Introduction
microRNAs (miRNAs) are a class of small, endogenous,
non-coding RNA molecules that are typically 20–25 nucleotides in
length. miRNAs negatively regulate specific gene products by
translational repression or mRNA degradation via binding to
partially or perfectly complementary sequences in the 3′
untranslated regions of target genes (1–3).
In human tumors, some miRNAs are upregulated and
function as oncogenes, while others are downregulated functioning
as tumor suppressor genes (TSGs). Recent studies have shown that
50% of miRNAs are located within fragile sites, thus supporting the
fact that many of these miRNAs may be lost during tumorigenesis
(1,4–14).
Consistent with this, significant data indicate that many miRNAs
exhibit decreased expression in tumors (1,5,6,8,10,13,15–25).
Thus, although miRNAs have been shown to be both pro- and
anti-tumorigenic, the majority seems to function as TSGs by
negatively regulating protein-coding oncogenes and genes regulating
cell proliferation and apoptosis (1,13,16,20–22,26–30).
The promoter regions of many genes, including a
number of TSGs, sometimes are embedded in CpG islands regions
within the DNA that are subject to methylation. In normal
condition, these regions tend to be unmethylated. However, in a
transformed setting, many of these CpG islands become
hypermethylated, resulting in silencing of gene expression.
Although hypermethylations of CpG islands has been mostly described
for protein-coding genes, a similar mechanism may be responsible
for silencing expression of miRNAs that possess antitumorigenic
properties; a mechanism such as this could potentially enhance
tumorigenesis (13,16,21,22,30–37).
Gastric cancer (GC) has a poor prognosis, in large
part, because patients often present with advanced disease.
Limitations of early diagnosis and effective therapies
unfortunately result in high lethality. Thus, additional research
to improve both detection and treatment of GC is critical. Several
studies have been performed examining the miRNA expression profile
of multiple tumor types (6,11,14,20,27,
28,34,38–42).
Evidence suggests that hypermethylation of CpG islands related with
the promoters of miRNA genes is a common event in GC (20,28,34,37,38,40,42–48).
Previously, we examined several miRNAs, including 19 downregulated
and seven upregulated, in GC (6).
Here, we specifically follow up on the downregulated miRNAs and
investigate the mechanism of their decreased expression. Four
down-regulated miRNAs contain CpG islands within 5,000 bp upstream
of the transcriptional start site, and these were selected as
initial candidate genes. We measured miRNA expression levels in GC
samples to validate the miRNA expression profile data. To assess
the importance of methylation in expression of these genes, we
treated GC cells with a demethylating agent. Based on these initial
results, miR-9 was selected for additional epigenetic research.
Thus, we studied the role of promoter methylation in regulating
miR-9 expression both in vitro and in vivo.
Materials and methods
Cell lines and animals
Cell lines used in this study included human GC cell
lines SGC-7901 and BGC-823 and normal gastric epithelium cell line
GES-1. The cells were purchased from the Centre of Cell Cultures of
Chinese Academy of Medical Sciences, Shanghai, China. All cell
lines were cultured in RPMI-1640 medium (Gibco, Grand Island, NY,
USA) supplemented with 10% fetal calf serum (FCS; Gibco), 100 U/ml
penicillin, 100 μg/ml streptomycin, 2 mM gluta-mine, and 1 mM
sodium pyruvate. Cells were housed in humidified incubators at 37°C
in an atmosphere with 5% CO2. Cells were maintained as a
monolayer by serial passaging after trypsinization with 0.1%
trypsin (Beyotime, Jiangsu, China).
Five to six-week-old male Balb/c nu-nu mice,
weighing 18–20 g, were purchased from the Shanghai Experimental
Animal Center of the Chinese Academy of Medical Sciences, China.
They were maintained in cages in a pathogen-free environment
(temperature 25–27°C, humidity 45–50%) and supplied with food and
water ad libitum. All animals received humane care in
accordance with institutional policies on Human Care and Use of
Laboratory Animals and with the approval of the Ethics Committee of
Chongqing Medical University.
GC samples
A total of 30 GC samples were obtained via surgery
from patients that had provided informed consent to the General
Surgery Department of the Second Affiliated Hospital, Chongqing
Medical University, Chongqing, China. Matched controls (non-cancer
gastric mucosa) were obtained from all patients. None of the
patients had received any pre-operative treatment.
Clinicopathologic information, such as age, gender, stage, grade,
pathological diagnosis, and lymph node metastasis, was available.
The study was approved by the Ethics Committee of Chongqing Medical
University.
miRNA microarray and bioinformatics
We previously profiled GC samples for miRNA
expression by microarray analysis (6). Data from that experiment prompted us
to focus on miRNAs that were decreased in GC since these miRNAs may
be functioning as tumor suppressors in the disease.
CpG Island Searcher (http://cpgislands.usc.edu/) and CpG plot (http://www.ebi.ac.uk/emboss/cpgplot) were used to
determine which miRNAs were embedded in or located near (<500 bp
5′-upstream) a CpG island. Over 90% of human miRNA promoters are
located 1,000 bp upstream of the mature miRNA (16,31).
Promoter miRNA gene clusters were predicted using a combination of
Promoter 2.0 (http://www.cbs.dtu.dk/services/Promoter/), Promoter
Scan (http://www-bimas.cit.nih.gov/molbio/proscan/), and
Neural Network Promoter Prediction (NNPP) (http://www.fruitfly.org/seq_tools/promoter.html).
RNA isolation and reverse
transcription
Total RNA was extracted using TRIzol (Sigma-Aldrich
Chemical Co., Milwaukee, WI, USA). Concentration and purity were
assessed with an ultraviolet spectrophotometer at wavelengths of
260 and 280 nm. RNA was reverse transcribed into cDNA using the
reverse transcription kit (Takara Bio, Inc., Dalian, China). RT
primers are listed in Table I. A
master mix (20 μl total) containing 5× PrimeScript Buffer (4 μl),
PrimeScript RT Enzyme Mix I (1 μl), RT specific primer (1 μl),
total RNA (1 μg), and nuclease-free water (13 μl) was prepared on
ice. The reaction was performed at 42°C for 15 min followed by 85°C
for 5 sec.
 | Table IReverse transcription primers. |
Table I
Reverse transcription primers.
| Gene name | Primer
sequence |
|---|
| U6 |
5′-CGCTTCACGAATTTGCGTGTCAT-3′ |
| hsa-miR-9 |
5′-GTCGTATCCAGTGCGTGTCGTGGAGTCGGCAATTGCACTGGATACGACTCATACAG-3′ |
| hsa-miR-433 |
5′-GTCGTATCCAGTGCGTGTCGTGGAGTCGGCAATTGCACTGGATACGACACACCG-3′ |
| hsa-miR-19b |
5′-GTCGTATCCAGTGCGTGTCGTGGAGTCGGCAATTGCACTGGATACGACTCAGTT-3′ |
| hsa-miR-370 |
5′-GTCGTATCCAGTGCGTGTCGTGGAGTCGGCAATTGCACTGGATACGACACCAGG-3′ |
Real-time quantitative PCR (RTQ-PCR)
RNA samples isolated from both GC tissues (n=30) and
cell lines were converted into cDNA and analyzed by RTQ-PCR. GC
cell lines and tumors from animal models following treatment with
5-aza-2′-deoxycytidine (5-AZA-CdR) or transfection with siRNA-DNMT1
were also examined by this method. RTQ-PCR was performed using the
SYBR-Green real-time PCR master mix kit (Takara Bio, Inc.) and the
IQ5 PCR instrument. In brief, a master mix (25 μl) was prepared on
ice with 12.5 μl 1× SYBR-Green buffer, 1 μl each primer, 2 μl cDNA,
and 8.5 μl nuclease-free water. The cDNA was initially denatured at
95°C for 30 sec followed by 40 cycles of denaturation at 95°C for 5
sec, annealing at 59°C for 30 sec, and extension at 72°C for 30
sec. Primer sequences were designed using software Primer 5.0, and
sequences are listed in Table II.
U6 snRNA served as an endogenous control. All experiments were
performed in triplicate.
 | Table IIRTQ-PCR primers. |
Table II
RTQ-PCR primers.
| Gene name | Primer
sequence | Annealing
temperature (°C) | Product length
(bp) |
|---|
| U6 | F:
5′-GCTTCGGCAGCACATATACTAAAAT-3′
R: 5′-CGCTTCACGAATTTGCGTGTCAT-3′ | 59 | 89 |
| hsa-miR-9 | F:
5′-GGGTCTTTGGTTATCTAGC-3′
R: 5′-TGCGTGTCGTGGAGTC-3′ | 59 | 63 |
| hsa-miR-433 | F:
5′-GGATCATGATGGGCTGGT-3′
R: 5′-CAGTGCGTGTCGTGGAGT-3′ | 59 | 64 |
| hsa-miR-19b | F:
5′-CGTGTGCAAATCCATGC-3′
R: 5′-CAGTGCGTGTCGTGGAG-3′ | 59 | 65 |
| hsa-miR-370 | F:
5′-GCCTGCTGAGATGGAATCTGATGTC-3′
R: 5′-CCAGTGCGTGTCGTAGAGTCATCAA-3′ | 59 | 63 |
Each run was accompanied by a melting curve analysis
to confirm the specificity of amplification and absence of primer
dimers. Relative quantification of miRNA expression was calculated
by the 2−ΔΔct method.
Bisulfite genomic sequencing PCR
(BSP)
DNA was isolated from GC tissues (human samples,
n=30; animal samples, n=10) and cell lines (including matched
normal controls). GC cell lines and tumor tissues from animal
models (n=10) following 5-AZA-CdR treatment or siRNA-DNMT1
transfection were also included. Genomic DNA was isolated using the
Genomic DNA Extraction kit (Takara Bio, Inc.) according to the
manufacturer’s instructions. Bisulfite modification (EZ DNA
Methylation-Gold™ kit, D5005/50; Zymo Research Corp., Irvine, CA,
USA) was performed to convert unmethylated cytosine to uracil;
methylated cytosine nucleotides are unaffected by the procedure.
Bisulfite-modified miRNA promoters were amplified using specially
designed primers listed in Table
III. The reaction volume 50 μl included 10× buffer (5 μl),
MgCl2 (2 μl), dNTP (1 μl), each primer (2 μl), DNA (5
μl), Platinum Taq (0.3 μl), and ddH2O (32.7 μl).
Amplification was carried out as follows: 5 min 95°C; 42 cycles of
30 sec at 95°C, 30 sec at 57°C, and 40 sec at 72°C; and a 10 min
final extension at 72°C. Per sample, five independent colonies for
each tested region were picked and sequenced. The extent of
methylation was assessed by identifying the number and position of
methylated cytosine residues.
 | Table IIISpecific BSP primers for miRNA-9. |
Table III
Specific BSP primers for miRNA-9.
| Gene name | Primer
sequence | Product length
(bp) | Tm (°C) |
|---|
| miR-9-1 | F:
5′-GGTAGAGTTAATTAGAGGATGGTTTG-3′
R: 5′-ACCAAAAATCACCCAAAATTATAAA-3′ | 498 | 57 |
| miR-9-2 | F:
5′-TGATTTTTGGTTTTTTTTGAAT-3′
R: 5′-TCCACTACCCTTCTCTAAAAAA-3′ | 504 | 58 |
5-AZA-CdR treatment in vitro and in
vivo
Cell lines were treated with 5 μM 5-AZA-CdR
(Sigma-Aldrich Chemical Co.) for 48 h.
Nude mice were subcutaneously injected with
107 GC cells on either side of the flank. Two weeks
post-implantation, tumors reached a size of ~1.2 cm3.
Orthotopic GC models (n=10) were constructed according to our
previously described protocol (49). Animals received intravenous
injection of 5-AZA-CdR at 0.6 mg/kg once per day for 4 weeks after
transplantation (50). Mice were
euthanized and fresh tumor fragments, free of any necrotic region,
were harvested and preserved in liquid nitrogen until use.
siRNA-DNMT1 synthesis and transfection
into cell lines and animal models in vivo
An siRNA-DNMT1 plasmid was synthesized by Jingsai
Bio Co., Ltd., (Hubei, China). Gastric cell lines were seeded at a
density of 2×105 cells/well in 6-well plates (Costar,
Cambridge, MA, USA) and cultured overnight. siRNA-DNMT1 plasmid (5
μl) was transfected into cells using Lipofectamine 2000 (Invitrogen
Life Technologies, Carlsbad, CA, USA) according to the
manufacturer’s instructions. Cells were incubated for 48 h, at
which time, the transfection efficiency was >80%.
Animal models (n=10) using transfected GC cell lines
were established as described above. Four weeks after implantation,
fresh tumor tissues were harvested and preserved as described.
Statistical analysis
Data are presented as mean ± SD. RTQ-PCR data were
analyzed by Student’s t-test. The correlation between miRNA
expression and clinicopathological factors was analyzed using
Fisher’s exact test. Differences in mean methylation levels were
analyzed using the χ2 test. Statistical significance was
set at p<0.05.
Results
Selection of candidate miRNAs of
interest
We previously profiled GC samples for miRNA
expression by microarray analysis (6). A total of 26 differentially expressed
miRNAs were identified, of which 19 were downregulated and seven
were upregulated. We prioritized studying miRNAs that were
repressed in GC and that had CpG islands within 5,000 bp upstream
of the transcription start site. The final list of potential target
genes was determined using a bioinformatics approach (51). Potential targets included miR-9,
miR-433, miR-19b, and miR-370 (Fig.
1A–D).
Validation of expression of four miRNAs
in GC tissues and cell lines
We measured expression of miR-9, miR-433, miR-19b,
and miR-370 and found that they were all strongly repressed in GC
samples compared to normal gastric mucosa. All miRNAs, except
miR-19b, displayed significant differences in expression
(p<0.05) (Fig. 2A). Compared to
the normal gastric epithelial cell line GES-1, the four tested
miRNAs all showed decreased expression in the GC cell lines
SGC-7901 and BGC-823. However, miR-9 was the only miRNA of the four
whose expression level was statistically significantly decreased
(p<0.05) (Fig. 2B).
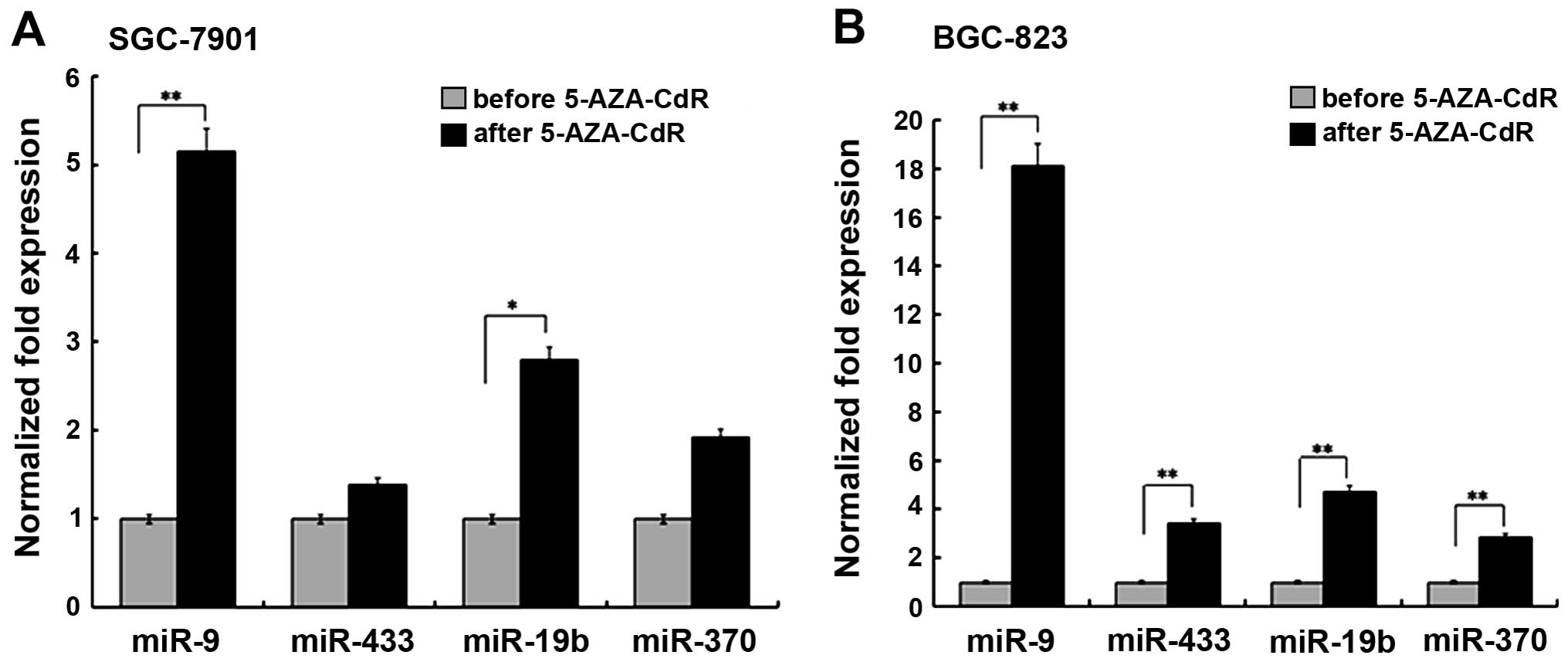
miRNA expression is increased following
treatment with demethylating agent
To assess the importance of methylation in
expression of the four candidate miRNAs, we examined their
expression in two GC cell lines following treatment with 5-AZA-CdR.
In both cell lines, miRNA expression was increased after
demethylation. In SGC-7901, miR-9 and miR-19b were both
significantly increased following 5-AZA-CdR treatment (p<0.01);
in contrast, the increased expression of miR-370 and miR-433 was
not statistically significant (Fig.
3A). In the BGC-823 cell line, statistical significance was
achieved for all four miRNAs (p<0.01) (Fig. 3B).
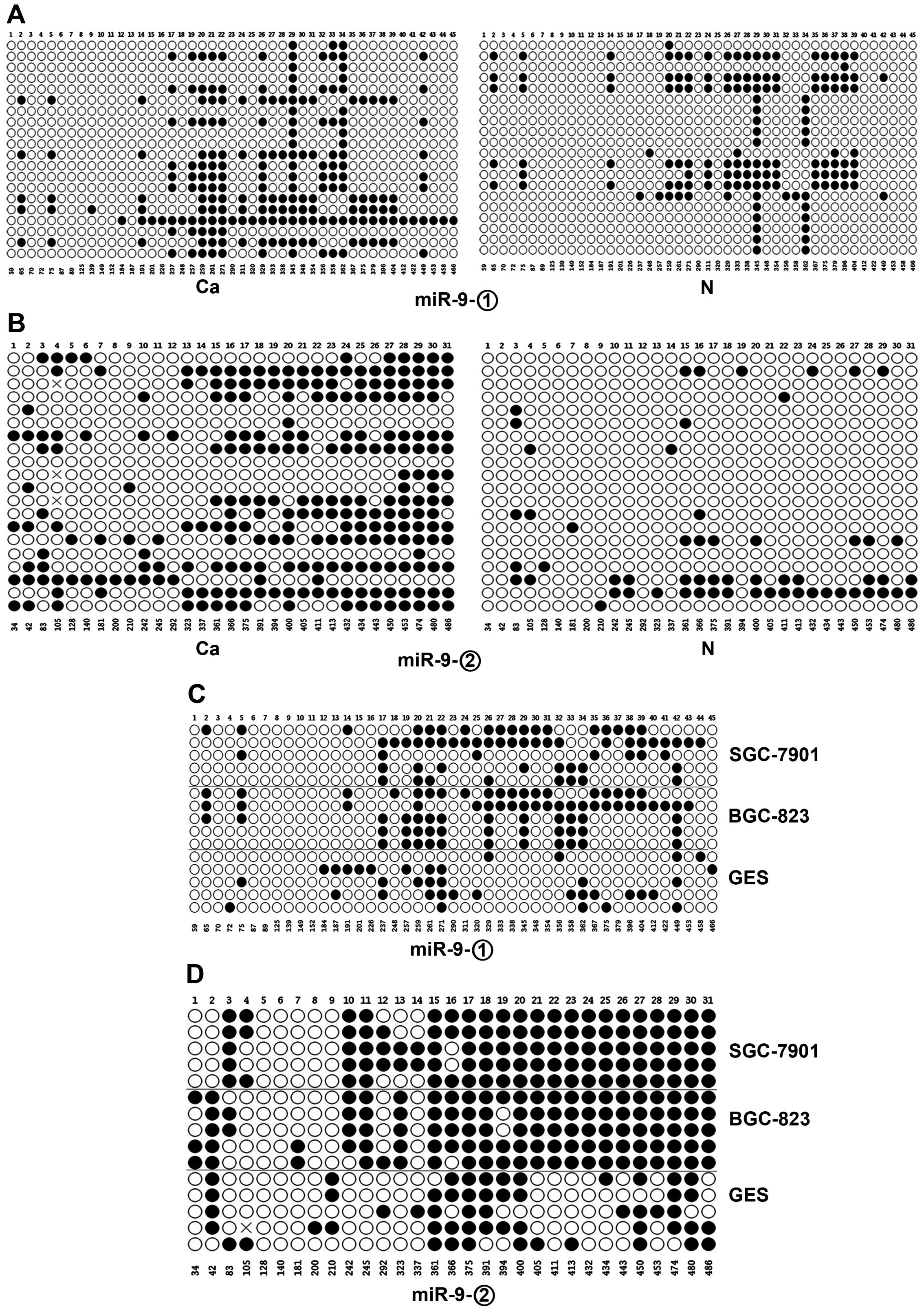
Analysis of DNA methylation in the miR-9
CpG island
Approximately 90% of human miRNA promoters are
located 1,000 bp upstream of the mature miRNA (16,31).
To identify promoters harboring CpG islands, a manual search of the
candidate miRNA promoters was performed via a bioinformatics
approach. miR-9 promoter was predicted to embed in CpG islands
based on this analysis (Fig. 4).
Moreover, we found that miR-9 was consistently deregulated in both
GC tissue and cell lines. Thus, miR-9 was chosen for further
study.
We investigated the methylation status of the miR-9
promoter (500–1,500 bp upstream of the transcription start site)
via amplification of two regions termed miR-9-1 and miR-9-2. CpG
island methylation of miR-9 in GC tissues was significantly higher
than the methylation level in normal gastric mucosa (p<0.05 and
p<0.005) (Fig. 5A and B). Cell
line data were consistent with this. That is, miR-9 methylation in
the GC cell lines SGC-7901 and BGC-823 was significantly higher
than methylation in normal controls (p<0.01 and p<0.005)
(Fig. 5C and D).
Demethylation, induced by either
5-AZA-CdR or siRNA-DNMT1, increases miR-9 expression in GC cell
lines
We assessed the importance of miR-9 methylation on
its expression by two methods. First, SGC-7901 and BGC-823 cells
were treated with 5-AZA-CdR. In both cell lines, methylation of
miR-9 CpG islands was decreased following treatment (p<0.05,
p<0.005) (Fig. 6A and B). This
was concomitant with increased miR-9 expression (p<0.01)
(Fig. 4A and B). SGC-7901 and
BGC-823 cells were also transfected with siRNA targeting DNMT1 as
another means of relieving DNA methylation. Consistent with the
5-AZA-CdR results, transfection of siRNA-DNMT1 decreased CpG island
methylation in both cell lines (p<0.05) (Fig. 6C and D) and increased expression of
miR-9 (p<0.01) (Fig. 6E and
F).
miR-9 methylation in the GC animal
model
We next explored miR-9 methylation in our orthotopic
GC animal model. In tumors, the degree of methylation of the
miR-9-1 CpG island was decreased following 5-AZA-CdR treatment, but
the change was not statistically significant. In contrast,
methylation of miR-9-2 was significantly decreased by 5-AZA-CdR
treatment (p<0.01) (Fig. 7A).
Although expression of miR-9 showed an increasing trend following
treatment, the change was not significant (Fig. 7B). Introduction of siRNA-DNMT1 into
our GC animal models significantly decreased the levels of
methylation of both miR-9-1 (p<0.01) and miR-9-2 (p<0.05)
(Fig. 7C). This occurred
concomitantly with increased miR-9 expression (p<0.01) (Fig. 7D).
Correlation between miRNAs and
clinicopathological features
We performed correlation analysis between miRNA
expression and several clinicopathological features, and the data
are listed in Table IV. Of note,
deregulation of miR-9 was positively correlated with tumor size
(p=0.026) and lymph node metastasis (p=0.041). miR-433 correlated
with gender (p=0.031), the position of local invasion (p=0.006),
grade (p=0.006), and lymph node metastasis (p=0.003). miR-19b was
found to correlate with gender (p=0.031), the position of tumor
involvement (p=0.001), and grade (p=0.031). Finally, miR-370
correlated with tumor position (p=0.001), grade (p=0.031), and
lymph node invasion (p=0.012).
 | Table IVCorrelation between miRNA and
clinicopathological features in gastric cancer (n=30). |
Table IV
Correlation between miRNA and
clinicopathological features in gastric cancer (n=30).
| Clinicopathological
features | Cases | miR-9 | p-value | miR-433 | p-value | miR-19b | p-value | miR-370 | p-value |
|---|
| |
| |
| |
| |
| |
|---|
| | LE | HE | | LE | HE | | LE | HE | | LE | HE | |
|---|
| Gender |
| Male | 23 | 22 | 1 | 0.128 | 22 | 1 | a0.031 | 22 | 1 | a0.031 | 21 | 2 | 0.225 |
| Female | 7 | 5 | 2 | | 4 | 3 | | 4 | 3 | | 5 | 2 | |
| Age |
| ≤50 years | 10 | 10 | 0 | 0.281 | 9 | 1 | 0.640 | 8 | 2 | 0.407 | 8 | 2 | 0.407 |
| >50 years | 20 | 17 | 3 | | 16 | 4 | | 18 | 2 | | 18 | 2 | |
| Tumor size |
| ≤3 cm | 13 | 9 | 4 | a0.026 | 10 | 3 | 0.367 | 12 | 1 | 0.409 | 12 | 1 | 0.409 |
| >3 cm | 17 | 17 | 0 | | 15 | 2 | | 14 | 3 | | 14 | 3 | |
| Tumor position |
| Lesser
curvature | 23 | 22 | 1 | 0.128 | 22 | 1 | b0.006 | 23 | 0 | b0.001 | 23 | 0 | b0.001 |
| Greater
curvature | 7 | 5 | 2 | | 3 | 4 | | 3 | 4 | | 3 | 4 | |
| Pathological
pattern |
| Squamous
carcinoma | 0 | 0 | 0 | None | 0 | 0 | None | 0 | 0 | None | 0 | 0 | None |
|
Adenocarcinoma | 30 | 27 | 3 | | 25 | 5 | | 26 | 4 | | 26 | 4 | |
| Pathological
grade |
| Well
differientiated | 7 | 5 | 2 | 0.128 | 3 | 4 | b0.006 | 4 | 3 | a0.031 | 4 | 3 | a0.031 |
| Poorly
differientiated | 23 | 22 | 1 | | 22 | 1 | | 22 | 1 | | 22 | 1 | |
| Lymph node
metastasis |
| Yes | 19 | 19 | 0 | a0.041 | 19 | 0 | b0.003 | 18 | 1 | 0.126 | 19 | 0 | a0.012 |
| No | 11 | 8 | 3 | | 6 | 5 | | 8 | 3 | | 7 | 4 | |
| Liver
metastasis |
| Yes | 0 | 0 | 0 | None | 0 | 0 | None | 0 | 0 | None | 0 | 0 | None |
| No | 30 | 27 | 3 | | 25 | 5 | | 26 | 4 | | 26 | 4 | |
| Peritoneum
dissemination |
| Yes | 0 | 0 | 0 | None | 0 | 0 | None | 0 | 0 | None | 0 | 0 | None |
| No | 30 | 27 | 3 | | 25 | 5 | | 26 | 4 | | 26 | 4 | |
| Clinical
stages |
| I, II | 29 | 26 | 3 | 0.900 | 24 | 5 | 0.833 | 25 | 4 | 0.867 | 25 | 4 | 0.867 |
| III, IV | 1 | 1 | 0 | | 1 | 0 | | 1 | 0 | | 1 | 0 | |
Discussion
miRNAs are heavily implicated in tumorigenesis in
multiple cancer types (7–14). We identified four candidate miRNAs
(miR-9, miR-433, miR-19b, and miR-370) whose expressions were all
reduced in GC tissues and cell lines compared to normal healthy
gastric epithelium. All of the miRNAs, except for miR-19b,
displayed significant differences in expression, validating the
previous miRNA profile data from GC patients. The only miRNA that
showed consistent downregulation in GC cell lines SGC-7901 and
BGC-823 (as in GC tissue) was miR-9. Recently, Du et al
showed that miRNAs in GC cell lines might not be repressed to the
same extent as they are in actual human tissue samples; in fact,
they state that most cell lines likely exhibit normal miRNA
expression (28). Their study may
provide an explanation as to why miR-433, miR-19b, and miR-370 were
not significantly repressed in the cell lines we examined. Many
other groups have identified miRNAs that are specifically
downregulated in GC samples (14,20,27,28,38,42,43,47).
The four candidate miRNAs included in this study had
CpG islands within 5,000 bp upstream of the transcriptional start
site. Thus, we hypothesized that expression of these miRNAs would
be increased following treatment with the demethylation agent
5-AZA-CdR. This proved true, as expression of all four miRNAs
(miR-9, miR-433, miR-19b, and miR-370) were increased after
5-AZA-CdR treatment; thus, methylation is an important epigenetic
regulatory mechanism governing expression of these miRNAs. In both
SGC-7901 and BGC-823 cells, the effect of 5-AZA-CdR on miR-9 and
miR-19b was the greatest, suggesting that these two miRNAs are
dominantly regulated by a methylation-dependent mechanism.
Interestingly, we found that the ability of miR-370 and miR-433 to
be demethylated was different in different GC cell lines.
Consistent with this, Guo et al reported that, following
5-AZA-CdR treatment of other GC lines (HGC-27 and MGC-803), miR-433
was re-expressed to different degrees (38). Thus, aberrant expression of miRNAs
in different tumor cell lines may result from tumor heterogeneity
(28). One study showed that miRNA
expression could be rescued by deacetylation even if miRNA
hypermethylation was maintained (25). The exact role of DNA
hypermethylation of miR-370 and miR-433 in SGC-7901 cells requires
additional research.
In cancer, many protein-coding genes with tumor
suppressor qualities are silenced by CpG island methylation. Here,
we examined if tumor suppressor miRNAs may be silenced in a similar
manner. Methylation of miR-9 at two promoter CpG islands was
significantly higher in GC tissues and cell lines compared to
normal controls. This finding supports our hypothesis that tumor
suppressor miRNAs can be silenced by DNA methylation in tumors,
similar to the silencing of protein-coding genes. We found that
miR-9 is epigenetically regulated by hypermethylation of
promoter-proximal CpG islands; this may be the dominant mechanism
of miR-9 silencing in GC.
miR-9 is the best characterized miRNA regulated by
methylation in cancer. Lehmann et al (35) showed that deregulation of
hsa-miR-9-1, mediated by CpG island methylation, was an early event
during breast tumorigenesis. Lujambio et al (31) determined that methylation-mediated
silencing of the miR-148a, miR-34b/c, and miR-9 promoters was
cancer-specific and closely correlated with lymph node metastasis.
Du et al (28) found that
hypermethylation repressed expression of seven miRNAs in GC;
interestingly the degree of miR-9 methylation was the most
significant among them. Another study also showed that miR-9
methylation correlated with decreased expression in GC (20). Taken together, a number of studies
have shown the importance of miRNA methylation in GC development
(20,34,38,52,53).
The data we present here contribute to this base of knowledge.
We validated, by two independent methods, that miR-9
expression is epigenetically regulated in vitro. The degree
of CpG island methylation of miR-9 was significantly decreased
after 5-AZA-CdR treatment or siRNA-DNMT1 in both GC cell lines and
animal models; this was concomitant with an increase in miR-9
expression. Our data are supported by the study of others
highlighting the importance of miRNA methylation (7,8,10,54–56).
We examined the importance of miR-9 methylation both
in cell lines and in animal models. In all cases, miR-9 methylation
was decreased and expression was increased following administration
of a demethylating agent. However, the changes in miR-9 methylation
and expression in vivo were not as dramatic as the in
vitro alterations. The discrepancies could be due to the method
of 5-AZA-CdR treatment in vitro versus in vivo,
including dosage, duration, and pathway. While some have reported
on 5-AZA-CdR treatment in mice, it is difficult to evaluate the
effects in different laboratories (57). To the best of our knowledge, this
is the first report describing the use of a demethylation drug in a
GC animal model. Administration of 5-AZA-CdR in GC animal models
likely requires further optimization. Although there were some
differences in treatment with 5-AZA-CdR compared to siRNA-DNMT1, we
effectively showed that miR-9 is epigenetically regulated by
hypermethylation in GC.
We found that our four candidate miRNAs were
significantly positively correlated with several
clinicopathological features. Low levels of miR-9 were associated
with tumor size, indicating that miR-9 may be an independent
diagnostic factor in GC. Deregulated expression of miR-9, miR-433,
and miR-370 was correlated with lymph node metastasis, which
contributed to poor prognosis. In addition, decreased expression of
these three miRNAs (miR-19b, miR-433, and miR-370) was shown to be
associated with less curvature of the stomach; this shape is the
most common position for both GC and poorly differentiated
carcinoma, which is the most common pathological type of late stage
GC. miR-19b and miR-433 positively correlated with male gender,
inferring that the aberrant expression of these two miRNAs might be
common events in male GC patients. Recently, Tsai et al
showed that miR-9 expression was associated with tumor grade,
metastasis, and survival rate in GC (20). Yanaihara et al (4) examined 104 lung cancer samples and
analyzed potential correlation between six over-expressed miRNAs
(miR-205, miR-99b, miR-203, miR-202, miR-102, and miR-204-prec) and
clinicopathological features; however, no miRNA was found to be
associated with gender. Similar analyses have been performed in
multiple tumor types (4,12,13).
In conclusion, we showed that four miRNAs were
downregulated in GC, and that their expression can be restored
following treatment with 5-AZA-CdR. Additionally, these four miRNAs
were positively correlated with several clinicopathological
features. Of the four candidate miRNAs, miR-19b and miR-370 are the
least studied in GC. To strengthen our claims, we performed
experiments with two different demethylation methods; together, our
data support that miR-9 is epigenetically silenced by CpG island
methylation in GC. Due to technical limitations, the in vivo
experiments should be revisited in more detail in the future.
Future studies should also focus on how miRNAs regulate oncogenesis
and which genes are the key miRNA targets.
Acknowledgements
We thank Professor Like Xiang for collecting the GC
samples. We also thank Juan Luo (MD) for technical advice on
treating GC samples and Xingxing Li (MD) and Yingying Zhang (MD)
for assistance with cell biology techniques. We are grateful to
Xiong Zhang (MM) for help with primer design.
References
|
1
|
Zhang B, Pan X, Cobb GP and Anderson TA:
microRNAs as oncogenes and tumor suppressors. Dev Biol. 302:1–12.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Finnegan EJ and Matzke MA: The small RNA
world. J Cell Sci. 116:4689–4693. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Weber B, Stresemann C, Brueckner B and
Lyko F: Methylation of human microRNA genes in normal and
neoplastic cells. Cell Cycle. 6:1001–1005. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Yanaihara N, Caplen N, Bowman E, et al:
Unique microRNA molecular profiles in lung cancer diagnosis and
prognosis. Cancer Cell. 9:189–198. 2006. View Article : Google Scholar
|
|
5
|
Wijnhoven BP, Michael MZ and Watson DI:
MicroRNAs and cancer. Br J Surg. 94:23–30. 2007. View Article : Google Scholar
|
|
6
|
Luo HC, Zhang ZZ, Zhang X, Ning B, Guo JJ,
Nie N, Liu B and Wu XL: MicroRNA expression signature in gastric
cancer. Chin J Cancer Res. 21:74–80. 2009. View Article : Google Scholar
|
|
7
|
Lu J, Getz G, Miska EA, et al: MicroRNA
expression profiles classify human cancers. Nature. 435:834–838.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Li Z, Lu J, Sun M, et al: Distinct
microRNA expression profiles in acute myeloid leukemia with common
translocations. Proc Natl Acad Sci USA. 105:15535–15540. 2008.
View Article : Google Scholar
|
|
9
|
Calin GA, Liu CG, Sevignani C, et al:
MicroRNA profiling reveals distinct signatures in B cell chronic
lymphocytic leukemias. Proc Natl Acad Sci USA. 101:11755–11760.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Volinia S, Calin GA, Liu CG, et al: A
microRNA expression signature of human solid tumors defines cancer
gene targets. Proc Natl Acad Sci USA. 103:2257–2261. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhang Z, Li Z, Gao C, et al: miR-21 plays
a pivotal role in gastric cancer pathogenesis and progression. Lab
Invest. 88:1358–1366. 2008. View Article : Google Scholar
|
|
12
|
Iorio MV, Ferracin M, Liu CG, et al:
MicroRNA gene expression deregulation in human breast cancer.
Cancer Res. 65:7065–7070. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Takamizawa J, Konishi H, Yanagisawa K, et
al: Reduced expression of the let-7 microRNAs in human lung cancers
in association with shortened postoperative survival. Cancer Res.
64:3753–3756. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Motoyama K, Inoue H, Nakamura Y, Uetake H,
Sugihara K and Mori M: Clinical significance of high mobility group
A2 in human gastric cancer and its relationship to let-7 microRNA
family. Clin Cancer Res. 14:2334–2340. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Varambally S, Cao Q, Mani RS, et al:
Genomic loss of microRNA-101 leads to overexpression of histone
methyltrans-ferase EZH2 in cancer. Science. 322:1695–1699. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Cimmino A, Calin GA, Fabbri M, et al:
miR-15 and miR-16 induce apoptosis by targeting BCL2. Proc Natl
Acad Sci USA. 102:13944–13949. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Costinean S, Zanesi N, Pekarsky Y, et al:
Pre-B cell proliferation and lymphoblastic leukemia/high-grade
lymphoma in E(mu)-miR155 transgenic mice. Proc Natl Acad Sci USA.
103:7024–7029. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
O’Donnell KA, Wentzel EA, Zeller KI, Dang
CV and Mendell JT: c-Myc-regulated microRNAs modulate E2F1
expression. Nature. 435:839–843. 2005.PubMed/NCBI
|
|
19
|
Murakami Y, Yasuda T, Saigo K, et al:
Comprehensive analysis of microRNA expression patterns in
hepatocellular carcinoma and non-tumorous tissues. Oncogene.
25:2537–2545. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Tsai KW, Liao YL, Wu CW, et al: Aberrant
hypermethylation of miR-9 genes in gastric cancer. Epigenetics.
6:1189–1197. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Tanaka N, Toyooka S, Soh J, et al:
Frequent methylation and oncogenic role of microRNA-34b/c in
small-cell lung cancer. Lung Cancer. 76:32–38. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Suh SO, Chen Y, Zaman MS, et al:
MicroRNA-145 is regulated by DNA methylation and p53 gene mutation
in prostate cancer. Carcinogenesis. 32:772–778. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Balaguer F, Link A, Lozano JJ, et al:
Epigenetic silencing of miR-137 is an early event in colorectal
carcinogenesis. Cancer Res. 70:6609–6618. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zhang Y, Wang X, Xu B, et al: Epigenetic
silencing of miR-126 contributes to tumor invasion and angiogenesis
in colorectal cancer. Oncol Rep. 30:1976–1984. 2013.PubMed/NCBI
|
|
25
|
Schiffgen M, Schmidt DH, von Rücker A,
Müller SC and Ellinger J: Epigenetic regulation of microRNA
expression in renal cell carcinoma. Biochem Biophys Res Commun.
436:79–84. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Michael MZ, O’ Connor SM, van Holst
Pellekaan NG, Young GP and James RJ: Reduced accumulation of
specific microRNAs in colorectal neoplasia. Mol Cancer Res.
1:882–891. 2003.PubMed/NCBI
|
|
27
|
Luo H, Zhang H, Zhang Z, et al:
Downregulated miR-9 and miR-433 in human gastric carcinoma. J Exp
Clin Cancer Res. 28:822009. View Article : Google Scholar
|
|
28
|
Du Y, Liu Z, Gu L, et al: Characterization
of human gastric carcinoma-related methylation of 9 miR CpG islands
and repression of their expressions in vitro and in vivo. BMC
Cancer. 12:2492012. View Article : Google Scholar
|
|
29
|
Minor J, Wang X, Zhang F, et al:
Methylation of microRNA-9 is a specific and sensitive biomarker for
oral and oropharyngeal squamous cell carcinomas. Oral Oncol.
48:73–78. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Tanaka T, Arai M, Wu S, et al: Epigenetic
silencing of microRNA-373 plays an important role in regulating
cell proliferation in colon cancer. Oncol Rep. 26:1329–1335.
2011.PubMed/NCBI
|
|
31
|
Lujambio A, Calin GA, Villanueva A, et al:
A microRNA DNA methylation signature for human cancer metastasis.
Proc Natl Acad Sci USA. 105:13556–13561. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Datta J, Kutay H, Nasser MW, et al:
Methylation mediated silencing of MicroRNA-1 gene and its role in
hepatocellular carcinogenesis. Cancer Res. 68:5049–5058. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Toyota M, Suzuki H, Sasaki Y, et al:
Epigenetic silencing of microRNA-34b/c and B-cell translocation
gene 4 is associated with CpG island methylation in colorectal
cancer. Cancer Res. 68:4123–4132. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Ando T, Yoshida T, Enomoto S, et al: DNA
methylation of microRNA genes in gastric mucosae of gastric cancer
patients: its possible involvement in the formation of epigenetic
field defect. Int J Cancer. 124:2367–2374. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Lehmann U, Hasemeier B, Christgen M, et
al: Epigenetic inactivation of microRNA gene hsa-mir-9-1 in human
breast cancer. J Pathol. 214:17–24. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Grady WM, Parkin RK, Mitchell PS, et al:
Epigenetic silencing of the intronic microRNA hsa-miR-342 and its
host gene EVL in colorectal cancer. Oncogene. 27:3880–3888. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Suzuki H, Maruyama R, Yamamoto E and Kai
M: DNA methylation and microRNA dysregulation in cancer. Mol Oncol.
6:567–578. 2012. View Article : Google Scholar
|
|
38
|
Guo LH, Li H, Wang F, Yu J and He JS: The
tumor suppressor roles of miR-433 and miR-127 in gastric cancer.
Int J Mol Sci. 14:14171–14184. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Wada R, Akiyama Y, Hashimoto Y, Fukamachi
H and Yuasa Y: miR-212 is downregulated and suppresses
methyl-CpG-binding protein MeCP2 in human gastric cancer. Int J
Cancer. 127:1106–1114. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Hashimoto Y, Akiyama Y, Otsubo T, Shimada
S and Yuasa Y: Involvement of epigenetically silenced microRNA-181c
in gastric carcinogenesis. Carcinogenesis. 31:777–784. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Shen R, Pan S, Qi S, Lin X and Cheng S:
Epigenetic repression of microRNA-129-2 leads to overexpression of
SOX4 in gastric cancer. Biochem Biophys Res Commun. 394:1047–1052.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Suzuki H, Yamamoto E, Nojima M, et al:
Methylation-associated silencing of microRNA-34b/c in gastric
cancer and its involvement in an epigenetic field defect.
Carcinogenesis. 31:2066–2073. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Rotkrua P, Akiyama Y, Hashimoto Y, Otsubo
T and Yuasa Y: MiR-9 downregulates CDX2 expression in gastric
cancer cells. Int J Cancer. 129:2611–2620. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Tsai KW, Wu CW, Hu LY, et al: Epigenetic
regulation of miR-34b and miR-129 expression in gastric cancer. Int
J Cancer. 129:2600–2610. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Zhu A, Xia J, Zuo J, et al: MicroRNA-148a
is silenced by hypermethylation and interacts with DNA
methyltransferase 1 in gastric cancer. Med Oncol. 29:2701–2709.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Li P, Chen X, Su L, et al: Epigenetic
silencing of miR-338-3p contributes to tumorigenicity in gastric
cancer by targeting SSX2IP. PloS One. 8:e667822013. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Lei H, Zou D, Li Z, et al:
MicroRNA-219-2-3p functions as a tumor suppressor in gastric cancer
and is regulated by DNA methylation. PLoS One. 8:e603692013.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Deng H, Guo Y, Song H, et al: MicroRNA-195
and microRNA-378 mediate tumor growth suppression by epigenetical
regulation in gastric cancer. Gene. 518:351–359. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Li Y, Li B, Zhang Y, Xiang CP, Li YY and
Wu XL: Serial observations on an orthotopic gastric cancer model
constructed using improved implantation technique. World J
Gastroenterol. 17:1442–1447. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Momparler RL: Pharmacology of
5-Aza-2′-deoxycytidine (decitabine). Semin Hematol. 42(Suppl 2):
S9–S16. 2005.
|
|
51
|
Takai D and Jones PA: The CpG island
searcher: a new WWW resource. In silico Biol. 3:235–240.
2003.PubMed/NCBI
|
|
52
|
Chen Q, Chen X, Zhang M, Fan Q, Luo S and
Cao X: miR-137 is frequently down-regulated in gastric cancer and
is a negative regulator of Cdc42. Dig Dis Sci. 56:2009–2016. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Yamamoto E, Suzuki H, Maruyama R and
Shinomura Y: Developing technologies for epigenomic analysis and
clinical application of molecular diagnosis. Rinsho Byori.
60:637–643. 2012.(In Japanese).
|
|
54
|
Langevin SM, Stone RA, Bunker CH, et al:
MicroRNA-137 promoter methylation is associated with poorer overall
survival in patients with squamous cell carcinoma of the head and
neck. Cancer. 117:1454–1462. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Furuta M, Kozaki KI, Tanaka S, Arii S,
Imoto I and Inazawa J: miR-124 and miR-203 are epigenetically
silenced tumor-suppressive microRNAs in hepatocellular carcinoma.
Carcinogenesis. 31:766–776. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Li Y, Kong D, Ahmad A, Bao B, Dyson G and
Sarkar FH: Epigenetic deregulation of miR-29a and miR-1256 by
isoflavone contributes to the inhibition of prostate cancer cell
growth and invasion. Epigenetics. 7:940–949. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Momparler RL: Epigenetic therapy of cancer
with 5-aza-2′-deoxy-cytidine (decitabine). Semin Oncol. 32:443–451.
2005.
|