Introduction
The Wnt ligand binds immediately to its receptor
gene, Frizzled, and this binding site is divided into the canonical
and non-canonical pathways (1,2).
Among them, the canonical pathway is dependent on β-catenin and
affects cancer cell adhesion. Wnt-1 proteins are secreted from
cells. Thereafter, Wnt-1 protein combines with Frizzled proteins
and lipoprotein-related protein 5/6 (LRP 5/6) of the two receptor
molecules. The Wnt-1 signaling pathway is dependent on β-catenin
(3–6). Wnt proteins bind the cysteine-rich
glycoprotein, acting only in a limited range of ligands, and focal
activation of the receptor-mediated signal transduction system acts
as an important regulator of cell proliferation and differentiation
factors (7). β-catenin is a factor
related to cell adhesion. In normal cells, downregulation of Wnt-1
signaling degrades β-catenin in the Axin-APC protein complex by
GSK-3β. However, cancer cells show upregulation of Wnt-1 signaling.
Therefore, cancer cells continue to grow and metastasis occurs. The
β-catenin degradation complex, an Axin degradation complex,
inhibits the activity of GSK3β when Wnt stimulation is external.
Therefore, phosphorylation of β-catenin is inhibited (8). Additionally, normal cells have
operating E-cadherin in the nucleus, but cancer cells degrade
E-cadherin. E-cadherin in the nucleus inhibits β-catenin and is
involved in intercellular adhesion, which plays an important role
in the maintenance of normal epithelial cells and epithelial
structures (9); catenin in the
cytoplasm and formation of the cadherin/catenin complex is
involved, and intercellular adhesion fails to progress to invasive
carcinoma (10,11). Change in cancer cell grade
increases metastasis when the expression of E-cadherin is lost
(12). Additionally, Snail,
typically a zinc finger transcription factor, inhibits E-cadherin
expression, which inhibits the cytosolic level of β-catenin in the
nucleus. Similar to Snail genes that are resistant to apoptosis,
invasion and migration of cancer cells promote the
epithelial-mesenchymal transition (EMT) and induce increased Snail
levels in the nucleus through upregulation of Wnt-1 signaling
(13). Therefore, β-catenin is not
degraded and cytoplasmic β-catenin levels increased, which
stabilize β-catenin by moving to the nucleus, and the Tcf/LEF
transcription factor regulates the expression of various genes.
Increases in cancer cell proliferation through the upregulation of
cell cycle-related proteins, c-myc and cyclin D, occur via
activated Tcf/LEF (14–17). In addition, representative
β-catenin target genes increase cell proliferation of ICAM-1 and
c-jun by increasing the expression of β-catenin and Tcf/LEF
(18,19). In a previous study, we confirmed
apoptosis of AGS cells by Capsosiphon fulvescens (20). Also, Cf-GP induced downregulation
of TGF-β1 signaling pathway in AGS cells. Therefore, we observed
inhibition of AGS cell proliferation through MTS assay (21). In this study, we observed the
downregulation of Wnt-1 signaling by Cf-GP and inhibition of AGS
cell proliferation by a G0/G1 phase arrest.
Materials and methods
Preparation of Cf-GP
The C. fulvescens (Cf) used in this
experiment was purchased in 2010 in Korea. Forty grams of Cf powder
was diluted with 1 liter of water. The Cf powder in diluted water
was stirred for 3 h at 80°C in heating mantle and centrifuged at
1,500 × g for 15 min at 4°C. Next, three volumes of 95% ethanol
were added, and precipitation was removed by vacuum filtration.
Supernatants were added to 80% ammonium sulfate and stirred for 24
h. Salt composition was then removed through a dialysis membrane
(Por Membrane MW 3,500 Da, Spcectrum Laboratories Inc., Rancho
Dominguez, CA, USA) for 1 day at 4°C. The concentrated solution was
then distributed into a 1.5-ml tube and stored at −70°C until use.
These samples were named C. fulvescens glycoprotein
(Cf-GP).
Cell culture
AGS human gastric cancer cell line (American Type
Culture Collection, Manassas, VA, USA) was maintained at 37°C in a
5% CO2 humidified atmosphere. Cells were cultured in
RPMI-1640 medium with 10% fetal bovine serum (FBS; Hyclone, Logan,
UT, USA), 100 U/ml penicillin and 100 mg/ml streptomycin. Cells
were cultured to 80% confluence in 100-mm diameter dishes. The
RPMI-1640 medium was replaced every day.
Cell attachment analysis
The cell attachment analysis was performed using 3
ml per 100-mm diameter dish in sterile 1% gelatin at 120°C for 20
min and then coated after storage at 4°C. Cells were cultured in
RPMI-1640 medium with 10% FBS and grown to 80% confluence in 100-mm
diameter dishes coated with 1% gelatin. Next, cells were treated
with Cf-GP of various concentrations and cultured for 24 h. The
unattached (apoptotic) cells were stained with trypan blue and live
cells were confirmed by microscopy at ×200 magnification.
mRNA expression analysis
AGS cells were seeded onto 6-well plates at
2×104 cells/well in 2 ml of medium. Cells were
maintained at 24 h and the medium was replaced with SFM. After 24
h, the SFM was replaced with Cf-GP (5, 10 or 20 μg/ml) for
24 h. Cells were treated with 1 ml TRIzol reagent (Invitrogen,
Carlsbad, CA, USA), and cDNA was synthesized using the Oligo(dT)
primer (iNtRON Biotechnology Inc., Seongnam, Korea). The
synthesized cDNA was added to 2X TOPsimple™ DyeMIX-nTaq (Enzynomics
Inc., Daejeon, Korea), and the primer (Table I) was added to 0.1%
diethylpyrocarbonate (DEPC) water. PCR products were separated on a
1% agarose gel and stained with RedSafe nucleic acid staining
solution (iNtRON Biotechnology Inc).
 | Table I.Oligonucleotide sequences of primer
pairs used for RT-PCR. |
Table I.
Oligonucleotide sequences of primer
pairs used for RT-PCR.
| Name | Sequence of primers
(5′→3′) |
|---|
| Wnt-1 | S:
TGC-ACG-CAC-ACG-CGC-GTA-CTG-CAC |
| A:
CAG-GAT-GGC-AAG-AGG-GTT-CAT-G |
| Frizzled | S:
CAG-AGC-GGG-GCA-GCA-GTA-CAA |
| A:
GCG-CGG-GCA-GGA-GAA-CTT |
| LRP5 |
S:CAG-CAC-CCG-GAA-GAT-CAT-TGT |
|
A:TCG-TTG-ATC-TCG-GTG-TTG-ACC |
| APC | S:
TAT-CTT-CAG-AAT-CAG-CCA-GGC-AC |
| A:
AAA-GTA-TCA-GCA-TCT-GGA-AGA-ACC |
| Axin | S:
ACC-GAA-AGT-ACA-TTC-TTG-ATA-AC |
| A:
TCC-ATA-CCT-GAA-CTC-TCT-GC |
| GSK-3β | S:
CAG-CAA-GGT-GAC-AAC-AGT-GG |
| A:
GGA-ACA-TAG-TCC-AGC-ACC-AGA |
| β-catenin | S:
GAA-ACG-GCT-TTC-AGT-TGA-GC |
| A:
CTG-GCC-ATA-TCC-ACC-AGA-GT |
| E-cadherin | S:
GAA-CAG-CAC-GTA-CAC-AGC-CCT |
| A:
GCA-GAA-GTG-TCC-CTG-TTC-CAG |
| Snail | S:
TAT-GCT-GCC-TTC-CCA-GGC-TTG |
| A:
ATG-TGC-ATC-TTG-AGG-GCA-CCC |
| Tcf | S:
TGA-CCT-CTC-TGG-CTT-CTA-CT |
| A:
TTG-ATG-GTT-GGC-TTC-TTG-GC |
| LEF-1 | S:
CCA-GCT-ATT-GTA-ACA-CCT-CA |
| A:
TTC-AGA-TGT-AGG-CAG-CTG-TC |
| ICAM-1 | S:
CAC-CTC-CTG-TGA-CCA-GCC-CA |
| A:
AAC-AGG-ACG-GTC-GCT-GAG-GG |
| c-jun | S:
ATG-ACT-GCA-AAG-ATG-GAA-ACG |
| A:
TCA-AAA-TGT-TTG-CAA-CTG-CTG-CG |
| c-myc | S:
CCA-GGA-CTG-TAT-GTG-GAG-CG |
| A:
CCT-GAG-GAC-CAG-TGG-GCT-GT |
| Cyclin D | S:
CTG-GCC-ATG-AAC-TAC-CTG-GA |
| A:
GTC-ACA-CTT-GAT-CAC-TCT-CC |
| β-actin | S:
CGT-ACC-ACT-GGC-ATC-GTG |
| A:
GTG-TTG-GCG-TAC-AGG-TCT-TTG |
Western blot analysis
AGS cells were cultured in 100-mm diameter dishes.
Cells were grown to 80% confluency and the medium was then replaced
with SFM for 4 h. The medium was then replaced with fresh SFM
containing Cf-GP (5, 10 or 20 μg/ml) for 24 h. For
collection, cells were washed with phosphate-buffered saline (PBS)
and then added to extraction lysis buffer (20 mM Tris, pH 7.5, 150
mM NaCl, 1 mM EDTA, 1 mM EGTA, 1% Triton X-100, 2.5 mM sodium
pyrophosphate, 1 mM β-glycerophosphate, 1 mM sodium orthovanadate,
1 μg/ml aprotinin, 1 μg/ml leupeptin, 1 μg/ml
pepstatin A, 0.25% Na-deoxycholate and 1 mM PMSF). To examine
protein expression, western blot analysis was performed by
separating proteins on a 10–15% sodium dodecyl sulfate
polyacrylamide gel electrophoresis (SDS-PAGE), and proteins were
then transferred onto an Immobilon-P transfer membrane (Millipore
Co., Billerica, MA, USA). The transferred membrane was blocked at
room temperature with 1% bovine serum albumin in TBS-T (10 mM
Tris-HCl, pH 7.5, 150 mM NaCl and 0.1% Tween-20), and then shaking
with the indicated primary antibodies (diluted 1:1,000):
anti-Wnt-1, anti-Frizzled, anti-LRP, anti-APC, anti-Axin,
anti-GSK-3β, anti-β-catenin, anti-E-cadherin, anti-Snail,
anti-LEF-1, anti-Tcf, anti-ICAM-1, anti-c-jun, anti-c-myc or
anti-cyclin D from Santa Cruz Biotechnology (Santa Cruz
Biotechnology Inc., Santa Cruz, CA, USA). The secondary antibodies,
horseradish peroxidase-conjugated goat, mouse and rabbit antibody
(1:10,000) from GE Healthcare Bio-Sciences (Piscataway, NJ, USA)
were then added. The reaction was terminated using SuperSignal West
Pico Chemiluminescent substrate (Thermo Fisher Scientific Inc.,
Rockford, IL, USA) and visualized by X-ray film (Kodak, Rochester,
NY, USA).
Cell cycle analysis
The rate of cell cycle phase by Cf-GP treatment was
determined using a Muse™ cell cycle kit from Millipore (EMD
Millipore Co., Billerica, MA, USA). Cells were cultured onto 6-well
plates grown to 80% confluency and treated with SFM or various
concentrations of Cf-GP (5, 10 or 20 μg/ml). After 24 h of
treatment, cells were collected in 1% FBS-RPMI-1640 media and Muse
cell cycle test reagent was added to each tube. Cells were mixed by
vortexing and the reaction was performed for 30 min at room
temperature in the dark. Following treatment, cells were stained to
determine G0/G1, S and G2/M phase rates of the cell cycle using a
Muse cell analyzer (EMD Millipore Co., Hayward, CA, USA).
Statistical analysis
Results of this experiment were compared using SPSS
ver.10.0 Programs (SPSS Inc., Chicago, IL, USA), and validated with
analysis of variance (ANOVA) and Duncan’s multiple range tests. A
p-value <0.05 was considered to indicate a statistically
significant difference. All data are expressed as the mean ±
standard deviation (SD).
Results
Effect of Cf-GP on the cell attachment
assay and apoptosis staining
Live cell motility and cell attachment analysis were
performed by coating 1% gelatin on 100-mm diameter dishes. In
addition, apoptotic cells were stained with trypan blue. Gelatin
was used as a representative extracellular matrix (ECM) component,
and motility of active cancer cells were well attached to the
gelatin (22). The cells were
seeded onto 1% gelatin-coated 100-mm diameter dishes and treated
with Cf-GP for 24 h. As a result, we observed decreased attachment
of AGS cells in a dose-dependent manner upon treatment with Cf-GP
(Fig. 1A). In general, apoptotic
cells are permeable. Therefore, dead cells will stain with trypan
blue and viable cells will not (23). As shown in Fig. 1B, the group treated with Cf-GP
displayed increased apoptotic cells compared to the control
group.
Effect of Cf-GP on the downregulation of
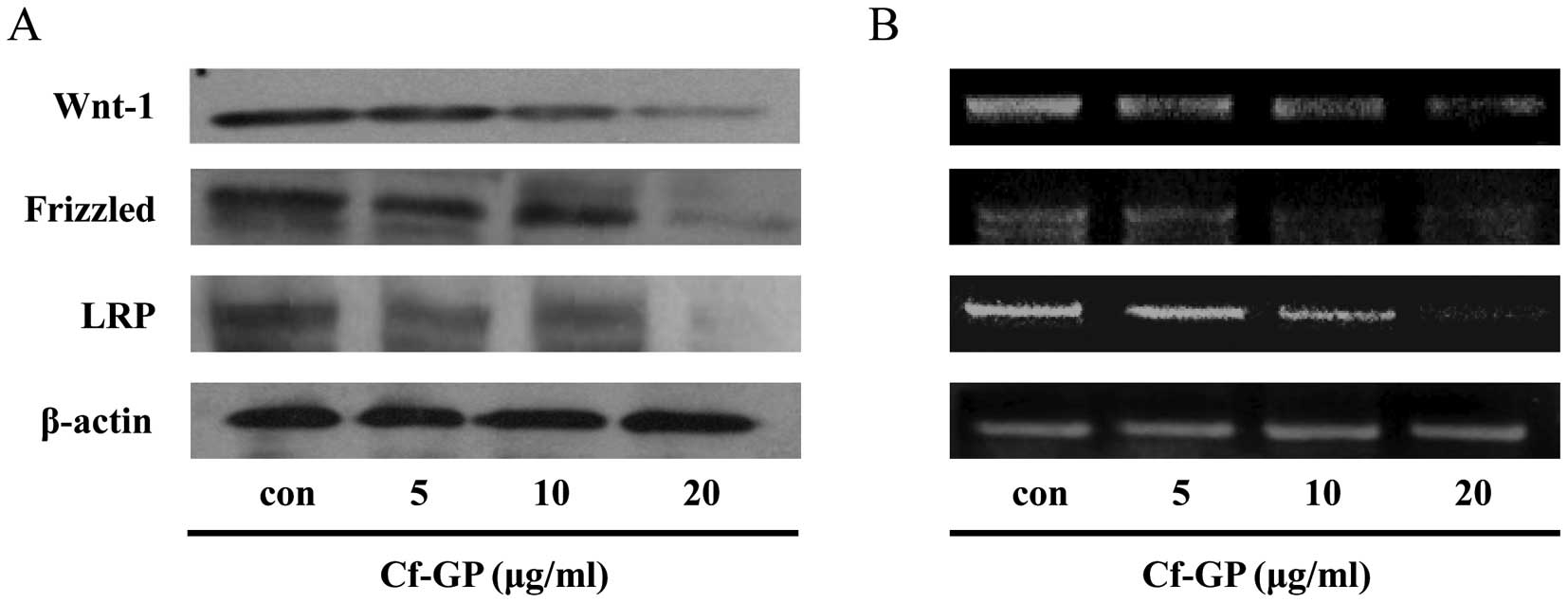
Wnt-1, Frizzled and LRP
The Wnt-1/Frizzled protein receptor demonstrates
that cell proliferation is associated with cancer cells (24). Therefore, inhibition of cancer cell
proliferation and attachment is required to suppress
Wnt-1/Frizzled/LRP. We observed the expression levels of these
genes by western blot analysis and RT-PCR. Cells were treated with
various concentration of Cf-GP (5, 10 or 20 μg/ml) for 24 h.
As a result, we observed a decrease in protein levels of Wnt-1,
Frizzled and LRP in a dose-dependent manner upon Cf-GP treatment
(Fig. 2A). Additionally, mRNA
expression levels revealed the same results (Fig. 2B). Expression of a sub-factor of
Wnt-1 signaling was due to reduced expression of a receptor that
was also observed in these experiments.
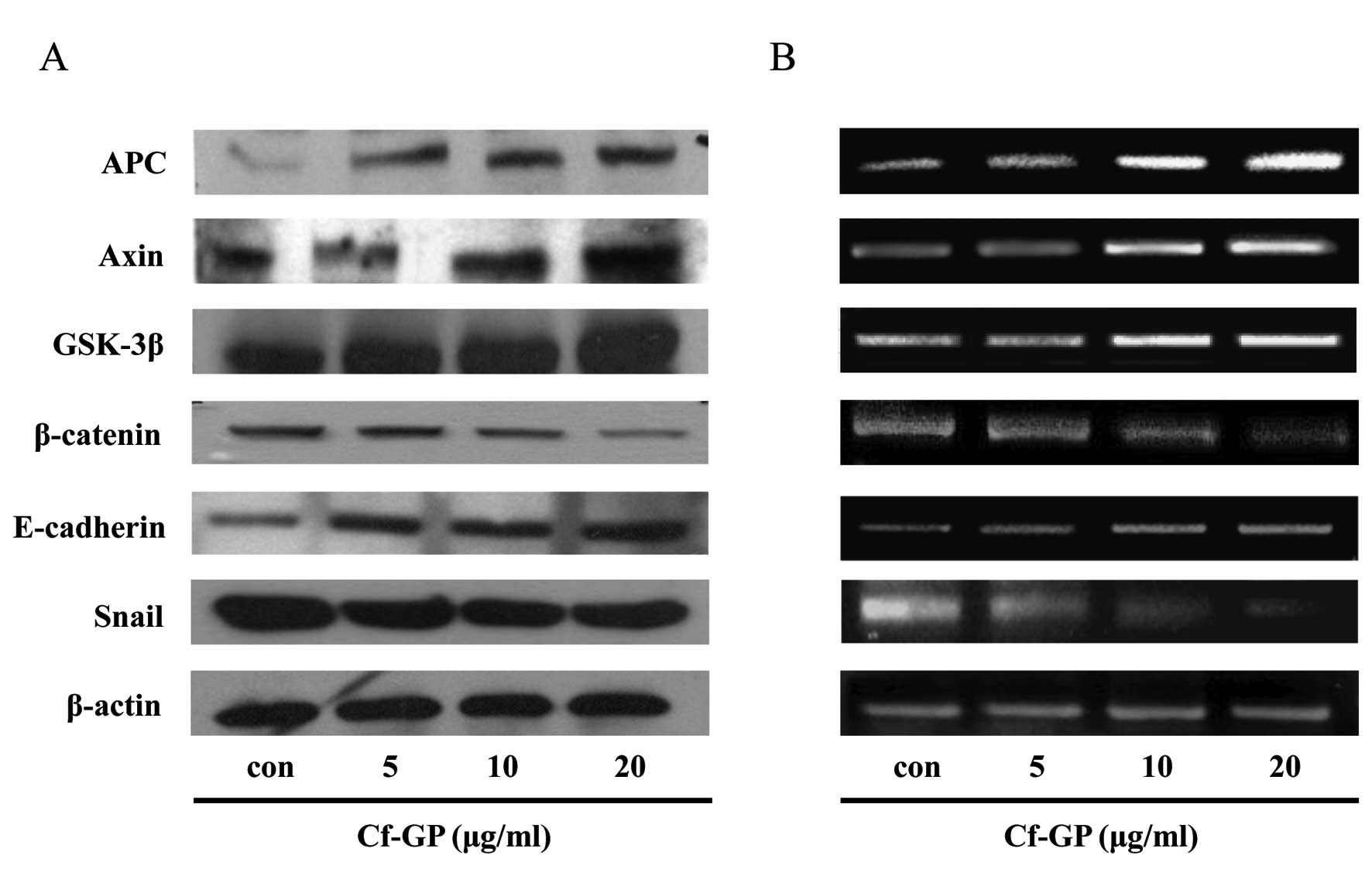
Effect of Cf-GP on the downregulation of
Wnt-1 signaling
The Wnt-1 receptor was activated in cancer cells and
altered the various sub-factors. Previously, we confirmed
inhibition of Wnt-1 and Frizzled and LRP receptors. Therefore,
expression levels of the sub-factors of these genes (APC, Axin,
GSK-3β and β-catenin) were measured in the same manner. The
β-catenin was degraded by APC, Axin and the GSK-3β complex in
normal cells. In addition, APC and Axin induced inhibition of
Wnt-1. However, these genes did not operate in cancer cells by
stimulated Wnt-1 and mutant Axin, and LRP combined with Wnt-1
activation. As a result, we confirmed increased expression of APC
and Axin. In addition, GSK-3β increased to decompose the role of
β-catenin by Cf-GP. Thus, a reduction in β-catenin is involved in
cell adhesion. Additionally, we observed increased E-cadherin,
which is involved in β-catenin inhibition and decreased Snail.
E-cadherin induced degration of β-catenin in normal cells. However,
almost all cancer cells have low level of E-cadherin by
upregulation of Snail. So, β-catenin degradation does not occur in
cancer cells (Fig. 3).
Effect of Cf-GP on the inhibition of
transcription factors and cell cycle genes
Target gene expression occurs following accumulation
of β-catenin in the nucleus and entering of the Tcf/LEF binding
factor. This promotes transcription of c-myc and cyclin D, which
promotes cell division by the β-catenin/Tcf/LEF complex. Like many
cancer cells, the expression of these genes does not arrest the
cell cycle. Therefore, we examined the expression levels of LEF,
Tcf, ICAM-1, c-jun, c-myc, and cyclin D by western blot analysis
and RT-PCR. Cells were treated with Cf-GP (5, 10 or 20
μg/ml) for 24 h. Next, cells were cultured under the same
conditions as the previous experiments. As a result, we observed
the downregulation of protein and mRNA levels of these genes in a
dose-dependent manner with Cf-GP (Fig.
4).
 | Figure 4.Effects of Cf-GP on the expression
levels of Tcf, LEF-1, ICAM-1, c-jun, c-myc and cyclin D. Cells were
treated with Cf-GP (5, 10 or 20 μg/ml) for 24 h. Gene
expression was determined by western blot analysis and RT-PCR. (A)
Western blot analysis using anti-Tcf, anti-LEF-1, anti-ICAM-1,
anti-c-jun, anti-c-myc, anti-cyclin D and anti-β-actin antibodies.
SDS-PAGE was performed on acrylamide gel. (B) cDNA and primers were
synthesized. PCR was then performed at the indicated annealing
temperatures. Reaction products were electrophoresed on a 1%
agarose gel and visualized with RedSafe reagent. |
Effect of Cf-GP on G0/G1 arrest of the
AGS cell cycle
Expression of c-myc and cyclin D did not properly
adjust Wnt-1 signal transduction in cancer cells. Therefore, the
cancer was generated by the promotion of proliferation and division
of cells. The order and progress of cell cycle events were
monitored during checkpoints of the cell cycle that occur during
the transition from G0/G1 to S phase. Progression of the cell
through the four phases (G0/G1-S-G2/M) of the cell cycle is
regulated by sequential activation. We used a cell cycle test
following the Muse cell cycle kit protocol and treated cells with
Cf-GP (5, 10 or 20 μg/ml) for 24 h. As a result, arrest of
G0/G1 phase with final concentration group (20 μg/ml) was
increased approximately 50% compared to the control group in AGS
cells (Fig. 5).
Discussion
Wnt proteins are involved in the cysteine-rich
secreted ligand coupled receptor-mediated signal transduction
pathway. The Wnt pathway is activated by binding to the cell
membrane receptor through autocrine or paracrine signals. The
original Wnt signal controls the development of organs, cell
proliferation, morphology and motility within vertebrates. The Wnt
pathway can be divided into two types. The protein family has a
composition of cysteine-rich glycoproteins that play important role
in proliferation and differentiation of cells. However, Wnt
stimulation causes the growth and proliferation of various cancer
cells (25,26). The Wnt signaling pathway has
separated into at least 19 types of Wnt genes involved in the
canonical and non-canonical pathways. One Wnt pathway is dependent
on β-catenin, also known as the ‘canonical Wnt pathway’, which is
activated by Wnt-1, 2, 3a and 10a, and decides the fate of cells.
The alternate β-catenin-independent pathway, is activated by Wnt-4,
5a and 11, which is referred to as the ‘non-canonical Wnt pathway’
or ‘Wnt/calcium pathway’; this pathway is involved in cell
polarity, adhesion and shape (27,28).
In this study, we focused on cell inhibition, cell motility and
proliferation through decreased β-catenin by downregulation of
Wnt-1. Normally, secreted Frizzled-related proteins (SFrp) bind
Wnt-1 proteins to induce and interfere with Wnt, Frizzled, and the
LRP complex in cells. Therefore, Wnt signaling will not occur
through the degradation of β-catenin by Axin, APC and GSK-3β
complex. However, cancer cells cannot operate the Axin/APC/GSK-3β
complex by overexpression of Wnt-1. So, β-catenin was not degraded
and thus accumulated in the nucleus, thereby promoting expression
of the transcription factor. In a previous study, we observed
inhibition of AGS cell proliferation through MTS assay by Cf-GP (5,
10 or 20 μg/ml) (21).
Decreased cell proliferation was evident upon Cf-GP treatment (5,
10 or 20 μg/ml) in a dose-dependent manner, and treatment at
high concentration of 20 μg/ml led to an approximately 40%
reduction compared to the untreated group. In this study, apoptotic
events were confirmed by staining cells with trypan blue. Trypan
blue moves into the cell by diffusion and exocytosis through
adenosine triphosphate (ATP) from viable cells that are discharged
to the outside of the cells and back. Living cells do not allow
trypan blue to enter cells. Therefore, apoptotic cells stained with
trypan blue by diffusion and motility was degraded. As shown in
Fig. 1, inhibition of motility and
induction of apoptosis was observed by Cf-GP (5, 10 or 20
μg/ml) in a dose-dependent manner.
Next, we confirmed the expression levels of
Wnt-1-related genes by western blot analysis and RT-PCR. As a
result, Wnt-1 and receptor proteins (Frizzled and LRP) decreased in
a dose-dependent manner upon treatment with Cf-GP (Fig. 2). In addition, based on the above
results, various gene factors were measured through downregulation
of Wnt-1 by Cf-GP. The expression levels of β-catenin decreased
based on western blot analysis and RT-PCR. Additionally, E-cadherin
and the Axin/APC/GSK-3β complex levels increased in a
dose-dependent manner by Cf-GP. This explains that upregulation of
E-cadherin and the Axin/APC/GSK-3β complex inhibits nucleus
accumulation of β-catenin. Also, we observed increased E-cadherin
by downregulation of Snail (Fig.
3). In the absence of β-catenin degradation, its accumulation
in the nucleus induced activation of transcription factors
(Tcf/LEF), which can have a significant effect on cell
proliferation (29,30). Therefore, it is important to
inhibit transcription factors (Tcf/LEF) in cancer cells. In a
previous experiment, we observed the inhibition of β-catenin and
Wnt-1. Therefore, we expected a reduction by expression of the
Tcf/LEF transcription factor activated in cancer cells, as well as
an increase in the expression of a variety of factors including
cyclin D, c-myc, ICAM-1 and c-jun, which are involved in the
promotion of cancer cell cycle and proliferation (31,32).
In the case of cell cycle-related genes, Cf-GP inhibits cyclin D,
c-myc, ICAM-1 and c-jun in AGS cells (Fig. 4). We then confirmed a change in
cell cycle phase, which was monitored during cell culture with
Cf-GP for 24 h. As shown in Fig.
5, the G0/G1 phase was approximately 18.4% in the control
groups, but Cf-GP treatment groups induced an increase in G0/G1
arrest (22.4, 59.5 and 73.1%, respectively). Therefore, the AGS
cell cycle was stagnant, and apoptosis was induced by Cf-GP in a
dose-dependent manner. C. fulvescens, green algae that grows
in Korea and other Asian coastal countries, has long been a healthy
food and bioactive material. Our previous results showed an
anticancer effect by Cf-GP through Fas signaling and apoptosis of
AGS cancer cells (33). In this
study, we confirmed the inhibition of AGS gastric cancer cell
migration by downregulating the Wnt-1 signaling pathway via Cf-GP.
Therefore, our results suggest potential for functional food and
therapeutic use of Cf-GP.
Acknowledgements
This research was supported by the
Basic Science Research Program through the National Research
Foundation of Korea (NRF) funded by the Ministry of Education,
Science and Technology (2012R1A6A1028677).
References
|
1.
|
Dann CE, Hsieh JC, Rattner A, Sharma D,
Nathans J and Leahy DJ: Insight into Wnt binding and signaling from
the structures of two Frizzled cysteine-rich domains. Nature.
412:86–90. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
2.
|
Hsieh JC, Ranttner A, Smallwood PM and
Nathans J: Biochemical characterization of Wnt-frizzled
interactions using a soluble, biologically active vertebrate Wnt
protein. Proc Natl Acad Sci USA. 96:3546–3551. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Cadigan KM and Nusse R: Wnt signaling: a
common theme in animal development. Genes Dev. 11:3286–3305. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Moon RT, Kohn AD, De Ferrari GV and Kaykas
A: Wnt and beta-catenin signaling: diseases and therapies. Nat Rev
Genet. 5:691–701. 2004. View
Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Nelson WJ and Nusse R: Convergence of Wnt,
beta-catenin, and cadherin pathways. Science. 303:1483–1487. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Miller JR, Hocking AM, Brown JD and Moon
RT: Mechanism and function of signal transduction by the
Wnt/beta-catenin and Wnt/Ca2+ pathways. Oncogene.
18:7860–7872. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
You L, Uematsu K, Xu Z, Mazieres J, Lee A,
McCormick F and Jablons DM: Inhibition of Wnt-1 signaling induces
apoptosis in β-catenin-deficient mesothelioma cells. Cancer Res.
64:3474–3478. 2004.
|
|
8.
|
Jho EH: Wnt signal transduction and its
involvement in human diseases. J Korean Endocr Soc. 20:306–318.
2005. View Article : Google Scholar
|
|
9.
|
Rubinfeld B, Albert I, Porfiri E, Fiol C,
Munemitsu S and Polakis P: Binding of GSK3β to the APC-β-catenin
complex and regulation of complex assembly. Science. 272:1023–1026.
1996.
|
|
10.
|
Rubinfeld B, Robbins P, El-Gamil M, Albert
I, Porfiri E and Polakis P: Stabilization of β-catenin by genetic
defects in melanoma cell lines. Science. 275:1790–1792. 1997.
|
|
11.
|
Liu C, Li Y, Semenov M, Han C, Baeg GH,
Tan Y, Zhang Z, Lin X and He X: Control of β-catenin
phosphorylation/degradation by a dual-kinase mechanism. Cell.
108:837–847. 2002.
|
|
12.
|
Moon RT, Boweman B, Boutros M and Perrimon
N: The promise and perils of Wnt signaling through β-catenin.
Science. 296:1644–1646. 2002.PubMed/NCBI
|
|
13.
|
Lee SY, Jeon HM, Ju MK, Kim CH, Jeong EK,
Park HG and Kang HS: Snail switches 5-FU-induced apoptosis to
necrosis through Akt/PKB activation and p53 down-regulation. J Life
Sci. 22:1018–1023. 2012. View Article : Google Scholar
|
|
14.
|
Wodarz A and Nusse R: Mechanisms of Wnt
signaling in development. Annu Rev Cell Dev Biol. 14:59–88. 1998.
View Article : Google Scholar
|
|
15.
|
Uthoff SM, Eichenberger MR, McAuliffe TL,
Hamilton CJ and Galandiuk S: Wingless type frizzled protein
receptor signaling and its putative role in human colon cancer. Mol
Carcinog. 31:56–62. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
16.
|
He TC, Sparks AB, Rago C, Hermeking H,
Zawel L, da Costa LT, Morin PJ, Vogelstein B and Kinzler KW:
Identification of c-MYC as a target of the APC pathway. Science.
281:1509–1512. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Tetsu O and McCormick F: β-catenin
regulates expression of cyclin D1 in colon carcinoma cells. Nature.
398:422–426. 1999.
|
|
18.
|
Pongracz JE and Stockley RA: Wnt signaling
in lung development and diseases. Respir Res. 26:7–15. 2006.
|
|
19.
|
Di Stefano A, Maestrelli P, Roggeri A,
Turato G, Calabro S, Potena A, Mapp CE, Ciaccia A, Covacev L,
Fabbri LM and Saetta M: Upregulation of adhesion molecules in the
bronchial mucosa of subjects with chronic obstructive bronchitis.
Am J Respir Crit Care Med. 149:803–810. 1994.PubMed/NCBI
|
|
20.
|
Kim YM, Kim IH and Nam TJ: Induction of
apoptosis signaling by a glycoprotein of Capsosiphon
fulvescens in AGS cell. Kor J Fish Aquat Sci. 44:216–224.
2011.
|
|
21.
|
Kim YM, Kim IH and Nam TJ: Capsosiphon
fulvescens glycoprotein reduces AGS gastric cancer cell
migration by downregulating transforming growth factor-β1 and
integrin expression. Int J Oncol. 43:1059–1065. 2013.
|
|
22.
|
Chea SC: Inhibitory effect of naringenin
on MMP-2, −9 activity and expression in HT-1080 cells. J Environ
Toxicol. 24:63–70. 2009.PubMed/NCBI
|
|
23.
|
Rezai KA, Farrokh-Siar L, Gasyna EM and
Ernest JT: Trypan blue induces apoptosis in human retinal pigment
epithelial cells. Am J Ophthalmol. 138:492–495. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
24.
|
He B, You L, Uematsu K, Xu Z, Lee AY,
Matsangou M, McCormick F and Jablons DM: A monoclonal antibody
against Wnt-1 induces apoptosis in human cancer cells. Neoplasia.
6:7–14. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
25.
|
Miller JR: The Wnts. Genome Biol.
3:Reviews3001. 2002.
|
|
26.
|
Van Gijn ME, Daemen MJ, Smits JF and
Blankestejin WM: The Wnt-frizzled cascade in cardiovascular
disease. Cardiovasc Res. 55:16–24. 2002.PubMed/NCBI
|
|
27.
|
Green JL, Kuntz SG and Sternberg PW: Ror
receptor tyrosine kinases: orphans no more. Trends Cell Biol.
18:536–544. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
28.
|
Angers S and Moon RT: Proximal events in
Wnt signal transduction. Nat Rev Mol Cell Biol. 10:468–477.
2009.PubMed/NCBI
|
|
29.
|
Clevers H: Wnt/β-catenin signaling in
development and disease. Cell. 127:469–480. 2006.
|
|
30.
|
MacDonal BT, Tamai K and He X:
Wnt/β-catenin signaling: components, mechanism, and diseases. Dev
Cell. 17:9–26. 2009.
|
|
31.
|
Kunnumakkara AB, Diagaradjane P, Anand P,
Harikumar KB, Deorukhkar A, Gelovani J, Guha S, Krishnan S and
Aggarwal BB: Curcumin sensitizes human colorectal cancer to
capecitabine by modulation of cyclin D1, COX-2, MMP-9, VEGF and
CXCR4 expression in an orthotopic mouse model. Int J Cancer.
125:2187–2197. 2009. View Article : Google Scholar
|
|
32.
|
Tharakan ST, Inamoto T, Sung B, Aggarwal
BB and Kamat AM: Curcumin potentiates the antitumor effects of
gemcitabine in an orthtopic model of human bladder cancer through
suppression of proliferative and angiogenic biomarkers. Biochem
Pharmacol. 79:218–228. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
33.
|
Kim YM, Kim IH and Nam TJ: Induction of
apoptosis signaling by glycoprotein of Capsosiphon
fulvescens in human gastric cancer (AGS) cells. Nutr Cancer.
64:761–769. 2012. View Article : Google Scholar : PubMed/NCBI
|