Introduction
Cancer is a major public health problem worldwide.
Epidemiologic and animal studies indicate that vegetables and
fruits with chemopreventive natural products, alone or in
combination with others, are associated with a reduced risk of
cancer development (1,2). For more than 15 years, we have been
screening natural phytochemical products in vegetables and fruits
for inhibitors of DNA metabolic enzymes, primarily mammalian DNA
polymerases (pols) and human DNA topoisomerases (topos).
Pols (DNA-dependent DNA polymerases, E.C. 2.7.7.7)
catalyze deoxyribonucleotide addition to the 3′-hydroxyl terminus
of primed double-stranded DNA (dsDNA) molecules (3). The human genome encodes at least 15
pols that function in cellular DNA synthesis (4,5).
Eukaryotic cells contain 3 replicative pols (α, δ and ɛ), one
mitochondrial pol (γ), and at least 11 non-replicative pols [β, ζ,
η, θ, ι, κ, λ, μ, ν, terminal deoxynucleotidyl transferase (TdT)
and REV1] (6,7). Pols have a highly conserved structure
and their overall catalytic subunits show little variance among
species. Conserved enzyme structures are usually preserved over
time because they perform important cellular functions that confer
evolutionary advantages. On the basis of sequence homology,
eukaryotic pols can be divided into 4 main families: A, B, X and Y
(6). Family A includes
mitochondrial pol γ as well as pols θ and ν. Family B includes pol
ζ and the 3 replicative pols α, δ and ɛ. Family X is comprised of
TdT and pols β, λ and μ. Family Y includes pols η, ι and κ in
addition to REV1.
Topos are nuclear enzymes that alter the DNA
topology required for the replication, transcription,
recombination, and segregation of daughter chromosomes (8). Eukaryotic cells have 2 types of
topos, I and II. Topo I catalyzes the passage of the DNA strand
through a transient single-strand break in the absence of any
high-energy cofactor. Topo II, in contrast, catalyzes the passage
of DNA double strands through a transient double-strand break in
the presence of ATP.
Selective inhibitors of pols and topos are
considered potentially useful anticancer, antiviral, antiparasitic
and antipregnancy agents because some are known to suppress human
cancer and normal cell proliferation and are cytotoxic (9–11).
We screened soybean isoflavones for these inhibitors. Legumes,
particularly soybeans, are the richest sources of isoflavones in
the human diet. Studies of soy isoflavones in populations that
regularly consume soy protein indicate that such populations have a
relatively low incidence of breast cancer and other common cancers.
This is because soy protein influences sex hormone metabolism and
biological activity through intracellular enzymes, protein
synthesis, growth factor actions, malignant cell proliferation,
cell differentiation and angiogenesis (12). Soy isoflavones also have some
important health-enhancing properties such as prevention of certain
cancers (13), lowering the risk
of cardiovascular diseases (14)
and improvement of bone health (15). In soybeans, isoflavones are present
as glycosides (bound to a sugar molecule). Fermentation or
digestion of soybeans or soy products results in the release of the
sugar molecule from the isoflavone glycoside, leaving an isoflavone
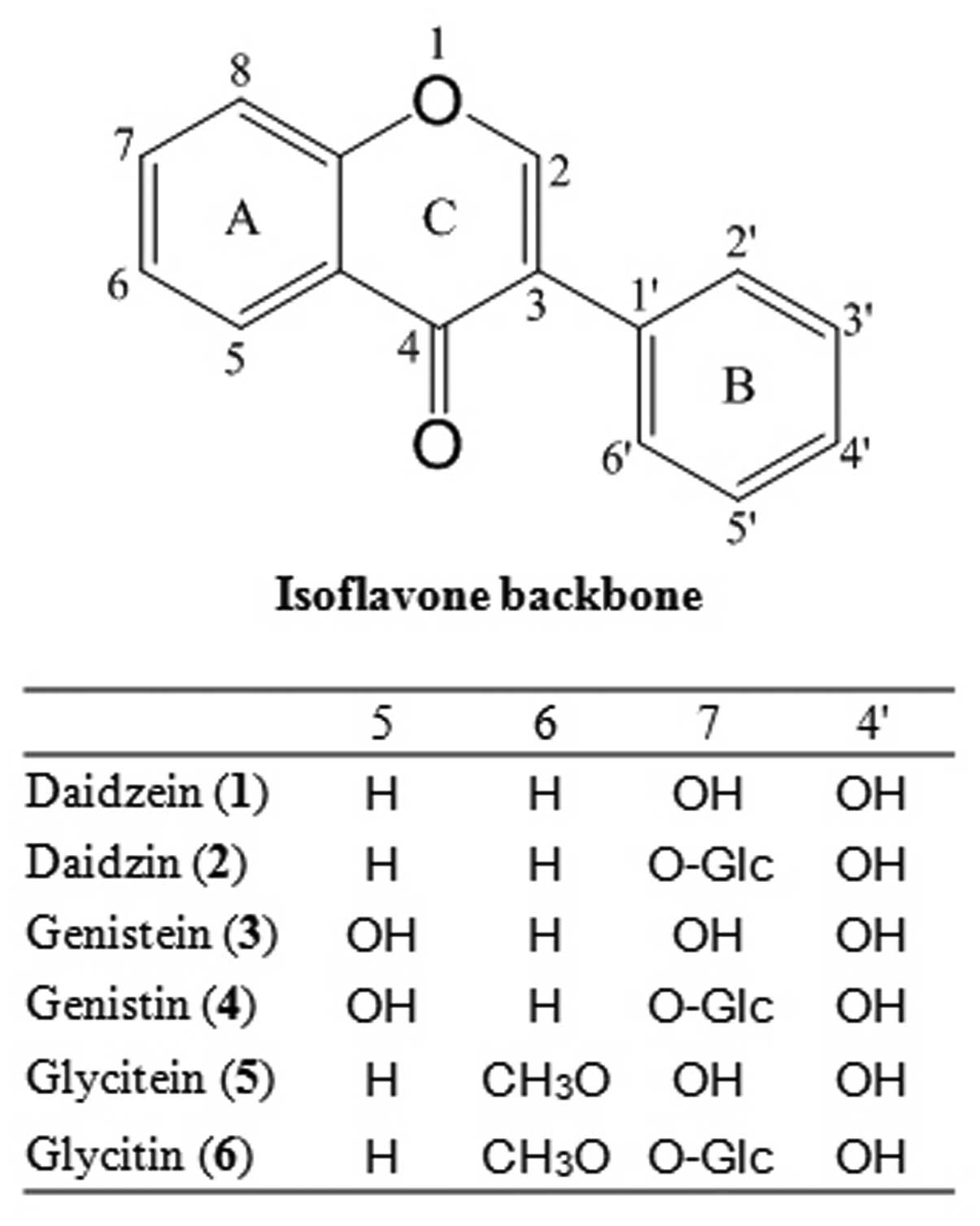
aglycone. The primary aglycones of soy isoflavones are daidzein,
genistein and glycitein, while the isoflavone glycosides are
daidzin, genistin and glycitin (Fig.
1).
The purpose of this study was to find novel
bioactivities of these 6 soy isoflavones. We investigated whether
these compounds inhibit DNA metabolic enzymes such as pols and
topos, or cellular proliferation processes such as DNA replication
of human large intestine cancer cells (HCT116). It is possible that
soy isoflavones have anticancer activity.
Materials and methods
Materials
Six soy isoflavones, daidzein (1), daidzin (2),
genistein (3), genistin (4), glycitein (5) and glycitin (6), were
purchased from Sigma-Aldrich Inc. (St. Louis, MO, USA; Fig. 1). The compounds, purified using
HPLC, were of analytical grade. A chemically synthesized DNA
template, poly(dA), was purchased from Sigma-Aldrich Inc. and a
customized oligo(dT)18 DNA primer was produced by
Sigma-Aldrich Japan K.K. (Hokkaido, Japan). Radioactive nucleotide
[3H]-labeled 2′-deoxythymidine-5′-triphosphate (dTTP; 43
Ci/mmol) was obtained from Moravek Biochemicals Inc. (Brea, CA,
USA). Supercoiled pBR322 plasmid dsDNA was obtained from Takara Bio
Inc. (Kyoto, Japan). All other reagents were analytical grade and
were obtained from Nacalai Tesque Inc. (Kyoto, Japan).
Enzymes
Pol α was purified from calf thymus by
immuno-affinity column chromatography, as described by Tamai et
al (16). Recombinant rat pol
β was purified from Escherichia coli JMpβ5, as described by
Date et al (17). Human pol
γ catalytic gene was cloned into pFastBac. The histidine-tagged
enzyme was expressed using the BACTO-BAC HT Baculovirus Expression
System, according to the supplier’s instructions (Life
Technologies, Frederick, MD) and was purified using ProBond resin
(Invitrogen Japan, Tokyo, Japan) (18). Human pols δ and ɛ were purified by
nuclear fractionation of human peripheral blood cancer cells
(Molt-4) using the second subunit of pol δ and ɛ-conjugated
affinity column chromatography, respectively (19). A truncated form of human pol η
(residues 1–511) tagged with His6 at its C-terminal was expressed
in E. coli cells and was purified as described by Kusumoto
et al (20). A recombinant
mouse pol ι that was tagged with His6 at its C-terminal was
expressed by E. coli and purified by Ni-NTA column
chromatography (unpublished data). A truncated form of pol κ
(residues 1–560) with 6X His-tags attached at the C-terminus was
overproduced in E. coli and purified as described by Ohashi
et al (21). Recombinant
human His-pol λ was overexpressed in E. coli and purified
according to a method described by Shimazaki et al (22). Recombinant human His-pol μ was
overexpressed in E. coli BL21 and purified using Glutathione
Sepharose™ 4B (GE Healthcare Bio-Science Corp., Piscataway, NJ,
USA) column chromatography using the same method as for pol λ
(22). Calf TdT, T7 RNA
polymerase, T4 polynucleotide kinase, and Bovine pancreas
deoxyribonuclease I were purchased from Takara Bio Inc. (Kyoto,
Japan). Purified human placenta topos I and II were purchased from
TopoGen Inc. (Columbus, OH, USA).
Measurement of pol activity
Reaction mixtures for calf pol α and rat pol β have
been described previously (23,24);
those for pol γ and for pols δ and ɛ were as described by Umeda
et al (18) and Ogawa et
al (25), respectively.
Reaction mixtures for pols η, ι and κ were the same as for pol α
and those for pols λ, μ and TdT were the same as for pol β. For the
pol reactions, poly(dA)/oligo(dT)18 (A/T, 2/1) and dTTP
were used as the DNA template-primer substrate and nucleotide
(dNTP; 2′-deoxynucleoside-5′-triphosphate) substrate, respectively.
For TdT reactions, oligo(dT)18 (3′-OH) and dTTP were
used as the DNA primer substrate and nucleotide substrate,
respectively.
Soy isoflavone compounds 1–6 were dissolved in
distilled dimethyl sulfoxide (DMSO) to various concentrations and
sonicated for 30 sec. Then, 4 μl aliquots were mixed with 16
μl of each enzyme (0.05 units) in 50 mM Tris-HCl at pH 7.5
that contained 1 mM dithiothreitol, 50% glycerol (by vol), and 0.1
mM ethylenediaminetetraacetic acid (EDTA). The mixtures were
maintained at 0°C for 10 min. Next, 8 μl of each
inhibitor-enzyme mixture was added to 16 μl of enzyme
standard reaction mixture and incubated at 37°C for 60 min. The
activity in samples without inhibitors was considered to be 100%
and the activity was determined for each inhibitor concentration
relative to the uninhibited activity. One unit of pol activity was
defined as the amount of each enzyme that catalyzed the
incorporation of 1 nmol dTTP into synthetic DNA template-primers in
60 min at 37°C and under normal reaction conditions (23,24).
Measurement of topo activity
The catalytic activity of topo I was determined by
detecting supercoiled plasmid DNA (form I) in its nicked form (form
II) (26). The topo I reaction was
performed in a 20 μl reaction mixture that contained 10 mM
Tris-HCl (pH 7.9), pBR322 DNA (250 ng), 1 mM EDTA, 150 mM NaCl,
0.1% bovine serum albumin (BSA), 0.l mM spermidine, 5% glycerol, 2
μl of one of the 6 test compounds 1–6 dissolved in DMSO, and
2 units of topo I. The catalytic activity of topo II was analyzed
in the same manner, except the reaction mixture contained 50 mM
Tris-HCl (pH 8.0), 120 mM KCl, 10 mM MgCl2, 0.5 mM ATP,
0.5 mM dithiothreitol, supercoiled pBR322 DNA (250 ng), and 2 units
of topo II (26). The reaction
mixtures were incubated at 37°C for 30 min, followed by digestion
with 1% sodium dodecyl sulfate (SDS) and 1 mg/ml proteinase K.
After digestion, 2 μl loading buffer, consisting of 5%
sarkosyl, 0.0025% bromophenol blue, and 25% glycerol, was added. To
study the binding of enzymes to DNA based on mobility shifts, the
same procedure was followed, but SDS denaturation and proteinase K
digestion were omitted. The mixtures were subjected to 1% agarose
gel electrophoresis in Tris/borate/EDTA buffer. Agarose gel was
stained with ethidium bromide (EtBr) and the DNA band shifts from
form I to form II by topos I and II were detected using an enhanced
chemiluminescence detection system (Perkin-Elmer Life Sciences
Inc., Waltham, MA, USA). Zero-D scan (Version 1.0, M & S
Instruments Trading Inc., Osaka, Japan) was used for densitometric
quantitation.
Other enzyme assays
Standard assays were used according to the
manufacturer’s instructions to measure the activities of T7 RNA
polymerase, mouse IMP dehydrogenase (type II), T4 polynucleotide
kinase, and bovine deoxyribonuclease I, as described by Nakayama
and Saneyoshi (27), Mizushina
et al (28), Soltis and
Uhlenbeck (29) and Lu and
Sakaguchi (30), respectively.
Thermal transition of DNA
Thermal profiles of the transition of dsDNA to
single-stranded DNA (ssDNA) with or without genistein were obtained
using a spectrophotometer (UV-2500, Shimadzu Corp., Kyoto, Japan)
equipped with a thermoelectric cell holder, as described previously
(31). Calf thymus DNA (6
μg/ml) was dissolved in 0.1 M sodium phosphate buffer (pH
7.0) that contained 1% DMSO. The solution temperature was
equilibrated at 75°C for 10 min, and then increased by 1°C at 2-min
intervals for each measurement point. Any change in the absorbance
of the compound at each temperature point was automatically
subtracted from that of the combined absorbance of the DNA and the
compound by the spectrophotometer.
Cell culture and measurement of cancer
cell viability
A human colon carcinoma cell line, HCT116, was
obtained from the American Type Culture Collection (Manassas, VA,
USA). HCT116 cells were cultured in McCoy’s 5A medium supplemented
with 10% fetal bovine serum, penicillin (100 U/ml), and
streptomycin (100 μg/ml) at 37°C in a humid atmosphere of 5%
CO2/95% air. For the cell viability assay, cells were
plated at 1×104 into each well of a 96-well microplate
with various concentrations of genistein. Cell viability was
determined by the WST-1 assay (32).
Cell cycle analysis
Cellular DNA content for cell cycle analysis was
determined as follows: aliquots of 3×105 HCT116 cells
were added to a 35-mm dish and incubated with a medium that
contained genistein [94.0 μM based on the 50% lethal dose
(LD50) value] for 24 h. Cells were then washed with
ice-cold PBS 3 times by centrifugation, fixed with 70% (v/v)
ethanol, and stored at −20°C. DNA was stained with PI
(3,8-diamino-5-[3-(diethylmethylammonio)propyl]-6-phenylphenanthridinium
diiodide) staining solution for at least 10 min at room temperature
in the dark. Intensity of fluorescence was measured using a
FACSCanto flow cytometer in combination with FACSDiVa software
(Becton-Dickinson Co., Franklin Lakes, NJ, USA).
Computational analysis
The molecular structures of compounds were
constructed using Discovery Studio (DS) 3.5 modeling software
(Accelrys Inc., San Diego, CA, USA). Energy minimization was
achieved using a salvation model and was calculated by the GBSW
(Generalized Born with simple switching) parameter using the
Minimization and Dynamics protocols contained within DS. The
calculation used a CHARMm (Chemistry at HARvard Macromolecular
Mechanics) force-field.
Results
Effect of soy isoflavones on the activity
of mammalian pols
The inhibitory activity of each soy isoflavone
toward mammalian pols was investigated using calf pol α, rat pol β,
human pol γ, and human pol κ. Pols α, β, γ and κ were used as
representatives of the B, X, A, and Y families of pols,
respectively (6,7). Assessment of the relative activity of
each pol at a set concentration (100 μM) of the 6 soy
isoflavones showed that none of the compounds had any effect on pol
inhibition, as no compound resulted in <90% relative activity of
the 4 pols (Fig. 2). These results
suggest that soy isoflavones do not influence the activities of
mammalian pol species. When activated DNA (bovine deoxyribonuclease
I-treated DNA) was used as the DNA template-primer substrate
instead of synthesized DNA [poly(dA)/oligo(dT)18
(A/T=2/1)] and dNTP was used as the nucleotide substrate instead of
dTTP, the inhibitory effects of these compounds did not change
(data not shown).
Effects of soy isoflavones on the
activity of human topos I and II
The inhibitory effects of each soy isoflavone were
examined against human topos I and II, which have ssDNA and dsDNA
nicking activity, respectively (8). None of the soy isoflavones at 100
μM influenced topo I nicking activity (Fig. 3). Even at concentration of greater
than 100 μM, these compounds had no effect on topo I
activity (data not shown). In contrast, 100 μM of genistein
(6) completely inhibited the
nicking activity of topo II, while the other compounds inhibited
topo II to a lesser extent or not at all (Fig. 3). These results suggest that
genistein is a potent human topo II inhibitor, but there are no
topo I inhibitors among the 6 soy isoflavones tested. Genistein was
therefore selected for further study.
Effects of genistein on the activity of
mammalian pols, topos and other DNA metabolic enzymes
Genistein did not affect the activity of any of the
eleven mammalian pol species tested in vitro (Table I). Genistein inhibited the activity
of human topo II with a 50% inhibitory concentration
(IC50) value of 37.5 μM (Table I).
 | Table I.IC50 values of genistein
for the activity of mammalian pols, topos and other DNA metabolic
enzymes. |
Table I.
IC50 values of genistein
for the activity of mammalian pols, topos and other DNA metabolic
enzymes.
| Enzymes | IC50
(μM) |
|---|
| Mammalian pols | |
| (A-family) | |
| Human pol
γ | >200 |
| (B-family) | |
| Calf pol α | >200 |
| Human pol
δ | >200 |
| Human pol
ɛ | >200 |
| (X-family) | |
| Rat pol β | >200 |
| Human pol
λ | >200 |
| Human pol
μ | >200 |
| Calf TdT | >200 |
| (Y-family) | |
| Human pol
η | >200 |
| Mouse pol
ι | >200 |
| Human pol
κ | >200 |
| Mammalian
topos | |
| Human topo I | >200 |
| Human topo
II | 37.5±2.5 |
| Other DNA metabolic
enzymes | |
| T7 RNA
polymerase | >200 |
| Mouse IMP
dehydrogenase (type II) | >200 |
| T4 polynucleotide
kinase | >200 |
| Bovine
deoxyribonuclease I | >200 |
Genistein had no influence on the activity of other
DNA metabolic enzymes such as T7 RNA polymerase, mouse IMP
dehydrogenase (type II), T4 polynucleotide kinase, and bovine
deoxyribonuclease I (Table I).
These results indicate that genistein should be specifically
classified as an inhibitor of human topo II.
Influence of genistein on the
hyperchromicity of dsDNA
Specific assays were performed to determine whether
genistein-induced inhibition resulted from the ability of the
compound to bind to DNA or to the enzyme. The interaction of
genistein with dsDNA was investigated by studying its thermal
transition. To accomplish this, the melting temperature (Tm) of
dsDNA in the presence of an excess of genistein (200 μM) was
measured using a spectrophotometer equipped with a thermoelectric
cell holder. As shown in Fig. 4,
when a typical intercalating compound, EtBr (15 μM), was
used as a positive control, a clear thermal transition (i.e., Tm)
was observed from 75 to 90°C. However, no such thermal transition
was observed when genistein was heated with dsDNA.
The question of whether the inhibitory effect of
genistein resulted from non-specific adhesion to human topo II or
from its selective binding to specific sites was investigated by
determining whether an excessive amount of nucleic acid [poly(rC)]
or protein (BSA) prevented the inhibitory effect of genistein.
Poly(rC) and BSA had little or no influence on the inhibition of
topo II by genistein (data not shown), suggesting that this
compound selectively bound to the topo II enzyme molecule. These
observations indicate that genistein does not act as a DNA
intercalating agent or as a template-primer substrate. Instead,
this compound binds directly to topo II and inhibits its
activity.
Collectively, these results suggest that genistein
may be a potent and specific inhibitor of human topo II. We
therefore investigated in more detail whether topo II inhibition by
genistein results in decreased human cancer cell proliferation.
Effect of genistein on cultured human
cancer cells
Topos have recently emerged as important cellular
targets for chemical intervention in the development of anticancer
agents. Genistein could therefore be useful in chemotherapy, and
thus, we investigated the cytotoxic effect of this compound against
the HCT116 human colon carcinoma cultured cell line. As shown in
Fig. 5A, 24 h of treatment with
genistein treatment suppressed HCT116 cell growth in a
dose-dependent manner, with a 50% lethal dose (LD50) of
94.0 μM. This LD50 is 2.5-fold higher than the
IC50 for topo II. This suggests that genistein may be able to
penetrate the cell membrane and reach the nucleus, where it may
inhibit the activity of topo II, which leads to suppression of cell
growth.
Next, we analyzed whether genistein affected the
cell cycle distribution of compound-treated HCT116 cells. The cell
cycle fraction was recorded after 24 h of treatment with a
concentration of genistein equal to its LD50. The ratio
of cells in each of 3 phases (i.e., G1, S, and G2/M) in the cell
cycle is shown in Fig. 5B.
Treatment with genistein significantly increased the population of
cells in the G2/M phase (2.57-fold increase of cells in G2/M
phases), did not significantly change the proportion of cells in
the G1 phase, and greatly decreased the percentage of cells in the
S phase. Etoposide, which is a classic topo II inhibitor, arrested
the cell cycle in the G2/M phase (1.80-fold increase of cells in
G2/M phase, data not shown). These results suggest that genistein
may be an effective inhibitor of topo II and halts the cell cycle
at the G2/M phase.
Discussion
Soybeans contain the highest concentrations of
isoflavones, at 1–3 mg/g, such as daidzein, genistein, glycitein
and their corresponding glycosides, such as daidzin, genistin and
glycitin, respectively, of foods consumed by humans. According to
USDA data (33), total soybean
isoflavones consist of 37% daidzein, 57% genistein and 6%
glycitein; therefore, the main component of soy isoflavone is
genistein. When ingested as part of the diet, genistin is readily
converted to its aglycone form, genistein. Genistin is hydrolyzed
by removal of the covalently bound glucose to form genistein.
Genistein is the form of the compound that is absorbed in the
intestine and is responsible for the biological activity of the
isoflavone. It was first demonstrated in 2002 that gut microflora
play a large role in the conversion of genistin to genistein
(34). It was later found that
enzymes present in the human small intestine and liver also have
the ability to convert the isoflavone. Hydrolysis starts very
quickly in the digestive system once genistin is ingested.
Conversion begins in the mouth and continues in the small
intestine. Moreover, both human saliva and intestinal cell-free
extract from mice can completely convert genistin to genistein
(34).
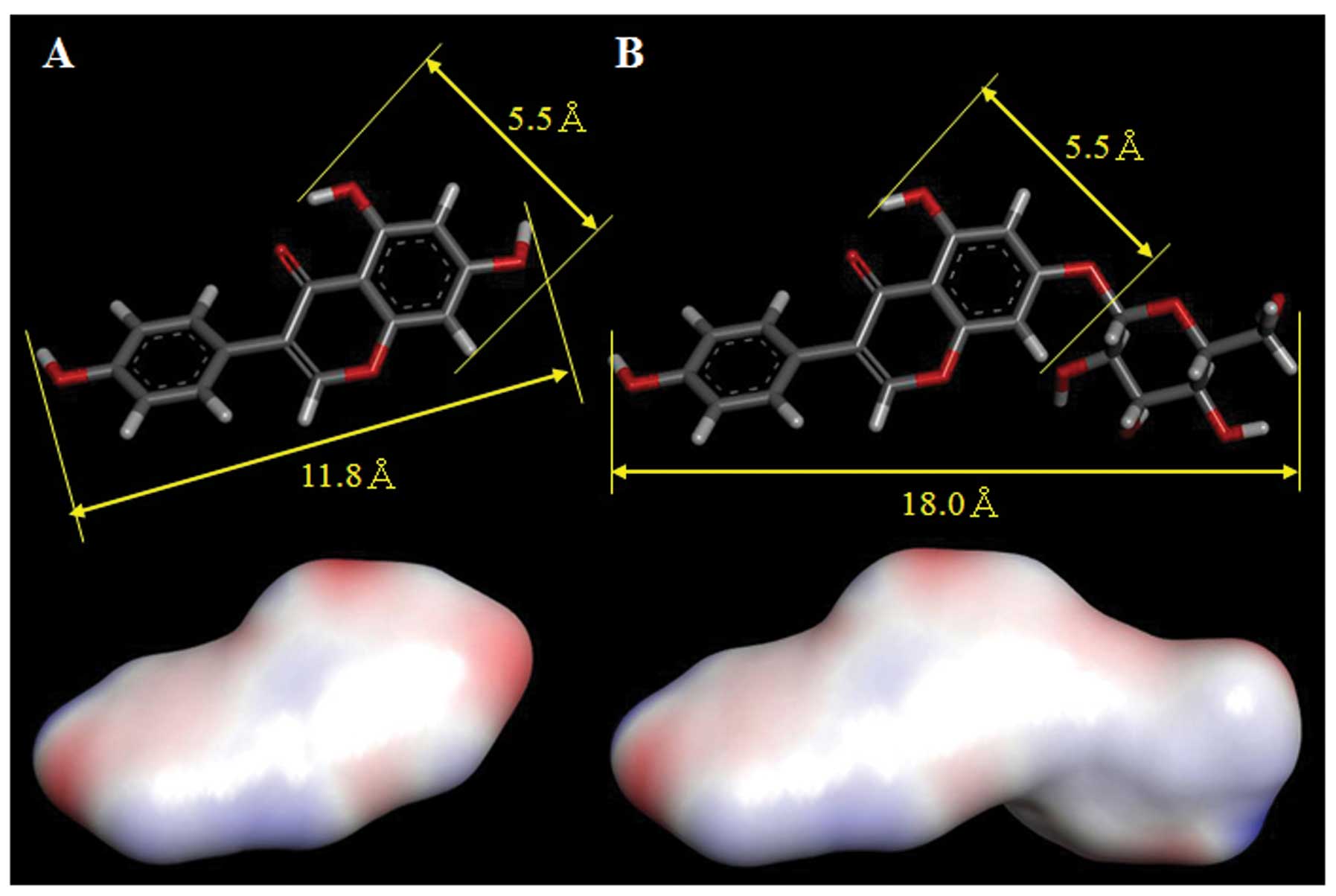
The three-dimensional structure of genistein and
genistin, from which the energy-minimized compounds were
calculated, were compared. The molecular length and width of these
compounds are indicated in the upper panels of Fig. 6. The width of genistein is the same
as that of genistin, but the length of genistein and genistin are
11.8 and 16.6 Å, respectively, a 1.4-fold difference. There is
likely an inhibitor binding pocket on the topo II protein surface,
and the width and length of this pocket might be approximately 5.5
and 11.8 Å, respectively, to accommodate genistein. The calculated
log P (Clog P) values (partition coefficients for octanol/water) of
genistein and genistin are different (Clog P=3.114±1.137 for
genistein and Clog P=0.942±0.912 for genistin), but these compounds
have nearly the same pKa (acid dissociation constant; pKa=6.51±0.20
for genistein and pKa=6.12±0.20 for genistin). The molecular
length, width, and hydrophobicity (Clog P and surface area of the
functional group negative/positive charges; the lower panels of
Fig. 6) of these compounds are
likely important for their bioactivity. Genistin is the
7-O-β-D-glucoside form of genistein and the conjugated glycoside
has a molecular length and hydrophobicity that are different from
that of the aglycone. Therefore, the aglycone structure (without a
sugar) must be important for topo II inhibition. The hydroxyl group
in 5-position of B ring of the isoflavone backbone is considered to
be the essential structural moiety of genistein
(4′,5,7-trihydroxyisoflavone) that is responsible for the observed
activity. This is because the other soy isoflavone aglycones,
daidzein (4′,7-dihydroxyisoflavone) and glycitein
(4′,7-dihydroxy-6-methoxyisoflavone) lack the hydroxyl group had no
inhibitory effect of topo II activity (Figs. 1 and 3).
Topo II inhibitors such as adriamycin, amsacrine,
ellipticine, saintopin, streptonigrin and terpentecin are
inter-calating agents that are thought to bind to the DNA molecule
directly and subsequently inhibit topo II activity indirectly.
These chemicals inhibit the DNA chain-rejoining reactions that are
catalyzed by topo II by stabilizing a tight topo II protein-DNA
complex called the ‘cleavable complex’. The possibility that
genistein also binds to DNA was examined by measuring the Tm of
dsDNA and no genistein was found to bind to dsDNA (Fig. 4). Thus, genistein must have
inhibited enzyme activity by interacting directly with the enzyme.
Topo inhibitors are categorized into 2 classes, ‘suppressors’,
which are believed to interact directly with the enzyme, and
‘poisons’, which stimulate DNA cleavage and intercalation (35). Genistein may be considered a
‘suppressor’ of topo functions rather than a conventional poison as
this compound does not appear to stabilize topo II protein-DNA
covalent complexes. Genistein may therefore be a new type of topo
II inhibitor.
Genistein, a major component of soy isoflavones, has
many physiological actions such as estrogen action, anti-oxidation,
mutation prevention, anti-infection, and the prevention
rehabilitation of heart-cerebrovascular disorders (36–38).
This suggests that genistein might be useful for health care
applications. In this study, we found that genistein causes human
cancer cell cytotoxicity by arresting the cell cycle at the G2/M
phase, and that it acts via the inhibition of topo II. Therefore,
soy isoflavones containing genistein are food components that have
potential for the prevention of cancer and promotion of health.
Abbreviations:
|
pol
|
DNA polymerase (E.C.2.7.7.7);
|
|
topo
|
DNA topoisomerase;
|
|
dsDNA
|
double-stranded DNA;
|
|
dTTP
|
2′-deoxythymidine-5′-triphosphate;
|
|
DMSO
|
dimethyl sulfoxide;
|
|
EDTA
|
ethylenediaminetetraacetic acid;
|
|
BSA
|
bovine serum albumin;
|
|
SDS
|
sodium dodecyl sulfate;
|
|
EtBr
|
ethidium bromide;
|
|
ssDNA
|
single-stranded DNA;
|
|
LD50
|
50% lethal dose,
|
|
DS
|
Discovery Studio;
|
|
IC50
|
50% inhibitory concentration;
|
|
Tm
|
melting temperature;
|
|
Clog P
|
calculated log P
|
Acknowledgements
This study was supported in part by
the Ministry of Education, Culture, Sports, Science and Technology,
Japan (MEXT)-Supported Program for the Strategic Research
Foundation at Private Universities, 2012–2016. Y.M. acknowledges
Grants-in-Aid for Scientific Research (C) (no. 24580205) from MEXT
and the Hyogo Science and Technology Association (Japan). I.K.
acknowledges a Grant-in-Aid for Young Scientists (B) (no. 23710262)
from MEXT.
References
|
1.
|
Liu RH: Potential synergy of
phytochemicals in cancer prevention: mechanism of action. J Nutr.
134:3479S–3485S. 2004.PubMed/NCBI
|
|
2.
|
Surh YJ: Cancer chemoprevention with
dietary phytochemicals. Nature Rev Cancer. 3:768–780. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Kornberg A and Baker TA: DNA Replication.
2nd edition. W.D. Freeman and Co; New York, NY: pp. 197–225.
1992
|
|
4.
|
Hubscher U, Maga G and Spadari S:
Eukaryotic DNA polymerases. Ann Rev Biochem. 71:133–163. 2002.
View Article : Google Scholar
|
|
5.
|
Bebenek K and Kunkel TA: Advances in
Protein Chemistry. Yang W: Elsevier; San Diego, CA: pp. 137–165.
2004, View Article : Google Scholar
|
|
6.
|
Lange SS, Takata K and Wood RD: DNA
polymerases and cancer. Nat Rev Cancer. 11:96–110. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Loeb LA and Monnat RJ Jr: DNA polymerases
and human disease. Nature Rev Genet. 9:594–604. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Wang JC: DNA topoisomerases. Ann Rev
Biochem. 65:635–692. 1996. View Article : Google Scholar
|
|
9.
|
Liu LF: DNA topoisomerase poisons as
antitumor drugs. Ann Rev Biochem. 58:351–375. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Sakaguchi K, Sugawara F and Mizushina Y:
Inhibitors of eukaryotic DNA polymerases. Seikagaku. 74:244–251.
2002.(In Japanese).
|
|
11.
|
Berdis AJ: DNA polymerases as therapeutic
targets. Biochemistry (Moscow). 47:8253–8260. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Heber D: Plant Foods and Phytochemicals in
human health. Berdanier CD, Dwyer JT and Feldman EB: CRC Press.
176–181. 2008.
|
|
13.
|
Barnes S and Messina M: The role of soy
products in reducing cancer risk. J Natl Cancer Inst. 83:541–546.
1991. View Article : Google Scholar
|
|
14.
|
Anderson JM, Johnstone BM and Cook-Newell
ME: Metaanalysis of the effects of soy protein intake on serum
lipids. N Engl J Med. 333:276–282. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Bahram HA, Alekel L, Hollis BW, Amin D,
Stacewicz-Sapuntzakis M, Guo P and Kukreja SC: Dietary soybean
proteins prevent bone loss in an ovariectomized rat model of
osteoporosis. J Nutr. 126:161–167. 1996.PubMed/NCBI
|
|
16.
|
Tamai K, Kojima K, Hanaichi T, Masaki S,
Suzuki M, Umekawa H and Yoshida S: Structural study of
immunoaffinity-purified DNA polymerase α-DNA primase complex from
calf thymus. Biochim Biophys Acta. 950:263–273. 1988.
|
|
17.
|
Date T, Yamaguchi M, Hirose F, Nishimoto
Y, Tanihara K and Matsukage A: Expression of active rat DNA
polymerase β in Escherichia coli. Biochemistry.
27:2983–2390. 1988.
|
|
18.
|
Umeda S, Muta T, Ohsato T, Takamatsu C,
Hamasaki N and Kang D: The D-loop structure of human mtDNA is
destabilized directly by 1-methyl-4-phenylpyridinium ion
(MPP+), a parkinsonism-causing toxin. Eur J Biochem.
267:200–206. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
19.
|
Oshige M, Takeuchi R, Ruike R, Kuroda K
and Sakaguchi K: Subunit protein-affinity isolation of
Drosophila DNA polymerase catalytic subunit. Protein Expr
Purif. 35:248–256. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
20.
|
Kusumoto R, Masutani C, Shimmyo S, Iwai S
and Hanaoka F: DNA binding properties of human DNA polymerase η:
implications for fidelity and polymerase switching of translesion
synthesis. Genes Cells. 9:1139–1150. 2004.
|
|
21.
|
Ohashi E, Murakumo Y, Kanjo N, Akagi J,
Masutani C, Hanaoka F and Ohmori H: Interaction of hREV1 with three
human Y-family DNA polymerases. Genes Cells. 9:523–531. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
22.
|
Shimazaki N, Yoshida K, Kobayashi T, Toji
S, Tamai T and Koiwai O: Over-expression of human DNA polymerase λ
in E. coli and characterization of the recombinant enzyme.
Genes Cells. 7:639–651. 2002.
|
|
23.
|
Mizushina Y, Tanaka N, Yagi H, Kurosawa T,
Onoue M, Seto H, Horie T, Aoyagi N, Yamaoka M, Matsukage A, Yoshida
S and Sakaguchi K: Fatty acids selectively inhibit eukaryotic DNA
polymerase activities in vitro. Biochim Biophys Acta. 1308:256–262.
1996. View Article : Google Scholar : PubMed/NCBI
|
|
24.
|
Mizushina Y, Yoshida S, Matsukage A and
Sakaguchi K: The inhibitory action of fatty acids on DNA polymerase
β. Biochim Biophys Acta. 1336:509–521. 1997.
|
|
25.
|
Ogawa A, Murate T, Suzuki M, Nimura Y and
Yoshida S: Lithocholic acid, a putative tumor promoter, inhibits
mammalian DNA polymerase β. Jpn J Cancer Res. 89:1154–1159.
1998.PubMed/NCBI
|
|
26.
|
Yonezawa Y, Tsuzuki T, Eitsuka T, Miyazawa
T, Hada T, Uryu K, Murakami-Nakai C, Ikawa H, Kuriyama I, Takemura
M, Oshige M, Yoshida H, Sakaguchi K and Mizushina Y: Inhibitory
effect of conjugated eicosapentaenoic acid on human DNA
topoisomerases I and II. Arch Biochem Biophys. 435:197–206. 2005.
View Article : Google Scholar
|
|
27.
|
Nakayama C and Saneyoshi M: Inhibitory
effects of 9-β-D-xylofuranosyladenine 5′-triphosphate on
DNA-dependent RNA polymerase I and II from cherry salmon
(Oncorhynchus masou). J Biochem (Tokyo). 97:1385–1389.
1985.
|
|
28.
|
Mizushina Y, Dairaku I, Yanaka N, Takeuchi
T, Ishimaru C, Sugawara F, Yoshida H and Kato N: Inhibitory action
of polyunsaturated fatty acids on IMP dehydrogenase. Biochimie.
89:581–590. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
29.
|
Soltis DA and Uhlenbeck OC: Isolation and
characterization of two mutant forms of T4 polynucleotide kinase. J
Biol Chem. 257:11332–11339. 1982.PubMed/NCBI
|
|
30.
|
Lu BC and Sakaguchi K: An endo-exonuclease
from meiotic tissues of the basidiomycete Coprinus cinereus:
ιts purification and characterization. J Biol Chem.
266:21060–21066. 1991.PubMed/NCBI
|
|
31.
|
Mizushina Y, Murakami C, Ohta K, Takikawa
H, Mori K, Yoshida H, Sugawara F and Sakaguchi K: Selective
inhibition of the activities of both eukaryotic DNA polymerases and
DNA topoisomerases by elenic acid. Biochem Pharmacol. 63:399–407.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
32.
|
Ishiyama M, Tominaga H, Shiga M, Sasamoto
K, Ohkura Y and Ueno K: A combined assay of cell viability and in
vitro cytotoxicity with a highly water-soluble tetrazolium salt,
neutral red and crystal violet. Biol Pharm Bull. 19:1518–1520.
1996. View Article : Google Scholar : PubMed/NCBI
|
|
33.
|
Isoflavones contents of food. http://www.isoflavones.info/isoflavones-content.php.
Accessed May 15, 2012.
|
|
34.
|
Coldham NG, Darby C, Hows M, King LJ,
Zhang AQ and Sauer MJ: Comparative metabolism of genistin by human
and rat gut microflora: detection and identification of the
end-products of metabolism. Xenobiotica. 22:45–62. 2001.PubMed/NCBI
|
|
35.
|
Christian B: Topoisomerase I poisons and
suppressors as anti-cancer drugs. Curr Med Chem. 7:39–58. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
36.
|
Davis TA, Mungunsukh O, Zins S, Day RM and
Landauer MR: Genistein induces radioprotection by hematopoietic
stem cell quiescence. Int J Radiat Biol. 84:713–726. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
37.
|
Lee YW and Lee WH: Protective effects of
genistein on proinflammatory pathways in human brain microvascular
endothelial cells. J Nutr Biochem. 19:819–825. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
38.
|
Okamura S, Sawada Y, Satoh T, Sakamoto H,
Saito Y, Sumino H, Takizawa T, Kogure T, Chaichantipyuth C, Higuchi
Y, Ishikawa T and Sakamaki T: Pueraria mirifica
phytoestrogens improve dyslipidemia in postmenopausal women
probably by activating estrogen receptor subtypes. Tohoku J Exp
Med. 216:341–351. 2008. View Article : Google Scholar
|