Introduction
Human urinary bladder cancer is considered an
increasingly significant public health issue in industrialized
countries, with a worldwide estimate of about two million patients
(1). Most of the patients have
recurrence after a complete transurethral prostatic resection,
which is the most serious problem in therapy (2–4). Men
have 3–4 times higher risk of bladder cancer than women, and it
increases with age (5). There are
several post-operative chemotherapeutic agents or immuno-therapy
for the prevention of recurrence. Although both medical and
surgical approaches have been investigated, bladder cancer is still
a recurrent disease (2–4). Therefore, new ways for the effective
control of bladder cancer recurrence is required.
The research on natural compounds for tumor growth
extinction or suppression showed a great potency and possibility in
cancer management. Methylsulfonylmethane (MSM), is a natural
organic sulfur from pine tree extract. MSM has not been developed
as an anticancer compound; it is used as functional food (6,7) with
no reported side-effects. MSM has a powerful anti-angiogenic and
anti-metastasis effect. It also has an inhibitory action for
canceration in vivo (8).
Many precedent studies defined reduction of angiogenesis and
inducement of cell death in various cancer cells, but studies on
human bladder cancer cells are relatively few (8–11).
Janus kinase (Jak) is tyrosin kinase which meditates
the signal pathway by STAT adjacent to cytoplasma (12). AG490 is a tyrosine kinase inhibitor
that has been extensively used for inhibiting Jak2 in vitro
and in vivo (13). AG490
and its derivatives have been widely used for inhibiting Jak2, as a
method of blocking STAT3 activation in vitro and in
vivo (14,15). AG490 has been shown to block Jak2
in patients with acute lymphoblastic leukemia and in genetically
active variants of Jak2 at relatively low concentrations (16). In addition, AG490 variants such as
WP1066 have been successfully used for treating cancers with active
Jak2 and STAT3 (17,18).
Signal transducer and activator of transcription
(STAT) is a family of seven different transcription factors that
play major roles in cytokine signaling (19). STATs are activated by
ligand-bindings to specific cell surface receptors, and after
tyrosine phosphorylation, dimerization and translocation to the
nucleus, directly regulate target genes (20,21).
Especially, STAT3, which was first identified as an acute-phase
response factor, was constitutively activated in many different
tumor cell lines and in human cancers including breast,
hematopoietic, head and neck, lung, prostate and ovarian cancers
(19,22). STAT3 is activated by growth factors
including EGF, TGF-α, IL-6, HGF and oncogenic kinases (12). In addition, this transcription
factor has been shown to regulate the expression of genes involved
in cell proliferation, anti-apoptosis and angiogenesis such as
cyclin D1 and VEGF (23). Its
phosphorylation is mediated through the activation of non-receptor
protein tyrosine kinases called Jak (12,24).
STAT3 is phosphorylated primarily by Janus kinase (Jak1 and 2) at
tyrosine 705 (25). Given the
importance of constitutively active STAT3 in tumor growth and
angiogenesis, targeting Jak2 has been considered a potentially good
therapeutic strategy for anticancer therapy (15,26).
Activated STAT3 proteins by cytokines and growth
factor binds to the promoter of various gene products involved in
anti-apoptosis (Bcl-2, Bcl-xL and survivin), proliferation (cyclin
D1) and angiogenesis (VEGF) (27).
VEGF is one of the most important growth factors involved in
vasculogenesis and angiogenesis (28). VEGF-R2 is the main receptor for
VEGFs, and its action is related to the activation of signaling
molecules such as PLCγ1, phosphoinositide-3 kinase (PI-3 kinase),
Akt, Src and ERK (29). The VEGF
gene and several other genes regulated by hypoxia and involved in
oxygen homeostasis are under the control of the transcription
factor HIF-1 (30). We have
reported that STAT3 modulates VEGF through HIF-1α (31). HIF-1 signaling pathway is known as
a major mechanism in hypoxia signaling (32).
In our present study, we evaluated the efficacy of
MSM together with AG490 not only for suppressing xenograft tumor
growth, but also for lung metastasis. We confirmed the involvement
of this drug combination in the suppression of STAT3 signaling both
in vivo and in vitro. The molecular mechanism of
Jak/STAT pathway inactivation was analyzed using the aggressive and
non-aggressive bladder cancer cell culture system.
Materials and methods
Antibodies and reagents
Penicillin-streptomycin solution and fetal bovine
serum (FBS) were purchased from HyClone (South Logan, UT).
RPMI-1640 was purchased from Sigma Chemical (St. Louis, MO).
Trypsin-EDTA (0.05%) was purchased from Gibco-BRL (Grand Island,
NY). STAT3, STAT5b, VEGF, VEGF-R2, IGF-1R, HIF-1α antibodies and
secondary antibody (goat anti-mouse and rabbit IgG-horseradish
peroxidase) were obtained from Santa Cruz Biotechnology (Santa
Cruz, CA). Phosphorylated STAT3 (Tyr705), phosphorylated JAK2 were
purchased from Cell Signaling Technology (Beverly, MA). Jak2 was
purchased from Millipore (Billerica, MA). Phosphorylated STAT5 was
purchased from Upstate Biotechnology (Lake Placid, NY). β-actin was
purchased from Sigma Chemical. The enhanced chemiluminescence (ECL)
detection kit was purchased from Amersham Pharmacia Biotech
(Piscataway, NJ). Restore™ Western Blot Stripping Buffer
and NE-PER kit were purchased from Pierce (Rockford, IL). The
electrophoretic mobility shift assay (EMSA) kit, oligonucleotide
probes (STAT3), luciferase assay substrates, and reporter lysis
buffer were purchased from Promega Corporation (Madison, WI).
FuGene6 transfection reagent was from Roche (Basel, Switzerland).
The RNeasy mini kit and Qiaprep spin miniprep kit were purchased
from Qiagen (Hilden, Germany). RT-PCR Premix kit and VEGF, IGF-1R,
18s primer for RT-PCR were synthesized by Bioneer (Dajeon, Korea).
Paraformaldehyde and mounting solution in immunohistochemistry
(IHC) were purchased from Dae Jung Chemicals & Metals Co.
(Shiheung, Korea) and Life Science (Mukilteo, WA). Triton X-100
were obtained from Sigma Chemical. Methylsulfonylmethane (Fig. 1) that was purchased from
Fluka/Sigma Co. (St. Louis, MO).
Cell culture and treatments
T24 and 253J-BV (gifts from Dr J.-H. Kim, Korea
University, Korea) human bladder cancer cell lines were maintained
in RPMI-1640 medium containing 10% FBS, 2 mM glutamine and 100 U/ml
penicillin. COS-7, monkey kidney cells were cultured in DMEM
containing 10% FBS, 2 mM glutamine, 100 U/ml penicillin and
incubated at 37°C in 5% CO2. At the start of each
experiment, the cells were resuspended in the medium at a density
of 2.5×105 cells/ml. Cells were treated for the
indicated duration with Jak2 inhibitor AG490 (Calbiochem, La Jolla,
CA) (Fig. 1) at 25 μM and
MSM at 300 mM and a combination of AG490 and MSM.
MTT assay
Cell viability was assayed by measuring blue
formazan that was metabolized from
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT)
by mitochondrial dehydrogenase, which is active only in live cells.
The day before the drug application, cells were seeded in 96-well
flat-bottomed microtiter plates (3,000–5,000 cells/well). Cells
were incubated for 24 h with various concentrations of drug
combination (AG490 and MSM). MTT (5 mg/ml) was added to each well
and incubated for 4 h at 37°C. The formazan product formed was
dissolved by adding 200 μl dimethylsulfoxide (DMSO) to each
well, and the plates were read at 550 nm. All measurements were
performed in triplicate, and each experiment was repeated at least
three times.
Western blot analysis
Whole-cell lysates were prepared by scraping cells
into 500 μl RIPA (radioimmunoprecipitation assay) lysis
buffer containing protease and phosphatase inhibitors and kept on
ice for 10 min. The lysate was centrifuged at 15,000 rpm for 10 min
at 4°C to clear the cellular debris. Protein concentrations were
measured using the Bradford method. Equal amounts of proteins were
resolved on SDS-PAGE and transferred onto nitrocellulose membrane.
The blots were blocked for 1 h with 5% skim milk. It was then
incubated over night at 4°C with the primary antibody followed by
washing with TBS-T and incubated for 1 h with the secondary
antibody. Detection was performed using the ECL detection kit and
LAS-4000 imaging device (Fujifilm, Japan).
Immunoprecipitation
Whole-cell lysates were prepared by scraping cells
into 500 μl RIPA lysis buffer containing protease and
phosphatase inhibitors and kept on ice for 10 min. The lysate was
centrifuged at 15,000 rpm for 10 min at 4°C to clear the cellular
debris. For immunoprecipitation, 500 μg of whole-cell
lysates was incubated with 3 μl anti-STAT3 antibodies for 3
h at 4°C. Protein-G-agarose beads were added and the mixture was
incubated overnight at 4°C on rocking platform. The mixture was
washed 4 times in 1X IP buffer and once in 0.1X IP buffer. It was
then resuspended in 1X Laemmli sample buffer and heat sample for 5
min. Immunoprecipitated proteins were subjected to western blot
analyses as above.
Reverse transcription polymerase chain
reaction (RT-PCR)
Total cellular RNA was extracted using RNeasy mini
kit (Qiagen) and quantified spectrophotometrically at 260 nm. cDNA
was synthesized from total RNA by reverse transcription at 42°C for
1 h and 80°C for 15 min using a first-strand cDNA synthesis kit
(Bioneer). The synthesized cDNA was used as a template for PCR
amplification. The primers were as follows: VEGF sense,
5′-AGGAGGGCAGAATCATCACG-3′; VEGF antisense,
5′-CAAGGCCCACAGGGATTTTCT-3′. The size of the amplified VEGF mRNA
fragment was 312 bp. The PCR condition was 94°C for 5 min
(denaturation), 30 cycles of 94°C for 1 min, 57°C for 1 min, and
72°C for 1 min followed by 72°C for 8 min. In addition, specific
primers for 18s RNA were used as control. The primers were sense:
5′-CGGCTACCA CATCCAAGGAA-3′ and antisense: 5′-CCGGCGTCCCTC
TTAATC-3′. PCR products were resolved by 1% agarose gel
electrophoresis and visualized by ethidium bromide staining.
Electrophoretic mobility shift assay
STAT3 DNA binding activity was detected using an
electrophoretic mobility shift assay (EMSA), in which a labeled
double-stranded DNA sequence was used as a DNA probe to bind an
active STAT3 protein in nuclear extracts. Nuclear protein extracts
were prepared with the Nuclear Extraction Kit (Panomics, AY2002).
The EMSA experiment was performed by incubating a biotin-labeled
transcription factor (TF-STAT3) probe with treated and untreated
nuclear extracts. Nuclear protein extracts were prepared with the
Nuclear Extraction Kit (Pierce). Reactions were resolved on a
non-denaturing 8% PAGE gel (Bio-Rad, Gangnam, Korea). The protein
gels were transferred to a nylon membrane and detected using
streptavidin-HRP and a chemiluminescent substrate.
Cotransfection and luciferase reporter
assay
Cells were co-transfected with various combinations
of the following constructs; wild-STAT3 (gifts from Dr M. Shong,
Chungnam National University, Korea); the VEGF reporter construct
containing 2.7 kb of the VEGF promoter region. Transfected cells
were washed with ice-cold PBS, lysed and lysates were used directly
to measure luciferase activity. The luciferase activity of each
sample was determined by measuring luminescence for 10 sec on a
Lumat LB 9507 luminometer (EG&G Berthold, TN). The experiments
were performed in triplicate, and similar results were obtained
from at least three independent experiments.
253J-BV xenograft animal model
All procedures for the animal experiment were
approved by the Committee on the Use and Care on Animals
(Institutional Animal Care and Use Committee, Seoul, Korea) and
performed in accordance with the institution guidelines. Male nude
mice (Orient Bio, Seongnam-Si, Korea) were subcutaneous injected
with highly metastatic human bladder cancer 253J-BV cells
(1×107). The mice were maintained under specific
pathogen-free conditions and used aged 5–8 weeks. The mice were
divided into 3 groups and treated with 3% MSM (n=5), 3% combination
(n=5) for 1 month. No treatment was given to the control group of
mice (n=5). The drug was administered as intragastric injections of
200 μl. Injections of 0.3 mg/kg body weight were given once
a day, for 4 weeks. Tumor volumes were periodically measured with
calipers. The tumor volume was calculated using the formula: tumor
(mm3) = maximal length (mm) x perpendicular width
(mm2)/2.
Hematoxylin and eosin (H&E)
staining
Histological analyses of xenografts were performed
using H&E staining. The animals were sacrificed and the
xenografts were harvested surgically. These xenografts were fixed
with 4% paraformaldehyde (Fisher Scientific, PA) and embedded in
paraffin, and consecutive 5-μm thick sections were made,
then deparaffinized and rehydrated with xylene, followed by a
washing with gradient of EtOH (100, 95, 90, 80 and 70) and staining
with H&E (Sigma Chemical).
Immunohistochemistry (IHC)
Formalin-fixed paraffin- embedded xenografts were
sliced into 5-μm thick tissue sections. These tissue
sections were deparaffinized, rehydrated with xylene, and washed
with 100, 95, 90, 80 and 70 EtOH, permeabilized with Triton X-100
(0.1%) and blocked with NGS (Normal Goat Serum in PBS 10%).
Incubated in a closed humid chamber with the STAT3, p-STAT3 and
VEGF antibody followed with the secondary antibody, Alexa Fluor 488
(rabbit) and Alexa Fluor 594 (mouse) (Invitrogen, Carlsbad, CA).
For nuclear staining, tissue sections were incubated with DAPI for
1 min and rinsed with PBS. The slides were then observed under a
fluorescence microscope.
Wound healing assay
253J-BV cells were plated on a 35-mm tissue culture
dish at a concentration of 1×105 cells/plate in
RPMI-1640 media containing 10% FBS and antibiotics. Monolayers were
scratched with a pipette tip and washed with PBS twice to remove
debris. Cells were treated with AG490, 300 mM MSM, and a
combination of AG490 and MSM. No treatment was given to the control
cells. Wound edges were photographed after 24-h incubation.
Metastatic animal model
Primary tumors were induced by a subcutaneous
injection of 253J-BV cells onto the flank of 5-week-old BALB/c nude
mice (Orient Bio). The mice were randomly placed into three groups
and treated with 300 mM MSM and a combination in distilled water as
intragastric injections of 200 μl. No treatment was given to
the control mice. Treatment was given for 1 month and then the mice
were euthanized and the lungs were removed. The number of
metastatic tumors on the lung surface was counted. The lungs were
fixed with 4% paraformaldehyde. The sections were stained with
H&E, metastatic nodules were counted, and the mean number of
nodules was recorded as the number of metastases.
Analysis of apoptosis
Fluorescein-conjugated Annexin V (Annexin V-FITC)
was used to quantitatively determine the percentage of cells
undergoing apoptosis. Treated cells were washed twice with cold PBS
and then resuspended in binding buffer at a concentration of
1×106 cells/ml. Annexin V-FITC (5 μl) and
propidium iodide (5 μl) were added to the suspended cells.
After incubation for 15 min at room temperature in the dark, the
percentage of apoptotic cells was analyzed by flow cytometry
(Becton-Dickinson FACScan, San Jose, CA). For positive controls, 10
μM camptothecin and 23 μM actinomycin D were
used.
Data analysis and statistics
The results of the experiments are expressed as mean
± SEM. Statistical analysis was performed by ANOVA-tests using the
SAS program.
Results
Combination of AG490 and MSM inhibits T24
and 253J-BV cell growth
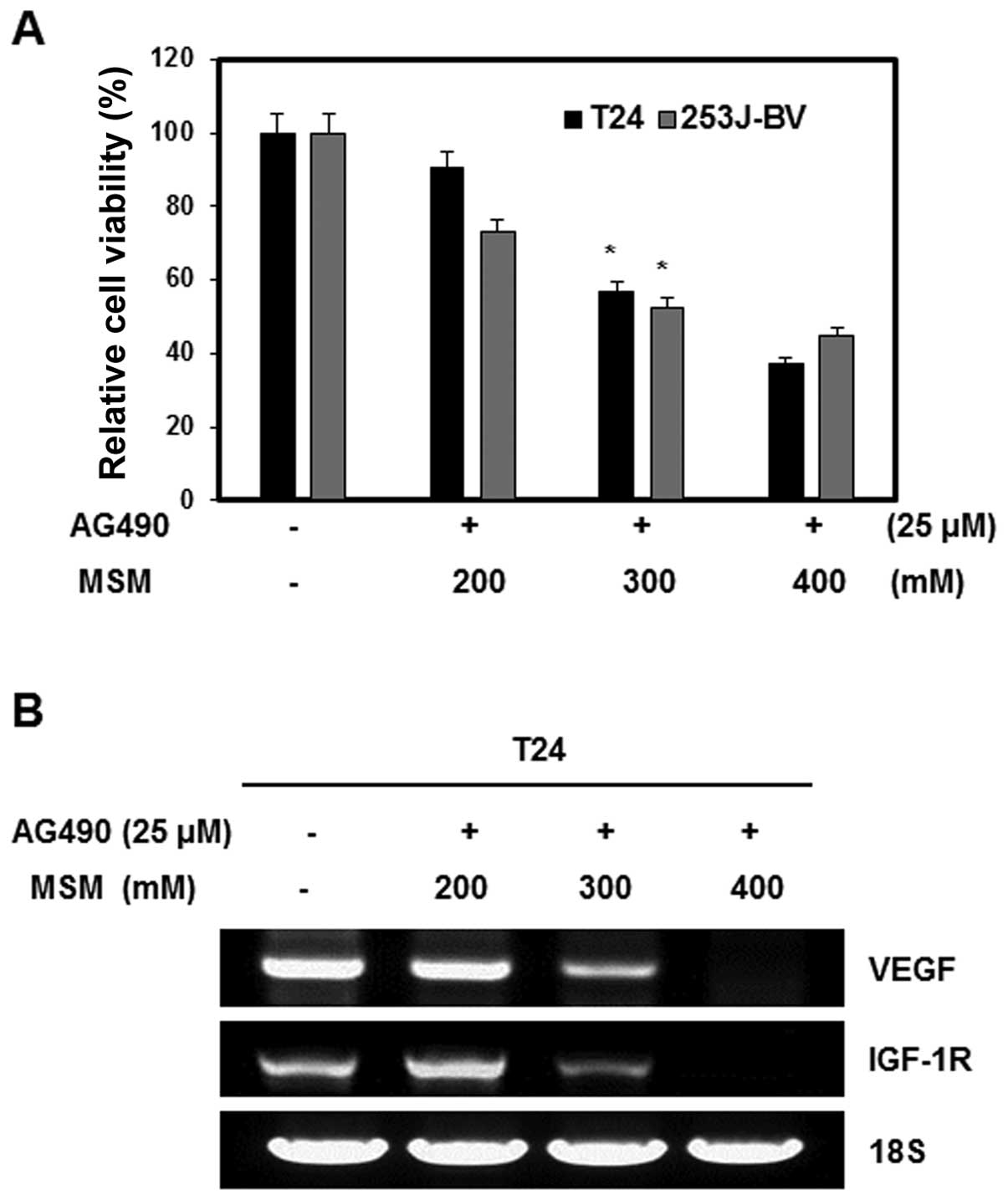
The effect of the combination of AG490 and MSM on
cell viability was examined by the MTT assay. Human bladder cancer
cell lines T24 and 253J-BV were exposed to different concentrations
of MSM and a fixed concentration of AG490 (25 μM). The
number of combination treated cells in the logarithmic phase of
growth was compared with that of the control cells. Combination
treatment inhibited the growth of T24 and 253J-BV cells in a
dose-dependent manner. The IC50 dosage of the
combination was 300 mM MSM with 25 μM AG490 for 24 h of
treatment. Higher concentration of MSM (400 mM) inhibited cell
growth by about 60% (Fig. 1A). To
determine the effect of the combination of AG490 and MSM on the
expression of VEGF and IGF-1R mRNA in T24 cells, total RNA was
extracted from cells treated with various concentrations of MSM.
This mRNA was examined using RT-PCR analysis. MSM decreased the
expression of VEGF and IGF-1R mRNA in a dose-dependent manner in 24
h. At a concentration of 300 and 400 mM MSM, the expression of VEGF
and IGF-1R mRNA was inhibited in T-24 cells (Fig. 1B).
Combination of AG490 with MSM suppresses
Jak2 activation in a time-dependent manner
Our next goal was to analyze the time-dependency of
the combination of AG490 and MSM on the protein expression of T24
cells and 253J-BV cells. The cells were incubated with 25 μM
AG490, 300 mM MSM and combination for different time periods. Total
cellular proteins were extracted and lysates were immunoblotted
with specific antibodies. As shown in Fig. 2A, there was a rapid fall in the
activation of Jak2 in the combination treated cells. This
inhibition was similar in both T24 and 235J-BV cells (Fig. 2A and C). The inhibition of Jak2
activation remained for 30 min and then gradually reversed, whereas
in the cells treated with AG490 or MSM alone, a mild, or no
inhibition on the Jak2 activation was found (Fig. 2A, panel 1). The time window on Jak2
inhibition shows better inhibition between 5–20 min in both T24 and
253J-BV cell lines (Fig. 2B and
D). Phospho-Jak2 was detected by immunoblot analysis with
anti-phosphotyrosine (4G10) antibody after Jak2
immuno-precipitation. We confirmed that combination of AG490 with
MSM suppressed Jak2 phosphorylation within 20 min (Fig. 2E).
Combination of AG490 with MSM suppresses
STAT3 activation time-dependently
Our next goal was to check the impact of Jak2
inhibition on the activation of STAT3. We observed a gross
inhibition of STAT3 activation within 3 h on exposure to the drug
combination whereas mild, or no inhibition on treatment with AG490
or MSM alone (Fig. 3A). The
inhibition of STAT3 activation was similar in both bladder cancer
cell lines (Fig. 3A and C). To
check STAT3 inhibition either by Jak2 or by any other tyrosine
kinase, we analyzed the expression and activation of AKT. As a
result, there was no alteration in the activation of AKT by
combination treatment (Fig. 3C).
Relative protein expression studies give a better view of STAT3
inhibition. Here it is found that inhibition of STAT3 activation in
both T24 and 253J-BV cell lines is between 3–6 h by the drug
combination (Fig. 3B and D). Cell
lystates were immunoprecipitated with anti-STAT3 antibody, followed
by immunoblot analysis with anti-phosphotyrosine (4G10) antibody.
These results demonstrate that the combination of AG490 with MSM
suppressed STAT3 phosphorylation within 5 h (Fig. 3E).
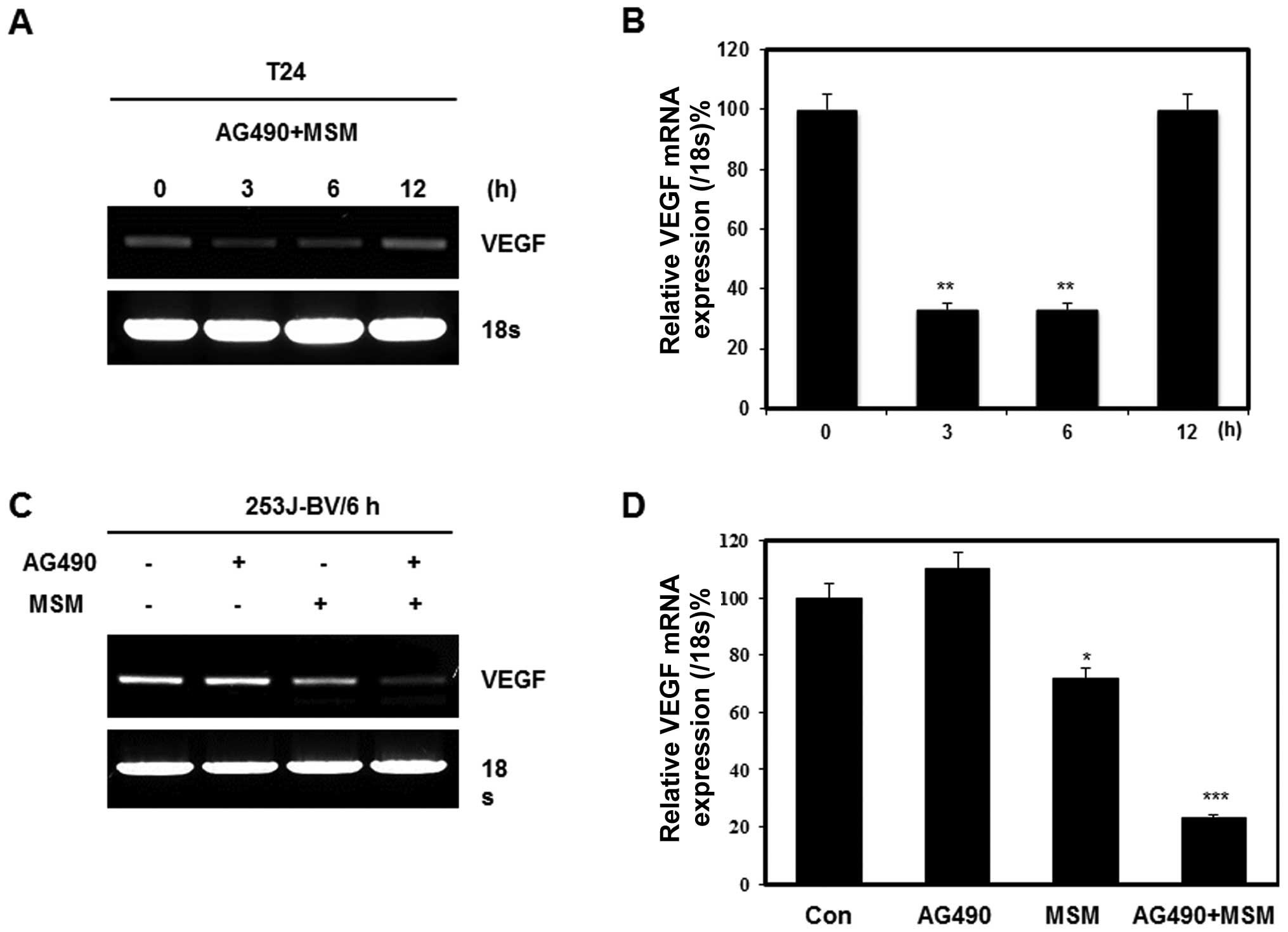
AG490 and MSM combination exposure leads
to the down-regulation of STAT3 target gene product VEGF
Our previous research showed that the best
inhibition of STAT3 phosphorylation was between 3 to 6 h. We
analyzed the expression of target genes of STAT3 after its
inhibition with combination of 25 μM AG490 and 300 mM MSM
for different periods of time. The RT-PCR analysis showed a
decrease in the transcription of VEGF mRNA (Fig. 4A). The results showed the
transcriptional level regulation of VEGF expression upto 6 h and
after that, it started to regain its expression. AG490 alone did
not show any role in transcriptional regulation of VEGF whereas a
40% inhibition was found on treatment with 300 mM MSM (Fig. 4C). The combination treatment
exhibited a synergistic effect and induced 80% inhibition on VEGF
compared with control (Fig. 4D).
Relative expression of VEGF showed a similar inhibition level by
combination treatment in both T24 and 253J-BV cell lines (Fig. 4B and D).
AG490 and MSM combination exposure leads
to down-regulation of STAT3 promoter activities
Activated STATs form dimers, translocate into the
nucleus, bind to specific response element in the promoters of
target genes and activate those genes (33). Using EMSA, we analyzed the binding
activity of p-STAT3 on VEGF gene promoter. As expected, scarce
DNA-STAT3 complex was observed in cells treated with combination of
25 μM AG490 with 300 mM MSM (Fig. 5A). The western blot analysis of
nuclear extracts showed a decreased level of p-STAT3 in cells
treated with the drug combination. Near normal or slight inhibition
of p-STAT3 level was observed in AG490 or MSM treated cells,
respectively (Fig. 5B). Apart from
this, the expression level of VEGF, VEGF-R2 and HIF-1α were
suppressed by the drug combination. We performed luciferase
reporter assays to confirm the inhibition of transcriptional
activities by combination treatment. As shown in Fig. 5C the relative luciferase activity
of STAT3/VEGF promoters were inhibited about 90% by combination
treatment. Treatment with AG490 only showed comparatively less
effect whereas MSM gave a significant inhibition in luciferase
activity, showing its role in the combination treatment.
AG490 and MSM combination downregulates
the expression of different oncoproteins
The role of AG490 and MSM combination on the
expression of different oncoproteins were compared with that of
AG490 and MSM alone. The T24 and 253J-BV cells were treated with
AG490, MSM and drug combination for 6 h and the level of protein
expression was assayed using western blot analysis. We found that
AG490 did not affect the expression of oncogenic proteins and the
expression level was close to the control level. MSM and the drug
combination treated cells showed decreased expression of
oncoproteins. MSM showed no regulation on the expression of STAT3
in bladder cancer cell lines, but in combination with AG490, it
regulated the expression as well as suppressed the phosphorylation
in both cell lines (Fig. 6). Other
oncogenic proteins such as VEGF-R2, p-STAT5, STAT5b, IGF-1R, HIF-1α
and AKT were also downregulated by AG490 and MSM combination
(Fig. 6A). In 253J-BV cells, the
expression of VEGF-R2, STAT5b, IGF-1R and HIF-1α were decreased,
but the phosphorylated level of STAT5b and IGF-1R were observed as
not altered in 253J-BV cells (Fig.
6B).
Combination of MSM and AG490 suppresses
tumor growth and induces cell death in human bladder cancer
xenografted mice
To confirm the in vivo role of the drug
combination and to analyze the synergistic effect of the drug
combination we included MSM as a reference trial drug. Tumor
xenografts were induced by subcutaneous administration of 253J-BV
(1×107) human bladder cancer cells. Drug treatment
started 2 weeks after the inoculation of cells, with dose of 0.3
mg/kg MSM or 25 μl/kg AG490 with 0.3 mg/kg MSM given once a
day. Tumor volumes were measured every day with calipers and
harvested during the 4th week. As shown in Fig. 7B, treatment with drug combination
led to a significant inhibition of tumor growth and decreased the
final tumor size by 32%, whereas, treatment with MSM did not result
in a significant reduction of the final tumor growth (110%).
Control group showed 150% increase in tumor growth (Fig. 7A). In the xenograft model,
apoptosis was associated with tumor necrosis. Histologic
examination of the xenografts using H&E staining showed
increased tumor cell death in animals treated with combination of
MSM and AG490 when compared to the MSM treated group and control
group (Fig. 7C).
Exposure of AG490 and MSM combination
leads to the downregulation of multiple oncogenic targets in
vivo
As shown Fig. 8A,
the level of oncogenic protein expression in AG490 and MSM
combination treated group decreased markedly compared to MSM
treated or control group. The expression levels of STAT3 and its
target gene VEGF were analyzed using an immuno-fluorescence
microscope. The results showed a decrease in the phosphorylation of
STAT3 and thereby decrease in the VEGF expression in AG490 and MSM
combination treated group compared to the other two groups
(Fig. 8B). Western blot analysis
of the xenografts showed a synergistic effect of the drug
combination over MSM. The drug combination effectively inhibited
the phosphorylation of STAT3 and VEGF (Fig. 8A). Phosphorylation of other
oncogenic proteins such as STAT5b and expression of IGF-1R were
also suppressed by the drug combination. The major angiogenic
factor VEGF and its receptor VEGF-R2 were slighly inhibited by the
combination treatment.
Combined treatment with AG490 and MSM
induces inhibition of cell migration
Inhibition of cell migration was determined by the
in vitro wound healing assay using 253J-BV cells. In wound
healing the cells detach from the substratum and move towards the
wounded area (9). We made a wound
on the confluent culture of 253J-BV cells and monitored the
migration of cells into the wound area using live cell microscopy.
The control cells showed a higher rate of migration to the wound
area. Mild inhibition on migration was observed in plates treated
with AG490 and MSM. In the samples treated with drug combination,
significant inhibition of migration was detected (Fig. 9).
Oral administration of AG490 and MSM
combination inhibits lung metastasis in nude mice
We investigated the possible impact of AG490 and MSM
combination in the regression of experimental lung metastasis in
vivo. Tumor xenografts were induced in male Balb/c nude mice
and were injected with 0.3 mg/kg MSM or 25 μl/kg AG490 with
0.3 mg/kg MSM once a day starting two weeks after the inoculation
of cells. After 4 weeks, the lungs were collected for histological
evaluation. As shown in Fig. 10A,
the metastatic cells were distinguishable on H&E stained
sections from the lung tissue as densely packed irregularly shaped
clusters. Enumeration of the area of metastatic nodes showed four
times higher incidence of metastasis in the control group (Fig. 10B).
Combined treatment with AG490 and MSM
induces apoptosis
MTT assay on T24 and 253J-BV showed that AG490 and
MSM combination had high levels of cytotoxic activity (Fig. 1A). To differentiate this from
necrosis and to confirm it as apoptosis, we performed Annexin
V-FITC flow cytometry. We quantitated the number of cells
undergoing apoptosis. Our results showed that combination of AG490
and MSM induced apoptosis in 43% of the 253J-BV cells (Fig. 11). The positive control
camptothecin (10 μM) and actinomycin D (23 μM)
induced apoptosis approximately 50 and 40%, respectively.
Discussion
Combination therapy is the approach used in the
treatment of many cancers that do not respond to current therapies.
This mode of therapy is shown to be safe and effective in humans.
Conventional therapies usually do not have a specific target,
instead they work on mass killing of cells. This often results in
severe side-effects. Development of target therapies have reduced
the side-effects and increased the efficacy in treatment.
Combination therapy has made an experimental breakthrough in the
targeted therapies. In our present study we used the combination of
AG490, a well-known inhibitor of Jak2 and methylsulfonylmethane, a
natural organic sulfur containing compound with no known
side-effects.
In this study, the efficacy of the drug combination
on the inhibition of bladder cancer xenograft growth and its
metastasis were analyzed both in vitro and in vivo.
In human bladder cancer cell lines T24 and 253J-BV, the drug
combination induced cell death at a combination of 25 μM
AG490 and 300 mM MSM. This concentration of combination was used
for further experiments.
Angiogenesis, the formation of new capillaries from
existing capillaries is a potential factor of tumor growth and
metastasis (34). Pathological
angiogenesis is characterized with rapid proliferation of blood
vessels and this is involved in various diseases (35). Targeting angiogenesis is the fourth
modality of anticancer therapy (36). We demonstrated that combination of
AG490 and MSM significantly inhibited angiogenesis and bladder
tumor xenograft growth under the valid dosage and treatment
time.
Constitutive activation of STAT3 is observed in
different types of tumors and promotes cell proliferation and
survival (37,38). STAT3 has become a critical
transcription activator biomarker in antigenic therapy of tumor
(39). In patients with
chemoresistance, STAT3 has been used as the major target for
increasing the chemosensitivity (40). STAT3 is upregulated by Jak2. So we
aimed to inhibit both Jak2 and STAT3 for anticancer activity. A
rapid suppression of Jak2 phosphorylation was observed after the
exposure of human bladder cancer cells to drug combination of AG490
and MSM. Inhibition of Jak2 activation inhibited the activation of
STAT3. In our study the maximum inhibition on STAT3 activation was
achieved at 3-6 h (Fig. 3). Also
we found that the drug combination has better capacity to regulate
the phosphorylation of STAT3 than the individual agents. It
indicates that the drug combination can synergistically inhibit
tumor growth more than the individual agents.
Targeting individual molecules for controlling tumor
growth is a challenging process and is usually not applicable in
different types of cancer. So we tried to inhibit the Jak2/STAT3
pathway, thereby achieving control over all its downstream target
genes. The effects of STAT3 inhibition on the downstream genes were
analyzed through VEGF expression. VEGF is an important
pro-angiogenic factor and induces endothelial cell proliferation
and migration (41). Targeting
VEGF signal pathway is also a major approach for the development of
drugs.
We found a concentration dependent decrease in VEGF
expression in bladder cancer cell lines. The regulation was maximal
at 6 h after exposure to the combination treatment. VEGF promoter
contains various transcription factor binding sites including STAT3
(42) as well as HIF-1α (43). Thus, the decrease in transcription
factor STAT3 constitutes a decrease in the transcriptional
activation of its target genes like VEGF. Maximal inhibition of
STAT3 phosphorylation occurred at 3–6 h; and this in turn resulted
in the suppression of VEGF expression. This ability of Jak2/STAT3
pathway in the downregulation of VEGF was confirmed using EMSA and
luciferase assay.
The combination treatment showed a higher degree of
regulation over different oncoproteins such as STAT5b, IGF-1R and
their phosphorylation. VEGF-R2 pathway is the main cascade involved
in angiogenesis. Inhibition of VEGF-R2 is the key strategy in
anti-angiogenic therapy of cancer (44). The expression of VEGF-R2 is found
decreased on exposure to combination treatment irrespective of the
cell type.
Cancer becomes lethal once it is metastatic. Cell
migration is an important step in the metastatic cascade, similar
to invasion and extravasation (45). As Jak2 and STAT3 are the effective
modulators for various cellular networks and processes, we analyzed
the role of drug combination on cell migration. For eliciting
various cellular processes we were in need of higher number of live
cells. So we reduced the concentration of MSM in the drug
combination to 200 mM which gave 80% cell viability (Fig. 1). Live cell microscopic observation
of the cells exposed to this combination showed, a reduction in
cell migration. A similar result was observed in the wound healing
assay. Therefore, we believe this combination of drugs has an
effective inhibitory role in the metastasis of bladder cancer.
Our in vivo experiments showed that oral
administration of combination of MSM and AG490 significantly
inhibited the growth of bladder cancer xenografts and lung
metastasis. The subcutaneous xenograft model revealed marked
reduction in tumor growth rate with the combination of MSM and
AG490 in the treated mice compared to controls. The number of
metastatic sites give a correlation on survival and response rate
of a therapeutic combination (46). Our drug combination induced cell
death in vivo, and decreased the incidence of metastasis to
the lungs. Lung is a common metastatic site of bladder cancer and
other urogenital cancers (47).
The mechanistic aspects of drug combination on the
suppression of tumor growth and inhibition of metastasis in the
xenografts were studied. IHC studies specific to p-STAT3 and VEGF
on xenografts showed a drastic decrease in expression on
combination treatment. Western blot analysis showed complete
inhibition of Jak2, STAT3 and regulation on the activation of other
oncogenic molecules such as STAT5b and downregulated the expression
of VEGF, VEGF-R2 and IGF-1R. This confirms the importance of drug
combination on the regulation of angiogenesis, cell migration,
growth inhibition and induction of apoptosis.
Our findings collectively suggest that therapy with
inhibitors of Jak2/STAT3 signaling like AG490 with MSM combination
may have a more potent antitumor activity than either treatment
alone in human bladder cancer. We have clearly demonstrated the
existence of a dose-dependent and cell type-independent inhibitory
effect of MSM and AG490 combination on cell proliferation, survival
and angiogenesis in human urinary bladder cancer cells. In
addition, the data showed the oral efficacy of drug combination on
the suppression of bladder cancer growth and metastasis. The
inhibition of the activated Jak2/STAT3 pathway, at least in part,
contributed to the anti-proliferative, anti-angiogenic effects, and
apoptosis. Therefore, this combination could be a novel basis for
small molecules targeting angiogenesis and of therapeutic
significance in the treatment of angiogenesis-related diseases.
Acknowledgements
This study was supported by the Konkuk
University, Seoul, Republic of Korea.
References
|
1.
|
Wu XR: Urothelial tumorigenesis: a tale of
divergent pathways. Nat Rev Cancer. 5:713–725. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
2.
|
Baselli EC and Greenberg RE: Intravesical
therapy for superficial bladder cancer. Oncology. 14:719–729.
2000.PubMed/NCBI
|
|
3.
|
Malmström P: Improved patient outcomes
with BCG immuno-therapy vs chemotherapy - Swedish and worldwide
experience. Eur Urol. 37(Suppl 1): S16–S20. 2000.PubMed/NCBI
|
|
4.
|
Sekine H, Ohya K, Kojima SI, Igarashi K
and Fukui I: Equivalent efficacy of mitomycin C plus doxorubicin
instillation to bacillus Calmette-Guerin therapy for carcinoma in
situ of the bladder. Int J Urol. 8:483–486. 2001. View Article : Google Scholar
|
|
5.
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2001. View Article : Google Scholar
|
|
6.
|
Lee JI, Min HK, Lee JW, Jeong JD, Ha YJ,
Kwack SC and Park JS: Change in the quality of loin from pigs
supplemented with dietary methyl sulfonyl methane during cold
storage. Korean J Food Sci Ani Resour. 29:299–237. 2009.
|
|
7.
|
Joung YH, Lim EJ, Darvin P, Chung SC, Jang
JW, Park KD, Lee HK, Kim HS, Park TK and Yang YM: MSM enhances GH
signaling via the Jak2/STAT5b pathway in osteoblast-like cells and
osteoblast differentiation through the activation of STAT5b in
MSCs. PLoS One. 7:e474772012. View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Lim EJ, Hong DY, Park JH, Joung YH, Pramod
D, Pak SH, Na YM, Hwang TS, Ye SK, Moon ES, Cho BW, Park KD, Lee HK
and Yang YM: Methylsulfonylmethane down-regulates the STAT3/VEGF
pathway in MDA-MB 231 cells and suppresses tumor growth of breast
cancer xenografts. PLoS One. 7:e333612011. View Article : Google Scholar
|
|
9.
|
Caron JM, Bannon M, Rosshirt L, Luis J,
Montaegudo L, Caron JM and Sternstein GM: Methyl sulfone induces
loss of metastatic properties and reemergence of normal phenotype
in a metastatic cloudman S-91 (M3) murine melanoma cell line. PLoS
One. 5:e117882010. View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Caron JM, Bannon M, Rosshirt L and
O’Donovan L: Methyl sulfone manifests anticancer activity in a
metastatic murine breast cancer cell line and in human breast
cancer tissue -part I: murine 4T1 (66cl-4) cell line. Chemotherapy.
59:14–23. 2013.PubMed/NCBI
|
|
11.
|
Caron JM, Monteagudo L, Sanders M, Bannon
M and Deckers PJ: Methyl sulfone manifests anticancer activity in a
metastatic murine breast cancer cell line and in human breast
cancer tissue - part 2: human breast cancer tissue. Chemotherapy.
59:24–34. 2013.
|
|
12.
|
Ihle JN: STATs: signal tranducers and
activators of transcription. Cell. 84:331–334. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Seo IA, Lee HK, Shin YK, Lee SH, Seo SY,
Park JW and Park HT: Janus kinase 2 inhibitor AG490 inhibits the
STAT3 signaling pathway by suppressing protein translation of
gp130. Korean J Physiol Pharmacol. 13:131–138. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Samanta AK, Lin H, Sun T, Kantarjian H and
Arlinghaus RB: Janus kinase 2: a critical target in chronic
myelogenous leukemia. Cancer Res. 66:6468–6472. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Miyamoto N, Sugita K, Goi K, Inukai T,
Lijima K, Tezuka T, Kojika S, Nakamura M, Kagami K and Nakazawa S:
The JAK2 inhibitor AG490 predominantly abrogates the growth of
human B-precursor leukemic cells with 11q23 translocation or
Philadelphia chromosome. Leukemia. 15:1758–1768. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Meydan N, Grunberger T, Dadi H, Shahar M,
Arpaia E, Lapidot Z, Leeder JS, Freedman M, Cohen A, Gazit A,
Levitzki A and Roifman CM: Inhibition of acute lymphoblastic
leukaemia by a Jak-2 inhibitor. Nature. 379:645–648. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Ferrajoli A, Faderl S, Van Q, Koch P,
Harris D, Liu Z, Hazan-Halevy I, Wang Y, Kantarjian HM, Priebe W
and Estrov Z: WP1066 disrupts Janus kinase-2 and induces
caspase-dependent apoptosis in acute myelogenous leukemia cells.
Cancer Res. 67:11291–11299. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Verstovsek S, Manshouri T, Quintás-Cardama
A, Harris D, Cortes J, Giles FJ, Kantarjian H, Priebe W and Estrov
Z: WP1066, a novel JAK2 inhibitor, suppresses proliferation and
induces apoptosis in erythroid human cells carrying the JAK2 V617F
mutation. Clin Cancer Res. 14:788–796. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19.
|
Turkson J and Jove R: STAT proteins: novel
molecular targets for cancer drug discovery. Oncogene.
19:6613–6626. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
20.
|
Darnell JE Jr: STATs and gene regulation.
Science. 277:1630–1635. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
21.
|
Bromberg JF: Activation of STAT proteins
and growth control. Bioessays. 23:161–169. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
22.
|
Fernandes A, Hamburger AW and Gerwin BI:
ErbB-2 kinase is required for constitutive stat 3 activation in
malignant human lung epithelial cells. Int J Cancer. 83:564–570.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
23.
|
Buettner R, Mora LB and Jove R: Activated
STAT signaling in human tumors provides novel molecular targets for
therapeutic intervention. Clin Cancer Res. 8:945–954.
2002.PubMed/NCBI
|
|
24.
|
Ren Z and Schaefer TS: ErbB-2 activates
Stat3 α in a Srcand JAK2-dependent manner. J Biol Chem.
277:38486–38493. 2002.
|
|
25.
|
Halachmi S, Aitken KJ, Szybowska M, Sabha
N, Dessouki S, Lorenzo A, Tse D and Bagli DJ: Role of signal
transducer and activator of transcription 3 (STAT3) in stretch
injury to bladder smooth muscle cells. Cell Tissue Res.
326:149–158. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
26.
|
Opdam FJ, Kamp M, de Bruijn R and Roos E:
Jak kinase activity is required for lymphoma invasion and
metastasis. Oncogene. 23:6647–6653. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
27.
|
Mijatovic T, Mathieu V, Gaussin JF, De
Neve N, Ribaucour F, Van Quaquebeke E, Dumont P, Darro F and Kiss
R: Cardenolide-induced lysosomal membrane permeabilization
demonstrates therapeutic benefits in experimental human non-small
cell lung cancers. Neoplasia. 8:402–412. 2006. View Article : Google Scholar
|
|
28.
|
Wu W, Shu X, Hovsepyan H, Mosteller RD and
Broek D: VEGF receptor expression and signaling in human bladder
tumors. Oncogene. 22:3361–3370. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
29.
|
Laramée M, Chabot C, Cloutier M, Stenne R,
Holgado-Madruga M, Wong AJ and Royal I: The scaffolding adapter
Gab1 mediates vascular endothelial growth factor signaling and is
required for endothelial cell migration and capillary formation. J
Biol Chem. 282:7758–7769. 2007.PubMed/NCBI
|
|
30.
|
Semenza GL, Jiang BH, Leung SW, Passantino
R, Concordet JP, Maire P and Giallongo A: Hypoxia response elements
in the aldolase A, enolase 1 and lactate dehydrogenase A gene
promoters contain essential binding sites for hypoxia-inducible
factor 1. J Biol Chem. 271:32529–32537. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
31.
|
Jung JE, Lee HG, Cho IH, Chung DH, Yoon
SH, Yang YM, Lee JW, Choi S, Park JW and Ye SK: STAT3 is a
potential modulator of HIF-1-mediated VEGF expression in human
renal carcinoma cells. FASEB J. 19:1296–1298. 2005.PubMed/NCBI
|
|
32.
|
Joung YH, Lim EJ, Lee MY, Park JH, Ye SK,
Park EU, Kim SY, Zhang Z, Lee KJ, Park DK, Park T, Moon WK and Yang
YM: Hypoxia activates the cyclin D1 promoter via the Jak2/STAT5b
pathway in breast cancer cells. Exp Mol Med. 37:353–364. 2007.
View Article : Google Scholar
|
|
33.
|
Joung YH, Park JH, Park TK, Lee CS, Kim
OH, Ye SK, Yang UM, Lee KJ and Yang YM: Hypoxia activates signal
transducers and activators of transcription 5 (STAT5) and increases
its binding activity to the GAS element in mammary epithelial
cells. Exp Mol Med. 35:350–357. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
34.
|
Ellis L, Hammers H and Pili R: Targeting
tumor angiogenesis with histone deacetylase inhibitors. Cancer
Lett. 280:145–153. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
35.
|
Folkman J: Angiogenesis: an organizing
principle for drug discovery? Nat Rev Drug Discov. 6:273–286. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
36.
|
Folkman J: Endogenous angiogenesis
inhibitors. APMIS. 112:496–507. 2004. View Article : Google Scholar
|
|
37.
|
Hodge DR, Hurt EM and Farrar WL: The role
of IL-6 and STAT3 in inflammation and cancer. Eur J Cancer.
41:2502–2512. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
38.
|
Haura EB, Turkson J and Jove R: Mechanisms
of disease: Insights into the emerging role of signal transducers
and activators of transcription in cancer. Nat Clin Pract Oncol.
2:315–324. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
39.
|
Chen SH, Murphy DA, Lassoued W, Thurston
G, Feldman MD and Lee WM: Activated STAT3 is a mediator and
biomarker of VEGF endothelial activation. Cancer Biol. 7:1994–2003.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
40.
|
Chen RJ, Ho YS, Guo HR and Wang YJ: Long
term nicotine exposure-induced chemoresistance is mediated by
activation of Stat3 and downregulation of ERK12 via nAChR and
beta-adrenoceptors in human bladder cancer cells. Toxicol Sci.
115:118–130. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
41.
|
McColl BK, Stacker SA and Achen MG:
Molecular regulation of the VEGF family-inducers of angiogenesis
and lymphangio-genesis. APMIS. 112:463–480. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
42.
|
Niu G, Wright KL, Huang M, Song L, Haura
E, Turkson J, Zhang S, Wang T, Sinibaldi D, Coppola D, Heller R,
Ellis LM, Karras J, Bromberg J, Pardoll D, Jove R and Yu H:
Constitutive Stat3 activity up-regulates VEGF expression and tumor
angio-genesis. Oncogene. 21:2000–2008. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
43.
|
Forsythe JA, Jiang BH, Iyer NV, Agani F,
Leung SW, Koos RD and Semenza GL: Activation of vascular
endothelial growth factor gene transcription by hypoxia-inducible
factor 1. Mol Cell Biol. 16:4604–4613. 1996.PubMed/NCBI
|
|
44.
|
Dong Y, Lu B, Zhang X, Zhang J, Lai L, Li
D, Wu Y, Song Y, Luo J, Pang X, Yi Z and Liu M: Cucurbitacin E, a
tetracyclic triterpenes compound from Chinese medicine, inhibits
tumor angiogenesis through VEGFR2-mediated Jak2-STAT3 signaling
pathway. Carcinogenesis. 31:2097–2104. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
45.
|
Guo W and Giancotti FG: Integrin
signalling during tumour progression. Nat Rev Mol Cell Biol.
5:816–826. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
46.
|
Parimoo D and Raghavan D: Progress in the
management of metastatic bladder cancer. Cancer Control. 7:347–356.
2000.PubMed/NCBI
|
|
47.
|
Raghavan D, Shipley WU, Garnick MB,
Russell PJ and Richie JP: Biology and management of bladder cancer.
N Engl J Med. 322:1129–1138. 1990. View Article : Google Scholar : PubMed/NCBI
|