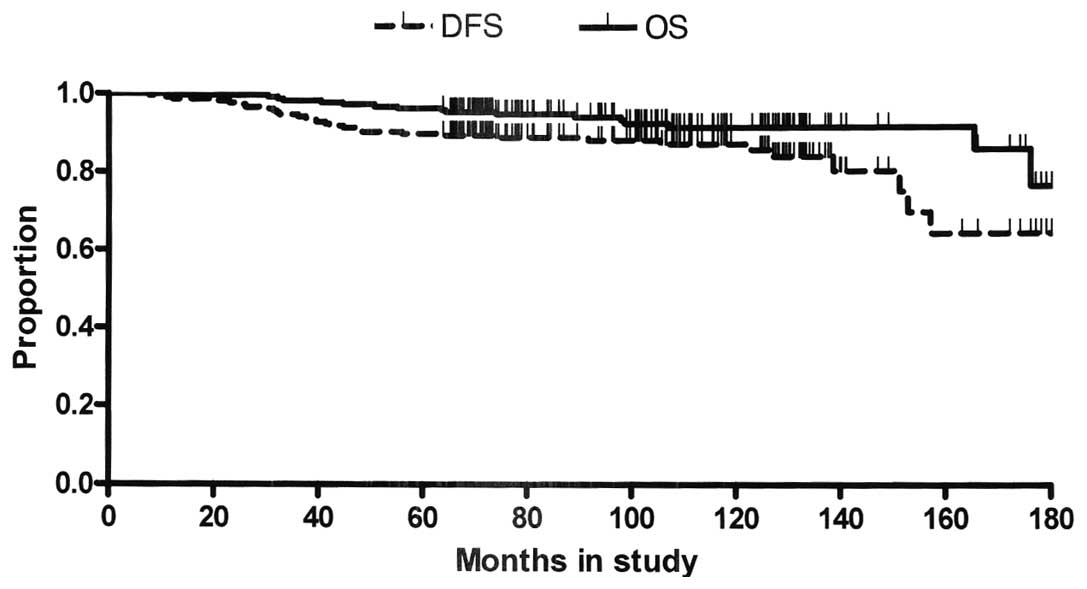
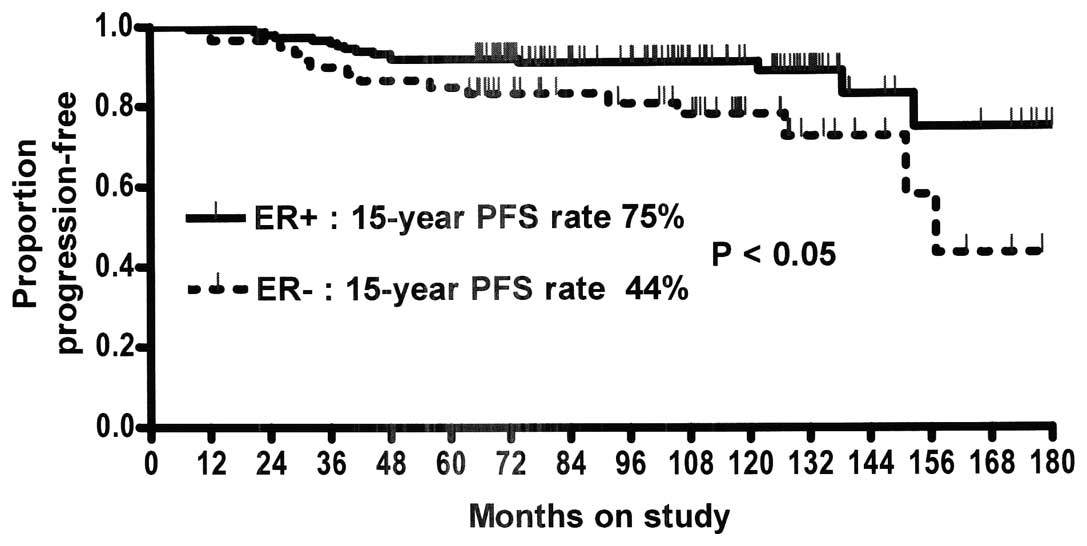
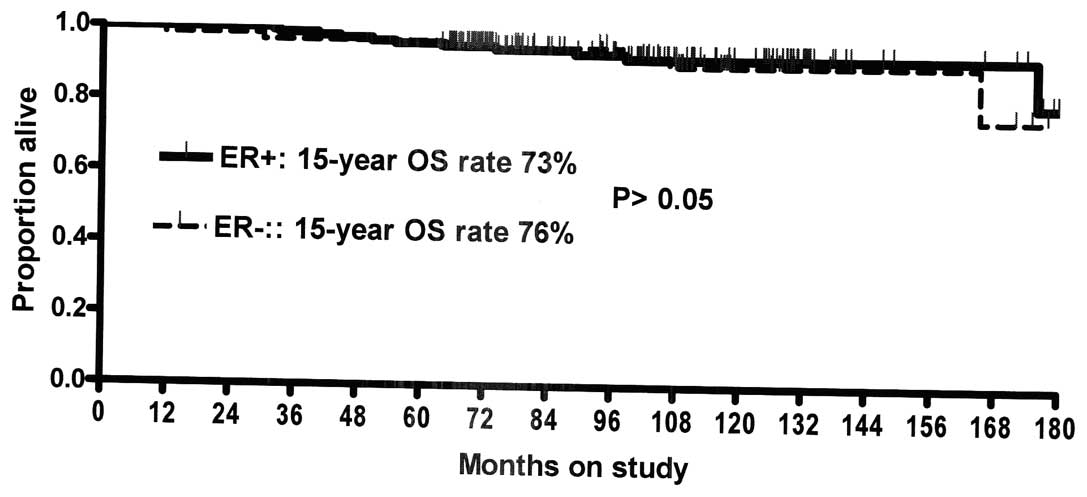
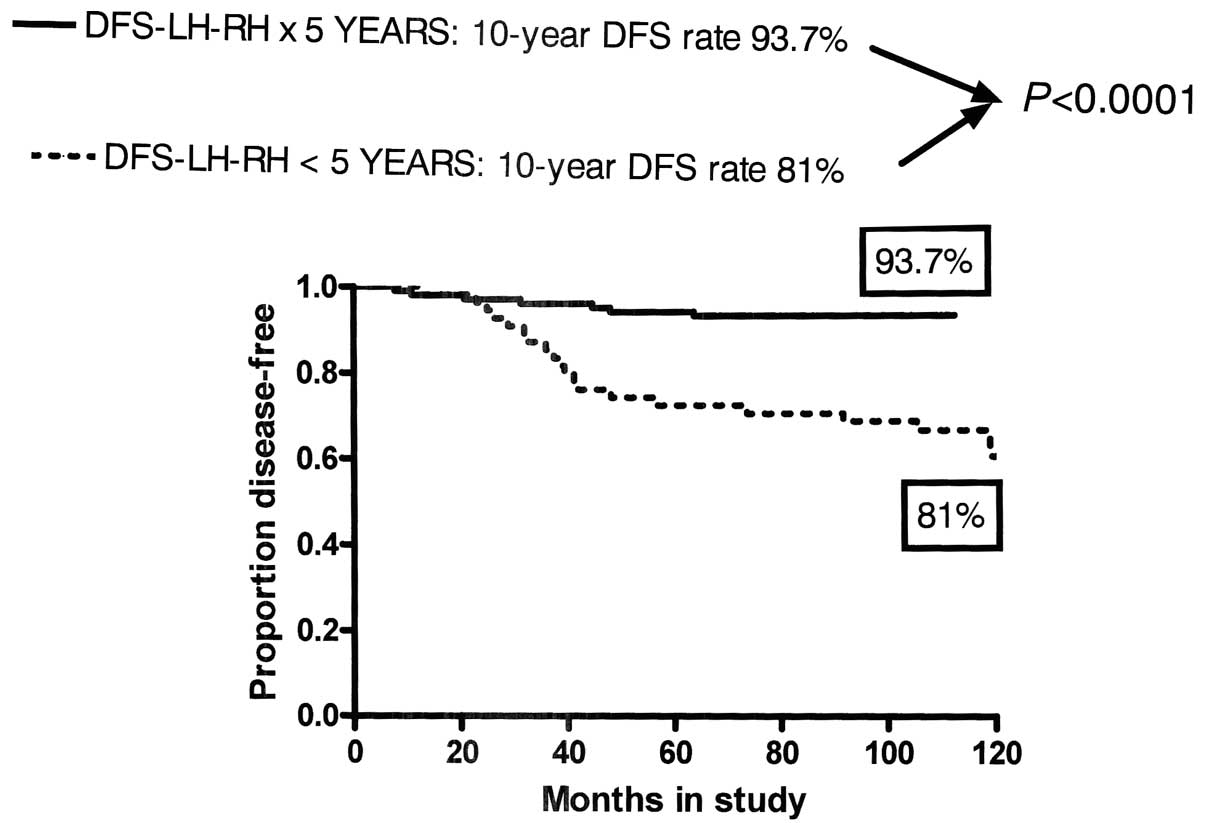
|
1
|
Beatson GT: On the treatment of inoperable
cases of carcinoma of the mamma: suggestions for a new method of
treatment with illustrative cases. Lancet ii. 104–107. 1896.
View Article : Google Scholar
|
|
2
|
Clarke MG: Ovarian ablation in breast
cancer 1896–1998. Milestones along hierarchy from evidence from
case report to Chochrane review. BMJ. 317:1246–1248. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Adjuvant chemotherapy for breast cancer.
NIH Consensus Statement Online. 5:1–19. 1985.
|
|
4
|
Bines J, Oleske DM and Cobleigh MA:
Ovarian function in premenopausal women treated with adjuvant
chemotherapy for breast cancer. J Clin Oncol. 14:1718–1729.
1996.PubMed/NCBI
|
|
5
|
Recchia F, Sica G, De Filippis S, Rosselli
M and Rea S: Goserelin as ovarian proitection in the adjuvant
treatment of premenopausal breast cancer: a phase II pilot study.
Anticancer Drugs. 13:417–424. 2001. View Article : Google Scholar
|
|
6
|
Recchia F, Saggio G, Amiconi G, Di Blasio
A, Cesta A, Candeloro G and Rea S: Gonadotropin-releasing hormone
analogues added to adjuvant chemotherapy protect ovarian function
and improve clinical outcomes in young women with early breast
carcinoma. Cancer. 106:514–523. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Early Breast Cancer Trialists’
Collaborative Group (EBCTCG). Effects of chemotherapy and hormonal
therapy for early breast cancer on recurrence and 15-year survival:
an overview of the randomised trials. Lancet. 365:1687–1717. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kaplan EL and Meier P: Nonparametric
estimation from incomplete observations. J Am Stat Ass. 53:457–481.
1958. View Article : Google Scholar
|
|
9
|
Therasse P, Arbuck SG, Eisenhauer EA, et
al: New guidelines to evaluate the response to treatment in solid
tumors: European Organization for Research and Treatment of Cancer,
National Cancer Institute of the United States, National Cancer
Institute of Canada. J Natl Cancer Inst. 92:205–216. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Endogenous Hormones and Breast Cancer
Collaborative Group. Key TJ, Appleby PN, Reeves GK, et al: Sex
hormones and risk of breast cancer in premenopausal women: a
collaborative reanalysis of individual participant data from seven
prospective studies. Lancet Oncol. 14:1009–1019. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Park IH, Han HS, Lee H, et al: Resumption
or persistence of menstruation after cytotoxic chemotherapy is a
prognostic factor for poor disease-free survival in premenopausal
patients with early breast cancer. Ann Oncol. 23:2283–2289. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ragaz J, Jackson SM, Le N, et al: Adjuvant
radiotherapy and chemotherapy in node-positive premenopausal women
with breast cancer. N Engl J Med. 337:956–962. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Toledano A, Azria D, Garaud P, et al:
Phase III trial of concurrent or sequential adjuvant
chemoradiotherapy after conservative surgery for early-stage breast
cancer: final results of the ARCOSEIN trial. J Clin Oncol.
25:405–410. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Meirow D, Biederman H, Anderson RA and
Wallace WH: Toxicity of chemotherapy and radiation on female
reproduction. Clin Obstet Gynecol. 53:727–739. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Overgaard M, Hansen PS, Overgaard J, et
al; for the Danish Breast Cancer Cooperative Group 82b Trial.
Postoperative radiotherapy in high-risk premenopausal women with
breast cancer who receive adjuvant chemotherapy. New Eng J Med.
337:949–955. 1997. View Article : Google Scholar
|
|
16
|
Huzarski T, Byrski T, Gronwald J, et al:
Ten-year survival in patients with BRCA1-negative and
BRCA1-positive breast cancer. J Clin Oncol. 31:3191–3196. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
De Placido S, De Laurentiis M, De Lena M,
et al: GOCSI Cooperative Group: A randomised factorial trial of
sequential doxorubicin and CMF vs CMF and chemotherapy alone vs
chemotherapy followed by goserelin plus tamoxifen as adjuvant
treatment of node-positive breast cancer. Br J Cancer. 92:467–474.
2005.PubMed/NCBI
|
|
18
|
International Breast Cancer Study Group
(IBCSG). Castiglione-Gertsch M, O’Neill A, Price KN, et al:
Adjuvant chemotherapy followed by goserelin versus either modality
alone for premenopausal lymph node-negative breast cancer: a
randomized trial. J Natl Cancer Inst. 95:1833–1846. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Roché H, Kerbrat P, Bonneterre J, et al:
Complete hormonal blockade versus epirubicin-based chemotherapy in
premenopausal, one to three node-positive, and hormone-receptor
positive, early breast cancer patients: 7-year follow-up results of
French Adjuvant Study Group 06 randomised trial. Ann Oncol.
17:1221–1227. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
LHRH-agonists in Early Breast Cancer
Overview Group. Cuzick J, Ambroisine L, Davidson N, Jakesz R,
Kaufmann M, Regan M and Sainsbury R: Use of
luteinising-hormone-releasing hormone agonists as adjuvant
treatment in premenopausal patients with hormone-receptor-positive
breast cancer: a meta-analysis of individual patient data from
randomised adjuvant trials. Lancet. 369:1711–1723. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Turner NH, Partridge A, Sanna G, Di Leo A
and Biganzoli L: Utility of gonadotropin-releasing hormone agonists
for fertility preservation in young breast cancer patients: the
benefit remains uncertain. Ann Oncol. 24:2224–2235. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Del Mastro L, Ceppi M, Poggio F, et al:
Gonadotropin-releasing hormone analogues for the prevention of
chemotherapy-induced premature ovarian failure in cancer women:
systematic review and meta-analysis of randomized trials. Cancer
Treat Rev. 40:675–683. 2014. View Article : Google Scholar
|
|
23
|
Pagani O, Regan MM, Walley BA, et al; TEXT
and SOFT Investigators and the International Breast Cancer Study
Group. Adjuvant exemestane with ovarian suppression in
premenopausal breast cancer. N Engl J Med. 371:107–118. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Moore HCF, Unger JM, Phillips KA, et al:
Phase III trial of LHRH analog during chemotherapy to reduce
ovarian failure in early-stage, hormone receptor-negative breast
cancer. In: ASCO Annual Meeting Presented; May 31, 2014; J Clin
Oncol. 32(5s): pp. LBA5052014
|
|
25
|
Davidson NE, O’Neill AM, Vukov AM, Osborne
CK, Martino S, White DR and Abeloff MD: Chemoendocrine therapy for
premenopausal women with axillary lymph node positive, steroid
hormone receptor-positive breast cancer: results from INT 0101
(E5188). J Clin Oncol. 23:5973–5982. 2005. View Article : Google Scholar : PubMed/NCBI
|