Introduction
Prostate cancer remains the second leading cause of
cancer death for men in the US. According to the National Cancer
Institute, it is estimated that there will be 238,590 new cases and
29,720 deaths from prostate cancer in 2013 (http://www.cancer.gov/cancertopics/types/prostate).
The cell-surface enzyme prostate-specific membrane antigen (PSMA)
is upregulated and strongly expressed on prostate cancer cells,
including those that are metastatic (1). Endothelial-expression of
prostate-specific membrane antigen (PSMA) in the neovasculature of
a variety of non-prostatic solid malignancies has also been
reported (2,3). PSMA is a type II membrane protein,
consisting of a cytoplasmic domain (1–19aa), transmembrane domain
(20–44aa) and extracellular domain (45–750aa), exhibits both
N-acetylated α-linked acidic dipeptidase (NAALADase) and folate
hydrolase (FOLH) activities, and constitutive or induced
internalization (4–6). These properties have allowed PSMA to
attract considerable attention as a target for antibody or small
molecule inhibitor-guided delivery of imaging and therapeutic
agents toward prostate cancer (7–15).
Furthermore, pilot studies support the position that PSMA is an
ideal biomarker for the targeted imaging and therapy of
PSMA-positive prostate cancer.
Exosomes are small vesicles (40–100 nm) secreted by
multiple normal tissue or pathological cells including cancers
containing proteins, mRNAs, microRNAs and lipids that are from
original cells (16). Through
exosome-carrying messages, cells can achieve cross-talk without
contacting each other (17). It
was noted that there are elevated exosome levels secreted by highly
advanced cancer cells enriched with tumor-markers (18). These studies may suggest that
tumor-secreting exosomes may play an important role in the
development and progression of cancer, serving as a potential
biomarker resource to develop non-invasive and dynamic approaches
for tumor diagnosis and prognostic evaluation of cancer treatment
(16–19).
In the present study, we sought to determine the
extent of PSMA enrichment in exosomes secreted by human prostate
cancer cells (PSMA-positive LNCaP), and whether the exosomal PSMA
is functionally active. Herein, our data revealed that tumor-marker
PSMA is strongly enriched in exosomes secreted by PSMA-positive
prostate cancer cells, and the exosomal PSMA maintains its
functional enzymatic activity despite of higher glycosylation
content. Therefore, tumor-related exosomal PSMA may serve as a
diagnostic or prognostic biomarker for prostate cancer.
Materials and methods
Cell lines and reagents
The human prostate cancer cell line LNCaP was
obtained from the American Type Culture Collection (Manassas, VA,
USA). CWR22Rv1 cells were obtained from Professor Henry F.
VanBrocklin (University of California, San Francisco, CA, USA).
Mouse monoclonal anti-human EpCAM antibody was obtained from Cell
Signaling Technology (Danvers, MA, USA). Mouse monoclonal anti-TSG
101 antibody (C-2) was obtained from Santa Cruz Biotechnology
(Santa Cruz, CA, USA). Mouse monoclonal anti-GAPDH antibody and
anti-α-tubulin antibody were obtained from Sigma-Aldrich (St.
Louis, MO, USA). Mouse monoclonal anti-CD9 antibody (LT-86A) was a
gift of Dr Davis at Washington State University (Pullman, WA, USA).
Mouse monoclonal anti-PSMA antibody 7E11 was graciously provided by
Cytogen Corporation (Princeton, NJ, USA). PNGase F was obtained
from New England Biolabs (Ipswich, MA, USA). Halt Protease
Inhibitor Cocktail (100X) was purchased from Thermo Fisher
Scientific (Rockford, IL, USA). All other chemicals and
cell-culture reagents were purchased from Fisher Scientific
(Sommerville, NJ, USA) or Sigma-Aldrich.
Cell culture
LNCaP and CWR22Rv1 were grown in T-75 flasks with
normal growth media [RPMI-1640 containing 10% heat-inactivated
fetal bovine serum (FBS), 100 units of penicillin and 100
μg/ml streptomycin] in a humidified incubator at 37°C with
5% CO2. Confluent cells were detached with a 0.25%
trypsin 0.53 mM EDTA solution for subculture growth.
Exosome isolation
Twenty flasks (each cell line) of prostate cancer
cells at 80% confluence (3 days), were subjected to washing twice
in 5 ml serum-free RPMI-1640 media, then replaced with 10 ml
serum-free RPMI-1640 media to continue growth for 48 h. A total of
100 ml of cell conditioned media were centrifuged at 300 × g for 10
min at 4°C to pellet the suspension cells. The supernatant media
was further cleared by centrifugation at 16,500 × g at 4°C for 30
min to remove protein aggregates and cell debris. The collected
supernatant was passed through 0.22 μm filter to remove the
>200 nm protein aggregates or vesicles. The filtered media was
concentrated to 60 ml using a 70 kDa MWCO Centricon Plus-20 filter
capsule (Millipore, Billerica, MA, USA). The concentrated media
were transferred to an ultracentrifuge tube for centrifugation at
100,000 × g for 70 min at 4°C to pellet exosomes. The isolated
exosomes were rinsed in 10 ml of PBS buffer, and centrifuged at
100,000 × g for 1 h at 4°C to be applied for the following
experiments.
Transmission electron microscopy
(TEM)
The isolated exosomes from LNCaP cells were fixed
with 50 μl of 4% formaldehyde for 15 min at room
temperature, and 5 μl of sample was loaded onto
carbon-coated copper grids and left for 20 min at room temperature.
The sample was washed three times in PBS and then fixed for 5 min
in 1% glutaraldehyde. After three washes, the exosome sample was
stained for 10 min with saturated aqueous uranyl, and dried after
removal of excess liquid. The samples were observed in a FEI Tecnai
T-20 at 200 kV and images were recorded using iTEM software
(Olympus, Münster, Germany).
Exosomal protein extraction and western
blot analysis
For protein extraction, the isolated exosomes were
re-suspended in 50 μl of lysis buffer (1% NP-40, 20 mM Tris
pH 8.0, 137 mM NaCl, 10% glycerol) supplemented with 1X Halt
Protease Inhibitor Cocktail, kept on ice for 15 min, then
centrifuged at 10,000 × g for 15 min. The supernatant was
collected, and stored at −80°C. The whole-cell protein extraction
was also performed as controls, according to our previous protocol
(20–22). Protein concentrations were
determined using Non-Interfering Protein Assay (G-Biosciences, St.
Louis, MO, USA). Western blot analysis was performed as described
previously with only minor modifications (22,23).
In brief, cellular proteins (30 μg) and exosomal proteins (5
μg) were loaded and separated on a NuPAGE™ 4–12% Bis-Tris
Gel (Invitrogen, Carlsbad, CA, USA) by electrophoresis for 60 min
at a constant 200 V under reducing conditions, and then transferred
to a 0.45-μm PVDF Immobilon-P Transfer Membrane (Millipore
Corporation, Bedford, MA, USA) at 400 mA for 120 min in a transfer
apparatus-Owl Bandit VEP-2 (Owl, Portsmouth, NH, USA) according to
the manufacturer’s instructions. Membranes were incubated with
primary antibody at corresponding dilution overnight at 4°C and
then with horseradish peroxidase conjugated-second antibody for 1 h
at room temperature. The immunoreactive bands were visualized using
Protein Detector TMB Western Blot Kit (KPL, Gaithersburg, MD, USA)
following the manufacturer’s instructions.
Deglycosylation analysis of PSMA
According to manufacturer’s guidance, cellular PSMA
and exosomal PSMA were subjected to denaturation in 1X denaturing
buffer for 10 min at 100°C, cooled and spun down. The denatured
proteins were mixed with PNGase F in 1X reaction buffer containing
1% NP-40 to incubate for 3 h at 37°C. Suitable amounts of
deglycosylated PSMAs were analyzed by western blotting, the equal
amounts of intact cellular PSMA and exosomal PSMA were loaded as
controls.
Enzymatic activity analysis
HPLC-based PSMA enzymatic activity analysis was
performed in triplicate as described previously with only minor
modifications (6,24). Working solutions of the substrate
{N-[4-(phenylazo)-benzoyl]-glutamyl-γ-glutamic acid, (PABGγG)} were
made at 10 μM in Tris buffer (50 mM, pH 7.4). Working
solutions of each protein sample were diluted at suitable
concentrations in Tris buffer (50 mM, pH 7.4 containing 1% Triton
X-100) to obtain ∼15% conversion (product/total substrate). A
typical incubation mixture (final volume 250 μl) was
prepared by the addition of 175 μl Tris buffer (50 mM, pH
7.4) and PABGγG (25 μl, 10 μM) in a test tube. The
enzymatic reaction was initiated by the addition of 25 μl of
the PSMA working solution. The reaction was allowed to proceed for
15 min with constant shaking at 37°C and terminated by the addition
of 25 μl methanolic TFA (2.5% trifluoroacetic acid by volume
in methanol) followed by vortexing. The quenched incubation mixture
was quickly buffered by the addition of 25 μl
K2HPO4 (0.1 M), vortexed, iced for 15 min,
and centrifuged (10 min at 7,000 × g). An 85 μl aliquot of
the resulting supernatant was subsequently quantified for the
proportions of substrate and product by HPLC as previously
described (25,26). Fractional enzymatic activity for
each protein sample was calculated from HPLC data. The relative
enzymatic activity (exosomal PSMA/cellular PSMA) was further
calculated.
Results
Validation of exosome isolation
protocol
TEM analysis clearly revealed that vesicle
morphology was cup or round shaped and the size (<100 nm) was
characteristic (27,28) for exosomes (Fig. 1). This result confirmed the
efficacy of our protocol for isolating exosomes from LNCaP
cells.
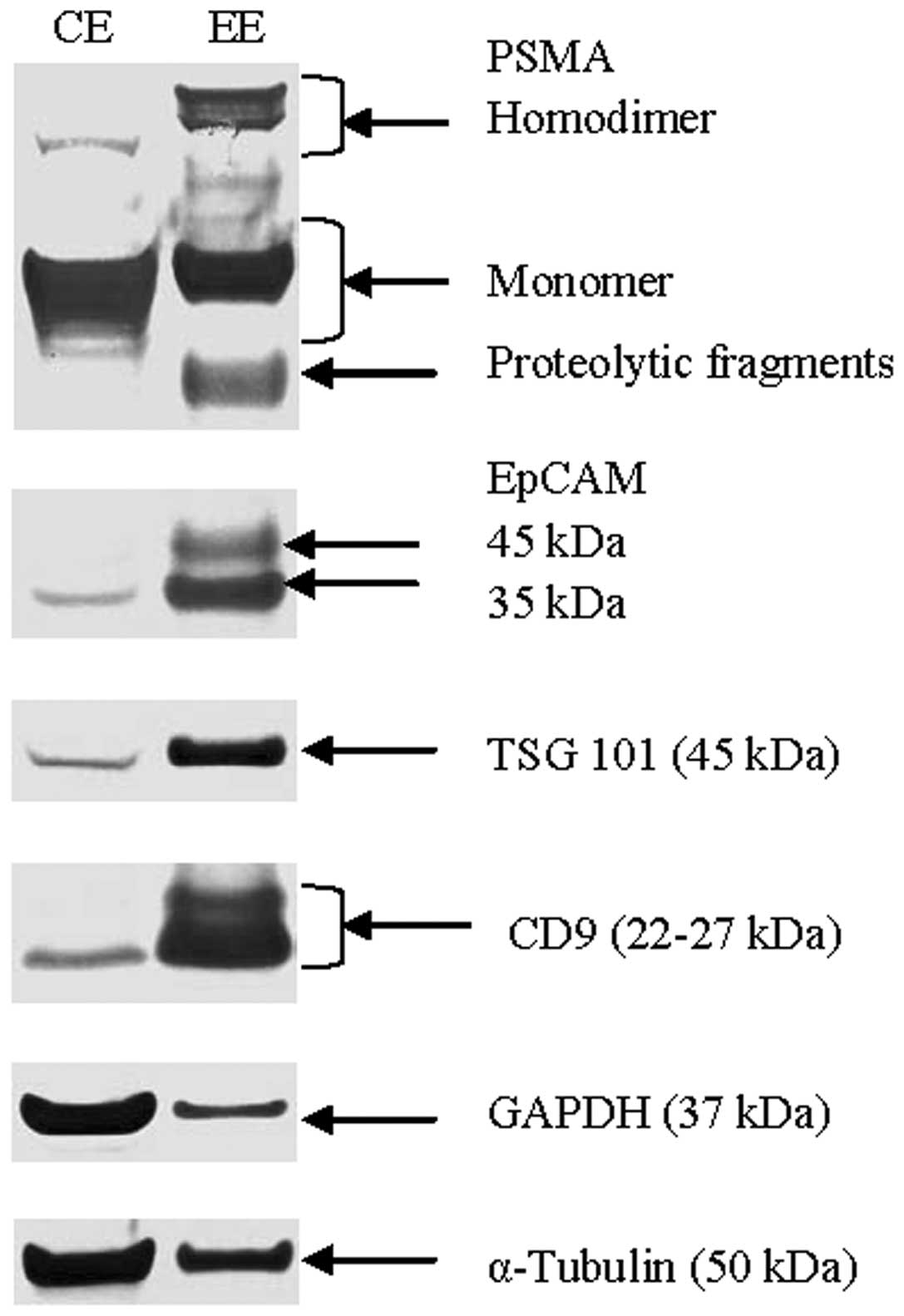
Enrichment of PSMA in exosomes
Western blot analysis data (Fig. 2) demonstrated exceptional
enrichment of exosomal markers (TSG 101, CD9) and epithelial cell
adhesion molecule (EpCAM), as well as moderately enriched prostate
tumor-marker PSMA in exosomes when compared to relatively stable
α-tubulin levels in cells and exosomes. Interestingly, exosomal
PSMA was also found to be highly enriched with an increase in
molecular weight when compared to the cell extract, and also
contained a small amount of proteolytic fragments (Fig. 2).
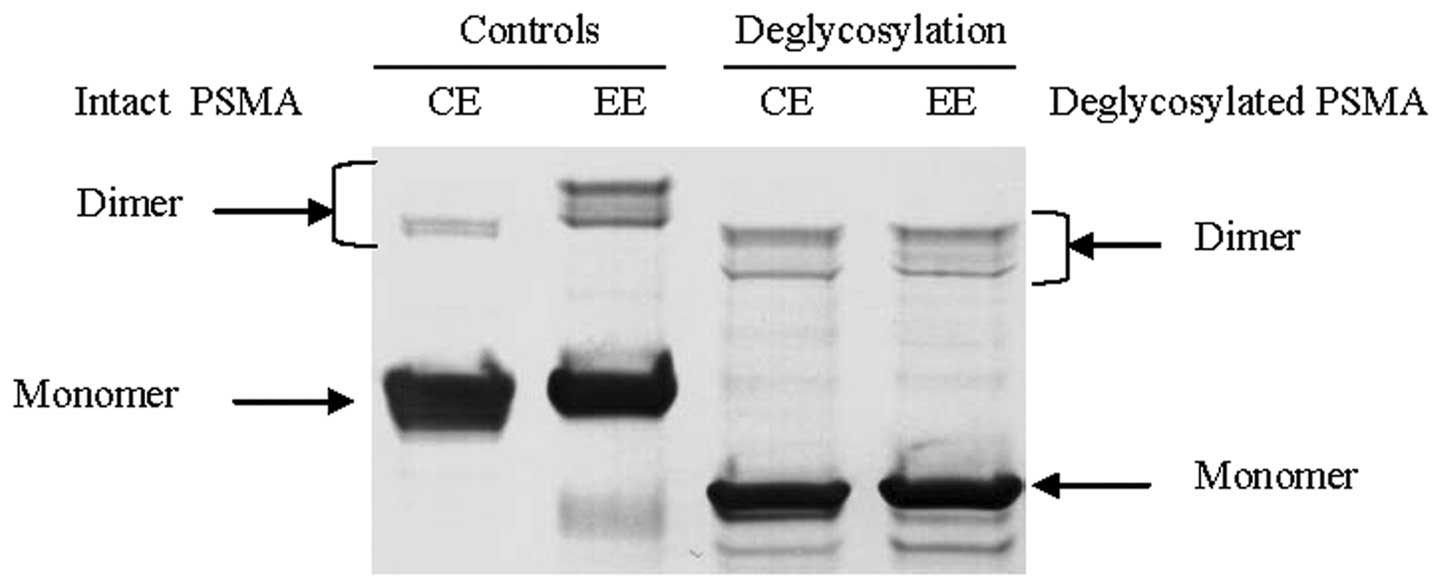
Highly glycosylated exosomal PSMA
To identify the source of PSMA’s perturbed molecular
weight, glycosylation analysis of cellular and exosomal PSMAs were
performed with PNGase F to remove all N-linked glycosylation from
PSMA. After deglycosylation, cellular and exosomal PSMAs exhibited
the same size of molecular weight on western blot analysis
(Fig. 3).
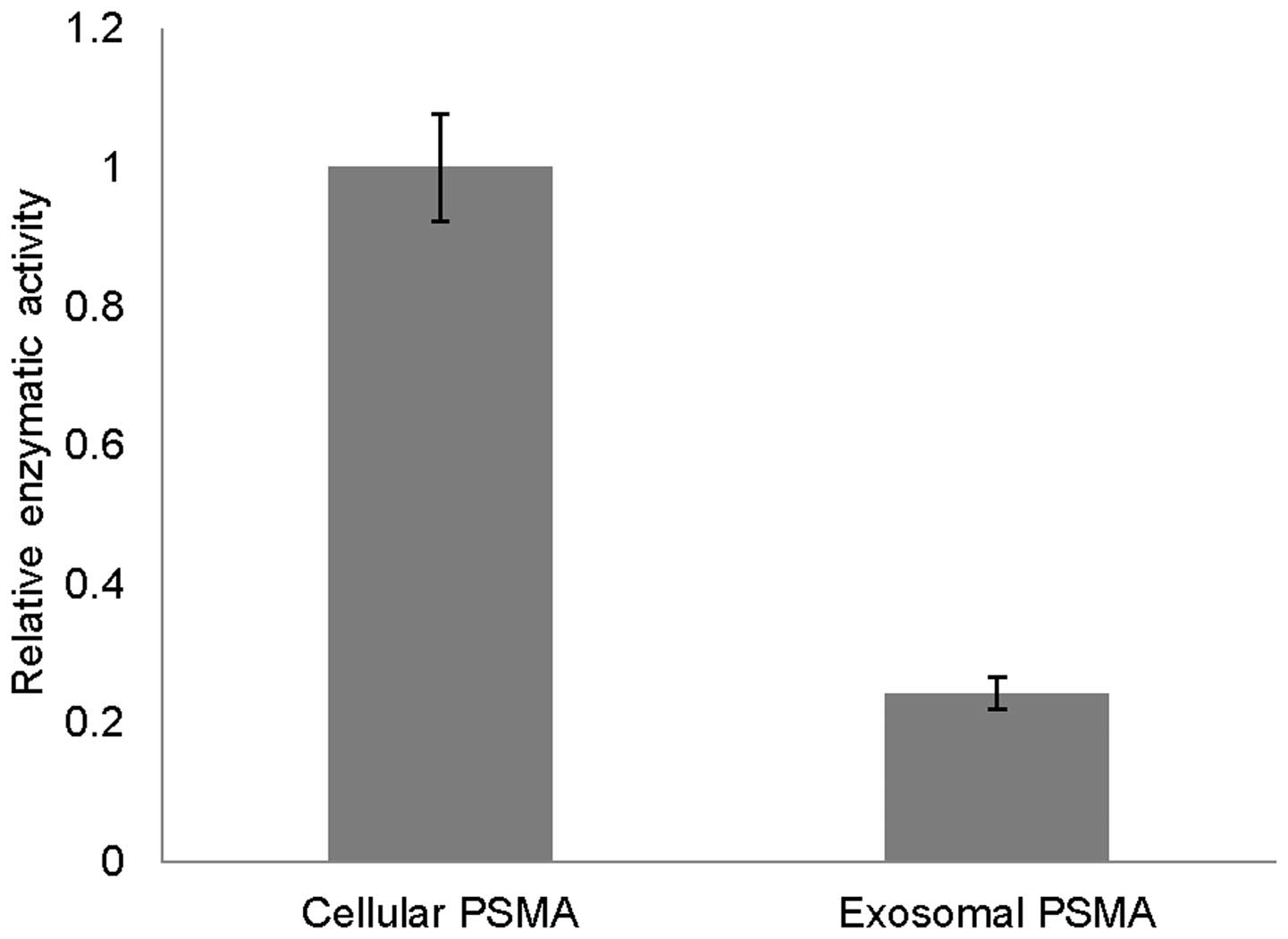
Retaining enzymatic activity of exosomal
PSMA
Equal amounts of exosomal and cellular PSMAs were
evaluated for their enzymatic activities using an HPLC-based, in
vitro enzyme assay. Exosomal PSMA retains ∼24% enzyme activity
of cellular PSMA (Fig. 4),
attributed to partial proteolysis (Fig. 2) and lower pH within endosomes
causing denaturation of internalized PSMA during exosome
formation.
Confirmation of enriched PSMA in
CWR22Rv1-derived exosomes
Employment of another PSMA-positive prostate cancer
cell line (CWR22Rv1) through western blot analysis (Fig. 5) further validated that
CWR22Rv1-derived exosomes were also enriched with highly
glycosylated PSMA analogous to LNCaP-derived exosomes. As controls,
the exosomal markers (CD9 and TSG 101) were also highly enriched.
Surprisingly, EpCAM was found to be at a low level in
CWR22Rv1-derived exosomes, but detected at a higher level in
CWR22Rv1 cells.
Discussion
A plethora of empirical data support that
tumor-derived exosomes can serve as cellular representatives or
messengers carrying multiple forms of tumor-associated information
including signaling molecules, tumor-markers and genetic factors,
which may be an untapped potential source of cancer biomarkers for
diagnostic or prognostic applications toward multiple cancer types
(16). For prostate cancer, our
study was carried out to explore whether prostate tumor-derived
exosomes were enriched with PSMA, because PSMA has been widely
studied and validated as an important biomarker for prostate
cancer. Thus, two PSMA-positive prostate cancer cell lines: LNCaP
(androgen-dependent) and CWR22Rv1 (androgen-independent) cells were
employed in the present project. Although it has been reported that
prostate tumor-derived exosomes can enrich biomarker PSMA (28,29),
by using both of LNCaP and CWR22Rv1 cells, our data further
confirmed the enrichment of exosomal PSMA without regard to
androgen-dependence or -independence of PSMA-positive prostate
cancer cells. To our surprise, our data revealed that exosomal PSMA
is highly glyco sylated, and still retains about 24% enzymatic
activity when compared to cellular PSMA. This evidence suggests
that the origin of exosomal PSMA may be from internalization of
mature (highly glycosylated) PSMA on the cell surface. The observed
diminished activity of PSMA may be due to partial proteolysis or
loss of native conformation under the low pH environment of
endosomes; a result of the internalization process prior to fusing
with multi-vesicular bodies (MVB) for exosome formation. Our data
also suggest that there may be alternative fates for internalized
PSMA: extracellular secretion through exosomes, recycling to the
membrane surface or lysosomal digestion (4,6,30).
Currently, there are three major approaches for
exosome isolation including ultracentrifugation, chemical
precipitation and affinity-binding beads (31,32)
which all have shortcomings. The first two approaches are void of
specificity, and the last one is dependent on the binding-target
protein. In example, EpCAM-based exosome-capture technology is not
selective, suffering from contamination of normal tissue-derived
exosomes, because EpCAM is widely expressed among a variety of
human epithelial tissues, cancers, progenitor and stem cells
(33). In contrast,
highly-expressed PSMA is only found in prostate cancer cells
(34). In fact, our group recently
reported successful capture of PSMA-positive prostate cancer cells
from blood samples using PSMA-based capture technology (35). Therefore, our data strongly support
the development of a novel PSMA-based exosome capture technology
platform for the accurate isolation of prostate tumor-derived
exosomes from normal tissue-related exosomes.
In summary, our present data support the concept
that prostate tumor-derived exosomes are highly enriched with
tumor-marker biomolecules (especially membrane proteins, such as
PSMA) representing characteristics of the original prostate cancer
cells. Furthermore, characterization of tumor-derived exosomes may
provide opportunities for the discovery of novel tumor-related
biomarkers. We expect that developing a highly efficient,
PSMA-based approach for tumor-derived exosome isolation will
accelerate the innovation of non-invasive diagnostic or prognostic
technologies for prostate cancer.
Acknowledgements
The authors thank Cytogen Corporation
(Princeton, NJ, USA) and Dr William C. Davis (Veterinary
Microbiology and Pathology at WSU, USA) for the gifts of the mouse
monoclonal anti-PSMA antibody 7E11 and anti-CD9 antibody,
respectively. The authors also extend their gratitude for technical
assistance from C. Davitt and V. Lynch-Holm at the WSU Franceschi
Microscopy and Imaging Center. This study was supported in part by
the National Institutes of Health (R01CA140617).
References
|
1.
|
Bacich DJ, Pinto JT, Tong WP and Heston
WD: Cloning, expression, genomic localization, and enzymatic
activities of the mouse homolog of prostate-specific membrane
antigen/NAALADase/folate hydrolase. Mamm Genome. 12:117–123. 2001.
View Article : Google Scholar
|
|
2.
|
Chang SS, Reuter VE, Heston WD and Gaudin
PB: Comparison of anti-prostate-specific membrane antigen
antibodies and other immunomarkers in metastatic prostate
carcinoma. Urology. 57:1179–1183. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Chang SS, O’Keefe DS, Bacich DJ, Reuter
VE, Heston WD and Gaudin PB: Prostate-specific membrane antigen is
produced in tumor-associated neovasculature. Clin Cancer Res.
5:2674–2681. 1999.PubMed/NCBI
|
|
4.
|
Liu H, Rajasekaran AK, Moy P, et al:
Constitutive and antibody-induced internalization of
prostate-specific membrane antigen. Cancer Res. 58:4055–4060.
1998.PubMed/NCBI
|
|
5.
|
Rajasekaran AK, Anilkumar G and
Christiansen JJ: Is prostate-specific membrane antigen a
multifunctional protein? Am J Physiol Cell Physiol. 288:C975–C981.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Liu T, Wu LY, Kazak M and Berkman CE:
Cell-surface labeling and internalization by a fluorescent
inhibitor of prostate-specific membrane antigen. Prostate.
68:955–964. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Tagawa ST, Beltran H, Vallabhajosula S, et
al: Anti-prostate-specific membrane antigen-based
radioimmunotherapy for prostate cancer. Cancer. 116:1075–1083.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Wolf P, Freudenberg N, Buhler P, et al:
Three conformational antibodies specific for different PSMA
epitopes are promising diagnostic and therapeutic tools for
prostate cancer. Prostate. 70:562–569. 2010.PubMed/NCBI
|
|
9.
|
Murphy GP, Greene TG, Tino WT, Boynton AL
and Holmes EH: Isolation and characterization of monoclonal
antibodies specific for the extracellular domain of prostate
specific membrane antigen. J Urol. 160:2396–2401. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Morris MJ, Pandit-Taskar N, Divgi CR, et
al: Phase I evaluation of J591 as a vascular targeting agent in
progressive solid tumors. Clin Cancer Res. 13:2707–2713. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Milowsky MI, Nanus DM, Kostakoglu L, et
al: Vascular targeted therapy with anti-prostate-specific membrane
antigen monoclonal antibody J591 in advanced solid tumors. J Clin
Oncol. 25:540–547. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Tsukamoto T, Wozniak KM and Slusher BS:
Progress in the discovery and development of glutamate
carboxypeptidase II inhibitors. Drug Discov Today. 12:767–776.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Kularatne SA, Zhou Z, Yang J, Post CB and
Low PS: Design, synthesis, and preclinical evaluation of
prostate-specific membrane antigen targeted (99m)Tc-radioimaging
agents. Mol Pharm. 6:790–800. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Lapi SE, Wahnishe H, Pham D, et al:
Assessment of an 18F-labeled phosphoramidate peptidomimetic as a
new prostate-specific membrane antigen-targeted imaging agent for
prostate cancer. J Nucl Med. 50:2042–2048. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Cho SY, Gage KL, Mease RC, et al:
Biodistribution, tumor detection, and radiation dosimetry of
18F-DCFBC, a low-molecular-weight inhibitor of prostate-specific
membrane antigen, in patients with metastatic prostate cancer. J
Nucl Med. 53:1883–1891. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Azmi AS, Bao B and Sarkar FH: Exosomes in
cancer development, metastasis, and drug resistance: a
comprehensive review. Cancer Metastasis Rev. May 25–2013.(Epub
ahead of print).
|
|
17.
|
Thery C: Exosomes: secreted vesicles and
intercellular communications. F1000 Biol Rep. 3:152011. View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Thompson CA, Purushothaman A, Ramani VC,
Vlodavsky I and Sanderson RD: Heparanase regulates secretion,
composition, and function of tumor cell-derived exosomes. J Biol
Chem. 288:10093–10099. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19.
|
Kharaziha P, Ceder S, Li Q and Panaretakis
T: Tumor cell-derived exosomes: a message in a bottle. Biochim
Biophys Acta. 1826:103–111. 2012.PubMed/NCBI
|
|
20.
|
Liu T, Mendes DE and Berkman CE: From AR
to c-Met: Androgen deprivation leads to a signaling pathway switch
in prostate cancer cells. Int J Oncol. 43:1125–1130.
2013.PubMed/NCBI
|
|
21.
|
Liu T, Wu LY, Fulton MD, Johnson JM and
Berkman CE: Prolonged androgen deprivation leads to downregulation
of androgen receptor and prostate-specific membrane antigen in
prostate cancer cells. Int J Oncol. 41:2087–2092. 2012.PubMed/NCBI
|
|
22.
|
Liu T, Wu LY and Berkman CE:
Prostate-specific membrane antigen-targeted photodynamic therapy
induces rapid cytoskeletal disruption. Cancer Lett. 296:106–112.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
23.
|
Liu T, Toriyabe Y and Berkman CE:
Purification of prostate-specific membrane antigen using
conformational epitope-specific antibody-affinity chromatography.
Protein Expr Purif. 49:251–255. 2006. View Article : Google Scholar
|
|
24.
|
Wu LY, Anderson MO, Toriyabe Y, et al: The
molecular pruning of a phosphoramidate peptidomimetic inhibitor of
prostate-specific membrane antigen. Bioorg Med Chem. 15:7434–7443.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
25.
|
Anderson MO, Wu LY, Santiago NM, et al:
Substrate specificity of prostate-specific membrane antigen. Bioorg
Med Chem. 15:6678–6686. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
26.
|
Maung J, Mallari JP, Girtsman TA, et al:
Probing for a hydrophobic a binding register in prostate-specific
membrane antigen with phenylalkylphosphonamidates. Bioorg Med Chem.
12:4969–4979. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
27.
|
Lehmann BD, Paine MS, Brooks AM, et al:
Senescence-associated exosome release from human prostate cancer
cells. Cancer Res. 68:7864–7871. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
28.
|
Hosseini-Beheshti E, Pham S, Adomat H, Li
N and Tomlinson Guns ES: Exosomes as biomarker enriched
microvesicles: characterization of exosomal proteins derived from a
panel of prostate cell lines with distinct AR phenotypes. Mol Cell
Proteomics. 11:863–885. 2012. View Article : Google Scholar
|
|
29.
|
Mitchell PJ, Welton J, Staffurth J, et al:
Can urinary exosomes act as treatment response markers in prostate
cancer? J Transl Med. 7:42009. View Article : Google Scholar : PubMed/NCBI
|
|
30.
|
Rajasekaran SA, Anilkumar G, Oshima E, et
al: A novel cytoplasmic tail MXXXL motif mediates the
internalization of prostate-specific membrane antigen. Mol Biol
Cell. 14:4835–4845. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
31.
|
Vlassov AV, Magdaleno S, Setterquist R and
Conrad R: Exosomes: current knowledge of their composition,
biological functions, and diagnostic and therapeutic potentials.
Biochim Biophys Acta. 1820:940–948. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
32.
|
Tauro BJ, Greening DW, Mathias RA, et al:
Comparison of ultracentrifugation, density gradient separation, and
immunoaffinity capture methods for isolating human colon cancer
cell line LIM1863-derived exosomes. Methods. 56:293–304. 2012.
View Article : Google Scholar
|
|
33.
|
Trzpis M, McLaughlin PM, de Leij LM and
Harmsen MC: Epithelial cell adhesion molecule: more than a
carcinoma marker and adhesion molecule. Am J Pathol. 171:386–395.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
34.
|
Ghosh A and Heston WD: Tumor target
prostate specific membrane antigen (PSMA) and its regulation in
prostate cancer. J Cell Biochem. 91:528–539. 2004. View Article : Google Scholar
|
|
35.
|
Wu LY, Liu T, Hopkins MR, Davis WC and
Berkman CE: Chemoaffinity capture of pre-targeted prostate cancer
cells with magnetic beads. Prostate. 72:1532–1541. 2012. View Article : Google Scholar : PubMed/NCBI
|