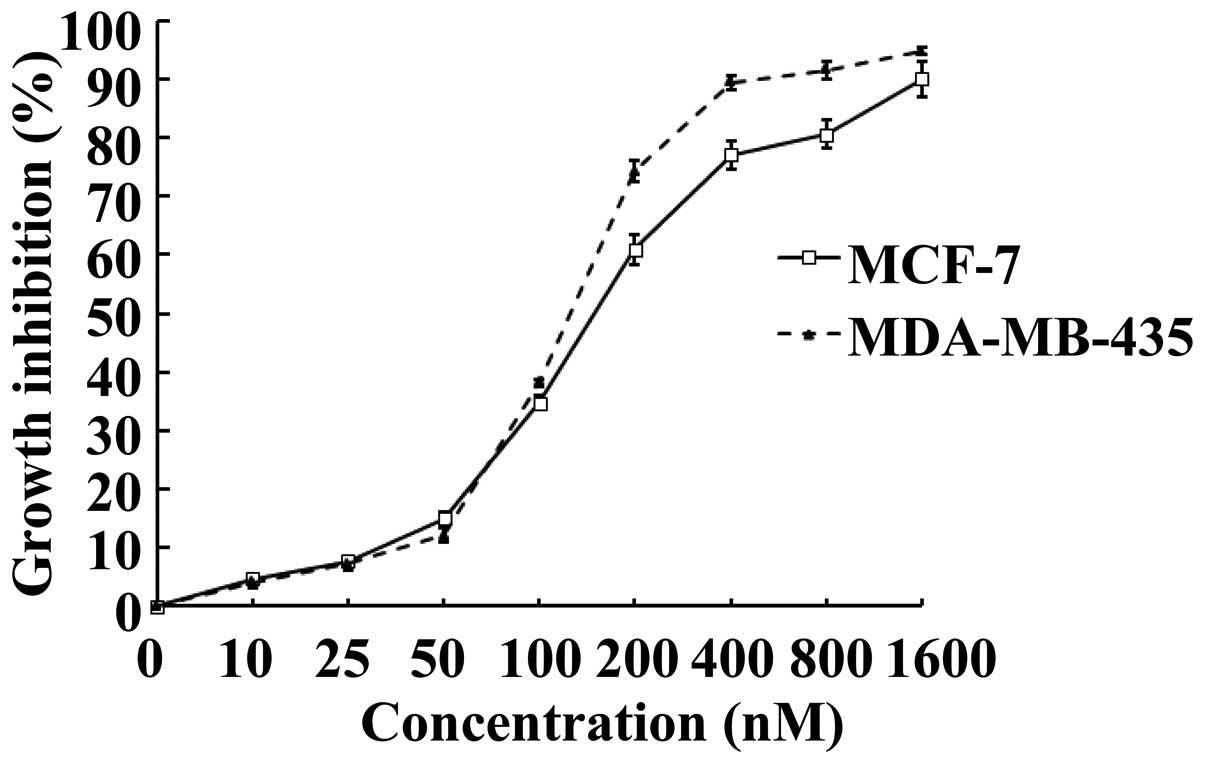
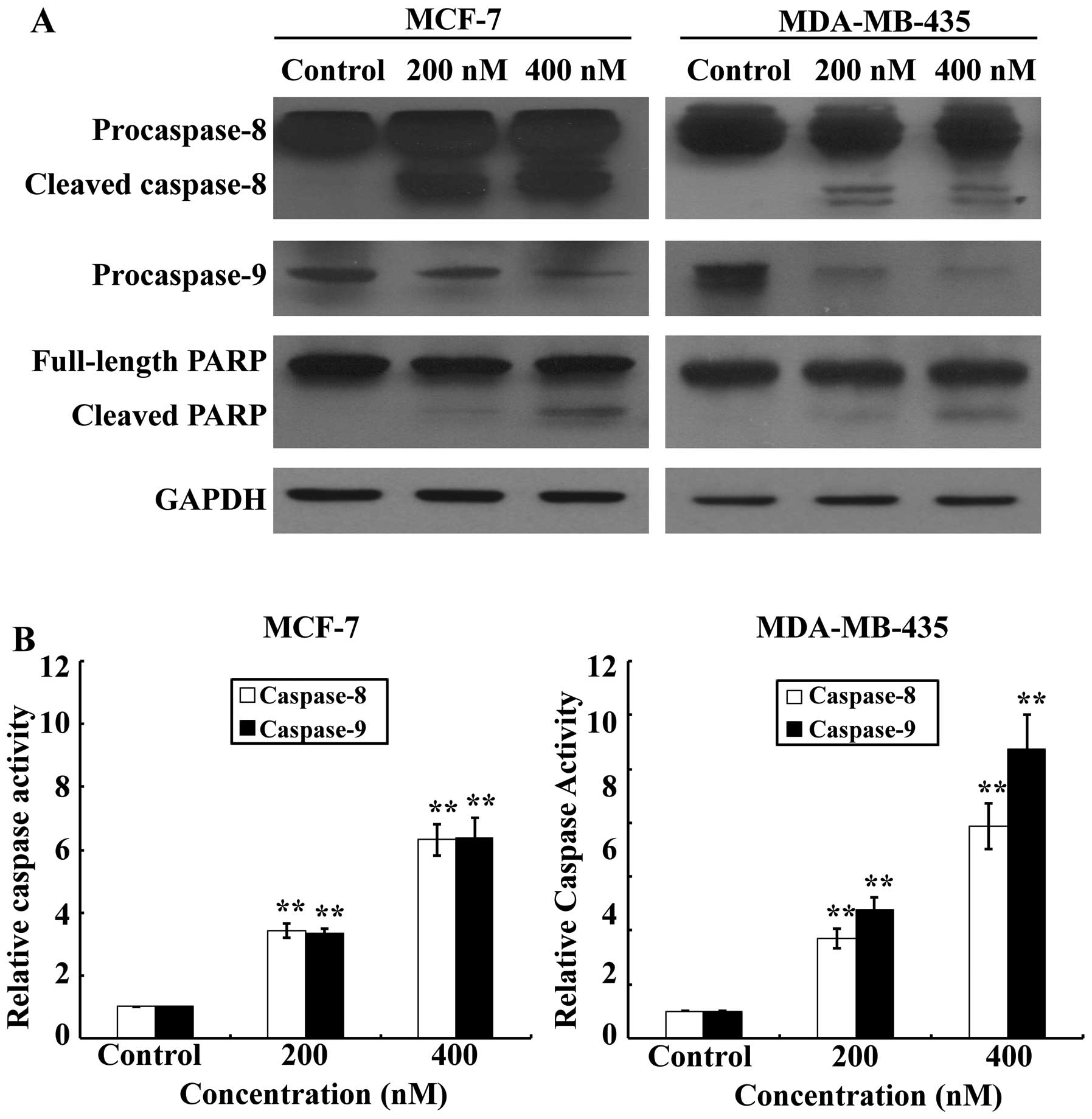
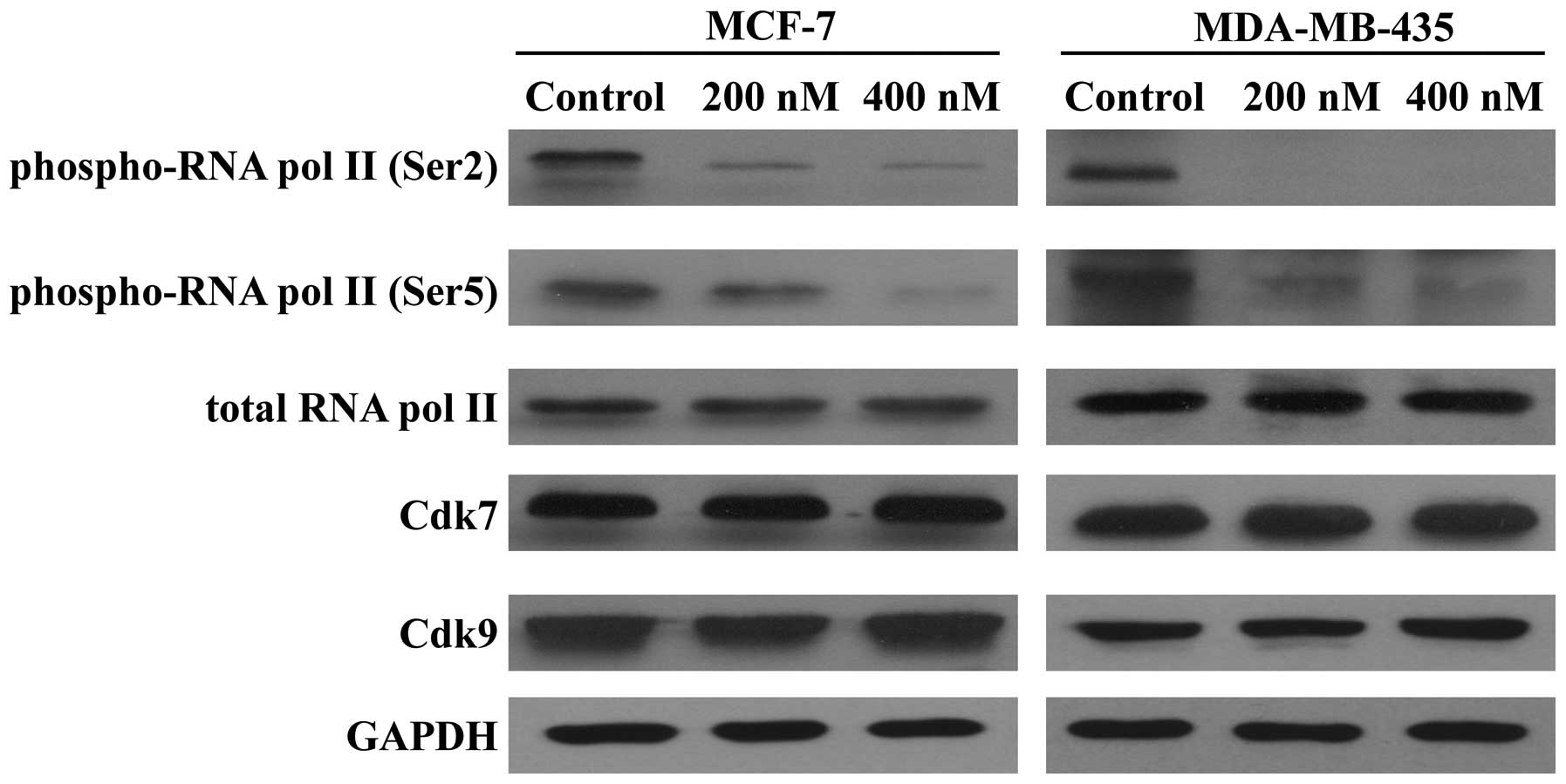
|
1
|
Friedenreich CM: Physical activity and
breast cancer: review of the epidemiologic evidence and biologic
mechanisms. Recent Results Cancer Res. 188:125–139. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Jafaar ZM, Litchfield LM, Ivanova MM,
Radde BN, Al-Rayyan N and Klinge CM: β-D-glucan inhibits
endocrine-resistant breast cancer cell proliferation and alters
gene expression. Int J Oncol. 44:1365–1375. 2014.
|
|
3
|
Perez EA: Impact, mechanisms, and novel
chemotherapy strategies for overcoming resistance to anthracyclines
and taxanes in metastatic breast cancer. Breast Cancer Res Treat.
114:195–201. 2009. View Article : Google Scholar
|
|
4
|
Gallorini M, Cataldi A and di Giacomo V:
Cyclin-dependent kinase modulators and cancer therapy. BioDrugs.
26:377–391. 2012.PubMed/NCBI
|
|
5
|
Canavese M, Santo L and Raje N: Cyclin
dependent kinases in cancer: potential for therapeutic
intervention. Cancer Biol Ther. 13:451–457. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Peterlin BM and Price DH: Controlling the
elongation phase of transcription with P-TEFb. Mol Cell.
23:297–305. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Larochelle S, Amat R, Glover-Cutter K, et
al: Cyclin-dependent kinase control of the initiation-to-elongation
switch of RNA polymerase II. Nat Struct Mol Biol. 19:1108–1115.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Cho SJ, Kim YJ, Surh YJ, Kim BM and Lee
SK: Ibulocydine is a novel prodrug Cdk inhibitor that effectively
induces apoptosis in hepatocellular carcinoma cells. J Biol Chem.
286:19662–19671. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wang S and Fischer PM: Cyclin-dependent
kinase 9: a key transcriptional regulator and potential drug target
in oncology, virology and cardiology. Trends Pharmacol Sci.
29:302–313. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lapenna S and Giordano A: Cell cycle
kinases as therapeutic targets for cancer. Nat Rev Drug Discov.
8:547–566. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
11
|
Diaz-Padilla I, Siu LL and Duran I:
Cyclin-dependent kinase inhibitors as potential targeted anticancer
agents. Invest New Drugs. 27:586–594. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Liu X, Shi S, Lam F, Pepper C, Fischer PM
and Wang S: CDKI-71, a novel CDK9 inhibitor, is preferentially
cytotoxic to cancer cells compared to flavopiridol. Int J Cancer.
130:1216–1226. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Shapiro GI: Preclinical and clinical
development of the cyclin-dependent kinase inhibitor flavopiridol.
Clin Cancer Res. 10:S4270–S4275. 2004. View Article : Google Scholar
|
|
14
|
Wang LM and Ren DM: Flavopiridol, the
first cyclin-dependent kinase inhibitor: recent advances in
combination chemotherapy. Mini Rev Med Chem. 10:1058–1070. 2012.
View Article : Google Scholar
|
|
15
|
Gojo I, Zhang B and Fenton RG: The
cyclin-dependent kinase inhibitor flavopiridol induces apoptosis in
multiple myeloma cells through transcriptional repression and
down-regulation of Mcl-1. Clin Cancer Res. 8:3527–3538. 2002.
|
|
16
|
Wittmann S, Bali P, Donapaty S, et al:
Flavopiridol down-regulates antiapoptotic proteins and sensitizes
human breast cancer cells to epothilone B-induced apoptosis. Cancer
Res. 63:93–99. 2003.PubMed/NCBI
|
|
17
|
Conroy A, Stockett DE, Walker D, et al:
SNS-032 is a potent and selective CDK 2, 7 and 9 inhibitor that
drives target modulation in patient samples. Cancer Chemother
Pharmacol. 64:723–732. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Chen R, Wierda WG, Chubb S, et al:
Mechanism of action of SNS-032, a novel cyclin-dependent kinase
inhibitor, in chronic lymphocytic leukemia. Blood. 113:4637–4645.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Xie G, Zhu X, Li Q, et al: SZ-685C, a
marine anthraquinone, is a potent inducer of apoptosis with
anticancer activity by suppression of the Akt/FOXO pathway. Br J
Pharmacol. 159:689–697. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Blagosklonny MV, Alvarez M, Fojo A and
Neckers LM: Bcl-2 protein downregulation is not required for
differentiation of multidrug resistant HL60 leukemia cells. Leuk
Res. 20:101–107. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Du Y, Shi A, Han B, et al: COX-2 silencing
enhances tamoxifen antitumor activity in breast cancer in
vivo and in vitro. Int J Oncol. 44:1385–1393.
2014.PubMed/NCBI
|
|
22
|
Riggins RB, Schrecengost RS, Guerrero MS
and Bouton AH: Pathways to tamoxifen resistance. Cancer Lett.
256:1–24. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhang B, Zhang X, Tang B, Zheng P and
Zhang Y: Investigation of elemene-induced reversal of tamoxifen
resistance in MCF-7 cells through oestrogen receptor α (ERα)
re-expression. Breast Cancer Res Treat. 136:399–406.
2012.PubMed/NCBI
|
|
24
|
Núñez M, Medina V, Cricco G, et al:
Glibenclamide inhibits cell growth by inducing G0/G1 arrest in the
human breast cancer cell line MDA-MB-231. BMC Pharmacol Toxicol.
14:62013.PubMed/NCBI
|
|
25
|
Jordan VC and Brodie AM: Development and
evolution of therapies targeted to the estrogen receptor for the
treatment and prevention of breast cancer. Steroids. 72:7–25. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Dickson MA and Schwartz GK: Development of
cell-cycle inhibitors for cancer therapy. Curr Oncol. 16:36–43.
2009.PubMed/NCBI
|
|
27
|
Rizzolio F, Tuccinardi T, Caligiuri I,
Lucchetti C and Giordano A: CDK inhibitors: from the bench to
clinical trials. Curr Drug Targets. 11:279–290. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
McInnes C: Progress in the evaluation of
CDK inhibitors as anti-tumor agents. Drug Discov Today. 13:875–881.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Malumbres M and Barbacid M: Cell cycle,
CDKs and cancer: a changing paradigm. Nat Rev Cancer. 9:153–166.
2009. View
Article : Google Scholar : PubMed/NCBI
|
|
30
|
Rudek MA, Bauer KS Jr, Lush RM III, et al:
Clinical pharmacology of flavopiridol following a 72-hour
continuous infusion. Ann Pharmacother. 37:1369–1374. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Byrd JC, Peterson BL, Gabrilove J, et al:
Treatment of relapsed chronic lymphocytic leukemia by 72-hour
continuous infusion or 1-hour bolus infusion of flavopiridol:
results from Cancer and Leukemia Group B study 19805. Clin Cancer
Res. 11:4176–4181. 2005. View Article : Google Scholar
|
|
32
|
Flinn IW, Byrd JC, Bartlett N, et al:
Flavopiridol administered as a 24-hour continuous infusion in
chronic lymphocytic leukemia lacks clinical activity. Leuk Res.
29:1253–1257. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Aklilu M, Kindler HL, Donehower RC, Mani S
and Vokes EE: Phase II study of flavopiridol in patients with
advanced colorectal cancer. Ann Oncol. 14:1270–1273. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Christian BA, Grever MR, Byrd JC and Lin
TS: Flavopiridol in the treatment of chronic lymphocytic leukemia.
Curr Opin Oncol. 19:573–578. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Byrd JC, Lin TS, Dalton JT, et al:
Flavopiridol administered using a pharmacologically derived
schedule is associated with marked clinical efficacy in refractory,
genetically high-risk chronic lymphocytic leukemia. Blood.
109:399–404. 2007. View Article : Google Scholar
|
|
36
|
Phelps MA, Lin TS, Johnson AJ, et al:
Clinical response and pharmacokinetics from a phase 1 study of an
active dosing schedule of flavopiridol in relapsed chronic
lymphocytic leukemia. Blood. 113:2637–2645. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Lin TS, Ruppert AS, Johnson AJ, et al:
Phase II study of flavopiridol in relapsed chronic lymphocytic
leukemia demonstrating high response rates in genetically high-risk
disease. J Clin Oncol. 27:6012–6018. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Heath EI, Bible K, Martell RE, Adelman DC
and Lorusso PM: A phase 1 study of SNS-032 (formerly BMS-387032), a
potent inhibitor of cyclin-dependent kinases 2, 7 and 9
administered as a single oral dose and weekly infusion in patients
with metastatic refractory solid tumors. Invest New Drugs.
26:59–65. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Tong WG, Chen R, Plunkett W, et al: Phase
I and pharmacologic study of SNS-032, a potent and selective Cdk2,
7, and 9 inhibitor, in patients with advanced chronic lymphocytic
leukemia and multiple myeloma. J Clin Oncol. 28:3015–3022. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Naumann K, Schmich K, Jaeger C, Kratz F
and Merfort I: Noxa/Mcl-1 balance influences the effect of the
proteasome inhibitor MG-132 in combination with anticancer agents
in pancreatic cancer cell lines. Anticancer Drugs. 23:614–626.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Li QQ, Lee RX, Liang H, Wang G, Li JM,
Zhong Y and Reed E: β-Elemene enhances susceptibility to cisplatin
in resistant ovarian carcinoma cells via downregulation of ERCC-1
and XIAP and inactivation of JNK. Int J Oncol. 43:721–728.
2013.
|
|
42
|
Martin AP, Mitchell C, Rahmani M, Nephew
KP, Grant S and Dent P: Inhibition of MCL-1 enhances lapatinib
toxicity and overcomes lapatinib resistance via BAK-dependent
autophagy. Cancer Biol Ther. 8:2084–2096. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Lima RT, Martins LM, Guimarães JE, Sambade
C and Vasconcelos MH: Specific downregulation of bcl-2 and xIAP by
RNAi enhances the effects of chemotherapeutic agents in MCF-7 human
breast cancer cells. Cancer Gene Ther. 11:309–316. 2004. View Article : Google Scholar : PubMed/NCBI
|