Introduction
Carbonic anhydrase (CA) is a ubiquitous enzyme of
fundamental physiological importance. As a catalyst of the
reversible inter-conversion between carbon dioxide and carbonic
acid (i.e., bicarbonate and proton), it facilitates many biological
processes dependent on intensive ion transport, acid-base balance
and biosynthetic reactions. The human body contains 15 different CA
isoforms mostly expressed in differentiated tissues, among which
carbonic anhydrase protein (CA IX) plays a special role as an
active component of the tumour phenotype (1).
CA IX possesses several attributes that support its
relevance for cancer biology: i) it is present in only few normal
tissues, namely in gastrointestinal epithelia (2); ii) it is expressed in a broad
spectrum of tumours, mostly carcinomas (3); iii) its expression is associated with
von Hippel Lindau (VHL) inactivation that occurs in a high
percentage of renal cell carcinomas (RCCs) (4); iv) it is linked to hypoxia and
associated with an aggressive phenotype of various non-RCC tumours
(5); v) it is strongly activated
on the transcriptional level by hypoxia inducible factor (HIF)
(predominantly HIF-1 isoform) via the HRE localized next to the
transcription initiation site (6);
vi) it is induced by hypoxia at the functional level (7,8);
vii) it is involved in signal transduction to the PI3K/Akt pathway
and from the hypoxia-activated protein kinase A (PKA) (8,9);
viii) it plays an active role in tumour biology as a component of
pH-regulating machinery that protects tumour cells from stress
induced by hypoxia and oncogenic metabolism, and thereby
contributes to tumour cell survival and treatment resistance
(10–12); ix) it also facilitates cell
dissociation, adhesion and migration/invasion (13–15);
x) it is exposed on the cell surface with its catalytic domain and
N-terminal proteoglycan region facing the extracellular space and
thus being accessible for ectodomain (ECD)-specific antibodies and
inhibitors of catalytic activity (7,16–18);
xi) it is a highly stable protein that can be shed in a
metalloproteinase-dependent manner (19–21);
and xii) there are several monoclonal antibodies, which are
specific for different domains of CA IX with excellent detection
and anticancer properties, and promising selective inhibitors of CA
IX enzyme activity are also under development (17,22–28).
CA IX exhibits particularly frequent, strong and
diffuse expression in clear cell renal cell carcinomas (ccRCC).
These tumours usually grow from lesion with mutations/deletions of
the VHL tumour suppressor gene that lead to functional inactivation
of the corresponding protein (29). VHL protein (pVHL) is a component of
an E3 ligase complex responsible for the normoxic degradation of
hydroxylated HIF-α subunits and subsequent inhibition of
HIF-mediated responses. Inactivation of pVHL results in a
constitutive activation of the HIF pathway and overexpression of
the HIF targets, including CA IX (4,6,30,31).
However, VHL-defective RCC tumours show a shift from HIF-1α toward
HIF-2α phenotype in the later stages of cancer progression and
therefore expression of CA IX (and other HIF-1 targets) decreases
(32). This is the reason for the
association of decreased CA IX expression with poor prognosis of
RCC patients, although the cut-off value of 85% of positive cells
suggests that the CA IX level may still be high enough to achieve
good therapeutic targeting (33).
Moreover, expression of CA IX can be further increased by treatment
with IL-2 or interferon (IFN)-γ, thus offering a strategy for
enrichment of the target density for the purpose of immunotherapy
(34,35).
The situation is different in non-RCC tumours, which
are not affected by VHL mutations. Here, the level and distribution
of CA IX correlate primarily with the presence of
microenvironmental hypoxia that results in stabilization and
activation of the HIF-α subunits (6). Indeed, CA IX is often detected in
perinecrotic areas and in areas more distant from perfused
vasculature and thus its expression is much more variable (from
focal to diffuse) and heterogeneous (from weak to strong) than in
RCC. This might complicate the immunotherapeutic targeting of such
tumours, although earlier studies in mouse models indicate that
this is not the case (17,36).
G250 monoclonal antibody (MAb) has been raised
against an RCC-associated antigen named G250, which was later
proven to be identical to CA IX (also called MN) (22,37,38).
The chimeric version of the antibody, cG250, functions principally
via antibody-dependent cell-cytotoxicity (ADCC). In Phase II
clinical studies in patients with metastatic RCC it showed
excellent accumulation in RCC, both primary and metastatic, and
increased median survival and overall survival rates (39–42).
The Phase I/II study of the combination therapy of cG250 with low
dose IFN-α indicated that it was safe, well-tolerated and with
clinical benefit for patients with progressive metastatic RCC
(43). The Phase III ARISER study
with cG250 monotherapy as an adjuvant treatment of nephrectomized
ccRCC patients who are at high-risk of disease recurrence, showed
that subjects with a high tumour CA IX score have a significantly
improved disease-free survival (44).
Successful clinical development of cG250 in RCC and
significance of CA IX as an intrinsic component of tumour hypoxia
in a broad spectrum of cancers promoted interest in the possible
application of a similar immunotherapeutic approach in non-RCC
tumours. However, before initiation of clinical studies, it was
important to better characterize the binding properties of cG250 to
CA IX antigen in different physiological conditions, its kinetics
and mode of internalization, fate and integrity of the internalized
antibody, and in vivo therapeutic effects of cG250 in a
non-RCC model. This report summarizes the results of such
characterization, which indicate that the therapeutic targeting of
non-RCC tumours with cG250 is feasible.
Materials and methods
Antibodies, inhibitors and recombinant
fusion proteins
The parental mouse MAb mG250 (IgG2a) and its
human-mouse IgG1 chimeric version cG250 (Rencarex/Girentuximab)
were provided by WILEX AG. The human CA IX PG domain-specific
monoclonal antibodies M75 and IV/18, and CA domain-specific
monoclonal antibodies VII/20, V/12 and V/10 were described
previously (24). The mouse CA
IX-specific MAb AM4 was also described earlier (45). Homosulfanilamide, a CA inhibitor
with an IC50 of 0.1 mM, determined as inhibition of extracellular
acidification mediated by CA IX in cell culture (7), was provided by Professor Claudiu T.
Supuran (University of Florence). GST-CA (GST fused to full-length
human CA IX protein) and GST-Car9 (GST fused to full-length mouse
CA IX protein) were described before (24,45).
Cells
For the experiments described in this report, we
used MDCK canine kidney cells permanently transfected with the
full-length human carbonic anhydrase (CA9) cDNA in the pSG5C
plasmid (MDCK-CA9) or with plasmids derived thereof encoding a CA
domain deletion variant (MDCK-ΔCA), a PG domain deletion variant
(MDCK-ΔPG) and a human alternatively spliced protein truncated in
the C-terminal part of the CA domain (MDCK-hAS). Mock-transfected
cells (MDCK-neo) were used as a negative control. The CGL3
tumourigenic cell line (HeLa x fibroblast hybrid) with high
normoxic expression of CA IX and moderate induction by hypoxia
(20) was kindly provided by
Professor Eric J. Stanbridge (University of California, Irvine).
Human HT-29 colorectal carcinoma cells (ATCC), which express CA IX
at high endogenous level, were used for in vivo experiments.
The cells were routinely cultured in DMEM with 10% FCS
(BioWhittaker, Inc.). Hypoxic treatments were done in a hypoxic
workstation (Ruskinn Technology, Ltd.) in a mixture of gases
containing 2% O2, 5% CO2, 10% H2,
and 83% N2.
ELISA
Microplate wells were coated overnight at 37°C with
the RIPA cell extracts diluted in PBS or 10 ng/well of GST fusion
proteins. After blocking with 10% FCS in PBS, the coated wells were
incubated with 10 μg/ml of mG250 or cG250, depending on the
experimental setting. Peroxidase-labelled pig anti-mouse IgG or
goat anti-human IgG (Sigma) were used as detectors.
ELISA for evaluation of mG250
cross-reactivity to CA I, II and XII
Microplate wells were coated overnight with the
following antigens diluted in PBS: purified CA I (200 ng/well),
purified CA II (100 ng/well) and recombinant CA XII (100 ng/well),
all kindly provided by Professor Seppo Parkkila (University of
Tampere, Finland). Then, the coated wells were incubated with mG250
and with polyclonal sera against CA I, CA II and CA XII (all
1:1,000), respectively, as positive controls. Peroxidase-labelled
pig anti-mouse IgG and pig anti-rabbit IgG diluted 1:5,000 (Sigma)
were used as detectors.
Competitive antibody-binding ELISA
An extract from MDCK-CA9 cells was adsorbed on
microplate wells at a concentration corresponding to 50% of maximal
binding of labelled MAbs. Coated plates were washed and saturated
with 10% FCS in PBS. Serial 2-fold dilutions of purified mouse MAbs
in 25 μl and a constant amount of biotinylated MAb in 25 μl were
added and incubated overnight at 4°C. The plates were washed and
peroxidase-labelled streptavidin (Pierce Biotechnology, Inc.) was
used as a detector.
Capture-detection ELISA
Microplate wells were coated with 50 μl/well of
individual purified mouse MAbs diluted in PBS (200 μg/ml). After
blocking, washing and incubation with the extract of MDCK-CA9 cells
(1:50 in PBS), the set of biotinylated antibodies (5 μg/ml) was
added. Binding of the detector MAbs was determined using
peroxidase-conjugated streptavidin. Results were expressed as
absorbance differences between the wells, in which CA IX antigen
was present or absent.
Competitive binding of mG250 and
inhibitor
CGL3 cells (with natural, hypoxia-induced expression
of CA IX) and MDCK-CA9 cells (with constitutive, ectopic expression
of CA XII) were plated in triplicates to wells of microplates and
allowed to form a confluent monolayer overnight. Then the cells
were transferred to hypoxia (2% O2) for 24 h. The
inhibitor homosulfanilamide was diluted in culture media and added
to the cells in increasing amounts together with a constant amount
of G250 MAb (200 μg/ml based on the saturation experiment) for the
last 6 h-period of the hypoxic incubation. Medium without the
inhibitor was added to the control sample. The cells were fixed
with methanol (5 min at −20°C) and the amount of mG250 MAb bound to
cells was determined by peroxidase-labelled anti-mouse IgG.
Immunoprecipitation
Tested mG250 MAb, M75 (as an anti-PG domain control)
and VII/10 (as an anti-CA domain control) were bound to a 25 μl 50%
suspension of Protein A Sepharose (Pharmacia) for 2 h at RT.
Biotinylated extracts of MDCK-CA9, MDCK-ΔCA, MDCK-ΔPG,
MDCK-hAS, and CGL3 cells (200 μl each), or media from biotinylated
CGL3 cells treated with PMA (activator of shedding) and MDCK-hAS
cells (1,500 μl) were pre-cleared with 20 μl of a 50% suspension of
Protein A Sepharose and then added to bound MAb. Immunocomplexes
collected on Protein A Sepharose were washed, boiled 5 min in
Laemmli loading buffer and separated by SDS-PAGE on a 10% gel.
Afterwards, the proteins were transferred onto a PVDF membrane and
detected with peroxidase-conjugated streptavidin (1:1,000; Pierce
Biotechnology, Inc.) followed by ECL.
Protein-A binding analysis by flow
cytometry
HT-29 cells were incubated with 100 μg/ml mG250
antibody for 1 h at 37°C to recruit the maximum of MAb to CA IX at
the cell surface. Subsequently, the cells were washed to remove
unbound antibody and left in fresh medium at 37°C for the
internalization of bound mG250 for different periods of time (0, 3,
24, 48 and 72 h). At the end of the internalization period, Fc
portions of the antibody remaining/recycled on/to the cell surface
were detected by Protein A conjugated with Alexa Fluor 488
(Invitrogen Life Technologies) diluted 1:100 for 2 h at 4°C to
prevent continued internalization. Data acquisition and analysis
were performed on a Guava flow cytometer using CytoSoft
software.
Immunofluorescence internalization
assay
Cells plated on sterile glass coverslips 24 h before
the experiment were incubated with 10 μg/ml cG250 antibody for 30
min at 4°C to recruit the MAb to CA IX at the cell surface.
Subsequently, the cells were washed to remove any unbound antibody
and transferred to 37°C for various time intervals to allow for the
internalization of CA IX-bound G250. Alternatively, the MAb was
left in the medium throughout the experiment. At the end of the
internalization period, the cells were washed and fixed in ice-cold
methanol at −20°C for 5 min and sequentially treated for 1 h at
37°C with anti-human Alexa-conjugated antibody (Invitrogen Life
Technologies) diluted 1:1,000 to detect internalized cG250.
Finally, the cells were thoroughly washed, mounted onto slides in
the Fluorescent Mounting Medium (Calbiochem), analysed with a Leica
DM4500 B microscope and photographed with a Leica DFC480 camera or
by a confocal laser-scanning microscope Zeiss LSM 510 Meta.
Animal experiments
The therapeutic effect of MAb G250 was investigated
in nude mice with tumour xenografts generated from HT-29 colorectal
carcinoma cells. Animal handling was approved by the Slovak
Veterinary Administration in accordance with EU regulations. For
the immunotherapy experiment, 3 × 105 HT-29 cells in 200
μl of PBS were grafted subcutaneously to the mouse back.
Immediately (within 1 h) after tumour cell grafting, one group of
mice received intravenous injections of 100 μg/dose of mG250 in PBS
and the other two groups received PBS without the MAb. All
subsequent injections were given into the tail vein twice a week
throughout the tumour growth (39 days). To get closer to the
situation in humans, where treatment is initiated only after
detection of the growing tumour, a delayed mG250 treatment was
started in the second group from day 10 after xenografting when the
first tumours became palpable. Then the regimen of the treatment
was the same as for the first group of animals. The third, control
group received only PBS injections at the same time points as the
antibody-treated animals. Tumour diameters were regularly measured
and recorded. On the day of sacrifice, tumours were dissected,
weighed and processed for immunohistochemistry. Serum samples were
also collected from all groups to detect the CA IX ECD.
ELISA detection of shed human CA IX ECD
in mouse serum samples
The capture antibody VII/38 (10 mg/ml, 100 ml/well)
was immobilized on the surface of microplate wells overnight at
4°C. After blocking and washing, mouse serum samples were diluted
1:2 in PBS and added to the coated wells (100 ml/well) for
overnight binding at 4°C. The attached antigen was then allowed to
react with biotinylated M75 MAb diluted 1:5,000 (200 ng/ml) in PBS.
The amount of bound detector antibody was determined after 1 h
incubation with the peroxidase-conjugated streptavidin (Pierce
Biotechnology, Inc.) using the peroxidase substrate ortho-phenylene
diamine (Sigma).
Results
Binding of cG250 MAb to CA IX deletion
variants
Earlier studies showed that G250 MAb interacts with
the native CA IX protein, but its domain specificity has remained
unknown (22). Thus, we first
analysed the binding of G250 MAb to CA IX variants with deletions
of various parts of the molecule. ELISA with these CA IX-related
antigens revealed that G250 MAb (both mouse and chimeric versions)
bound only to those variants that contained an intact CA domain,
including GST-CA and extracts from MDCK-CA9 and MDCK-ΔPG cells
(Fig. 1A). Accordingly, mG250 MAb
was capable of immunoprecipitating only the full-length IX CA
protein and its ΔPG variant (Fig.
1B), but not the ΔCA variant lacking the catalytic domain, as
was shown previously for other CA domain-specific MAbs (24). A competitive antibody-binding ELISA
was then performed to analyse mutual relationships among these MAbs
and derive the position of mG250 on the epitope map of the CA IX
antigen. Only the CA domain-specific antibodies VII/20, V/12 and
V/10 were able to compete for the binding to CA IX with labelled
mG250 to an extent similar to the homologous competition by
non-labelled mG250 (Fig. 1C). A
capture-detection assay corroborated the relationships between the
antibodies with minor differences attributable to the different
arrangement of the assay (Fig.
1D).
 | Figure 1G250 monoclonal antibody (MAb)
binding properties - domain specificity. (A) The target domain of
the parental murine mG250 and chimeric cG250 MAbs was determined by
ELISA using the following antigens: a recombinant fusion protein
composed of the catalytic domain of carbonic anhydrase protein (CA
IX) (aa 191–397) fused to GST (GST-CA) and GST alone, extract of
transfected MDCK cells expressing the full-length CA IX (MDCK-CA9),
or the deletion variant lacking the PG domain (MDCK-ΔPG), or the
deletion variant lacking the carbonic anhydrase (CA) domain
(MDCK-ΔCA). (B) The CA domain specificity of G250 MAb was confirmed
by immunoprecipitation of the full-length human carbonic anhydrase
gene (CA9) and its deletion variants from biotinylated cell
extracts with G250 MAb as well as with M75 and V/10 MAbs as
controls as described in Materials and methods. (C) The competitive
ELISA was performed with the biotin-labeled G250 competing for
binding to CA IX antigen from MDCK-CA9 cell extract against
increasing concentrations of the simultaneously added non-labeled
CA IX-specific antibodies characterized previously by Zatovicova
et al, 2003, including the antibodies M75 and 18 specific
for the PG domain and the antibodies 10, 12, 20, 28, 32 and 38
specific for the CA domain. The extent of competition was expressed
in % relative to the G250 binding in the absence of competitor. (D
and E) The relative position of the antigenic site of G250 among
the other CA IX-specific monoclonal antibodies was schematically
illustrated on Chequer-board maps of antigenic sites delineated on
the basis of (D) the competitive binding ELISA and (E)
capture-detection ELISA described in Materials and methods. Results
are expressed (D) as percentage of absorbance measured in the
absence of competitor antibody or (E) as absorbance resulting from
the cooperative binding of capture-detector antibodies, which
indicates spatial separation between the epitopes of the paired
antibodies. (F) Schematic illustration of relative positions of the
binding sites for the monoclonal antibodies deduced on the basis of
their domain specificity and mutual binding relationships. Scissors
indicate the region of the ectodomain (ECD) cleavage. |
Binding of cG250 MAb to naturally
occurring CA IX variants, orthologs and other CA isoforms
CA IX protein exists in three naturally occurring
forms, namely the full-length protein, the ECD shed to
extracellular space and the alternatively spliced (AS) variant that
is partially intracellular and partially secreted (20,21,46).
The CA IX ECD can be detected in body fluids of cancer patients and
appears to have a prognostic/monitoring value (47–53).
On the other hand, the truncated AS variant is produced at a low
level independently of hypoxia and tumour phenotype and for
immunotherapy its interaction with CA IX antibodies is not
desirable due to possible interference with the binding of the
clinically relevant CA IX molecules (46). Indeed, ELISA and
immunoprecipitation of CA IX variants from extracts of transfected
cells or natural CA IX expressors demonstrated that mG250 MAb
recognizes only the full-length CA IX protein and its extracellular
domain shed to medium, but not the AS variant lacking the
C-terminal part of the catalytic domain (Fig. 2A and B).
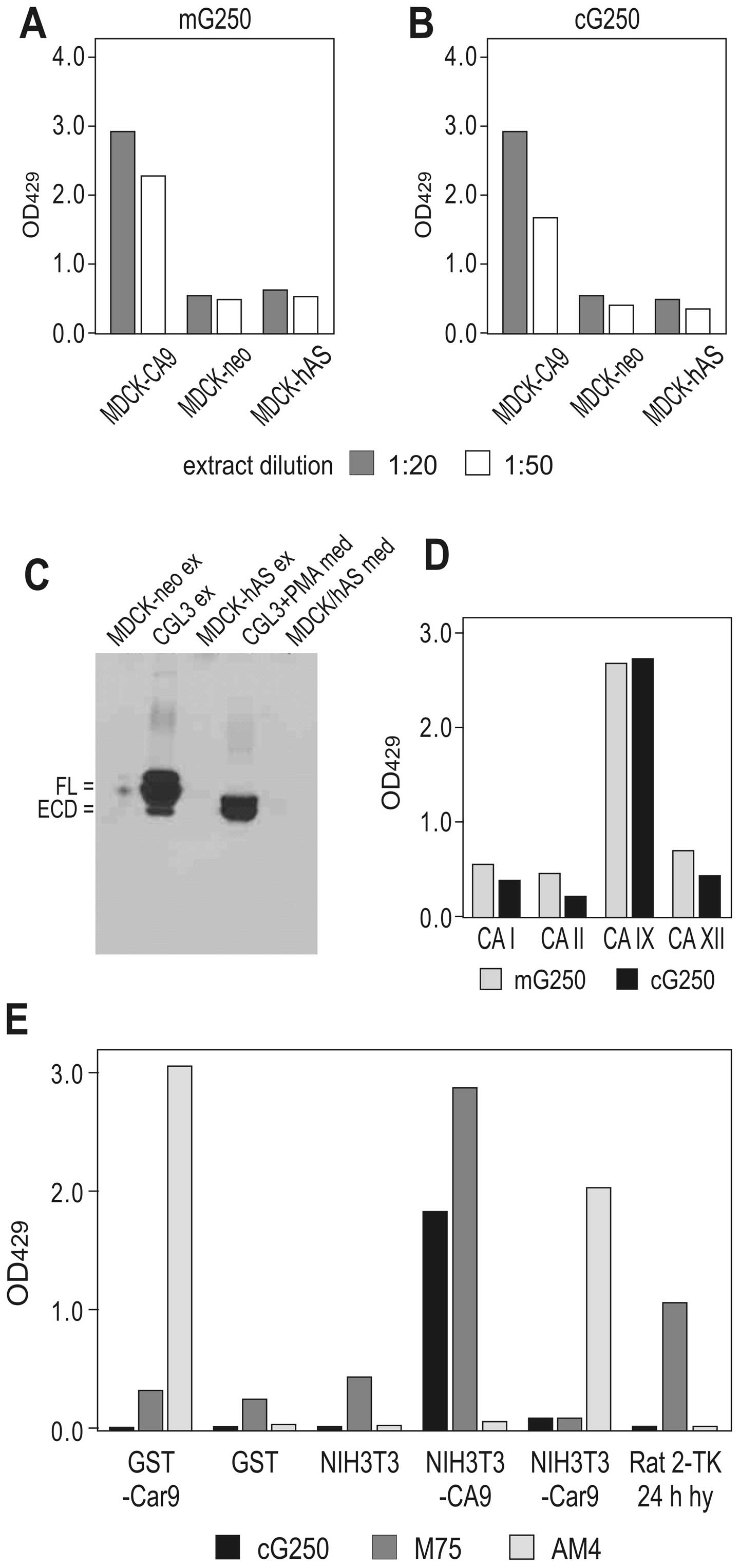 | Figure 2G250 monoclonal antibody (MAb)
binding properties - antigen/isoform specificity. (A and B) Binding
of the G250 MAb (its mouse and chimeric versions) to the
full-length carbonic anhydrase protein (CA ) protein versus the
alternatively spliced variant was determined by ELISA using the
extracts of MDCK cells transfected with the full-length human
carbonic anhydrase gene (CA9) cDNA (MDCK-CA9), its splicing variant
lacking the exons 8 and 9 (MDCK-hAS), and mock-transfected control
(MDCK-neo). (C) Ability of mG250 MAb to recognize the full-length
CA (FL) and its ectodomain (ECD), but not the alternatively spliced
variant (hAS) was demonstrated by immunoprecipitation using the
extracts and media from CGL3 cells naturally expressing CA and from
the transfected MDCK cells (MDCK-hAS), followed by the western
blotting with the M75 MAb. (D) CA isoform specificity of the mG250
and cG250 MAbs was proven by ELISA using purified antigens of the
CA I, II, IX and XII isoenzymes as described in Materials and
methods. (E) cG250 specificity for the human CA was shown by ELISA
using the mouse CA fused to GST (GST-Car9), extracts of NIH3T3
cells transfected either with the human CA9 cDNA or with the mouse
rodent carbonic anhydrase gene (Car9) cDNA, and hypoxic Rat
2-TK-cells naturally expressing rat CA. M75 MAb, which recognizes
both human and rat CA IX, and MAb AM4, which recognizes mouse CA
IX, were used for control. |
Since G250 binds to the CA domain, we analyzed
whether it can also recognize related CA isoforms (particularly
those that could be co-expressed with CA IX in tumour cells). We
used ELISA to test the binding of G250 MAb (both mouse and chimeric
versions) to purified CA I, CA II, and CA XII antigens, and found
that G250 did not bind any of the CA isoforms tested (Fig. 2C). Moreover, neither mouse nor rat
CA IX orthologs interacted with G250 suggesting that it
specifically reacts with the human CA IX antigen (Fig. 2D). Therefore, it can be
investigated in animal models with human tumour xenografts, but not
with natural, induced, syngeneic tumours or mouse/rat
xenografts.
Binding of cG250 MAb to CA IX in presence
of CA inhibitor
The CA domain of CA IX is a globular structure
containing the enzyme active site that is located in a large
conical cavity with the catalytic zinc at the bottom (54). This active site can accommodate
different types of carbonic anhydrase inhibitors (CAIs). Since CA
IX activity plays an important role in tumour biology, these
inhibitors have been investigated as clinically promising tools for
detection and therapeutic targeting of hypoxia-activated CA IX
(11,18,55).
We previously showed that the FITC-conjugated homosulfanilamide
inhibitor (FITC-CAI) binds to CA IX expressed in hypoxic cells
(7). Here we examined whether
interaction of cG250 MAb to the catalytic domain of CA IX is
affected by FITC-CAI binding to the active site. To this end we
used a competitive ELISA on monolayers of hypoxic cells, namely
CGL3 cells with natural CA IX expression and MDCK-CA9 cells with
ectopic CA IX expression. FITC-CAI was added in increasing amounts
together with a constant amount of cG250 MAb (200 μg/ml based on
the saturation experiment, data not shown). CAI did not block the
internalization of cG250 either (Fig.
3C). Moreover, in both cell lines, FITC-CAI did not reduce
cG250 MAb binding (Fig. 3A and B),
indicating that cG250 does not bind in or close to the active site,
but rather interacts with the backbone of the catalytic domain.
This suggests that CA IX-expressing hypoxic tumours can be
potentially subjected to a combination treatment with cG250 and
CAI.
Internalization of cG250: co-localization
with CA IX, the effect of cell density and hypoxia
Binding of cG250 MAb to CA IX was shown to trigger
receptor-mediated internalization, a process that has particular
impact on the outcome of immunotherapy targeted to cancer-related
antigens (56). Since we wanted to
learn more about the temporal and mechanistic aspects of cG250
internalization, we followed the localization and fate of the
internalized antibody for different time periods and under
different conditions.
To analyze whether cG250 co-localizes with CA IX
during the internalization path, the MAb was first recruited to the
cell surface CA IX at 4°C, then unbound antibody was washed away,
internalization was initiated at 37°C and allowed to proceed for 3,
6, 24 and 48 h. In all internalization periods, staining signals of
cG250 antibody and CA IX antigen overlapped (Fig. 4A) suggesting that cG250 antibody
remains associated with the antigen. Interestingly, intracellular
staining of both cG250 and CA IX prevailed up till 24 h of
internalization, whereas samples incubated for 48 h showed the
membrane staining, apparently due to recycling of the cG250-CA IX
complex back to the cell surface. Moreover, the analysis of cG250
internalization under hypoxia (2% O2) and in acidic
extracellular pH revealed that cG250 enters the intracellular space
independently of these physiological conditions and confirms that
it also remains associated with CA IX inside the cell (Fig. 4B). Interestingly, cG250 antibody
remained associated with the cells at least for 3 days, even when
the cells were split and re-plated (data not shown).
The distribution of immunotherapeutic antibodies in
solid tumours reflects different physiological barriers including
high cell density (57). We
therefore examined internalization of cG250 in a highly packed cell
monolayer under normoxia and hypoxia, respectively, using MDCK-CA9
cells with the constitutive expression of CA IX and CGL3 cells with
high endogenous expression of CA IX. We found that cG250 MAb was
unable to internalize into dense cells under normoxia, whereas
hypoxia facilitated internalization presumably due to release of
tight cell-cell contacts (Fig.
4C). This suggests that CA IX present on the surface of hypoxic
and/or HIF-activated cancer cells can mediate antibody-induced
internalization despite high local cell density.
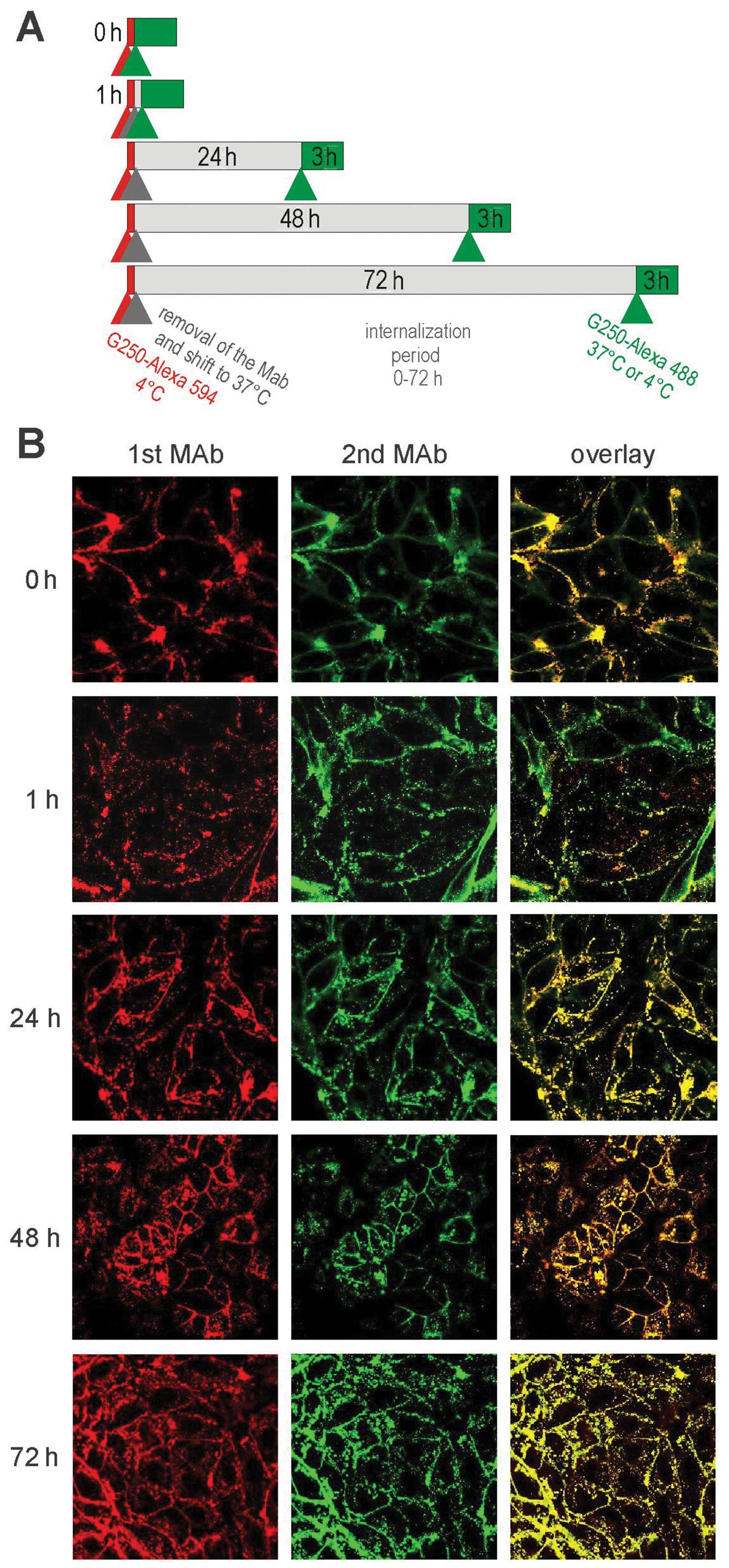
Consecutive internalization cycles of
cG250 MAb
We then investigated whether cG250 MAb is able to
undergo consecutive internalization cycles via newly produced
and/or unoccupied CA IX antigen present on the cell surface. The
experimental scheme utilizing consequent green- and red-labelled
G250 MAbs was set as shown in Fig.
5A. Confocal microscopic analysis revealed that both antibodies
bound to CA IX and internalized irrespective of the time interval
between their addition to cells (see Fig. 5). Interestingly, there were no big
differences in the subcellular localization of the 1st versus 2nd
antibody, and both antibodies showed considerable co-localization.
A slight difference could be seen in the 1-h sample, where the red
signal seemed to dominate in the intracellular space, while the
green signal prevailed on the cell surface. In the samples with 24
and 72 h internalization period, both red and green signals
overlapped, while a slight red intracellular staining was visible
again in the sample with the 48 h internalization period. These
very subtle differences might reflect partly asynchronous movement
(e.g., time-shift in internalization-recycling) of the red and
green MAbs in the cells.
cG250 internalization pathway, recycling
and integrity of Fc fragment
To gain insight into the fate of internalized cG250
we used several inhibitors and markers of the internalization
pathways. We could see that the pattern of cG250 internalization is
very similar to transferrin (TR) a molecule known to enter cells in
a clathrin-dependent manner (Fig.
6A). Consistent with this, nystatin, a cholesterol-aggregating
inhibitor of clathrin-independent endocytosis, did not have any
effect on cG250 internalization (data not shown). Moreover, no
major differences in cG250 internalization patterns were observed
following treatment with the lysosomotropic agent concanavalin A
(data not shown). Indeed, cG250 MAb showed no or only a minor
overlap with LysoTracker Red lysosomal marker in support of the
idea that the major part of the antibody escapes lysosomal
degradation and enters the recycling pathway (Fig. 6B). Finally, we performed
internalization of cG250 in the presence of nocodazole, a
microtubule-disrupting agent, which reduces the transit of
internalized molecules to the perinuclear compartment and thereby
inhibits their recycling. This led to loss of perinuclear
localization of cG250, diminished recycling to the plasma membrane
and to its diffuse distribution throughout the entire cytoplasm
(Fig. 6C).
 | Figure 6Internalization path of G250. (A)
CGL3 cells were incubated at 37°C in medium containing G250
monoclonal antibody (MAb) and Alexa 568-labeled transferrin (TR).
Membrane-bound TR and mG250 were acid-stripped either immediately
before fixation (at 30 min and 1 h intervals) or after 3 h (for 24
h interval) and the rest of the incubation was done with
TR/G250-free medium. Then the cells were fixed, treated with Alexa
488 anti-mouse IgG to detect mG250, washed, and analyzed by
confocal microscopy. (B) MDCK-CA9 cells were incubated with the
FITC-conjugated mG250 MAb for 30 min at 4°C, then were transferred
to 37°C for 3 h and 75 nM LysoTracker Red was added to the medium
with mG250-FITC MAb 30 min before the end of the experiment.
Finally, the cells were washed and immediately subjected to
confocal analysis. (C) MDCK-CA9 cells were treated first with 5 μM
nocodazole for 1 h, then 3 μg/ml of the mG250 MAb was allowed to
bind to cells for 30 min at 4°C, the cells were transferred to 37°C
for 3 h to permit internalization. Unbound antibody was washed and
cell surface-bound antibody was acid-stripped, the fresh medium
(with or without nocodazole) was added to one pair of dishes to
continue the intracellular processing of the mG250 MAb for
additional 69 h. At the end of the internalization period, cells
were washed, fixed, incubated with Alexa 488 anti-mouse IgG, washed
again and analyzed by confocal microscopy. |
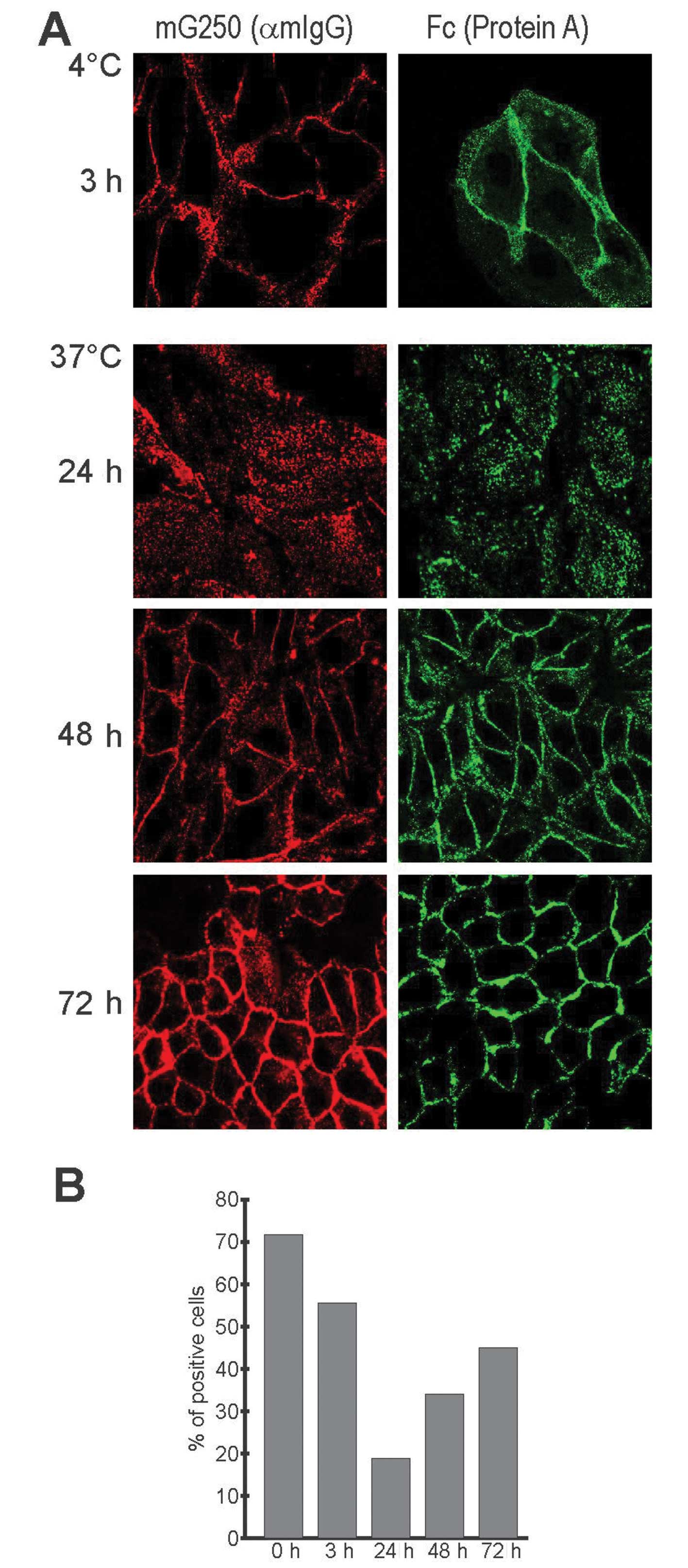
We further evaluated the internalized/recycled cG250
MAb with respect to the integrity of its Fc portion, which mediates
ADCC response, i.e., directs cytotoxic activities of effector cells
against the target cell. We first examined the capacity of cG250 to
bind Protein A in immunofluorescence analysis, which showed that
the antibody can bind Protein A at each point of the
internalization cycle, i.e., initially on the cell surface, then
inside the cells and finally on the cell surface again (Fig. 7A). Flow cytometric analysis of
HT-29 cells that highly express CA IX revealed that the percentage
of Protein A-positive cells (in which Protein A was bound to cG250
MAb attached to CA IX at the surface of living cells) decreased
from initial 72 to 55% after 3 h internalization and to 18% after
24 h (Fig. 7B). This decline
agrees with the strength of the intracellular signal observed in
immunofluorescence and suggests a reduction of the antibody on the
cell surface as a consequence of internalization. On the other
hand, gradual elevation of the Protein A-binding cells to 31% after
48 h and 45% after 72 h suggests that the antibody recycled back to
the plasma membrane, although it did not reach the initial level
(Fig. 7B). This reduction could be
due either to partial degradation of the internalized antibody or,
more conceivably, to cell division during the long internalization
periods that resulted in the relative dilution of the staining
signal. Undoubtedly, these results confirm that the recycled
antibody possesses an intact Fc portion.
In vivo anti-tumour effects of G250 in a
non-RCC xenograft model
In order to learn whether G250-based immunotherapy
can be useful in tumours other than RCC, we investigated its
anticancer effect against HT-29 colorectal carcinoma xenografts
implanted in nude mice. This model was chosen because HT-29 cells
display high expression of CA IX even under normoxia. Although
hypoxic induction of CA IX in monolayer culture of HT-29 cells is
relatively low, its distribution in xenografts displays a typical
hypoxic pattern with high perinecrotic expression of CA IX and low
or no expression around vessels as demonstrated earlier (36).
The xenografted animals were subjected to immediate
as well as to delayed treatment with mG250 as described above in
Materials and methods. Both groups showed a significant reduction
of tumour volume and tumour weight when compared to control,
placebo-treated animals suggesting that mG250 is capable of
eliciting not only a protective, but also a real therapeutic
anti-cancer effect in a setting similar to the treatment of cancer
patients (Fig. 8A and B).
We also wanted to find out whether the presence of
the HT-29 tumour is reflected in serum levels of the CA IX ECD. For
this purpose, we adopted a capture-detection ELISA using a
combination of the CA domain-specific VII/38 MAb and the PG
domain-specific M75 MAb. The analysis revealed detectable serum
levels of the CA IX ECD in tumour-bearing animals (Fig. 8C). Although levels of CA IX ECD did
not fully correlate with the weights of tumours (conceivably due to
differences in tumour tissue heterogeneity, extent of hypoxia and
intratumoural levels of CA IX) these data principally support the
monitoring potential of CA IX serum levels.
Discussion
In this study, we describe novel data that clarify
the binding characteristics and biological properties of the
therapeutic MAb cG250 (INN: Girentuximab) and provide the
experimental evidence supporting its potential usefulness in
immunotherapy of tumours other than RCC.
We show here that the G250 MAb possesses several
attributes favourable to its clinical application in targeting of
the CA IX antigen, which has a unique tumour-associated expression
pattern and biological relevance for the tumour phenotype. These
attributes include i) specific recognition of the human CA IX
protein, but not the other CA isoforms; ii) binding to the
catalytic domain without interfering with its small molecule active
site inhibitors; and iii) no binding to the alternatively spliced
variant of CA IX. Thus, our data prove the high CA IX antigen
specificity and selectivity of the G250 MAb, which is in line with
the observations of excellent antibody tolerability and safety in
RCC patients included in G250 immunotherapy-based clinical trials
(23). The data also suggest that
G250-mediated immunotherapy can be potentially combined with the
therapy based on inhibition of CA IX activity. The same conclusion
can be applied to the use of G250 in CA IX-related imaging of
primary and metastatic tumour lesions.
Moreover, G250 can induce receptor-mediated
internalization, as also described earlier (56). However, we found that the CA
IX-mediated internalization induced by G250 can proceed in
consecutive cycles and that in contrast to other endocytosed
ligand-receptor or antibody-antigen complexes, G250-CA IX complex
has an exceptionally long intracellular persistence of 48–72 h.
Throughout this period, antibody-antigen interaction seems to
remain undisturbed and the complex can then recycle back to the
cell surface in its principally intact form with the preserved Fc
part of the G250 MAb. These findings are important for the
understanding of the therapeutic efficacy of the antibody. Firstly,
the consecutive internalization can contribute to better
utilization of the therapeutic antibody, which does not immediately
bind to the antigen and remains in its free form in the
pericellular space, or which arrives later from the circulation.
Secondly, long intracellular persistence can potentially modulate
the intracellular signalling of CA IX, since it is known that
regulatory receptors can either extend or cease their signalling
from endosomes (58). At present
we do not have enough data to support or exclude this assumption.
Third, recycling and cell surface exposure of the G250 antibody
with the intact Fc fragment can allow for prolonged ADCC response,
which represents its principal anticancer mode of action (59,60).
Recycling of intact G250 can also explain its long-lasting effects
in patients (61).
The Phase III clinical trial ‘ARISER’ conducted in
patients with non-metastatic ccRCC showed significant benefit of
prolonged disease-free survival of ~22 month in patients with high
CA IX score in the resected primary tumours compared to patients in
the placebo arm (44). Thus, the
situation in G250-mediated immunotherapy of RCC seems promising in
patients with tumours with high CA IX score. Frequent and strong
expression of CA IX is observed in a high percentage of tumour
cells in RCC tissues (22,33). But, can we expect similar or any
anticancer effect in patients with other tumour types? There, CA IX
expression is heterogeneous (with respect to both loco-regional
distribution and cellular expression levels) owing to its principal
link with hypoxia (5).
Importantly, more frequent and intense expression is seen in more
hypoxic and aggressive tumours. According to the generally accepted
view, hypoxic tumour areas are poorly accessible by antibodies and
drugs, because of their greater diffusion distance from the
functional blood vessels as well as to the increased cell density
(57). However, CA IX expression
is not limited to perinecrotic regions, but rather extends toward
less distant areas with less severe hypoxia. The median distance
between a blood vessel and the beginning of CA IX expression was 80
μm (range, 40–140) in head and neck carcinoma and bladder carcinoma
(62,63) and ~90 μm in non-small-cell lung
carcinoma (64). Thus, CA IX is
found between the borders of HIF-1α zone and a zone of EF5 chemical
marker of hypoxia suggesting that CA IX induction requires lower
oxygen levels than HIF-1α, but higher than EF5 (or pimonidazole).
These intermediate, moderately hypoxic tissue areas are known to
contain viable cells that are adaptable to hypoxic stress,
resistant to conventional therapy, and represent the principal
source of metastatic precursors (65). Indeed, it was demonstrated that the
cells expressing CA IX belong to a broader perinecrotic area and
are viable, clonogenic and resistant to killing by ionizing
radiation (66). Therefore, it
appears that the G250 antibody does not need to diffuse to the most
remote distances from the irregular but leaky tumour blood vessels
to reach its target. Moreover, hypoxia is known to facilitate
metastasis through the promotion of epithelial-mesenchymal
transition, which involves initial destabilization of cell-cell
contacts via modulated expression of cell adhesion molecules and
extracellular matrix-degrading proteases (67). This can also contribute to lower
cell density and reduced matrix stiffness, and hence to better
penetration of the antibody across the tumour tissue as well as to
its improved endocytosis. Indeed, earlier studies demonstrated that
other CA IX-specific antibodies, namely the PG domain-binding MAb
M75 as well as the CA domain-binding MAb VII/20, exhibit excellent
tumour uptake and therapeutic efficacy, respectively, in the mouse
models with non-RCC tumour xenografts containing hypoxic regions
(17,36). Similarly, the present study showed
a considerable anticancer effect of G250 MAb in the non-RCC
setting.
Taking all these circumstances together it is
imaginable that the targeting of the intermediate (less distant),
CA IX-positive, moderately hypoxic tumour cell subpopulation by
cG250-mediated immunotherapy could eliminate the most aggressive
and dangerous components of tumour tissue and potentially prevent,
reduce, or delay the onset of the metastatic process, which is the
main cause of death from cancer. Although additional extensive
experimentation is needed to support this proposal, the recent
study offers the rationale and substantiates the direction of the
research towards the application of cG250 in immunotherapy of
non-RCC cancer.
To conclude, we showed that G250 MAb (Girentuximab)
that is primarily evaluated as an immunotherapy for kidney cancer,
recognizes the catalytic domain of the hypoxia-induced CA IX
protein. We found that the antibody G250 internalizes via
clathrin-coated vesicles and recycles to cell surface via
perinuclear compartment. This leads to long intracellular
persistence and consecutive internalization cycles. The recycled
antibody maintains intact Fc portion potentially capable of
continuous induction of ADCC response and high therapeutic
efficacy. Finally, we showed that G250-mediated immunotherapy is
effective against HT-29 colorectal carcinoma xenografts that
display heterogeneous, hypoxia-related expression of CA IX. These
results support potential therapeutic usefulness of the G250 MAb in
hypoxic tumours other than RCCs.
Acknowledgements
This study was supported by WILEX AG, and by the
Slovak Scientific Grant Agency VEGA (2/0134/12 and 2/0081/14).
There are the following potential conflicts of interest: i) the
authors J. Pastorek, S. Pastorekova, and M. Zatovicova are
inventors of patents related to CA IX; and ii) the study was partly
funded by WILEX AG company, reputation of which may be affected by
the publication of the study and the authors W. Schmalix, V.
Boettger, P. Bevan are employees of that company.
Abbreviations:
|
ADCC
|
antibody-dependent cell-mediated cyto
toxicity
|
|
AS
|
alternative splicing
|
|
CA IX
|
carbonic anhydrase protein
|
|
CA9
|
human carbonic anhydrase gene
|
|
Car9
|
rodent carbonic anhydrase gene
|
|
CAI
|
carbonic anhydrase inhibitor
|
|
ECD
|
ectodomain
|
|
HIF
|
hypoxia inducible factor
|
|
IL-1
|
interleukin-1
|
|
IFN
|
interferon
|
|
MAb
|
monoclonal antibody
|
|
PKA
|
protein kinase A
|
|
RCC
|
renal cell carcinoma
|
|
VHL
|
von Hippel Lindau
|
References
|
1
|
Pastorekova S, Parkkila S, Pastorek J and
Supuran CT: Carbonic anhydrases: current state of the art,
therapeutic applications and future prospects. J Enzyme Inhib Med
Chem. 19:199–229. 2004.PubMed/NCBI
|
|
2
|
Pastoreková S, Parkkila S, Parkkila AK,
Opavský R, Zelník V, Saarnio J and Pastorek J: Carbonic anhydrase
IX, MN/CA IX: analysis of stomach complementary DNA sequence and
expression in human and rat alimentary tracts. Gastroenterology.
112:398–408. 1997.PubMed/NCBI
|
|
3
|
Pastorekova S, Parkkila S and Zavada J:
Tumor-associated carbonic anhydrases and their clinical
significance. Adv Clin Chem. 42:167–216. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Mandriota SJ, Turner KJ, Davies DR, Murray
PG, Morgan NV, Sowter HM, Wykoff CC, Maher ER, Harris AL, Ratcliffe
PJ and Maxwell PH: HIF activation identifies early lesions in VHL
kidneys: evidence for site-specific tumor suppressor function in
the nephron. Cancer Cell. 1:459–468. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Potter C and Harris AL: Hypoxia inducible
carbonic anhydrase IX, marker of tumour hypoxia, survival pathway
and therapy target. Cell Cycle. 3:164–167. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wykoff CC, Beasley NJ, Watson PH, Turner
KJ, Pastorek J, Sibtain A, Wilson GD, Turley H, Talks KL, Maxwell
PH, et al: Hypoxia-inducible regulation of tumor-associated
carbonic anhydrases. Cancer Res. 60:7075–7083. 2000.PubMed/NCBI
|
|
7
|
Svastová E, Hulíková A, Rafajová M,
Zat’ovicová M, Gibadulinová A, Casini A, Cecchi A, Scozzafava A,
Supuran CT, Pastorek J and Pastoreková S: Hypoxia activates the
capacity of tumour-associated carbonic anhydrase IX to acidify
extracellular pH. FEBS Lett. 577:439–445. 2004.PubMed/NCBI
|
|
8
|
Ditte P, Dequiedt F, Svastova E, Hulikova
A, Ohradanova-Repic A, Zatovicova M, Csaderova L, Kopacek J,
Supuran CT, Pastorekova S and Pastorek J: Phosphorylation of
carbonic anhydrase IX controls its ability to mediate extracellular
acidification in hypoxic tumors. Cancer Res. 71:7558–7567. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Dorai T, Sawczuk IS, Pastorek J, Wiernik
PH and Dutcher JP: The role of carbonic anhydrase IX overexpression
in kidney cancer. Eur J Cancer. 41:2935–2947. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Chiche J, Ilc K, Laferrière J, Trottier E,
Dayan F, Mazure NM, Brahimi-Horn MC and Pouysségur J:
Hypoxia-inducible carbonic anhydrase IX and XII promote tumour cell
growth by counteracting acidosis through the regulation of the
intracellular pH. Cancer Res. 69:358–368. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Dubois L, Peeters S, Lieuwes NG, Geusens
N, Thiry A, Wigfield S, Carta F, McIntyre A, Scozzafava A, Dogné
JM, et al: Specific inhibition of carbonic anhydrase IX activity
enhances the in vivo therapeutic effect of tumour irradiation.
Radiother Oncol. 99:424–431. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
McIntyre A, Patiar S, Wigfield S, Li JL,
Ledaki I, Turley H, Leek R, Snell C, Gatter K, Sly WS, et al:
Carbonic anhydrase IX promotes tumour growth and necrosis in vivo
and inhibition enhances anti-VEGF therapy. Clin Cancer Res.
18:3100–3111. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Svastová E, Zilka N, Zat’ovicová M,
Gibadulinová A, Ciampor F, Pastorek J and Pastoreková S: Carbonic
anhydrase reduces E-cadherin-mediated adhesion of MDCK cells via
interaction with beta-catenin. Exp Cell Res. 290:332–345.
2003.PubMed/NCBI
|
|
14
|
Svastova E, Witarski W, Csaderova L, Kosik
I, Skvarkova L, Hulikova A, Zatovicova M, Barathova M, Kopacek J,
Pastorek J and Pastorekova S: Carbonic anhydrase IX interacts with
bicarbonate transporters in lamellipodia and increases cell
migration via its catalytic domain. J Biol Chem. 287:3392–3402.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Csaderova L, Debreova M, Radvak P, Stano
M, Vrestiakova M, Kopacek J, Pastorekova S and Svastova E: The
effect of carbonic anhydrase IX on focal contacts during cell
spreading and migration. Front Physiol. 4:2712013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Závada J, Závadová Z, Pastoreková S,
Ciampor F, Pastorek J and Zelník V: Expression of MaTu-MN protein
in human tumor cultures and in clinical specimens. Int J Cancer.
54:268–274. 1993.PubMed/NCBI
|
|
17
|
Zatovicova M, Jelenska L, Hulikova A,
Csaderova L, Ditte Z, Ditte P, Goliasova T, Pastorek J and
Pastorekova S: Carbonic anhydrase IX as an anticancer therapy
target: preclinical evaluation of internalizing monoclonal antibody
directed to catalytic domain. Curr Pharm Des. 16:3255–3263. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lou Y, McDonald PC, Oloumi A, Chia S,
Ostlund C, Ahmadi A, Kyle A, Auf dem Keller U, Leung S, Huntsman D,
et al: Targeting tumor hypoxia: suppression of breast tumour growth
and metastasis by novel carbonic anhydrase IX inhibitors. Cancer
Res. 71:3364–3376. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Rafajová M, Zatovicová M, Kettmann R,
Pastorek J and Pastoreková S: Induction by hypoxia combined with
low glucose or low bicarbonate and high posttranslational stability
upon reoxygenation contribute to carbonic anhydrase IX expression
in cancer cells. Int J Oncol. 24:995–1004. 2004.PubMed/NCBI
|
|
20
|
Zatovicova M, Sedlakova O, Svastova E,
Ohradanova A, Ciampor F, Arribas J, Pastorek J and Pastorekova S:
Ectodomain shedding of the hypoxia-induced carbonic anhydrase IX is
a metalloprotease-dependent process regulated by TACE/ADAM17. Br J
Cancer. 93:1267–1276. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zatovičová M and Pastorekova S: Modulation
of cell surface density of carbonic anhydrase IX by shedding of the
ectodomain and endocytosis. Acta Virol. 57:257–264. 2013.PubMed/NCBI
|
|
22
|
Oosterwijk E, Ruiter DJ, Hoedemaeker PJ,
Pauwels EK, Jonas U, Zwartendijk J and Warnaar SO: Monoclonal
antibody G 250 recognizes a determinant present in renal-cell
carcinoma and absent from normal kidney. Int J Cancer. 38:489–494.
1986. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Oosterwijk-Wakka JC, Boerman OC, Mulders
PF and Oosterwijk E: Application of monoclonal antibody G250
recognizing carbonic anhydrase IX in renal cell carcinoma. Int J
Mol Sci. 14:11402–11423. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zat’ovicová M, Tarábková K, Svastová E,
Gibadulinová A, Mucha V, Jakubícková L, Biesová Z, Rafajová M,
Ortova Gut M, Parkkila S, et al: Monoclonal antibodies generated in
carbonic anhydrase IX-deficient mice recognize different domains of
tumour-associated hypoxia-induced carbonic anhydrase IX. J Immunol
Methods. 282:117–134. 2003.
|
|
25
|
Pastorek J and Pastorekova S: Molecular
mechanisms regulating expression and function of cancer-associated
anhydrase IX. The Tumour Microenvironment. Bagley RG: Springer New
York: Humana Press, NY; pp. 59–90. 2010, View Article : Google Scholar
|
|
26
|
Xu C, Lo A, Yammanuru A, Tallarico AS,
Brady K, Murakami A, Barteneva N, Zhu Q and Marasco WA: Unique
biological properties of catalytic domain directed human anti-CAIX
antibodies discovered through phage-display technology. PLoS One.
5:e96252010. View Article : Google Scholar
|
|
27
|
Murri-Plesko MT, Hulikova A, Oosterwijk E,
Scott AM, Zortea A, Harris AL, Ritter G, Old L, Bauer S, Swietach P
and Renner C: Antibody inhibiting enzymatic activity of
tumour-associated carbonic anhydrase isoform IX. Eur J Pharmacol.
657:173–183. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Supuran CT: Carbonic anhydrases: novel
therapeutic applications for inhibitors and activators. Nat Rev
Drug Discov. 7:168–181. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Gnarra JR, Tory K, Weng Y, Schmidt L, Wei
MH, Li H, Latif F, Liu S, Chen F, Duh FM, et al: Mutations of the
VHL tumour suppressor gene in renal carcinoma. Nat Genet. 7:85–90.
1994. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ivanov SV, Kuzmin I, Wei MH, Pack S, Geil
L, Johnson BE, Stanbridge EJ and Lerman MI: Down-regulation of
transmembrane carbonic anhydrases in renal cell carcinoma cell
lines by wild-type von Hippel-Lindau transgenes. Proc Natl Acad Sci
USA. 95:12596–12601. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Wiesener MS, Münchenhagen PM, Berger I,
Morgan NV, Roigas J, Schwiertz A, Jürgensen JS, Gruber G, Maxwell
PH, Löning SA, et al: Constitutive activation of hypoxia-inducible
genes related to overexpression of hypoxia-inducible factor-1alpha
in clear cell renal carcinomas. Cancer Res. 61:5215–5222.
2001.PubMed/NCBI
|
|
32
|
Raval RR, Lau KW, Tran MGB, Sowter HM,
Mandriota SJ, Li JL, Pugh CW, Maxwell PH, Harris AL and Ratcliffe
PJ: Contrasting properties of hypoxia-inducible factor 1 (HIF-1)
and HIF-2 in von Hippel-Lindau-associated renal cell carcinoma. Mol
Cell Biol. 25:5675–5686. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Bui MH, Seligson D, Han KR, Pantuck AJ,
Dorey FJ, Huang Y, Horvath S, Leibovich BC, Chopra S, Liao SY, et
al: Carbonic anhydrase IX is an independent predictor of survival
in advanced renal clear cell carcinoma: implications for prognosis
and therapy. Clin Cancer Res. 9:802–811. 2003.PubMed/NCBI
|
|
34
|
Brouwers AH, Frielink C, Oosterwijk E,
Oyen WJ, Corstens FH and Boerman OC: Interferons can upregulate the
expression of the tumor associated antigen G250-MN/CA IX, a
potential target for (radio)immunotherapy of renal cell carcinoma.
Cancer Biother Radiopharm. 18:539–547. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Atkins M, Regan M, McDermott D, Mier J,
Stanbridge E, Youmans A, Febbo P, Upton M, Lechpammer M and
Signoretti S: Carbonic anhydrase IX expression predicts outcome of
interleukin 2 therapy for renal cancer. Clin Cancer Res.
11:3714–3721. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Chrastina A, Závada J, Parkkila S, Kaluz
S, Kaluzová M, Rajcáni J, Pastorek J and Pastoreková S:
Biodistribution and pharmacokinetics of 125I-labeled monoclonal
antibody M75 specific for carbonic anhydrase IX, an intrinsic
marker of hypoxia, in nude mice xenografted with human colorectal
carcinoma. Int J Cancer. 105:873–881. 2003. View Article : Google Scholar
|
|
37
|
Pastorek J, Pastoreková S, Callebaut I,
Mornon JP, Zelník V, Opavský R, Zat’ovicová M, Liao S, Portetelle
D, Stanbridge EJ, et al: Cloning and characterization of MN, a
human tumor-associated protein with a domain homologous to carbonic
anhydrase and a putative helix-loop-helix DNA binding segment.
Oncogene. 9:2877–2888. 1994.
|
|
38
|
Grabmaier K, Vissers JL, De Weijert MC,
Oosterwijk-Wakka JC, Van Bokhoven A, Brakenhoff RH, Noessner E,
Mulders PA, Merkx G, Figdor CG, et al: Molecular cloning and
immunogenicity of renal cell carcinoma-associated antigen G250. Int
J Cancer. 85:865–870. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Oosterwijk E, Bander NH, Divgi CR, Welt S,
Wakka JC, Finn RD, Carswell EA, Larson SM, Warnaar SO, Fleuren GJ,
et al: Antibody localization in human renal cell carcinoma: a phase
I study of monoclonal antibody G250. J Clin Oncol. 11:738–750.
1993.PubMed/NCBI
|
|
40
|
Steffens MG, Boerman OC, Oosterwijk-Wakka
JC, Oosterhof GO, Witjes JA, Koenders EB, Oyen WJ, Buijs WC,
Debruyne FM, Corstens FH and Oosterwijk E: Targeting of renal cell
carcinoma with iodine-131-labeled chimeric monoclonal antibody
G250. J Clin Oncol. 15:1529–1537. 1997.PubMed/NCBI
|
|
41
|
Bleumer I, Knuth A, Oosterwijk E, Hofmann
R, Varga Z, Lamers C, Kruit W, Melchior S, Mala C, Ullrich S, et
al: A phase II trial of chimeric monoclonal antibody G250 for
advanced renal cell carcinoma patients. Br J Cancer. 90:985–990.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Bleumer I, Oosterwijk E, Oosterwijk-Wakka
JC, Völler MC, Melchior S, Warnaar SO, Mala C, Beck J and Mulders
PF: A clinical trial with chimeric monoclonal antibody WX-G250 and
low dose interleukin-2 pulsing scheme for advanced renal cell
carcinoma. J Urol. 175:57–62. 2006. View Article : Google Scholar
|
|
43
|
Siebels M, Rohrmann K, Oberneder R,
Stahler M, Haseke N, Beck J, Hofmann R, Kindler M, Kloepfer P and
Stief C: A clinical phase I/II trial with the monoclonal antibody
cG250 (RENCAREX®) and interferon-alpha-2a in metastatic
renal cell carcinoma patients. World J Urol. 29:121–126. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Belldegrun AS, Chamie K, Kloepfer P, Fall
B, Bevan P, Störkel S, Wilhelm O and Pantuck AJ: ARISER: a
randomized double blind phase III study to evaluate adjuvant cG250
treatment versus placebo in patients with high-risk ccRCC - results
and implications for adjuvant clinical trials. J Clin Oncol.
31(Suppl; abs. 4507)2013.
|
|
45
|
Takacova M, Barathova M, Hulikova A,
Ohradanova A, Kopacek J, Parkkila S, Pastorek J, Pastorekova S and
Zatovicova M: Hypoxia-inducible expression of the mouse carbonic
anhydrase IX demonstrated by new monoclonal antibodies. Int J
Oncol. 31:1103–1110. 2007.PubMed/NCBI
|
|
46
|
Barathova M, Takacova M, Holotnakova T,
Gibadulinova A, Ohradanova A, Zatovicova M, Hulikova A, Kopacek J,
Parkkila S, Supuran CT, et al: Alternative splicing variant of the
hypoxia marker carbonic anhydrase IX expressed independently of
hypoxia and tumour phenotype. Br J Cancer. 98:129–136. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Závada J, Závadová Z, Zat’ovicová M, Hyrsl
L and Kawaciuk I: Soluble form of carbonic anhydrase IX (CA IX) in
the serum and urine of renal carcinoma patients. Br J Cancer.
89:1067–1071. 2003.
|
|
48
|
Hyrsl L, Zavada J, Zavadova Z, Kawaciuk I,
Vesely S and Skapa P: Soluble form of carbonic anhydrase IX (CA IX)
in transitional cell carcinoma of urinary tract. Neoplasma.
56:298–302. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Zhou GX, Ireland J, Rayman P, Finke J and
Zhou M: Quantification of carbonic anhydrase IX expression in serum
and tissue of renal cell carcinoma patients using enzyme-linked
immunosorbent assay: prognostic and diagnostic potentials. Urology.
75:257–261. 2010. View Article : Google Scholar
|
|
50
|
Kock L, Mahner S, Choschzick M, Eulenburg
C, Milde-Langosch K, Schwarz J, Jaenicke F, Müller V and Woelber L:
Serum carbonic anhydrase IX and its prognostic relevance in vulvar
cancer. Int J Gynecol Cancer. 21:141–148. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Müller V, Riethdorf S, Rack B, Janni W,
Fasching PA, Solomayer E, Aktas B, Kasimir-Bauer S, Zeitz J, Pantel
K, et al: Prospective evaluation of serum tissue inhibitor of
metalloproteinase 1 and carbonic anhydrase IX in correlation to
circulating tumor cells in patients with metastatic breast cancer.
Breast Cancer Res. 13:R712011.
|
|
52
|
Gigante M, Li G, Ferlay C, Perol D, Blanc
E, Paul S, Zhao A, Tostain J, Escudier B, Negrier S and Genin C:
Prognostic value of serum CA9 in patients with metastatic clear
cell renal cell carcinoma under targeted therapy. Anticancer Res.
32:5447–5451. 2012.PubMed/NCBI
|
|
53
|
Takacova M, Bartosova M, Skvarkova L,
Zatovicova M, Vidlickova I, Csaderova L, Barathova M, Breza J Jr,
Bujdak P, Pastorek J, et al: Carbonic anhydrase IX is a clinically
significant tissue and serum biomarker associated with renal cell
carcinoma. Oncol Lett. 5:191–197. 2013.PubMed/NCBI
|
|
54
|
Alterio V, Hilvo M, Di Fiore A, Supuran
CT, Pan P, Parkkila S, Scaloni A, Pastorek J, Pastorekova S, Pedone
C, et al: Crystal structure of the catalytic domain of the
tumor-associated human carbonic anhydrase IX. Proc Natl Acad Sci
USA. 106:16233–16238. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Dubois L, Lieuwes NG, Maresca A, Thiry A,
Supuran CT, Scozzafava A, Wouters BG and Lambin P: Imaging of CA IX
with fluorescent labelled sulfonamides distinguishes hypoxic and
(re)-oxygenated cells in a xenograft tumour model. Radiother Oncol.
92:423–428. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Dürrbach A, Angevin E, Poncet P, Rouleau
M, Chavanel G, Chapel A, Thierry D, Gorter A, Hirsch R, Charpentier
B, et al: Antibody-mediated endocytosis of G250 tumor-associated
antigen allows targeted gene transfer to human renal cell carcinoma
in vitro. Cancer Gene Ther. 6:564–571. 1999.PubMed/NCBI
|
|
57
|
Grantab R, Sivananthan S and Tannock IF:
The penetration of anticancer drugs through tumour tissue as a
function of cellular adhesion and packing density of tumour cells.
Cancer Res. 66:1033–1039. 2006. View Article : Google Scholar
|
|
58
|
Sorkin A and von Zastrow M: Endocytosis
and signalling: intertwining molecular networks. Nat Rev Mol Cell
Biol. 10:609–622. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Surfus JE, Hank JA, Oosterwijk E, Welt S,
Lindstrom MJ, Albertini MR, Schiller JH and Sondel PM:
Anti-renal-cell carcinoma chimeric antibody G250 facilitates
antibody-dependent cellular cytotoxicity with in vitro and in vivo
interleukin-2-activated effectors. J Immunother Emphasis Tumor
Immunol. 19:184–191. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Liu Z, Smyth FE, Renner C, Lee FT,
Oosterwijk E and Scott AM: Anti-renal cell carcinoma chimeric
antibody G250: cytokine enhancement of in vitro antibody-dependent
cellular cytotoxicity. Cancer Immunol Immunother. 51:171–177. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Divgi CR, Pandit-Taskar N, Jungbluth AA,
Reuter VE, Gönen M, Ruan S, Pierre C, Nagel A, Pryma DA, Humm J, et
al: Preoperative characterisation of clear-cell renal carcinoma
using iodine-124-labelled antibody chimeric G250
(124I-cG250) and PET in patients with renal masses: a
phase I trial. Lancet Oncol. 8:304–310. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Beasley NJ, Wykoff CC, Watson PH, Leek R,
Turley H, Gatter K, Pastorek J, Cox GJ, Ratcliffe P and Harris AL:
Carbonic anhydrase IX, an endogenous hypoxia marker, expression in
head and neck squamous cell carcinoma and its relationship to
hypoxia, necrosis and microvessel density. Cancer Res.
61:5262–5267. 2001.PubMed/NCBI
|
|
63
|
Turner KJ, Crew JP, Wykoff CC, Watson PH,
Poulsom R, Pastorek J, Ratcliffe PJ, Cranston D and Harris AL: The
hypoxia-inducible genes VEGF and CA9 are differentially regulated
in superficial vs invasive bladder cancer. Br J Cancer.
86:1276–1282. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Swinson DE, Jones JL, Cox G, Richardson D,
Harris AL and O’Byrne KJ: Hypoxia-inducible factor-1 alpha in non
small cell lung cancer: relation to growth factor, protease and
apoptosis pathways. Int J Cancer. 111:43–50. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Harris AL: Hypoxia - a key regulatory
factor in tumour growth. Nat Rev Cancer. 2:38–47. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Olive PL, Aquino-Parsons C, MacPhail SH,
Laio S, Raleigh JA, Lerman MI and Stanbridge EJ: Carbonic anhydrase
9 as an endogenous marker for hypoxic cells in cervical cancer.
Cancer Res. 61:8924–8929. 2001.PubMed/NCBI
|
|
67
|
Thiery JP and Sleeman JP: Complex networks
orchestrate epithelial-mesenchymal transitions. Nat Rev Mol Cell
Biol. 7:131–142. 2006. View Article : Google Scholar : PubMed/NCBI
|