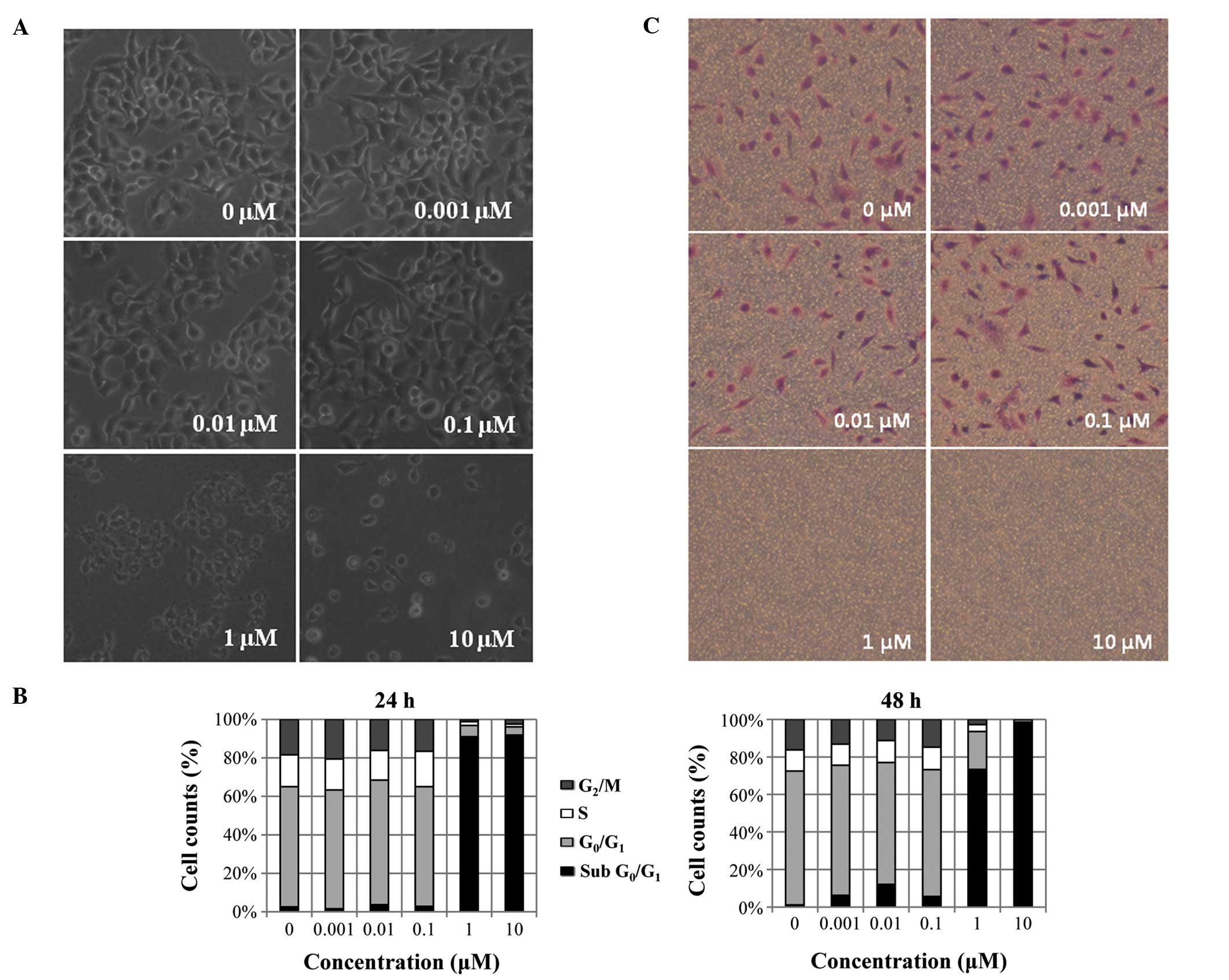
|
1.
|
Bosch-Presegue L and Vaquero A: The dual
role of sirtuins in cancer. Genes Cancer. 2:648–662. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
2.
|
Carafa V, Nebbioso A and Altucci L:
Sirtuins and disease: the road ahead. Front Pharmacol. 3:42012.
View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
North BJ, Marshall BL, Borra MT, Denu JM
and Verdin E: The human Sir2 ortholog, SIRT2, is an
NAD+-dependent tubulin deacetylase. Mol Cell.
11:437–444. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Vaquero A, Scher MB, Lee DH, et al: SirT2
is a histone deacetylase with preference for histone H4 Lys 16
during mitosis. Genes Dev. 20:1256–1261. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Stunkel W and Campbell RM: Sirtuin 1
(SIRT1): the misunderstood HDAC. J Biomol Screen. 16:1153–1169.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Bonezzi K, Belotti D, North BJ, et al:
Inhibition of SIRT2 potentiates the anti-motility activity of
taxanes: implications for antineoplastic combination therapies.
Neoplasia. 14:846–854. 2012.PubMed/NCBI
|
|
7.
|
Napper AD, Hixon J, McDonagh T, et al:
Discovery of indoles as potent and selective inhibitors of the
deacetylase SIRT1. J Med Chem. 48:8045–8054. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Bedalov A, Gatbonton T, Irvine WP,
Gottschling DE and Simon JA: Identification of a small molecule
inhibitor of Sir2p. Proc Natl Acad Sci USA. 98:15113–15118. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Mai A, Massa S, Lavu S, et al: Design,
synthesis, and biological evaluation of sirtinol analogues as class
III histone/protein deacetylase (Sirtuin) inhibitors. J Med Chem.
48:7789–7795. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Heltweg B, Gatbonton T, Schuler AD, et al:
Antitumor activity of a small-molecule inhibitor of human silent
information regulator 2 enzymes. Cancer Res. 66:4368–4377. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Outeiro TF, Kontopoulos E, Altmann SM, et
al: Sirtuin 2 inhibitors rescue alpha-synuclein-mediated toxicity
in models of Parkinson's disease. Science. 317:516–519.
2007.PubMed/NCBI
|
|
12.
|
Trapp J, Meier R, Hongwiset D, Kassack MU,
Sippl W and Jung M: Structure-activity studies on suramin analogues
as inhibitors of NAD+-dependent histone deacetylases
(sirtuins). ChemMedChem. 2:1419–1431. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Lain S, Hollick JJ, Campbell J, et al:
Discovery, in vivo activity, and mechanism of action of a
small-molecule p53 activator. Cancer Cell. 13:454–463. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Lara E, Mai A, Calvanese V, et al:
Salermide, a Sirtuin inhibitor with a strong cancer-specific
proapoptotic effect. Oncogene. 28:781–791. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Kalle AM, Mallika A, Badiger J, Alinakhi,
Talukdar P and Sachchidanand: Inhibition of SIRT1 by a small
molecule induces apoptosis in breast cancer cells. Biochem Biophys
Res Commun. 401:13–19. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Nebbioso A, Pereira R, Khanwalkar H, et
al: Death receptor pathway activation and increase of ROS
production by the triple epigenetic inhibitor UVI5008. Mol Cancer
Ther. 10:2394–2404. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Zhang Q, Zeng SX, Zhang Y, et al: A small
molecule Inauhzin inhibits SIRT1 activity and suppresses tumour
growth through activation of p53. EMBO Mol Med. 4:298–312. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Machlowitz RA, Fisher WP, Betsey McKay S,
Tytell AA and Charney J: Xanthothricin, a new antibiotic. Antibiot
Chemother. 4:259–261. 1954.PubMed/NCBI
|
|
19.
|
Black TH: An improved, large-scale
synthesis of xanthothricin and reumycin. J Heterocyclic Chem.
24:1373–1375. 1987. View Article : Google Scholar
|
|
20.
|
Spinks D, Shanks EJ, Cleghorn LA, et al:
Investigation of trypanothione reductase as a drug target in
Trypanosoma brucei. ChemMedChem. 4:2060–2069. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
21.
|
Todorovic N, Giacomelli A, Hassell JA,
Frampton CS and Capretta A: Microwave-assisted synthesis of
3-arylpyrimido[5,4-e][1,2,4]triazine-5,7(1H,6H)-dione libraries:
derivatives of toxoflavin. Tetrahedron Lett. 51:6037–6040.
2010.
|
|
22.
|
Peck B, Chen CY, Ho KK, et al: SIRT
inhibitors induce cell death and p53 acetylation through targeting
both SIRT1 and SIRT2. Mol Cancer Ther. 9:844–855. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23.
|
Lu X, Magrane G, Yin C, Louis DN, Gray J
and Van Dyke T: Selective inactivation of p53 facilitates mouse
epithelial tumor progression without chromosomal instability. Mol
Cell Biol. 21:6017–6030. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
24.
|
Vaziri H, Dessain SK, Ng Eaton E, et al:
hSIR2(SIRT1) functions as an NAD-dependent p53 deacetylase. Cell.
107:149–159. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
25.
|
Venkatachalam CM, Jiang X, Oldfield T and
Waldman M: LigandFit: a novel method for the shape-directed rapid
docking of ligands to protein active sites. J Mol Graph Model.
21:289–307. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
26.
|
Finnin MS, Donigian JR and Pavletich NP:
Structure of the histone deacetylase SIRT2. Nat Struct Biol.
8:621–625. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
27.
|
Wu S, Skolnick J and Zhang Y: Ab initio
modeling of small proteins by iterative TASSER simulations. BMC
Biol. 5:172007. View Article : Google Scholar : PubMed/NCBI
|
|
28.
|
Brooks BR, Bruccoleri RE, Olafson BD,
States DJ, Swaminathan S and Karplus M: CHARMM: A program for
macromolecular energy, minimization, and dynamics calculations. J
Comput Chem. 4:187–217. 1983. View Article : Google Scholar
|
|
29.
|
Song S-H, Lee M-O, Lee J-S, Oh J-S, Cho
S-U and Cha H-J: Sirt1 promotes DNA damage repair and cellular
survival. Biomol Ther. 19:282–287. 2011. View Article : Google Scholar
|
|
30.
|
van Veen AG and Mertens WK: Das
Toxoflavin, der gelbe Giftstoff der Bongkrek. Rec Trav Chim.
53:398–404. 1934.PubMed/NCBI
|
|
31.
|
Latuasan HE and Berends W: On the origin
of the toxicity of toxoflavin. Biochim Biophys Acta. 52:502–508.
1961. View Article : Google Scholar : PubMed/NCBI
|
|
32.
|
Goh KC, Wang H, Yu N, et al: PLK1 as a
potential drug target in cancer therapy. Drug Dev Res. 62:349–361.
2004. View Article : Google Scholar
|
|
33.
|
Hayward DG, Newbatt Y, Pickard L, et al:
Identification by high-throughput screening of viridin analogs as
biochemical and cell-based inhibitors of the cell cycle-regulated
nek2 kinase. J Biomol Screen. 15:918–927. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
34.
|
Burns S, Travers J, Collins I, et al:
Identification of small-molecule inhibitors of protein kinase B
(PKB/AKT) in an AlphaScreen™ high-throughput screen. J Biomol
Screen. 11:822–827. 2006.PubMed/NCBI
|