Introduction
miRNAs are small non-coding RNAs that regulate gene
expression by associating with the nucleotides in the 3’
untranslated regions (UTRs) of target mRNAs and inhibiting protein
translation (1) or by directing
mRNA degradation (2). miRNAs play
important roles in cell proliferation, differentiation, cell cycle
and apoptosis (3,4). Each miRNA can potentially regulate
hundreds of mRNAs. In particular, miRNAs that regulate cell
proliferation are associated with various disorders including
cancer (5). Numerous studies have
revealed differences in the expression of various miRNAs between
tumors and normal tissues (6),
suggesting that miRNAs can function as tumor suppressors or
oncogenes in human cancers.
Human interferons (HuIFNs) are a family of cytokines
that have pleiotropic biological functions, including antiviral and
antitumor effects (7–9). They also regulate basic cellular
functions including growth, differentiation, and immunoreactivity
(10–12). HuIFNs induce a cascade of events
leading to an increase in the expression of various genes,
including those responsible for the biological effects of IFNs
(10–14). Based in part on the differential
use of unique receptors through which they mediate their biological
effects, three classes of HuIFNs have been distinguished: HuIFN-α
and -β are grouped together as type I IFN; HuIFN-γ is a type II
IFN; and HuIFN-λ is a type III IFN. HuIFN-β is useful as an
anti-neoplastic drug sensitizer when administered in combination
with nitrosoureas (15), although
the role of miRNAs in this function is not clear.
In a previous study, we reported that inhibition of
cell viability by HuIFN-β was mediated by miR-431 (16). Medulloblastoma is the most common
malignant tumor of the central nervous system in children (17). Conventional treatment of
medulloblastoma consists of surgery, radiation and chemotherapy.
Despite successful treatments, aggressive adverse effects result in
neurological and endocrine complications in many survivors
(18). Glioblastoma is highly
invasive, proliferative and vascularized (19). Despite aggressive treatment, such
as surgery, radiotherapy and chemotherapy, the median survival is
<16 months (20). Therefore, it
is important to understand the mechanisms associated with the
development and progression of glioblastoma.
In this study, we focused renewed attention on the
miR-431. We investigated the relationship between miR-431
expression and the antineoplastic effects of HuIFN-β using
medulloblastoma and glioblastoma cell lines, because HuIFN-β is
used clinically as an antineoplastic drug. Our goal was to identify
antineoplastic mechanism of miR-431 associated with antineoplastic
effects of HuIFN-β in medulloblastoma and glioblastoma.
Materials and methods
Cell lines and culture conditions
Human medulloblastoma cell line TE671 was provided
by the Department of Molecular Genetics, Kitasato University
Graduate School of Medical Sciences. Another medulloblastoma cell
line, ONS-76, and glioblastoma cell lines A172 and U251-MG were
purchased from the Japanese Health Science Research Resources Bank.
ONS-76 cell line was maintained in RPMI-1640 medium supplemented
with 10% fetal bovine serum (FBS, Sanko Junyaku Co., Ltd., Tokyo,
Japan). TE671 and A172 cell lines were maintained in Dulbecco’s
modified Eagle’s medium supplemented with 10% FBS. U251-MG cell
line was maintained in Eagle’s minimal essential medium
supplemented with 10% FBS. Cell lines were treated with recombinant
HuIFN-β (PeproTech., Rocky Hill, NJ, USA) dissolved in medium just
before use. Cells were grown in medium containing
0.01×105, 0.5×105 or 1.0×105 IU/ml
recombinant HuIFN-β for 24 h or 48 h, with replacement of fresh
medium and recombinant HuIFN-β every 24 h. All cell lines were
cultured at 37°C in a humidified atmosphere containing 5%
CO2.
Measurement of cell viability
Cell viability was measured from the average of 6
MTS assays per sample, quantified by absorbance at 490 nm in a
microplate colorimeter using the CellTiter 96 AQueous One Solution
Reagent (Promega, Madison, WI, USA) according to the manufacturer’s
instructions, for each time point.
RNA extraction
Total RNA was extracted using TRIzol reagent
(Invitrogen) according to the manufacturer’s instructions.
Evaluation of HuIFN-β-induced changes in
miRNA expression levels using quantitative RT-PCR
Quantitative RT-PCR for miR-431 was carried out
using the Taqman MicroRNA Assay (Applied Biosystems, Foster City,
CA, USA). Thirty nanograms of total RNA from medulloblastoma and
glioblastoma cells were reverse transcribed using the TaqMan
Reverse Transcription kit (Applied Biosystems) according to the
manufacturer’s protocol. For normalization, each miRNA was
amplified on the same plate with the reference miRNA, RNU6B, and we
calculated changes in expression levels relative to this
standard.
Transfection of miRNA molecules
miRNA precursors that mimic miR-431 and a control
nonspecific miRNA (Pre-miR Negative Control) were obtained from
Ambion (Austin, TX, USA). Using siPORT NeoFX (Ambion), miRNA
precursors were transfected into ONS-76, TE671, A172 and U251-MG
cells, according to the manufacturer’s protocol.
Estimation of miR-431 target gene
expression
Medulloblastoma (ONS-76 and TE671) and glioblastoma
(A172 and U251-MG) cell lines were grown in medium containing
1.0×105 IU/ml recombinant HuIFN-β for 48 h. RNA was
extracted at 48 h of treatment, cDNA was synthesized using
SuperScript III First-Strand Synthesis SuperMix (Invitrogen), and
predicted targets gene for miR-431 were identified using TargetScan
(http://www.targetscan.org). Steady-state
expression levels of SOCS6 mRNA was evaluated by quantitative
RT-PCR using Fast SYBER Green Master Mix and the following
oligonucleotide primers and annealing temperatures: SOCS6,
5′-AAGAATTCATCCCTTGGATTAGGT AAC-3′ (forward) and
5′-CAGACTGGAGGTCGTGGAA-3′ (reverse) at 60°C. Expression of each
gene was normalized to a GAPDH control: 5′-ACCCACTCCTCCACCTTTG-3′
(forward) and 5′-CTCTTGTGCTCTTGCTGGG-3′ (reverse).
Western blotting
Cells grown to 80% confluence were treated with
HuIFN-β or pre-miR-431. After treatment, cells were washed three
times with ice-cold PBS. Equal amounts of protein were separated by
SDS-PAGE and transferred to polyvinylidene fluoride membranes
(ATTO, Tokyo, Japan). Membranes were blocked in 5% non-fat dried
milk in PBS-Tween-20 and incubated with primary antibody. The
following antibodies were used: SOCS6, JAK1 and total Akt1/2/3
(Santa Cruz Biotechnology, Santa Cruz, CA, USA); and STAT1 p-STAT1,
STAT2, p-STAT2, p-Akt (phospho-Akt, Ser473), p44/42 MAPK
(Erk1/2), and phosphor-p44/42 MAPK (Erk1/2) (Cell Signaling
Technology, Danvers, MA, USA). GAPDH (Sigma, St. Louis, MO, USA)
was used as an internal control. Secondary antibodies were
conjugated to horseradish peroxidase and immunoreactive proteins
were detected using the ECL-plus system (Amersham, Piscataway, NJ,
USA).
Results
Effect of HuIFN-β on viability of
medulloblastoma and glioblastoma cells
HuIFN-β was added to culture medium at a
concentration 0.01×105, 0.5×105 or
1.0×105 IU/ml for 24 h or 48 h. HuIFN-β suppressed
viability of medulloblastoma and glioblastoma cell lines in a dose-
and time-dependent manner (Fig.
1).
Effect of HuIFN-β on miR-431
expression
Based on the results shown in Fig. 1, we analyzed the expression levels
of miR-431 in medulloblastoma and glioblastoma cell lines after 48
h treating with HuIFN-β (1.0×105 IU/ml). HuIFN-β
significantly decreased miR-431 expression in all cell lines
compared with untreated control cell lines (Fig. 2).
Examination of the role of miR-431 in
cell viability
In order to examine whether decreased expression of
miR-431 results in reduced cell viability, medulloblastoma and
glioblastoma cells were treated with HuIFN-β (1.0×105
IU/ml) and transiently transfected with miR-431. Cell proliferation
was not suppressed at 48 h (Fig.
3).
Search for miR-431 target genes
Predicted target genes for miR-431 were identified
with TargetScan. There were 166 conserved targets for miR-431.
Among these genes, we focused on SOCS6, which is related to cell
viability. A schematic representation of SOCS6 mRNAs,
showing the predicted miR-431 binding sites located in their 3’
UTR, is shown in Fig. 4A.
Upregulation of miR-431 target genes
Treating medulloblastoma and glioblastoma cells with
HuIFN-β (1.0×105 IU/ml) also significantly increased
SOCS6 expression after 48 h relative to control cells
(Fig. 4B). However, when these
cells were treated with both HuIFN-β (1.0×105 IU/ml) and
transiently transfected with miR-431, SOCS6 expression was
significantly suppressed (Fig.
4B).
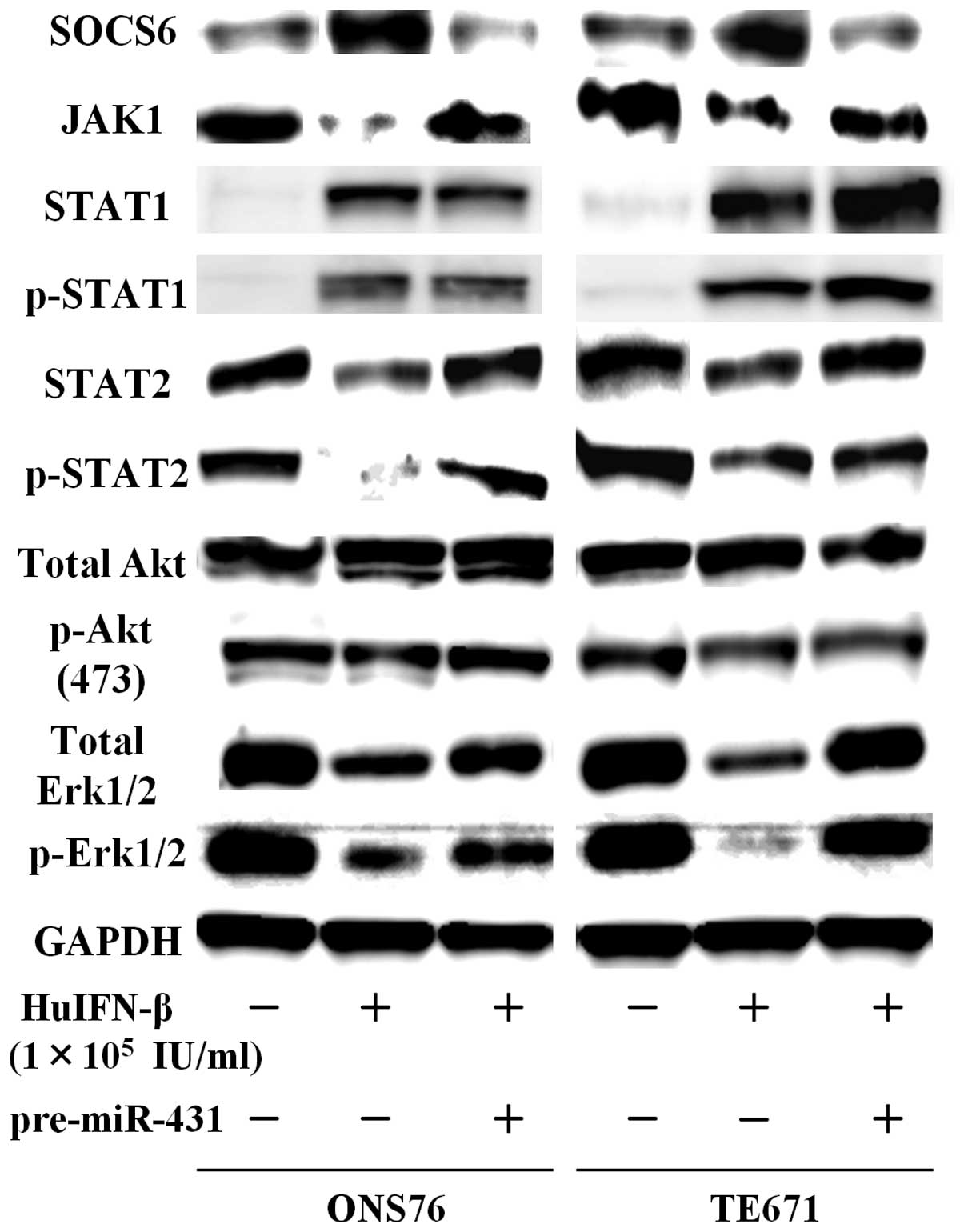
Examination of JAK-STAT signaling
pathways
When medulloblastoma and glioblastoma cells were
treated with HuIFN-β (1.0×105 IU/ml), an increase in
protein levels of SOCS6 was consistently observed after 48 h
(Figs. 5 and 6). The addition of HuIFN-β
(1.0×105 IU/ml) and transiently transfected with miR-431
significantly reduced expression of SOCS6 in medulloblastoma and
glioblastoma cells (Figs. 5 and
6). We investigated the effects of
HuIFN-β-mediated SOCS6 upregulation via suppression of
miR-431 on JAK-STAT, phosphoinositide 3-kinase (PI3K)-Akt and
mitogen-activated protein kinase (MAPK) phosphorylation. In
medulloblastoma and glioblastoma cells, protein levels of JAK1,
STAT2 and p-STAT2 were reduced after 48 h treatment with HuIFN-β
(1.0×105 IU/ml), while protein levels of STAT1 and
p-STAT1 were not reduced (Figs. 5
and 6). As for PI3K-Akt and MAPK
phosphorylation, when ONS76 and TE671 cells were treated with
HuIFN-β (1.0×105 IU/ml), there was a reduction in total
extracellular signal-regulated kinase (Erk)1/2 and p-Erk1/2, while
the levels of total Akt and p-Akt Ser473 were not
significantly reduced (Fig. 5).
When A172 and U251-MG cells were treated with HuIFN-β
(1.0×105 IU/ml), there was a reduction in the levels of
total Akt and p-Akt Ser473, while the levels of total
Erk1/2 and p-Erk1/2 were not significantly reduced (Fig. 6). Upregulation of miR-431 by
transient transfection with miR-431 and simultaneous treatment with
HuIFN-β (1.0×105 IU/ml) reversed the suppression of
expression of these genes (Figs. 5
and 6).
Discussion
miRNAs are known as playing an important role in
antitumor activity. Differential expression of miRNAs is observed
in a variety of cancers. Changes in miRNA expression in cancer lead
to dysregulation of cell proliferation, which results in
tumorigenesis (21,22). Several studies have reported
dysregulation of cell proliferation in medulloblastoma and
glioblastoma via downregulation of target genes by changes in miRNA
expression. For example, in medulloblastoma, miR-124, miR-129 and
miR-383 inhibit cell proliferation through down-regulation of their
respective target genes, which are SLC16A1 for miR-124 (23), CDK6 for miR-124 and miR-129
(24,25), and PRDX3 for miR-383 (26). In glioblastoma, miR-124, miR-134
and miR-137 inhibit cell proliferation through downregulation of
their respective target genes, which are PPP1R13L or SOS1 for
miR-124 (27,28), Nanog for miR-134 (29), and RTVP-1 for miR-137 (30).
IFN-β is used as a therapeutic agent for a variety
of neoplasms. For example, it is frequently used to treat melanoma,
medulloblastoma and glioblastoma in adjuvant therapy. Although its
molecular mechanisms remain unclear, Chawla-Sarkar et al
suggested that one of the antitumor effects of IFN-β in melanoma
cells involves apoptosis (31).
Yoshino et al also suggested that IFN-β mediated
cytotoxicity, including apoptosis, in glioblastoma cells might
involve upregulation of IFN regulatory factor (IRF)-1 and IRF-2
(9).
Based on these conclusions, we investigated the
interaction between expression of miRNAs and treatment with IFN-β,
using medulloblastoma and glioblastoma cell lines, because these
factors both have antitumor effects. We previously reported that
miR-431 expression was upregulated by addition of HuIFN-β, using a
non-cancer HuIFN-β sensitive cell line (RSa) and its variant
HuIFN-β resistant cell line (F-IFr) (16). The change in miR-431 expression
induced by addition of HuIFN-β was involved in inhibition of cell
viability (16). Therefore, we
focused renewed attention on the function of miR-431 in
medulloblastoma and glioblastoma cells.
First, we confirmed the effect of HuIFN-β on
inhibition of cell proliferation in medulloblastoma and
glioblastoma cells. Then, we showed using quantitative RT-PCR that
miR-431 expression in both cell lines treated with HuIFN-β was
significantly decreased.
In addition, we confirmed that cell proliferation
was not suppressed after 48 h treatment with HuIFN-β and transient
transfection with miR-431. These results suggest that miR-431 plays
an important role in regulating proliferation by HuIFN-β in
medulloblastoma and glioblastoma cells. We then sought to determine
whether miRNA-regulated signaling pathways modulated cell
viability. Previous studies revealed that some signaling pathways
that affect cell viability are regulated by miRNA expression
(32–35). We decided to focus on one of these,
the JAK-STAT signaling pathway. The factors that regulate JAK-STAT
signaling pathways and are involved in cell proliferation in cancer
have been investigated by others (36,37).
For numerous cancers, activation of JAK-STAT signaling pathway
contributes to cell proliferation. Therefore, suppression of this
pathway should inhibit cancer cell proliferation. In gastric
cancer, OPB-31121, a novel small molecular inhibitor, inhibits
JAK-STAT signaling pathway and has antitumor effects (37). Thus, JAK-STAT signaling pathway can
affect cell proliferation.
In the present study, we examined the miR-431 target
genes that are suspected of suppressing cell viability, and focused
on SOCS6, which is a functional target of miR-431. SOCS6 is a
suppressor of cytokine signaling that belongs to the
cytokine-induced STAT inhibitors, and it mediates cytokine-induced
signaling. According to quantitative RT-PCR analysis and western
blot analysis, although SOCS6 is upregulated in medulloblastoma and
glioblastoma cells treated with HuIFN-β, it is downregulated in
cells treated with HuIFN-β and transiently transfected with
miR-431. These observations suggest that decreased miR-431
expression increases SOCS6 expression in medulloblastoma and
glioblastoma cells treated with HuIFN-β. Therefore, we hypothesized
that miR-431-mediated SOCS6 upregulation would be accompanied by
the inhibition of JAK-STAT signaling. HuIFN-β suppressed this
signaling pathway in medulloblastoma and glioblastoma cells via
suppression of JAK1 and STAT2. In addition, we also considered the
involvement of the PI3K-Akt and MAPK pathways, as part of the
JAK-STAT signaling pathway, in cell proliferation. The MAPK pathway
was only inhibited in medulloblastoma cells. In contrast, the
PI3K-Akt pathway was only inhibited in glioblastoma cells.
There are several clinical studies on the
therapeutic use of IFN-β for various malignant tumors. For example,
NC65 tumors (a human renal cell carcinoma) treated with recombinant
HuIFN-β did not shrink and failed to undergo apoptosis (38). This study indicates that there are
many hurdles facing the use of HuIFN-β as an anticancer agent. In
contrast, combination therapy with IFN-β and ranimustine has been
particularly useful for the treatment of malignant gliomas in Japan
(39). A recent study investigated
IFN-β monotherapy and combination therapy with IFN-β and
temozolomide, a relatively new alkylating agent. This combination
therapy was significantly associated with a favorable outcome
(40). Our results suggest that
decreased miR-431 expression in medulloblastoma and glioblastoma
cells, in combination with a PI3K-Akt or MAPK inhibitor, may be
able to suppress tumor cell proliferation more effectively.
In conclusion, our results demonstrate that miR-431,
which is downregulated by HuIFN-β in medulloblastoma and
glioblastoma cells, contributes to suppression of the JAK1 and
STAT2 via upregulation of SOCS6. Combination therapy with
downregulation of miR-431 and a PI3K-Akt or MAPK inhibitor may
represent a more effective strategy for the treatment of IFN-β
resistant medulloblastoma and glioblastoma.
Acknowledgements
The authors thank Mrs. Chihomi Sato
for her excellent technical assistance. This study was supported by
the Smoking Research Foundation, and the Japan Society for the
Promotion of Science (JSPS) KAKENHI Grant number 24701002.
References
|
1.
|
Du T and Zamore PD: MicroPrimer: the
biogenesis and function of microRNA. Development. 132:4645–4652.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
2.
|
He L and Hannon GJ: MicroRNAs: small RNAs
with a big role in gene regulation. Nat Rev Genet. 5:522–531. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Ambros V: The functions of animal
microRNAs. Nature. 431:350–355. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Bartel DP: MicroRNAs: Genomics,
biogenesis, mechanism, and function. Cell. 116:281–297. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Lawler S and Chiocca EA: Emerging
functions of microRNAs in glioblastoma. J Neurooncol. 92:297–306.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Lu J, Getz G, Miska EA, et al: MicroRNA
expression profiles classify human cancers. Nature. 2435:834–838.
2005. View Article : Google Scholar
|
|
7.
|
Pfeffer LM: Mechanisms of Interferon
Action. CRC Press; Boca Raton, FL: 1987
|
|
8.
|
Saito R, Mizuno M, Hatano M, et al: Two
different mechanisms of apoptosis resistance observed in
interferon-β induced apoptosis of human glioma cells. J Neurooncol.
67:273–280. 2004.
|
|
9.
|
Yoshino A, Katayama Y, Yokoyama T, et al:
Therapeutic implication of interferon regulatory factor 1 (IRF-1)
and IRF-2 in diffusely infiltrating astrocytomas (DIA): response to
IFN-β in glioblastoma cells and prognostic value for DIA. J
Neurooncol. 74:249–260. 2005.PubMed/NCBI
|
|
10.
|
Petska S, Langer AJ, Zoon K, et al:
Interferons and their action. Annu Rev Biochem. 56:727–777. 1987.
View Article : Google Scholar
|
|
11.
|
Taniguchi T and Takaoka A: The
interferon-alpha/beta system in antiviral responses: a multimodal
machinery of gene regulation by the IRF family of transcription
factors. Curr Opin Immunol. 14:111–116. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Biron CA: Interferon alpha and beta as
immune regulators - a new look. Immunity. 14:661–664. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Revel M and Chebath J:
Interferon-activated genes. Trends Biochem Sci. 11:166–170. 1986.
View Article : Google Scholar
|
|
14.
|
Williams BR: Transcriptional regulation of
interferon-stimulated genes. Eur J Biochem. 200:1–11. 1991.
View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Yoshida J, Kajita Y, Wakabayashi T, et al:
Long-term follow-up results of 175 patients with malignant glioma:
importance of radical tumor resection and post-operative adjuvant
therapy with interferon, ACNU and radiation. Acta Neurochir.
127:55–59. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Tanaka T, Sugaya S, Kita K, et al:
Inhibition of cell viability by human IFN-β is mediated by
microRNA-431. Int J Oncol. 40:1470–1476. 2012.
|
|
17.
|
Packer RJ and Vezina G: Management of and
prognosis with medulloblastoma: therapy at a crossroads. Arch
Neurol. 65:1419–1424. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Ribi K, Relly C, landolt MA, et al:
Outcome of medulloblastoma in children: long-term complications and
quality of life. Neuropediatrics. 36:357–365. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
19.
|
Furnari FB, Fenton T, Bachoo RM, et al:
Malignant astrocytic glioma: genetics, biology, and paths to
treatment. Genes Dev. 21:2683–2710. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20.
|
Wen PY and Kesari S: Malignant gliomas in
adults. N Engl J Med. 359:492–507. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21.
|
Calin GA and Croce CM: MicroRNA signatures
in human cancers. Nat Rev Cancer. 6:857–866. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
22.
|
Cho WC: OncomiRs: the discovery and
progress of microRNAs in cancers. Mol Cancer. 6:602007. View Article : Google Scholar : PubMed/NCBI
|
|
23.
|
Li KK, Pang JC, Ching AK, et al: miR-124
is frequently down-regulated in medulloblastoma and is a negative
regulator of SLC16A1. Hum Pathol. 40:1234–1243. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
24.
|
Silber J, Hashizume R, Felix T, et al:
Expression of miR-124 inhibits growth of medulloblastoma cells.
Neuro Oncol. 15:83–90. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25.
|
Wu J, Qian J, Li C, et al: miR-129
regulates cell proliferation by downregulating Cdk6 expression.
Cell Cycle. 9:1809–1818. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
26.
|
Wang XM, Zhang SF, Cheng ZQ, et al:
MicroRNA383 regulates expression of PRDX3 in human
medulloblastomas. Zhonghua Bing Li Xue Za Zhi. 41:547–552. 2012.(In
Chinese).
|
|
27.
|
Zhao WH, Wu SQ and Zhang YD:
Downregulation of miR-124 promotes the growth and invasiveness of
glioblastoma cells involving upregulation of PPP1R13L. Int J Mol
Med. 32:101–107. 2013.PubMed/NCBI
|
|
28.
|
Lv Z and Yang L: miR-124 inhibits the
growth of glioblastoma through the downregulation of SOS1. Mol Med
Rep. 8:345–349. 2013.PubMed/NCBI
|
|
29.
|
Niu CS, Yang Y and Cheng CD: MiR-134
regulates the proliferation and invasion of glioblastoma cells by
reducing Nanog expression. Int J Oncol. 42:1533–1540.
2013.PubMed/NCBI
|
|
30.
|
Bier A, Giladi N, Kronfeld N, et al:
MicroRNA-137 is down-regulated in glioblastoma and inhibits the
stemness of glioma stem cells by targeting RTVP-1. Oncotarget.
4:665–676. 2013.PubMed/NCBI
|
|
31.
|
Chawla-Sarkar M, Leaman DW and Borden EC:
Preferential induction of apoptosis by interferon (IFN)-beta
compared with IFN-alpha2: correlation with TRAIL/Apo2L induction in
melanoma cell lines. Clin Cancer Res. 7:1821–1831. 2001.PubMed/NCBI
|
|
32.
|
Chou YT, Lin HH, Lien YU, et al: EGFR
promotes lung tumori-genesis by activating miR-7 through a
Ras/ERK/Myc pathway that targets the Ets2 transcriptional repressor
ERF. Cancer Res. 70:8822–8831. 2010. View Article : Google Scholar
|
|
33.
|
Guo C, Sah JF, Beard L, et al: The
Non-coding RNA, miR-126, suppresses the growth of neoplastic cells
by targeting phosphatidylinositol 3-kinase signaling and is
frequently lost in colon cancers. Gene Chromosomes Cancer.
47:939–946. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
34.
|
Kefas B, Godlewski J, Comeau L, et al:
microRNA-7 inhibits the epidermal growth factor receptor and the
Akt pathway and is down-regulated in glioblastoma. Cancer Res.
68:3566–3572. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
35.
|
Teramo A, Gattazzo C, Passeri F, et al:
Intrinsic and extrinsic mechanisms contribute to maintain the
JAK/STAT pathway aberrantly activated in T-type large granular
lymphocyte leukemia. Blood. 121:3843–3854. S12013. View Article : Google Scholar : PubMed/NCBI
|
|
36.
|
Gao W, Xu J, Liu L, et al: A
systematic-analysis of predicted miR-21 targets identifies a
signature for lung cancer. Biomed Pharmacother. 66:21–28. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
37.
|
Kim MJ, Nam HJ, Kim HP, et al: OPB-31121,
a novel small molecular inhibitor, disrupts the JAK2/STAT3 pathway
and exhibits an antitumor activity in gastric cancer cells. Cancer
Lett. 335:145–152. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
38.
|
Yamamoto K, Mizutani Y, Nakanishi H, et
al: Significant antitumor activity of cationic multilamellar
liposomes containing human interferon-β gene in combination with
5-fluorouracil against human renal cell carcinoma. Int J Oncol.
33:565–571. 2008.
|
|
39.
|
Wakabayashi T, Hatano N, Kajita Y, et al:
Initial and maintenance combination treatment with interferon-beta,
MCNU (Ranimustine), and radiotherapy for patients with previously
untreated malignant glioma. J Neurooncol. 49:57–62. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
40.
|
Motomura K, Natsume A, Kishida Y, et al:
Benefits of interferon-β and temozolomide combination therapy for
newly diagnosed primary glioblastoma with the unmethylated MGMT
promoter. Cancer. 117:1721–1730. 2011.
|