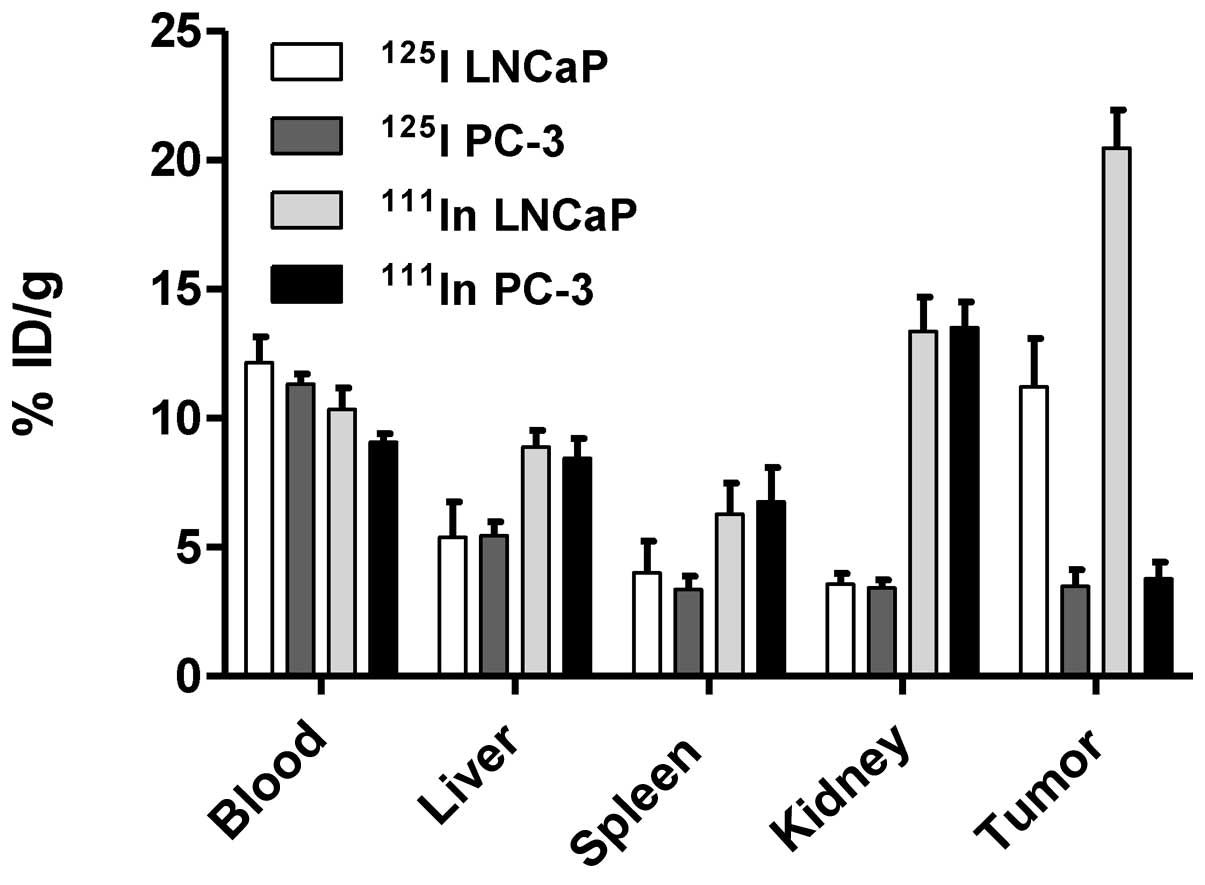
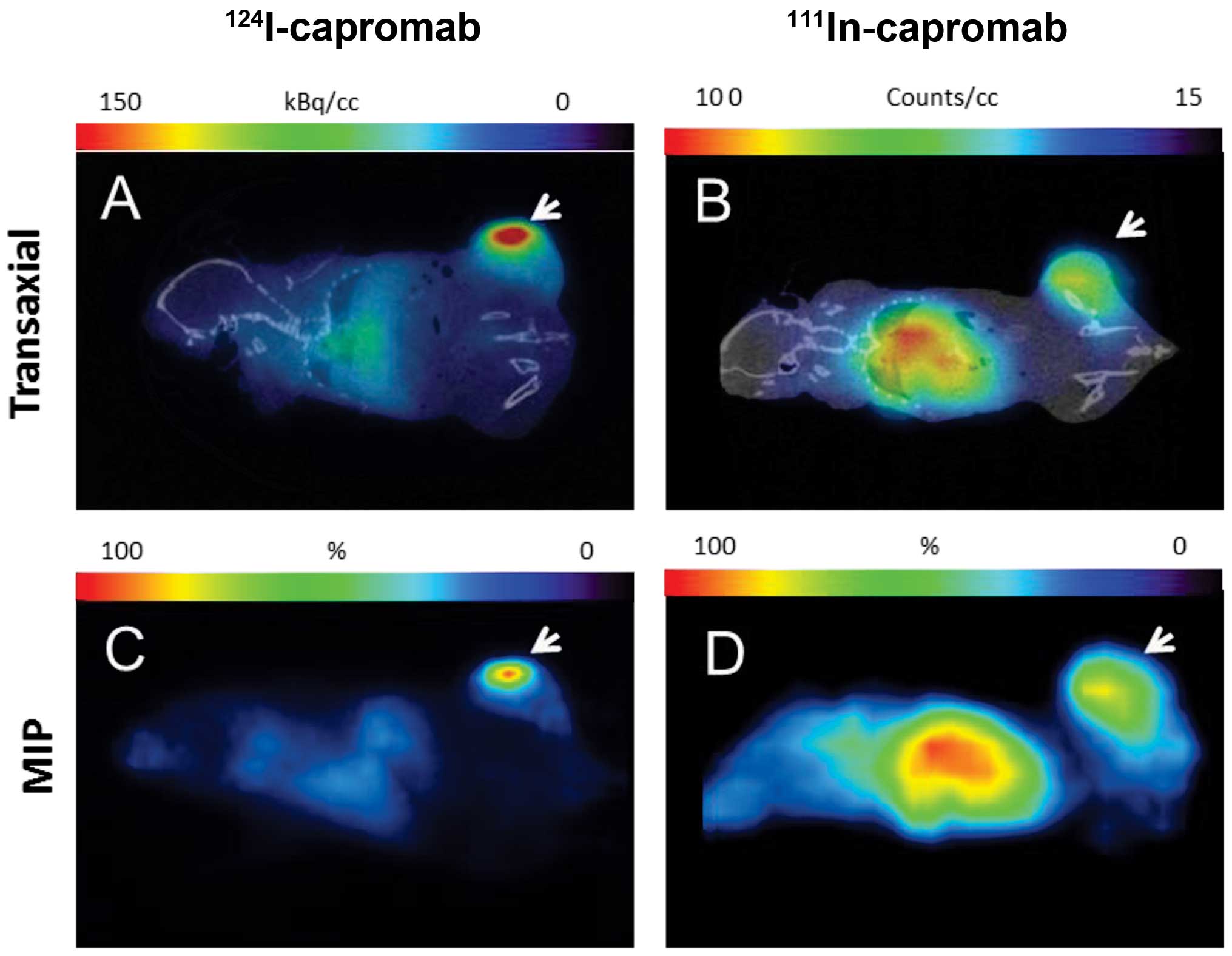
|
1.
|
Takahashi N, Inoue T, Lee J, Yamaguchi T
and Shizukuishi K: The roles of PET and PET/CT in the diagnosis and
management of prostate cancer. Oncology. 2:226–233. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
2.
|
Lawrentschuk N, Davis ID, Bolton D-M and
Scott AM: Positron emission tomography and molecular imaging of the
prostate: an update. BJU Int. 97:923–931. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Beresford MJ, Gillatt D, Benson RJ and
Ajithkumar T: A systematic review of the role of imaging before
salvage radio-therapy for post-prostatectomy biochemical
recurrence. Clin Oncol (R Coll Radiol). 22:46–55. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Schilling D, Schlemmer HP, Wagner PH, et
al: Histological verification of 11C-choline-positron
emission/computed tomography-positive lymph nodes in patients with
biochemical failure after treatment for localized prostate cancer.
BJU Int. 102:446–451. 2008.
|
|
5.
|
Vees H, Buchegger F, Albrecht S, et al:
18F-choline and/or 11C-acetate positron
emission tomography: detection of residual or progressive
subclinical disease at very low prostate-specific antigen values
(<1 ng/ml) after radical prostatectomy. BJU Int. 99:1415–1420.
2007. View Article : Google Scholar
|
|
6.
|
Lilja H, Ulmert D and Vickers AJ:
Prostate-specific antigen and prostate cancer: prediction,
detection and monitoring. Nat Rev Cancer. 8:268–278. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Raff AB, Gray A and Kast WM: Prostate stem
cell antigen: A prospective therapeutic and diagnostic target.
Cancer Lett. 277:126–132. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Silver DA, Pellicer I, Fair WR, Heston WD
and Cordon-Cardo C: Prostate-specific membrane antigen expression
in normal and malignant human tissues. Clin Cancer Res. 3:81–85.
1997.PubMed/NCBI
|
|
9.
|
Ulmert D, Evans MJ, Holland JP, et al:
Imaging androgen receptor signaling with a radiotracer targeting
free prostate-specific antigen. Cancer Discov. 2:320–327. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Manyak MJ: Indium-111 capromab pendetide
in the management of recurrent prostate cancer. Expert Rev
Anticancer Ther. 8:175–181. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Miyamoto DT, Lee RJ, Stott SL, et al:
Androgen receptor signaling in circulating tumor cells as a marker
of hormonally responsive prostate cancer. Cancer Discov.
2:995–1003. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Eder M, Eisenhut M, Babich J and Haberkorn
U: PSMA as a target for radiolabelled small molecules. Eur J Nucl
Med Mol Imaging. 40:819–823. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Manya MJ, Hinkle GH, Olsen JO, et al:
Immunoscintigraphy with indium-111-capromab pendetide: evaluation
before definitive therapy in patients with prostate cancer.
Urology. 54:1058–1063. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Van Dongen GA and Vosjan MJ:
Immuno-positron emission tomography: shedding light on clinical
antibody therapy. Cancer Biother Radiopharm. 25:375–385.
2010.PubMed/NCBI
|
|
15.
|
Brouwers A, Verel I, van Eerd J, et al:
PET radioimmunoscintigraphy of renal cell cancer using
89Zr-labeled cG250 monoclonal antibody in nude rats.
Cancer Biother Radiopharm. 19:155–163. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Nagengast WB, de Vries EG, Hospers GA, et
al: In vivo VEGF imaging with radiolabeled bevacizumab in a human
ovarian tumor xenograft. J Nucl Med. 48:1313–1319. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Perk LR, Stigter-van Walsum M, Visser GW,
et al: Quantitative PET imaging of Met-expressing human cancer
xenografts with 89Zr-labelled monoclonal antibody DN30.
Eur J Nucl Med Mol Imaging. 35:1857–1867. 2008. View Article : Google Scholar
|
|
18.
|
Dijkers EC, Kosterink JG, Rademaker AP, et
al: Development and characterization of clinical-grade
89Zr-trastuzumab for HER2/neu immunoPET imaging. J Nucl
Med. 50:974–981. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19.
|
Nagengast WB, de Korte MA, Oude Munnink
TH, et al: 89Zr-bevacizumab PET of early antiangiogenic
tumor response to treatment with HSP90 inhibitor NVP-AUY922. J Nucl
Med. 51:761–767. 2010. View Article : Google Scholar
|
|
20.
|
Orlova A, Wållberg H, Stone-Elander S and
Tolmachev V: On the selection of a tracer for PET imaging of
HER2-expressing tumors: direct comparison of a
124I-labeled affibody molecule and trastuzumab in a
murine xenograft model. J Nucl Med. 50:417–425. 2009. View Article : Google Scholar
|
|
21.
|
Dijkers EC, Oude Munnink TH, Kosterink JG,
et al: Biodistribution of 89Zr-trastuzumab and PET
imaging of HER2-positive lesions in patients with metastatic breast
cancer. Clin Pharmacol Ther. 87:586–592. 2010.
|
|
22.
|
Divgi CR, Pandit-Taskar N, Jungbluth AA,
et al: Preoperative characterisation of clear-cell renal carcinoma
using iodine-124-labelled antibody chimeric G250
(124I-cG250) and PET in patients with renal masses: a
phase I trial. Lancet Oncol. 8:304–310. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
23.
|
Ruggiero A, Holland JP, Hudolin T, et al:
Targeting the internal epitope of prostate-specific membrane
antigen with 89Zr-7E11 immuno-PET. J Nucl Med.
52:1608–1615. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24.
|
Holland JP, Divilov V, Bander NH,
Smith-Jones PM, Larson SM and Lewis JS: 89Zr-DFO-J591
for immunoPET of prostate-specific membrane antigen expression in
vivo. J Nucl Med. 51:1293–1300. 2010. View Article : Google Scholar
|
|
25.
|
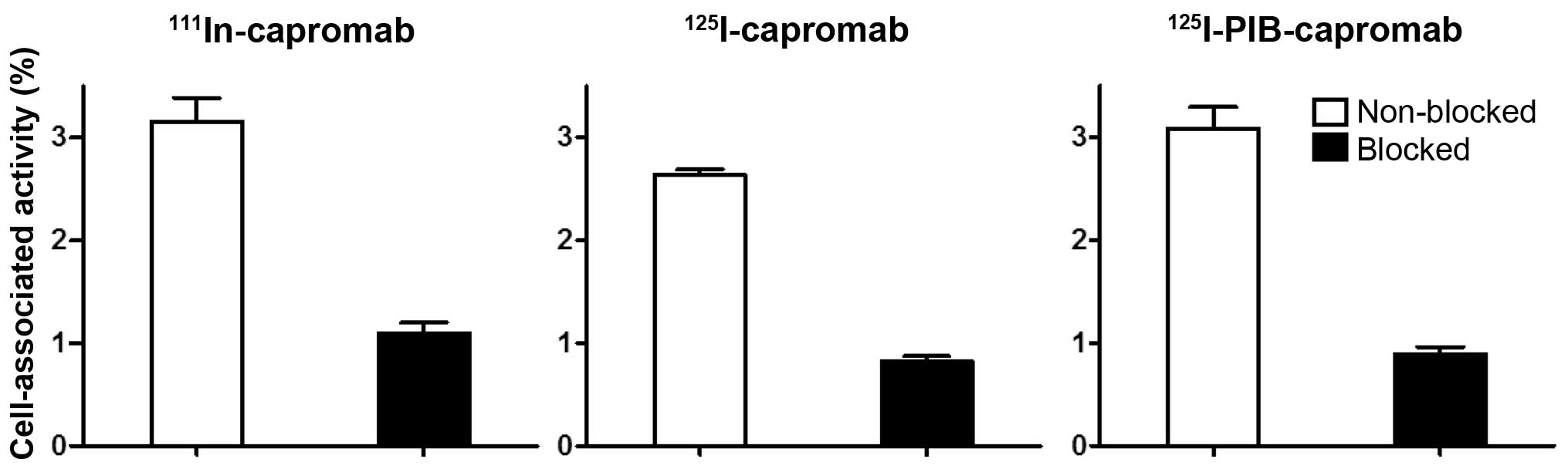
Barren RJ III, Holmes EH, Boynton AL,
Misrock SL and Murphy GP: Monoclonal antibody 7E11.C5 staining of
viable LNCaP cells. Prostate. 30:65–68. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
26.
|
Tolmachev V and Orlova A: Influence of
labelling methods on biodistribution and imaging properties of
radiolabelled peptides for visualisation of molecular therapeutic
targets. Curr Med Chem. 17:2636–2355. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27.
|
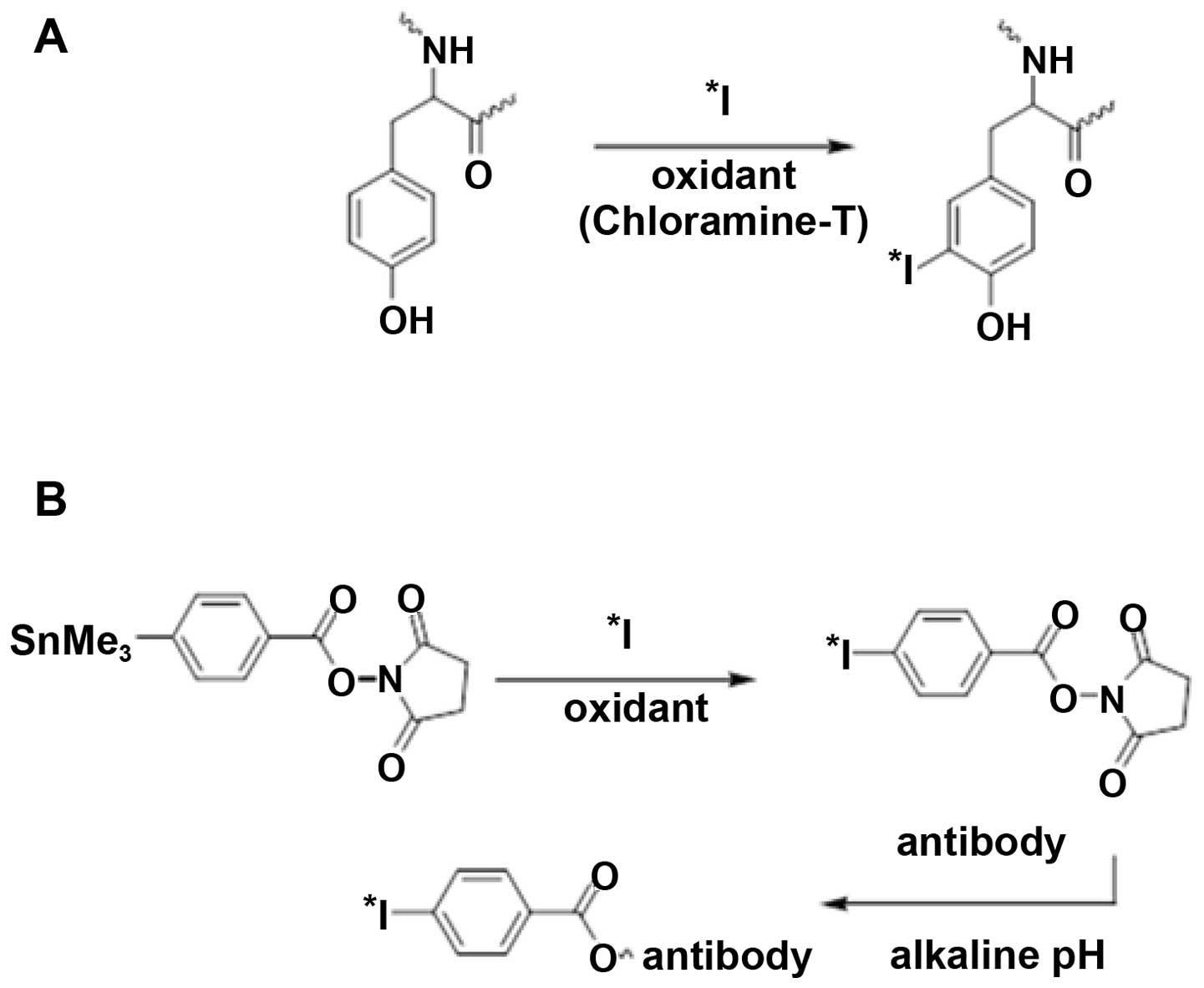
Koziorowski J, Henssen C and Weinreich R:
A new convenient route to radioiodinated N-succinimidyl 3- and
4-iodobenzoate, two reagents for iodination of proteins. Appl
Radiat Isot. 49:955–959. 1998. View Article : Google Scholar
|
|
28.
|
Lindmo T, Boven E, Cuttila F, Fedorko J
and Bunn PA Jr: Determination of immunoractive fraction of
radiolabeled monoclonal antibody by linear extrapolation to binding
at infinite antigen excess. J Immunol Methods. 72:77–89. 1984.
View Article : Google Scholar
|
|
29.
|
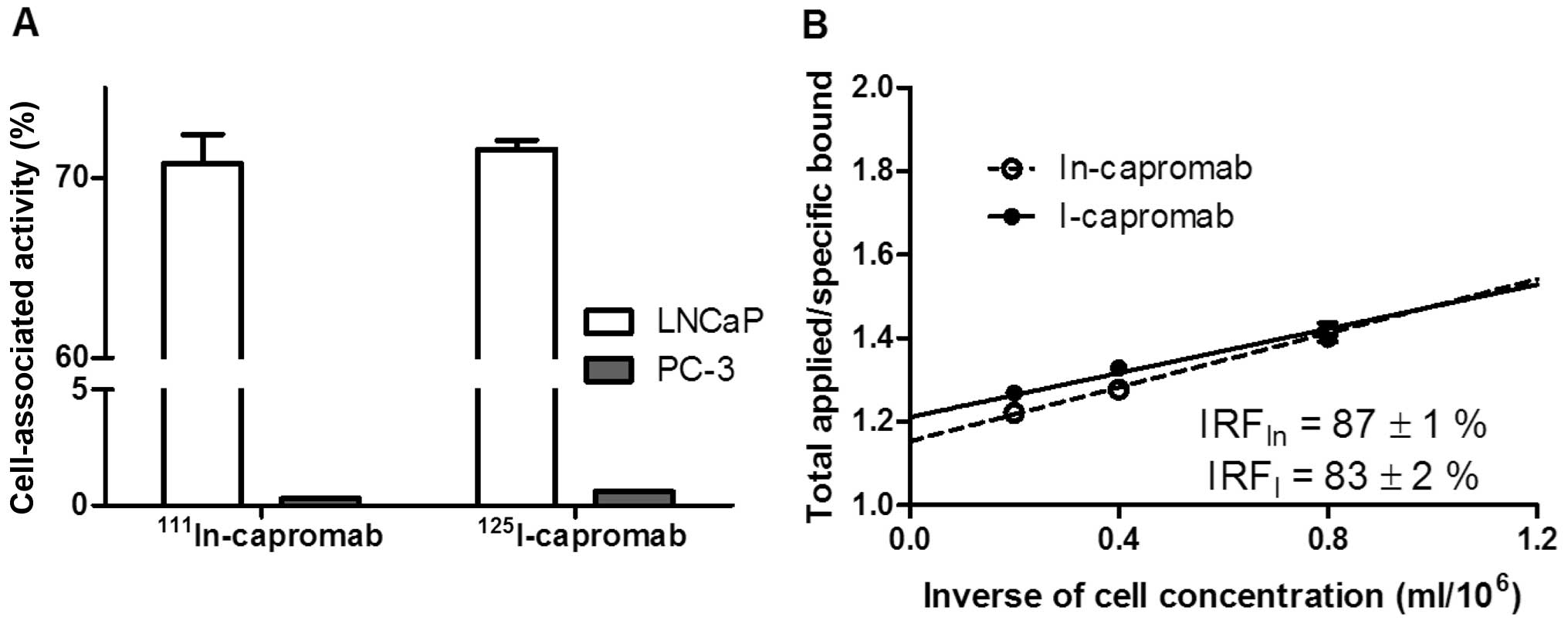
Khaw BA, Cooney J, Edgington T and Strauss
HW: Differences in experimental tumor localization of dual-labeled
monoclonal antibody. J Nucl Med. 27:1293–1239. 1986.
|
|
30.
|
Meijs WE, Haisma HJ, Klok RP, van Gog FB,
Kievit E, Pinedo HM and Herscheid JD: Zirconium-labeled monoclonal
antibodies and their distribution in tumor-bearing nude mice. J
Nucl Med. 38:112–118. 1997.PubMed/NCBI
|
|
31.
|
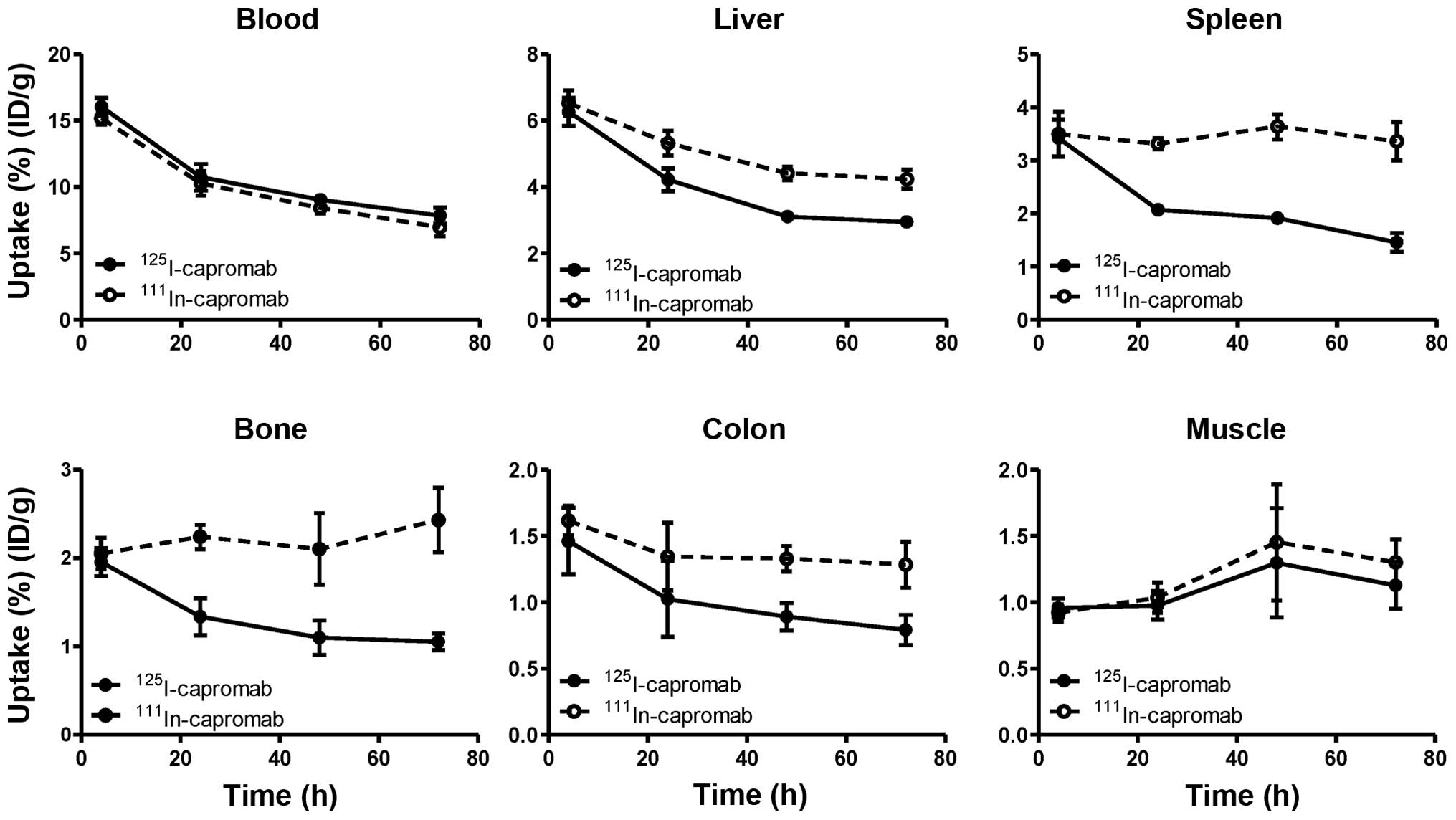
Malmberg J, Sandström M, Wester K,
Tolmachev V and Orlova A: Comparative biodistribution of imaging
agents for in vivo molecular profiling of disseminated prostate
cancer in mice bearing prostate cancer xenografts: focus on
111In- and 125I-labeled anti-HER2 humanized
monoclonal trastuzumab and ABY-025 affibody. Nucl Med Biol.
38:1093–1102. 2011. View Article : Google Scholar : PubMed/NCBI
|