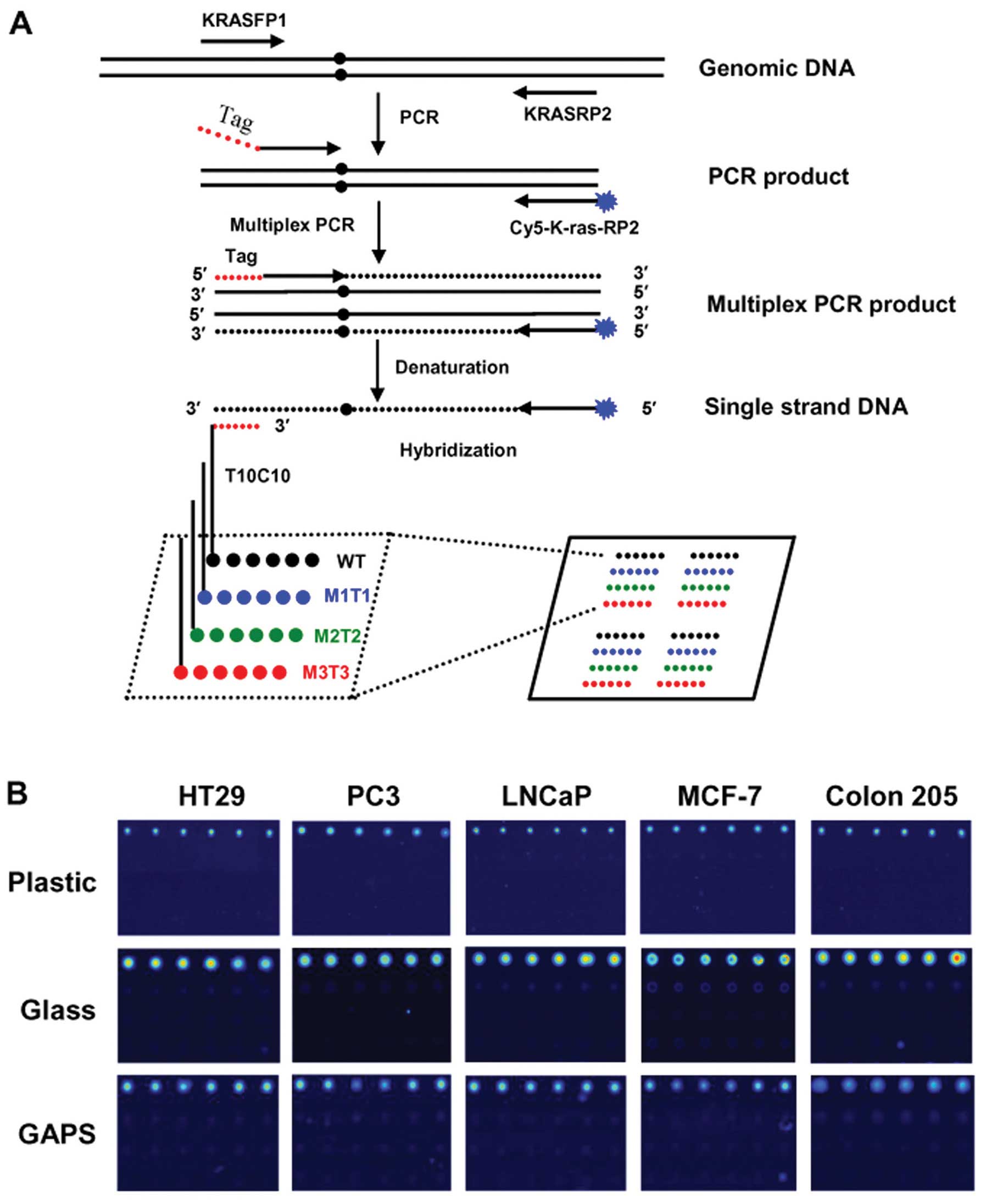
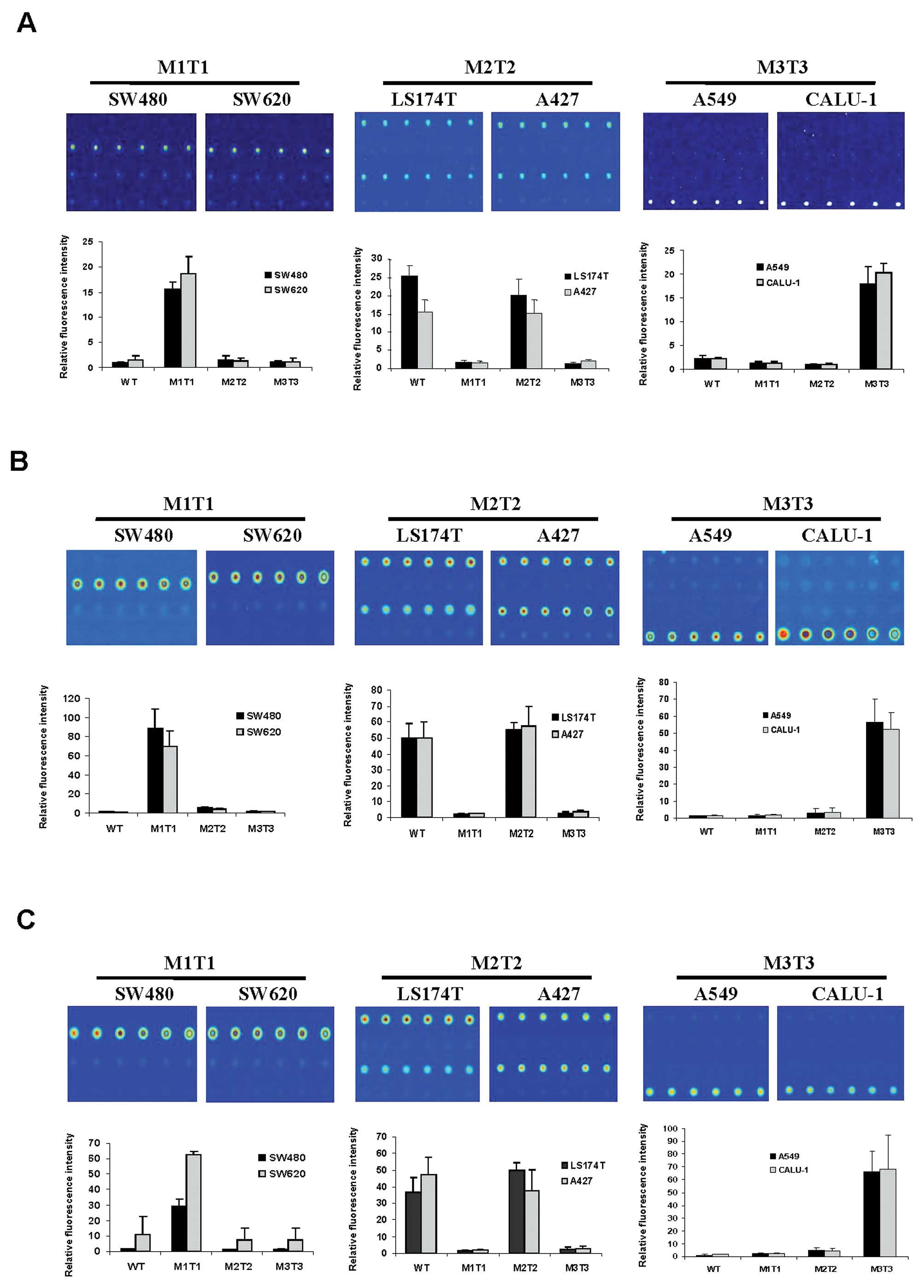
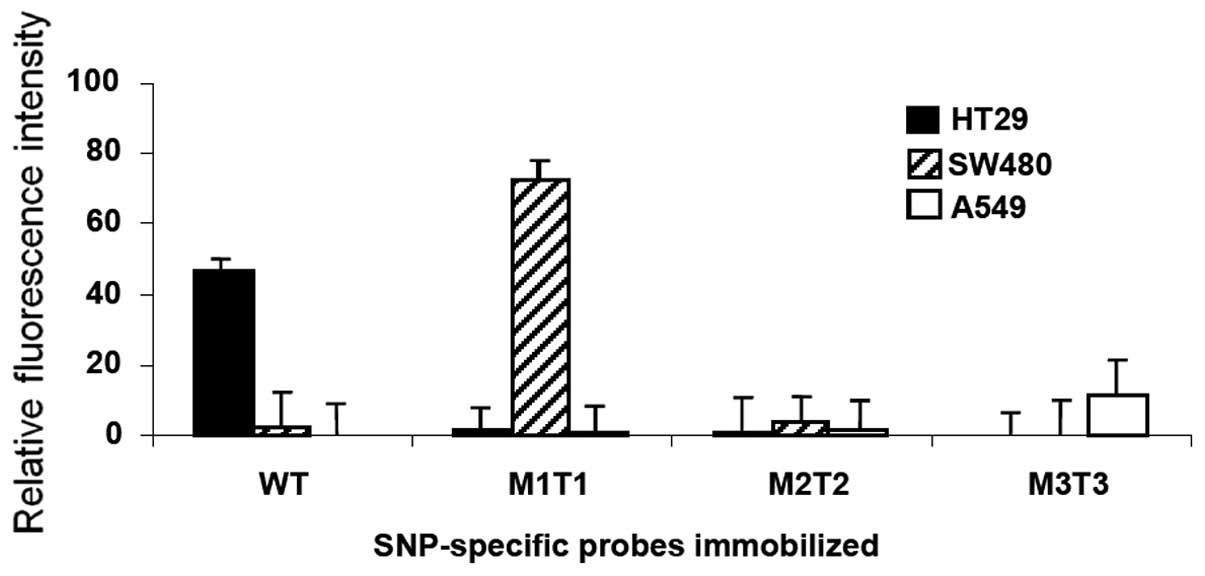
|
1
|
Landis S, Murray T, Bolden S and Wingo P:
Cancer statistics. CA Cancer J Clin. 48:6–29. 1998.
|
|
2
|
Moerkerk P, Arends J, van Driel M, de
Bruine A, de Goeij A and ten Kate J: Type and number of Ki-ras
point mutations relate to stage of human colorectal cancer. Cancer
Res. 54:3376–3378. 1994.PubMed/NCBI
|
|
3
|
Sidransky D, Tokino T, Hamilton S, et al:
Identification of ras oncogene mutations in the stool of patients
with curable colorectal tumors. Science. 256:102–105. 1992.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Winawer S, Fletcher R, Miller L, et al:
Colorectal cancer screening: clinical guidelines and rationale.
Gastroenterology. 112:594–642. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Rex D, Rahmani E, Haseman J, Lemmel G,
Kaster S and Buckley J: Relative sensitivity of colonoscopy and
barium enema for detection of colorectal cancer in clinical
practice. Gastroenterology. 112:17–23. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hardcastle J, Chamberlain J, Robinson M,
Moss S, Amar S and Balfour T: Randomised controlled trial of
faecal-occult-blood screening for colorectal cancer. Lancet.
348:1472–1477. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lieberman D and Weiss D: One-time
screening for colorectal cancer with combined fecal occult-blood
testing and examination of the distal colon. N Engl J Med.
345:555–560. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Fearon ER: Molecular genetics of
colorectal cancer. Ann NY Acad Sci. 768:101–110. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Burt RW: Colon cancer screening.
Gastroenterology. 119:837–853. 2000. View Article : Google Scholar
|
|
10
|
Hoffman M: Getting a handle on Ras
activity. Science. 255:1591992. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kiaris H and Spandidos DA: Mutations of
ras genes in human tumours (Review). Int J Oncol. 7:413–421.
1995.
|
|
12
|
Shirasawa S, Furuse M, Yokoyama N and
Sasazuki T: Altered growth of human colon cancer cell lines
disrupted at activated Ki-ras. Science. 260:85–88. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Jacobson D and Mills N: A highly sensitive
assay for mutant ras genes and its application to the study of
presentation and relapse genotypes in acute leukemia. Oncogene.
9:553–563. 1994.PubMed/NCBI
|
|
14
|
Nishikawa T, Maemura K, Hirata I, et al: A
simple method of detecting K-ras point mutations in stool samples
for colorectal cancer screening using one-step polymerase chain
reaction/restriction fragment length polymorphism analysis. Clin
Chim Acta. 318:107–112. 2002. View Article : Google Scholar
|
|
15
|
Markman B, Javier F, Capdevila J and
Tabernero J: EGFR and KRAS in colorectal cancer. Adv Clin Chem.
51:71–119. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Siena S, Sartore-Bianchi A, Di
Nicolantonio F, Balfour J and Bardelli A: Biomarkers predicting
clinical outcome of epidermal growth factor receptor-targeted
therapy in metastatic colorectal cancer. J Natl Cancer Inst.
101:1308–1324. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Linardou H, Dahabreh J, Kanaloupiti D, et
al: Assessment of somatic K-RAS mutations as a mechanism associated
with resistance to EGFR-targeted agents: A systematic review and
meta-analysis of studies in advanced non-small-cell lung cancer and
metastatic colorectal cancer. Lancet Oncol. 9:962–972. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Benvenuti S, Sartore-Bianchi A, Di
Nicolantonio F, et al: Oncogenic activation of the RAS/RAF
signaling pathway impairs the response of metastatic colorectal
cancers to anti-epidermal growth factor receptor antibody
therapies. Cancer Res. 67:2643–2648. 2007. View Article : Google Scholar
|
|
19
|
Lee C, Chen H and Liu H: Favorable
response to erlotinib in a lung adenocarcinoma with both epidermal
growth factor receptor exon 19 deletion and K-ras G13D mutations. J
Clin Oncol. 28:e111–e112. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
De Roock W, Jonker D, Nicolantonio F, et
al: Association of KRAS p. G13D mutation with outcome in patients
with chemotherapy-refractory metastatic colorectal cancer treated
with cetuximab. J Am Med Assoc. 304:1812–1820. 2010.PubMed/NCBI
|
|
21
|
Allegra C, Jessup J, Somerfield M, et al:
American Society of Clinical Oncology provisional clinical opinion:
testing for KRAS gene mutations in patients with metastatic
colorectal carcinoma to predict response to anti-epidermal growth
factor receptor monoclonal antibody therapy. J Clin Oncol.
27:2091–2096. 2009. View Article : Google Scholar
|
|
22
|
Toyooka S, Tsukuda K, Ouchida M, et al:
Detection of codon 61 point mutations of the K-ras gene in lung and
colorectal cancers by enriched PCR. Oncol Rep. 10:1455–1459.
2003.PubMed/NCBI
|
|
23
|
Nollau P, Moser C, Weinland G and Wagener
C: Detection of K-ras mutations in stools of patients with
colorectal cancer by mutant-enriched PCR. Int J Cancer. 66:332–336.
1996. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Luo J, Chan E, Shih C, et al: Detection of
rare mutant K-ras DNA in a single-tube reaction using peptide
nucleic acid as both PCR clamp and sensor probe. Nucleic Acids Res.
34:e122006. View Article : Google Scholar
|
|
25
|
Mixich F, Ioana M, Voinea F, Saftoiu A and
Ciurea T: Noninvasive detection through REMS-PCR technique of K-ras
mutations in stool DNA of patients with colorectal cancer. J
Gastrointestin Liver Dis. 16:5–10. 2007.PubMed/NCBI
|
|
26
|
Dieterle C, Conzelmann M, Linnemann U and
Berger M: Detection of isolated tumor cells by polymerase chain
reaction-restriction fragment length polymorphism for K-ras
mutations in tissue samples of 199 colorectal cancer patients. Clin
Cancer Res. 10:641–650. 2004. View Article : Google Scholar
|
|
27
|
Lopez-Crapez E, Chypre C, Saavedra J,
Marchand J and Grenier J: Rapid and large-scale method to detect
K-ras gene mutations in tumor samples. Clin Chem. 43:936–942.
1997.PubMed/NCBI
|
|
28
|
Maekawa M, Nagaoka T, Taniguchi T, et al:
Three-dimensional microarray compared with PCR-single-strand
conformation polymorphism analysis/DNA sequencing for mutation
analysis of K-ras codons 12 and 13. Clin Chem. 50:1322–1327. 2004.
View Article : Google Scholar
|
|
29
|
Parsons B, Marchant-Miros K, Delongchamp
R, et al: ACB-PCR quantification of K-RAS codon 12 GAT and GTT
mutant fraction in colon tumor and non-tumor tissue. Cancer Invest.
28:364–375. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Hirschhorn J, Sklar P, Lindblad-Toh K, et
al: SBE-TAGS: an array-based method for efficient single-nucleotide
polymorphism genotyping. Proc Natl Acad Sci USA. 97:12164–12169.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Dufva M, Petersen J, Stoltenborg M,
Birgens H and Christensen C: Detection of mutations using
microarrays of poly(C)10-poly(T)10 modified DNA probes immobilized
on agarose films. Anal Biochem. 352:188–197. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Gudnason H, Dufva M, Bang D and Wolff A:
An inexpensive and simple method for thermal stable immobilization
of DNA on an unmodifed glass surface: UV linking of poly (T)
10-poly (C) 10 tagged DNA probes. Bio Technique. 45:261–271.
2008.PubMed/NCBI
|
|
33
|
Winzeler E, Shoemaker D, Astromoff A, et
al: Functional characterization of the S. cerevisiae genome
by gene deletion and parallel analysis. Science. 285:901–906.
1999.
|
|
34
|
Fan J, Chen X, Halushka M, et al: Parallel
genotyping of human SNPs using generic high-density oligonucleotide
tag arrays. Genome Res. 10:853–860. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Pastinen T, Raitio M, Lindroos K, Tainola
P, Peltonen L and Syvanen AC: A system for specific,
high-throughput genotyping by allele-specific primer extension on
microarrays. Genome Res. 10:1031–1042. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Lleonart M, Ramony Cajal S, Groopman J and
Friesen M: Sensitive and specific detection of K-ras mutations in
colon tumors by short oligonucleotide mass analysis. Nucleic Acids
Res. 32:e532004. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Finkelstein S, Sayegh R, Christensen S and
Swalsky P: Genotypic classification of colorectal adenocarcinoma.
Biologic behavior correlates with K-ras-2 mutation type. Cancer.
71:3827–3838. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Chang Y, Yeh K, Chang T, et al: Fast
simultaneous detection of K-RAS mutations in colorectal cancer. BMC
Cancer. 9:1792009. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Huang J, Mehrens D, Wiese R, et al:
High-throughput genomic and proteomic analysis using microarray
technology. Clin Chem. 47:1912–1916. 2001.PubMed/NCBI
|
|
40
|
Kim I, Kang H, Jang S, et al:
Oligonucleotide microarray analysis of distinct gene expression
patterns in colorectal cancer tissues harboring BRAF and K-ras
mutations. Carcinogenesis. 27:392–404. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
The World Cancer Report - the major
findings. Cent Eur J Public Health. 11:177–179. 2003.
|
|
42
|
Fumagalli D, Gavin P, Taniyama Y, et al: A
rapid, sensitive, reproducible and cost-effective method for
mutation profiling of colon cancer and metastatic lymph nodes. BMC
Cancer. 10:1012010. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Chien C, Chen S, Liu C, Lee C, Yang R and
Huang C: Correlation of K-ras codon 12 mutations in human feces and
ages of patients with colorectal cancer (CRC). Transl Res.
149:96–102. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Cerottini J, Caplin S, Saraga E, Givel J
and Benhattar J: The type of K-ras mutation determines prognosis in
colorectal cancer. Am J Surg. 175:198–202. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Cho K and Vogelstein B: Genetic
alterations in the adenomacarcinoma sequence. Cancer. 70:1727–1731.
1992. View Article : Google Scholar : PubMed/NCBI
|