|
1
|
Bickeboller M, Tagscherer KE, Kloor M, et
al: Functional characterization of the tumor-suppressor MARCKS in
colorectal cancer and its association with survival. Oncogene.
24–March;2014.(Epub ahead of print). View Article : Google Scholar
|
|
2
|
Brooks G, Brooks SF and Goss MW: MARCKS
functions as a novel growth suppressor in cells of melanocyte
origin. Carcinogenesis. 17:683–689. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
3
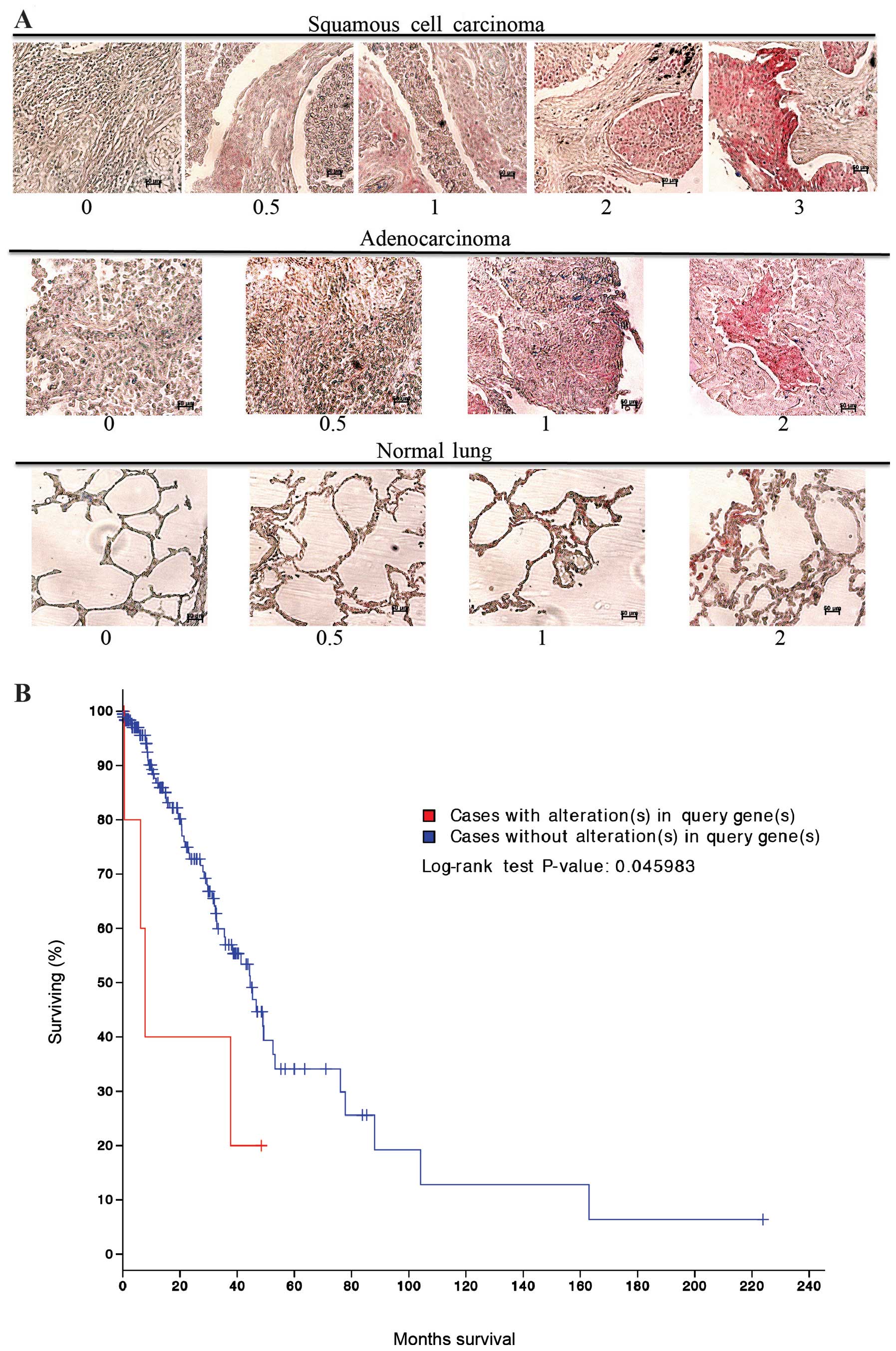
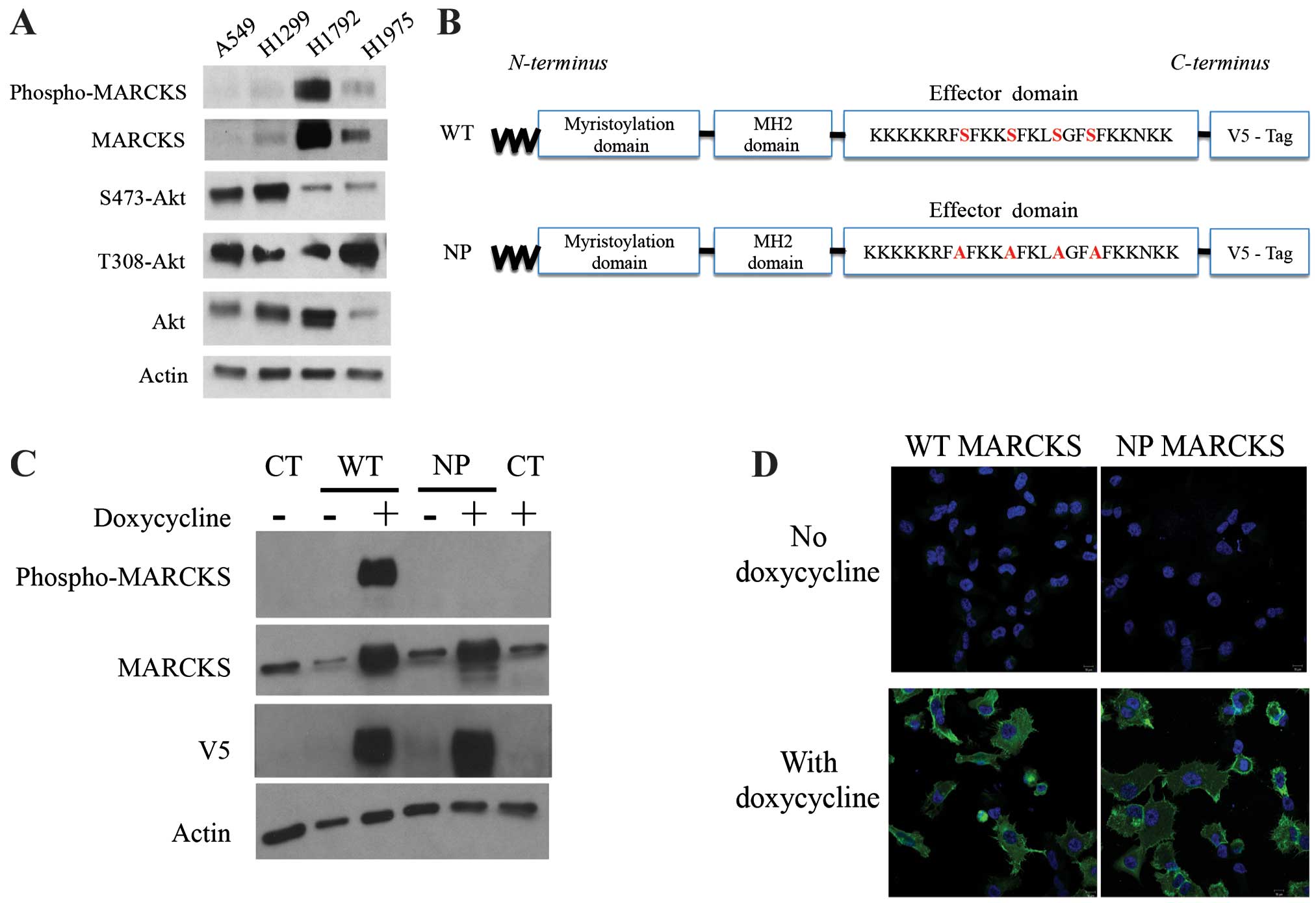
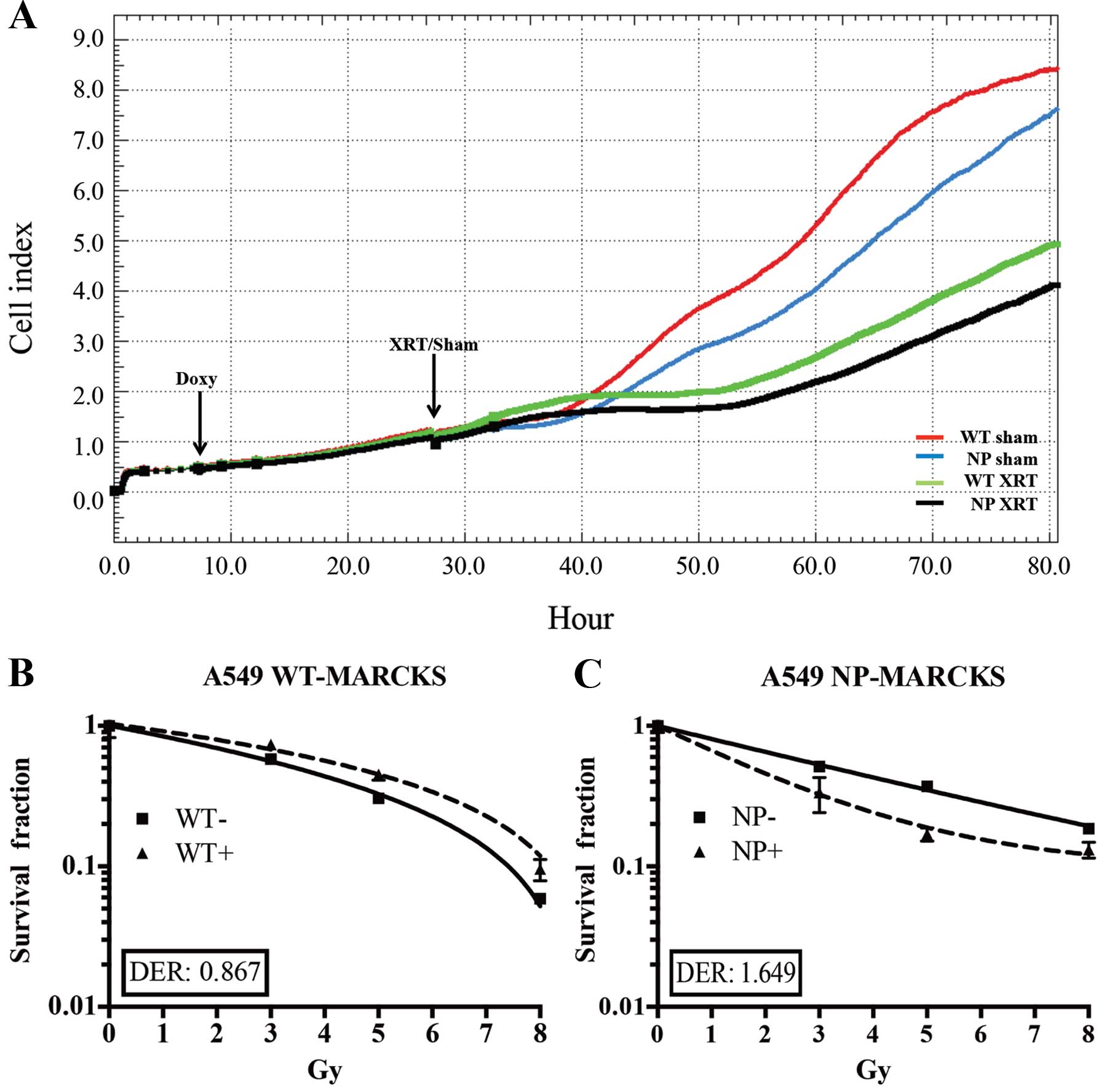
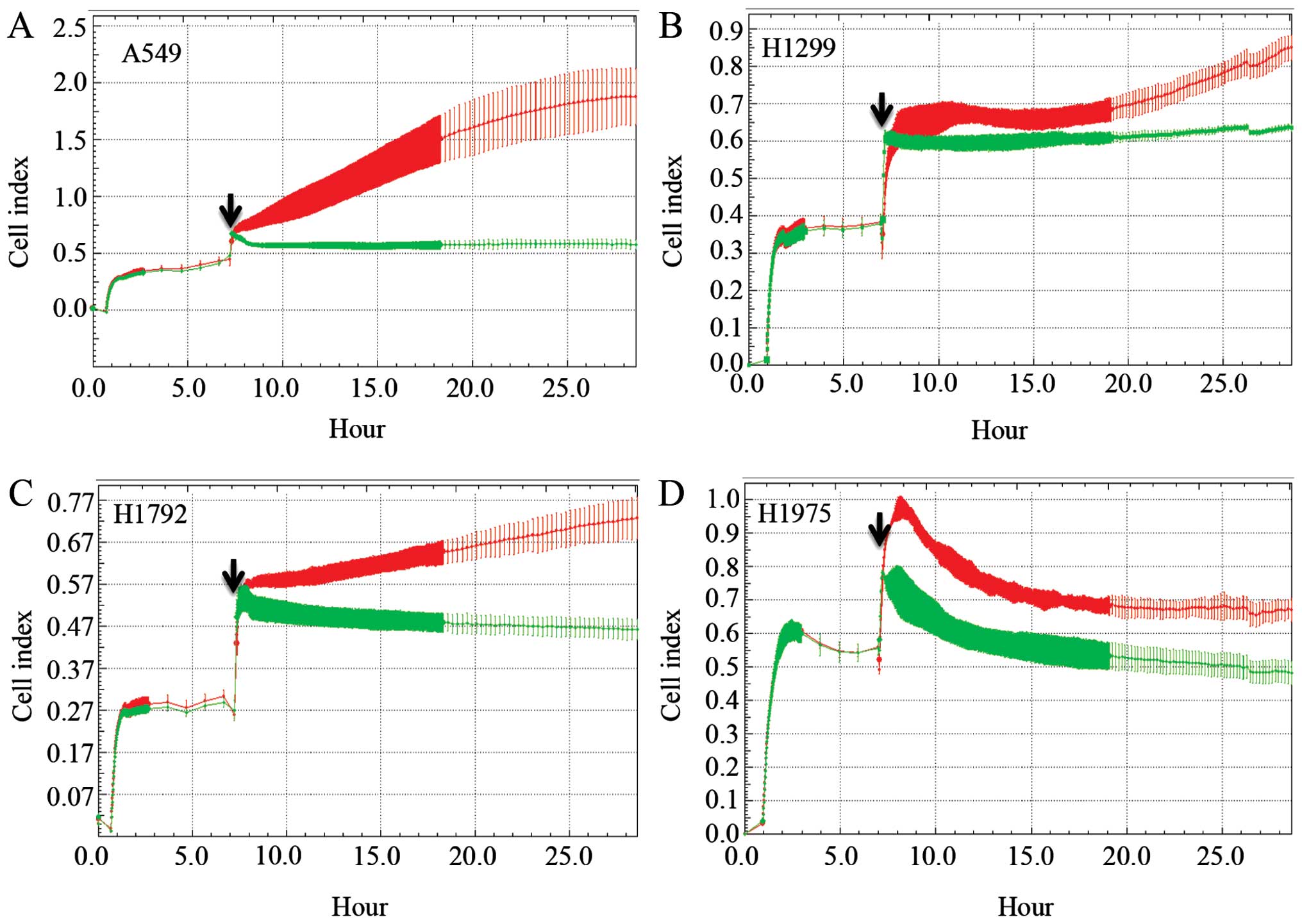
|
Jarboe JS, Anderson JC, Duarte CW, et al:
MARCKS regulates growth and radiation sensitivity and is a novel
prognostic factor for glioma. Clin Cancer Res. 18:3030–3041. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Li T, Li D, Sha J, Sun P and Huang Y:
MicroRNA-21 directly targets MARCKS and promotes apoptosis
resistance and invasion in prostate cancer cells. Biochem Biophys
Res Commun. 383:280–285. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Masaki T, Tokuda M, Yoshida S, et al:
Comparison study of the expressions of myristoylated alanine-rich C
kinase substrate in hepatocellular carcinoma, liver cirrhosis,
chronic hepatitis, and normal liver. Int J Oncol. 26:661–671.
2005.PubMed/NCBI
|
|
6
|
Browne BC, Hochgräfe F, Wu J, et al:
Global characterization of signalling networks associated with
tamoxifen resistance in breast cancer. FEBS J. 280:5237–5257. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Techasen A, Loilome W, Namwat N, et al:
Myristoylated alanine-rich C kinase substrate phosphorylation
promotes cholangiocarcinoma cell migration and metastasis via the
protein kinase C-dependent pathway. Cancer Sci. 101:658–665. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Janku F, Garrido-Laguna I, Petruzelka LB,
Stewart DJ and Kurzrock R: Novel therapeutic targets in non-small
cell lung cancer. J Thorac Oncol. 6:1601–1612. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Newton AC: Lipid activation of protein
kinases. J Lipid Res. 50(Suppl): S266–S271. 2009. View Article : Google Scholar :
|
|
10
|
Engelman JA: Targeting PI3K signalling in
cancer: opportunities, challenges and limitations. Nat Rev Cancer.
9:550–562. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ellena JF, Burnitz MC and Cafiso DS:
Location of the myristoylated alanine-rich C-kinase substrate
(MARCKS) effector domain in negatively charged phospholipid
bicelles. Biophys J. 85:2442–2448. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wang J, Gambhir A, Hangyas-Mihalyne G,
Murray D, Golebiewska U and McLaughlin S: Lateral sequestration of
phosphatidylinositol 4,5-bisphosphate by the basic effector domain
of myristoylated alanine-rich C kinase substrate is due to
nonspecific electrostatic interactions. J Biol Chem.
277:34401–34412. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Tanabe A, Kamisuki Y, Hidaka H, Suzuki M,
Negishi M and Takuwa Y: PKC phosphorylates MARCKS Ser159 not only
directly but also through RhoA/ROCK. Biochem Biophys Res Commun.
345:156–161. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Thelen M, Rosen A, Nairn AC and Aderem A:
Regulation by phosphorylation of reversible association of a
myristoylated protein kinase C substrate with the plasma membrane.
Nature. 351:320–322. 1991. View
Article : Google Scholar : PubMed/NCBI
|
|
15
|
Clarke PR, Siddhanti SR, Cohen P and
Blackshear PJ: Okadaic acid-sensitive protein phosphatases
dephosphorylate MARCKS, a major protein kinase C substrate. FEBS
Lett. 336:37–42. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Stumpo DJ, Graff JM, Albert KA, Greengard
P and Blackshear PJ: Molecular cloning, characterization, and
expression of a cDNA encoding the ‘80- to 87-kDa’ myristoylated
alanine-rich C kinase substrate: a major cellular substrate for
protein kinase C. Proc Natl Acad Sci USA. 86:4012–4016. 1989.
View Article : Google Scholar
|
|
17
|
Hanada S, Kakehashi A, Nishiyama N, et al:
Myristoylated alanine-rich C-kinase substrate as a prognostic
biomarker in human primary lung squamous cell carcinoma. Cancer
Biomark. 13:289–298. 2013.PubMed/NCBI
|
|
18
|
Chen CH, Thai P, Yoneda K, Adler KB, Yang
PC and Wu R: A peptide that inhibits function of Myristoylated
Alanine-Rich C Kinase Substrate (MARCKS) reduces lung cancer
metastasis. Oncogene. 33:3696–3706. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Graff JM, Rajan RR, Randall RR, Nairn AC
and Blackshear PJ: Protein kinase C substrate and inhibitor
characteristics of peptides derived from the myristoylated
alanine-rich C kinase substrate (MARCKS) protein phosphorylation
site domain. J Biol Chem. 266:14390–14398. 1991.PubMed/NCBI
|
|
20
|
Solly K, Wang X, Xu X, Strulovici B and
Zheng W: Application of real-time cell electronic sensing (RT-CES)
technology to cell-based assays. Assay Drug Dev Technol. 2:363–372.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Nam HY, Han MW, Chang HW, et al:
Radioresistant cancer cells can be conditioned to enter senescence
by mTOR inhibition. Cancer Res. 73:4267–4277. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Jarboe JS, Jaboin JJ, Anderson JC, et al:
Kinomic profiling approach identifies Trk as a novel radiation
modulator. Radiother Oncol. 103:380–387. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Tu T, Thotala D, Geng L, Hallahan DE and
Willey CD: Bone marrow X kinase-mediated signal transduction in
irradiated vascular endothelium. Cancer Res. 68:2861–2869. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Yang ES, Wang H, Jiang G, et al:
Lithium-mediated protection of hippocampal cells involves
enhancement of DNA-PK-dependent repair in mice. J Clin Invest.
119:1124–1135. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
25
|
Nowsheen S, Bonner JA and Yang ES: The
poly(ADP-Ribose) polymerase inhibitor ABT-888 reduces
radiation-induced nuclear EGFR and augments head and neck tumor
response to radiotherapy. Radiother Oncol. 99:331–338. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Yang ES, Nowsheen S, Wang T, Thotala DK
and Xia F: Glycogen synthase kinase 3beta inhibition enhances
repair of DNA double-strand breaks in irradiated hippocampal
neurons. Neuro Oncol. 13:459–470. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Gao J, Aksoy BA, Dogrusoz U, et al:
Integrative analysis of complex cancer genomics and clinical
profiles using the cBio-Portal. Sci Signal. 6:pl12013.
|
|
28
|
Cerami E, Gao J, Dogrusoz U, et al: The
cBio cancer genomics portal: an open platform for exploring
multidimensional cancer genomics data. Cancer Discov. 2:401–404.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ohmori S, Sakai N, Shirai Y, et al:
Importance of protein kinase C targeting for the phosphorylation of
its substrate, myristoylated alanine-rich C-kinase substrate. J
Biol Chem. 275:26449–26457. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Franken NA, Rodermond HM, Stap J, Haveman
J and van Bree C: Clonogenic assay of cells in vitro. Nat Protoc.
1:2315–2319. 2006. View Article : Google Scholar
|
|
31
|
Chen X and Rotenberg SA: PhosphoMARCKS
drives motility of mouse melanoma cells. Cell Signal. 22:1097–1103.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Disatnik MH, Boutet SC, Pacio W, et al:
The bi-directional translocation of MARCKS between membrane and
cytosol regulates integrin-mediated muscle cell spreading. J Cell
Sci. 117:4469–4479. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Yarmola EG, Edison AS, Lenox RH and Bubb
MR: Actin filament cross-linking by MARCKS: characterization of two
actin-binding sites within the phosphorylation site domain. J Biol
Chem. 276:22351–22358. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Kalwa H and Michel T: The MARCKS protein
plays a critical role in phosphatidylinositol 4,5-bisphosphate
metabolism and directed cell movement in vascular endothelial
cells. J Biol Chem. 286:2320–2330. 2011. View Article : Google Scholar :
|