Introduction
The Escherichia coli purine nucleoside
phosphorylase/2-fluoro-2-deoxyadenosine suicide system has been
found to have powerful killing and bystander effects on tumor cells
(1). However, several drawbacks to
cancer suicide/gene therapy remain to be resolved, including the
side-effects of this therapy (2).
The human telomerase reverse transcriptase (hTERT) promoter has
been widely used to drive the specific expression of therapeutic
genes for the treatment of tumor cells (3,4).
However, the transcriptional activity of the hTERT promoter is
weaker than that of the conventional CMV promoter, which results in
an insufficient therapeutic effect (5).
The pSFV1 eukaryotic expression vector (Invitrogen,
USA), which is based on the Semliki Forest virus (SFV) replicon, is
a self-replicating RNA vector with a high level of expression
efficiency (6). After transfection
of the SFV-based DNA vector, an initial plus-strand full-length RNA
driven by the CMV promoter is transcribed in the nucleus,
translocated into the cytoplasm and then translated into the
replicase complex of SFV. The replicase complex directly initiates
the replication cascade and consequently, high-level transcription
of exogenous genes occurs in the cytoplasm. Death is induced in
host cells transfected by this SFV-based DNA vector and a large
amount of the protein expressed from the vector is released, which
eliminates the potential genotoxic risks of exogenous DNA (7–9).
Studies have shown that protein expression based on the alphavirus
replicase complex is several times greater than the protein
expression driven by the conventional CMV promoter (8). However, the transfection efficiency
of the plasmid is significantly reduced because its size is ∼12 kb;
hence, its application in disease research has been greatly limited
(10).
Several types of anaerobes have great potential as
vectors for carrying plasmids into cells (11–13).
In previous studies, attenuated Salmonella typhimurium
SL7207 was used as an effective vehicle for transporting plasmids
into cells. Because this organism is an aroA-defective anaerobe
that exhibits limited proliferation within cells, the plasmids are
released upon the death of the bacterium, which results in high
expression of the exogenous genes (14,15).
Therefore, in this study, we designed and
constructed a new SFV-based DNA vector carrying a replicase gene
under the control of the hTERT promoter, which ensure targeted and
powerful gene expression in tumor cells. To our knowledge, this
study is the first to use transfer of this big plasmid into tumor
cells with SL7207 as a vehicle to achieve high levels of expression
of the ePNP gene in the cytoplasm. We expect that administration of
this live recombinant bacterial vaccine together with the prodrug
F-dAdo could provide a new strategy for clinical therapy of solid
tumors.
Materials and methods
Animals, bacterial strains, plasmids
and cells
Female C57BL/6J mice (age 6–8 weeks) and a feeding
site were provided by the Laboratory Animal Center of Xiamen
University. The attenuated S. typhimurium strains LB5000 and
SL7207 were obtained from ATCC (Rockville, MD, USA). The plasmid
pSFV1 was generously provided by Professor Zhuozhuang Lu (Chinese
Center for Disease Control and Prevention, China). The plasmids
pGL3-hTERT-luc, pCI-neo, pIRES and pIRES-GFP were provided by Dr
Hanbing He (School of Life Sciences, Sichuan University, China).
Murine B16 and LLC cells and human WI-38 cells were purchased from
the Shanghai Cell Bank of the Chinese Academy of Science and grown
in Dulbecco’s modified Eagle medium (DMEM) with 10% FBS and
antibiotic-antimycotic mix (Gibco BRL, USA).
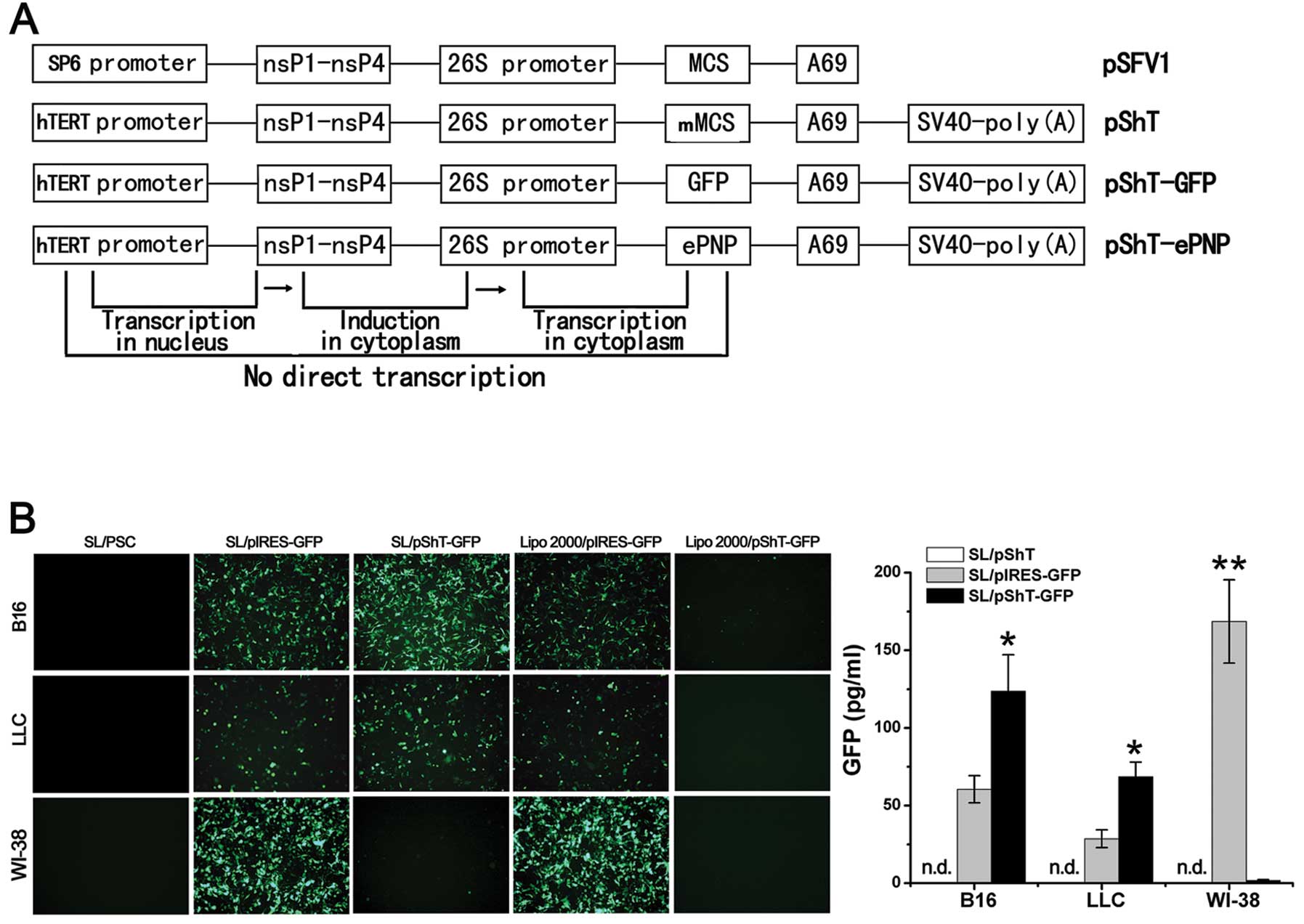
Vector construction and transformation
of S. typhimurium with plasmids
The plasmid pSFV1 was used as the basic plasmid. The
hTERT promoter sequence was amplified from plasmid pGL3-hTERT-luc
and used to replace the SP6 promoter in plasmid pSFV1 by
overlapping polymerase chain reaction (PCR). A strong
transcription-termination signal, the SV40 poly(A) sequence
amplified from plasmid pCI-neo was inserted downstream of the
multiple cloning site by overlap PCR and the restriction
endonuclease sites BamHI and ClaI were introduced
into the multiple cloning site simultaneously. The plasmid pShT was
then constructed successfully. The ePNP and GFP genes were
amplified from the Escherichia coli genome and the plasmid
pIRES-GFP, respectively and cloned into the multiple cloning site
of the plasmid pShT to construct the plasmids pShT-ePNP and
pShT-GFP (Fig. 1A). Similarly, the
ePNP gene was cloned into plasmid pIRES to construct the plasmid
pIRES-ePNP. All recombinant plasmids were analyzed by restriction
enzyme digestion and sent for sequencing to Shanghai Biological
Engineering Co. (SBEC, China). These plasmids were then transformed
into attenuated S. typhimurium LB5000 by electroporation
(12.5 kV, 1 impulse, 4.8 ms) by using a Gene Pulser II apparatus
(Bio-Rad, USA). The plasmids were obtained from the positive clones
and then introduced into S. typhimurium SL7207 under the
same conditions used for LB5000. The recombinant bacteria were
identified as SL/pShT, SL/pShT-ePNP, SL/pIRES-ePNP, SL/pShT-GFP and
SL/pIRES-GFP and were amplified in LB medium and stored at −80°C
for subsequent experiments.
Bacterial infection and gene
expression
Recombinant bacteria (20 μl) SL/pShT,
SL/pShT-GFP and SL/pIRES-GFP were seeded into 200 ml of LB medium
containing ampicillin (100 μg/ml) and were grown at 37°C for
16 h. The bacterial count was adjusted to 2×108 cfu/ml
by using an automatic urinary sediment analyzer (Sysmex, Japan).
Murine B16 cells and LLC cells and human WI-38 cells
(2×105 cells/well) were seeded into 6-well plates until
the cells reached 70–80% confluence. Next, 100 μl of
recombinant bacteria was added when the multiplicity of infection
(MOI) was 100. After incubation for 2 h, the cells were washed
twice and new medium containing tetracycline (10 mg/l) was added
for further culturing. Simultaneously, 1 μmol of plasmid
pIRES-GFP or pSh-TGFP was transfected into cells using
Lipofectamine 2000 (Lipo2000, Invitrogen). Forty-eight hours later,
the expression efficiency of GFP was observed by fluorescence
microscopy and the expression level was quantitatively analyzed
according to the handling procedures for the GFP quantification kit
(Biovision, USA). Forty-eight hours after infection with SL/pShT,
SL/pShT-ePNP and SL/pIRES-ePNP, the infected cells were harvested
and ePNP gene expression was monitored by reverse
transcriptase-polymerase chain reaction (RT-PCR).
Efficiency of F-dAdo conversion to
F-Ade
To determine the efficiency of F-dAdo (Sigma, USA)
conversion to 2-fluoroadenine (F-Ade), the cells (2×105
cells/well) were seeded onto 6-well plates. Twenty-four hours after
infection, the medium was changed and F-dAdo was added until a
final concentration of 80 μg/ml was achieved. After another
48 h, the cell supernatant was harvested for boiling, followed by
centrifugation at 12,000 g for 5 min. The supernatant was again
harvested and then analyzed by high-performance liquid
chromatography (HPLC) (16).
Cytotoxicity assays
B16, LLC and WI-38 cells were infected with
recombinant bacteria at various MOIs. Twenty-four hours after
infection, F-dAdo was added until a final concentration of 80
μg/ml was achieved. Seventy-two hours after F-Ado addition,
the cell counting kit-8 (CCK-8, Dojindo, Japan) was used. The
optical density (OD) difference was measured 1 h later at 450 nm.
The relative survival rate of cells was calculated using the
following formula: (Asample −
Ablank)/(Acontrol − Ablank) × 100%
(17). The relative survival rates
of infected cells (MOI = 100) at different F-Ado concentrations
(0–160 μg/ml) were observed by using the CCK-8 as described
above. Similarly, following incubation with 80 μg/ml F-dAdo,
the relative survival rates of infected cells (MOI = 100) at
different time-points were also calculated as described above.
In vivo gene expression and analysis
of antitumor effects
C57BL/6J mice were used according to the guidelines
for administration to lab animals, issued by the Ministry of
Science and Technology (Beijing, China) and an animal care and use
protocol approved by Xiamen University. Tumors were established by
subcutaneous inoculation with B16 melanoma cells (100 μl
containing 1×106 cells). When the tumor volume reached
100 mm3, the following experiments were conducted. Four
B16 melanoma-bearing mice were sacrificed 4 days after oral
administration of 5×107 cfu of SL/pIRESGFP, SL/pShT-GFP,
or SL/pShT and phosphate-buffered saline (PBS) (as a control).
Tumor tissues were excised and cut into frozen sections for the
detection of GFP expression. B16 melanoma-bearing mice were divided
into 5 groups of 13 mice each. The mice in each group were orally
administered 1 ml PBS containing 5×107 cfu of SL,
SL/pShT, SL/pIRES-ePNP, or SL/pShT-ePNP or were a1dministered 1 ml
PBS as the control. F-dAdo (10 mg/kg) dissolved in 0.5 ml PBS was
injected intraperitoneally 3 times daily for 3 consecutive days,
beginning at 48 h after the administration of recombinant bacteria.
This schedule was counted as a single course and 3 consecutive
courses were administered (18).
Tumor diameters were measured using calipers every 2 days and tumor
volumes were calculated using the following formula: volume =
length × width2 × 0.52. On the fourth day of the first
course, 3 mice in each group were sacrificed and tumor and other
organs were excised. The expression of ePNP gene in tumors for each
group was examined by RT-PCR. Simultaneously, cell suspensions
prepared from the tumor, heart, liver, spleen and lung in SL/pShT,
SL/pShT-ePNP and SL/pIRES-ePNP groups were spread on LB agar plates
containing ampicillin (100 μg/ml) to analyze the bacterial
distribution in vivo. At the end of the third course, 5 mice
in each group were sacrificed and the tumor specimens were
subjected to histopathological analysis and TUNEL staining (Roche,
Switzerland).
To evaluate the specificity of this therapeutic
vaccine, on the fourth day of the first course, the expression of
the ePNP gene in tumor tissue and various organs of B16
tumor-bearing mice in SL/pShT group (n=3) was detected by RT-PCR
assays. To evaluate the safty of this therapeutic vaccine,
peripheral blood was drawn from the orbital region of 5 mice in
each group at the end of the third course for biochemical and
hematological assays performed using a DXC 800 biochemical
auto-analyzer (Beckman, USA) and XS-1000i hematology analyzer
(Sysmex, Japan). The heart, liver, spleen and lungs were also
subjected to histopathological analysis for safty evaluation. When
the tumor size reached 4,500 mm3, the other 5 B16
melanoma-bearing mice from each group were sacrificed and the time
until sacrifice was defined as the survival time.
Statistical analysis
One-way analysis of variance (ANOVA) was used to
evaluate the experimental data and the groups were compared using
Dunnet’s t-test. The log-rank test was used to analysis the
survival times of the mice. Differences were considered significant
at P<0.05.
Results
In vitro expression of exogenous genes
of the pShT plasmid carried by SL7207
A series of plasmids was constructed using
commercialized pSFV-1 as a template. Sequencing confirmed that all
plasmids were successfully constructed. B16, LLC and WI-38 cells
were infected with SL/pShT, SL/pShT-GFP, or SL/pIRES-GFP and
transfected with the plasmids pIRES-GFP and pShT-GFP by using
Lipofectamine 2000. Forty-eight hours later, fluorescence
microscopy analysis helped detect high levels of GFP expression
from B16 and LLC cells in the SL/pIRES-GFP and SL/pShT-GFP groups,
whereas WI-38 cells showed detectable GFP expression only in the
SL/pIRES-GFP group and almost no GFP expression in the SL/pShT-GFP
group, which indicates that plasmid pShT was tumor-targeted
(Fig. 1B). We also observed a high
level of GFP expression in the Lipo2000/pIRES-GFP group for B16 and
LLC cells, whereas almost no GFP expression was detected for the
Lip2000/pShT-GFP group (Fig. 1B).
Quantitative analysis showed the highest GFP expression in the
SL/pShT-GFP group, which was ∼2.0 and 2.4 times higher than that of
the SL/pIRES-GFP group for B16 cells and LLC cells, respectively
(Fig. 1B, P<0.05). Total RNA
was extracted from the infected cells and ePNP mRNA was detected by
RT-PCR. A band was detected at 750 bp in the SL/pIRES-ePNP and
SL/pShT-ePNP groups for B16 and LLC cells, whereas almost no
expression was detected for WI-38 cells (Fig. 2). These results demonstrated that a
series of constructed plasmids based on pSFV-1 could be effectively
transported into tumor cells by SL7207 and expressed there in a
targeted manner. Moreover, the expression efficiency was
considerably better than that of traditional eukaryotic expression
vectors such as pIRES.
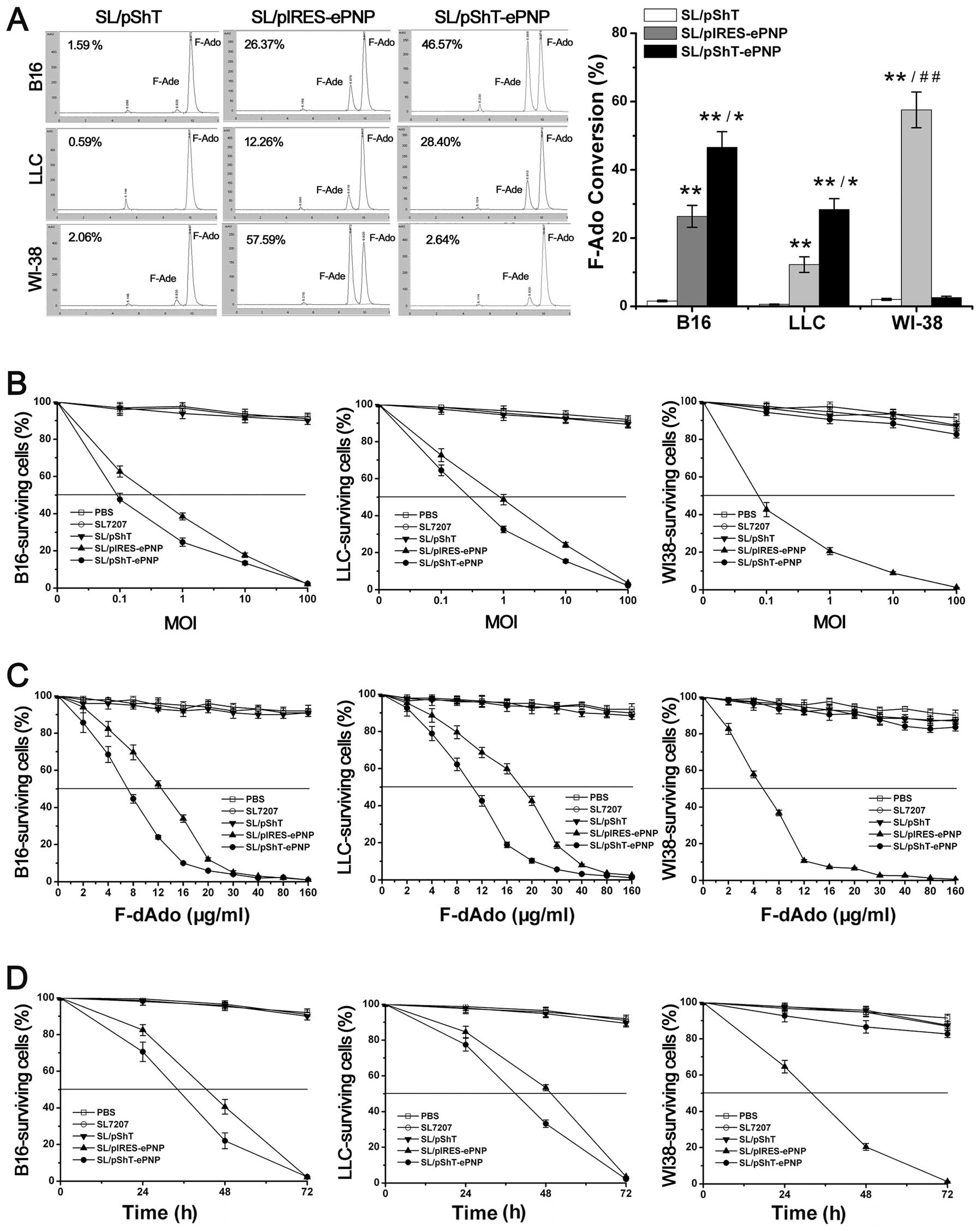
Effects of F-dAdo on B16 cells
infected by SL7207 carrying various plasmids
To observe the effect of the prodrug F-dAdo on tumor
cells, we used HPLC to monitor the ratio of F-dAdo conversion.
Forty-eight hours after F-dAdo addition, the ratio of F-dAdo
conversion for B16 and LLC cells in the SL/pShT-ePNP group reached
46.57 and 28.40%, respectively, whereas that of the SL/pIRES-PNP
group was only 26.37 and 12.26%, respectively. Significant
differences were observed between the SL/pShT-ePNP group and the
SL/pIRES-PNP group for B16 and LLC cells (Fig. 3A, P<0.05). Moreover, WI-38 cells
in the SL/pShT-ePNP group showed no sensitivity to F-Ado, which
indicates that the plasmid pShT-ePNP was only functional in tumor
cells.
We used different concentrations of recombinant
bacteria to infect cells. In the experiment on B16 cells, when the
MOI was 0.1 and 10, the cell viability in the SL/pShT-ePNP group
was 47.65 and 13.45%, respectively (P<0.05) and that in the
SL/pIRES-PNP group was 62.65 and 17.68%, respectively (P<0.05).
Similar results were obtained for LLC cells. In contrast, in the
control WI-38 cells, the cell viability in the SL/pShT-ePNP group
did not differ with increase in the MOI (Fig. 3B). We used the half-inhibitory
concentration (IC50) to evaluate the cytotoxicity of the
suicide gene system. After addition of different concentrations of
F-Ado, the B16 cells in the SL/pShT-ePNP and SL/pIRES-ePNP groups
showed significantly decreased viability whereas those in the PBS,
SL and SL/pShT groups did not appear to be affected. The lowest
IC50 was seen in the SL/pShT-ePNP group (6.86
μg/ml) and the IC50 of the SL/pIRES-ePNP group
was 12.34 μg/ml (P<0.05). Similar results were also
obtained for LLC cells. In contrast, in the control WI-38 cells,
there was no significant cytotoxicity in any group except for the
SL/pIRES-PNP group (Fig. 3C). When
the MOI of the recombinant bacteria was 100 and the concentration
of F-Ado was 80 μg/ml, it was observed that as the treatment
time increased, the cell viability for B16 and LLC cells in the
SL/pShT-ePNP group decreased, whereas no such result was observed
for the control WI-38 cells (Fig.
3D). These results indicate that the plasmid pShT-ePNP can be
effectively delivered into cells by SL7207, expressed specifically
in tumor cells and exert cytotoxicity when F-Ado is added.
Furthermore, the cytotoxicity observed increased over time and was
concentration-dependent.
In vivo expression of ePNP in tumors
in B16 melanoma-bearing mice
Four tumor-bearing mice were orally administered
PBS, SL/pShT, SL/pShT-GFP, or SL/pIRES-GFP and sacrificed 4 days
later. The tumors were immediately excised, sectioned and frozen.
High levels of GFP expression were observed in the SL/pShT-GFP or
SL/pIRES-GFP group on fluorescence microscopy analysis. The PBS and
SL/pShT groups were used as the controls (Fig. 4A). Tumor tissues of the treated
mice were dispersed into cell suspensions and total RNA was
extracted for RT-PCR analysis. Bands were observed at 750 bp in the
SL/pShT-ePNP and SL/pIRES-ePNP groups, which indicates that ePNP
was successfully expressed in tumor tissue (Fig. 4B). These results demonstrate that
the plasmid constructed, that is, pShT-ePNP was transferred into
tumor cells by oral administration of SL7207 and was expressed
successfully in vivo.
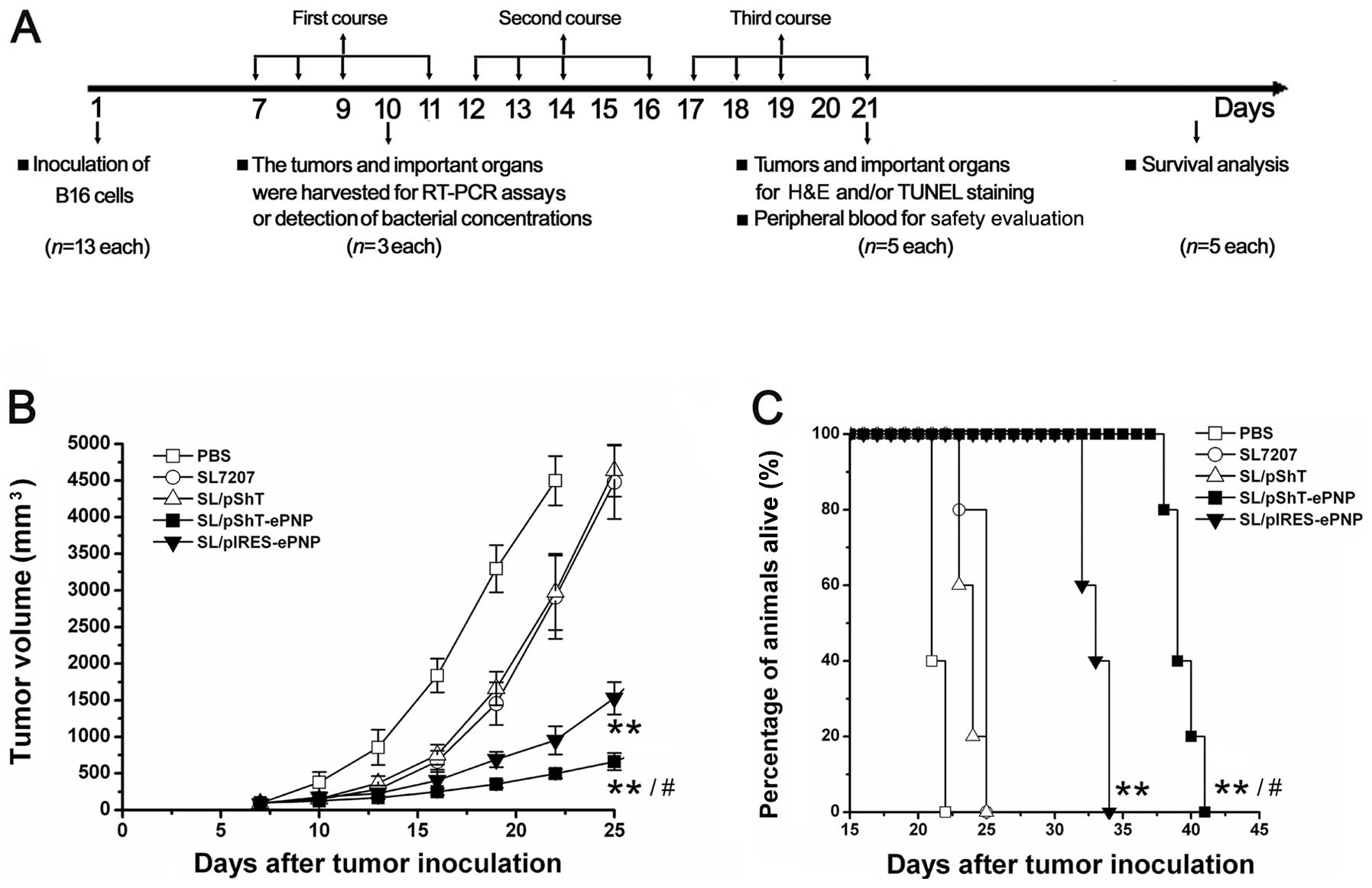
Therapeutic efficacy of SL/pShT-ePNP for B16
melanoma-bearing mice. After oral administration of recombinant
bacteria and intraperitoneal injection of F-dAdo, tumor volume was
measured every 2 days and the data were used to draw a tumor growth
curve (Fig. 5B). No significant
differences were observed among groups at the beginning of
treatment (P>0.05). Three days after the entire treatment was
completed, a pairwise comparison was performed among these groups.
The average volumes of tumors from the SL/pIRES-ePNP and
SL/pShT-ePNP groups were significantly lower than the volumes of
tumors from the other groups. Tumors from the SL/pShT-ePNP group
exhibited the lowest volume. There were significant differences
between the SL/pShT-ePNP group and the SL/pIRES-ePNP group
(P<0.05). A survival analysis curve for B16 tumor-bearing mice
is shown in Fig. 5C. The survival
time of each group was analyzed by a log-rank test and the results
indicated significant differences among the groups. The survival
time of the SL/pShT-ePNP group was significantly greater than that
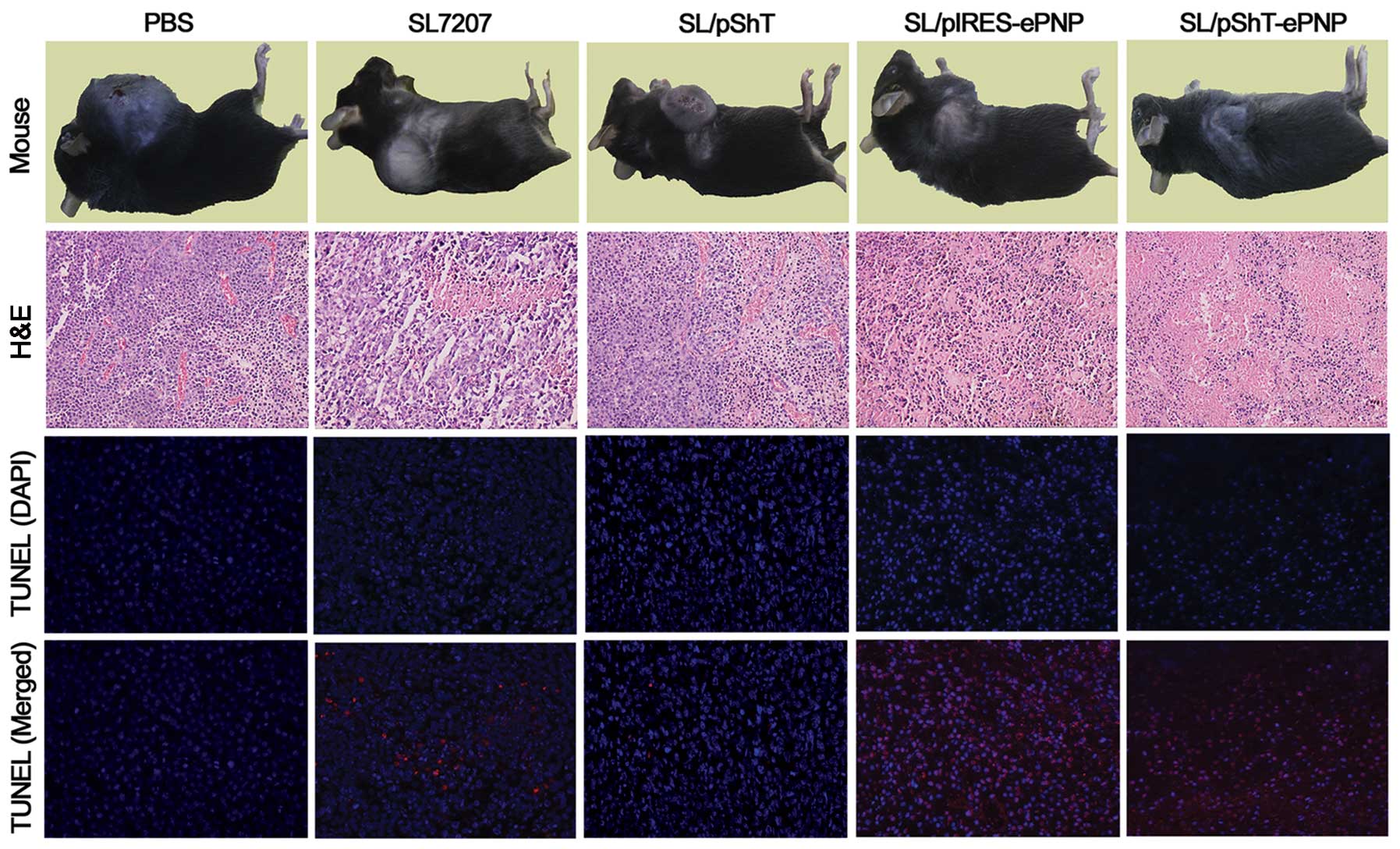
of the other groups. The pathological results showed large-scale
coagulation necrosis and apoptotic cells in the tumor tissues of
the SL/pShT-ePNP group and this phenomenon was also seen in other
groups to different degrees except in the PBS control group, in
which no obvious changes were seen (Fig. 6). Thus, the SL/pShT-ePNP vaccine
was effective in treating B16 melanoma-bearing mice and prolonging
their survival.
Target specificity and safety analysis
of SL/pShT-ePNP therapeutic vaccine
Tumor tissue and various important organs of B16
melanoma-bearing mice that were orally administered recombinant
bacteria were dispersed into cell suspensions and cultured on LB
plates. The bacterial concentrations in the heart, liver, spleen
and lung were very low. The concentration of bacteria in the
SL/pShT-ePNP group was very high in tumors (≤7.8×107
cfu/ml) and the ratio of the concentration in the tumors to that in
the liver was ∼27,000:1 (Fig. 7A,
P<0.01). The RT-PCR results for tumors and organs in the
SL/pShT-ePNP group showed that expression of the ePNP gene was only
present in tumors; expression of the ePNP gene could not be
detected in other tissues (Fig.
7B). Pathological analyses of various important organs were
performed; no significant pathological changes were detected
(Fig. 7C). Neither
SL/pShT-ePNP-treated mice nor control mice had any abnormal results
for the blood tests or liver function tests (Table I), which indicates that the
SL/pShT-ePNP therapeutic system is safe and effective for tumor
treatment.
 | Table IBiochemical and hematological
analyses. |
Table I
Biochemical and hematological
analyses.
| Items | PBS | SL7207 | SL/pShT | SL/pIRES-ePNP | SL/pShT-ePNP |
|---|
| TP (g/l) | 57.55±3.89 | 55.68±3.55 | 57.20±4.02 | 56.72±3.80 | 57.23±4.12 |
| ALB (g/l) | 30.25±5.23 | 31.27±4.86 | 31.43±5.08 | 32.72±4.79 | 32.58±4.25 |
| GOT (U/l) | 41.3±21.7 | 42.5±20.5 | 42.8±21.6 | 43.8±22.7 | 40.0±20.7 |
| GPT (U/l) | 123.8±21.8 | 120.6±22.9 | 122.4±23.7 | 125.7±24.2 | 124.7±23.1 |
| GLU (mmol/l) | 3.65±0.42 | 3.72±0.45 | 3.53±0.48 | 3.74±0.56 | 3.76±0.49 |
| BUN (mmol/l) | 8.59±1.38 | 8.67±1.20 | 8.53±1.23 | 8.78±1.30 | 8.74±1.32 |
| CREA
(μmmol/l) | 38.42±4.37 | 38.64±4.68 | 39.52±4.88 | 38.77±5.99 | 37.56±4.60 |
| WBC
(109/l) | 8.42±2.96 | 11.37±3.50 | 12.03±4.05 | 13.20±3.46 | 12.47±4.63 |
| RBC
(109/l) | 7.30±0.78 | 7.18±0.63 | 6.89±0.56 | 7.02±0.74 | 7.20±0.67 |
| HGB (g/l) | 122.35±18.42 | 127.59±17.93 | 130.57±22.04 | 129.58±19.50 | 132.47±17.88 |
| PLT
(109/l) | 538.95±150.37 | 582.32±163.24 | 576.37±153.47 | 566.49±132.57 | 542.70±148.32 |
Discussion
To our knowledge, our study is the first to combine
a targeted virus replicon vector with attenuated S.
typhimurium for tumor treatment. We cloned the suicide gene
ePNP into the targeted alphavirus replicon-based vector pShT to
express the ePNP gene with high efficiency and enhanced target
specificity in tumor cells. When the prodrug F-Ado was added,
massive cytotoxicity was induced. Both in vitro and in
vivo experiments confirmed the efficiency and specificity of
this system.
Previous studies on suicide gene therapy for tumors
have often used the hTERT promoter to target the therapeutic effect
to tumor cells (19,20). However, all the tumor-specific
promoters had poor expression efficiency that limited the
expression of the suicide gene in tumor cells and affected the
efficacy of these therapies (5).
Therefore, alphavirus replicon was induced to improve the
expression efficacy of the hTERT promoter. We constructed the
alphavirus replicon-based vectors pShT-ePNP and pShT-GFP. However,
when we transfected the plasmid pShT-GFP using Lipofectamine 2000,
almost no fluorescence was seen after 48 h, which indicates very
low transfection efficiency. We speculated that the size of
pShT-GFP reaches 12 kb; therefore, conventional transfection agents
may not be able to transfect it into cells with sufficient
efficiency (21). Thus, we had to
find some other way to effectively solve this problem.
The anaerobic attenuated S. typhimurium
SL7207, which was engineered to knock out the aroA gene, grows in
clusters in tumor tissues and invades tumor cells (22,23).
However, these bacteria cannot grow in tumor cells for a long time
and when they eventually die within the cell, they release their
contents into the cell, including plasmids that can be transcribed
and expressed in the cell (24,25).
Therefore, we used electrotransformation to place pShT-GFP and
pShT-ePNP plasmids into attenuated S. typhimurium that would
then carry the plasmid into tumor cells (26). Theoretically, after the vector is
transferred into host cells, the hTERT promoter controls the
transcription of the alphavirus replicon, which is further
translated into a virus replicase complex. The exogenous genes
under transcriptional control by the 26S subpromoter combined with
replicase complex would be largely transcribed in the cytoplasm and
a large amount of double-stranded RNA would be produced (8,27).
Apoptosis of the host cell would then be induced in a short period
to eliminate the risk of long-term existence of DNA fragments in
cells and ensure safety (28).
In vitro and in vivo experiments showed that when
SL7207 was used as a vehicle, the pShT-GFP plasmid was effectively
transferred into tumor cells and the GFP expression level was
significantly higher in the SL/pShT-GFP group than in the
SL/pIRES-GFP group. Meanwhile, the control WI-38 cells showed
almost no GFP expression in vitro. Therefore, we believe
that the plasmid pShT could targetly express exogenous genes in
tumor cells and has higher levels of expression efficiency via
SL7207 compared with ordinary vectors.
RT-PCR results also confirmed that the ePNP gene was
expressed successfully in tumor cells in vitro. We found
that when the MOI was 10, the B16 cells viability was only 13.45%.
However, when we used SL/pShT-GFP to infect B16 cells under the
same conditions, flow cytometry showed that only 13.86% of the
cells had GFP expression (data not shown). Combining these 2
results, we speculate that because the ePNP/F-Ado system has a
powerful bystander effect, successful transfection of only a small
fraction of cells would be sufficient to induce massive cell death
(1,29). We also observed that as the
concentration of F-Ado and the treatment time increased, the
cytotoxicity of the ePNP/F-Ado system proportionally increased.
Additionally, HPLC results showed that F-Ado was effectively
converted and could induce significant cytotoxicity from the early
stage of treatment, possibly because the plasmid pShT expresses
ePNP in a burst to effectively convert F-Ado at the early stage.
These results show that the plasmid pShT-ePNP expresses ePNP
specifically in tumor cells and in comparison with conventional
CMV-promoter-based eukaryotic expression vectors, the expression
efficiency is highly increased.
Because F-dAdo has a relatively short half-life
(26), we chose to administer
F-dAdo 2 days after oral administration of recombinant bacteria.
F-dAdo was given 3 times a day for 3 days (18). At 4 days after oral administration
of recombinant bacteria, the bacterial count in tumor tissues was
∼27,000 times higher than that in the liver and other normal
tissues, which indicates that SL7207 tends to aggregate in tumor
tissues but still exists in normal tissues at a low concentration.
The bacteria effectively aggregated in tumor tissues; however,
because we applied the drug 2 days after oral administration of
recombinant bacteria, we cannot exclude the possibility that other
tissues were exposed to the cytotoxic effect. Therefore, to ensure
clinical safety, we used the tumor-specific
hTERT-promoter-containing pShT-ePNP plasmid (30). At the end of treatment, the safety
evaluation indicated that SL/pShT-ePNP is highly safe because of
its dual-level target specificity. The system effectively exerts a
therapeutic effect on tumors; in particular, it may be effective
for intratumoral sites that are not reachable by conventional
chemoradiotherapy.
We found that SL/pShT-ePNP was effective in
inhibiting tumor growth and prolonging the life of tumor-bearing
mice; H&E staining and apoptosis assays also showed that tumors
in the SL/pShT-ePNP group had significantly more necrosis and
apoptosis than that in the other groups. We therefore speculate
that the alphavirus replication enzyme compensated for the low
efficiency of expression by hTERT in the pShT-ePNP therapeutic
system. However, SL/pShT-ePNP did not cure the tumor despite its
good therapeutic effect. We speculate that clearance of tumors
relies on strong cytotoxic T lymphocyte responses that target tumor
cells (31,32). Therefore, in subsequent studies, we
intend to use highly immunogenic proteins (such as Mycobacterium
tuberculosis heat shock protein 70) or interleukin-12 to
enhance the clearance of tumor cells (33,34).
In conclusion, the recombinant live vaccines that we
designed combined the advantages of various therapeutic vaccines
reported in previous studies. We constructed a tumor-targeted
alphavirus replicon-based vector, pShT-ePNP, which highly expresses
exogenous genes and delivered this plasmid into tumors by using
attenuated S. typhimurium. In vitro and in vivo
experiments both confirmed the high efficiency and high specificity
of ePNP expression in tumor cells. When F-Ado was added, the
ePNP/F-Ado system exerted significant therapeutic effects with
respect to tumors. Our studies further confirmed a lack of adverse
effects in SL/pShT-ePNP treated mice. Thus, we established that
this vaccine is potentially useful for cancer therapy.
Acknowledgements
We gratefully thank every one of
Pathology Department and Central Laboratory, Xiamen Maternal and
Child Health Hospital for their sincere help. This study was
supported by The National Natural Science Foundation of China (no.
30471603/H1014).
References
|
1
|
Afshar S, Olafsen T, Wu AM and Morrison
SL: Characterization of an engineered human purine nucleoside
phosphorylase fused to an anti-her2/neu single chain Fv for use in
ADEPT. J Exp Clin Cancer Res. 28:1472009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Fukazawa T, Matsuoka J, Yamatsuji T, Maeda
Y, Durbin ML and Naomoto Y: Adenovirus-mediated cancer gene therapy
and virotherapy (Review). Int J Mol Med. 25:3–10. 2010.PubMed/NCBI
|
|
3
|
Gu J, Kagawa S, Takakura M, Kyo S, Inoue
M, Roth JA and Fang B: Tumor-specific transgene expression from the
human telomerase reverse transcriptase promoter enables targeting
of the therapeutic effects of the Bax gene to cancers. Cancer Res.
60:5359–5364. 2000.
|
|
4
|
Fakhoury J, Nimmo GA and Autexier C:
Harnessing telomerase in cancer therapeutics. Anticancer Agents Med
Chem. 7:475–483. 2007. View Article : Google Scholar
|
|
5
|
Song JS: Activity of the human telomerase
catalytic subunit (hTERT) gene promoter could be increased by the
SV40 enhancer. Biosci Biotechnol Biochem. 68:1634–1639. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Liljeström P and Garoff H: A new
generation of animal cell expression vectors based on the Semliki
Forest virus replicon. Biotechnology. 9:1356–1361. 1991.PubMed/NCBI
|
|
7
|
Diciommo DP and Bremner R: Rapid, high
level protein production using DNA-based Semliki Forest virus
vectors. J Biol Chem. 273:18060–18066. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kohno A, Emi N, Kasai M, Tanimoto M and
Saito H: Semliki Forest virus-based DNA expression vector:
transient protein production followed by cell death. Gene Ther.
5:415–418. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Quetglas JI, Ruiz-Guillen M, Aranda A,
Cassales E, Bezunartea J and Smerdou C: Alphavirus vectors for
cancer therapy. Virus Res. 153:179–196. 2010. View Article : Google Scholar
|
|
10
|
Wang WL, Xu HL, Lu RJ and Ruan L: A
comparative study on SFV-based DNA vaccine and the conventional DNA
vaccine. Chin J Virol. 18:325–331. 2002.
|
|
11
|
Juárez-Rodríguez MD, Arteaga-Cortés LT,
Kader R, Curtiss R III and Clark-Curtiss JE: Live attenuated
Salmonella vaccines against Mycobacterium
tuberculosis with antigen delivery via the type III secretion
system. Infect Immun. 80:798–814. 2012.
|
|
12
|
Hegazy WA and Hensel M: Salmonella
enterica as a vaccine carrier. Future Microbiol. 7:111–127.
2012. View Article : Google Scholar
|
|
13
|
Loessner H and Weiss S: Bacteria-mediated
DNA transfer in gene therapy and vaccination. Expert Opin Biol
Ther. 4:157–168. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Jarosz M, Jazowiecka-Rakus J, Cichoń T, et
al: Therapeutic antitumor potential of endoglin-based DNA vaccine
combined with immunomodulatory agents. Gene Ther. 12:1038–1046.
2012.PubMed/NCBI
|
|
15
|
Moreno M, Kramer MG, Yim L and Chabalgoity
JA: Salmonella as live Trojan horse for vaccine development and
cancer gene therapy. Curr Gene Ther. 10:56–76. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Sorscher EJ, Peng S, Bebok Z, Allan PW,
Bennett LL Jr and Parker WB: Tumor cell bystander killing in
colonic carcinoma utilizing the E. coli Deo D gene and generation
of toxic purines. Gene Ther. 1:233–238. 1994.PubMed/NCBI
|
|
17
|
Mosmann T: Rapid colorimetric assay for
cellular growth and survival: application to proliferation and
cytotoxicity assays. J Immunol Methods. 65:551983. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Parker WB, Allan PW, Hassan AE, Secrist JA
III, Sorscher EJ and Waud WR: Antitumor activity of
2-fluoro-2’-deoxyadenosine against tumors that express
Escherichia coli purine nucleoside phosphorylase. Cancer
Gene Ther. 10:23–29. 2003.
|
|
19
|
Wang W, Jin B, Li W, et al: Targeted
antitumor effect induced by hTERT promoter mediated ODC antisense
adenovirus. Mol Biol Rep. 37:3239–3247. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Hashimoto Y, Tazawa H, Teraishi F, et al:
The hTERT promoter enhances the antitumor activity of an oncolytic
adenovirus under a hypoxic microenvironment. PLoS One.
7:e392922012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhang J, Wild J, Bieler K and Graf M:
Comparative study of expression and humoral immunogenicity of HIV-1
group specific antigen Pr55∼(gag) by conventional plasmid vectors
versus semliki forest virus-derived vectors. Chin J Virol.
18:529–536. 2002.
|
|
22
|
Brown A, Hormaeche CE, Demarco de
Hormaeche R, Winther M, Dougan G, Maskell DJ and Stocker BA: An
attenuated aroA Salmonella typhimurium vaccine elicits
humoral and cellular immunity to cloned beta-galactosidase in mice.
J Infect Dis. 155:86–92. 1987.
|
|
23
|
Hoiseth SK and Stocker BA:
Aromatic-dependent Salmonella typhimurium are non-virulent
and effective as live vaccines. Nature. 291:238–239.
1981.PubMed/NCBI
|
|
24
|
Darji A, Guzman CA, Gerstel B, et al: Oral
somatic transgene vaccination using attenuated S.
typhimurium. Cell. 91:765–775. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zeng S, Zhang J, Zhang J, et al:
Suppression of murine melanoma growth by a vaccine of attenuated
Salmonella carrying heat shock protein 70 and Herpes simplex
virus-thymidine kinase genes. Oncol Rep. 27:798–806.
2012.PubMed/NCBI
|
|
26
|
Fu W, Lan H, Li S, Han X, Gao T and Ren D:
Synergistic antitumor efficacy of suicide/ePNP gene and
6-methylpurine 2′-deoxyriboside via Salmonella against murine
tumors. Cancer Gene Ther. 15:474–484. 2008.PubMed/NCBI
|
|
27
|
Leitner WW, Hwang LN, deVeer MJ, et al:
Alphavirus-based DNA vaccine breaks immunological tolerance by
activating innate antiviral pathways. Nat Med. 9:33–39. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Gil J, Alcami J and Esteban M: Induction
of apoptosis by double-stranded-RNA-dependent protein kinase (PKR)
involves the alpha subunit of eukaryotic translation initiation
factor 2 and NF-kappaB. Mol Cell Biol. 19:4653–4663. 1999.
|
|
29
|
Xie X, Guo J, Kong Y, et al: Targeted
expression of Escherichia coli purine nucleoside
phosphorylase and Fludara® for prostate cancer therapy.
J Gene Med. 13:680–691. 2011.PubMed/NCBI
|
|
30
|
Ueki H, Watanabe M, Kaku H, et al: A novel
gene expression system for detecting viable bladder cancer cells.
Int J Oncol. 41:135–140. 2012.PubMed/NCBI
|
|
31
|
Ambade AV, Joshi GV and Mulherkar R:
Effect of suicide gene therapy in combination with immunotherapy on
antitumour immune response and tumour regression in a xenograft
mouse model for head and neck squamous cell carcinoma. Indian J Med
Res. 132:415–422. 2010.PubMed/NCBI
|
|
32
|
Kuriyama S, Tsujinoue H and Yoshiji H:
Immune response to suicide gene therapy. Methods Mol Med.
90:353–369. 2004.PubMed/NCBI
|
|
33
|
Dong B, Sun L, Wu X, et al: Vaccination
with TCL plus MHSP65 induces anti-lung cancer immunity in mice.
Cancer Immunol Immunother. 59:899–908. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Yamazaki M, Straus FH, Messina M, et al:
Adenovirus-mediated tumor-specific combined gene therapy using
Herpes simplex virus thymidine/ganciclovir system and murine
interleukin-12 induces effective antitumor activity against
medullary thyroid carcinoma. Cancer Gene Ther. 11:8–15. 2004.
View Article : Google Scholar
|