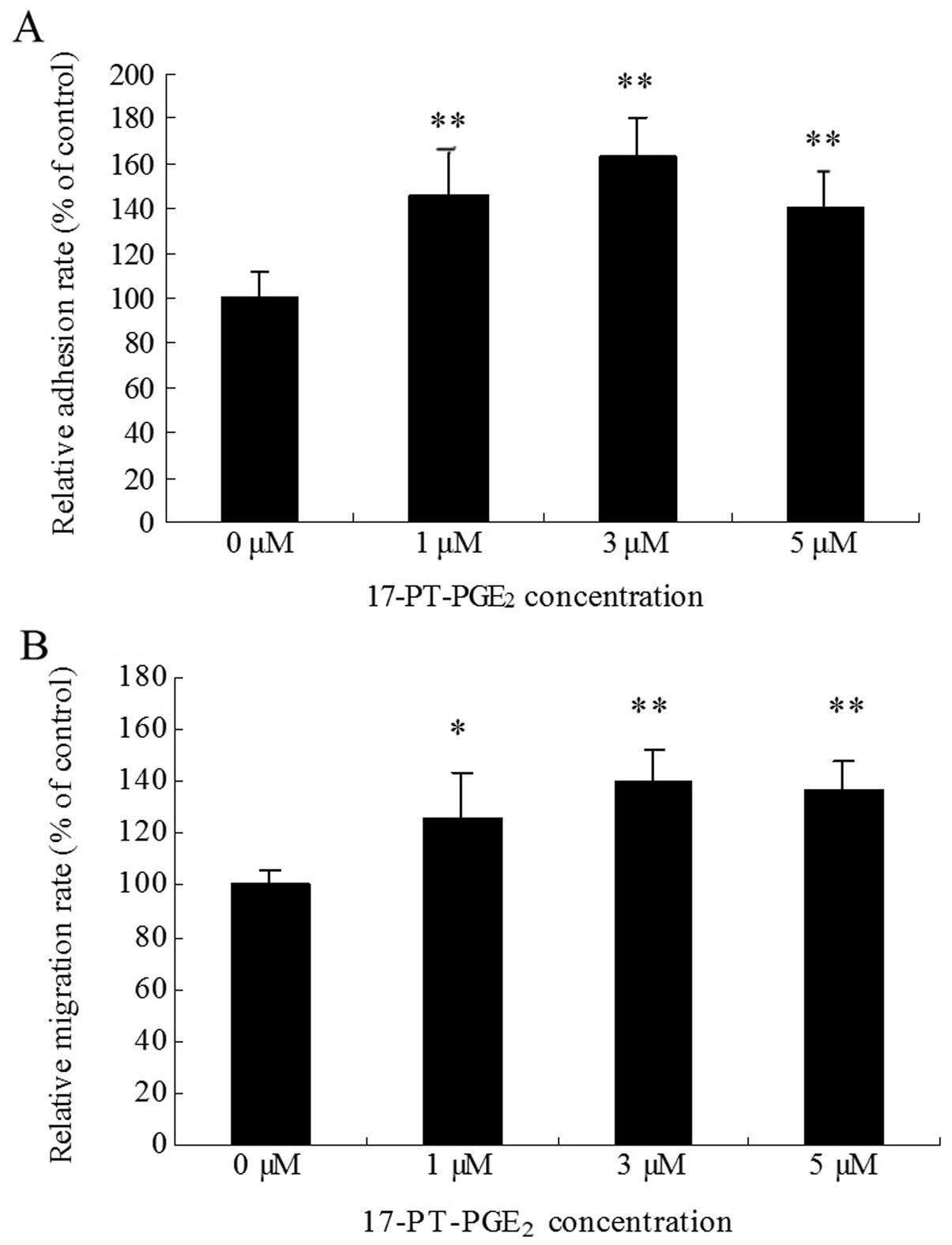
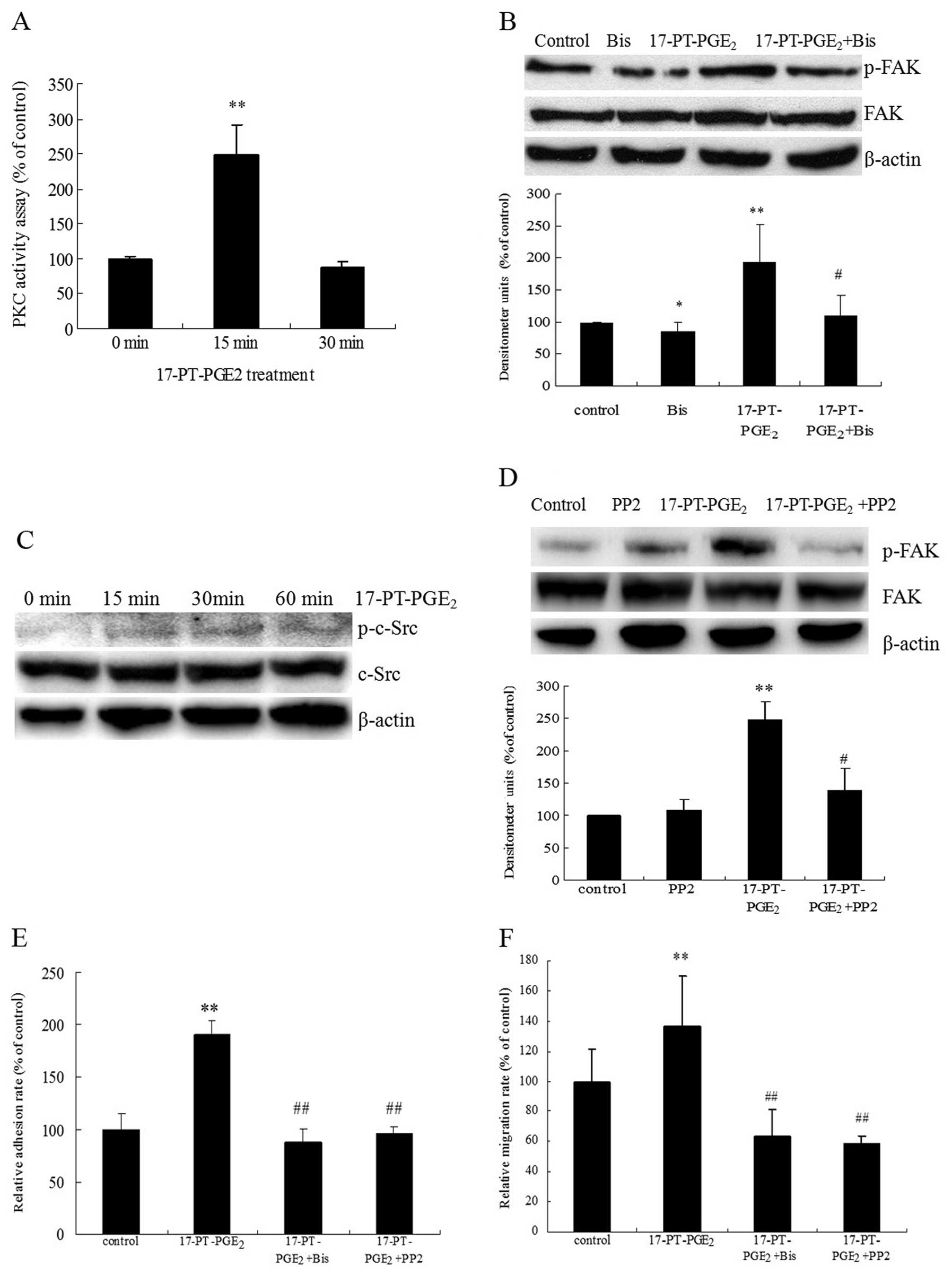
|
1.
|
Wu T: Cyclooxygenase-2 in hepatocellular
carcinoma. Cancer Treat Rev. 32:28–44. 2006. View Article : Google Scholar
|
|
2.
|
Uchino K, Tateishi R, Shiina S, Kanda M,
Masuzaki R, Kondo Y, Goto T, Omata M, Yoshida H and Koike K:
Hepatocellular carcinoma with extrahepatic metastasis: clinical
features and prognostic factors. Cancer. 117:4475–4483. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Pang RW, Joh JW, Johnson PJ, Monden M,
Pawlik TM and Poon RT: Biology of hepatocellular carcinoma. Ann
Surg Oncol. 15:962–971. 2008. View Article : Google Scholar
|
|
4.
|
Mon NN, Ito S, Senga T and Hamaguchi M:
FAK signaling in neoplastic disorders: a linkage between
inflammation and cancer. Ann NY Acad Sci. 1086:199–212. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Meng XN, Jin Y, Yu Y, Bai J, Liu GY, Zhu
J, Zhao YZ, Wang Z, Chen F, Lee KY and Fu SB: Characterisation of
fibronectin-mediated FAK signalling pathways in lung cancer cell
migration and invasion. Br J Cancer. 101:327–334. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Kwiatkowska A, Kijewska M, Lipko M, Hibner
U and Kaminska B: Downregulation of Akt and FAK phosphorylation
reduces invasion of glioblastoma cells by impairment of MT1-MMP
shuttling to lamellipodia and downregulates mMPs expression.
Biochim Biophys Acta. 1813:655–667. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Chan KT, Cortesio CL and Huttenlocher A:
FAK alters invadopodia and focal adhesion composition and dynamics
to regulate breast cancer invasion. J Cell Biol. 185:357–370. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Guan JL: Integrin signaling through FAK in
the regulation of mammary stem cells and breast cancer. IUBMB Life.
62:268–276. 2010.PubMed/NCBI
|
|
9.
|
Mitra SK and Schlaepfer DD:
Integrin-regulated FAK-Src signaling in normal and cancer cells.
Curr Opin Cell Biol. 18:516–523. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Yuan Z, Zheng Q, Fan J, Ai KX, Chen J and
Huang XY: Expression and prognostic significance of focal adhesion
kinase in hepatocellular carcinoma. J Cancer Res Clin Oncol.
136:1489–1496. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Chen JS, Huang XH, Wang Q, Chen XL, Fu XH,
Tan HX, Zhang LJ, Li W and Bi J: FAK is involved in invasion and
metastasis of hepatocellular carcinoma. Clin Exp Metastasis.
27:71–82. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Bai XM, Zhang W, Liu NB, Jiang H, Lou KX,
Peng T, Ma J, Zhang L, Zhang H and Leng J: Focal adhesion kinase:
important to prostaglandin E2-mediated adhesion, migration and
invasion in hepatocellular carcinoma cells. Oncol Rep. 21:129–136.
2009.PubMed/NCBI
|
|
13.
|
Xia D, Wang D, Kim SH, Katoh H and DuBois
RN: Prostaglandin E2 promotes intestinal tumor growth via DNA
methylation. Nat Med. 18:224–226. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Harding P and LaPointe MC: Prostaglandin
E2 increases cardiac fibroblast proliferation and increases cyclin
D expression via EP1 receptor. Prostaglandins Leukot Essent Fatty
Acids. 84:147–152. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Kim JI, Lakshmikanthan V, Frilot N and
Daaka Y: Prostaglandin E2 promotes lung cancer cell migration via
EP4-betaArrestin1-c-Src signalsome. Mol Cancer Res. 8:569–577.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Wu J, Zhang Y, Frilot N, Kim JI, Kim WJ
and Daaka Y: Prostaglandin E2 regulates renal cell carcinoma
invasion through the EP4 receptor-Rap GTPase signal transduction
pathway. J Biol Chem. 286:33954–33962. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Boie Y, Stocco R, Sawyer N, Slipetz DM,
Ungrin MD, Neuschäfer-Rube F, Püschel GP, Metters KM and Abramovitz
M: Molecular cloning and characterization of the four rat
prostaglandin E2 prostanoid receptor subtypes. Eur J Pharmacol.
340:227–241. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
O’Callaghan G, Kelly J, Shanahan F and
Houston A: Prostaglandin E2 stimulates Fas ligand expression via
the EP1 receptor in colon cancer cells. Br J Cancer. 99:502–512.
2008.PubMed/NCBI
|
|
19.
|
Rundhaug JE, Simper MS, Surh I and Fischer
SM: The role of the EP receptors for prostaglandin E2 in skin and
skin cancer. Cancer Metastasis Rev. 30:465–480. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20.
|
Liu H, Xiao J, Yang Y, Liu Y, Ma R, Li Y,
Deng F and Zhang Y: COX-2 expression is correlated with VEGF-C,
lymphangiogenesis and lymph node metastasis in human cervical
cancer. Microvasc Res. 82:131–140. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21.
|
Han C, Michalopoulos GK and Wu T:
Prostaglandin E2 receptor EP1 transactivates EGFR/MET receptor
tyrosine kinases and enhances invasiveness in human hepatocellular
carcinoma cells. J Cell Physiol. 207:261–270. 2006. View Article : Google Scholar
|
|
22.
|
Liu JF, Fong YC, Chang CS, Huang CY, Chen
HT, Yang WH, Hsu CJ, Jeng LB, Chen CY and Tang CH: Cyclooxygenase-2
enhances alpha2beta1 integrin expression and cell migration via EP1
dependent signaling pathway in human chondrosarcoma cells. Mol
Cancer. 9:432010. View Article : Google Scholar : PubMed/NCBI
|
|
23.
|
Bai XM, Jiang H, Ding JX, Peng T, Ma J,
Wang YH, Zhang L, Zhang H and Leng J: Prostaglandin E2 upregulates
survivin expression via the EP1 receptor in hepatocellular
carcinoma cells. Life Sci. 86:214–223. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24.
|
Leng J, Han C, Demetris AJ, Michalopoulos
GK and Wu T: Cyclooxygenase-2 promotes hepatocellular carcinoma
cell growth through Akt activation: evidence for Akt inhibition in
celecoxib-induced apoptosis. Hepatology. 38:756–768. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
25.
|
Wendum D, Masliah J, Trugnan G and Fléjou
JF: Cyclooxygenase-2 and its role in colorectal cancer development.
Virchows Arch. 445:327–333. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
26.
|
Zha S, Yegnasubramanian V, Nelson WG,
Isaacs WB and De Marzo AM: Cyclooxygenases in cancer: progress and
perspective. Cancer Lett. 215:1–20. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
27.
|
Alfranca A, López-Oliva JM, Genís L,
López-Maderuelo D, Mirones I, Salvado D, Quesada AJ, Arroyo AG and
Redondo JM: PGE2 induces angiogenesis via MT1-MMP-mediated
activation of the TGFbeta/Alk5 signaling pathway. Blood.
112:1120–1128. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
28.
|
Zhang Y and Daaka Y: PGE2 promotes
angiogenesis through EP4 and PKA Cγ pathway. Blood. 118:5355–5364.
2011.PubMed/NCBI
|
|
29.
|
Cui W, Yu CH and Hu KQ: In vitro and in
vivo effects and mechanisms of celecoxib-induced growth inhibition
of human hepatocellular carcinoma cells. Clin Cancer Res.
11:8213–8221. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
30.
|
Wu T, Leng J, Han C and Demetris AJ: The
cyclooxygenase-2 inhibitor celecoxib blocks phosphorylation of Akt
and induces apoptosis in human cholangiocarcinoma cells. Mol Cancer
Ther. 3:299–307. 2004.PubMed/NCBI
|
|
31.
|
Boonmasawai S, Akarasereenont P,
Techatraisak K, Thaworn A, Chotewuttakorn S and Palo T: Effects of
selective COX-inhibitors and classical NSAIDs on endothelial cell
proliferation and migration induced by human cholangiocarcinoma
cell culture. J Med Assoc Thai. 92:1508–1515. 2009.PubMed/NCBI
|
|
32.
|
Tsai WC, Hsu CC, Chou SW, Chung CY, Chen J
and Pang JH: Effects of celecoxib on migration, proliferation and
collagen expression of tendon cells. Connect Tissue Res. 48:46–51.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
33.
|
Thorat MA, Morimiya A, Mehrotra S, Konger
R and Badve SS: Prostanoid receptor EP1 expression in breast
cancer. Mod Pathol. 21:15–21. 2008. View Article : Google Scholar
|
|
34.
|
Han C and Wu T: Cyclooxygenase-2-derived
prostaglandin E2 promotes human cholangiocarcinoma cell growth and
invasion through EP1 receptor-mediated activation of the epidermal
growth factor receptor and Akt. J Biol Chem. 280:24053–24063. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
35.
|
Surh I, Rundhaug JE, Pavone A, Mikulec C,
Abel E, Simper M and Fischer SM: The EP1 receptor for prostaglandin
E2 promotes the development and progression of malignant murine
skin tumors. Mol Carcinog. 51:553–564. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
36.
|
Yang SF, Chen MK, Hsieh YS, Chung TT,
Hsieh YH, Lin CW, Su JL, Tsai MH and Tang CH: Prostaglandin E2/EP1
signaling pathway enhances intercellular adhesion molecule 1
(ICAM-1) expression and cell motility in oral cancer cells. J Biol
Chem. 285:29808–29816. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
37.
|
Yamamoto D, Sonoda Y, Hasegawa M,
Funakoshi-Tago M, Aizu-Yokota E and Kasahara T: FAK overexpression
upregulates cyclin D3 and enhances cell proliferation via the PKC
and PI3-kinase-Akt pathways. Cell Signal. 15:575–583. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
38.
|
von Sengbusch A, Gassmann P, Fisch KM,
Enns A, Nicolson GL and Haier J: Focal adhesion kinase regulates
metastatic adhesion of carcinoma cells within liver sinusoids. Am J
Pathol. 166:585–596. 2005.PubMed/NCBI
|
|
39.
|
Hess AR and Hendrix MJ: Focal adhesion
kinase signaling and the aggressive melanoma phenotype. Cell Cycle.
5:478–480. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
40.
|
Cox BD, Natarajan M, Stettner MR and
Gladson CL: New concepts regarding focal adhesion kinase promotion
of cell migration and proliferation. J Cell Biochem. 99:36–52.
2006.PubMed/NCBI
|
|
41.
|
Bosco R, Melloni E, Celeghini C, Rimondi
E, Vaccarezza M and Zauli G: Fine tuning of protein kinase C (PKC)
isoforms in cancer: shortening the distance from the laboratory to
the bedside. Mini Rev Med Chem. 11:185–199. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
42.
|
Rosse C, Linch M, Kermorgant S, Cameron
AJ, Boeckeler K and Parker PJ: PKC and the control of localized
signal dynamics. Nat Rev Mol Cell Biol. 11:103–112. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
43.
|
Wu TT, Hsieh YH, Wu CC, Hsieh YS, Huang CY
and Liu JY: Overexpression of protein kinase C alpha mRNA in human
hepatocellular carcinoma: a potential marker of disease prognosis.
Clin Chim Acta. 382:54–58. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
44.
|
Wu TT, Hsieh YH, Hsieh YS and Liu JY:
Reduction of PKC alpha decreases cell proliferation, migration, and
invasion of human malignant hepatocellular carcinoma. J Cell
Biochem. 103:9–20. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
45.
|
Siesser PM and Hanks SK: The signaling and
biological implications of FAK overexpression in cancer. Clin
Cancer Res. 12:3233–3237. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
46.
|
Lau GM, Lau GM, Yu GL, Gelman IH, Gutowski
A, Hangauer D and Fang JW: Expression of Src and FAK in
hepatocellular carcinoma and the effect of Src inhibitors on
hepatocellular carcinoma in vitro. Dig Dis Sci. 54:1465–1474. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
47.
|
Fischer OM, Hart S, Gschwind A and Ullrich
A: EGFR signal transactivation in cancer cells. Biochem Soc Trans.
31:1203–1208. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
48.
|
Buchanan FG, Wang D, Bargiacchi F and
DuBois RN: Prostaglandin E2 regulates cell migration via the
intracellular activation of the epidermal growth factor receptor. J
Biol Chem. 278:35451–35457. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
49.
|
Wang SE, Xiang B, Zent R, Quaranta V,
Pozzi A and Arteaga CL: Transforming growth factor β induces
clustering of HER2 and integrins by activating Src-FAK and receptor
association to the cytoskeleton. Cancer Res. 69:475–482. 2009.
|
|
50.
|
Aponte M, Jiang W, Lakkis M, Li MJ,
Edwards D, Albitar L, Vitonis A, Mok SC, Cramer DW and Ye B:
Activation of PAF-receptor and pleiotropic effects on tyrosine
phospho-EGFR/Src/FAK/Paxillin in ovarian cancer. Cancer Res.
68:5839–5848. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
51.
|
Calandrella SO, Barrett KE and Keely SJ:
Transactivation of the epidermal growth factor receptor mediates
muscarinic stimulation of focal adhesion kinase in intestinal
epithelial cells. J Cell Physiol. 203:103–110. 2005. View Article : Google Scholar
|