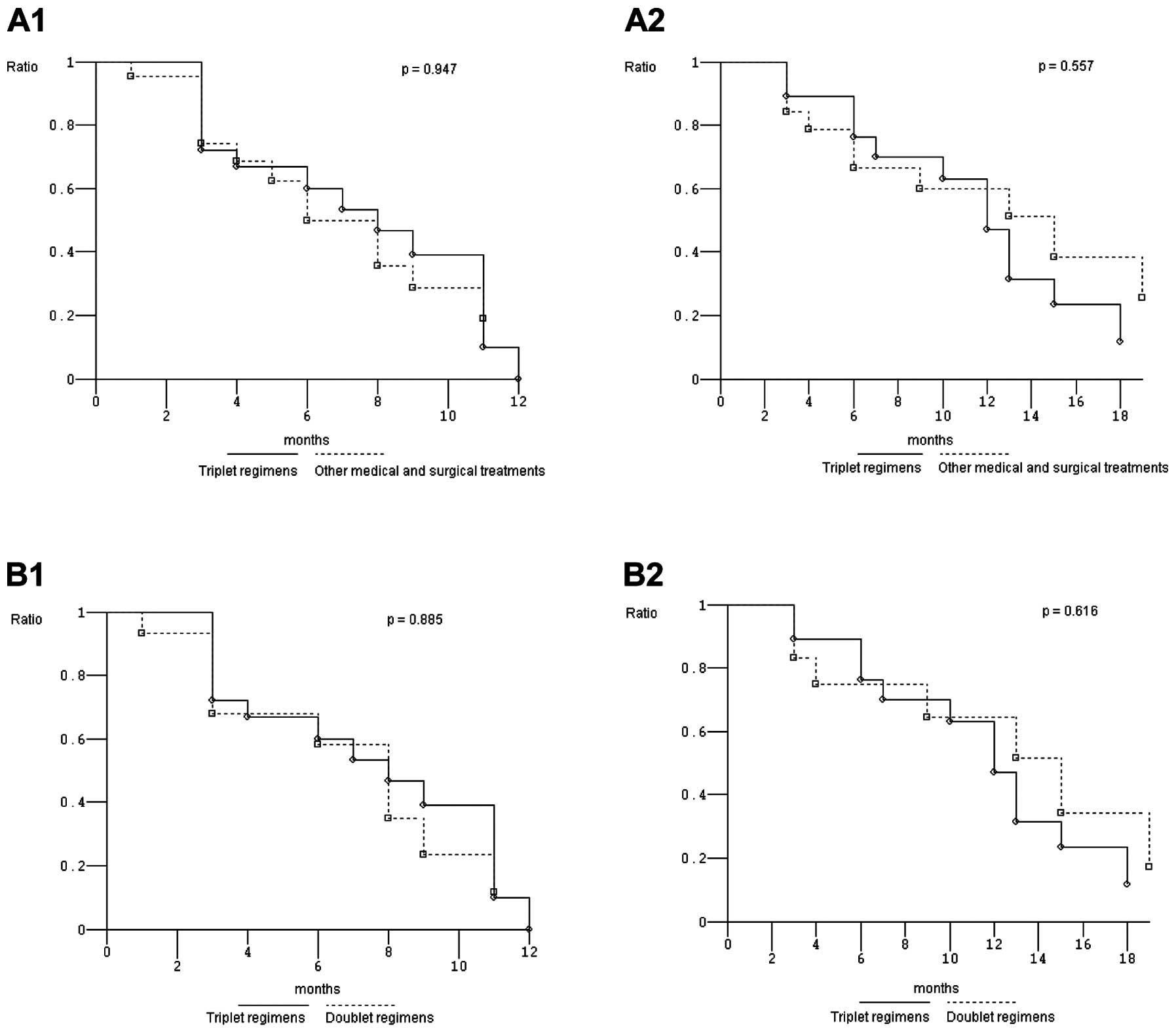
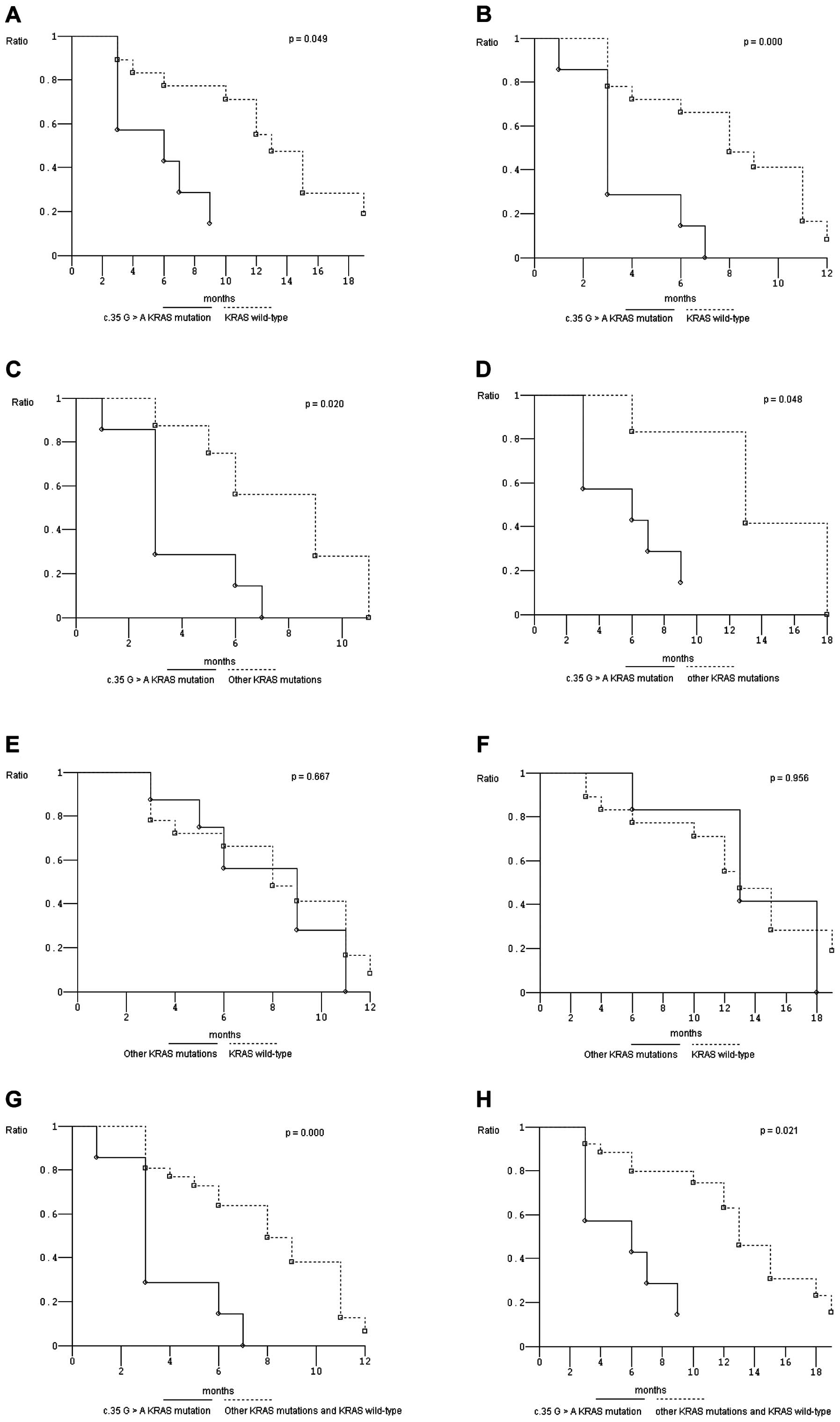
|
1.
|
Schmoll HJ, Van Cutsem E, Stein A,
Valentini V, Glimelius B, Haustermans K, Nordlinger B, van de Velde
CJ, Balmana J, Regula J, Nagtegaal ID, Beets-Tan RG, Arnold D,
Ciardiello F, Hoff P, Kerr D, Köhne CH, Labianca R, Price T,
Scheithauer W, Sobrero A, Tabernero J, Aderka D, Barroso S, Bodoky
G, Douillard JY, El Ghazaly H, Gallardo J, Garin A, Glynne-Jones R,
Jordan K, Meshcheryakov A, Papamichail D, Pfeiffer P, Souglakos I,
Turhal S and Cervantes A: ESMO Consensus Guidelines for management
of patients with colon and rectal cancer. A personalized approach
to clinical decision making. Ann Oncol. 23:2479–2516. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
2.
|
Ficorella C, Bruera G, Cannita K, Porzio
G, Lanfiuti Baldi P, Tinari N, Natoli C and Ricevuto E: Triplet
chemotherapy in patients with metastatic colorectal cancer: toward
the best way to safely administer a highly active regimen in
clinical practice. Clin Colorectal Cancer. 11:229–237. 2012.
View Article : Google Scholar
|
|
3.
|
Bruera G, Cannita K, Giuliante F, Lanfiuti
Baldi P, Vicentini R, Marchetti P, Nuzzo G, Antonucci A, Ficorella
C and Ricevuto E: Effectiveness of liver metastasectomies in
patients with metastatic colorectal cancer treated with FIr-B/FOx
triplet chemotherapy plus bevacizumab. Clin Colorectal Cancer.
11:119–126. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Bruera G, Cannita K, Di Giacomo D, Lamy A,
Troncone G, Dal Mas A, Coletti G, Frébourg T, Sabourin JC, Tosi M,
Ficorella C and Ricevuto E: Prognostic value of KRAS genotype in
metastatic colorectal cancer (MCRC) patients treated with intensive
triplet chemotherapy plus bevacizumab (FIr-B/FOx) according to
extension of metastatic disease. BMC Med. 10:1352012. View Article : Google Scholar
|
|
5.
|
Bruera G and Ricevuto E: Intensive
chemotherapy of meta-static colorectal cancer: weighing between
safety and clinical efficacy. Evaluation of Masi G, Loupakis F,
Salvatore L, et al: Bevacizumab with FOLFOXIRI (irinotecan,
oxaliplatin, fluorouracil, and folinate) as first-line treatment
for metastatic colorectal cancer: a phase 2 trial. Expert Opin Biol
Ther. 11:21–824. 2011.PubMed/NCBI
|
|
6.
|
Masi G, Vasile E, Loupakis F, Cupini S,
Fornaro L, Baldi G, Salvatore L, Cremolini C, Stasi I, Brunetti I,
Fabbri MA, Pugliesi M, Trenta P, Granetto C, Chiara S, Fioretto L,
Allegrini G, Crinò L, Andreuccetti M and Falcone A: Randomized
trial of two induction chemotherapy regimens in metastatic
colorectal cancer: an updated analysis. J Natl Cancer Inst.
103:21–30. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Bruera G, Santomaggio A, Cannita K,
Lanfiuti Baldi P, Tudini M, De Galitiis F, Mancini M, Marchetti P,
Antonucci A, Ficorella C and Ricevuto E: ‘Poker’ association of
weekly alternating 5-fluorouracil, irinotecan, bevacizumab and
oxaliplatin (FIr-B/FOx) in first line treatment of metastatic
colorectal cancer: a phase II study. BMC Cancer. 10:5672010.
|
|
8.
|
Masi G, Loupakis F, Salvatore L, Fornaro
L, Cremolini C, Cupini S, Ciarlo A, Del Monte F, Cortesi E, Amoroso
D, Granetto C, Fontanini G, Sensi E, Lupi C, Andreuccetti M and
Falcone A: Bevacizumab with FOLFOXIRI (irinotecan, oxaliplatin,
fluorouracil, and folinate) as first-line treatment for metastatic
colorectal cancer: a phase 2 trial. Lancet Oncol. 11:845–852. 2010.
View Article : Google Scholar
|
|
9.
|
Garufi C, Torsello A, Tumolo S, Ettorre
GM, Zeuli M, Campanella C, Vennarecci G, Mottolese M, Sperduti I
and Cognetti F: Cetuximab plus chronomodulated irinotecan,
5-fluorouracil, leucovorin and oxaliplatin as neoadiuvant
chemotherapy in colorectal liver metastases: POCHER trial. Br J
Cancer. 103:1542–1547. 2010. View Article : Google Scholar
|
|
10.
|
Extermann M, Overcash J, Lyman GH, Parr J
and Balducci L: Comorbidity and functional status are independent
in older cancer patients. J Clin Oncol. 16:1582–1587.
1998.PubMed/NCBI
|
|
11.
|
Folprecht G, Cunningham D, Ross P,
Glimelius B, Di Costanzo F, Wils J, Scheithauer W, Rougier P,
Aranda E, Hecker H and Kohne CH: Efficacy of 5-fluorouracil-based
chemotherapy in elderly patients with metastatic colorectal cancer:
a pooled analysis of clinical trials. Ann Oncol. 15:1330–1338.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Popescu RA, Norman A, Ross PJ, Parikh B
and Cunningham D: Adjuvant or palliative chemotherapy for
colorectal cancer in patients 70 years or older. J Clin Oncol.
17:2412–2418. 1999.PubMed/NCBI
|
|
13.
|
Chiara S, Nobile MT, Vincenti M, Lionetto
R, Gozza A, Barzacchi MC, Sanguineti O, Repetto L and Rosso R:
Advanced colorectal cancer in the elderly: results of consecutive
trials with 5-fluorouracil-based chemotherapy. Cancer Chemother
Pharmacol. 42:336–340. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Folprecht G, Seymour MT, Saltz L,
Douillard JY, Hecker H, Stephens RJ, Maughan TS, Van Cutsem E,
Rougier P, Mitry E, Schubert U and Kohne CH:
Irinotecan/fluorouracil combination in first-line therapy of older
and younger patients with meta-static colorectal cancer: combined
analysis of 2,691 patients in randomized controlled trials. J Clin
Oncol. 26:1443–1451. 2008. View Article : Google Scholar
|
|
15.
|
Mitry E, Douillard JY, Van Cutsem E,
Cunningham D, Magherini E, Mery-Mignard D, Awad L and Rouigier P:
Predictive factors of survival in patients with advanced colorectal
cancer: an individual data analysis of 602 patients included in
irinotecan phase III trials. Ann Oncol. 15:1013–1017. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Goldberg RM, Tabah-Fisch I, Bleiberg H, de
Gramont A, Tournigand C, Andre T, Rothenberg ML, Green E and
Sargent DJ: Pooled analysis of safety and efficacy of oxaliplatin
plus fluorouracil/leucovorin administered bimonthly in elderly
patients with colorectal cancer. J Clin Oncol. 24:4085–4091. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Pallis AG, Papamichael D, Audisio R,
Peeters M, Folprecht G, Lacombe D and Van Cutsem E: EORTC Elderly
Task Force experts’ opinion for the treatment of colon cancer in
older patients. Cancer Treat Rev. 36:83–90. 2010.
|
|
18.
|
Papamichael D, Audisio R, Horiot JC,
Glimelius B, Sastre J, Mitry E, Van Cutsem E, Gosney M, Kohne CH
and Aapro M: Treatment of the elderly colorectal cancer patient:
SIOG expert recommendations. Ann Oncol. 20:5–16. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19.
|
Audisio RA and Papamichael D: Treatment of
colorectal cancer in older patients. Nat Rev Gastroenterol Hepatol.
9:716–725. 2012. View Article : Google Scholar
|
|
20.
|
Figer A, Perez-Staub N, Carola E,
Tournigand C, Lledo G, Flesch M, Barcelo R, Cervantes A, André T,
Colin P, Louvet C and de Gramont A: FOLFOX in patients aged between
76 and 80 years with metastatic colorectal cancer an exploratory
cohort of the OPTIMOX1 study. Cancer. 110:2666–2671.
2007.PubMed/NCBI
|
|
21.
|
Hurwitz HI, Fehrenbacher L, Novotny WF,
Cartwright T, Hainsworth J, Meropol NJ, Heim W, Berlin J, Baron A,
Griffing S, Holmgren E, Ferrara N, Fyfe G, Rogers B, Ross M and
Kabbinavar FF: Bevacizumab plus irinotecan, fluorouracil, and
leucovorin for metastatic colorectal cancer. N Engl J Med.
350:2335–2342. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
22.
|
Kabbinavar FF, Hurwitz HI, Yi J, Sarkar S
and Rosen O: Addition of bevacizumab to fluorouracil-based
first-line treatment of metastatic colorectal cancer: pooled
analysis of cohorts of older patients from two randomized clinical
trials. J Clin Oncol. 27:199–205. 2008. View Article : Google Scholar
|
|
23.
|
Cassidy J, Saltz LB, Giantonio BJ,
Kabbinavar FF, Hurwitz HI and Rohr UP: Effect of bevacizumab in
older patients with metastatic colorectal cancer: pooled analysis
of four randomized studies. J Cancer Res Clin Oncol. 136:737–743.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
24.
|
Kozloff MF, Berlin J, Flynn PJ, Kabbinavar
F, Ashby M, Dong W, Sing AP and Grothey A; for the BRiTE
Investigators: Clinical outcomes in elderly patients with
metastatic colorectal cancer receiving bevacizumab and
chemotherapy: results from the BRiTE observational cohort study.
Oncology. 78:329–339. 2010. View Article : Google Scholar
|
|
25.
|
Van Cutsem E, Rivera F, Berry S,
Kretzschmar A, Michael M, Di Bartolomeo M, Mazier MA, Canon JL,
Georgoulias V, Peeters M, Bridgewater J and Cunningham D; on behalf
of the First BEAT investigators: Safety and efficacy of first-line
bevacizumab with FOLFOX, XELOX, FOLFIRI and fluoropyrimidines in
metastatic colorectal cancer: the BEAT study. Ann Oncol.
20:1842–1847. 2009.PubMed/NCBI
|
|
26.
|
Souglakos J, Andrulakis N, Syrigos K,
Polyzos A, Ziras N, Athanasiadis A, Kalolyris S, Tsousis S,
Kouroussis CH, Vamvakas L, Kalykaki A, Samonis G, Mavroudis D and
Georgoulias V: FOLFOXIRI (folin acid, 5-fluorouracil, oxaliplatin
and irinotecan) vs FOLFIRI (folin acid, 5-fluorouracil and
irinotecan) as first-line treatment in metestatic colorectal cancer
(MCC): a multicentre randomised phase III trial from the Hellenic
Oncology Research Group (HORG). Br J Cancer. 94:798–805. 2006.
|
|
27.
|
Vamvakas L, Athanasiadis A, Karampeazis A,
Kakolyris S, Polyzos A, Kouroussis C, Ziras N, Kalbakis K,
Georgoulias V and Souglakos J: Clinical outcome of elderly patients
with metastatic colorectal cancer treated with FOLFOXIRI versus
FOLFIRI: Subgroup analysis of a randomized phase III trial from the
Hellenic Oncology Research Group (HORG). Crit Rev Oncol Hematol.
76:61–70. 2010. View Article : Google Scholar
|
|
28.
|
Meyerhardt JA, Jackson McCleary N,
Niedzwiecki D, Hollis D, Venook A, Mayer R and Goldberg R: Impact
of age and comorbidities on treatment effect, tolerance, and
toxicity in metastatic colorectal cancer (mCRC) patients treated on
CALGB 80203. J Clin Oncol. 27(Suppl 15)4038:2009
|
|
29.
|
Douillard J, Siena S, Cassidy J, Tabernero
J, Burkes R, Barugel M, Humblet Y, Bodoky G, Cunningham D, Jassem
J, Rivera F, Kocàkova I, Ruff P, Blasinska-Morawiec M, Smakal M,
Canon JL, Rother M, Oliner KS, Wolf M and Gansert J: Randomized,
phase III trial of Panitumumab with infusional fluorouracil,
leicovorin, and oxaliplatin (FOLFOX4) versus FOLFOX4 alone as
first-line treatment in patients with previously untreated
metastatic colorectal cancer: the PRIME trial. J Clin Oncol.
28:4697–4705. 2010. View Article : Google Scholar
|
|
30.
|
Sargent DJ, Kohne CH, Kelly Sanoff H, Bot
BM, Seymour MT, de Gramont A, Porschen R, Saltz LB, Rougier P,
Tournigand C, Douillard JY, Stephens RJ, Grothey A and Goldberg RM:
Pooled safety and efficacy analysis examining the effect of
performance status on outcomes in nine first-line treatment trials
using individual data from patients with metastatic colorectal
cancer. J Clin Oncol. 27:1948–1955. 2009. View Article : Google Scholar
|
|
31.
|
Seymour MT, Thompson LC, Wasan HS,
Middleton G, Brewster AE, Shepherd SF, O’Mahony MS, Maughan TS,
Parmar M and Langley RE; on behalf of the FOCUS2 Investigators, and
the National Cancer Research Institute Colorectal Cancer Clinical
Studies Group: Chemotherapy options in elderly and frail patients
with metastatic colorectal cancer (MRC FOCUS2): an open-label,
randomised factorial trial. Lancet. 377:1749–1759. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
32.
|
Andreyev HJ, Norman AR, Cunningham D,
Oates J, Dix BR, Iacopetta BJ, Young Y, Walsh T, Ward R, Hawkins N,
Beranek M, Jandik P, Benamouzig R, Jullian E, Laurent-Puig P,
Olschwang S, Muller O, Hoffmann I, Rabes HM, Zietz C, Troungos C,
Valavanis C, Yuen ST, Ho JWC, Croke CT, O’Donoghue DP, Giaretti W,
Rapallo A, Russo A, Bazan V, et al: Kirsten ras mutations in
patients with colorectal cancer: the ‘RASCAL II’ study. Br J
Cancer. 85:692–696. 2001.
|
|
33.
|
Normanno N, Tejpar S, Morbillo F, De Luca
A, Van Cutsem E and Ciardiello F: Implication of KRAS status and
EGFR-targeted therapies in metastatic CRC. Nat Rev Clin Oncol.
6:519–527. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
34.
|
De Roock W, Claes B, Bernasconi D, De
Schutter J, Biesmans B, Fountzilas G, Kalogeras KT, Kotoula V,
Papamichael D, Laurent-Puig P, Penault-Llorca F, Rougier P,
Vincenzi B, Santini D, Tonini G, Cappuzzo F, Frattini M, Molinari
F, Saletti P, De Dosso S, Martini M, Bardelli A, Siena S,
Sartore-Bianchi A, Tabernero J, Macarulla T, Di Fiore F, Gangloff
AO, Ciardiello F, Pfeiffer P, et al: Effects of KRAS, BRAF, NRAS,
and PIK3CA mutations on the efficacy of cetuximab plus chemotherapy
in chemotherapy-refractory metastatic colorectal cancer: a
retrospective consortium analysis. Lancet Oncol. 11:753–762.
2010.PubMed/NCBI
|
|
35.
|
Guerrero S, Casanova I, Farrè L, Mazo A,
Capellà G and Mangues R: K-ras codon 12 mutation induces higher
level of resistance to apoptosis and predisposition to
anchorage-independent growth than codon 13 mutation or
proto-oncogene overexpression. Cancer Res. 60:6750–6756. 2000.
|
|
36.
|
Ince WL, Jubb AM, Holden SN, Holmgren EB,
Tobin P, Sridhar M, Hurwitz HI, Kabbinavar F, Novotny WF, Hillan KJ
and Koeppen H: Association of K-ras, B-raf, and p53 status with the
treatment effect of bevacizumab. J Natl Cancer Inst. 97:981–989.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
37.
|
Hurwitz HI, Yi J, Ince W, Novotny WF and
Rosen O: The clinical benefit of bevacizumab in metastatic
colorectal cancer is independent of K-ras mutation status: analysis
of a phase III study of bevacizumab with chemotherapy in previously
untreated metastatic colorectal cancer. Oncologist. 14:22–28. 2009.
View Article : Google Scholar
|
|
38.
|
Van Cutsem E, Köhne CH, Làng I, Folprecht
G, Nowacki MP, Cascinu S, Shchepotin I, Maurel J, Cunningham D,
Tejpar S, Schlichting M, Zubel A, Celik I, Rougier P and Ciardiello
F: Cetuximab plus irinotecan, fluorouracil, and leucovorin as
first-line treatment for metastatic colorectal cancer: updated
analysis of overall survival according to tumor KRAS and BRAF
mutation status. J Clin Oncol. 29:2011–2019. 2011.
|
|
39.
|
Bokemeyer C, Bondarenko I, Hartmann JT, de
Braud F, Schuch G, Zubel A, Celik I, Schlichting M and Koralewski
P: Efficacy according to biomarker status of cetuximab plus
FOLFOX-4 as first-line treatment for metastatic colorectal cancer:
the OPUS study. Ann Oncol. 22:1535–1546. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
40.
|
Bruera G, Cannita K, Di Giacomo D, Lamy A,
Frébourg T, Sabourin JC, Tosi M, Alesse E, Ficorella C and Ricevuto
E: Worse prognosis of KRAS c.35 G>A mutant metastatic colorectal
cancer (MCRC) patients treated with intensive triplet chemotherapy
plus bevacizumab (FIr-B/FOx). BMC Med. 11:592013.
|
|
41.
|
Morelli MF, Santomaggio A, Ricevuto E,
Cannita K, De Galitiis F, Tudini M, Bruera G, Mancini M,
Pelliccione M, Calista F, Guglielmi F, Martella F, Lanfiuti Baldi
P, Porzio G, Russo A, Gebbia N, Iacobelli S, Marchetti P and
Ficorella C; on behalf of CINBO (Consorzio Interuniversitario
Nazionale per la Bio-Oncologia): Triplet schedule of weekly
5-fluorouracil and alternating irinotecan or oxaliplatin in
advanced colorectal cancer: a dose-finding and phase II study.
Oncol Rep. 23:1635–1640. 2010.PubMed/NCBI
|
|
42.
|
Ficorella C, Ricevuto E, Morelli MF,
Morese R, Cannita K, Cianci G, Di Rocco ZC, De Galitiis F, De Tursi
M, Tinari N, Iacobelli S and Marchetti P: Increased tolerability of
bimonthly 12-hour timed flat infusion 5-fluorouracil/irinotecan
regimen in advanced colorectal cancer: a dose-finding study. Oncol
Rep. 15:1345–1350. 2006.
|
|
43.
|
Therasse P, Arbuck SG, Eisenhauer EA,
Wanders J, Kaplan RS, Rubinstein L, Verweij J, Glabbeke MV, van
Oosterom AT, Christian MC and Gwyther SG: New guidelines to
evaluate the response to treatment in solid tumors: European
Organization for Research and Treatment of Cancer, National Cancer
Institute of the United States, National Cancer Institute of
Canada. J Natl Cancer Inst. 92:205–216. 2000. View Article : Google Scholar
|
|
44.
|
Kaplan EL and Meier P: Nonparametric
estimation of incomplete observations. J Am Statist Assoc.
53:457–481. 1958. View Article : Google Scholar
|
|
45.
|
Peto R and Peto J: Asymptomatically
efficient rank invariant test procedures. J R Stat Soc A.
135:185–207. 1972. View Article : Google Scholar
|
|
46.
|
Tebbutt NC, Wilson K, Gebski VJ, Cummins
MM, Zannino D, van Hazel GA, Robinson B, Broad A, Ganju V, Ackland
SP, Forgeson G, Cunningham D, Saunders MP, Stockler MR, Chua Y,
Zalcberg JR, Simes RJ and Price TJ: Capecitabine, bevacizumab, and
mitomycin in first-line treatment of metastatic colorectal cancer:
results of the Australasian Gastrointestinal Trials Group
randomized phase III MAX study. J Clin Oncol. 28:3191–3198. 2010.
View Article : Google Scholar
|
|
47.
|
Bruera G, Cannita K, Giordano AV,
Vicentini R, Ficorella C and Ricevuto E: Effectiveness and safety
of intensive triplet chemotherapy plus bevacizumab, FIr-B/FOx, in
young-elderly Metastatic Colorectal Cancer (MCRC) patients. BioMed
Res Int. 2013:1432732013. View Article : Google Scholar
|
|
48.
|
Figueras J, Ramos E, López-Ben S, Torras
J, Albiol M, Llado L, González HD and Rafecas A: Surgical treatment
of liver metastases from colorectal carcinoma in elderly patients.
When is it worthwhile? Clin Transl Oncol. 9:392–400. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
49.
|
Diaz-Rubio E, Gomez-Espana A, Massuti B,
Sastre J, Reboredo M, Manzano JL, Rivera F, Safont Mj, Montagut C,
lez EG, Benavides M, Marcuello E, Cervantes A, de Prado PM,
Fernandez-Martos C, Arrivi A, Bando I and Aranda E; on behalf of
the Spanish Cooperative Group for the Treatment of Digestive Tumors
(TTD): Role of Kras status in patients with metastatic colorectal
cancer receiving first-line chemotherapy plus bevacizumab: a TTD
group cooperative study. PLoS One. 7:e473452012. View Article : Google Scholar : PubMed/NCBI
|