Introduction
Despite years of research and development of various
therapies, cancer remains one of the major causes of mortality
worldwide. Breast cancer is a leading cause of cancer-related
deaths amongst women globally. Recently, the increasing incidence
of breast cancer has slowed down, but this varies between
countries, due to differences in reproductive and hormonal factors,
and the availability of early diagnostic services (1). There has also been an increase in the
breast cancer mortality rate, which is probably caused by improper
treatment, the poor prognosis related to metastatic cancer, and
recurrence of cancer after surgery (2,3).
Metastatic breast cancer could spread beyond the original organ, to
bone, liver, lung, and brain, causing secondary cancers; for
example, breast cancer cells that metastasize is considered
invasive breast cancer, not lung cancer.
Tumor metastasis proceeds by sequential and
selective steps including cell adhesion, uncontrolled
proliferation, formation of the malignant phenotype, detachment
from the primary site, invasion into the connective tissue and
circulation, and extravasation into the organ parenchyma (4,5). A
crucial step during migration and invasion, which results in a
secondary tumor at a distant site is the degradation of
environmental barriers, such as the extracellular matrix and the
basement membrane, by various proteolytic enzymes, called matrix
metalloproteinases (MMPs). MMPs are a family of secretory
membrane-anchored proteases and are directly activated by the
serine protease plasmin, which is produced from plasminogen by the
serine protease urokinase-type plasminogen activator-1 (uPA-1)
(6). It has been found that MMP-2,
-9 and uPA-1 are highly expressed in cancerous organs, including
breast tissue, and, together, they are associated with invasiveness
and progression of breast cancer (7–11).
Tumor necrosis factor-α (TNF-α) also regulates tumor remodeling by
stimulating cell motility and invasion, via induction of MMPs
(8,12,13).
The activities of MMPs and of proteolytic uPA-1 are modulated by
tissue inhibitors of metalloproteinases (TIMPs) and plasminogen
activator inhibitor (PAI), respectively; this protects the basement
membrane against excessive degradation (14–16).
Therefore, regulation of the expression of MMPs and TIMPs and/or
regulation of uPA-1-mediated migration or invasion could be
considered as a potential treatment for preventing or inhibiting
cancer metastasis.
Exploration of novel therapeutic drugs for the
treatment of advanced, recurrent, and metastatic breast cancer
carries a high priority. Many studies have reported that natural
products, dietary phytochemicals, such as sulforaphane and
isothiocyanates from broccoli and watercress (17,18),
tea catechins (19), genistein,
apigenin (20), ganoderic acid
from the Ganoderma lucidum mushroom (21), and Phellinus linteus
(22,23) have a variety of anticancer,
anti-invasive, and anti-metastatic activities. Naematolma (Syn.
Hypoloma) spp. are basidiomycete that are known to produce an
antitumor compound, clavaric acid (24–26).
In our previous studies, we reported the high-performance liquid
chromatography (HPLC) profiles and biological functions including,
anti-oxidative, anti-inflammatory, and anticancer activities
against various cancer cells of N. sublateritium extracts
(27,28). Particularly, among fractions
sequentially prepared from N. sublateritium ethanol extract,
hexane fractions (HFNS) and the dichloromethane fraction of N.
sublateritium exhibited the ability to inhibit cell
proliferation and viability of triple-negative breast cancer cell
line (TNBC), MDA-MB-231 (27).
These observation led us to consider whether the
activity of HFNS also extend to modulation of metastatic potential.
Thus, in the present study, we investigated the effects of HFNS on
the motility and migration of MDA-MB-231 cells along with its
ability to modulate the regulatory proteins MMP-2, MMP-9, uPA-1,
TIMP, and PAI. Furthermore, the influence of HFNS on the MAPK
signaling, which is related to MMPs activation (29) through regulation of the
transcriptional factors nuclear factor-κB (NFκB) and activator
protein-1 (AP-1), was also investigated. The results of our study
indicate the significant potential of HFNS as a preventive agent
against the occurrence or metastasis of TNBC.
Materials and methods
Materials
The human breast cancer cell line, MDA-MB-231 was
obtained from the Korean Cell Line Bank (Seoul, Korea). RPMI-1640
medium, fetal bovine serum (FBS), and antibiotics were purchased
from Gibco (Grand Island, NY, USA). Human TNF-α, 3-(4,
5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT),
dimethyl sulfoxide (DMSO) and anti β-actin antibody were purchased
from Sigma (St. Louis, MO, USA). Antibodies against MMP-2, MMP-9,
TIMP-1, TIMP-2, PAI-1, phospho-JNK1/2, phospho-ERK1/2, and
phospho-p38 were obtained from Cell Signaling Technology (Beverly,
MA, USA). Antibodies against uPA-1, JNK1/2, ERK, and p38 were
purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA).
Cultrex basement membrane extract (BME) cell invasion assay kit and
gelatin precast gels were from Trevigen Inc. (Gathersburg, MD, USA)
and Bio-Rad (Hercules, CA, USA), respectively.
Preparation of HFNS
The ethanolic extract of N. sublateritium was
prepared as described (28). The
extract was sequentially fractionated using the organic solvents,
hexane, dichloromethane, n-butanol and ethyl acetate. The hexane
fraction was concentrated, passed through a 0.2-μm filter and dried
using a freeze-drier. Dried HFNS was reconstituted in vehicle for
cell culture studies. The HPLC chromatogram of HFNS confirmed the
non-polar characteristics of the extract at late retention times
(27)
Proliferation and viability assay
Monolayer cultures of MDA-MB-231 cells were
maintained in RPMI-1640 medium supplemented with 10% (v/v) FBS and
antibiotics. Cells were maintained in an atmosphere of 95% air and
5% CO2 at 37°C. The effect of HFNS on cell proliferation
and viability was determined by the MTT colorimetric method and
propidium iodide (PI) staining, respectively (27). Cells were treated with DMSO
(control) or HFNS for 24 or 48 h. Results are expressed as the
ratio of the number of live cells with HFNS treatment relative to
that observed after DMSO-treatment.
Wound-healing assay
Cells were plated in a 6-well plate and allowed to
form a confluent monolayer for 24 h. Cells were then serum starved
for 24 h and the monolayer in each cell was scratched with a
pipette tip, and washed with serum-free medium to remove floating
cells. Cells were then treated with TNF-α (20 ng/ml) in the
presence of various concentrations of HFNS for 18 h. The cells were
allowed to migrate across the scratch, and photographed at three
randomly selected sites per well through an inverted microscope
(x40 magnification).
Invasion assay
The invasion assay was performed using
Cultrex® 96-well BME Cell invasion assay kit. Briefly,
duplicate transwell chambers with 8-μm pore polycarbonate filters
were coated with 50 μl of ice-cold 0.8X BME in coat buffer and
incubated overnight at 37°C. To monitor cell migration,
1×105 cells were seeded on to BME-coated filter, which
was then inserted into the upper chamber, containing serum-free
media. The lower chamber was filled with 500 μl of medium
containing TNF-α, as well as various concentrations of HFNS. The
control well contained media only. After incubation for 24 h, the
cells on the underside of the filter were quantified using
Calcein-AM according to the assay kit manual. Cells that had
migrated to the bottom of the membrane were visualized and counted
using an inverted fluorescence microscope.
Gelatin zymography assay
The activities of MMP-2 and MMP-9 medium released
from MDA-MB-231 cells into the medium were measured using a gelatin
zymography protease assay. Cells were serum starved for 18 h and
then treated with TNF-α or TNF-α combined with HFNS for the
indicated time or concentration for 12 h. The cell culture medium
was then collected and concentrated using Microcon YM-10 filters
(Millipore, Billerica, MA, USA). The concentrated sample was
subjected to electrophoresis on 7% polyacrylamide gels containing
gelatin, in the absence of a reducing agent.
Immunoblotting
Cells were treated with different HFNS
concentrations in the presence of TNF-α for the indicated time. The
cell culture medium was collected and concentrated. Cell lysates
were prepared by centrifugation at 12,000 × g for 20 min, as
previously described (18).
Proteins were resolved on SDS-PAGE and transferred onto a PVDF
membrane. Immunoblotting was performed with antibodies specific for
uPA-1 PAI-1, TIMP-1, TIMP-2, MMP-2 and MMP-9. Changes in total
protein level and phospho-JNK1/2, phospho-ERK1/2, and phospho-p38
levels were also determined using specific antibodies. The
immunoreactive bands were visualized using enhanced
chemiluminescence. The band intensity was quantified using a
densitometer followed by normalization to the density of
β-actin.
Semi-quantitative reverse
transcription-PCR
MDA-MB-231 cells were treated with TNF-α or TNF-α
plus HFNS of the indicated concentration and for the indicated
time, and then total RNA was extracted using the BCP Phase
Separation Reagent (MRC Inc., Cincinnati, OH, USA). The RNA was
converted to cDNA using a reverse transcription-PCR kit containing
oligo(dT) primers (Intron Biotech., Seoul, Korea) according to the
manufacturer’s protocol (28). The
primers and PCR conditions are listed in Table I. PCR products were electrophoresed
on 1% agarose gels and visualized under ultraviolet light after
ethidium bromide staining.
 | Table IPCR primers. |
Table I
PCR primers.
| Target genes | Primer
sequences | Size (bp) | Annealing
temperature (°C) | Cycle |
|---|
| PAI-1 | Sense |
5′-TGCTGGTGAATGCCCTCTACT-3′ | 399 | 58 | 28 |
| Antisense |
5′-TAGAGAACCTGGGAATGACCG-3′ | | | |
| uPA-1 | Sense |
5′-CACGCAAGGGGAGATGAA-3′ | 341 | 58 | 28 |
| Antisense |
5′-AAGTCACCACCAAAATGCTGT-3′ | | | |
| TIMP-1 | Sense |
5′-CTTCCACAGGTCCCACAACC-3′ | 304 | 60 | 30 |
| Antisense |
5′-GCCTCGGGAGCCAGGGCTG-3′ | | | |
| TIMP-2 | Sense |
5′-GATGCACATCACCCTCTGTGA-3′ | 196 | 52 | 30 |
| Antisense |
5′-AGAACATCAACGGGCAC-3′ | | | |
| MMP-9 | Sense |
5′-GCACGACGTCTTCCAGTACC-3′ | 130 | 58 | 28 |
| Antisense |
5′-ACCTATGACATCCTGCAGTGC-3′ | | | |
| β-actin | Sense |
5′-AGCAGAGAATGGAAAGTCAAA | 490 | 55 | a |
| Antisense |
5′-ATGCTGCTTACATGTCTCGAT-3′ | | | |
Electrophoretic mobility shift assay for
AP-1 and NFκB
Cells were treated with the different HFNS
concentrations in the presence of TNF-α. Nuclear extracts of the
cells were prepared and the proteins were subjected to
electrophoretic mobility shift assays (EMSA) as previously
described (28). Briefly, 2 μg of
nuclear extract was combined with 0.25 mg/ml
poly(dI)-poly(dC)-non-specific competitor in Gel Shift binding
buffer (20% glycerol, 5 mM MgCl2, 2.5 mM EDTA, 2.5 mM
DTT, 250 mM NaCl, and 50 mM Tris-HCl); to this was added IRDye
700-labeled AP-1 or NFκB oligonucleotide (LI-COR Inc., Lincoln, NE,
USA). After incubation at room temperature for 30 min, the
protein-DNA complexes were separated from the free probe on a
pre-run 8% polyacrylamide gel. The signal was then detected and
quantified using the Odyssey Infrared Imaging System (LI-COR
Inc.).
Statistical analysis
Differences in the measured variables between the
control and HFNS-treated groups were determined using a one-way
analysis of variance (ANOVA) followed by Dunnett’s or Bonferroni’s
test for multiple comparisons. P-values of <0.05 were considered
significant.
Results
Effect of HFNS on the viability and
proliferation of TNF-α-stimulated MDA-MB-231 cells
In a previous study, we observed that HFNS
significantly inhibited growth of various human cancer cell lines
(27). The HFNS concentration
required for 50% inhibition of viability and proliferation of
MDA-MB-231 cells was 200 μg/ml for a 24 h treatment. To verify the
effects of HFNS, MDA-MB-231 cells were exposed to different
concentrations of HFNS and were then stimulated with TNF-α for 24
or 48 h. As shown in Fig. 1, the
viability of MDA-MB-231 cells was not affected by up to 100 μg/ml
HFNS treatment, indicating that these were non-cytotoxic
concentrations of HFNS in the presence of TNF-α. A significant
decrease in cell viability was observed after a 24 h exposure of
200 μg/ml HFNS: cell viability was reduced by ~39.7% relative to
that of cells treated with TNF-α only (Fig. 1A). The MTT assay indicated that
proliferation of MDA-MB-231 cells was markedly inhibited by HFNS
treatment, even at 50 μg/ml for 24 h, confirming the
anti-proliferating activity of HFNS on MDA-MB-231 cells (Fig. 1B). These results suggested that the
change in cell viability observed at 100 μg/ml HFNS can be
attributed mainly to the inhibition of proliferation. Moreover,
HFNS could regulate the metastatic properties of MDA-MB-231 cells
without causing significant cell death.
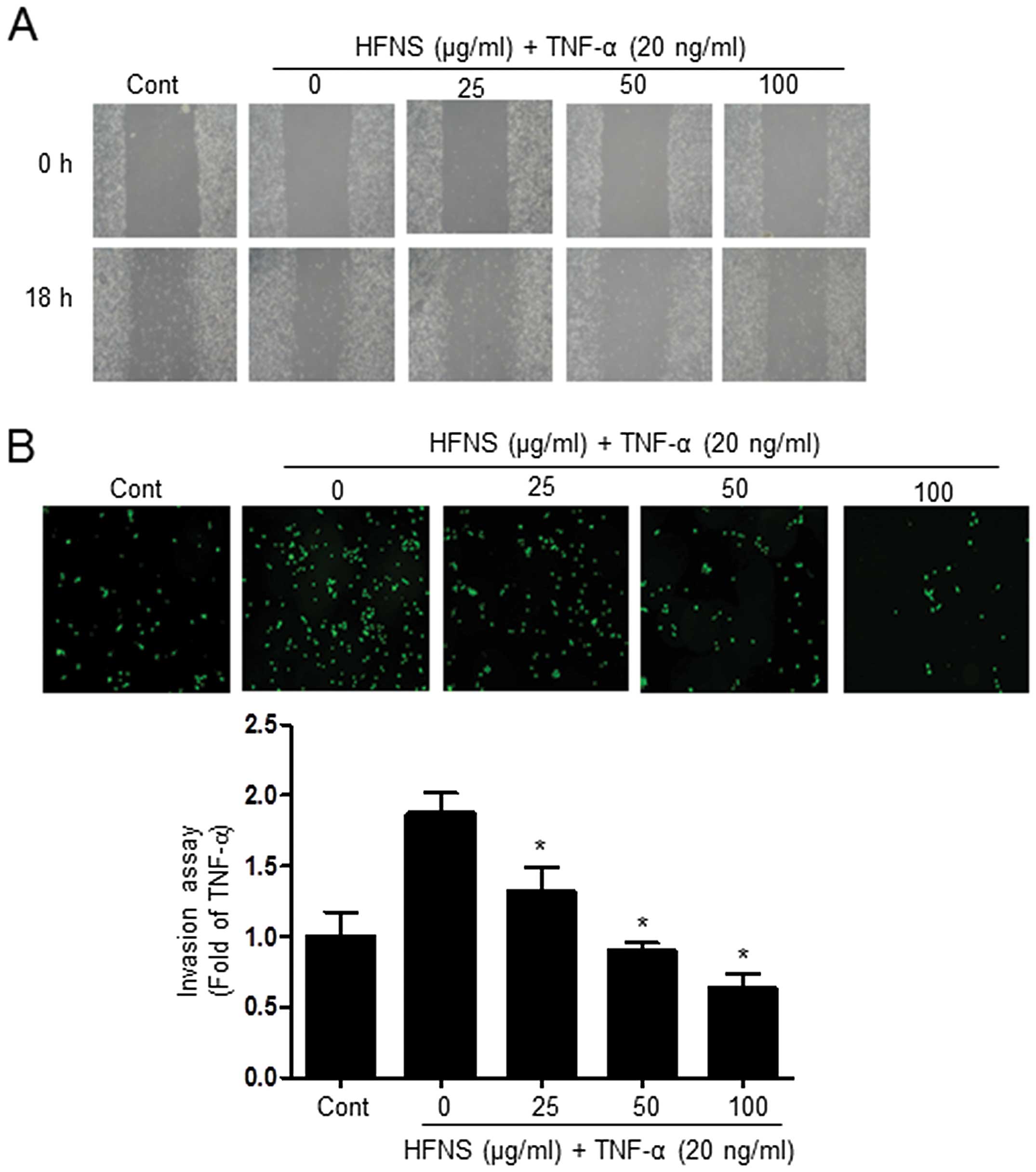
HFNS inhibits migration and invasion of
TNF-α-stimulated MDA-MB-231 cells
Next, we investigated the effects of HFNS on
TNF-α-stimulated MDA-MB-231 cells, considering the highly invasive
nature of TNBC. We investigated whether HFNS could inhibit the
migration and invasive potential of TNF-α-stimulated MDA-MB-231
cells, using a wound-healing repair assay and an in vitro
transwell assay, respectively. After cell monolayers were wounded
in the repair assay, TNF-α-treated cells migrated to the cleared
area, but treatment with HFNS dose-dependently inhibited
TNF-α-stimulated migration of MDA-MB-231 cells (Fig. 2A). Moreover, the invasive activity
of MDA-MB-231 cells was significantly regulated by HFNS treatment
in a dose-dependent manner. As shown in Fig. 2B, in the transwell chamber assay,
HFNS induced a decrease in the fluorescence of MDA-MB-231 invasive
cells in the lower chamber, reached through a Matrigel-coated
membrane. More specifically, the fluorescent intensity was reduced
by 51.4% relative to that seen with TNF-α-treated control cells
following a 24 h exposure to 50 μg/ml of HFNS. These results
indicated that HFNS has an inhibitory effect on the migration and
invasiveness of TNF-α-stimulated MDA-MB-231 cells.
Inhibition of MMP activity by HFNS
Because MMPs have emerged as critical regulators of
metastasis, by their role in degrading the basement membrane, we
investigated the effect of HFNS treatment on the activity of
secreted MMP-9 and MMP-2 in TNF-α-stimulated MDA-MB-231 cells. The
effect of HFNS on the gelatinase activity of MMP-2 and MMP-9 was
analyzed using zymogram gels containing gelatin, the preferred
substrate. The activity of MMPs present in the cell culture
supernatant was identified by digestion of the substrate at a
molecular weight corresponding to that attribute to specific MMPs
(MMP-9: 92 kDa), which is seen as a clear band.
As can be seen in Fig.
3, in conditioned medium from cells treated with only TNF-α,
the intensity of the MMP-9 band was decreased in a dose- and
time-dependent manner. Treatment of cells with 100 μg/ml HFNS
resulted in a rapid decrease in the intensity of this band (~34.2%
decrease, compared to the levels of the TNF-α-treated control
cells) within 2 h, indicating inhibition of MMP-9 activity. In
contrast, no detectable amount of MMP-2 activity was seen with
either TNF-α or HFNS treatment (Fig.
3B). This suggests that the inhibitory activity of HFNS on the
migration and invasion of MDA-MB-231 cells may be due mainly to
inhibition of MMP-9 activity.
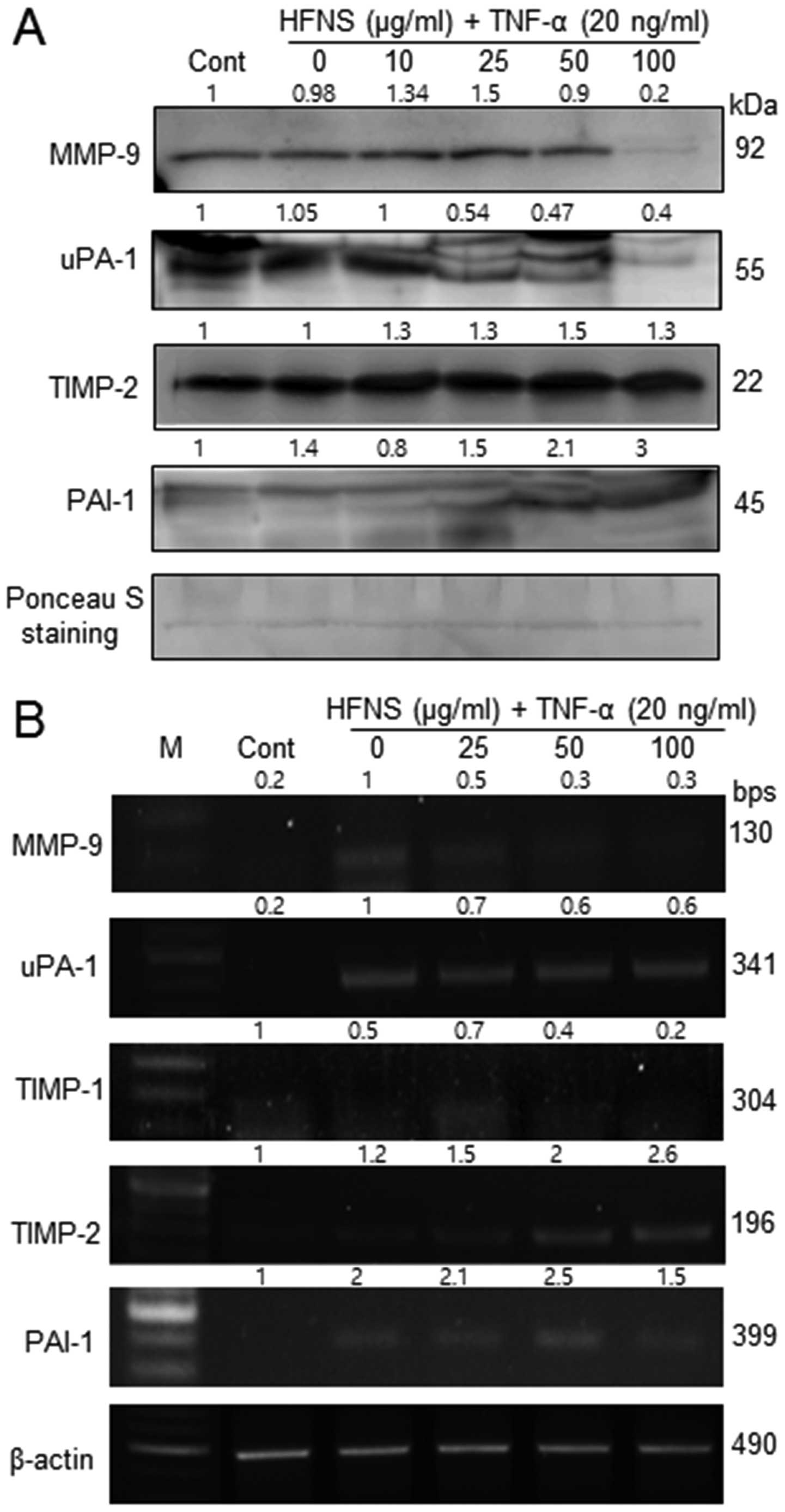
Regulation of MMP-9, TIMP-2, uPA-1, and
PAI-1 expression by HFNS treatment
Because HFNS treatment resulted in inhibition of
MMP-9 activity, we also evaluated the secretion of MMP-9, MMP-2,
uPA-1, and the MMP inhibitors, viz., TIMP-1, TIMP-2, and PAI-1 in
MDA-MB-231 cells. In immunoblot analysis, the levels of MMP-9 and
uPA-1 were significantly decreased by HFNS treatment, in accordance
with the decreased MMP-9 activity. There were no noticeable changes
in MMP-2 levels in TNF-α- or HFNS-treated MDA-MB-231 cells (data
not shown).
The level of MMPs and uPA-1 are inversely related to
the levels of TIMPs and PAIs, respectively. Interestingly, the
treatment with HFNS induced a modest increase in levels of TIMP-2
and PAI-1 in a dose-dependent manner, although the change in the
level of TIMP-2 was not statistically significant. However,
upregulated TIMP2 and PAI-1 at transcript level was
also observed by semi-quantitative RT-PCR analysis after treatment
of cells with HFNS (Fig. 4B).
Inhibition of MAPK signaling pathways by
HFNS treatment
Previous reports have demonstrated that natural
product-derived agents could regulate metastasis through
suppression of MAPKs (13,29,30).
To gain insights into the mechanism underlying the anti-metastatic
effects of HFNS in MDA-MB-231 cells, we investigated the MAPK
signaling pathways, and determined the effects of HFNS on JNK,
p38-MAPK, and ERK1/2 activation. In response to TNF-α stimulation,
JNK and p38 proteins were phosphorylated within 30 min and the
levels reached ~2.7- and 5.1-fold those in the control cells,
respectively (Fig. 5A). The
activation of p38 MAPK was prolonged for >2 h after exposure to
TNF-α-simulated MDA-MB-231 cells (Fig.
5A), although JNK activation declined gradually. However, cells
exposed to 100 μg/ml HFNS exhibited a rapid and complete
inactivation of JNK and p38, which was not induced by changes in
their total protein level (Fig.
5A). In contrast, phosphorylation of ERK was not affected by
HFNS treatment or by the ERK inhibitor PD98059 (Fig. 5). These results indicated that
HFNS-mediated supression of MAPKs was selective for the regulation
of metastatic MDA-MB-231 cells.
 | Figure 5The inhibitory effects of HFNS on
TNF-α-stimulated MAP kinases that regulate MMP-9. Immunoblotting of
phospho-JNK1/2 (P-JNK1/2), phospho-p38 (P-p38), and phospho-ERK1/2
(P-ERK1/2) in MDA-MB-231 cells. (A) Cells were pre-incubated with
100 μg/ml HFNS for the indicated time and were then stimulated with
TNF-α. (B) Cells were pretreated with SP600125 (30 μM, JNK1/2
inhibitor), SB202190 (20 μM, p38 MAPK inhibitor), or PD98059 (50
μM, MEK1-ERK1/2 inhibitor) for 2 h and were then stimulated with
TNF-α for the indicated time. Each blot was stripped and reprobed
with anti-JNK1/2, p-38, or ERK1/2 antibody to correct for
differences in protein levels. Medium conditioned by, and lysates
of MDA-MB-231 cells that had been treated with HFNS or the above
inhibitors were evaluated by gelatin zymography and immunoblot for
MMP-9, respectively. Densitometric scanning data after correction
for actin loading control are shown on top of the bands.
Immunoblotting of each protein was done at least twice using
independently prepared lysates and the results were similar.
Representative data from a single experiment are shown. |
Furthermore, we confirmed the functional
significance of MAPK downregulation using pharmacologic inhibitors
of JNK1/2 (SP600125), p38 MAPK (SB202190), and ERK1/2 (PD98059).
The activation of MAPKs in response to TNF-α was attenuated by
pretreatment of cells with SP600125 or SB202190, but not PD98059,
indicating inactivation of JNK1/2 or p38, but not ERK1/2,
respectively. The pharmacological inactivation of MAPK also caused
downregulated expression and enzymatic inactivation of MMP-9 as
shown in Fig. 5B. The results were
comparable to those of HFNS-treated MDA-MB-231 cells. Collectively,
these results pointed toward an important regulatory role for
JNK1/2 and p38 MAPK anti-metastatic effect of HFNS on MDA-MB-231
human breast cancer cells.
HFNS inhibits AP-1 and NFκB DNA binding
activities
The expression of MMP-encoding genes is regulated by
the transcription factors AP-1 or NFκB (3,11,21,29),
which are upregulated by TNF-α in a variety of cancer cell lines,
including breast cancer cells (28,31,32).
To confirm whether the repressive effect of HFNS on TNF-α-induced
MMP-9 or uPA-1 expression is mediated via AP-1 or NFκB motifs,
TNF-α-stimulated MDA-MB-231 cells were dose-dependently treated
with HFNS for 18 h. The DNA-binding activity of these transcription
factors was then determined using nuclear extracts and EMSA, TNF-α
treatment caused an increase in AP-1 and NFκB binding activity
(Fig. 6A). The activity of NFκB
was markedly inhibited at treatment of cells with 100 μg/ml HFNS;
more specifically, the initial TNF-α-induced activation was
followed by 25.4% inhibition (Fig.
6B). The binding activity of AP-1 was sustained at first and
then declined to 27.1% of that in the TNF-α-treated control after
treatment with 200 μg/ml HFNS (Fig.
6B). Based on these results, it is plausible that HFNS
treatment inhibits the binding of transcription factors to their
DNA response element in response to TNF-α signaling, leading to
downregulation of MMP-9 expression.
Discussion
Regulation of metastasis in breast cancer has been a
major goal for successful treatment because most breast
cancer-related deaths are due to advanced disease and progressive
metastasis. The present study first revealed that HFNS prevents
invasive potential of the TNF-α activated human MDA-MB-231 breast
cancer cells; regulation of invasion is important to prevent tumor
reoccurrence. HFNS treatment significantly induced inhibition of
cell proliferation and migration of MDA-MB-231 cells. This
correlated with a decrease in protein levels of MMP-9, and uPA-1,
leading to inhibition of these protease activities; concurrently,
levels of TIMP-2, and PAI-1, which are involved in the MAPK
signaling pathway were upregulated. In addition, we demonstrated
that HFNS suppressed TNF-α-mediated MMP-9 activation by decreasing
AP-1 or NFκB DNA-binding activity.
As described in previous invasive model studies
using breast cancer cells, the protective activities of natural
products against metastasis were proposed to be related to
regulation of MMPs and TIMPs, and indicated a correlation between
MMP expression levels and aggressiveness of tumor cell growth and
metastatic potential of such cells (3,9,11,14,33).
Therefore, inhibition of invasion mediated by MMPs and uPA-1 could
be an important strategy in the prevention of cancer metastasis.
Among MMPs, MMP-9 and MMP-2 are considered to play critical roles
in tumor invasion and metastasis (34). Our present study findings also
demonstrated that HFNS decreased the activity or protein levels of
MMP-9 in a dose- and time-dependent manner. In contrast, the
treatment with HFNS had no effect on MMP-2 expression and activity
in TNF-α-stimulated MDA-MB-231 cells. This may be due to tighter
regulation of MMP-2; in contrast, other zymogen MMPs are cleaved
and activated, and show the ability to activate themselves or other
members of this family. MMP-2 is the most commonly expressed MMP in
normal tissues (34). Furthermore,
differences between tissues and cell types can account for
variation of activated MMPs, at least in part, although the
expression and secretion of MMPs is controlled in a similar manner
in the MAPKs signaling pathways (35). For example, our previous results
showed that HFNS exhibited anticancer effect against MDA-MB 231
cells, while having no significant effect on other cancer cell
lines, viz., HeLa (cervical carcinoma), HT29 (colonic
adenocarcinoma), and breast MCF-7 (breast adenocarcinoma) (27).
Additionally, a number of dietary phytochemicals
including extracts or single compounds derived from natural
products have been found to target regulatory proteins, including
NFκB, AP-1, and MAPKs. Some studies have reported that the
cis-acting elements of human MMP-9 include NFκB, SP-1 and
AP-1 elements (13,21,29);
thus, exposure to phytochemicals may cause DNA binding activities
of NFκB, or AP-1, which can then regulate MMP-9 expression
(35). Interestingly, our present
results showed that the binding activity of both AP-1 and NFκB was
increased in TNF-α-stimulated human breast cancer MDA-MB-231 cells.
HFNS treatment significantly suppressed the TNF-α-induced increase
in the binding activity of both AP-1 and NFκB, confirming
transcriptional regulation of MMP-9 via motifs corresponding to
NFκB or AP-1 binding sites. We also demonstrated that HFNS
treatment inhibited phosphorylation of p38, and JNK1/2 and that
this resulted in a concurrent reduction in the levels of MMPs and
uPA-1, indicating a possible mechanism of inhibition of MMPs or
uPA-1 synthesis by HFNS. These results present the first systematic
investigation of the molecular mechanism underlying the
anti-metastatic effects of HFNS. These observations confirm and
expand the reported anticancer action of N.
sublateritium.
Functional compounds identified in N.
sublateritium are predominantly polysaccharides, triterpenoids,
steroids and lipid molecules such as ceramide (24,25,36).
HFNS contains non-polar compounds (27), including tripenoid, clavaric acid,
with antitumor activity, which has been shown to significantly
inhibit the metastatic ability of MDA-MB-231 cells, which represent
TNBC. For effective targeted therapy against TNBC, it has been
suggested that chemotherapy be used as first-line therapy (37). Because therapeutic options in both
early and late stage breast cancer are significantly affected by
the expression of the estrogen receptor, progesterone receptor, and
HER-2/Neu. Given the lack of established molecular targets and the
adverse clinical outcome typical in patients with TNBC, there is a
clear need for continued development of therapies using
chemotherapeutic agents derived from natural products.
In conclusion, HFNS treatment inhibits metastatic
steps including migration and invasion in TNF-α-stimulated
MDA-MB-231 cells. This is achieved by regulation of the activities
of migration and invasion-associated proteinases and their
inhibitors. The anti-metastatic effect of HFNS is mediated by
suppression of MAPKs signaling pathways and NFκB/AP-1 DNA-binding
activities. Taken together, our results indicated that HFNS may be
a potential therapeutic approach to the treatment of TNBC.
Acknowledgements
This study was supported by the National Research
Foundation of Korea (NRF) grant funded by the Ministry of Science,
ICT and Future Planning (2012R1A1A3015385, 2007-0054932), Korea.
This study was also supported by ‘Forest Science & Technology
Projects (no. S120911L110000)’ Korea Forest Service (KFS).
References
|
1
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar
|
|
2
|
Kreusel KM, Bechrakis NE, Wiegel T, Krause
L and Foerster MH: Incidence and clinical characteristics of
symptomatic choroidal metastasis from lung cancer. Acta Ophthalmol.
86:515–519. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Yang HL, Kuo YH, Tsai CT, et al:
Anti-metastatic activities of Antrodia camphorata against human
breast cancer cells mediated through suppression of the MAPK
signaling pathway. Food Chem Toxicol. 49:290–298. 2011. View Article : Google Scholar
|
|
4
|
Fidler IJ: Orthotopic implantation of
human colon carcinomas into nude mice provides a valuable model for
the biology and therapy of metastasis. Cancer Metastasis Rev.
10:229–243. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Yilmaz M, Christofori G and Lehembre F:
Distinct mechanisms of tumor invasion and metastasis. Trends Mol
Med. 13:535–541. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Sliva D, English D, Lyons D and Lloyd FP
Jr: Protein kinase C induces motility of breast cancers by
upregulating secretion of urokinase-type plasminogen activator
through activation of AP-1 and NF-kappaB. Biochem Biophys Res
Commun. 290:552–557. 2002. View Article : Google Scholar
|
|
7
|
Zheng H, Takahashi H, Murai Y, et al:
Expressions of MMP-2, MMP-9 and VEGF are closely linked to growth,
invasion, metastasis and angiogenesis of gastric carcinoma.
Anticancer Res. 26:3579–3583. 2006.PubMed/NCBI
|
|
8
|
Westermarck J and Kahari VM: Regulation of
matrix metalloproteinase expression in tumor invasion. FASEB J.
13:781–792. 1999.PubMed/NCBI
|
|
9
|
Quaranta M, Daniele A, Coviello M, et al:
MMP-2, MMP-9, VEGF and CA 15.3 in breast cancer. Anticancer Res.
27:3593–3600. 2007.
|
|
10
|
Folgueras AR, Pendas AM, Sanchez LM and
Lopez-Otin C: Matrix metalloproteinases in cancer: from new
functions to improved inhibition strategies. Int J Dev Biol.
48:411–424. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Look M, van Putten W, Duffy M, et al:
Pooled analysis of prognostic impact of uPA and PAI-1 in breast
cancer patients. Thromb Haemost. 90:538–548. 2003.PubMed/NCBI
|
|
12
|
Rosen EM, Goldberg ID, Liu D, et al: Tumor
necrosis factor stimulates epithelial tumor cell motility. Cancer
Res. 51:5315–5321. 1991.PubMed/NCBI
|
|
13
|
Hagemann T, Wilson J, Kulbe H, et al:
Macrophages induce invasiveness of epithelial cancer cells via
NF-kappa B and JNK. J Immunol. 175:1197–1205. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Chazaud B, Ricoux R, Christov C, Plonquet
A, Gherardi RK and Barlovatz-Meimon G: Promigratory effect of
plasminogen activator inhibitor-1 on invasive breast cancer cell
populations. Am J Pathol. 160:237–246. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Stetler-Stevenson WG: Tissue inhibitors of
metalloproteinases in cell signaling: metalloproteinase-independent
biological activities. Sci Signal. 1:re62008. View Article : Google Scholar
|
|
16
|
Lijnen HR: Pleiotropic functions of
plasminogen activator inhibitor-1. J Thromb Haemost. 3:35–45. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Rose P, Huang Q, Ong CN and Whiteman M:
Broccoli and watercress suppress matrix metalloproteinase-9
activity and invasiveness of human MDA-MB-231 breast cancer cells.
Toxicol Appl Pharmacol. 209:105–113. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Choi S and Singh SV: Bax and Bak are
required for apoptosis induction by sulforaphane, a cruciferous
vegetable-derived cancer chemopreventive agent. Cancer Res.
65:2035–2043. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ho YC, Yang SF, Peng CY, Chou MY and Chang
YC: Epigallocatechin-3-gallate inhibits the invasion of human oral
cancer cells and decreases the productions of matrix
metalloproteinases and urokinase-plasminogen activator. J Oral
Pathol Med. 36:588–593. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Seo HS, DeNardo DG, Jacquot Y, et al:
Stimulatory effect of genistein and apigenin on the growth of
breast cancer cells correlates with their ability to activate ER
alpha. Breast Cancer Res Treat. 99:121–134. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Jiang J, Grieb B, Thyagarajan A and Sliva
D: Ganoderic acids suppress growth and invasive behavior of breast
cancer cells by modulating AP-1 and NF-kappaB signaling. Int J Mol
Med. 21:577–584. 2008.PubMed/NCBI
|
|
22
|
Sliva D, Jedinak A, Kawasaki J, Harvey K
and Slivova V: Phellinus linteus suppresses growth,
angiogenesis and invasive behaviour of breast cancer cells through
the inhibition of AKT signalling. Br J Cancer. 98:1348–1356. 2008.
View Article : Google Scholar
|
|
23
|
Kim HG, Yoon DH, Lee WH, et al:
Phellinus linteus inhibits inflammatory mediators by
suppressing redox-based NF-kappaB and MAPKs activation in
lipopolysaccharide-induced RAW 264.7 macrophage. J Ethnopharmacol.
114:307–315. 2007. View Article : Google Scholar
|
|
24
|
Godio RP, Fouces R, Gudina EJ and Martin
JF: Agrobacterium tumefaciens-mediated transformation of the
antitumor clavaric acid-producing basidiomycete Hypholoma
sublateritium. Curr Genet. 46:287–294. 2004. View Article : Google Scholar
|
|
25
|
Godio RP, Fouces R and Martin JF: A
squalene epoxidase is involved in biosynthesis of both the
antitumor compound clavaric acid and sterols in the basidiomycete
H. sublateritium. Chem Biol. 14:1334–1346. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Godio RP and Martin JF: Modified
oxidosqualene cyclases in the formation of bioactive secondary
metabolites: biosynthesis of the antitumor clavaric acid. Fungal
Genet Biol. 46:232–242. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Choi S, Jang HJ, Choi JY, Kim MS, Lee YR,
Kim HS, Choi SW, Jeon BH, Won SI, Kim TW and Choi JW: Antioxidant
and anticancer activity of fractions of the ethanol extract of
Naematoloma sublateritium. J Med Plants Res. 6:92012.
|
|
28
|
Lee YR, Kim KM, Jeon BH, Choi JW and Choi
S: The n-butanol fraction of Naematoloma sublateritium
suppresses the inflammatory response through downregulation of
NF-kappaB in human endothelial cells. Int J Mol Med. 29:801–808.
2012.
|
|
29
|
Kajanne R, Miettinen P, Mehlem A, et al:
EGF-R regulates MMP function in fibroblasts through MAPK and AP-1
pathways. J Cel Physiol. 212:489–497. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Cohen M, Meisser A, Haenggeli L and
Bischof P: Involvement of MAPK pathway in TNF-alpha-induced MMP-9
expression in human trophoblastic cells. Mol Hum Reprod.
12:225–232. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Leber TM and Balkwill FR: Regulation of
monocyte MMP-9 production by TNF-alpha and a tumour-derived soluble
factor (MMPSF). Br J Cancer. 78:724–732. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Stuelten CH, DaCosta Byfield S, Arany PR,
Karpova TS, Stetler-Stevenson WG and Roberts AB: Breast cancer
cells induce stromal fibroblasts to express MMP-9 via secretion of
TNF-alpha and TGF-beta. J Cell Sci. 118:2143–2153. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Bachmeier BE, Nerlich AG, Lichtinghagen R
and Sommerhoff CP: Matrix metalloproteinases (MMPs) in breast
cancer cell lines of different tumorigenicity. Anticancer Res.
21:3821–3828. 2001.PubMed/NCBI
|
|
34
|
John A and Tuszynski G: The role of matrix
metalloproteinases in tumor angiogenesis and tumor metastasis.
Pathol Oncol Res. 7:14–23. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Lee EJ, Kim WJ and Moon SK: Cordycepin
suppresses TNF-alpha-induced invasion, migration and matrix
metalloproteinase-9 expression in human bladder cancer cells.
Phytother Res. 24:1755–1761. 2010. View
Article : Google Scholar : PubMed/NCBI
|
|
36
|
Yaoita Y, Matsuki K, Iijima T, et al: New
sterols and triterpenoids from four edible mushrooms. Chem Pharm
Bull. 49:589–594. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Chavez KJ, Garimella SV and Lipkowitz S:
Triple negative breast cancer cell lines: one tool in the search
for better treatment of triple negative breast cancer. Breast Dis.
32:35–48. 2010.PubMed/NCBI
|