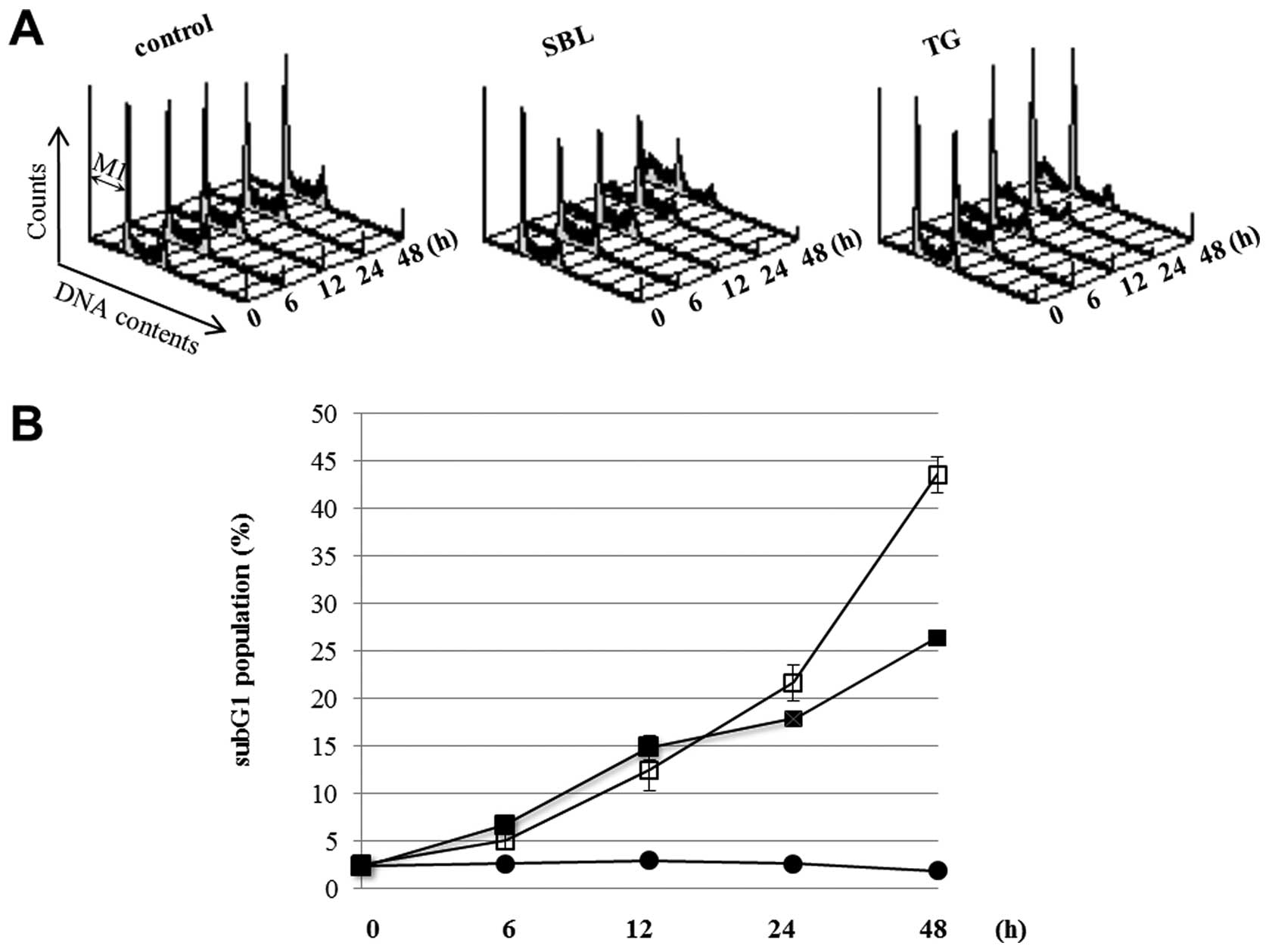
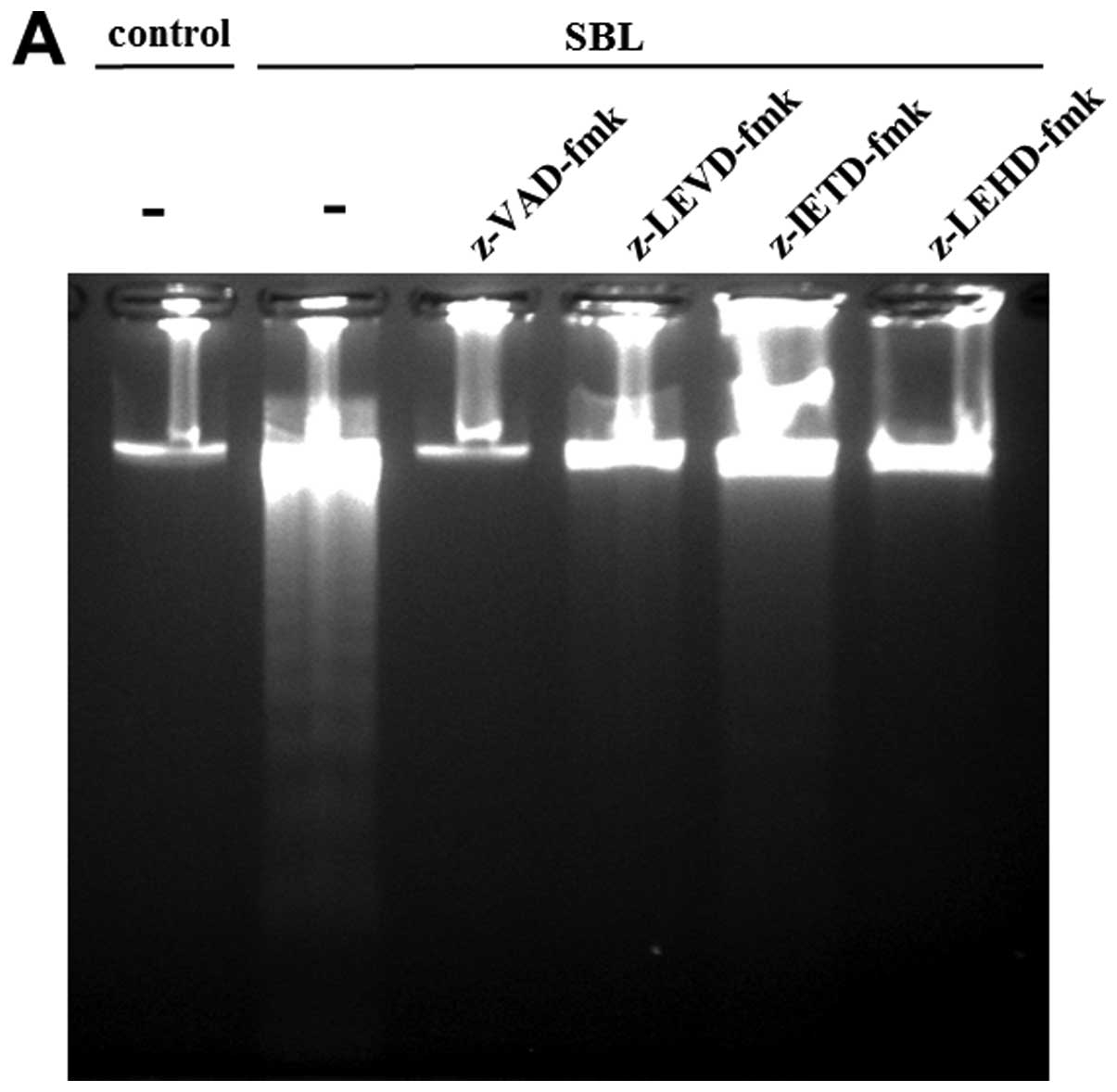
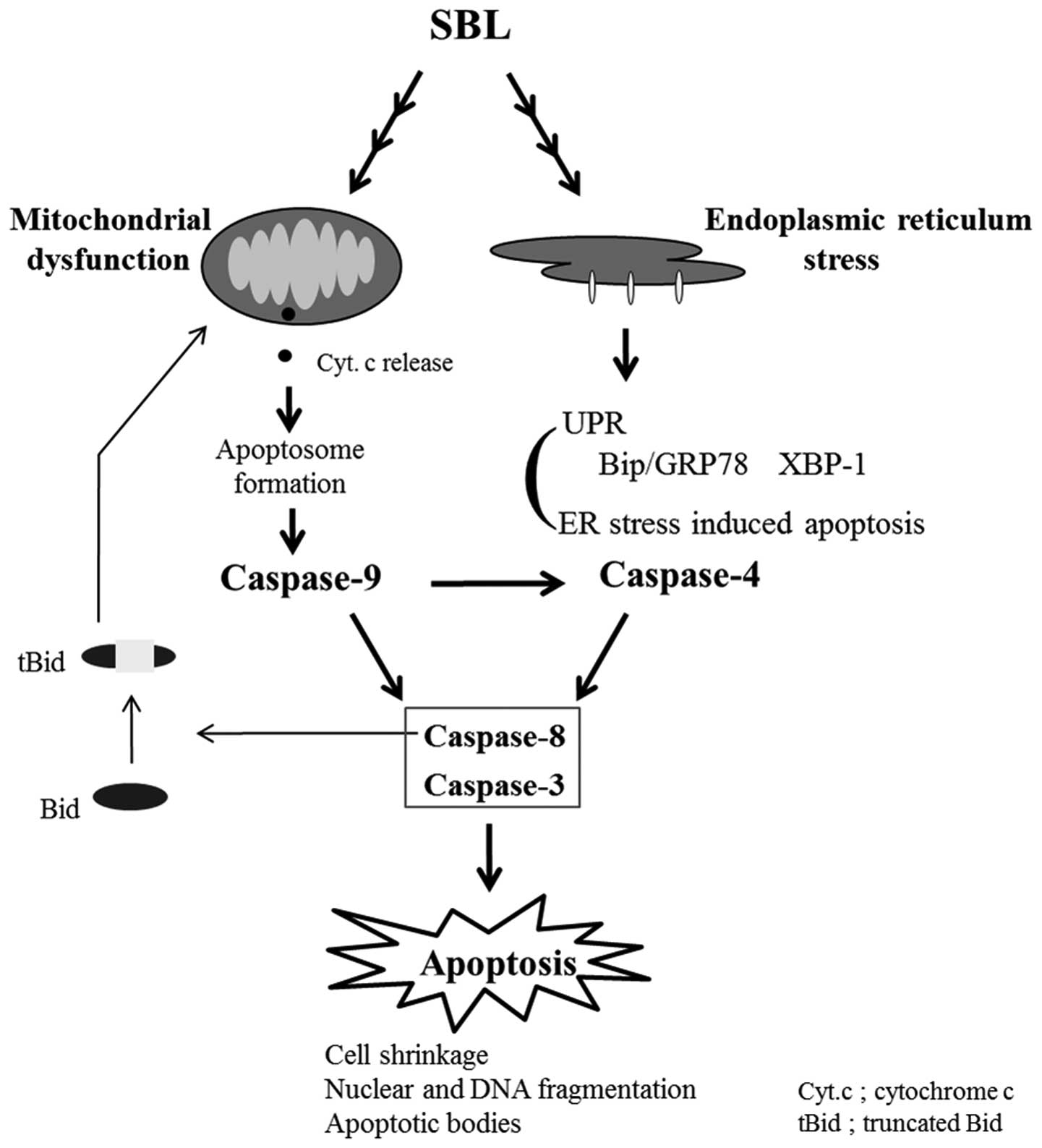
|
1.
|
Muller M, Strand S, Hug H, Heinemann EM,
Walczak H, Hofmann WJ, Stremmel W, et al: Drug-induced apoptosis in
hepatoma cells is mediated by the CD95 (APO-1/Fas) receptor/ligand
system and involves activation of wild-type p53. J Clin Invest.
99:403–413. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
2.
|
Fulda S, Sieverts H, Friesen C, Herr I and
Debatin KM: The CD95 (APO-1/Fas) system mediates drug-induced
apoptosis in neuroblastoma cells. Cancer Res. 57:3823–3829.
1997.PubMed/NCBI
|
|
3.
|
Kerr JF, Wyllie AH and Currie AR:
Apoptosis: a basic biological phenomenon with wide-ranging
implications in tissue kinetics. Br J Cancer. 26:239–257. 1972.
View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Arends MJ and Wyllie AH: Apoptosis:
mechanisms and roles in pathology. Int Rev Exp Pathol. 32:223–254.
1991. View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Ashkenazi A and Dixit VM: Apoptosis
control by death and decoy receptors. Curr Opin Cell Biol.
11:255–260. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Ashkenazi A and Dixit VM: Death receptors:
signaling and modulation. Science. 281:1305–1308. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Zou H, Henzel WJ, Liu X, Lutschg A and
Wang X: Apaf-1, a human protein homologous to C. elegans CED-4,
participates in cytochrome c-dependent activation of caspase-3.
Cell. 90:405–413. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Green DR: Apoptotic pathways: the roads to
ruin. Cell. 94:695–698. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Schroder M and Kaufman RJ: ER stress and
the unfolded protein response. Mutat Res. 569:29–63. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Martinez IM and Chrispeels MJ: Genomic
analysis of the unfolded protein response in Arabidopsis
shows its connection to important cellular processes. Plant Cell.
15:561–576. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Pakula TM, Laxell M, Huuskonen A, Uusitalo
J, Saloheimo M and Penttila M: The effects of drugs inhibiting
protein secretion in the filamentous fungus Trichoderma
reesei. Evidence for down-regulation of genes that encode
secreted proteins in the stressed cells. J Biol Chem.
278:45011–45020. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Harding HP, Zhang Y and Ron D: Protein
translation and folding are coupled by an
endoplasmic-reticulum-resident kinase. Nature. 397:271–274. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Kadowaki H, Nishitoh H and Ichijo H:
Survival and apoptosis signals in ER stress: the role of protein
kinases. J Chem Neuroanat. 28:93–100. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Csordas G, Thomas AP and Hajnoczky G:
Quasi-synaptic calcium signal transmission between endoplasmic
reticulum and mitochondria. EMBO J. 18:96–108. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Tabas I and Ron D: Integrating the
mechanisms of apoptosis induced by endoplasmic reticulum stress.
Nat Cell Biol. 13:184–190. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Nakagawa T, Zhu H, Morishima N, Li E, Xu
J, Yankner BA and Yuan J: Caspase-12 mediates
endoplasmic-reticulum-specific apoptosis and cytotoxicity by
amyloid-beta. Nature. 403:98–103. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Imai Y, Soda M, Inoue H, Hattori N, Mizuno
Y and Takahashi R: An unfolded putative transmembrane polypeptide,
which can lead to endoplasmic reticulum stress, is a substrate of
Parkin. Cell. 105:891–902. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Araki E, Oyadomari S and Mori M:
Endoplasmic reticulum stress and diabetes mellitus. Intern Med.
42:7–14. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
19.
|
Nawrocki ST, Carew JS, Dunner K Jr, Boise
LH, Chiao PJ, Huang P, Abbruzzese JL, et al: Bortezomib inhibits
PKR-like endoplasmic reticulum (ER) kinase and induces apoptosis
via ER stress in human pancreatic cancer cells. Cancer Res.
65:11510–11519. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
20.
|
Gills JJ, Lopiccolo J, Tsurutani J,
Shoemaker RH, Best CJ, Abu-Asab MS, Borojerdi J, et al: Nelfinavir,
a lead HIV protease inhibitor, is a broad-spectrum, anticancer
agent that induces endoplasmic reticulum stress, autophagy, and
apoptosis in vitro and in vivo. Clin Cancer Res. 13:5183–5194.
2007. View Article : Google Scholar
|
|
21.
|
Gallerne C, Prola A and Lemaire C: Hsp90
inhibition by PU-H71 induces apoptosis through endoplasmic
reticulum stress and mitochondrial pathway in cancer cells and
overcomes the resistance conferred by Bcl-2. Biochim Biophys Acta.
1833:1356–1366. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22.
|
Kawauchi H, Sakakibara F and Watanabe K:
Agglutinins of frog eggs: a new class of proteins causing
preferential agglutination of tumor cells. Experientia. 31:364–365.
1975. View Article : Google Scholar : PubMed/NCBI
|
|
23.
|
Sakakibara F, Kawauchi H, Takayanagi G and
Ise H: Egg lectin of Rana japonica and its receptor
glycoprotein of Ehrlich tumor cells. Cancer Res. 39:1347–1352.
1979.
|
|
24.
|
Nitta K, Takayanagi G, Kawauchi H and
Hakomori S: Isolation and characterization of Rana
catesbeiana lectin and demonstration of the lectin-binding
glycoprotein of rodent and human tumor cell membranes. Cancer Res.
47:4877–4883. 1987.PubMed/NCBI
|
|
25.
|
Titani K, Takio K, Kuwada M, Nitta K,
Sakakibara F, Kawauchi H, Takayanagi G, et al: Amino acid sequence
of sialic acid binding lectin from frog (Rana catesbeiana)
eggs. Biochemistry. 26:2189–2194. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
26.
|
Kamiya Y, Oyama F, Oyama R, Sakakibara F,
Nitta K, Kawauchi H, Takayanagi Y, et al: Amino acid sequence of a
lectin from Japanese frog (Rana japonica) eggs. J Biochem.
108:139–143. 1990.PubMed/NCBI
|
|
27.
|
Nitta K, Oyama F, Oyama R, Sekiguchi K,
Kawauchi H, Takayanagi Y, Hakomori S, et al: Ribonuclease activity
of sialic acid-binding lectin from Rana catesbeiana eggs.
Glycobiology. 3:37–45. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
28.
|
Okabe Y, Katayama N, Iwama M, Watanabe H,
Ohgi K, Irie M, Nitta K, et al: Comparative base specificity,
stability, and lectin activity of two lectins from eggs of Rana
catesbeiana and R. japonica and liver ribonuclease from
R catesbeiana. J Biochem. 109:786–790. 1991.PubMed/NCBI
|
|
29.
|
Nitta K, Ozaki K, Ishikawa M, Furusawa S,
Hosono M, Kawauchi H, Sasaki K, et al: Inhibition of cell
proliferation by Rana catesbeiana and Rana japonica
lectins belonging to the ribonuclease superfamily. Cancer Res.
54:920–927. 1994.PubMed/NCBI
|
|
30.
|
Nitta K, Ozaki K, Tsukamoto Y, Furusawa S,
Ohkubo Y, Takimoto H, Murata R, et al: Characterization of a
Rana catesbeiana lectin-resistant mutant of leukemia P388
cells. Cancer Res. 54:928–934. 1994.
|
|
31.
|
Nitta K, Ozaki K, Tsukamoto Y, Hosono M,
Ogawakonno Y, Kawauchi H, Takayanagi Y, et al: Catalytic lectin
(leczyme) from bullfrog (Rana catesbeiana) eggs. Int J
Oncol. 9:19–23. 1996.PubMed/NCBI
|
|
32.
|
Tatsuta T, Hosono M, Sugawara S, Kariya Y,
Ogawa Y, Hakomori S and Nitta K: Sialic acid-binding lectin
(leczyme) induces caspase-dependent apoptosis mediated
mitochondrial pertubation in Jurkat cells. Int J Oncol.
43:1402–1412. 2013.
|
|
33.
|
Nakamura M, Gotoh T, Okuno Y, Tatetsu H,
Sonoki T, Uneda S, Mori M, et al: Activation of the endoplasmic
reticulum stress pathway is associated with survival of myeloma
cells. Leuk Lymphoma. 47:531–539. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
34.
|
Travers KJ, Patil CK, Wodicka L, Lockhart
DJ, Weissman JS and Walter P: Functional and genomic analyses
reveal an essential coordination between the unfolded protein
response and ER-associated degradation. Cell. 101:249–258. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
35.
|
Shen X, Ellis RE, Sakaki K and Kaufman RJ:
Genetic interactions due to constitutive and inducible gene
regulation mediated by the unfolded protein response in C
elegans. PLoS Genet. 1:e372005. View Article : Google Scholar : PubMed/NCBI
|
|
36.
|
Yoshida H, Matsui T, Hosokawa N, Kaufman
RJ, Nagata K and Mori K: A time-dependent phase shift in the
mammalian unfolded protein response. Dev Cell. 4:265–271. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
37.
|
Harding HP, Zhang Y, Bertolotti A, Zeng H
and Ron D: Perk is essential for translational regulation and cell
survival during the unfolded protein response. Mol Cell. 5:897–904.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
38.
|
Scheuner D, Song B, McEwen E, Liu C,
Laybutt R, Gillespie P, Saunders T, et al: Translational control is
required for the unfolded protein response and in vivo glucose
homeostasis. Mol Cell. 7:1165–1176. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
39.
|
Ma Y, Brewer JW, Diehl JA and Hendershot
LM: Two distinct stress signaling pathways converge upon the CHOP
promoter during the mammalian unfolded protein response. J Mol
Biol. 318:1351–1365. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
40.
|
Oyadomari S and Mori M: Roles of
CHOP/GADD153 in endoplasmic reticulum stress. Cell Death Differ.
11:381–389. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
41.
|
Hitomi J, Katayama T, Eguchi Y, Kudo T,
Taniguchi M, Koyama Y, Manabe T, et al: Involvement of caspase-4 in
endoplasmic reticulum stress-induced apoptosis and Abeta-induced
cell death. J Cell Biol. 165:347–356. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
42.
|
Rudy A, Lopez-Anton N, Dirsch VM and
Vollmar AM: The cephalostatin way of apoptosis. J Nat Prod.
71:482–486. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
43.
|
Zong WX, Li C, Hatzivassiliou G, Lindsten
T, Yu QC, Yuan J and Thompson CB: Bax and Bak can localize to the
endoplasmic reticulum to initiate apoptosis. J Cell Biol.
162:59–69. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
44.
|
Hung JH, Su IJ, Lei HY, Wang HC, Lin WC,
Chang WT, Huang W, et al: Endoplasmic reticulum stress stimulates
the expression of cyclooxygenase-2 through activation of NF-kappaB
and pp38 mitogen-activated protein kinase. J Biol Chem.
279:46384–46392. 2004. View Article : Google Scholar : PubMed/NCBI
|