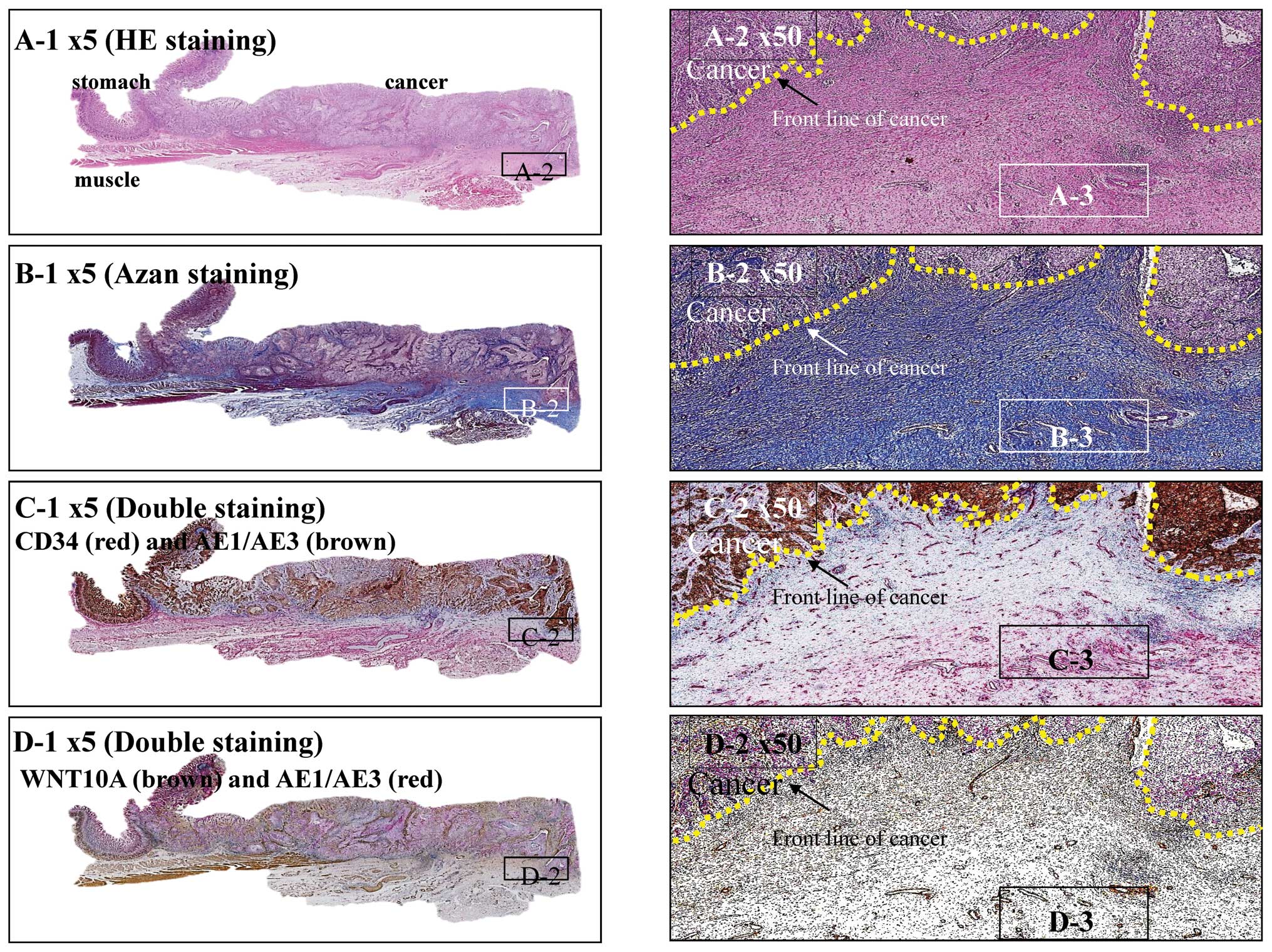
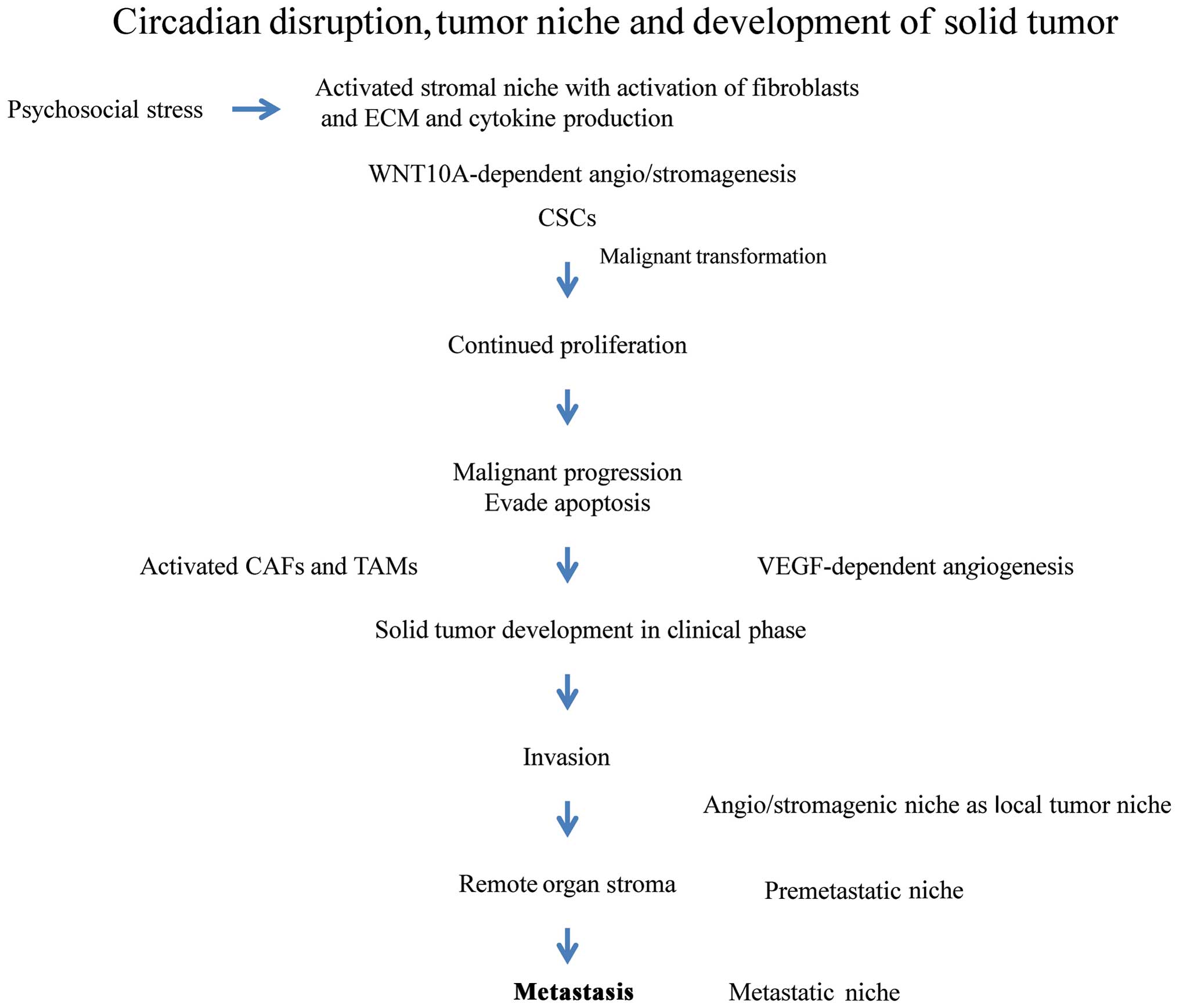
|
1.
|
Antoni MH, Lutgendorf SK, Cole SW, et al:
The influence of bio-behavioural factors on tumour biology:
pathways and mechanisms. Nat Rev Cancer. 6:240–248. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
2.
|
Lutgendorf SK, Sood AK and Antoni MH: Host
factors and cancer progression: biobehavioral signaling pathways
and interventions. J Clin Oncol. 28:4094–4099. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Costa G, Haus E and Stevens R: Shift work
and cancer-considerations on rationale, mechanisms, and
epidemiology. Scand J Work Environ Health. 36:163–179. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Kolstad HA: Nightshift work and risk of
breast cancer and other cancers - a critical review of the
epidemiologic evidence. Scand J Work Environ Health. 34:5–22. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
McGregor BA and Antoni MH: Psychological
intervention and health outcomes among women treated for breast
cancer: a review of stress pathways and biological mediators. Brain
Behav Immun. 23:159–166. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Fu L and Lee CC: The circadian clock:
pacemaker and tumour suppressor. Nat Rev Cancer. 3:350–361. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Yu EA and Weaver DR: Disrupting the
circadian clock: gene-specific effects on aging, cancer, and other
phenotypes. Aging (Albany, NY). 3:479–493. 2011.PubMed/NCBI
|
|
8.
|
Borovski T, De Sousa E, Melo F, Vermeulen
L and Medema JP: Cancer stem cell niche: the place to be. Cancer
Res. 71:634–639. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Li L and Xie T: Stem cell niche: structure
and function. Annu Rev Cell Dev Biol. 21:605–631. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
McAllister SS and Weinberg RA: Tumor-host
interactions: a far-reaching relationship. J Clin Oncol.
28:4022–4028. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Lu P, Weaver VM and Werb Z: The
extracellular matrix: a dynamic niche in cancer progression. J Cell
Biol. 196:395–406. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Provenzano PP, Inman DR, Eliceiri KW, et
al: Collagen density promotes mammary tumor initiation and
progression. BMC Med. 6:112008. View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Coghlin C and Murray GI: Current and
emerging concepts in tumour metastasis. J Pathol. 222:1–15. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Zhou Y, Yan H, Guo M, et al: Reactive
oxygen species in vascular formation and development. Oxid Med Cell
Longev. 2013:3749632013. View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Kessenbrock K, Plaks V and Werb Z: Matrix
metalloproteinases: regulators of the tumor microenvironment. Cell.
141:52–67. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Kohno K, Uchiumi T, Niina I, et al:
Transcription factors and drug resistance. Eur J Cancer.
41:2577–2586. 2005. View Article : Google Scholar
|
|
17.
|
Kalluri R and Zeisberg M: Fibroblasts in
cancer. Nat Rev Cancer. 6:392–401. 2006. View Article : Google Scholar
|
|
18.
|
Cirri P and Chiarugi P:
Cancer-associated-fibroblasts and tumour cells: a diabolic liaison
driving cancer progression. Cancer Metastasis Rev. 31:195–208.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
19.
|
Bhowmick NA, Neilson EG and Moses HL:
Stromal fibroblasts in cancer initiation and progression. Nature.
432:332–337. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
20.
|
Karnoub AE, Dash AB, Vo AP, et al:
Mesenchymal stem cells within tumour stroma promote breast cancer
metastasis. Nature. 449:557–563. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
21.
|
Mishra PJ, Mishra PJ, Humeniuk R, et al:
Carcinoma-associated fibroblast-like differentiation of human
mesenchymal stem cells. Cancer Res. 68:4331–4339. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22.
|
Quante M, Tu SP, Tomita H, et al: Bone
marrow-derived myofibroblasts contribute to the mesenchymal stem
cell niche and promote tumor growth. Cancer Cell. 19:257–272. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
23.
|
Liu S, Ginestier C, Ou SJ, et al: Breast
cancer stem cells are regulated by mesenchymal stem cells through
cytokine networks. Cancer Res. 71:614–624. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24.
|
Cabarcas SM, Mathews LA and Farrar WL: The
cancer stem cell niche - there goes the neighborhood? Int J Cancer.
129:2315–2327. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25.
|
Yasuniwa Y, Izumi H, Wang KY, et al:
Circadian disruption accelerates tumor growth and
angio/stromagenesis through a Wnt signaling pathway. PLoS One.
5:e153302010. View Article : Google Scholar : PubMed/NCBI
|
|
26.
|
Fodde R and Brabletz T: Wnt/beta-catenin
signaling in cancer stemness and malignant behavior. Curr Opin Cell
Biol. 19:150–158. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
27.
|
Pagès F, Galon J, Dieu-Nosjean MC, et al:
Immune infiltration in human tumors: a prognostic factor that
should not be ignored. Oncogene. 29:1093–1102. 2010.PubMed/NCBI
|
|
28.
|
Mantovani A, Germano G, Marchesi F, et al:
Cancer-promoting tumor-associated macrophages: new vistas and open
questions. Eur J Immunol. 41:2522–2525. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
29.
|
Bateman A: Growing a tumor stroma: a role
for granulin and the bone marrow. J Clin Invest. 121:516–519. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
30.
|
Bohring A, Stamm T, Spaich C, et al:
WNT10A mutations are a frequent cause of a broad spectrum of
ectodermal dysplasias with sex-biased manifestation pattern in
heterozygotes. Am J Hum Genet. 85:97–105. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
31.
|
Russell SB, Russell JD, Trupin KM, et al:
Epigenetically altered wound healing in keloid fibroblasts. J
Invest Dermatol. 130:2489–2496. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
32.
|
Schäfer M and Werner S: Cancer as an
overhealing wound: an old hypothesis revisited. Nat Rev Mol Cell
Biol. 9:628–638. 2008.PubMed/NCBI
|
|
33.
|
Mediavilla MD, Sanchez-Barcelo EJ, Tan DX,
Manchester L and Reiter RJ: Basic mechanisms involved in the
anti-cancer effects of melatonin. Curr Med Chem. 17:4462–4481.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
34.
|
Torigoe T, Izumi H, Ishiguchi H, et al:
Cisplatin resistance and transcription factors. Curr Med Chem
Anticancer Agents. 5:15–27. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
35.
|
Tanabe M, Izumi H, Ise T, et al:
Activating transcription factor 4 increases the cisplatin
resistance of human cancer cell lines. Cancer Res. 63:8592–8595.
2003.PubMed/NCBI
|
|
36.
|
Igarashi T, Izumi H, Uchiumi T, et al:
Clock and ATF4 transcription system regulates drug resistance in
human cancer cell lines. Oncogene. 26:4749–4760. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
37.
|
Miyamoto N, Izumi H, Miyamoto R, et al:
Transcriptional regulation of activating transcription factor 4
under oxidative stress in retinal pigment epithelial ARPE-19/HPV-16
cells. Invest Ophthalmol Vis Sci. 52:1226–1234. 2011. View Article : Google Scholar
|
|
38.
|
Miyamoto N, Izumi H, Noguchi T, et al:
Tip60 is regulated by circadian transcription factor clock and is
involved in cisplatin resistance. J Biol Chem. 283:18218–18226.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
39.
|
Lévi F, Okyar A, Dulong S, Innominato PF
and Clairambault J: Circadian timing in cancer treatments. Annu Rev
Pharmacol Toxicol. 50:377–421. 2010.
|
|
40.
|
Sun Y, Campisi J, Higano C, et al:
Treatment-induced damage to the tumor microenvironment promotes
prostate cancer therapy resistance through WNT16B. Nat Med.
18:1359–1368. 2012. View
Article : Google Scholar : PubMed/NCBI
|
|
41.
|
Guo B, Chatterjee S, Li L, et al: The
clock gene, brain and muscle Arnt-like 1, regulates adipogenesis
via Wnt signaling pathway. FASEB J. 26:3453–3463. 2012. View Article : Google Scholar : PubMed/NCBI
|