Introduction
Thioredoxin domain containing 5 (TXNDC5) protein,
also named ERp46, has a protein disulfide isomerase (PDI) domain
that exhibits a high sequence similarity to thioredoxin, a catalyst
of the rate limiting reaction of disulfide bond formation,
isomerization and reduction (1,2).
Yeast complementation tests showed that TXNDC5 can conduct PDI
functions in vivo (3).
Indirect immunofluorescence microscopy and subcellular
fractionation studies have shown that TXNDC5 is present both in the
endoplasmic reticulum and in the plasma membrane (4). TXNDC5 is highly expressed in
endothelial cells during hypoxic conditions, and it plays important
roles in anti-oxidative injury, anti-anoxia-induced apoptosis and
the promotion of cell proliferation (1,2).
Recent studies reported that TXNDC5 is overexpressed
in some tumors including the cervix, uterus, stomach and lung
cancers (5). Wang et al
also found that TXNDC5 was significantly upregulated in colorectal
cancer tissues compared with normal mucosa (6). Vincent et al demonstrated that
62% of non-small cell lung carcinomas exhibit an increase in TXNDC5
expression compared with normal lung tissue (7). TXNDC5 has been reported to be
involved in cancer progression. Zhang et al inserted TXNDC5
cDNA into the gastric cell line HFE145. They demonstrated that
TXNDC5 promotes growth, proliferation and invasion of gastric cells
(8). Thus, TXNDC5 can be thought
of as a tumor-enhancing gene. However, there is no comprehensive
investigation reported on TXNDC5 expression in various tumor types,
and not much is known about the functional roles of the TXNDC5 in
the tumorigenic process.
In the present study, we investigated the expression
of TXNDC5 in various tumors. We also investigated the role of
TXNDC5 in cell proliferation and migration of cultured tumor cell
lines using small interfering RNAs. In addition, we determined
whether common polymorphisms in TXNDC5 are associated with various
tumors using microarray. To our knowledge, this is the first study
to examine this potential association of the genes with tumors.
Materials and methods
Western blot analysis
Breast cancer (n=4), gastric adenocarcinoma (n=4)
and rectal cancer (n=4) tissue samples were collected during
excision surgery in Shandong Provincial Qianfoshan Hospital (Jinan,
China). Parallel healthy tissues 5 cm from the tumors were also
collected during the excision surgery. The tumor diagnosis was
verified by histological methods, and pathological categorization
was performed according to the World Health Organization (WHO)
classification system. All patients signed informed consents, and
this study was approved by the ethics committee of Shandong
Provincial Qianfoshan Hospital.
Two hundred micrograms of the tumor tissues from
gastric adenocarcinoma, rectal cancer, breast cancer and their
parallel healthy tissues were homogenized in Cell Lysis Solution
(Sigma) and centrifuged at 16,000 × g for 5 min at 4˚C. The total
protein was separated by sodium dodecyl sulfate-polyacryl-amide gel
electrophoresis (SDS-PAGE) and trans-blotted onto nitrocellulose
membranes (Amersham, Piscataway, NJ, USA). Western blot analysis
was conducted using antibodies against human TXNDC5 at a 2,000-fold
dilution overnight at 4˚C. The anti-TXNDC5 antibodies that were
used were raised in goats using the oligopeptide SLHRFVLSQAKDEL
from TXNDC5. The western signals were visualized using the Protein
Detector BCIP/NBT Western Blot kit (Beyotime, Haimen, China)
following the manufacturer’s instructions. A separate membrane that
was prepared by the same protocol was probed with an anti-GAPDH
antibody (Santa Cruz Biotechnology, Santa Cruz, CA, USA) to
normalize the sample loading.
Immunohistochemistry
Tissue arrays were obtained commercially from
Chaoying Bioscience (Shanxi, China). The array slides contained
tumor and normal samples from each of the following tissues: breast
(n=208), cervical (n=33), colon carcinoma (n=50), esophageal cancer
(n=76), gastric carcinoma (n=84), hepatocellular (n=73), ovarian
(n=46), pancreatic cancer (n=24), prostate cancer (n=54), rectal
(n=29) and small cell lung (n=100). Clinical data, including the
age, sex, clinical pathological diagnosis, and origin of every
participant, were provided by the manufacturer. Tissue sections
were de-paraffinized and re-hydrated by standard procedures. Before
the anti-TXNDC5 antibodies were applied, the tissue sections were
heated at 95˚C for 10 min in citrate buffer solution (Sigma) for
antigen recovery followed by incubation with an endogenous
peroxidase inhibitor (Maixin-Bio, Fuzhou, China) for 30 min at room
temperature. After washing with PBS buffer (NaCl 0.132 M,
K2HPO4 0.0066 M, KH2PO4
0.0015 M in distilled water, pH 7.6), the sections were incubated
with an antibody directed against TXNDC5 (Abcam) overnight at 4˚C.
Immunoreactions were processed using the UltraSensitive™ S-P kit
(Maixin-Bio) according to the manufacturer's instructions.
Immunoreactive signals were visualized using the DAB substrate,
which stains the target protein yellow. Cell structures were
counterstained with hematoxylin.
To determine the antibody specificity and optimize
the antibody dilution, the tissue samples were incubated with goat
pre-immune serum (Maixin-Bio) or treated with the modification
buffer without addition of antibody.
Inhibiting TXNDC5 expression in cultured
tumor cell lines with siRNAs
HeLa cells (originally from human cervical cancer)
and U2OS cells (originally from human osteosarcoma) were cultured
in McCoy's 5A medium (Gibco-BRL, Carlsbad, CA, USA) supplemented
with 100 U/ml penicillin, 100 mg/ml streptomycin (Gibco-BRL) and
15% (v/v) fetal bovine serum (Gibco-BRL). siRNA oligonucleotides
targeting TXNDC5 (target mRNA sequence:
5′-CACATACAGGCTTAAGCTCTA-3′) were designed and synthesized by
Qiagen (Hilden, Germany). Cultured tumor cells were transfected
with 100 nM of the siRNA using the HiPerFect transfection reagent
(Qiagen) according to the manufacturer's protocol. The cells were
harvested for analysis 48 h following the transfection. Mm/Hs-MAPK1
siRNA (AATGCTGACTCCAAAGCTCTG) and AllStars Negative Control siRNA,
which were provided with the kit, were used as positive and
negative controls, respectively, in parallel transfections.
Mm/Hs_MAPK1 Control siRNA is a positive control targeting both the
human and mouse MAPK1 genes. AllStars Negative Control siRNA is the
most thoroughly tested and validated negative control siRNA.
Cell proliferation assay
U2OS and HeLa cells were seeded onto 96-well culture
plates and incubated until they reached 80% confluence. The
cultures were then treated with TXNDC5 siRNA as described above. An
MTT [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide]
assay was performed 48 h later by adding 100 μl of 1 mg/ml
MTT (Amresco, Solon, OH, USA) mixed with culture media into each
well and incubating for 4 h at 37˚C in the dark. The MTT solution
was then removed, and the cells were washed twice in PBS followed
by air drying. The resulting MTT-formazan products were extracted
with 100 μl DMSO in the dark at room temperature, and their
absorbance was measured at 490 nm with a spectrophotometer. The
data were obtained from three independent experiments.
Cell migration assay
A cell migration assay was conducted in a Transwell
apparatus (Costar Corning, Corning, NY, USA). Cells were seeded in
the upper compartment of the Transwell apparatus while the lower
compartment was filled with DMEM with 10% FBS, and the plates were
incubated at 37˚C for 24 h until the cells reached 80% confluence.
At this point, the cells were transfected with 100 nM of TXNDC5
siRNA, and the cells were incubated for an additional 48 h in the
incubator. The upper surface of the insert was wiped with cotton
swabs to remove non-invading cells, and the bottom surface of the
insert was stained with Giemsa. The number of cells that invaded
through the membrane was counted in 5 random fields at ×100
magnification. The data were obtained from three independent
experiments.
Statistical analysis of the data was performed using
the SPSS V.16 software (SPSS, Chicago, IL, USA). Multiple
comparisons were conducted with ANOVA, and a t-test was used to
assess significant differences between two groups. P-values that
were <0.05 were considered significant. The errors in the data
are shown as standard deviation (SD).
Genomic DNA isolation, SNP selection
Genomic DNA was extracted from whole blood samples
with the Omega E-Z 96 Blood DNA kit (Omega Bio-Tek, Norcross, GA,
USA) according to the manufacturer's protocol. After extraction,
the genomic DNA was diluted to a final concentration of 15–20
ng/μl for the genotyping assays.
Tag single nucleotide polymorphisms (tagSNPs) across
the TXNDC5 gene were determined by searching the HapMap database.
Only SNPs with minor allele frequency greater (MAF) than 5% with a
pair-wise r2 ≥0.8 were considered. Ninty-six SNPs that
span 185,000 bases of the chromosome 6 were selected. The SNP
information including locus name, gene symbol, coordinate, location
and coding status is shown in Table
I.
 | Table I.SNPs selected for illumina
microarray. |
Table I.
SNPs selected for illumina
microarray.
| Locus name | Gene symbol | Coordinate | Location | Coding status |
|---|
| rs1044104 | BMP6/TXNDC5 | 7881311 | COMPLEX | |
| rs9505298 | BMP6/TXNDC5 | 7881449 | COMPLEX | |
| rs41302895 | TXNDC5/BMP6 | 7881754 | COMPLEX | |
| rs1043784 | TXNDC5/BMP6 | 7881931 | COMPLEX | |
| rs7764128 | BMP6/TXNDC5 | 7882205 | COMPLEX | |
| rs8643 | BMP6/TXNDC5 | 7883073 | COMPLEX | |
| rs9502656 | TXNDC5 | 7883386 | CODING | NONSYN |
| rs35264740 | TXNDC5 | 7883865 | INTRON | |
| rs35871461 | TXNDC5 | 7884291 | INTRON | |
| rs2277105 | TXNDC5 | 7884652 | CODING | NONSYN |
| rs35126514 | TXNDC5 | 7885048 | INTRON | |
| rs1225936 | TXNDC5 | 7885184 | INTRON | |
| rs1225937 | TXNDC5 | 7885302 | INTRON | |
| rs11758961 | TXNDC5 | 7885797 | INTRON | |
| rs1225938 | TXNDC5 | 7886534 | INTRON | |
| rs454654 | TXNDC5 | 7886639 | INTRON | |
| rs11962800 | TXNDC5 | 7886905 | INTRON | |
| rs9505301 | TXNDC5 | 7887131 | INTRON | |
| rs372578 | TXNDC5 | 7887223 | INTRON | |
| rs7740689 | TXNDC5 | 7888066 | INTRON | |
| rs89715 | TXNDC5 | 7888168 | INTRON | |
| rs7745225 | TXNDC5 | 7888251 | INTRON | |
| rs378963 | TXNDC5 | 7888328 | INTRON | |
| rs45441296 | TXNDC5 | 7889033 | CODING | NONSYN |
| rs34782746 | TXNDC5 | 7889254 | INTRON | |
| rs7746818 | TXNDC5 | 7889466 | INTRON | |
| rs71559189 | TXNDC5 | 7889796 | CODING | NONSYN |
| rs60084141 | TXNDC5 | 7889894 | INTRON | |
| rs1225947 | TXNDC5 | 7890121 | INTRON | |
| rs13873 | TXNDC5 | 7891160 | INTRON | |
| rs73365786 | TXNDC5 | 7891230 | INTRON | |
| rs7771314 | TXNDC5 | 7891403 | INTRON | |
| rs34599679 | TXNDC5 | 7891514 | INTRON | |
| rs1225949 | TXNDC5 | 7891673 | INTRON | |
| rs3734589 | TXNDC5 | 7891775 | INTRON | |
| rs9502658 | TXNDC5 | 7891947 | CODING | SYNON |
| rs35365768 | TXNDC5 | 7892037 | INTRON | |
| rs1225950 | TXNDC5 | 7892143 | INTRON | |
| rs11759946 | TXNDC5 | 7892360 | INTRON | |
| rs72829238 | TXNDC5 | 7892575 | INTRON | |
| rs7749719 | TXNDC5 | 7894695 | INTRON | |
| rs58711083 | TXNDC5 | 7895348 | CODING | NONSYN |
| rs443861 | TXNDC5 | 7896491 | INTRON | |
| rs369086 | TXNDC5 | 7898875 | INTRON | |
| rs408014 | TXNDC5 | 7899394 | INTRON | |
| rs13218143 | TXNDC5 | 7899573 | INTRON | |
| rs383084 | TXNDC5 | 7899657 | INTRON | |
| rs1225954 | TXNDC5 | 7900028 | INTRON | |
| rs1225955 | TXNDC5 | 7900709 | INTRON | |
| rs6933089 | TXNDC5 | 7900856 | INTRON | |
| rs13209404 | TXNDC5 | 7909967 | INTRON | |
| rs13210097 | TXNDC5/MUTED | 7911345 | INTERGENIC | |
| rs9502663 | TXNDC5/MUTED | 7911474 | INTERGENIC | |
| rs3812162 | TXNDC5/MUTED | 7911702 | INTERGENIC | |
| rs34066135 | MUTED/TXNDC5 | 7911855 | INTERGENIC | |
| rs72829251 | TXNDC5/MUTED | 7911982 | INTERGENIC | |
| rs1632346 | TXNDC5/MUTED | 7913546 | INTERGENIC | |
| rs1743634 | TXNDC5/MUTED | 7916207 | INTERGENIC | |
| rs9505309 | MUTED/TXNDC5 | 7917528 | INTERGENIC | |
| rs6922018 | MUTED/TXNDC5 | 7918311 | INTERGENIC | |
| rs6923488 | MUTED/TXNDC5 | 7918405 | INTERGENIC | |
| rs1594467 | TXNDC5/MUTED | 7920361 | INTERGENIC | |
| rs419588 | MUTED/TXNDC5 | 7920808 | INTERGENIC | |
| rs365936 | MUTED/TXNDC5 | 7920904 | INTERGENIC | |
| rs1237879 | TXNDC5/MUTED | 7932261 | INTERGENIC | |
| rs627957 | MUTED/TXNDC5 | 7936475 | INTERGENIC | |
| rs155487 | MUTED/TXNDC5 | 7938773 | INTERGENIC | |
| rs10484327 | TXNDC5/MUTED | 7942566 | INTERGENIC | |
| rs7764884 | MUTED/TXNDC5 | 7970540 | INTERGENIC | |
| rs7763447 | MUTED/TXNDC5 | 7973380 | INTERGENIC | |
| rs4959462 | MUTED/TXNDC5 | 7975135 | INTERGENIC | |
| rs6597292 | TXNDC5/MUTED | 7975259 | INTERGENIC | |
| rs197119 | TXNDC5/MUTED | 7976745 | INTERGENIC | |
| rs6597293 | TXNDC5/MUTED | 7987883 | INTERGENIC | |
| rs11754300 | TXNDC5/MUTED | 7988766 | INTERGENIC | |
| rs7744601 | TXNDC5/MUTED | 7988910 | INTERGENIC | |
| rs2567226 | MUTED/TXNDC5 | 7993977 | INTERGENIC | |
| rs12204273 | TXNDC5/MUTED | 8002705 | INTERGENIC | |
| rs9392182 | MUTED/TXNDC5 | 8009035 | INTERGENIC | |
| rs2207720 | MUTED | 8019197 | INTRON | |
| rs9392189 | MUTED | 8021532 | INTRON | |
| rs2815128 | MUTED | 8023462 | INTRON | |
| rs2815142 | MUTED | 8043546 | INTRON | |
| rs2743992 | MUTED | 8054722 | INTRON | |
| rs2294436 | MUTED | 8057688 | INTRON | |
| rs2743991 | MUTED | 8060175 | INTRON | |
| rs12663430 | MUTED | 8060534 | INTRON | |
| rs7763203 | MUTED | 8061872 | INTRON | |
| rs9405369 | MUTED | 8062437 | INTRON | |
| rs12207627 | MUTED | 8062532 | INTRON | |
| rs2743989 | MUTED | 8064035 | INTRON | |
| rs2743987 | MUTED | 8064303 | INTRON | |
| rs35991100 | EEF1E1/MUTED | 8064677 | INTERGENIC | |
| rs9328453 | MUTED/EEF1E1 | 8065127 | INTERGENIC | |
| rs2815155 | MUTED/EEF1E1 | 8065230 | INTERGENIC | |
| rs12660697 | EEF1E1/MUTED | 8065707 | INTERGENIC | |
| rs7751386 | MUTED/EEF1E1 | 8066414 | INTERGENIC | |
Genotyping using Illumina 384-SNP
VeraCode microarray
We performed genotyping using a custom-designed
Illumina 384-SNP VeraCode microarray (Illumina). Peripheral blood
samples were collected from patients with breast cancer (n=281; 281
female; aged 26–80 years; mean, 49.3 years), cervical carcinoma
(n=197; 197 female; aged 29–61 years; mean, 54.4 years), esophageal
carcinoma (n=221; 30 female; aged 39–86 years; mean, 60.8 years),
gastric carcinoma (n=308; 64 female; aged 31–80 years; mean 58.2
years) and liver cancer (n=202; 30 female; aged 30–79 years; mean,
55.9 years). A total of 374 (125 female, aged 24–58 years) healthy
individuals with a mean age of 40.2 years were blood donors. All
patients signed informed consents, and this study was approved by
the ethics committee of Shandong Provincial Qianfoshan Hospital.
The genotyping was conducted with the BeadXpress Reader using the
Illumina VeraCode GoldenGate Assay kit. A total of 500 ng of sample
DNA were used per assay. Genotype clustering and calling were
performed using BeadStudio software (Illumina). This study was
completed at Beijing Institute of Genomics of Chinese Academy of
Sciences, which provided technical service for the genotyping.
The genotyping quality was examined by a detailed
quality control procedure consisting of a >95% successful call
rate, duplicate calling of the genotypes, internal positive control
samples and Hardy-Weinberg Equilibrium (HWE) testing. The SNPs were
analyzed for association by comparing the MAF between the cases and
controls. Dominant and recessive models were considered with
respect to the minor allele. Associations of the SNPs with the
diseases were evaluated using odds ratios (OR) with 95% confidence
intervals (CI). Fisher's exact test was used for comparisons
between categorical variables. P-values <0.05 were considered to
be statistically significant. Genotypic associations were assessed
using Plink v1.07 (http://pngu.mgh.harvard.edu/purcell/plink/) and SHEsis
(http://analysis.bio-x.cn/myAnalysis.php) software
(9,10). Multiple-test correction, including
genomic-control correction, Bonferroni single-step correction, Holm
step-down correction and Sidak single-step correction, were
performed by Plink v1.07. Linkage disequilibrium (LD), coefficient
(D' and r2) and haplotype were estimated by software
Haploview 4.2 (http://www.broad.mit.edu/mpg/haploview/) software
(11).
Results
TXNDC5 expression in various tumor
tissues
Western blots revealed a protein with a molecular
weight of 50 kDa in each of the tumor tissues and parallel normal
tissues. The expression level was normalized using GAPDH as a
reference. Compared with the expression in the parallel normal
tissues, significantly increased TXNDC5 expression was detected in
the gastric adenocarcinoma, rectal cancer and breast cancer
tissues. These results were consistently observed in all the
samples of these tumor types. The results are shown in Fig. 1.
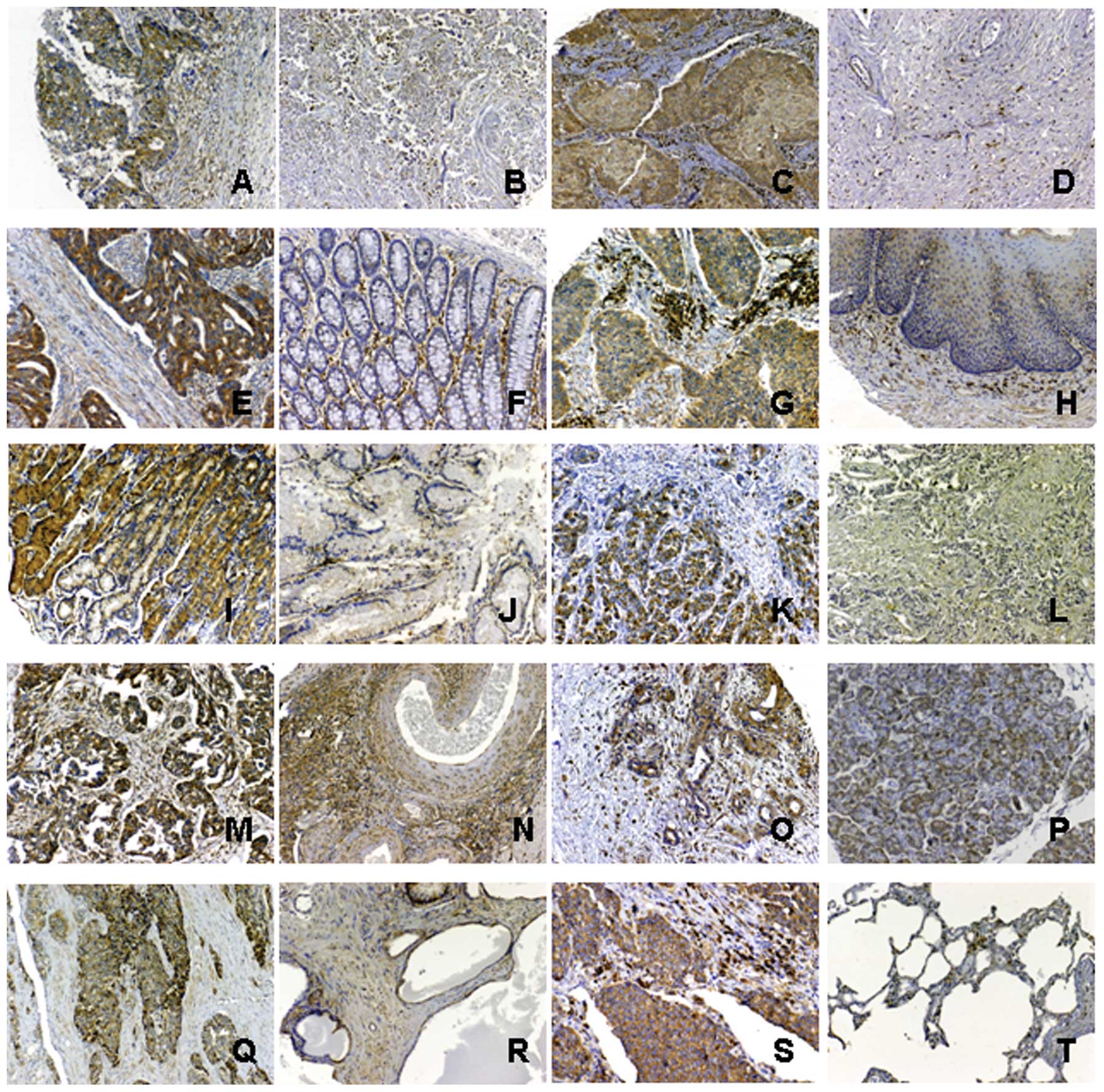
Immunohistochemistry was used to detect the
expression of TXNDC5 in a panel of tumor tissues. TXNDC5 was
significantly expressed in the tumor tissues from breast invasive
ductal carcinomas (n=170). The immuno-signal was observed in both
tumor cells and mesenchymal cells in these tissues. TXNDC5 was not
detected in the corresponding normal tissues except in some
mesenchymal cells (n=38). TXNDC5 was significantly expressed in the
tumor tissues from cervical squamous cell carcinomas (n=22). The
immuno-signal was observed in both tumor cells and mesenchymal
cells in these tissues. TXNDC5 was not detected in the parallel
normal tissues (n=6) and tissues from cervical adenocarcinomas
(n=2) except in some mesenchymal cells. The expression of TXNDC5
was also observed in mesenchymal cells from the chronically
inflamed mucosa of the uterus, but the signal density was relative
low (n=3). TXNDC5 was significantly expressed in the tumor tissues
from colon carcinomas (n=18) and rectal cancers (n=23). Normal
tissue from the colon (n=32) and the rectum (n=6) also showed
TXNDC5 expression. The protein was expressed in both the tumor
cells and mesenchymal cells, but the number of TXNDC5-positive
mesenchymal cells was relatively low in the normal tissues. TXNDC5
expression was detected in tissues from esophageal squamous cell
carcinomas (n=38) but not in healthy esophageal tissue (n=28). Its
expression was also very strong in mesenchymal cells from the
chronically inflamed mucosa of the esophagus (n=10). TXNDC5
expression was detected in gastric carcinoma (n=43). The expression
was present in both the tumor cells and mesenchymal cells of the
tumor tissues. TXNDC5 expression was also detected in mesenchymal
cells from the inflamed gastric mucosa (n=23). TXNDC5 was not
detected in healthy gastric tissues except in some mesenchymal
cells (n=18). TXNDC5 was detected in hepatocellular carcinomas
(n=51), but it was not present in samples from patients with
chronic hepatitis cirrhosis (n=22). TXNDC5 was significantly
expressed in tissues from ovarian papillary serous carcinomas
(n=14), ovarian endometrioid adenocarcinomas (n=6) and ovarian
clear cell carcinomas (n=2). Expression was detected in both tumor
cells and mesenchymal cells of these tumor tissues. TXNDC5 was also
detected in some mesenchymal cells of normal ovary tissues (n=24).
TXNDC5 was significantly expressed in tumor cells and mesenchymal
cells from carcinomas of the prostate (n=48). TXNDC5 expression was
also detected in some mesenchymal cells and endothelial cells from
prostates undergoing hyperplasia (n=6). TXNDC5 was expressed in
tumor cells and mesenchymal cells from pancreatic cancers (n=12).
The expression was also detected in cells of the normal pancreatic
tissues (n=12). TXNDC5 showed strong expression in tumor cells and
mesenchymal cells from undifferentiated cell carcinomas of the lung
(n=80). TXNDC5 expression was also detected in some mesenchymal
cells of normal lung tissues (n=20). Immunostaining of TXNDC5 was
observed in the cytoplasm of tumor cells and mesenchymal cells of
both tumor tissues and the health tissues. These
immunohistochemical results are shown in Fig. 2.
Cell proliferation, migration and
invasion in the presence of TXNDC5 siRNA
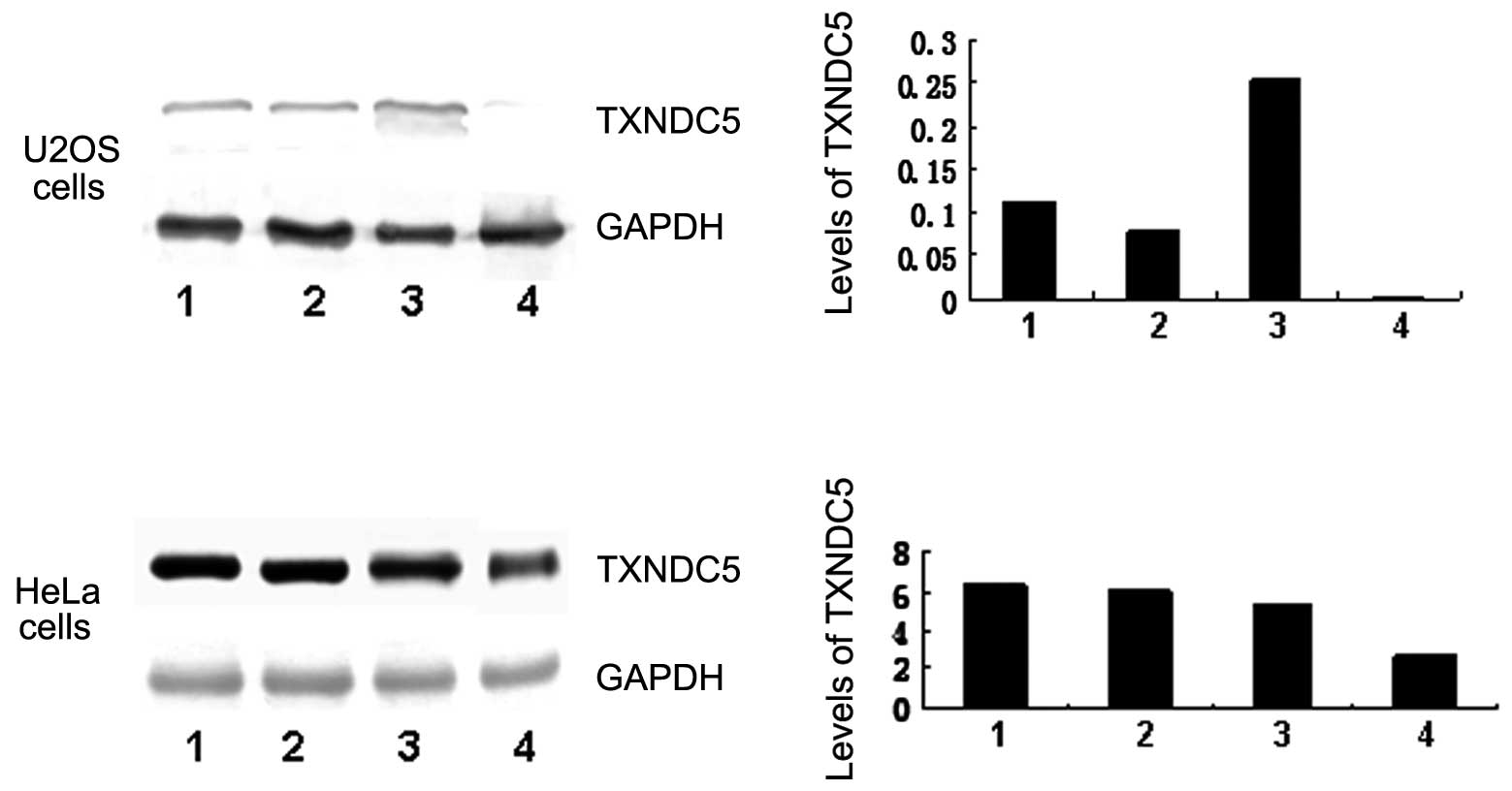
Cultured HeLa and U2OS cells were transfected with
100 nM siRNA. TXNDC5 expression in transfected U2OS was decreased
considerably compared with untransfected cells. TXNDC5 expression
was also mildly decreased in the siRNA-transfected HeLa cells.
TXNDC5 levels were not significantly changed in the positive
controls, negative controls or untransfected cells. The result is
seen in Fig. 3.
A cell proliferation assay was performed using the
U2OS cells and HeLa cells that were transfected with TXNDC5 siRNA.
U2OS cells transfected with 100 nM TXNDC5 targeted siRNA exhibited
a significant decrease in cell proliferation when compared with the
positive and negative controls. Cell proliferation was mildly
decreased in HeLa cells transfected with TXNDC5 targeted siRNA
compared with untransfected cells. The data shown were obtained
from three independent experiments. The result is shown in Fig. 4.
The migratory ability of U2OS cells and HeLa cells
was examined using a 2-compartment Transwell system. Migration of
both cell lines was significantly decreased when TXNDC5 expression
was suppressed by TXNDC5 siRNA transfection. The data shown here
were obtained from three independent experiments. The result is
shown in Fig. 5.
Genotyping SNPs located in TXNDC5
Ninety-seven SNPs across TXNDC5 gene were genotyped
using illumina micro-array. All of the SNPs yielded genotypic data,
and the study sample success rate was 98.1%. SNPs except rs1225938,
rs408014, rs1225954, rs2207720 and rs2743989 were in Hardy-Weinberg
equilibrium (P>0.05) within the health samples. SNPs rs9502656,
rs35264740, rs35871461, rs2277105, rs35126514, rs11758961,
rs7740689, rs34782746, rs7746818, rs73365786, rs34599679,
rs9502658, rs35365768, rs7749719, rs13218143, rs6933089, rs9502663,
rs34066135, rs35991100, rs9328453 and rs7751386 did not show
polymorphisms in the studied subjects. The differences in allele
and genotype frequencies between the cases and controls were
compared. The case-control analysis showed a significant difference
in allele frequency and genotype frequency for rs41302895 between
breast cancer patients and health controls. The allele frequency
and genotype frequency for rs9505298, rs41302895, rs7771314 and
rs155487 provided statistically significant evidence for an
association with cervical carcinoma. The case-control analysis
showed a significant difference in allele frequency and genotype
frequency for rs1225950 and rs2815128 between esophageal carcinoma
patients and health controls. The analysis also showed a
significant difference in allele frequency and genotype frequency
for rs13210097 and rs9392182 between liver cancer patients and the
controls. In addition, the allele frequency for rs383084,
rs1632346, rs1237879 and rs11754300 provided statistically
significant evidence for an association with cervical carcinoma,
esophageal carcinoma, gastric carcinoma and liver cancer,
respectively. The genotype frequency for rs13873, rs1632346,
rs9505309, rs1594467 and rs2815142 provided statistically
significant evidence for an association with esophageal carcinoma,
gastric carcinoma and breast cancer. Following multiple-test
correction, these SNPs rs9505298 and rs7771314 still had
significant difference in allelic frequency and genotypic frequency
between cervical carcinoma patients and the controls; SNPs
rs1632346, rs9505309, rs2815128 and rs2815142 still had significant
difference in allelic frequency and genotypic frequency between
esophageal carcinoma patients and the controls; SNPs rs13210097,
rs11754300, rs9392182 and rs2815128 still had significant
difference in allelic frequency and genotypic frequency between
liver cancer patients and the controls. The above result is shown
in Table II. The Illumina
microarray data has been submitted to NCBI Gene Expression Omnibus
(GEO), a public functional genomics data repository supporting
MIAME-compliant data submissions. The record was approved and
assigned GEO accession number (GSE39428).
 | Table II.Allele and genotype frequencies in a
case control (n=384) cohort of patients with various tumors. |
Table II.
Allele and genotype frequencies in a
case control (n=384) cohort of patients with various tumors.
| Breast cancer
(n=281) | Cervical carcinoma
(n=197) | Esophageal
carcinoma (n=221) | Gastric carcinoma
(n=308) | Liver cancer
(n=202) |
|---|
| rs9505298 | | | | | | | | | | | | | | | |
| Allele | A | G | | A | G | | A | G | | A | G | | A | G | |
| Allele case
(frequency) | 0 (0.000) | 420 (1.000) | | 16 (0.049) | 312 (0.951) | | 0 (0.000) | 316 (1.000) | | 0 (0.000) | 498 (1.000) | | 0 (0.000) | 348 (1.000) | |
| Allele control
(frequency) | 1 (0.002) | 565 (0.998) | | 1 (0.002) | 565 (0.998) | | 1 (0.002) | 565 (0.998) | | 1 (0.002) | 565 (0.998) | | 1 (0.002) | 565 (0.998) | |
| Odds ratio [95%
CI] | | 28.974360
[3.824260–219.523117] | | | |
| Fisher’s
p-value | 0.388806 | 7.27E-07 | 0.454724 | 0.348059 | 0.432756 |
| Genotype | A/G | G/G | | A/G | G/G | | A/G | G/G | | A/G | G/G | | A/G | G/G | |
| Genotype case
(frequency) | 0 (0.000) | 210 (1.000) | | 16 (0.098) | 148 (0.902) | | 0 (0.000) | 158 (1.000) | | 0 (0.000) | 249 (1.000) | | 0 (0.000) | 174 (1.000) | |
| Genotype control
(frequency) | 1 (0.004) | 282 (0.996) | | 1 (0.004) | 282 (0.996) | | 1 (0.004) | 282 (0.996) | | 1 (0.004) | 282 (0.996) | | 1 (0.004) | 282 (0.996) | |
| Odds ratio [95%
CI] | | 30.486486
[4.003584–232.148453] | | | |
| Fisher’s
p-value | 0.388565 | 5.66E-07 | 0.454468 | 0.347832 | 0.432504 |
| HWE for case
(Fisher’s p-value) | 1 | 0.511384 | 1 | 1 | 1 |
| HWE for control
(Fisher’s p-value) | 0.976248 | 0.976248 | 0.976236 | 0.976236 | 0.976236 |
|
| rs41302895 | | | | | | | | | | | | | | | |
| Allele | A | T | | A | T | | A | T | | A | T | | A | T | |
| Allele case
(frequency) | 3 (0.005) | 551 (0.995) | | 2 (0.006) | 326 (0.994) | | 1 (0.002) | 415 (0.998) | | 606 (1.000) | | | 1 (0.003) | 397 (0.997) | |
| Allele control
(frequency) | 0 (0.000) | 746 (1.000) | | 0 (0.000) | 746 (1.000) | | 0 (0.000) | 746 (1.000) | | 746 (1.000) | | | 0 (0.000) | 746 (1.000) | |
| Odds ratio [95%
CI] | | | | | |
| Fisher’s
p-value | 0.04425 | 0.032828 | 0.180402 | | 0.17085 |
| Genotype | A/T | T/T | | A/T | T/T | | A/T | T/T | | A/T | T/T | | A/T | T/T | |
| Genotype case
(frequency) | 3 (0.011) | 274 (0.989) | | 2 (0.012) | 162 (0.988) | | 1 (0.005) | 207 (0.995) | | 303 (1.000) | | | 1 (0.005) | 198 (0.995) | |
| Genotype control
(frequency) | 0 (0.000) | 373 (1.000) | | 0 (0.000) | 373 (1.000) | | 0 (0.000) | 373 (1.000) | | 373 (1.000) | | | 0 (0.000) | 373 (1.000) | |
| Odds ratio [95%
CI] | | | | | |
| Fisher’s
p-value | 0.044004 | 0.032665 | 0.180214 | | 0.170662 |
| HWE for case
(Fisher’s p-value) | 0.927801 | 0.937381 | 0.972279 | | 0.971656 |
| HWE for control
(Fisher’s p-value) | 1 | 1 | 1 | | 1 |
|
| rs13873 | | | | | | | | | | | | | | | |
| Allele | A | C | | A | C | | A | C | | A | C | | A | C | |
| Allele case
(frequency) | 317 (0.574) | 235 (0.426) | | 184 (0.571) | 138 (0.429) | | 262 (0.633) | 152 (0.367) | | 359 (0.594) | 245 (0.406) | | 242 (0.611) | 154 (0.389) | |
| Allele control
(frequency) | 436 (0.586) | 308 (0.414) | | 436 (0.586) | 308 (0.414) | | 436 (0.586) | 308 (0.414) | | 436 (0.586) | 308 (0.414) | | 436 (0.586) | 308 (0.414) | |
| Odds ratio [95%
CI] | 0.952918
[0.762409–1.191032] | 0.941896
[0.722936–1.227174] | 1.217649
[0.950749–1.559474] | 1.035125
[0.832102–1.287682] | 1.110092
[0.865227–1.424254] |
| Fisher’s
p-value | 0.671733 | 0.657439 | 0.118635 | 0.756631 | 0.411361 |
| Genotype | A/A | A/C | C/C | A/A | A/C | C/C | A/A | A/C | C/C | A/A | A/C | C/C | A/A | A/C | C/C |
| Genotype case
(frequency) | 89 (0.322) | 139 (0.504) | 48 (0.174) | 52 (0.323) | 80 (0.497) | 29 (0.180) | 78 (0.377) | 106 (0.512) | 23 (0.111) | 108 (0.358) | 143 (0.474) | 51 (0.169) | 74 (0.374) | 94 (0.475) | 30 (0.152) |
| Genotype control
(frequency) | 134 (0.360) | 168 (0.452) | 70 (0.188) | 134 (0.360) | 168 (0.452) | 70 (0.188) | 134 (0.360) | 168 (0.452) | 70 (0.188) | 134 (0.360) | 168 (0.452) | 70 (0.188) | 134 (0.360) | 168 (0.452) | 70 (0.188) |
| Odds ratio [95%
CI] | | | | | |
| Fisher’s
p-value | 0.419488 | 0.612423 | 0.048781 | 0.77025 | 0.547495 |
| HWE for case
(Fisher’s p-value) | 0.618517 | 0.854105 | 0.142498 | 0.754469 | 0.986746 |
| HWE for control
(Fisher’s p-value) | 0.181888 | 0.181888 | 0.181888 | 0.181888 | 0.181888 |
|
| rs7771314 | | | | | | | | | | | | | | | |
| Allele | A | G | | A | G | | A | G | | A | G | | A | G | |
| Allele case
(frequency) | 512 (0.931) | 38 (0.069) | | 297 (0.905) | 31 (0.095) | | 386 (0.928) | 30 (0.072) | | 559 (0.925) | 45 (0.075) | | 377 (0.938) | 25 (0.062) | |
| Allele control
(frequency) | 704 (0.944) | 42 (0.056) | | 704 (0.944) | 42 (0.056) | | 704 (0.944) | 42 (0.056) | | 704 (0.944) | 42 (0.056) | | 704 (0.944) | 42 (0.056) | |
| Odds ratio [95%
CI] | 0.803828
[0.510832–1.264876] | 0.571573
[0.352441–0.926951] | 0.767614
[0.472762–1.246359] | 0.741098
[0.479720–1.144890] | 0.899659
[0.539883–1.499189] |
| Fisher’s
p-value | 0.344369 | 0.021973 | 0.283753 | 0.17568 | 0.684758 |
| Genotype | A/A | A/G | G/G | A/A | A/G | G/G | A/A | A/G | G/G | A/A | A/G | G/G | A/A | A/G | G/G |
| Genotype case
(frequency) | 237 (0.862) | 38 (0.138) | 0 (0.000) | 133 (0.811) | 31 (0.189) | 0 (0.000) | 180 (0.865) | 26 (0.125) | 2 (0.010) | 259 (0.858) | 41 (0.136) | 2 (0.007) | 177 (0.881) | 23 (0.114) | 1 (0.005) |
| Genotype control
(frequency) | 334 (0.895) | 36 (0.097) | 3 (0.008) | 334 (0.895) | 36 (0.097) | 3 (0.008) | 334 (0.895) | 36 (0.097) | 3 (0.008) | 334 (0.895) | 36 (0.097) | 3 (0.008) | 334 (0.895) | 36 (0.097) | 3 (0.008) |
| Odds ratio [95%
CI] | | | | | |
| Fisher’s
p-value | 0.089854 | 0.006548 | 0.551574 | 0.276654 | 0.734752 |
| HWE for case
(Fisher’s p-value) | 0.218464 | 0.181388 | 0.341388 | 0.787079 | 0.787742 |
| HWE for control
(Fisher’s p-value) | 0.076551 | 0.076551 | 0.076551 | 0.076551 | 0.076551 |
|
| rs1225950 | | | | | | | | | | | | | | | |
| Allele | C | G | | C | G | | C | G | | C | G | | C | G | |
| Allele case
(frequency) | 306 (0.732) | 112 (0.268) | | 184 (0.697) | 80 (0.303) | | 254 (0.804) | 62 (0.196) | | 368 (0.736) | 132 (0.264) | | 258 (0.741) | 90 (0.259) | |
| Allele control
(frequency) | 414 (0.734) | 150 (0.266) | | 414 (0.734) | 150 (0.266) | | 414 (0.734) | 150 (0.266) | | 414 (0.734) | 150 (0.266) | | 414 (0.734) | 150 (0.266) | |
| Odds ratio [95%
CI] | 0.989907
[0.743747–1.317538] | 0.833333
[0.603818–1.150090] | 1.484339
[1.062199–2.074244] | 1.010101
[0.768917–1.326936] | 1.038647
[0.766316–1.407758] |
| Fisher’s
p-value | 0.944559 | 0.26708 | 0.020307 | 0.942443 | 0.806914 |
| Genotype | C/C | C/G | G/G | C/C | C/G | G/G | C/C | C/G | G/G | C/C | C/G | G/G | C/C | C/G | G/G |
| Genotype case
(frequency) | 110 (0.526) | 86 (0.411) | 13 (0.062) | 65 (0.492) | 54 (0.409) | 13 (0.098) | 101 (0.639) | 52 (0.329) | 5 (0.032) | 133 (0.532) | 102 (0.408) | 15 (0.060) | 94 (0.540) | 70 (0.402) | 10 (0.057) |
| Genotype control
(frequency) | 157 (0.557) | 100 (0.355) | 25 (0.089) | 157 (0.557) | 100 (0.355) | 25 (0.089) | 157 (0.557) | 100 (0.355) | 25 (0.089) | 157 (0.557) | 100 (0.355) | 25 (0.089) | 157 (0.557) | 100 (0.355) | 25 (0.089) |
| Odds ratio [95%
CI] | | | | | |
| Fisher’s
p-value | 0.314443 | 0.471077 | 0.045302 | 0.273912 | 0.354813 |
| HWE for case
(Fisher’s p-value) | 0.479611 | 0.71725 | 0.585119 | 0.430145 | 0.517263 |
| HWE for control
(Fisher’s p-value) | 0.123289 | 0.123289 | 0.123289 | 0.123289 | 0.123289 |
|
| rs383084 | | | | | | | | | | | | | | | |
| Allele | A | G | | A | G | | A | G | | A | G | | A | G | |
| Allele case
(frequency) | 337 (0.677) | 161 (0.323) | | 138 (0.595) | 94 (0.405) | | 234 (0.718) | 92 (0.282) | | 305 (0.657) | 159 (0.343) | | 233 (0.685) | 107 (0.315) | |
| Allele control
(frequency) | 389 (0.668) | 193 (0.332) | | 389 (0.668) | 193 (0.332) | | 389 (0.668) | 193 (0.332) | | 389 (0.668) | 193 (0.332) | | 389 (0.668) | 193 (0.332) | |
| Odds ratio [95%
CI] | 1.038512
[0.804736–1.340201] | 0.728382
[0.532190–0.996899] | 1.261931
[0.938074–1.697597] | 0.951723
[0.735335–1.231786] | 1.080388
[0.811125–1.439038] |
| Fisher’s
p-value | 0.771498 | 0.047435 | 0.123874 | 0.706935 | 0.597015 |
| Genotype | A/A | A/G | G/G | A/A | A/G | G/G | A/A | A/G | G/G | A/A | A/G | G/G | A/A | A/G | G/G |
| Genotype case
(frequency) | 109 (0.438) | 119 (0.478) | 21 (0.084) | 37 (0.319) | 64 (0.552) | 15 (0.129) | 80 (0.491) | 74 (0.454) | 9 (0.055) | 92 (0.397) | 121 (0.522) | 19 (0.082) | 75 (0.441) | 83 (0.488) | 12 (0.071) |
| Genotype control
(frequency) | 128 (0.440) | 133 (0.457) | 30 (0.103) | 28 (0.440) | 133 (0.457) | 30 (0.103) | 128 (0.440) | 133 (0.457) | 30 (0.103) | 128 (0.440) | 133 (0.457) | 30 (0.103) | 128 (0.440) | 133 (0.457) | 30 (0.103) |
| Odds ratio [95%
CI] | | | | | |
| Fisher’s
p-value | 0.731139 | 0.080489 | 0.184464 | 0.316614 | 0.480111 |
| HWE for case
(Fisher’s p-value) | 0.145561 | 0.119392 | 0.123661 | 0.016308 | 0.085421 |
| HWE for control
(Fisher’s p-value) | 0.596699 | 0.596699 | 0.596699 | 0.596699 | 0.596699 |
|
| rs13210097 | | | | | | | | | | | | | | | |
| Allele | A | C | | A | | | A | | | A | | | A | C | |
| Allele case
(frequency) | 553 (0.998) | 1 (0.002) | | 328 (1.000) | | | 416 (1.000) | | | 606 (1.000) | | | 399 (0.993) | 3 (0.007) | |
| Allele control
(frequency) | 748 (1.000) | 0 (0.000) | | 748 (1.000) | | | 748 (1.000) | | | 748 (1.000) | | | 748 (1.000) | 0 (0.000) | |
| Odds ratio [95%
CI] | | | | | |
| Fisher’s
p-value | 0.245121 | | | | 0.018032 |
| Genotype | A/A | A/C | | A/A | | | A/A | | | A/A | | | A/A | A/C | |
| Genotype case
(frequency) | 276 (0.996) | 1 (0.004) | | 164 (1.000) | | | 208 (1.000) | | | 303 (1.000) | | | 198 (0.985) | 3 (0.015) | |
| Genotype control
(frequency) | 374 (1.000) | 0 (0.000) | | 374 (1.000) | | | 374 (1.000) | | | 374 (1.000) | | | 374 (1.000) | 0 (0.000) | |
| Odds ratio [95%
CI] | | | | | |
| Fisher’s
p-value | 0.244939 | | | | 0.017882 |
| HWE for case
(Fisher’s p-value) | 0.975979 | | | | 0.915087 |
| HWE for control
(Fisher’s p-value) | 1 | | | | 1 |
|
| rs1632346 | | | | | | | | | | | | | | | |
| Allele | A | G | | A | G | | A | G | | A | G | | A | G | |
| Allele case
(frequency) | 395 (0.721) | 153 (0.279) | | 219 (0.672) | 107 (0.328) | | 262 (0.639) | 148 (0.361) | | 399 (0.661) | 205 (0.339) | | 272 (0.680) | 128 (0.320) | |
| Allele control
(frequency) | 527 (0.706) | 219 (0.294) | | 527 (0.706) | 219 (0.294) | | 527 (0.706) | 219 (0.294) | | 527 (0.706) | 219 (0.294) | | 527 (0.706) | 219 (0.294) | |
| Odds ratio [95%
CI] | 1.072850
[0.840343–1.369689] | 0.850538
[0.642966–1.125122] | 0.735653
[0.569595–0.950123] | 0.808821
[0.642219–1.018643] | 0.883065
[0.679109–1.148273] |
| Fisher’s
p-value | 0.57258 | 0.256573 | 0.018534 | 0.071254 | 0.35326 |
| Genotype | A/A | A/G | G/G | A/A | A/G | G/G | A/A | A/G | G/G | A/A | A/G | G/G | A/A | A/G | G/G |
| Genotype case
(frequency) | 138 (0.504) | 119 (0.434) | 17 (0.062) | 71 (0.436) | 77 (0.472) | 15 (0.092) | 82 (0.400) | 98 (0.478) | 25 (0.122) | 139 (0.460) | 121 (0.401) | 42 (0.139) | 87 (0.435) | 98 (0.490) | 15 (0.075) |
| Genotype control
(frequency) | 183 (0.491) | 161 (0.432) | 29 (0.078) | 183 (0.491) | 161 (0.432) | 29 (0.078) | 183 (0.491) | 161 (0.432) | 29 (0.078) | 183 (0.491) | 161 (0.432) | 29 (0.078) | 183 (0.491) | 161 (0.432) | 29 (0.078) |
| Odds ratio [95%
CI] | | | | | |
| Fisher’s
p-value | 0.739121 | 0.489006 | 0.055888 | 0.035656 | 0.396877 |
| HWE for case
(Fisher’s p-value) | 0.190717 | 0.363169 | 0.604189 | 0.064218 | 0.074958 |
| HWE for control
(Fisher’s p-value) | 0.432268 | 0.432268 | 0.432268 | 0.432268 | 0.432268 |
|
| rs9505309 | | | | | | | | | | | | | | | |
| Allele | A | C | | A | C | | A | C | | A | C | | A | C | |
| Allele case
(frequency) | 416 (0.754) | 136 (0.246) | | 228 (0.695) | 100 (0.305) | | 285 (0.688) | 129 (0.312) | | 428 (0.709) | 176 (0.291) | | 297 (0.739) | 105 (0.261) | |
| Allele control
(frequency) | 541 (0.727) | 203 (0.273) | | 541 (0.727) | 203 (0.273) | | 541 (0.727) | 203 (0.273) | | 541 (0.727) | 203 (0.273) | | 541 (0.727) | 203 (0.273) | |
| Odds ratio [95%
CI] | 1.147766
[0.892090–1.476719] | 0.855527
[0.643295–1.137777] | 0.828999
[0.637147–1.078620] | 0.912494
[0.718952–1.158136] | 1.061368
[0.806301–1.397124] |
| Fisher’s
p-value | 0.283655 | 0.283241 | 0.162381 | 0.451472 | 0.671045 |
| Genotype | A/A | A/C | C/C | A/A | A/C | C/C | A/A | A/C | C/C | A/A | A/C | C/C | A/A | A/C | C/C |
| Genotype case
(frequency) | 155 (0.562) | 106 (0.384) | 15 (0.054) | 81 (0.494) | 66 (0.402) | 17 (0.104) | 103 (0.498) | 79 (0.382) | 25 (0.121) | 148 (0.490) | 132 (0.437) | 22 (0.073) | 111 (0.552) | 75 (0.373) | 15 (0.075) |
| Genotype control
(frequency) | 191 (0.513) | 159 (0.427) | 22 (0.059) | 191 (0.513) | 159 (0.427) | 22 (0.059) | 191 (0.513) | 159 (0.427) | 22 (0.059) | 191 (0.513) | 159 (0.427) | 22 (0.059) | 191 (0.513) | 159 (0.427) | 22 (0.059) |
| Odds ratio [95%
CI] | | | | | |
| Fisher’s
p-value | 0.477105 | 0.186931 | 0.030767 | 0.705791 | 0.406832 |
| HWE for case
(Fisher’s p-value) | 0.56972 | 0.517616 | 0.112183 | 0.310054 | 0.637994 |
| HWE for control
(Fisher’s p-value) | 0.136728 | 0.136728 | 0.136728 | 0.136728 | 0.136728 |
|
| rs1594467 | | | | | | | | | | | | | | | |
| Allele | A | G | | A | G | | A | G | | A | G | | A | G | |
| Allele case
(frequency) | 499 (0.907) | 51 (0.093) | | 307 (0.936) | 21 (0.064) | | 392 (0.947) | 22 (0.053) | | 564 (0.940) | 36 (0.060) | | 377 (0.938) | 25 (0.062) | |
| Allele control
(frequency) | 700 (0.941) | 44 (0.059) | | 700 (0.941) | 44 (0.059) | | 700 (0.941) | 44 (0.059) | | 700 (0.941) | 44 (0.059) | | 700 (0.941) | 44 (0.059) | |
| Odds ratio [95%
CI] | 0.615014
[0.404372–0.935382] | 0.918912
[0.537166–1.571952] | 1.120000
[0.661541–1.896179] | 0.984762
[0.625265–1.550952] | 0.947886
[0.571136–1.573159] |
| Fisher’s
p-value | 0.022061 | 0.757486 | 0.672991 | 0.947174 | 0.83595 |
| Genotype | A/A | A/G | G/G | A/A | A/G | G/G | A/A | A/G | G/G | A/A | A/G | G/G | A/A | A/G | G/G |
| Genotype case
(frequency) | 225 (0.818) | 49 (0.178) | 1 (0.004) | 144 (0.878) | 19 (0.116) | 1 (0.006) | 185 (0.894) | 22 (0.106) | 0 (0.000) | 265 (0.883) | 34 (0.113) | 1 (0.003) | 176 (0.876) | 25 (0.124) | 0 (0.000) |
| Genotype control
(frequency) | 330 (0.887) | 40 (0.108) | 2 (0.005) | 330 (0.887) | 40 (0.108) | 2 (0.005) | 330 (0.887) | 40 (0.108) | 2 (0.005) | 330 (0.887) | 40 (0.108) | 2 (0.005) | 330 (0.887) | 40 (0.108) | 2 (0.005) |
| Odds ratio [95%
CI] | | | | | |
| Fisher’s
p-value | 0.03487 | 0.954656 | 0.570847 | 0.90088 | 0.490036 |
| HWE for case
(Fisher’s p-value) | 0.328021 | 0.669345 | 0.419405 | 0.934711 | 0.347133 |
| HWE for control
(Fisher’s p-value) | 0.514897 | 0.514897 | 0.514897 | 0.514897 | 0.514897 |
|
| rs419588 | | | | | | | | | | | | | | | |
| Allele | A | G | | A | G | | A | G | | A | G | | A | G | |
| Allele case
(frequency) | 329 (0.600) | 219 (0.400) | | 197 (0.601) | 131 (0.399) | | 269 (0.659) | 139 (0.341) | | 393 (0.653) | 209 (0.347) | | 251 (0.640) | 141 (0.360) | |
| Allele control
(frequency) | 491 (0.658) | 255 (0.342) | | 491 (0.658) | 255 (0.342) | | 491 (0.658) | 255 (0.342) | | 491 (0.658) | 255 (0.342) | | 491 (0.658) | 255 (0.342) | |
| Odds ratio [95%
CI] | 0.780208
[0.620975–0.980273] | 0.781005
[0.597518–1.020836] | 1.005070
[0.779177–1.296450] | 0.976574
[0.779084–1.224125] | 0.924514
[0.715839–1.194018] |
| Fisher’s
p-value | 0.03299 | 0.070216 | 0.968945 | 0.837073 | 0.547587 |
| Genotype | A/A | A/G | G/G | A/A | A/G | G/G | A/A | A/G | G/G | A/A | A/G | G/G | A/A | A/G | G/G |
| Genotype case
(frequency) | 99 (0.361) | 131 (0.478) | 44 (0.161) | 63 (0.384) | 71 (0.433) | 30 (0.183) | 88 (0.431) | 93 (0.456) | 23 (0.113) | 131 (0.435) | 131 (0.435) | 39 (0.130) | 71 (0.362) | 109 (0.556) | 16 (0.082) |
| Genotype control
(frequency) | 161 (0.432) | 169 (0.453) | 43 (0.115) | 161 (0.432) | 169 (0.453) | 43 (0.115) | 161 (0.432) | 169 (0.453) | 43 (0.115) | 161 (0.432) | 169 (0.453) | 43 (0.115) | 161 (0.432) | 169 (0.453) | 43 (0.115) |
| Odds ratio [95%
CI] | | | | | |
| Fisher’s
p-value | 0.101981 | 0.102703 | 0.995155 | 0.817159 | 0.057581 |
| HWE for case
(Fisher’s p-value) | 0.951802 | 0.211276 | 0.83269 | 0.489103 | 0.003715 |
| HWE for control
(Fisher’s p-value) | 0.893346 | 0.893346 | 0.893346 | 0.893346 | 0.893346 |
|
| rs1237879 | | | | | | | | | | | | | | | |
| Allele | A | G | | A | G | | A | G | | A | G | | A | G | |
| Allele case
(frequency) | 382 (0.910) | 38 (0.090) | | 292 (0.896) | 34 (0.104) | | 278 (0.891) | 34 (0.109) | | 442 (0.888) | 56 (0.112) | | 314 (0.902) | 34 (0.098) | |
| Allele control
(frequency) | 523 (0.924) | 43 (0.076) | | 523 (0.924) | 43 (0.076) | | 523 (0.924) | 43 (0.076) | | 523 (0.924) | 43 (0.076) | | 523 (0.924) | 43 (0.076) | |
| Odds ratio [95%
CI] | 0.826507
[0.523909–1.303879] | 0.706107
[0.440416–1.132084] | 0.672253
[0.419006–1.078562] | 0.648935
[0.427617–0.984798] | 0.759307
[0.474061–1.216188] |
| Fisher’s
p-value | 0.412156 | 0.146988 | 0.098048 | 0.041023 | 0.250839 |
| Genotype | A/A | A/G | G/G | A/A | A/G | G/G | A/A | A/G | G/G | A/A | A/G | G/G | A/A | A/G | G/G |
| Genotype case
(frequency) | 178 (0.848) | 26 (0.124) | 6 (0.029) | 131 (0.804) | 30 (0.184) | 2 (0.012) | 122 (0.782) | 34 (0.218) | 0 (0.000) | 199 (0.799) | 44 (0.177) | 6 (0.024) | 140 (0.805) | 34 (0.195) | 0 (0.000) |
| Genotype control
(frequency) | 241 (0.852) | 41 (0.145) | 1 (0.004) | 241 (0.852) | 41 (0.145) | 1 (0.004) | 241 (0.852) | 41 (0.145) | 1 (0.004) | 241 (0.852) | 41 (0.145) | 1 (0.004) | 241 (0.852) | 41 (0.145) | 1 (0.004) |
| Odds ratio [95%
CI] | | | | | |
| Fisher’s
p-value | 0.057413 | 0.293086 | 0.117619 | 0.062881 | 0.275863 |
| HWE for case
(Fisher’s p-value) | 0.000333 | 0.849041 | 0.126615 | 0.070221 | 0.153187 |
| HWE for control
(Fisher’s p-value) | 0.591745 | 0.591745 | 0.591745 | 0.591745 | 0.591745 |
|
| rs155487 | | | | | | | | | | | | | | | |
| Allele | A | G | | A | G | | A | G | | A | G | | A | G | |
| Allele case
(frequency) | 338 (0.790) | 90 (0.210) | | 251 (0.765) | 77 (0.235) | | 293 (0.809) | 69 (0.191) | | 490 (0.809) | 116 (0.191) | | 314 (0.781) | 88 (0.219) | |
| Allele control
(frequency) | 614 (0.821) | 134 (0.179) | | 614 (0.821) | 134 (0.179) | | 614 (0.821) | 134 (0.179) | | 614 (0.821) | 134 (0.179) | | 614 (0.821) | 134 (0.179) | |
| Odds ratio [95%
CI] | 0.819616
[0.608227–1.104473] | 0.711409
[0.518442–0.976200] | 0.926734
[0.671573–1.278841] | 0.921880
[0.699907–1.214252] | 0.778724
[0.576112–1.052591] |
| Fisher’s
p-value | 0.190843 | 0.034479 | 0.643274 | 0.562727 | 0.103376 |
| Genotype | A/A | A/G | G/G | A/A | A/G | G/G | A/A | A/G | G/G | A/A | A/G | G/G | A/A | A/G | G/G |
| Genotype case
(frequency) | 134 (0.626) | 70 (0.327) | 10 (0.047) | 99 (0.604) | 53 (0.323) | 12 (0.073) | 118 (0.652) | 57 (0.315) | 6 (0.033) | 196 (0.647) | 98 (0.323) | 9 (0.030) | 120 (0.597) | 74 (0.368) | 7 (0.035) |
| Genotype control
(frequency) | 251 (0.671) | 112 (0.299) | 11 (0.029) | 251 (0.671) | 112 (0.299) | 11 (0.029) | 251 (0.671) | 112 (0.299) | 11 (0.029) | 251 (0.671) | 112 (0.299) | 11 (0.029) | 251 (0.671) | 112 (0.299) | 11 (0.029) |
| Odds ratio [95%
CI] | | | | | |
| Fisher’s
p-value | 0.387012 | 0.04714 | 0.896369 | 0.794685 | 0.207896 |
| HWE for case
(Fisher’s p-value) | 0.824919 | 0.197918 | 0.781399 | 0.435216 | 0.277603 |
| HWE for control
(Fisher’s p-value) | 0.724417 | 0.724417 | 0.724417 | 0.724417 | 0.724417 |
|
| rs11754300 | | | | | | | | | | | | | | | |
| Allele | A | G | | A | G | | A | G | | A | G | | A | G | |
| Allele case
(frequency) | 522 (0.949) | 28 (0.051) | | 314 (0.963) | 12 (0.037) | | 401 (0.964) | 15 (0.036) | | 583 (0.965) | 21 (0.035) | | 393 (0.978) | 9 (0.022) | |
| Allele control
(frequency) | 711 (0.951) | 37 (0.049) | | 711 (0.951) | 37 (0.049) | | 711 (0.951) | 37 (0.049) | | 711 (0.951) | 37 (0.049) | | 711 (0.951) | 37 (0.049) | |
| Odds ratio [95%
CI] | 0.970163
[0.586216–1.605578] | 1.361697
[0.700615–2.646559] | 1.391186
[0.754162–2.566290] | 1.444712
[0.836380–2.495510] | 2.272386
[1.085471–4.757141] |
| Fisher’s
p-value | 0.906186 | 0.360836 | 0.288681 | 0.184938 | 0.025504 |
| Genotype | A/A | A/G | G/G | A/A | A/G | G/G | A/A | A/G | G/G | A/A | A/G | G/G | A/A | A/G | G/G |
| Genotype case
(frequency) | 248 (0.902) | 26 (0.095) | 1 (0.004) | 152 (0.933) | 10 (0.061) | 1 (0.006) | 193 (0.928) | 15 (0.072) | 0 (0.000) | 281 (0.930) | 21 (0.070) | 0 (0.000) | 192 (0.955) | 9 (0.045) | 0 (0.000) |
| Genotype control
(frequency) | 338 (0.904) | 35 (0.094) | 1 (0.003) | 338 (0.904) | 35 (0.094) | 1 (0.003) | 338 (0.904) | 35 (0.094) | 1 (0.003) | 38 (0.904) | 35 (0.094) | 1 (0.003) | 338 (0.904) | 35 (0.094) | 1 (0.003) |
| Odds ratio [95%
CI] | | | | | |
| Fisher’s
p-value | 0.975378 | 0.391545 | 0.507032 | 0.349313 | 0.082882 |
| HWE for case
(Fisher’s p-value) | 0.719959 | 0.085207 | 0.589576 | 0.531354 | 0.745435 |
| HWE for control
(Fisher’s p-value) | 0.925595 | 0.925595 | 0.925595 | 0.925595 | 0.925595 |
|
| rs9392182 | | | | | | | | | | | | | | | |
| Allele | A | T | | A | T | | A | T | | A | T | | A | T | |
| Allele case
(frequency) | 15 (0.027) | 539 (0.973) | | 8 (0.024) | 320 (0.976) | | 11 (0.026) | 405 (0.974) | | 15 (0.025) | 591 (0.975) | | 18 (0.045) | 382 (0.955) | |
| Allele control
(frequency) | 16 (0.022) | 728 (0.978) | | 16 (0.022) | 728 (0.978) | | 16 (0.022) | 728 (0.978) | | 16 (0.022) | 728 (0.978) | | 16 (0.022) | 728 (0.978) | |
| Odds ratio [95%
CI] | 1.266234
[0.620566–2.583685] | 0.137500
[0.481912–2.684941] | 1.235802
[0.568066–2.688433] | 1.154822
[0.566214–2.355319] | 2.143979
[1.081050–4.252019] |
| Fisher’s
p-value | 0.51564 | 0.768608 | 0.592785 | 0.691996 | 0.025692 |
| Genotype | A/T | T/T | | A/T | T/T | | A/T | T/T | | A/T | T/T | | A/T | T/T | |
| Genotype case
(frequency) | 15 (0.054) | 262 (0.946) | | 8 (0.049) | 156 (0.951) | | 11 (0.053) | 197 (0.947) | | 1 (0.003) | 13 (0.043) | 289 (0.954) | 18 (0.090) | 182 (0.910) | |
| Genotype control
(frequency) | 16 (0.043) | 356 (0.957) | | 16 (0.043) | 356 (0.957) | | 16 (0.043) | 356 (0.957) | | 0 (0.000) | 16 (0.043) | 356 (0.957) | 16 (0.043) | 356 (0.957) | |
| Odds ratio [95%
CI] | 1.273855
[0.618652–2.622972] | 1.141026
[0.478360–2.721674] | 1.242386
[0.565451–2.729717] | | 2.200549
[1.096349–4.416858] |
| Fisher’s
p-value | 0.51042 | 0.76599 | 0.588306 | 0.540804 | 0.023464 |
| HWE for case
(Fisher’s p-value) | 0.643263 | 0.748857 | 0.695286 | 0.67166 | 0.505183 |
| HWE for control
(Fisher’s p-value) | 0.67166 | 0.67166 | 0.67166 | 0.67166 | 0.67166 |
|
| rs2815128 | | | | | | | | | | | | | | | |
| Allele | A | C | | A | C | | A | C | | A | C | | A | C | |
| Allele case
(frequency) | 521 (0.944) | 31 (0.056) | | 311 (0.948) | 17 (0.052) | | 378 (0.913) | 36 (0.087) | | 566 (0.937) | 38 (0.063) | | 376 (0.935) | 26 (0.065) | |
| Allele control
(frequency) | 709 (0.948) | 39 (0.052) | | 709 (0.948) | 39 (0.052) | | 709 (0.948) | 39 (0.052) | | 709 (0.948) | 39 (0.052) | | 09 (0.948) | 39 (0.052) | |
| Odds ratio [95%
CI] | 0.924473
[0.569200–1.501494] | 1.006305
[0.560607–1.806347] | 0.577574
[0.361025–0.924012] | 0.819316
[0.517106–1.298143] | 0.795487
[0.476849–1.327042] |
| Fisher’s
p-value | 0.750925 | 0.9832 | 0.020754 | 0.39542 | 0.380029 |
| Genotype | A/A | A/C | C/C | A/A | A/C | C/C | A/A | A/C | C/C | A/A | A/C | C/C | A/A | A/C | C/C |
| Genotype case
(frequency) | 246 (0.891) | 29 (0.105) | 1 (0.004) | 148 (0.902) | 15 (0.091) | 1 (0.006) | 176 (0.850) | 26 (0.126) | 5 (0.024) | 266 (0.881) | 34 (0.113) | 2 (0.007) | 179 (0.891) | 18 (0.090) | 4 (0.020) |
| Genotype control
(frequency) | 335 (0.896) | 39 (0.104) | 0 (0.000) | 335 (0.896) | 39 (0.104) | 0 (0.000) | 335 (0.896) | 39 (0.104) | 0 (0.000) | 335 (0.896) | 39 (0.104) | 0 (0.000) | 335 (0.896) | 39 (0.104) | 0 (0.000) |
| Odds ratio [95%
CI] | | | | | |
| Fisher’s
p-value | 0.506791 | 0.290525 | 0.007158 | 0.269184 | 0.020997 |
| HWE for case
(Fisher’s p-value) | 0.88304 | 0.374024 | 0.002649 | 0.432248 | 0.000232 |
| HWE for control
(Fisher’s p-value) | 0.287409 | 0.287409 | 0.287409 | 0.287409 | 0.287409 |
|
| rs2815142 | | | | | | | | | | | | | | | |
| Allele | A | G | | A | G | | A | G | | A | G | | A | G | |
| Allele case
(frequency) | 500 (0.912) | 48 (0.088) | | 303 (0.924) | 25 (0.076) | | 368 (0.893) | 44 (0.107) | | 556 (0.917) | 50 (0.083) | | 364 (0.915) | 34 (0.085) | |
| Allele control
(frequency) | 676 (0.909) | 68 (0.091) | | 676 (0.909) | 68 (0.091) | | 676 (0.909) | 68 (0.091) | | 676 (0.909) | 68 (0.091) | | 676 (0.909) | 68 (0.091) | |
| Odds ratio [95%
CI] | 1.047830
[0.711458–1.543238] | 1.219172
[0.755885–1.966408] | 0.841313
[0.564001–1.254973] | 1.118580
[0.763431–1.638945] | 1.076923
[0.699882–1.657084] |
| Fisher’s
p-value | 0.81302 | 0.415912 | 0.396662 | 0.565176 | 0.736049 |
| Genotype | A/A | A/G | | A/A | A/G | G/G | A/A | A/G | G/G | A/A | A/G | G/G | A/A | A/G | G/G |
| Genotype case
(frequency) | 226 (0.825) | 48 (0.175) | | 140 (0.854) | 23 (0.140) | 1 (0.006) | 167 (0.811) | 34 (0.165) | 5 (0.024) | 256 (0.845) | 44 (0.145) | 3 (0.010) | 169 (0.849) | 26 (0.131) | 4 (0.020) |
| Genotype control
(frequency) | 304 (0.817) | 68 (0.183) | | 304 (0.817) | 68 (0.183) | 0 (0.000) | 304 (0.817) | 68 (0.183) | 0 (0.000) | 304 (0.817) | 68 (0.183) | 0 (0.000) | 304 (0.817) | 68 (0.183) | 0 (0.000) |
| Odds ratio [95%
CI] | 1.053180
[0.700601–1.583194] | | | | |
| Fisher’s
p-value | 0.803254 | 0.160138 | 0.009735 | 0.072223 | 0.00756 |
| HWE for case
(Fisher’s p-value) | 0.112105 | 0.958205 | 0.052935 | 0.476929 | 0.02084 |
| HWE for control
(Fisher’s p-value) | 0.052419 | 0.052419 | 0.052419 | 0.052419 | 0.052419 |
Discussion
In the present study, immunohistochemistry detected
significant expression of TXNDC5 in breast invasive ductal
carcinoma, cervical squamous cell carcinoma, esophageal squamous
cell carcinomas, gastric carcinoma, hepatocellular carcinoma,
ovarian papillary serous carcinoma, prostate cancer and
undifferentiated cell carcinoma of the lung tissues. TXNDC5
expression was also quantitatively assessed by western blot
analysis. In comparison with parallel normal tissues, TXNDC5
expression was significantly increased in the tumor tissues of
breast cancers, gastric adenocarcinomas and rectal cancers. This
observation is in accordance with the results of
immunohistochemistry, showing that TXNDC5 expression is increased
in many tumor tissues. This is the first comprehensive
investigation of TXNDC5 expression in various tumor types.
Tumors are thought to have decreased supply of
oxygen, leading to hypoxia and hypo-perfusion (12,13).
TXNDC5 expression is upregulated by hypoxia and has a protective
effect on endothelial cells by inducing the activities of
chaperones important for protein folding of hypoxia-induced
anti-apoptotic molecules (1,2). In
this study, HeLa and U2OS cells were treated with anti-TXNDC5
siRNA. Decreased growth and migration of these cells were observed
when TXNDC5 expression was knocked down. This result suggests that
TXNDC5 is involved in the proliferative and migration of tumor
cells. Zhang et al also reported that TXNDC5 had a strong
effect on gastric cell proliferation and could enhance the invasive
capability of gastric cancer cells (8). We recently cultured synovial
fibroblasts from patients with rheumatoid arthritis and incubated
the cells with TXNDC5-siRNA or CoCl2, a chemical
inducing hypoxia. Increased cell proliferation, cell migration and
TXNDC5 expression were observed in the synovial fibroblasts
following incubation with 1 μM CoCl2; however, this effect
was decreased when TXNDC5 expression was inhibited with 100 nM
siRNA (14). Together, these data
suggest that hypoxia induces TXNDC5 expression and that inhibition
of TXNDC5 expression prevents hypoxia-induced cell proliferation
and migration.
TXNDC5 has been genetically mapped to chromo-some
6p24.3. The gene encoding TXNDC5 is approximately 845.2 kbp, and it
is divided into 13 exons. By genotyping 97 tagSNPs located in the
TXNDC5 region, illumina microarray demonstrated significant
association between TXNDC5 DNA polymorphisms and susceptibility to
cervical carcinoma, esophageal carcinoma and liver cancer in a
Chinese population. This is the first report to demonstrate the
genetic effect of TXNDC5 on the tumor risk. To determine whether
variations in the TXNDC5 gene contributed to the risk of developing
non-segmental vitiligo, Jeong et al conducted a case-control
association study within a Korean population. They genotyped seven
SNPs and found that three exonic SNPs (rs1043784, rs7764128 and
rs8643) were statistically associated with non-segmental vitiligo.
The haplotypes AGG and GAA, consisting of rs1043784, rs7764128 and
rs8643, demonstrated a significant association with the disease
(15). Lin et al reported
that SNP rs13873 and haplotype rs1225934-rs13873 of BMP6-TXNDC5
genes were significantly associated with schizophrenia (16). We recently found that rs443861 has
an association with rheumatoid arthritis using Taqman SNP assay and
illumine microassay (17). In the
present study, SNPs rs1043784, rs7764128 and rs8643 did not show
significant association with tumors. These reports suggest that
TXNDC5 contribute to the risk of many diseases.
In conclusion, the present study showed that the
expression of TXNDC5 is increased in many tumors. This study also
found that TXNDC5 is involved in the proliferation and migration of
tumor cells, acting as a tumor-enhancing gene. Moreover, the
genetic effect of TXNDC5 was revealed on cervical carcinoma,
esophageal carcinoma and liver cancer risk. The present findings
are hopefully useful for understanding further the tumorigenic
process.
Acknowledgements
This study was supported by the
National Natural Science Foundation of China (NTFC) (81171990,
81373218) and the Shandong Taishan Scholarship.
References
|
1.
|
Edman JC, Ellis L, Blacher RW, Roth RA and
Rutter WJ: Sequence of protein disulphide isomerase and
implications of its relationship to thioredoxin. Nature.
317:267–270. 1985. View
Article : Google Scholar : PubMed/NCBI
|
|
2.
|
Freedman RB, Hirst TR and Tuite MF:
Protein disulphide isomerase: building bridges in protein folding.
Trends Biochem Sci. 19:331–336. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Knoblach B, Keller BO, Groenendyk J,
Aldred S, Zheng J, Lemire BD, Li L and Michalak M: ERp19 and ERp46,
new members of the thioredoxin family of endoplasmic reticulum
proteins. Mol Cell Proteomics. 2:1104–1109. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Charlton HK, Webster J, Kruger S, Simpson
F, Richards AA and Whitehead JP: ERp46 binds to AdipoR1, but not
AdipoR2, and modulates adiponectin signaling. Biochem Biophys Res
Commun. 392:234–239. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Sullivan DC, Huminiecki L, Moore JW, Boyle
JJ, Poulsom R, Creamer D, Barker J and Bicknell R: EndoPDI, a novel
protein-disulfide isomerase-like protein that is preferentially
expressed in endothelial cells acts as a stress survival factor. J
Biol Chem. 47:47079–47088. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Wang Y, Ma Y, Lü B, Xu E, Huang Q and Lai
M: Differential expression of mimecan and thioredoxin
domain-containing protein 5 in colorectal adenoma and cancer: a
proteomic study. Exp Biol Med (Maywood). 232:1152–1159. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Vincent EE, Elder DJ, Phillips L, Heesom
KJ, Pawade J, Luckett M, Sohail M, May MT, Hetzel MR and Tavaré
JML: Overexpression of the TXNDC5 protein in non-small cell lung
carcinoma. Anticancer Res. 31:1577–1582. 2011.PubMed/NCBI
|
|
8.
|
Zhang L, Hou Y, Li N, Wu K and Zhai J: The
influence of TXNDC5 gene on gastric cancer cell. J Cancer Res Clin
Oncol. 136:1497–1505. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
The International HapMap Consortium:
Integrating ethics and science in the International HapMap Project.
Nat Rev Genet. 5:467–475. 2004. View
Article : Google Scholar
|
|
10.
|
Barrett JC, Fry B, Maller J and Daly MJ:
Haploview analysis and visualization of LD and haplotype maps.
Bioinformatics. 21:263–265. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Li Z, Zhang Z, He Z, Tang W, Li T, Zeng Z,
He L and Shi Y: A partition-ligation-combination-subdivision EM
algorithm for haplotype inference with multiallelic markers: update
of the SHEsis. Cell Res. 19:519–523. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Policastro LL, Ibañez IL, Notcovich C,
Durán HA and Podhajcer OL: The tumor microenvironment:
characterization, redox considerations and novel approaches for
ROS-targeted gene therapy. Antioxid Redox Signal. 19:854–895. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Dang CV: Links between metabolism and
cancer. Genes Dev. 26:877–890. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Wang L, Zheng Y, Xu H, Yan X and Chang X:
Investigate pathogenic mechanism of TXNDC5 in rheumatoid arthritis.
PLoS One. 8:e533012013. View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Jeong KH, Shin MK, Uhm YK, Kim HJ, Chung
JH and Lee MH: Association of TXNDC5 gene polymorphisms and
susceptibility to nonsegmental vitiligo in the Korean population.
Br J Dermatol. 162:759–764. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Lin SH, Liu CM, Liu YL, Shen-Jang Fann C,
Hsiao PC, Wu JY, Hung SI, Chen CH, Wu HM, Jou YS, Liu SK, Hwang TJ,
Hsieh MH, Chang CC, Yang WC, Lin JJ, Chou FH, Faraone SV, Tsuang
MT, Hwu HG and Chen WJ: Clustering by neurocognition for fine
mapping of the schizophrenia susceptibility loci on chromosome 6p.
Genes Brain Behav. 8:785–794. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Chang X, Zhao Y, Yan X, Pan J, Fang K and
Wang L: Investigating a pathogenic role for TXNDC5 in rheumatoid
arthritis. Arthritis Res Ther. 13:R122011. View Article : Google Scholar : PubMed/NCBI
|