Introduction
Breast cancer in young women is associated with a
poor prognosis; in the absence of adjuvant treatment, approximately
half of the patients will die of metastatic disease. Ovarian
ablation was the first described form of endocrine therapy for
treating advanced breast cancer (1). Previous trials have also suggested
that ovarian ablation with surgery or radiation therapy could
reduce the risk of recurrence in young premenopausal women
(2). However, in recent years,
adjuvant chemotherapy has become the standard treatment for young
premenopausal patients with breast cancer that have a high risk of
systemic disease (3).
One important aspect of chemotherapy is the
toxicity, which can induce premature ovarian failure (POF). POF was
reported in 68% of women after cyclophosphamide, methotrexate, and
5-fluorouracil (CMF) regimens, and a POF higher rate was observed
in women treated with anthracycline-based regimens (4). This may be a serious problem for
young women in reproductive ages. Preserving fertility during
adjuvant chemotherapy has been an ongoing endeavor, since 1987
(data on file), when a nulliparous, just married, 33-year-old
woman, with high risk of breast cancer underwent
anthracycline-based adjuvant chemotherapy. She was treated with
buserelin, a luteinizing-hormone releasing hormone (LH-RH) agonist,
once daily in a nasal spray, during 6 months of chemotherapy. After
chemotherapy and radiation therapy, she completed two full term
pregnancies.
In the following years, we conducted a clinical
trial in premenopausal patients with early breast cancer, where
another LH-RH agonist, goserelin was given subcutaneously (3.6 mg)
every 28 days for 1 year, in addition to adjuvant chemotherapy. In
the early course of that study, we found that the treatment was
well tolerated and it protected long-term ovarian function
(5). In the second part of the
study, additional patients were recruited to receive 11.25 mg
goserelin every 84 days for 2 years. After a median follow-up of 48
months, patients that had received goserelin for 2 years showed a
lower rate of POF compared to controls, which was the main
objective of the study. Those patients also had a statistically
significant (P<0.005) improvement in disease-free survival (DFS)
compared to patients treated for 1 year (6). Furthermore, we found that the
majority of local-regional and systemic recurrences occurred during
the second year of the study in patients with
estrogen-receptor-positive (ER+) tumors. Therefore, we
amended the protocol: the LH-RH analogue therapy was continued for
two more years, and an aromatase inhibitor was added to the regimen
for patients with ER+ tumors. With this background, and
according to the latest data from the literature (7), the last 100 patients that entered the
study received anthracycline-taxane-based chemotherapy, concomitant
with goserelin therapy, continued for 5 years. Here, we report the
long-term clinical outcome of all 200 patients treated with ovarian
protection during chemotherapy, focusing on the outcome of patients
treated with goserelin for 5 years or less.
Materials and methods
Patients
Eligibility in the trial required documented
premenopausal status with follicle stimulating hormone (FSH) levels
<10 mU/ml, 17-β estradiol levels between 20 and 693 pg/ml, and
progesterone levels between 0.15 and 28 ng/ml. Inclusion criteria
comprised histologically proven breast cancer confined to the
breast and ipsilateral axilla (tumor-node-metastasis
classifications: T2–T3, N0–N3 and M0), unilateral or bilateral,
with positive or negative expression of the estrogen and
progesterone receptors (ER+ and PGR+ or
ER− and PGR−, respectively). Receptor
expression was determined by immunohistochemical testing of tumor
biopsies; results were considered positive when expression was
detected in >10% of tumor cells. All included women had
undergone a modified radical mastectomy or breast-conserving
surgery plus full axillary node dissection, followed by radiation
therapy. All patients had Eastern Co-operative Oncology Group
scores of 0 to 1 for performance status. Macroscopic metastatic
spread of the disease was excluded according to the usual criteria.
The following laboratory parameters were required: granulocyte
count ≥2,000/μl, platelet count ≥100,000/μl, hematocrit ≥30%, total
bilirubin and alanine aminotransferase (AST) levels ≤1.5 times the
upper limit of normal, serum creatinine concentration ≤1.8 mg/dl,
and left ventricular ejection fraction ≥50%. Patients were excluded
when they had histologically-documented metastases or malignancies
other than curatively treated skin or cervical cancer. All patients
provided written informed consent. Patients underwent clinical
follow-up examinations every 6 months. The study was performed
according to the Declaration of Helsinki, after obtaining local
Ethics Committee approval.
Treatment plan
The treatment of the first 100 patients was
previously described (5,6). At the start of the study, 3 weeks
after surgery, and 1 week before starting chemotherapy, the second
group of 100 patients received 11.4 mg goserelin every 84 days for
5 years. Chemotherapy consisted of 4 courses of
anthracycline-taxane therapy (epirubicin, 75 mg/m2 and
docetaxel, 75 mg/m2), followed by 6 courses of CMF (600
mg/m2 cyclophosphamide, 600 mg/m2
5-fluorouracil, and 40 mg/m2 methotrexate). Both
regimens were repeated every 3 weeks, while the latter regimen was
administered concomitant with radiation therapy.
Eleven patients with >10 positive axillary nodes
and a median age of 37 years, were treated with high-dose
chemotherapy and autologous, peripheral blood progenitor cell
(PBPC) transplantations (6). For
this protocol, 4 weeks after the fourth course of
anthracycline-based chemotherapy, one course of high-dose
chemotherapy (carboplatin, etopside and melphalan) was delivered
together with previously collected progenitor cells. One to 2
months after PBPC transplantation, patients received 6 courses of
CMF chemotherapy, administered every 21 days concomitant with
radiation therapy.
Twenty patients with tumors positive for the
oncogene, c-erb-B2, received trastuzumab. Following completion of
chemotherapy, all patients with ER+ tumors received the
aromatase inhibitor, exemestane (10 mg daily), for 5 years. After
aromatase inhibitor treatment, 28 high-risk patients with
ER+ tumors received 20 mg tamoxifen for 5 more years
(7).
During the study, all patients received supplements
of vitamin D, calcium and bisphosphonates. Radiation therapy was
delivered to all patients (74% treated with segmental mastectomies
and 26% treated with modified radical mastectomy) concomitant with
CMF, after the anthracycline-based chemotherapy.
Statistical analysis
The date of relapse was defined as the day that
recurrent disease was diagnosed. DFS was defined as the time from
the first LH-RH dose to the diagnosis of any relapse, the
appearance of a second primary cancer, or death, whichever occurred
first. DFS and overall survival (OS) were estimated with the
Kaplan-Meier, product-limit method (8). Survival curves of sub-groups of
patients were compared with the log-rank test. Adverse events were
monitored based on standard World Health Organization criteria
(9).
Data analyses were performed in March 2014. All
patients were evaluated according to the intention-to-treat
principle.
Results
Patient characteristics
The present study included 200 patients that had
been diagnosed with adenocarcinoma of the breast, stage PT2-3a,
N-/+, M0 and had undergone a modified radical mastectomy or breast
conserving surgery plus a full axillary node dissection. The first
cohort of patients entered the study between September 1993 and
August 2002; the second cohort entered between September 2002 and
June 2007. The baseline demographics and tumor characteristics of
the first and second cohorts are shown in Table I. All patients were fully evaluated
for POF. All patients had a good performance status, and the median
age was 43 years. All women were premenopausal at the time of
breast cancer diagnosis; they had normal levels of gonadotropins,
estradiol and progesterone. The first cohort comprised 42% of
patients with N0; in the second part of the trial, the proportion
with negative axillary nodes increased to 50%. This increase was
probably due to the lack of age-specific screening procedures and
to recent refinements in diagnostic procedures. However, although
this was not a randomized study, no statistically significant
difference was observed between the two cohorts of patients in
hormone receptor status (P<0.001), in tumor histology
(P<0.001) or in clinical stage (P<0.001) at diagnosis. The
first and second cohorts included 11 and 10 patients, respectively,
with more than ten positive axillary nodes. Treatment compliance
was excellent; all patients received the assigned treatments of the
LH-RH analogue, chemotherapy, radiotherapy and hormonal
therapy.
 | Table IPatient and tumor characteristics. |
Table I
Patient and tumor characteristics.
| Characteristics | N | N |
|---|
| Patients groups | 1–100 | 101–200 |
| Age (years) |
| Median | 43 | 43 |
| Range | 27–50 | 26–45 |
| Hormone receptors
status |
| ER+ | 52 | 56 |
| ER− | 48 | 44 |
| Tumor histology |
| Ductal
infiltrating | 76 | 85 |
| Lobular
infiltrating | 13 | 10 |
| Other | 11 | 5 |
| Grading |
| G1–G2 | 44 | 51 |
| G3 | 56 | 49 |
| Clinical stage |
| II A | 48 | 51 |
| II B | 16 | 13 |
| III A | 11 | 8 |
| III B | 16 | 18 |
| III C | 9 | 10 |
| Nodes |
| 0 | 42 | 50 |
| 1–3 | 40 | 32 |
| 4–9 | 7 | 8 |
| >10 | 11 | 10 |
| Type of primary
surgery |
| Mastectomy | 32 | 29 |
|
Quadrantectomy | 68 | 71 |
Fertility, time to progression and
survival
After a median follow-up of 105 months (range,
65–180), all patients had completed chemotherapy and LH-RH analogue
treatment. When treatment was discontinued, a total of 132 women
(66%) resumed normal menses, with appropriate levels of FSH, LH,
17-β estradiol and progesterone. Moreover, all women under 40 years
old resumed normal menses, including 10 women treated with
high-dose chemotherapy and PBPC transplantation. One of the
patients treated with high-dose chemotherapy and PBPC
transplantation became pregnant and elected to terminate the
pregnancy. Five years after chemotherapy and radiotherapy, 6
patients had 7 babies.
Six patients experienced local disease recurrence
after a median of 40 months (range, 26–111). Recurrences were
treated with additional loco-regional and systemic therapies, as
necessary. All patients are currently disease-free, after a median
of 90 months. Three women experienced contra-lateral breast cancer,
with ER− and PR− tumors. They were salvaged
with radical mastectomy, and all three patients have remained
disease-free to date. Another 25 patients (12.5%) experienced
systemic recurrences: 10 died within the first 5 years, and the
other 15 patients lived for a median of 98 months (range, 74–170
months). At 5 years, 89.5% of patients were free from breast cancer
recurrence at a distant site. For patients with recurrences, the
median survival times were 50 months for those with ER+
and 59 months for those with ER− tumors. The actuarial
median time to progression and overall survival rates of all 200
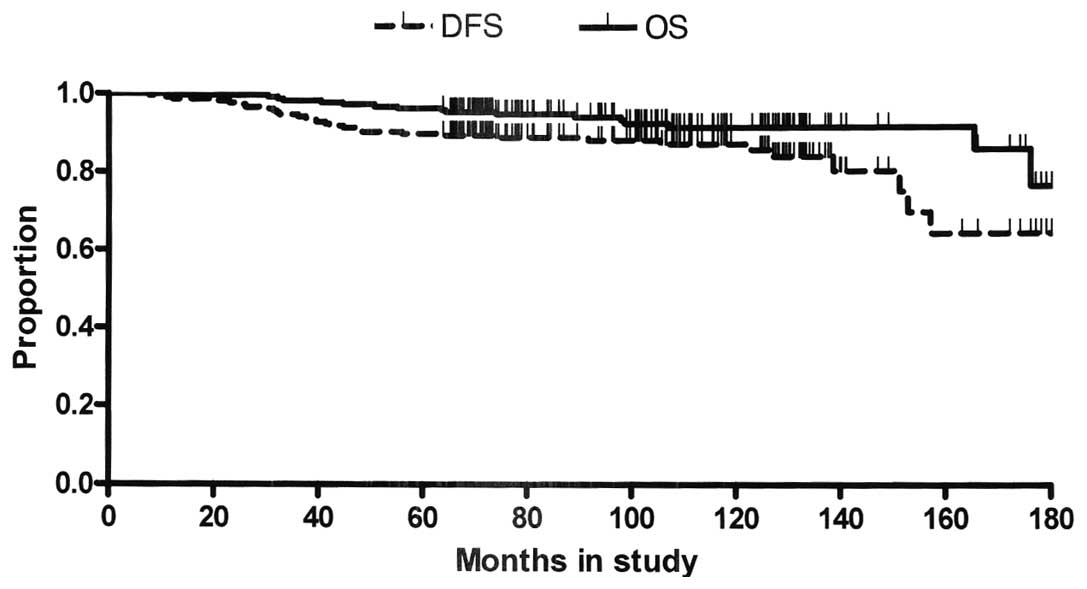
patients (Fig. 1) have not been
reached yet, as 64% of patients were disease free and 73% were
alive when the analysis of the data was performed. The projected
DFS and OS rates at 10 years were 86 and 91%, respectively.
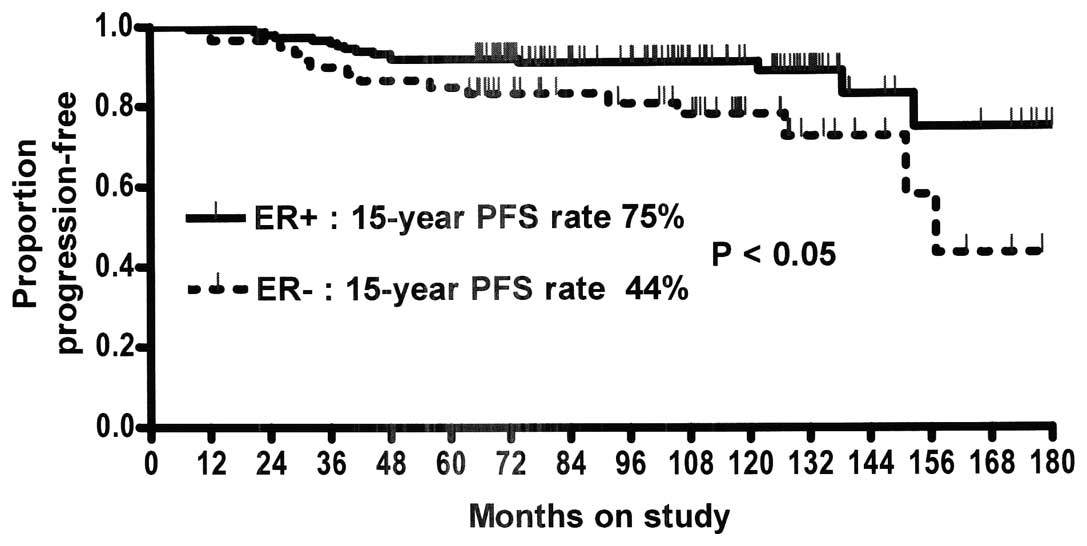
Patients with ER+ tumors showed a statistically
significant improvement in the 15-year DFS rate compared to
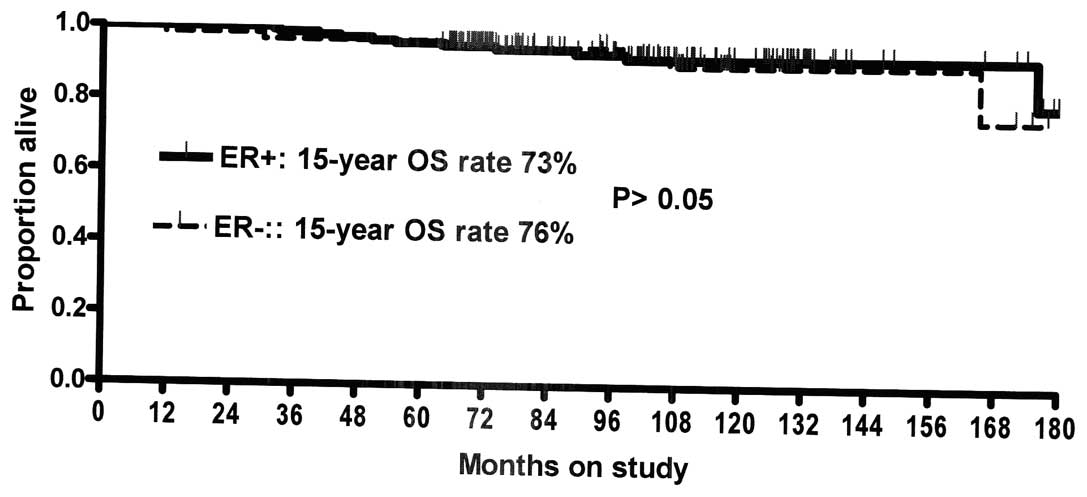
patients with ER− tumors (Fig. 2). However, these groups showed no
statistically significant difference in their 15-year OS rate
(Fig. 3). Patients with positive
and negative node tumors showed no statistically significant
difference in DFS and OS; however, patients with >5 positive
axillary nodes had higher DFS (P<0.005) and OS (P<0.005)
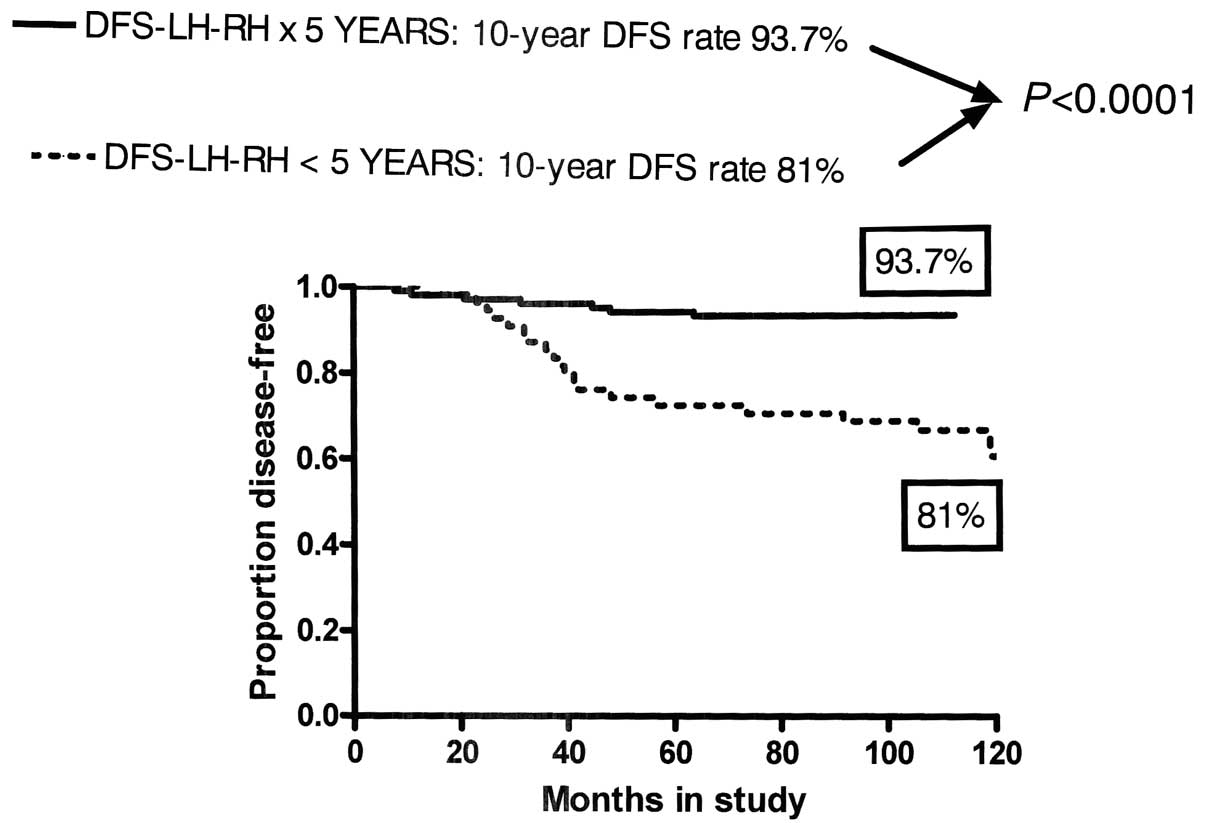
rates than patients with <5 involved nodes. Although there was
no statistically significant difference in baseline
characteristics, patients treated with the analogue for 5 years
showed highly significant improvements in both DFS (P<0.0001)
and OS (P<0.001) compared to patients treated for <5 years
(Figs. 4 and 5).
Toxicity
Adverse events reported during the chemotherapeutic
treatment are shown in Table
II.
 | Table IIGrade 3 and 4 toxicity. |
Table II
Grade 3 and 4 toxicity.
| Type of
therapy |
|---|
|
|
|---|
| LH-RH analogue (200
patients) No. (%) |
Anthracycline-taxanes (200 patients) No.
(%) | CMF + XRT (200
patients) No. (%) | HD-CT (68 patients)
No. (%) |
|---|
| Haematologic |
| Leukopenia | 0 | 64 (32%) | 6 (3%) | 68 (100%) |
|
Thrombocytopenia | 0 | 18 (9%) | 0 | 68 (100%) |
| Anemia | 0 | 18 (9%) | 0 | 4 (6%) |
|
Gastrointestinal |
|
Nausea-vomiting | 0 | 38 (19%) | 26 (13%) | 18 (26%) |
| Diarrhea | 0 | 12 (6%) | 6 (3%) | 9 (13%) |
| Mucositis | 0 | 10 (5%) | 16 (8%) | 0 |
| Infection | 0 | 4 (2%) | 0 | 3 (4%) |
| Neurotoxicity grade
2 | 0 | 20 (10%) | 0 | 0 |
| Alopecia | 0 | 200 (100%) | 0 | 68 (100%) |
| Osteopenia | 40 (20%) | | | |
| Hot flushes | 180 (90%) | 0 | 0 | 0 |
Goserelin
Fifty-six patients that received goserelin (56%)
complained of hot flushes.
CMF chemotherapy
No unexpected toxicity occurred during the
administration of CMF chemotherapy, and all 200 patients completed
the scheduled treatment. Hematological toxicity grade 2 occurred in
23% of patients. Grade 2 diarrhea occurred in 10% of patients, and
31% of patients reported nausea and vomiting. There were no
treatment-related deaths.
Anthracycline-taxane based
chemotherapy
Grades 3–4 hematological toxicity occurred in 30% of
patients treated with anthracycline-taxane based chemotherapy.
Gastrointestinal toxicity symptoms were observed in 5% (diarrhea)
and 3% (mucositis) of patients. Severe nausea and vomiting occurred
in 13% of patients. Infection was reported in 3% of patients. Grade
3 alopecia was observed in all patients. No significant cardiac
dysfunction was observed in any patient, and there were no
treatment-related deaths.
High-dose chemotherapy
Nausea and vomiting and other gastrointestinal
toxicity occurred in 30% of patients, but they only reached grades
2–3 severities, due to the appropriate use of antiemetic
medications. Grade 4 neutropenia and thrombocytopenia were observed
in all patients. An absolute neutrophil count of
<5×103/ml was observed for a median of 4.5 days
(range, 3–5 days), and a platelet count of <20×103/ml
occurred for a median of 1 day (range, 0–3 days). Six patients
required a platelet transfusion (median of 2 units). Anemia, which
occurred infrequently, due to the use of erythropoietin, occurred
in 20% of patients. Six patients had fevers >38°C for a median
of 3 days (range, 0–6). Grade 2 mucositis occurred in 6 patients,
and grade 3 diarrhea occurred in 4 patients (20%). One patient had
a documented infection, where the blood culture was positive for
staphylococcus epidermidis. Three patients reported bone
pain for a median 2-day duration. There were no treatment-related
deaths.
Discussion
Due to the high risk of recurrence in premenopausal
breast cancer, we attempted to achieve the best results by
combining all available treatment modalities, including surgery,
hormonal therapy, chemotherapy and radiation therapy, as
appropriate. After the surgical procedure, one week before starting
adjuvant chemotherapy, an LH-RH analogue was administered to the
second cohort of 100 patients. This therapy was continued for 5
years for a dual purpose: first, to protect the ovaries from the
damage of chemotherapy, and second, to reduce the amount of
circulating estrogen, a fundamental element for breast cancer cell
survival (10). In fact, it has
been shown that the hormonal surge associated with resumption or
persistence of menses, after chemotherapy is a negative prognostic
factor for DFS in premenopausal patients with early breast cancer
(11). Radiation therapy was
administered after chemotherapy, concurrent with the CMF regimen,
to guarantee complete sterilization of the axilla and
supraclavicular region (12,13).
The use of chemotherapy in the adjuvant treatment of
premenopausal breast cancer presents a trade-off between the
benefit of reducing the odds of recurrence and the risk of damaging
ovarian function and subsequent POF. Women have a fixed number of
oocytes, which decreases with age (14). Hence, the severity of ovarian
damage induced by chemotherapy depends on the age of the patient,
the type of drug used, and the duration of exposure. Alkylating
agents run the highest risk of ovarian damage, due to their highly
reactive molecules. Agents that present intermediate risk include
antibiotics, taxanes, anthracyclines and platinum derivates. Agents
that are considered low risk include bleomycin, vinka alkaloids,
and antimetabolites, such as 5-fluorouracil and methotrexate.
In recent years, progress in premenopausal breast
cancer treatment has enabled some improvement in clinical outcome.
In fact, in 1997, a randomized trial that enrolled 852 high-risk
patients treated with combined CMF, chemotherapy, and radiation
therapy, showed a 10-year OS rate of 54% (15). In 2013, another trial with 3,312
patients that did not carry BRCA1, were treated with
anthracycline-taxane-based chemotherapy and showed a 10-year OS
rate of 82.2% (16).
We used anthracycline-based adjuvant chemotherapy,
because it has been shown to be more effective than CMF, and it
offered the best results in premenopausal patients when
administered in the sequence of adriamycin-CMF-goserelin (17). Additionally, the Early Breast
Cancer Trialists’ Collaborative Group has shown that 6 months of
anthracycline-based poly-chemotherapy (e.g., with fluorouracil,
cyclophosphamide and either doxorubicin or epirubicin) reduced the
annual breast cancer-related death rate by ~38% in women younger
than 50 years of age. Those results were largely independent of
tamoxifen use, estrogen receptor status, nodal status, or other
tumor characteristics. Furthermore, for ER+ disease
only, 5 years of adjuvant tamoxifen reduced the annual breast
cancer-related death rate by 31% (7).
Several studies have addressed the role of LH-RH
analogues during adjuvant chemotherapy. Adjuvant chemotherapy with
CMF was compared directly with goserelin in the treatment of
premenopausal, node-negative breast cancer. Patients with
ER− tumors achieved higher DFS rates when they received
CMF than when they received goserelin alone (84 vs. 73%). In
contrast, in patients with ER+ disease, chemotherapy
alone and goserelin alone produced similar results (18). Complete hormonal blockade with an
LH-RH analogue and tamoxifen for three years was compared to the
fluorouracil, epirubicin, and cyclophosphamide regimen in a group
of 333 patients with intermediate risk breast cancer. That
randomized study found no statistically significant difference in
either DFS or OS between the two groups of patients (19). In another study, goserelin alone
did not significantly reduce recurrence or death after recurrence
in patients with hormone-receptor-positive cancers; however, the
addition of LH-RH agonists to tamoxifen, chemotherapy, or both
reduced recurrence by 12.7% and death after recurrence by 15.1%. In
that group of patients, the addition of LH-RH agonists showed
similar efficacy to chemotherapy alone. However, the duration of
LH-RH analogue administration was not assessed. Moreover, no trial
had assessed an LH-RH agonist vs. chemotherapy. However, LH-RH
agonists were shown to be ineffective in hormone-receptor-negative
tumors (20).
Previous studies have reported conflicting results
regarding the utility of LH-RH analogues for avoiding POF. A
recently published study found that it was uncertain whether LH-RH
agonists were useful for preserving fertility in young patients
with breast cancer (21). A recent
meta-analysis calculated a pooled risk estimate and found a highly
significant reduction in the risk of POF (OR, 0.43; 95% CI,
0.22–0.84; P=0.013) in patients that received LH-RH analogues
(22).
More recently, attention has focused on the
potential effects of LH-RH analogues on survival of premenopausal
patients with breast cancer. Two recent studies have shed light on
the utility of LH-RH analogues in treating premenopausal patients
with breast cancers that were ER+ (23) and ER− (24). In the first study (2003–2011),
4,690 premenopausal women with hormone-receptor-positive early
breast cancer were randomized to receive either exemestane plus
ovarian suppression or tamoxifen plus ovarian suppression. After a
median follow-up of 68 months, patients that received exemestane
plus ovarian suppression showed significant reductions in
recurrence compared to patients that received tamoxifen plus
ovarian suppression. Moreover, the 5-year DFS rates were 91.1 and
87.3%, respectively (P<0.001), and OS rates were 95.9 and 96.9%,
respectively (P=0.37). However, it should be noted that 57.8% of
patients in that study (23) had
negative axillary nodes. In addition, a large proportion of
patients in that study (1,996 patients) had low risk of recurrence
and did not receive chemotherapy.
The second study was the phase III Prevention of
Early Menopause Study (POEMS)/Southwest Oncology Group (SWOG) S0230
(2004 to 2011). That study evaluated the rate of POF in
premenopausal women, aged 18–49, with stage I to IIIA
ER− and PR− breast cancers (24). After considering all the drop outs,
the remaining fully evaluable patients included 69 randomized to
chemotherapy alone and 66 randomized to chemotherapy plus
goserelin. At 2 years, 21% of patients treated with chemotherapy
had POF, and only 7.5% of patients treated with chemotherapy plus
goserelin had POF, which represented a 70% reduction. Patients
treated with chemotherapy alone had 4-year DFS and OS rates of 78
and 82%, respectively; patients treated with chemotherapy plus
goserelin had 4-year DFS and OS rates of 89 and 92%,
respectively.
Although the patients in the present study were not
randomized, they reflected a population of patients at mid-high
risk for breast cancer that were recruited sequentially in a
general community hospital. We analyzed the potential benefit of
LH-RH analogues in preventing POF with a long follow-up; the median
was 105 months (range, 65–180). For comparison, the two studies
described above had median follow-ups of 68 months (23) and 49.2 months (24). Our 4-year DFS and OS rates in
patients with ER− tumors were 84 and 96%, respectively.
Our 5-year DFS and OS rates in patients with ER+ tumors
were 92 and 96 %, respectively. The entire cohort had 10-year DFS
and OS rates of 85 and 91%, respectively, and 15-year DFS and OS
rates of 64 and 73%, respectively. Among our cohort of 200
patients, 142 patients with N+ and ER+ tumors
had 9-year DFS and OS rates of 91 and 92%, respectively. These
rates were consistent with a cohort of 507 patients, described in
2005, that had 9-year DFS and OS rates of 68 and 76%, respectively
(25).
In conclusion, the present phase II trial had a much
longer follow-up than previous studies of this kind. We confirmed
the results obtained in a previous randomized trial (23) for premenopausal patients with
hormone receptor positive tumors, which showed that the addition of
an LH-RH analogue to adjuvant chemotherapy, followed by examestane,
provided a new treatment option that reduced the risk of
recurrence. We also confirmed previous findings that the same
treatment reduced the risk of breast cancer recurrence in
premenopausal patients with hormone receptor negative tumors
(24).
References
|
1
|
Beatson GT: On the treatment of inoperable
cases of carcinoma of the mamma: suggestions for a new method of
treatment with illustrative cases. Lancet ii. 104–107. 1896.
View Article : Google Scholar
|
|
2
|
Clarke MG: Ovarian ablation in breast
cancer 1896–1998. Milestones along hierarchy from evidence from
case report to Chochrane review. BMJ. 317:1246–1248. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Adjuvant chemotherapy for breast cancer.
NIH Consensus Statement Online. 5:1–19. 1985.
|
|
4
|
Bines J, Oleske DM and Cobleigh MA:
Ovarian function in premenopausal women treated with adjuvant
chemotherapy for breast cancer. J Clin Oncol. 14:1718–1729.
1996.PubMed/NCBI
|
|
5
|
Recchia F, Sica G, De Filippis S, Rosselli
M and Rea S: Goserelin as ovarian proitection in the adjuvant
treatment of premenopausal breast cancer: a phase II pilot study.
Anticancer Drugs. 13:417–424. 2001. View Article : Google Scholar
|
|
6
|
Recchia F, Saggio G, Amiconi G, Di Blasio
A, Cesta A, Candeloro G and Rea S: Gonadotropin-releasing hormone
analogues added to adjuvant chemotherapy protect ovarian function
and improve clinical outcomes in young women with early breast
carcinoma. Cancer. 106:514–523. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Early Breast Cancer Trialists’
Collaborative Group (EBCTCG). Effects of chemotherapy and hormonal
therapy for early breast cancer on recurrence and 15-year survival:
an overview of the randomised trials. Lancet. 365:1687–1717. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kaplan EL and Meier P: Nonparametric
estimation from incomplete observations. J Am Stat Ass. 53:457–481.
1958. View Article : Google Scholar
|
|
9
|
Therasse P, Arbuck SG, Eisenhauer EA, et
al: New guidelines to evaluate the response to treatment in solid
tumors: European Organization for Research and Treatment of Cancer,
National Cancer Institute of the United States, National Cancer
Institute of Canada. J Natl Cancer Inst. 92:205–216. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Endogenous Hormones and Breast Cancer
Collaborative Group. Key TJ, Appleby PN, Reeves GK, et al: Sex
hormones and risk of breast cancer in premenopausal women: a
collaborative reanalysis of individual participant data from seven
prospective studies. Lancet Oncol. 14:1009–1019. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Park IH, Han HS, Lee H, et al: Resumption
or persistence of menstruation after cytotoxic chemotherapy is a
prognostic factor for poor disease-free survival in premenopausal
patients with early breast cancer. Ann Oncol. 23:2283–2289. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ragaz J, Jackson SM, Le N, et al: Adjuvant
radiotherapy and chemotherapy in node-positive premenopausal women
with breast cancer. N Engl J Med. 337:956–962. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Toledano A, Azria D, Garaud P, et al:
Phase III trial of concurrent or sequential adjuvant
chemoradiotherapy after conservative surgery for early-stage breast
cancer: final results of the ARCOSEIN trial. J Clin Oncol.
25:405–410. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Meirow D, Biederman H, Anderson RA and
Wallace WH: Toxicity of chemotherapy and radiation on female
reproduction. Clin Obstet Gynecol. 53:727–739. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Overgaard M, Hansen PS, Overgaard J, et
al; for the Danish Breast Cancer Cooperative Group 82b Trial.
Postoperative radiotherapy in high-risk premenopausal women with
breast cancer who receive adjuvant chemotherapy. New Eng J Med.
337:949–955. 1997. View Article : Google Scholar
|
|
16
|
Huzarski T, Byrski T, Gronwald J, et al:
Ten-year survival in patients with BRCA1-negative and
BRCA1-positive breast cancer. J Clin Oncol. 31:3191–3196. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
De Placido S, De Laurentiis M, De Lena M,
et al: GOCSI Cooperative Group: A randomised factorial trial of
sequential doxorubicin and CMF vs CMF and chemotherapy alone vs
chemotherapy followed by goserelin plus tamoxifen as adjuvant
treatment of node-positive breast cancer. Br J Cancer. 92:467–474.
2005.PubMed/NCBI
|
|
18
|
International Breast Cancer Study Group
(IBCSG). Castiglione-Gertsch M, O’Neill A, Price KN, et al:
Adjuvant chemotherapy followed by goserelin versus either modality
alone for premenopausal lymph node-negative breast cancer: a
randomized trial. J Natl Cancer Inst. 95:1833–1846. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Roché H, Kerbrat P, Bonneterre J, et al:
Complete hormonal blockade versus epirubicin-based chemotherapy in
premenopausal, one to three node-positive, and hormone-receptor
positive, early breast cancer patients: 7-year follow-up results of
French Adjuvant Study Group 06 randomised trial. Ann Oncol.
17:1221–1227. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
LHRH-agonists in Early Breast Cancer
Overview Group. Cuzick J, Ambroisine L, Davidson N, Jakesz R,
Kaufmann M, Regan M and Sainsbury R: Use of
luteinising-hormone-releasing hormone agonists as adjuvant
treatment in premenopausal patients with hormone-receptor-positive
breast cancer: a meta-analysis of individual patient data from
randomised adjuvant trials. Lancet. 369:1711–1723. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Turner NH, Partridge A, Sanna G, Di Leo A
and Biganzoli L: Utility of gonadotropin-releasing hormone agonists
for fertility preservation in young breast cancer patients: the
benefit remains uncertain. Ann Oncol. 24:2224–2235. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Del Mastro L, Ceppi M, Poggio F, et al:
Gonadotropin-releasing hormone analogues for the prevention of
chemotherapy-induced premature ovarian failure in cancer women:
systematic review and meta-analysis of randomized trials. Cancer
Treat Rev. 40:675–683. 2014. View Article : Google Scholar
|
|
23
|
Pagani O, Regan MM, Walley BA, et al; TEXT
and SOFT Investigators and the International Breast Cancer Study
Group. Adjuvant exemestane with ovarian suppression in
premenopausal breast cancer. N Engl J Med. 371:107–118. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Moore HCF, Unger JM, Phillips KA, et al:
Phase III trial of LHRH analog during chemotherapy to reduce
ovarian failure in early-stage, hormone receptor-negative breast
cancer. In: ASCO Annual Meeting Presented; May 31, 2014; J Clin
Oncol. 32(5s): pp. LBA5052014
|
|
25
|
Davidson NE, O’Neill AM, Vukov AM, Osborne
CK, Martino S, White DR and Abeloff MD: Chemoendocrine therapy for
premenopausal women with axillary lymph node positive, steroid
hormone receptor-positive breast cancer: results from INT 0101
(E5188). J Clin Oncol. 23:5973–5982. 2005. View Article : Google Scholar : PubMed/NCBI
|