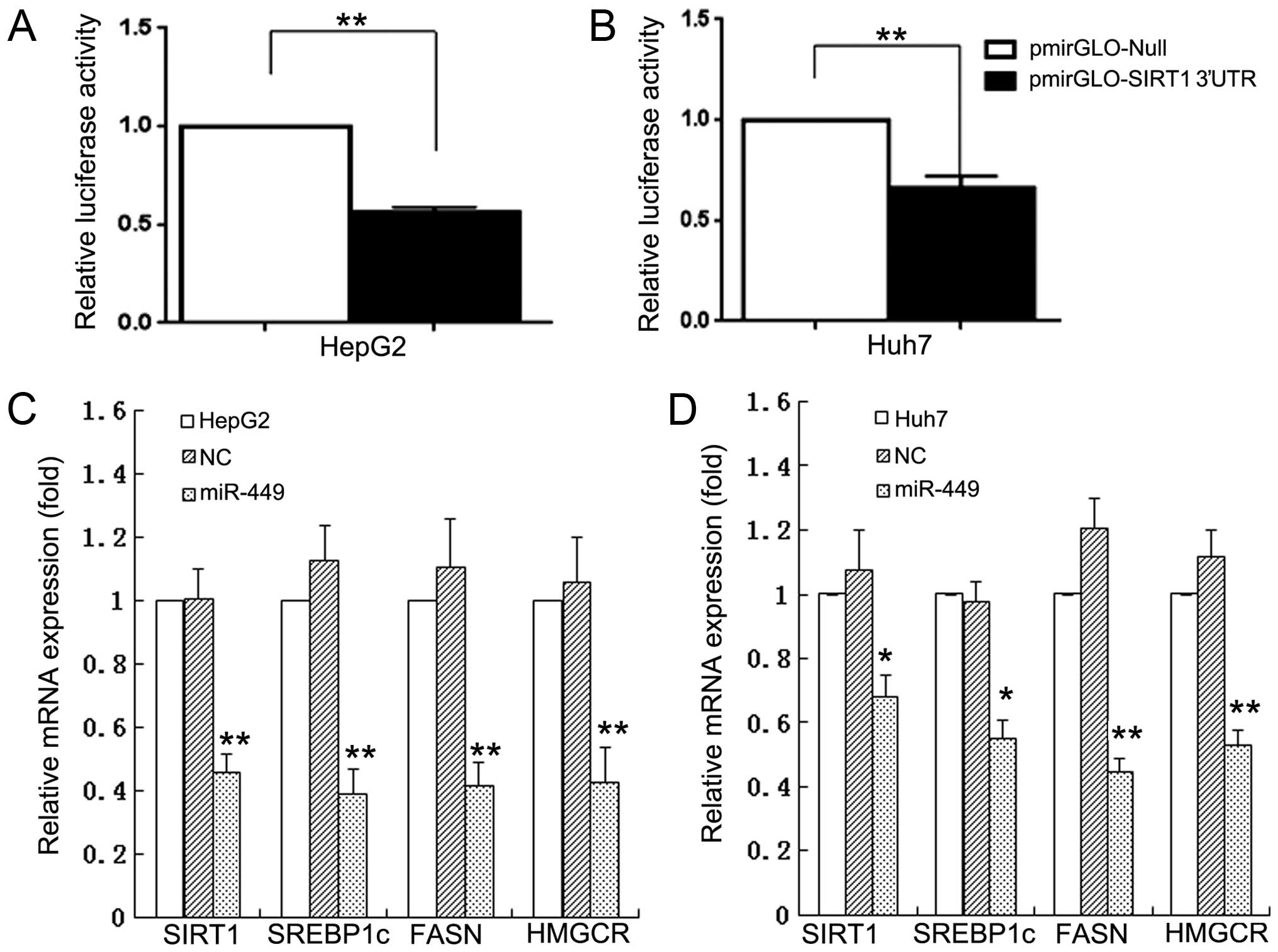
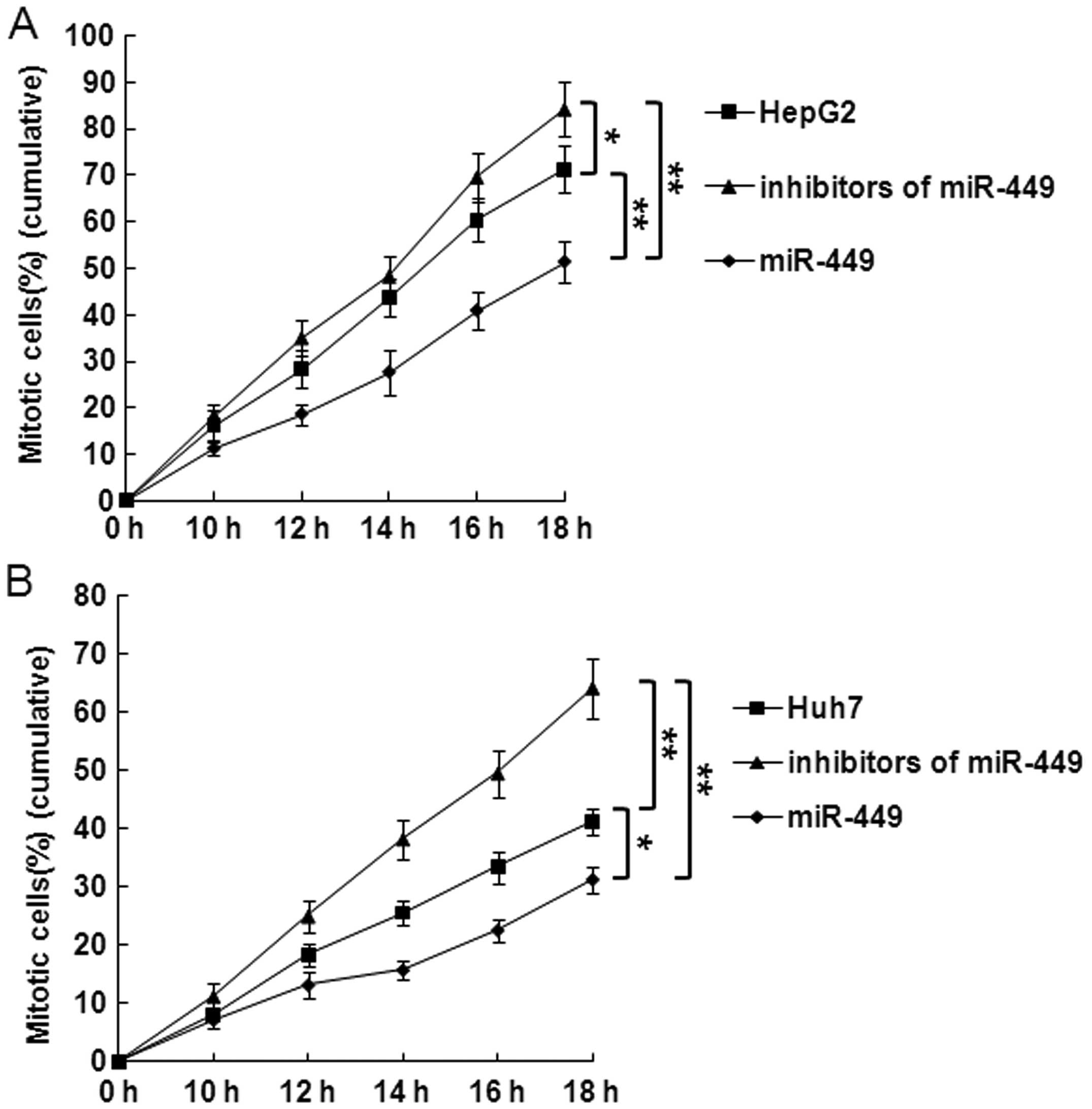
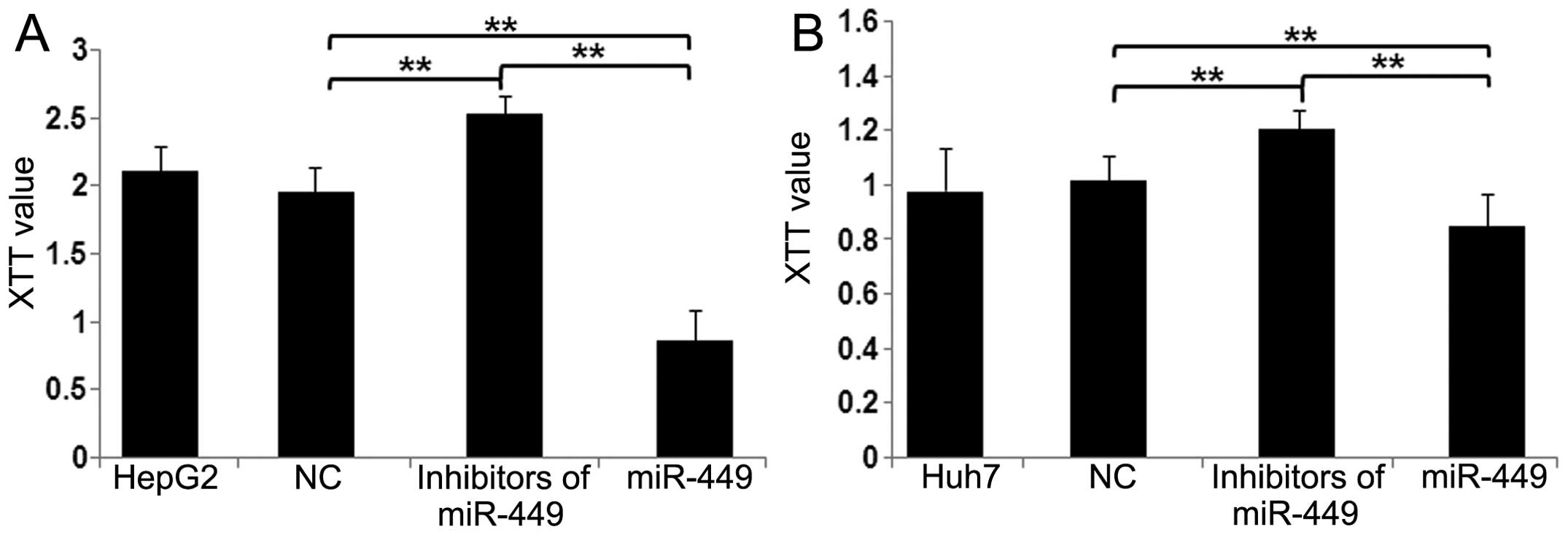
|
1
|
Herold C, Reck T, Fischler P, et al:
Prognosis of a large cohort of patients with hepatocellular
carcinoma in a single European centre. Liver. 22:23–28. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Okuda K: Hepatocellular carcinoma. J
Hepatol. 32:225–237. 2000. View Article : Google Scholar
|
|
3
|
Bhalla KN: Epigenetic and chromatin
modifiers as targeted therapy of hematologic malignancies. J Clin
Oncol. 23:3971–3993. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Dowman JK, Hopkins LJ, Reynolds GM, et al:
Development of hepatocellular carcinoma in a murine model of
nonalcoholic steatohepatitis induced by use of a high-fat/fructose
diet and sedentary lifestyle. Am J Pathol. 184:1550–1561. 2014.
View Article : Google Scholar
|
|
5
|
Karagozian R, Derdák Z and Baffy G:
Obesity-associated mechanisms of hepatocarcinogenesis. Metabolism.
63:607–617. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Qin H and Ruan ZH: The role of
monoacylglycerol lipase (MAGL) in the cancer progress. Cell Biochem
Biophys. Mar 16–2014.(Epub ahead of print).
|
|
7
|
Pralhada Rao R, Vaidyanathan N, Rengasamy
M, Mammen Oommen A, Somaiya N and Jagannath MR: Sphingolipid
metabolic pathway: an overview of major roles played in human
diseases. J Lipids. 2013:1789102013.PubMed/NCBI
|
|
8
|
Lee RC, Feinbaum RL and Ambros V: The
C. elegans heterochronic gene lin-4 encodes small RNAs with
antisense complementarity to lin-14. Cell. 75:843–854. 1993.
|
|
9
|
Xu P, Vernooy SY, Guo M and Hay BA: The
Drosophila microRNA Mir-14 suppresses cell death and is
required for normal fat metabolism. Curr Biol. 13:790–795.
2003.
|
|
10
|
Wightman B, Ha I and Ruvkun G:
Post-transcriptional regulation of the heterochronic gene lin-14 by
lin-4 mediates temporal pattern formation in C. elegans.
Cell. 75:855–862. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Reinhart BJ, Slack FJ, Basson M, et al:
The 21-nucleotide let-7 RNA regulates developmental timing in
Caenorhabditis elegans. Nature. 403:901–906. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Brennecke J, Hipfner DR, Stark A, Russell
RB and Cohen SM: Bantam encodes a developmentally regulated
microRNA that controls cell proliferation and regulates the
proapoptotic gene hid in Drosophila. Cell. 113:25–36. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hatfield SD, Shcherbata HR, Fischer KA,
Nakahara K, Carthew RW and Ruohola-Baker H: Stem cell division is
regulated by the microRNA pathway. Nature. 435:974–978. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Michael MZ, O’ Connor SM, van Holst
Pellekaan NG, Young GP and James RJ: Reduced accumulation of
specific microRNAs in colorectal neoplasia. Mol Cancer Res.
1:882–891. 2003.PubMed/NCBI
|
|
15
|
Calin GA, Sevignani C, Dumitru CD, et al:
Human microRNA genes are frequently located at fragile sites and
genomic regions involved in cancers. Proc Natl Acad Sci USA.
101:2999–3004. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Iorio MV, Ferracin M, Liu CG, et al:
MicroRNA gene expression deregulation inhumanbreast cancer. Cancer
Res. 65:7065–7070. 2005. View Article : Google Scholar
|
|
17
|
Lu J, Getz G, Miska EA, et al: MicroRNA
expression profiles classify human cancers. Nature. 435:834–838.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
He L, Thomson JM, Hemann MT, et al: A
microRNA polycistron as a potential human oncogene. Nature.
435:828–833. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Volinia S, Calin GA, Liu CG, et al: A
microRNA expression signature of human solid tumors defines cancer
gene targets. Proc Natl Acad Sci USA. 103:2257–2261. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Pekarsky Y, Santanam U, Cimmino A, et al:
Tcl1 expression in chronic lymphocytic leukemia is regulated by
miR-29 and miR-181. Cancer Res. 66:11590–11593. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Slack FJ and Weidhaas JB: MicroRNAs as a
potential magic bullet in cancer. Future Oncol. 2:73–82. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Cimmino A, Calin GA, Fabbri M, et al:
miR-15 and miR-16 induce apoptosis by targeting BCL2. Proc Natl
Acad Sci USA. 102:13944–13949. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Johnson SM, Grosshans H, Shingara J, et
al: RAS is regulated by the let-7 microRNA family. Cell.
120:635–647. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Takamizawa J, Konishi H, Yanagisawa K, et
al: Reduced expression of the let-7 microRNAs in human lung cancers
in association with shortened postoperative survival. Cancer Res.
64:3753–3756. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Mayr C, Hemann MT and Bartel DP:
Disrupting the pairing between let-7 and Hmga2 enhances oncogenic
transformation. Science. 315:1576–1579. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Morris JP and McManus MT: Slowing down the
Ras lane: miRNAs as tumor suppressors? Sci STKE.
16:412005.PubMed/NCBI
|
|
27
|
He L, He X, Lowe SW and Hannon GJ:
MicroRNAs join the p53 network - another piece in the
tumour-suppression puzzle. Nat Rev Cancer. 7:819–822. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Lizé M, Klimke A and Dobbelstein M:
MicroRNA-449 in cell fate determination. Cell Cycle. 10:2874–2882.
2011.PubMed/NCBI
|
|
29
|
Lizé M, Pilarski S and Dobbelstein M:
E2F1-inducible microRNA 449a/b suppresses cell proliferation and
promotes apoptosis. Cell Death Differ. 17:452–458. 2010.PubMed/NCBI
|
|
30
|
Hida Y, Kubo Y, Murao K and Arase S:
Strong expression of a longevity-related protein, SIRT1, in Bowen’s
disease. Arch Dermatol Res. 299:103–106. 2007.PubMed/NCBI
|
|
31
|
Yeung F, Hoberg JE, Ramsey CS, et al:
Modulation of NF-kappaB-dependent transcription and cell survival
by the SIRT1 deacetylase. EMBO J. 23:2369–2380. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Walker AK, Yang F, Jiang K, et al:
Conserved role of SIRT1 orthologs in fasting-dependent inhibition
of the lipid/cholesterol regulator SREBP. Genes Dev. 24:1403–1417.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Rodgers JT and Puigserver P:
Fasting-dependent glucose and lipid metabolic response through
hepatic sirtuin 1. Proc Natl Acad Sci USA. 104:12861–12866. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Ponugoti B, Kim DH, Xiao Z, et al: SIRT1
deacetylates and inhibits SREBP-1C activity in regulation of
hepatic lipid metabolism. J Biol Chem. 285:33959–33970. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Truman JP, García-Barros M, Obeid LM and
Hannun YA: Evolving concepts in cancer therapy through targeting
sphingolipid metabolism. Biochim Biophys Acta. Dec 30–2013.(Epub
ahead of print).
|
|
36
|
Zhou B, Li C, Qi W, et al: Downregulation
of miR-181a upregulates sirtuin-1 (SIRT1) and improves hepatic
insulin sensitivity. Diabetologia. 55:2032–2043. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Ford J, Jiang M and Milner J:
Cancer-specific functions of SIRT1 enable human epithelial cancer
cell growth and survival. Cancer Res. 65:10457–10463. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Hamamoto R, Furukawa Y, Morita M, et al:
SMYD3 encodes a histone methyltransferase involved in the
proliferation of cancer cells. Nat Cell Biol. 6:731–740. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Wang H, Liu H, Chen K, et al: SIRT1
promotes tumorigenesis of hepatocellular carcinoma through
PI3K/PTEN/AKT signaling. Oncol Rep. 28:311–318. 2012.PubMed/NCBI
|
|
40
|
Eberle D, Hegarty B, Bossard P, Ferre P
and Foufelle F: SREBP transcription factors: master regulators of
lipid homeostasis. Biochimie. 86:839–848. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Dif N, Euthine V, Gonnet E, et al: Insulin
activates human sterol-regulatory-element-binding protein-1c
(SREBP-1c) promoter through SRE motifs. Biochem J. 400:179–188.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Guillet-Deniau I, Mieulet V, Le Lay S, et
al: Sterol regulatory element binding protein-1c expression and
action in rat muscles: insulin-like effects on the control of
glycolytic and lipogenic enzymes and UCP3 gene expression.
Diabetes. 51:1722–1728. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Rome S, Lecomte V, Meugnier E, et al:
Microarray analyses of SREBP-1a and SREBP-1c target genes identify
new regulatory pathways in muscle. Physiol Genomics. 34:327–337.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Giandomenico V, Simonsson M, Gronroos E
and Ericsson J: Coactivator dependent acetylation stabilizes
members of the SREBP family of transcription factors. Mol Cell
Biol. 23:2587–2599. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Calkin AC and Tontonoz P: Transcriptional
integration of metabolism by the nuclear sterol-activated receptors
LXR and FXR. Nat Rev Mol Cell Biol. 13:213–224. 2012.PubMed/NCBI
|
|
46
|
Yoshikawa T, Shimano H, Amemiya-Kudo M, et
al: Identification of liver X receptor-retinoid X receptor as an
activator of the sterol regulatory element-binding protein 1c gene
promoter. Mol Cell Biol. 21:2991–3000. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Repa JJ, Liang G, Ou J, et al: Regulation
of mouse sterol regulatory element-binding protein-1c gene
(SREBP-1c) by oxysterol receptors, LXRalpha and LXRbeta. Genes Dev.
14:2819–2830. 2000. View Article : Google Scholar
|
|
48
|
Cozzone D, Debard C, Dif N, et al:
Activation of liver X receptors promotes lipid accumulation but
does not alter insulin action in human skeletal muscle cells.
Diabetologia. 49:990–999. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Swinnen JV, Heemers H, van de Sande T, et
al: Androgens, lipogenesis and prostate cancer. J Steroid Biochem
Mol Biol. 92:273–279. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Yamashita T, Honda M, Takatori H, et al:
Activation of lipogenic pathway correlates with cell proliferation
and poor prognosis in hepatocellular carcinoma. J Hepatol.
50:100–110. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Di Vizio D, Solomon KR and Freeman MR:
Cholesterol and cholesterol-rich membranes in prostate cancer: an
update. Tumori. 94:633–639. 2008.PubMed/NCBI
|
|
52
|
Swinnen JV, Brusselmans K and Verhoeven G:
Increased lipogenesis in cancer cells: new players, novel targets.
Curr Opin Clin Nutr Metab Care. 9:358–365. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Freeman MR, Cinar B and Lu ML: Membrane
rafts as potential sites of nongenomic hormonal signaling in
prostate cancer. Trends Endocrinol Metab. 16:273–279. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Fernandez-Hernando C, Suarez Y, Rayner KJ
and Moore KJ: MicroRNAs in lipid metabolism. Curr Opin Lipidol.
22:86–92. 2011. View Article : Google Scholar
|
|
55
|
Krutzfeldt J and Stoffel M: MicroRNAs: a
new class of regulatory genes affecting metabolism. Cell Metab.
4:9–12. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Henry JC, Azevedo-Pouly AC and Schmittgen
TD: MicroRNA replacement therapy for cancer. Pharm Res.
28:3030–3042. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Garzon R, Marcucci G and Croce CM:
Targeting microRNAs in cancer: rationale, strategies and
challenges. Nat Rev Drug Discov. 9:775–789. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Bader AG, Brown D and Winkler M: The
promise of microRNA replacement therapy. Cancer Res. 70:7027–7030.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Lee J and Kemper JK: Controlling SIRT1
expression by microRNAs in health and metabolic disease. Aging
(Albany, NY). 2:527–534. 2010.PubMed/NCBI
|
|
60
|
Firestein R, Blander G, Michan S, et al:
The SIRT1 deacetylase suppresses intestinal tumorigenesis and colon
cancer growth. PLoS One. 3:e20202008. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Bae HJ, Noh JH, Kim JK, et al:
MicroRNA-29c functions as a tumor suppressor by direct targeting
oncogenic SIRT1 in hepatocellular carcinoma. Oncogene.
33:2557–2567. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Wang RH, Sengupta K, Li C, et al: Impaired
DNA damage response, genome instability, and tumorigenesis in SIRT1
mutant mice. Cancer Cell. 14:312–323. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Huffman DM, Grizzle WE, Bamman MM, et al:
SIRT1 is significantly elevated in mouse and human prostate cancer.
Cancer Res. 67:6612–6618. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Jang KY, Kim KS, Hwang SH, et al:
Expression and prognostic significance of SIRT1 in ovarian
epithelial tumours. Pathology. 41:366–371. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Cha EJ, Noh SJ, Kwon KS, et al: Expression
of DBC1 and SIRT1 is associated with poor prognosis of gastric
carcinoma. Clin Cancer Res. 15:4453–4459. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Stunkel W, Peh BK, Tan YC, et al: Function
of the SIRT1 protein deacetylase in cancer. Biotechnol J.
2:1360–1368. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Chen J, Zhang B, Wong N, et al: Sirtuin 1
is upregulated in a subset of hepatocellular carcinomas where it is
essential for telomere maintenance and tumor cell growth. Cancer
Res. 71:4138–4149. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Noh JH, Jung KH, Kim JK, et al: Aberrant
regulation of HDAC2 mediates proliferation of hepatocellular
carcinoma cells by deregulating expression of G1/S cell cycle
proteins. PLoS One. 6:e281032011. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Xie HJ, Noh JH, Kim JK, et al: HDAC1
inactivation induces mitotic defect and caspase-independent
autophagic cell death in liver cancer. PLoS One. 7:e342652012.
View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Buurman R, Gürlevik E, Schäffer V, et al:
Histone deacetylases activate hepatocyte growth factor signaling by
repressing microRNA-449 in hepatocellular carcinoma cells.
Gastroenterology. 143:811–820. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Huang WC, Li X, Liu J, Lin JT and Chung
LW: Activation of androgen receptor, lipogenesis and oxidative
stress converged by SREBP-1 is responsible for regulating growth
and progression of prostate cancer cells. Mol Cancer Res.
10:133–142. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Menendez JA, Decker JP and Lupu R: In
support of fatty acid synthase (FAS) as a metabolic oncogene:
extracellular acidosis acts in an epigenetic fashion activating FAS
gene expression in cancer cells. J Cell Biochem. 94:1–4. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Baron A, Migita T, Tang D and Loda M:
Fatty acid synthase: a metabolic oncogene in prostate cancer? J
Cell Biochem. 91:47–53. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Li Y, Xu J, Chen H, et al: Comprehensive
analysis of the functional microRNA-mRNA regulatory network
identifies miRNA signatures associated with hepatoma malignant
progression. Nucleic Acids Res. 41:e2032013. View Article : Google Scholar : PubMed/NCBI
|