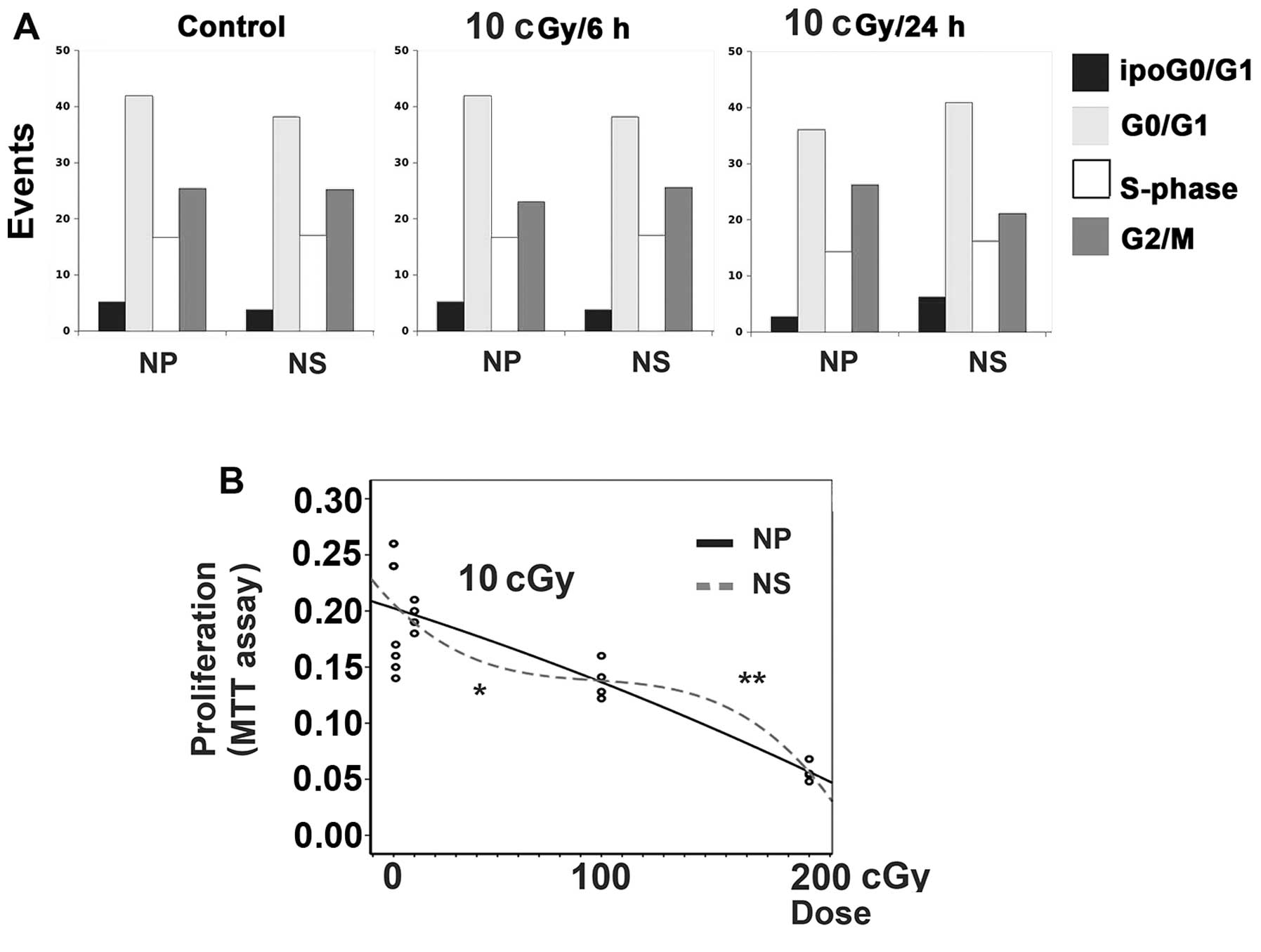
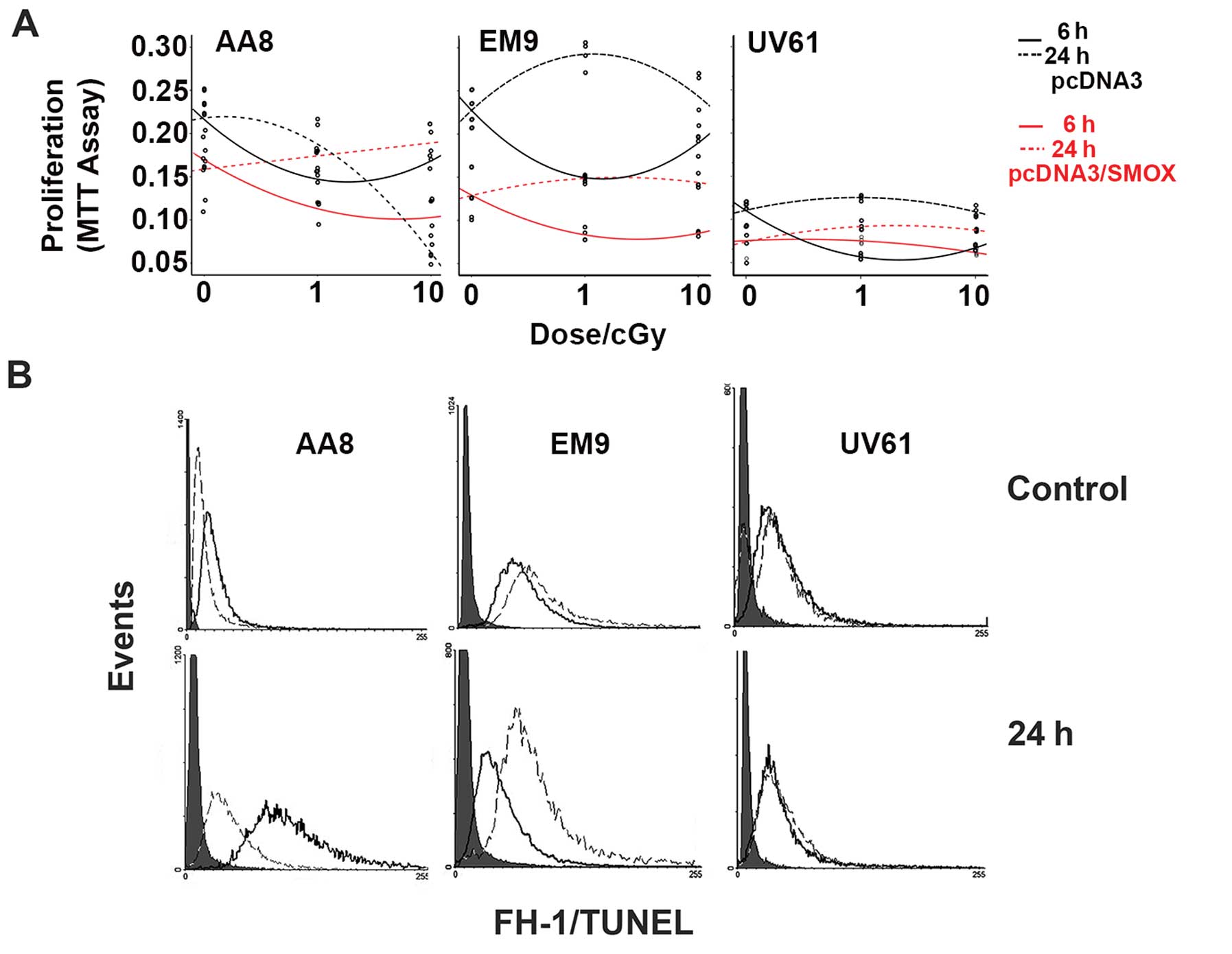
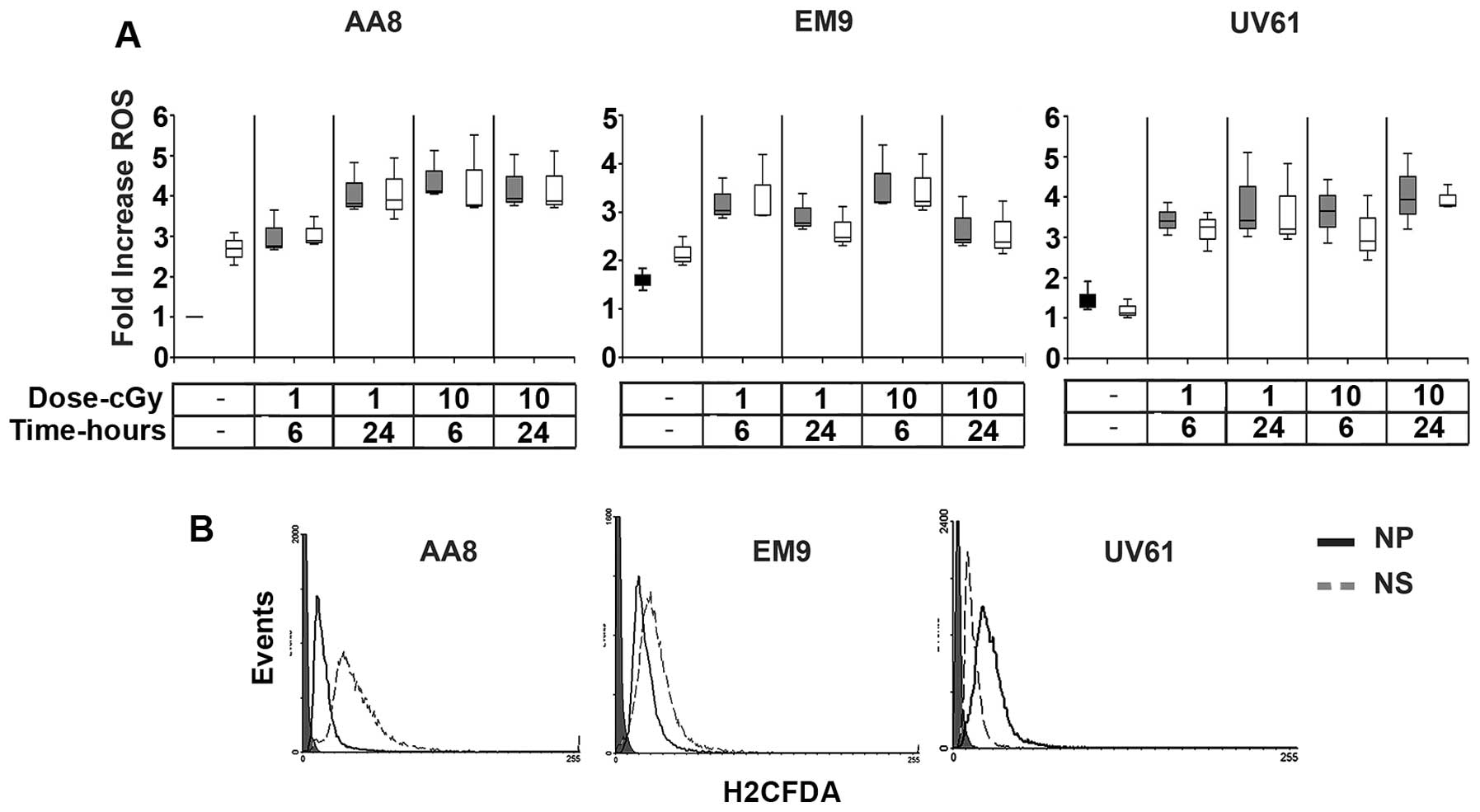
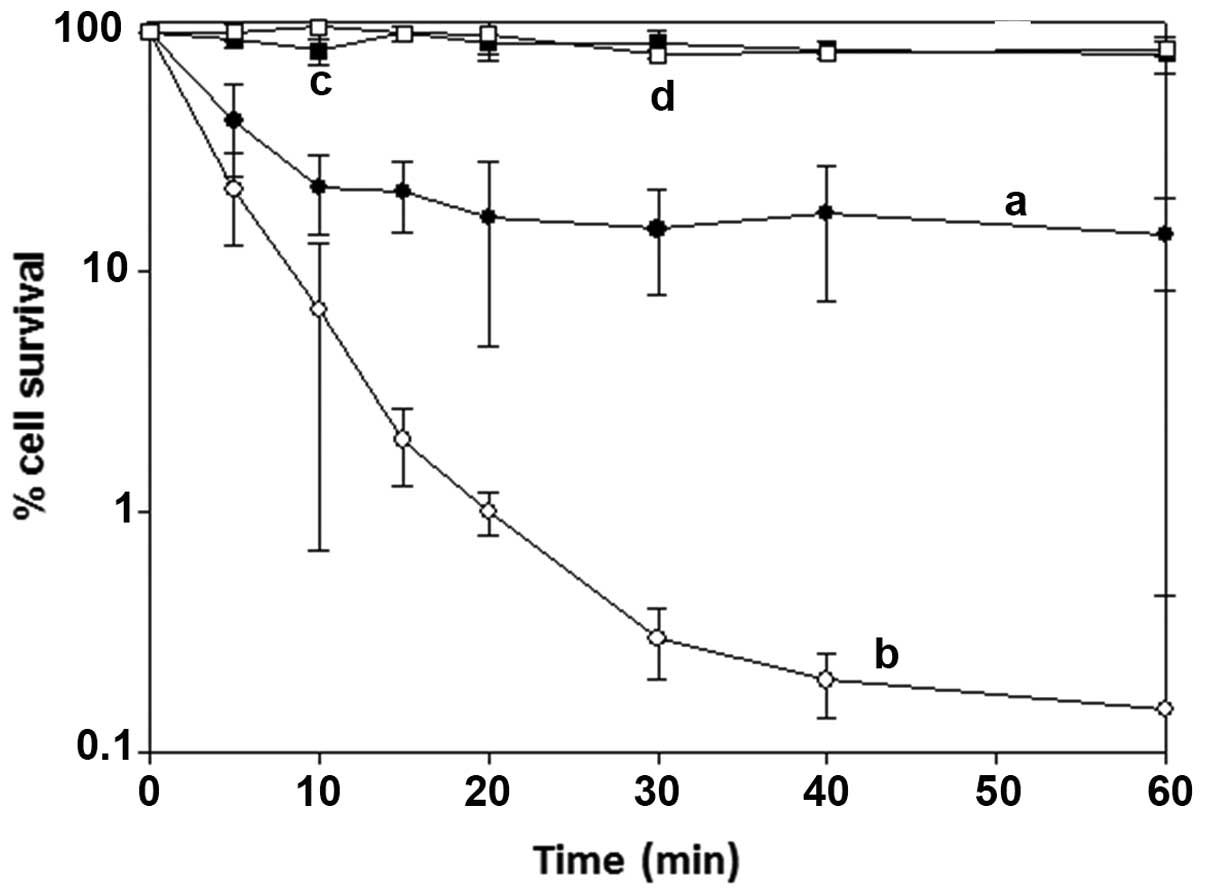
|
1
|
Ames BN, Shigenaga MK and Hagen TM:
Oxidants, antioxidants and the degenerative diseases of aging. Proc
Natl Acad Sci USA. 90:7915–7922. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Chance B, Sies H and Boveris A:
Hydroperoxide metabolism in mammalian organs. Physiol Rev.
59:527–605. 1979.PubMed/NCBI
|
|
3
|
Schumacker PT: Reactive oxygen species in
cancer cells: live by the sword, die by the sword. Cancer Cell.
10:175–176. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ghavami S, Eshragi M, Ande SR, et al:
S100A8/A9 induces autophagy and apoptosis via ROS-mediated
cross-talk between mitochondria and lysosomes that involves BNIP3.
Cell Res. 20:314–331. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wong CH, Iskandar KB, Yadav SK, Hirpara
JL, Loh T and Pervaiz S: Simultaneous induction of non-canonical
autophagy and apoptosis in cancer cells by ROS-dependent ERK and
JNK activation. PLoS One. 5:e99962010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kamata H, Honda S, Maeda S, et al:
Reactive oxygen species promote TNFalpha-induced death and
sustained JNK activation by inhibiting MAP kinase phosphatases.
Cell. 120:649–661. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wallace HM, Fraser AV and Hughes A: A
perspective of polyamine metabolism. Biochem J. 376:1–14. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Casero RA Jr and Marton LJ: Targeting
polyamine metabolism and function in cancer and other
hyperproliferative diseases. Nat Rev Drug Discov. 6:373–390. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ploszaj T, Motyl T, Zimowska W, Skierski J
and Zwierzchowski L: Inhibition of ornithine decarboxylase by
α-difluoromethylornithine induces apoptosis in HC11 mouse mammary
epithelial cells. Amino Acids. 19:483–496. 2000.
|
|
10
|
Seiler N and Raul F: Polyamines and
apoptosis. J Cell Mol Med. 9:623–642. 2005. View Article : Google Scholar
|
|
11
|
Amendola R, Cervelli M, Fratini E,
Polticelli F, Sallustio DE and Mariottini P: Spermine metabolism
and anticancer therapy. Cur Cancer Drug Targets. 9:118–130. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Calcabrini A, Arancia G, Marra M, et al:
Enzymatic oxidation products of spermine induce greater cytotoxic
effects on human multidrug-resistant colon carcinoma cells (LoVo)
than on their wild-type counterparts. Int J Cancer. 99:43–52. 2002.
View Article : Google Scholar
|
|
13
|
Arancia G, Calcabrini A, Marra M, et al:
Mitochondrial alterations induced by serum amine oxidase and
spermine on human multidrug resistant tumor cells. Amino Acids.
26:273–282. 2004. View Article : Google Scholar
|
|
14
|
Pledgie A, Huang Y, Hacker A, et al:
Spermine oxidase SMO(PAOh1), Not N1-acetylpolyamine oxidase PAO, is
the primary source of cytotoxic H2O2 in
polyamine analog-treated human breast cancer cell lines. J Biol
Chem. 280:39843–39851. 2005.PubMed/NCBI
|
|
15
|
Bianchi M, Bellini A, Cervelli M, et al:
Chronic sub-lethal oxidative stress by spermine oxidase over
activity induces continuous DNA repair and hypersensitivity to
radiation exposure. Biochim Biophys Acta. 1773:774–783. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Amendola R, Bellini A, Cervelli M, et al:
Direct oxidative DNA damage, apoptosis and radio sensitivity by
spermine oxidase activities in mouse neuroblastoma cells. Biochim
Biophys Acta Rev Cancer. 1775:15–24. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Agostinelli E, Arancia G, Dalla Vedova L,
et al: The biological functions of polyamine oxidation products by
amine oxidises: perspectives of clinical applications. Amino Acids.
27:347–358. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Agostinelli E, Dalla Vedova L, Belli F,
Condello M, Arancia G and Seiler N: Sensitization of human colon
adenocarcinoma cells (LoVo) to reactive oxygen species by
lysosomotropic compounds. Int J Oncol. 29:947–955. 2006.PubMed/NCBI
|
|
19
|
Agostinelli E, Condello M, Molinari A,
Tempera G, Viceconte N and Arancia G: Cytotoxicity of spermine
oxidation products to multidrug resistant melanoma cells (M14
ADR2): sensitisation by MDL 72527, a lysosomotropic compound. Int J
Oncol. 35:485–498. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Soares FA, Shaugnessy SG, MacLarkey WR and
Orr FW: Quantification and morphologic demonstration of reactive
oxygen species produced by Walker 256 tumor cells in vitro and
during metastasis in vivo. Lab Invest. 71:480–489. 1994.PubMed/NCBI
|
|
21
|
Thompson LH, Brookman KW, Dillehay LE, et
al: A CHO-cell strain having hypersensitivity to mutagens, a defect
in DNA strand-break repair and an extraordinary baseline frequency
of sister-chromatid exchange. Mutat Res. 95:427–440. 1982.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Thompson LH, Salazar EP, Brookman KW, et
al: Recent progress with the DNA repair mutants of Chinese hamster
ovary cells. J Cell Sci. (Suppl 6): 97–110. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Pelicano H, Carney D and Huang P: ROS
stress in cancer cells and therapeutic implication. Drug Resist
Updat. 7:97–110. 2004. View Article : Google Scholar
|
|
24
|
Grandi M, Geroni C and Giuliani FC:
Isolation and characterization of a human colon adenocarcinoma cell
line resistant to doxorubicin. Br J Cancer. 54:515–518. 1986.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Turini P, Sabatini S, Befani O, et al:
Purification of serum amine oxidase. Anal Biochem. 125:294–298.
1982. View Article : Google Scholar
|
|
26
|
Bahn S, Mimmack M, Ryan M, et al: Neuronal
target genes of the neuron-restrictive silencer factor in
neurospheres derived from fetuses with Down’s syndrome: a gene
expression study. Lancet. 359:310–315. 2002.PubMed/NCBI
|
|
27
|
Sharmin S, Sakata K, Kashiwagi K, et al:
Polyamine cytotoxicity in the presence of bovine serum amine
oxidase. Biochem Biophys Res Commun. 282:228–235. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Regulus P, Duroux B, Bayle PA, et al:
Oxidation of the sugar moiety of DNA by ionizing radiation or
bleomycin could induce the formation of a cluster DNA lesion. Proc
Natl Acad Sci USA. 104:14032–14037. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Eccles LJ, Lomax ME and O’Neill P:
Hierarchy of lesion processing governs the repair, double-strand
break formation and mutability of three-lesion clustered DNA
damage. Nucleic Acids Res. 38:1123–1134. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Hitomi K, Iwai S and Tainer JA: The
intricate structural chemistry of base excision repair machinery:
implications for DNA damage recognition, removal and repair. DNA
Repair. 6:410–428. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Zharkov DO: Base excision DNA repair. Cell
Mol Life Sci. 65:1544–1565. 2008. View Article : Google Scholar
|
|
32
|
Maxwell CA, Fleisch MC, Sylvain V, et al:
Targeted and nontargeted effects of ionizing radiation that impact
genomic instability. Cancer Res. 68:8304–8831. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Agostinelli E, Belli F, Molinari A, et al:
Toxicity of enzymatic oxidation products of spermine to human
melanoma cells (M14): sensitization by heat and MDL 72527. Biochim
Biophys Acta. 1763:1040–1050. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Agostinelli E and Seiler N: Lysosomotropic
compounds and spermine enzymatic oxidation products in cancer
therapy (Review). Int J Oncol. 31:473–484. 2007.PubMed/NCBI
|
|
35
|
Agostinelli E and Seiler N:
Non-irradiation-derived reactive oxygen species (ROS) and cancer.
Therapeutic implications. Amino Acids. 31:341–355. 2006. View Article : Google Scholar : PubMed/NCBI
|