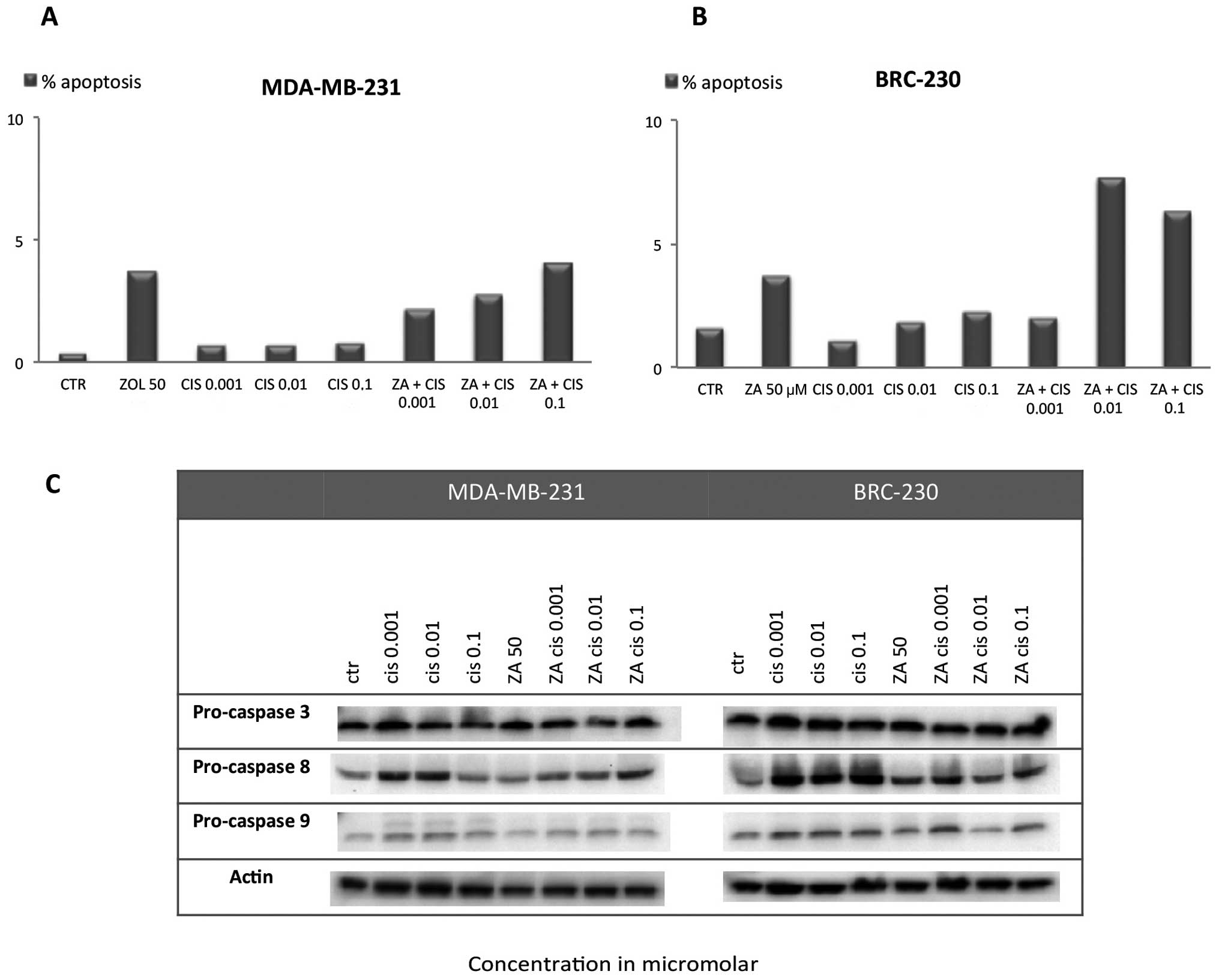
Apoptosis induction
Assessment of apoptosis by TUNEL assay showed that
both single drug exposure and the ZA and Cis combination induced a
small, not statistically significant increase in apoptotic cell
percentage with respect to control in both cell lines. In
MDA-MB-231, the apoptotic cell percentage did not exceed 5% in any
of the Cis concentrations used alone or in combination with ZA
(Fig. 5A). In BRC-230 the
percentage of apoptosis reached 7.7 and 6.3% after the combination
of ZA and Cis 0.01 or 0.1 μM, respectively (Fig. 5B). These data are in agreement with
western blot analysis of caspase-3, -8 and -9. We did not observe a
substantial increase in the cleaved form of the three caspases or a
decrease in pro-caspase levels after any of the treatments
(Fig. 5C).
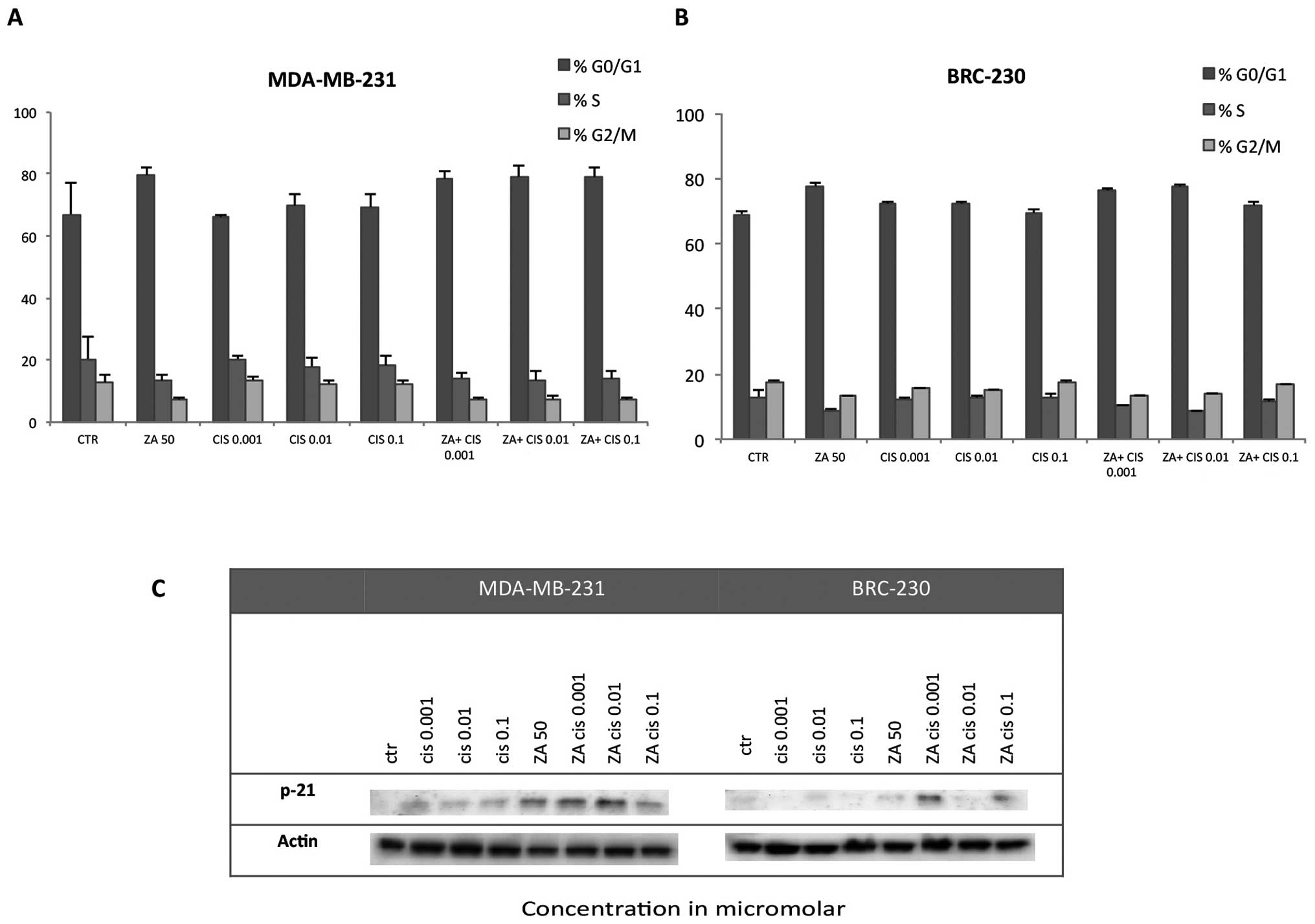
Cell cycle perturbation
The combination of ZA and Cis did not produce a
significant block of the cell cycle in the G0–G1 or G2 phases in
either triple-negative cell line. A slight increment with respect
to control was observed in the percentage of cells in G0–G1 after
treatment with ZA alone and also in combination with all Cis
concentrations in MDA-MB-231 (Fig.
6A) and with Cis 0.001 and 0.01 μm in BRC-230 (Fig. 6B). These findings were confirmed by
western blot analysis of p-21 in which the protein was found to be
upregulated with respect to control after treatment with ZA and Cis
at any tested dose in the MDA-MB-231 cell line, but only at the
lowest Cis doses for BRC-230 (Fig.
6C).
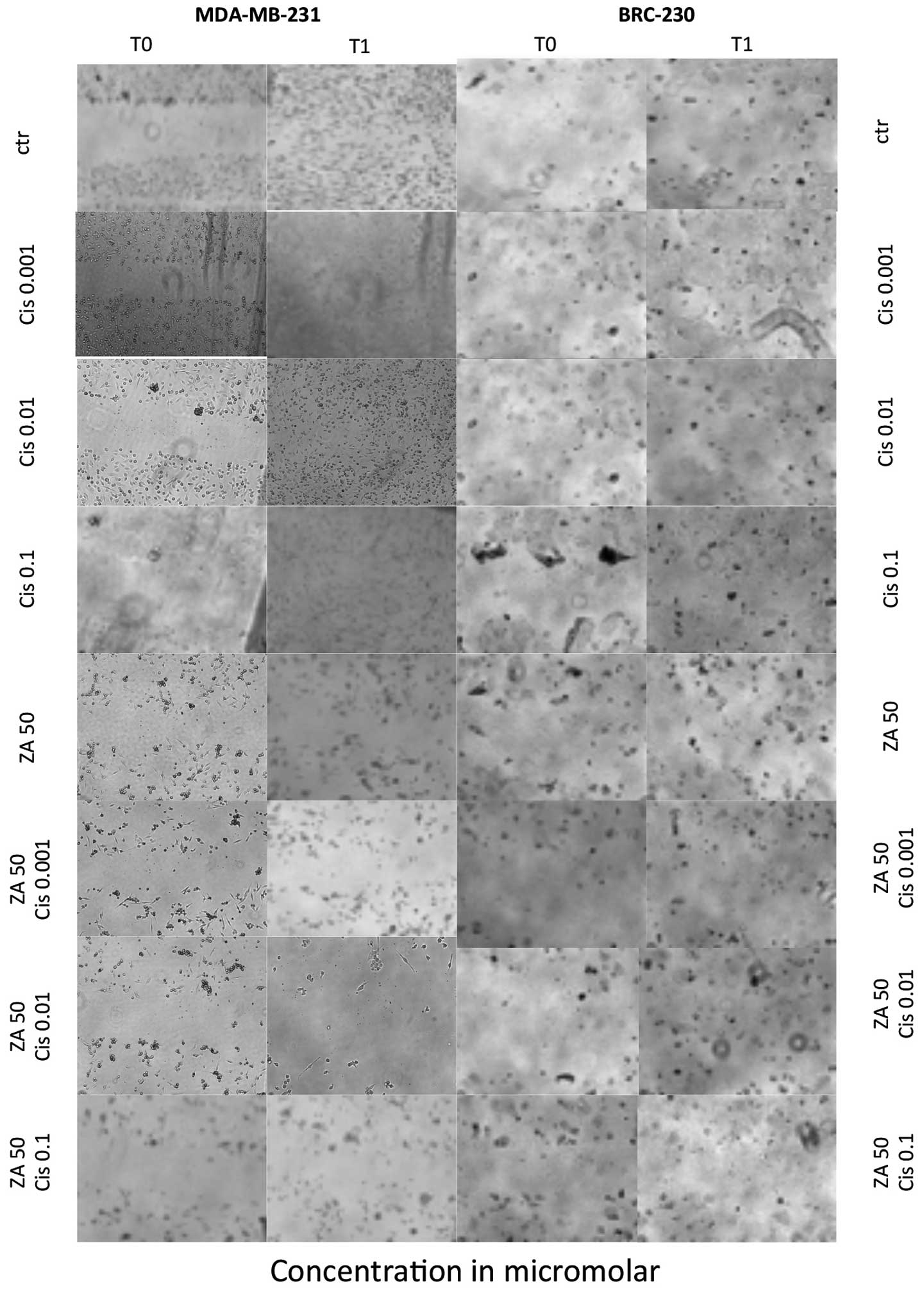
Effect on migration ability
Treatment of cells with the combination of ZA and
Cis resulted in a decreased migration rate with respect to control
cells. Such a reduction was detected using the scratch assay in
both triple-negative cell lines. Untreated cells and cells exposed
to Cis alone closed the scratch wound by migration, whereas cells
treated with ZA alone or in combination with any of the Cis
concentrations did not migrate properly and were unable to close
the wound (Fig. 7).
Discussion
In the present study, we evaluated the in
vitro effects of different doses of Cis and ZA, alone or in
combination, in breast cancer cell lines. ZA was found to have a
direct antitumor activity on breast cancer cells, which is in
agreement with results from previous studies (12,16,18).
Drug concentrations and exposure times used in our study were
chosen on the basis of both literature data (32–34)
and results from a preclinical investigation carried out in our
laboratory (35), which
highlighted that ZA is more effective in triple-negative lines and
that a cytocidal effect is reached only in these cells.
We chose platinum compounds to evaluate the
potential synergic effect of ZA and chemotherapeutic agents as
conventional chemotherapy for breast cancer often uses DNA-damaging
drugs to prevent proliferation and stimulate apoptosis of cancer
cells, especially in TNBC (36).
Initially we performed parallel experiments with carboplatin (1, 10
and 100 μM) and Cis (0.1, 1 and 10 μM). As the
combination of ZA and carboplatin did not produce additive or
synergic effects, we decided to focus on the Cis combination. In
the triple-negative cell lines, Cis produced a synergistic effect
with ZA in MDA-MB-231, whereas an additive effect was reached in
BRC-230. No activity was observed in the other two lines, possibly
due to their low sensitivity to these drugs. This finding confirms
the results of our previous study which highlighted the greater
sensitivity of triple-negative cells to ZA. Such sensitivity can be
attributed to genetic alterations of oncogenic pathways. K-Ras and
BRAF are mutated in the MDA-MB-231 cell line and consequently the
K-Ras pathway is constitutively active. BRC-230 has a genetic
amplification of EGFR that leads to an overexpression of the
protein. We hypothesized that these triple-negative cells are more
sensitive to ZA because this drug, inhibiting the mevalonate
pathway, may produce a block in the K-Ras pathway which is
overactivated in these cells. The hormone receptor-positive (MCF-7)
and HER2-expressing (SKBR3) lines, not presenting alterations in
BRAF, K-Ras or EGFR genes, are less sensitive. Furthermore, we
observed that the two triple-negative lines showed different
sensitivity to ZA and Cis, in agreement with a previous study
characterizing a cohort of triple-negative breast cancer subtypes
with different drug sensitivity (37). Lehmann et al(37) identified 6 subtypes distinguishable
by their molecular profiles. The MDA-MB-231 cell line is a
mesenchymal stem-like subtype enriched in genes involved in EMT
transition and growth pathways, resistant to Cis and sensitive to
NVP-BEZ235 and dasatinib. Notably, our results suggest that ZA
sensitizes MDA-MB-231 to Cis, in contrast to Cis alone, which did
not exert any effect on cell proliferation or survival. We do not
have any information on the BRC-230 subtype as this cell line was
isolated in our laboratory. Further molecular characterizations are
ongoing.
To our knowledge, this is the first study to
describe a synergistic effect of ZA in association with Cis in
breast cancer cell lines, whereas the combination of these two
drugs has already been studied in osteosarcoma (38) and lung cancer cells (39).
We also observed a high inhibition of cell
proliferation in MDA-MB-231 when exposed to ZA in association with
low concentrations of Cis. Based on these results, we further
evaluated two lower concentrations (0.001 and 0.01 μM) of
Cis, both of which produced a greater synergistic effect. Finally,
we investigated the molecular mechanisms involved in the
synergistic/additive effects observed. Assessment of apoptosis
showed that the combination of ZA and Cis induced a small, not
statistically significant increase in the percentage of apoptotic
cells in both cell lines. Furthermore, we performed
chemosensitivity analyses on a primary culture from a bone
metastasis specimen as well and the obtained results were
concordant with data of a cell line, MCF-7, that has the same
pattern of HER-2 and hormonal receptor status of the primary tumor
of the bone metastasis specimen. This is a key finding for the
confirmation of our in vitro experiments. Further evaluation
on a primary culture obtained from a triple negative bone lesion is
warranted.
The principal molecular mechanism involved appears
to be that of proliferation control. Although only a slight
increment in the percentage of cells in the G1 phase was detected,
an important decrease in p-MAPK, Mcl-1 and p-mTOR expression and an
increase in p21 were observed. P-MAPK is part of the mevalonate
pathway and our results thus support previous findings that ZA
exerts its effect by modulating this pathway (6). In addition to its anti-apoptotic
effect, Mcl-1 has been found to be involved in cell cycle and
proliferation regulation (40) and
has also been reported to be modulated by ZA in prostate cancer
cell lines (35). mTOR is
critically involved in the mediation of cell survival and
proliferation, and a number of clinical trials have been conducted
on everolimus, a new mTOR inhibitor, in metastatic breast cancer
(41). Furthermore, the
PI3K/Akt/mTOR pathway is involved in chemotherapeutic drug
resistance and response to radiation in breast cancer cells
(42). A previous study
highlighted that mTOR inhibitors have the potential to overcome
drug resistance from topoisomerase II in solid tumors (43) and ZA is capable of enhancing mTOR
inhibition in osteosarcoma cells (44). Finally, we know that MDA-MB-231 is
a mesenchymal stem-like subtype cell line that is responsive to
mTOR inhibitors but resistant to Cis (37). Taking all these facts into
consideration, we can hypothesize that mTOR pathway inhibition
plays an important role in ZA anticancer activity and in its
ability to overcome MDA-MB-231 resistance to Cis. Further research
is warranted to identify new molecular targets to use in
preclinical and clinical trials, particularly in TNBC where such
targeted therapies are lacking.
In conclusion, our results confirm that ZA exerts a
direct antitumor activity on human breast cancer cell lines, as
previously described in vitro(12,16,18)
in mouse models (45) and as
reported in postmenopausal women of the Azure clinical trial
(46). Furthermore, we observed
that ZA produced a synergistic/additive effect on Cis in
triple-negative cell lines, whereas no effect was exerted on the
hormone receptor-positive or HER2-expressing lines. Investigating
the molecular mechanisms involved, it was concluded that control of
proliferation pathways is possibly the key to the action of the
drug combination. p21, pMAPK and mTOR pathways were found to be
regulated, especially at lower doses of Cis. Although further
research is required to elucidate the molecular mechanisms in
question, several new potential targets have come to light.
Finally, it would be interesting to test the study schedules, first
on xenograft models and then in a clinical setting, in an attempt
to increase the currently limited options available for
triple-negative breast cancer patients. In fact, the synergistic
effect exerted by the combination could enable Cis dosages to be
reduced, thus minimizing side-effects associated with this
chemotherapeutic agent.
Acknowledgements
The authors thank Gráinne Tierney and
Ursula Elbling for editing the manuscript.
References
|
1
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar
|
|
2
|
Coleman RE and Rubens RD: The clinical
course of bone metastases from breast cancer. Br J Cancer.
55:61–66. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Saad F, Adachi JD, Brown JP, Canning LA,
Gelmon KA, Josse RG and Pritchard KL: Cancer treatment-induced bone
loss in breast and prostate cancer. J Clin Oncol. 26:5465–5476.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Coleman RE: Risks and benefits of
bisphosphonates. Br J Cancer. 98:1736–1740. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Coleman RE and McCloskey EV:
Bisphosphonates in oncology. Bone. 49:71–76. 2011. View Article : Google Scholar
|
|
6
|
Amin D, Cornell SA, Gustafson SK, Needle
SJ, Ullrich JW, Bilder GE and Perrone MH: Bisphosphonates used for
the treatment of bone disorders inhibit squalene synthase in
cholesterol biosynthesis. J Lipid Res. 33:1657–1663.
1992.PubMed/NCBI
|
|
7
|
Van Beek E, Pieterman E, Cohen L, Lowick C
and Papapoulos S: Farnesyl pyrophosphatase synthase is the
molecular target of nitrogen-containing bisphosphonates. Biochem
Biophys Res Commun. 264:108–111. 1999.
|
|
8
|
Coxon FP, Helfrich MH, Van't Hof R, Sebti
S, Ralston SH, Hamilton A and Rogers MJ: Protein
geranylgeranylation is required for osteoclast formation, function
and survival: inhibition by bisphosphonates and GGTI-298. J Bone
Miner Res. 15:1467–1476. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Rogers MJ, Gordon S, Benford HL, Coxon FP,
Luckman SP, Monkkonen J and Frith JC: Cellular and molecular
mechanisms of action of bisphosphonates. Cancer. 88:2961–2978.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Benford HL, McGowan NW, Helfrich MH,
Nuttall ME and Rogers MJ: Visualization of bisphosphonate-induced
caspase-3 activity in apoptotic osteoclasts in vitro. Bone.
28:465–473. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Jagdev SP, Coleman RE, Shipman CM, Rostami
HA and Croucher PI: The bisphosphonate, ZA, induces apoptosis of
breast cancer cells: evidence for synergy with paclitaxel. Br J
Cancer. 84:1126–1134. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Senaratne SG, Pirianov G, Mansi JL, Arnett
T and Colston KW: Bisphosphonates induce apoptosis in human breast
cancer cell lines. Br J Cancer. 82:1459–1468. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Derenne S, Amiot M, Barille S, et al:
Zoledronate is a potent inhibitor of myeloma cell growth and
secretion of IL-6 and MMP-1 by the tumoral environment. J Bone
Miner Res. 14:2048–2056. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lee MV, Fong EM, Singer FR and Guenette
RS: Bisphosphonate treatment inhibits the growth of prostate cancer
cells. Cancer Res. 61:2602–2608. 2001.PubMed/NCBI
|
|
15
|
Shipman CM, Rogers MJ, Apperley JF,
Russell RG and Croucher PI: Bisphosphonates induce apoptosis in
human myeloma cell lines: a novel anti-tumour activity. Br J
Haematol. 98:665–672. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Van der Pluijm G, Vloedgraven H, van Beek
EJ, van der Wee-Pals L, Lowik C and Papapoulos S: Bisphosphonates
inhibit the adhesion of breast cancer cells to bone matrices in
vitro. J Clin Invest. 98:698–705. 1996.
|
|
17
|
Boissier S, Magnetto S, Frappart L, Cuzin
B, Ebetino FH, Delmas PD and Clezardin P: Bisphosphonates inhibit
prostate and breast carcinoma cell adhesion to unmineralized and
mineralized bone extracellular matrices. Cancer Res. 57:3890–3894.
1997.PubMed/NCBI
|
|
18
|
Boissier S, Ferreras M, Peyruchaud O, et
al: Bisphosphonates inhibit breast and prostate carcinoma cell
invasion, an early event in the formation of bone metastases.
Cancer Res. 60:2949–2954. 2000.PubMed/NCBI
|
|
19
|
Wood J, Bonjean K, Ruetz S, Bellahcene A,
Devy L and Foidart JM: Novel antiangiogenic effects of the
bisphosphonate compound zoledronic acid. J Pharmacol Exp Ther.
302:1055–1061. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Neville-Webbe HL, Rostami-Hodjegan A,
Evans CA, Coleman RE and Holen I: Sequence- and schedule-dependent
enhancement of ZA induced apoptosis by doxorubicin in breast and
prostate cancer cells. Int J Cancer. 113:364–371. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Neville-Webbe HL, Evans CA, Coleman RE and
Holen I: Mechanisms of the synergistic interaction between the
bisphosphonate ZA and the chemotherapy agent paclitaxel in breast
cancer cells in vitro. Tumour Biol. 27:92–103. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Vogt U, Bielawski KP, Bosse U and
Schlotter CM: Breast tumour growth inhibition in vitro
through the combination of
cyclophosphamide/metotrexate/5-fluorouracil,
epirubicin/cyclophosphamide, epirubicin/paclitaxel, and
epirubicin/docetaxel with the bisphosphonates ibandronate and
zoledronic acid. Oncol Rep. 12:1109–1114. 2004.
|
|
23
|
Zhao X, Xu X, Guo L, et al: Biomarker
alterations with metronomic use of low-dose ZA for breast cancer
patients with bone metastases and potential clinical significance.
Breast Cancer Res Treat. 124:733–743. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Valentin MD, da Silva SD, Privat M,
Alaoui-Jamali M and Bignon YJ: Molecular insights on basal-like
breast cancer. Breast Cancer Res Treat. 134:21–30. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Sirohi B, Arnedos M, Popat S, et al:
Platinum-based chemotherapy in triple-negative breast cancer. Ann
Oncol. 19:1847–1852. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Amadori D, Bertoni L, Flamigni A, et al:
Establishment and characterization of a new cell line from primary
human breast carcinoma. Breast Cancer Res Treat. 28:251–260. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Skehan P, Storeng R, Scudiero D, et al:
New colorimetric cytotoxicity assay for anticancer-drug screening.
J Natl Cancer Inst. 82:1107–1112. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Monks A, Scudiero D, Skehan P, et al:
Feasibility of a high-flux anticancer drug screen using a diverse
panel of cultured human tumor cell lines. J Natl Cancer Inst.
83:757–766. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kern DH, Morgan CR and Hildebrand-Zanki
SU: In vitro pharmacodynamics of 1-beta-D-arabinofuranosylcytosine:
synergy of antitumor activity with
cis-diamminedichloroplatinum(II). Cancer Res. 48:117–121.
1988.PubMed/NCBI
|
|
30
|
Romanelli S, Perego P, Pratesi G, Carenini
N, Tortoreto M and Zunino F: In vitro and in vivo interaction
between cisplatin and topotecan in ovarian carcinoma systems.
Cancer Chemother Pharmacol. 41:385–390. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Hahnel A, Wichmann H, Kappler M, Kotzsch
M, Vordermark D, Taubert H and Bache M: Effects of osteopontin
inhibition on radiosensitivity of MDA-MB-231 breast cancer cells.
Radiat Oncol. 5:822010. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Senaratne SG, Mansi JL and Colston KW: The
bisphosphonate ZA impairs Ras membrane [correction of impairs
membrane] localisation and induces cytochrome c release in breast
cancer cells. Br J Cancer. 86:1479–1486. 2002.
|
|
33
|
Rachner TD, Singh SK, Schoppet M, et al:
ZA induces apoptosis and changes the TRAIL/OPG ratio in breast
cancer cells. Cancer Lett. 287:109–116. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Guise TA: Antitumor effects of
bisphosphonates: promising preclinical evidence. Cancer Treat Rev.
34:19–24. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Fabbri F, Brigliadori G, Carloni S, et al:
Zoledronic acid increases docetaxel cytotoxicity through pMEK and
Mcl-1 inhibition in a hormone-sensitive prostate carcinoma cell
line. J Transl Med. 6:432008. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Curigliano G and Goldhirsch A:
Triple-negative subtype: new ideas for the poorest prognosis breast
cancer. J Natl Cancer Inst Monogr. 108–110. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Lehmann BD, Bauer JA, Chen X, Sanders ME,
Chakravarthy AB, Shyr Y and Pietenpol JA: Identification of human
triple-negative breast cancer subtypes and preclinical models for
selection of targeted therapies. J Clin Invest. 121:2750–2767.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Benassi MS, Chiechi A, Ponticelli F, et
al: Growth inhibition and sensitization to cisplatin by zoledronic
acid in osteosarcoma cells. Cancer Lett. 250:194–205. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Ozturk OH, Bozcuk H, Burgucu D, Ekinci D,
Ozdogan M, Akca S and Yildiz M: Cisplatin cytotoxicity is enhanced
with zoledronic acid in A549 lung cancer cell line: preliminary
results of an in vitro study. Cell Biol Int. 31:1069–1071. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Fujise K, Zhang D, Liu J and Yeh ET:
Regulation of apoptosis and cell cycle progression by MCL1.
Differential role of proliferating cell nuclear antigen. J Biol
Chem. 275:39458–39465. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Ellard SL, Clemons M, Gelmon KA, et al:
Randomized phase II study comparing two schedules of everolimus in
patients with recurrent/metastatic breast cancer: NCI Clinical
Trials Group IND.163. J Clin Oncol. 27:4536–4541. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Steelman LS, Navolanic P, Chappell WH, et
al: Involvement of Akt and mTOR in chemotherapeutic- and
hormonal-based drug resistance and response to radiation in breast
cancer cells. Cell Cycle. 10:3003–3015. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Gaur S, Chen L, Yang L, Wu X, Un F and Yen
Y: Inhibitors of mTOR overcome drug resistance from topoisomerase
II inhibitors in solid tumors. Cancer Lett. 311:20–28. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Moriceau G, Ory B, Mitrofan L, et al:
Zoledronic acid potentiates mTOR inhibition and abolishes the
resistance of osteosarcoma cells to RAD001 (Everolimus): pivotal
role of the prenylation process. Cancer Res. 70:10329–10339. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Ottewell PD, Deux B, Mönkkönen H, Cross S,
Coleman RE, Clezardin P and Holen I: Differential effect of
doxorubicin and zoledronic acid on intraosseous versus extraosseous
breast tumor growth in vivo. Clin Cancer Res. 14:4658–4666. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Coleman RE, Winter MC, Cameron D, et al:
The effects of adding zoledronic acid to neoadjuvant chemotherapy
on tumour response: exploratory evidence for direct anti-tumour
activity in breast cancer. Br J Cancer. 102:1099–1105. 2010.
View Article : Google Scholar : PubMed/NCBI
|