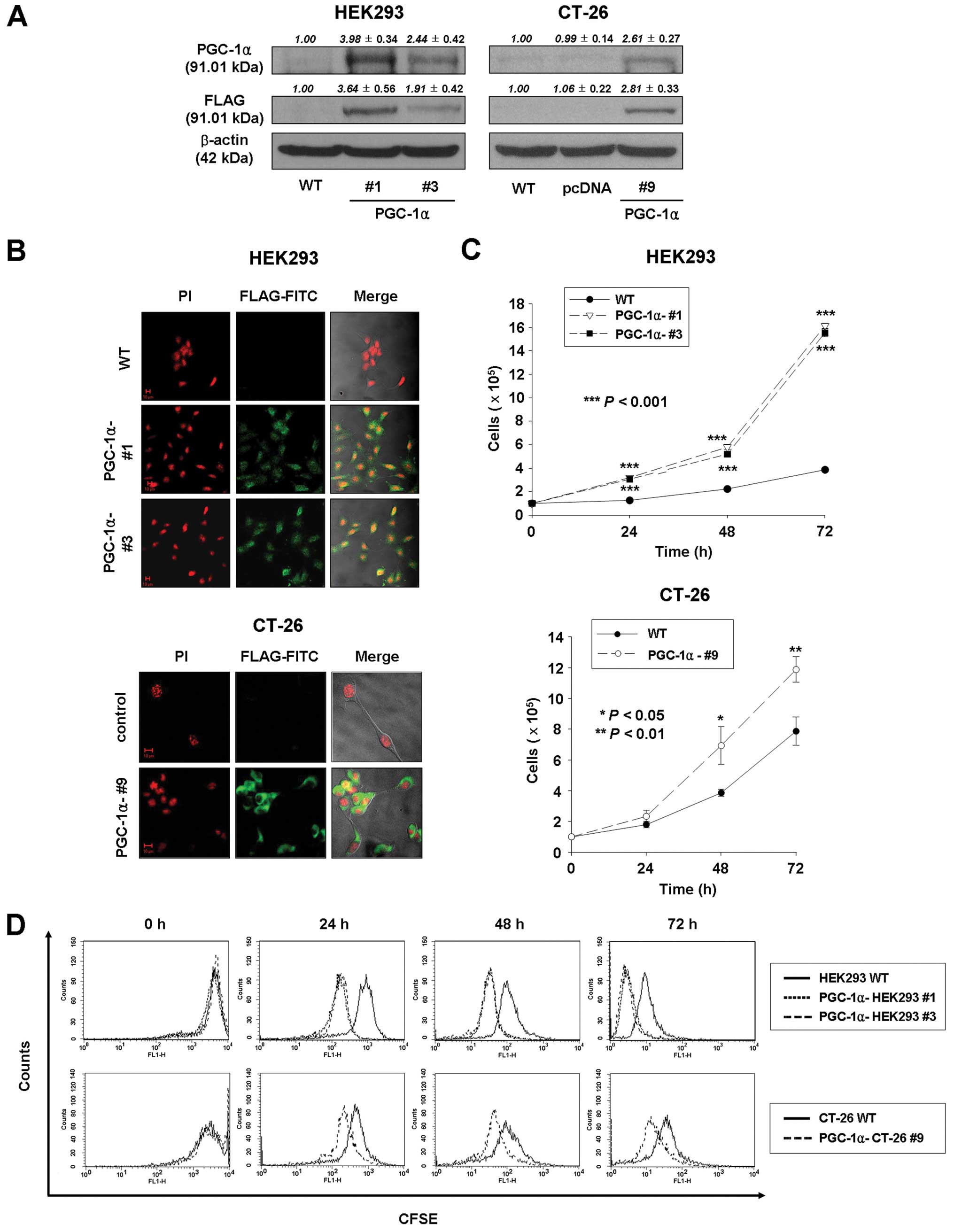
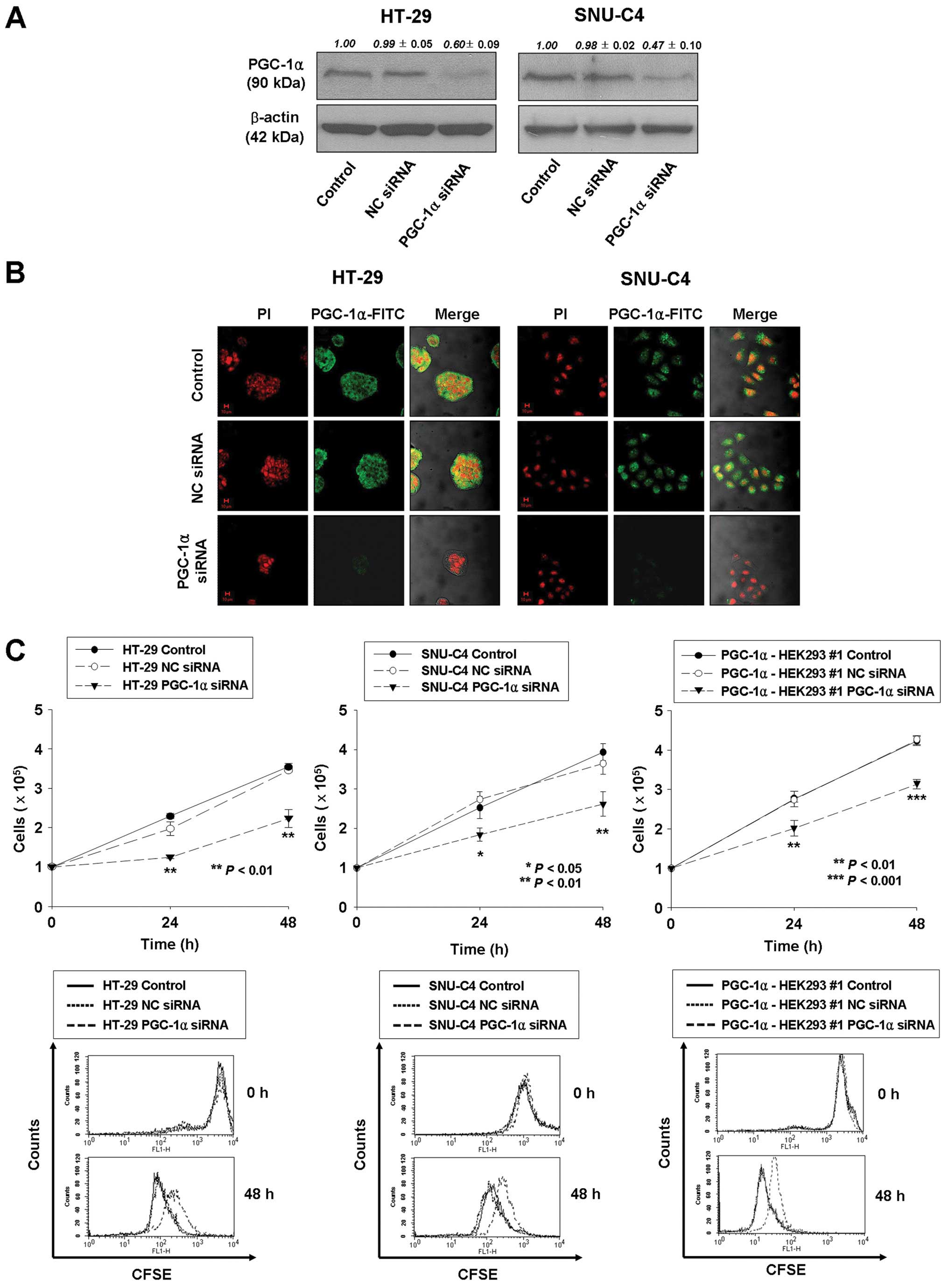
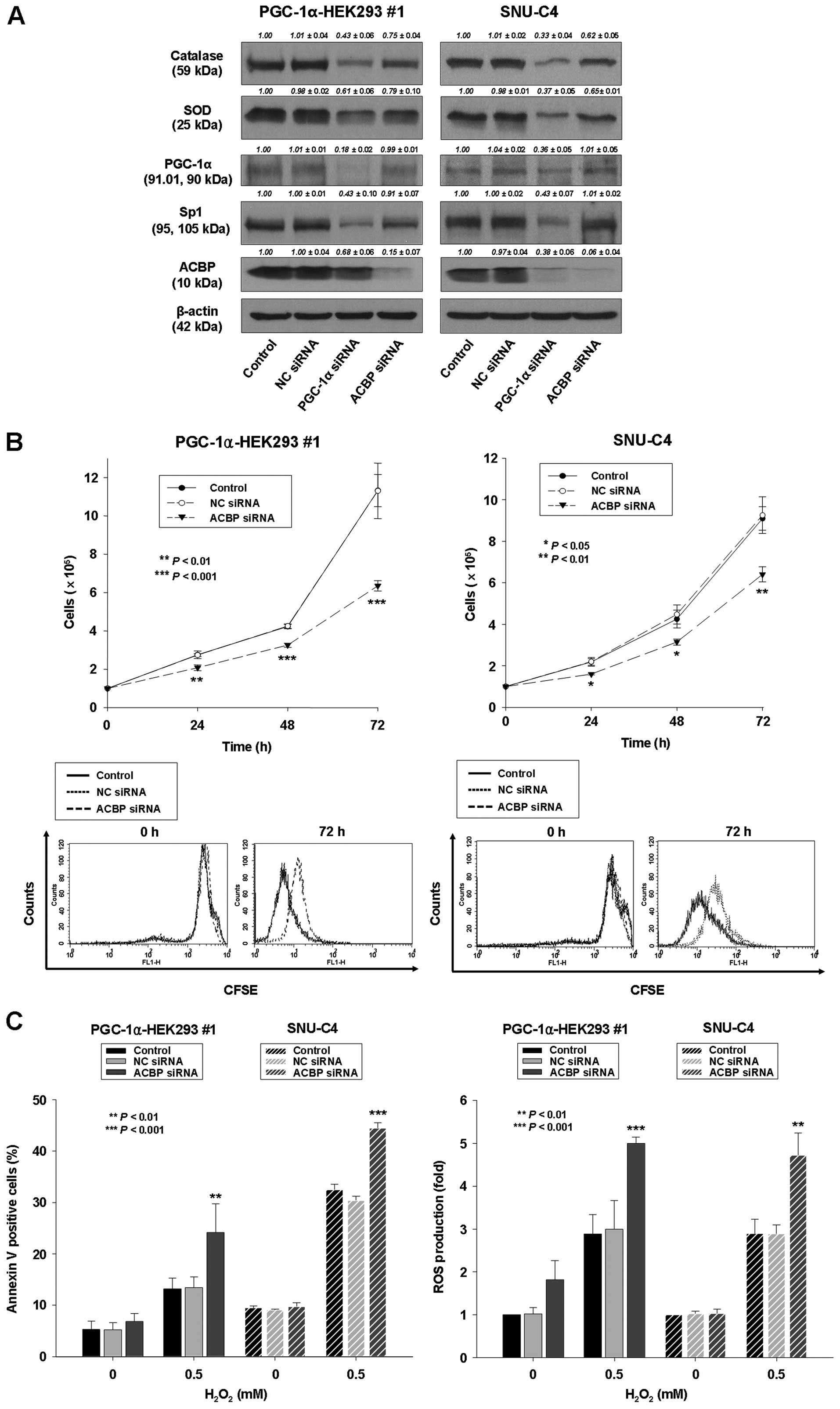
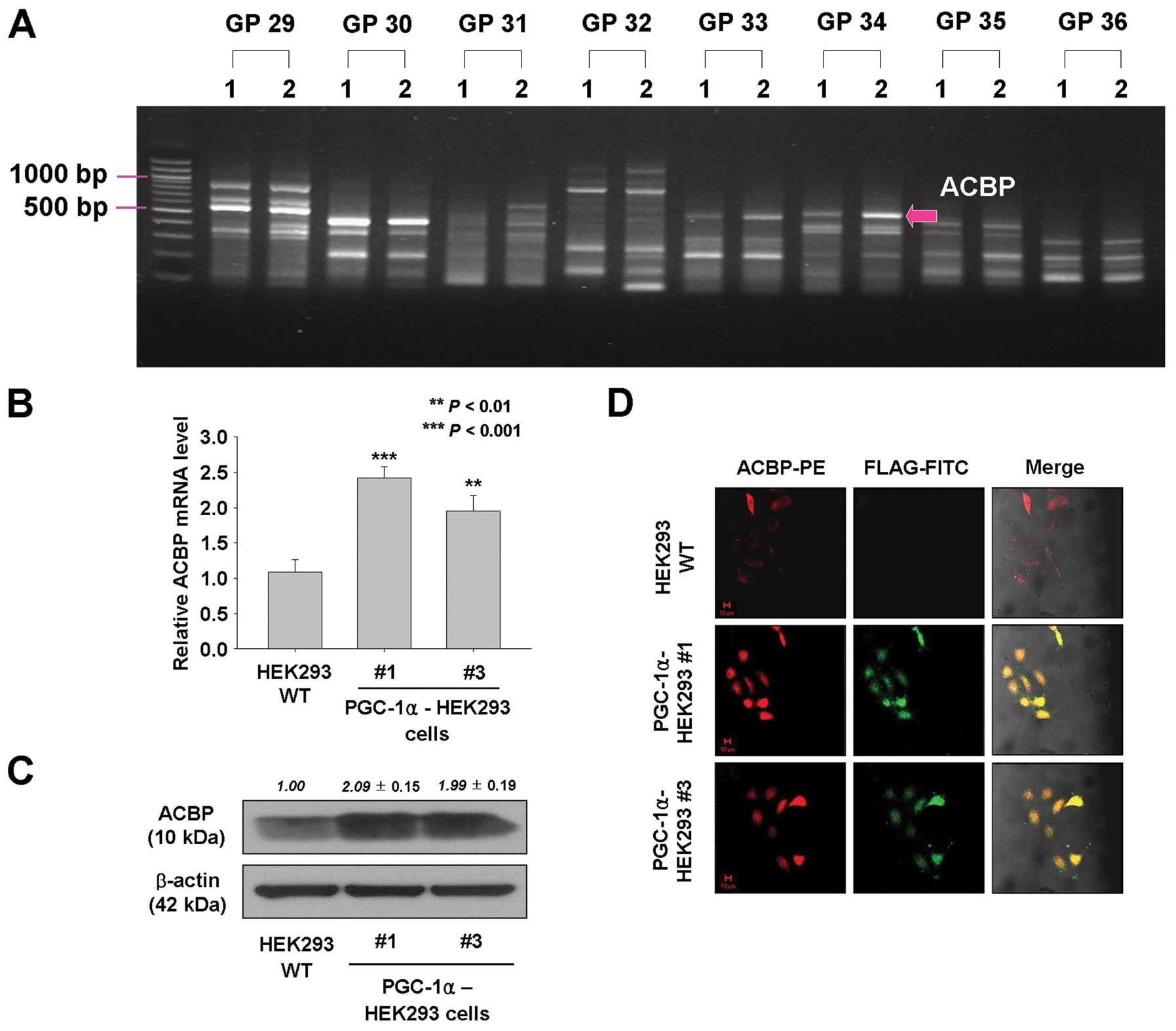
|
1
|
Puigserver P, Wu ZD, Park CW, Graves R,
Wright M and Spiegelman BM: A cold-inducible coactivator of nuclear
receptors linked to adaptive thermogenesis. Cell. 92:829–839. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Finck BN and Kelly DP: PGC-1 coactivators:
inducible regulators of energy metabolism in health and disease. J
Clin Invest. 116:615–622. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
3
|
Handschin C and Spiegelman BM: Peroxisome
proliferator-activated receptor γ coactivator 1 coactivators,
energy homeostasis, and metabolism. Endocr Rev. 27:728–735. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wu Z, Puigserver P, Andersson U, Zhang C,
Adelmant G, Mootha V, Troy A, Cinti S, Lowell B, Scarpulla RC and
Spiegelman BM: Mechanisms controlling mitochondrial biogenesis and
respiration through the thermogenic coactivator PGC-1. Cell.
98:115–124. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Vega RB, Huss JM and Kelly DP: The
coactivator PGC-1 cooperates with peroxisome proliferator-activated
receptor α in transcriptional control of nuclear genes encoding
mitochondrial fatty acid oxidation enzymes. Mol Cell Biol.
20:1868–1876. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wang YX, Lee CH, Tiep S, Yu RT, Ham J,
Kang H and Evans RM: Peroxisome-proliferator-activated receptor δ
activates fat metabolism to prevent obesity. Cell. 113:159–170.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Herzig S, Long F, Jhala US, Hedrick S,
Quinn R, Bauer A, Rudolph D, Schutz G, Yoon C, Puigserver P,
Spiegelman B and Montminy M: CREB regulates hepatic gluconeogenesis
through the coactivator PGC-1. Nature. 413:179–183. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Yoon JC, Puigserver P, Chen GX, Donovan J,
Wu ZD, Rhee J, Adelmant G, Stafford J, Kahn CR, Granner DK, Newgard
CB and Spiegelman BM: Control of hepatic gluconeogenesis through
the transcriptional coactivator PGC-1. Nature. 413:131–138. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lin J, Wu H, Tarr PT, Zhang CY, Wu Z, Boss
O, Michael LF, Puigserver P, Isotani E, Olson EN, Lowell BB,
Bassel-Duby R and Spiegelman BM: Transcriptional co-activator
PGC-1α drives the formation of slow-twitch muscle fibres. Nature.
418:797–801. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
St-Pierre J, Drori S, Uldry M, Silvaggi
JM, Rhee J, Jäger S, Handschin C, Zheng K, Lin J, Yang W, Simon DK,
Bachoo R and Spiegelman BM: Suppression of reactive oxygen species
and neurodegeneration by the PGC-1 transcriptional coactivators.
Cell. 127:397–408. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Liu C, Li S, Liu T, Borjigin J and Lin JD:
Transcriptional coactivator PGC-1α integrates the mammalian clock
and energy metabolism. Nature. 447:477–481. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Knutti D, Kaul A and Kralli A: A
tissue-specific coactivator of steroid receptors, identified in a
functional genetic screen. Mol Cell Biol. 20:2411–2422. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Tcherepanova I, Puigserver P, Norris JD,
Spiegelman BM and McDonnell DP: Modulation of estrogen receptor-α
transcriptional activity by the coactivator PGC-1. J Biol Chem.
275:16302–16308. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Delerive P, Wu YF, Burris TP, Chin WW and
Suen CS: PGC-1 functions as a transcriptional coactivator for the
retinoid X receptors. J Biol Chem. 277:3913–3917. 2002. View Article : Google Scholar
|
|
15
|
Schreiber SN, Knutti D, Brogli K, Uhlmann
T and Kralli A: The transcriptional coactivator PGC-1 regulates the
expression and activity of the orphan nuclear receptor
estrogen-related receptor α (ERRα). J Biol Chem. 278:9013–9018.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Jiang WG, Douglas-Jones A and Mansel RE:
Expression of peroxisome-proliferator activated receptor-γ (PPAR-γ)
and the PPARγ co-activator, PGC-1, in human breast cancer
correlates with clinical outcomes. Int J Cancer. 106:752–757. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Watkins G, Douglas-Jones A, Mansel RE and
Jiang WG: The localisation and reduction of nuclear staining of
PPARγ and PGC-1 in human breast cancer. Oncol Rep. 12:483–488.
2004.PubMed/NCBI
|
|
18
|
Feilchenfeldt J, Bründler MA, Soravia C,
Tötsch M and Meier CA: Peroxisome proliferator-activated receptors
(PPARs) and associated transcription factors in colon cancer:
reduced expression of PPARγ-coactivator 1 (PGC-1). Cancer Lett.
203:25–33. 2004. View Article : Google Scholar
|
|
19
|
Zhang Y, Ba Y, Liu C, Sun G, Ding L, Gao
S, Hao J, Yu Z, Zhang J, Zen K, Tong Z, Xiang Y and Zhang CY:
PGC-1α induces apoptosis in human epithelial ovarian cancer cells
through a PPARγ-dependent pathway. Cell Res. 17:363–373. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Cowell RM, Talati P, Blake KR,
Meador-Woodruff JH and Russell JW: Identification of novel targets
for PGC-1α and histone deacetylase inhibitors in neuroblastoma
cells. Biochem Biophys Res Commun. 379:578–582. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Shiota M, Yokomizo A, Tada Y, Inokuchi J,
Tatsugami K, Kuroiwa K, Uchiumi T, Fujimoto N, Seki N and Naito S:
Peroxisome proliferator-activated receptor γ coactivator-1α
interacts with the androgen receptor (AR) and promotes prostate
cancer cell growth by activating the AR. Mol Endocrinol.
24:114–127. 2010. View Article : Google Scholar
|
|
22
|
Kang W, Nielsen O, Fenger C, Leslie G,
Holmskov U and Reid KB: Induction of DMBT1 expression by reduced
ERK activity during a gastric mucosa differentiation-like process
and its association with human gastric cancer. Carcinogenesis.
26:1129–1137. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kim YJ, Kwak CI, Gu YY, Hwang IT and Chun
JY: Annealing control primer system for identification of
differentially expressed genes on agarose gels. Biotechniques.
36:424–430. 2004.PubMed/NCBI
|
|
24
|
Shin SW, Seo CY, Han H, Han JY, Jeong JS,
Kwak JY and Park JI: 15d-PGJ2 induces apoptosis by
reactive oxygen species-mediated inactivation of Akt in leukemia
and colorectal cancer cells and shows in vivo antitumor activity.
Clin Cancer Res. 15:5414–5425. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Radkov SA, Kellam P and Boshoff C: The
latent nuclear antigen of Kaposi sarcoma-associated herpesvirus
targets the retinoblastoma-E2F pathway and with the oncogene H-ras
transforms primary rat cells. Nat Med. 6:1121–1127. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Valle I, Alvarez-Barrientos A, Arza E,
Lamas S and Monsalve M: PGC-1α regulates the mitochondrial
antioxidant defense system in vascular endothelial cells.
Cardiovasc Res. 66:562–573. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Sandberg MB, Bloksgaard M, Duran-Sandoval
D, Duval C, Staels B and Mendrup S: The gene encoding
acyl-CoA-binding protein is subject to metabolic regulation by both
sterol regulatory element-binding protein and peroxisome
proliferator-activated receptor α in hepatocytes. J Biol Chem.
280:5258–5266. 2005. View Article : Google Scholar
|
|
28
|
Bhalla K, Hwang BJ, Dewi RE, Qu L, Twaddel
W, Fang H, Vafai SB, Vazquez F, Puigserver P, Boros L and Girrun
GD: PGC-1α promotes tumor growth by inducing gene expression
programs supporting lipogenesis. Cancer Res. 71:6888–6898. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Diplock AT, Rice-Evans CA and Burdon RH:
Is there a significant role for lipid peroxidation in the causation
of malignancy and for antioxidants in cancer prevention? Cancer
Res. 54:1952–1965. 1994.
|
|
30
|
Lee JM: Inhibition of p53-dependent
apoptosis by the KIT tyrosine kinase: regulation of mitochondrial
permeability transition and reactive oxygen species generation.
Oncogene. 17:1653–1662. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Guha M, Bai W, Nadler JL and Natarajan R:
Molecular mechanisms of tumor necrosis factor gene expression in
monocytic cells via hyperglycemia-induced oxidant stress-dependent
and -independent pathways. J Biol Chem. 275:17728–17739. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Borboni P, Condorelli L, De Stefanis P,
Sesti G and Lauro R: Modulation of insulin secretion by diazepam
binding inhibitor and its processing products. Neuropharmacology.
30:1399–1403. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Chen ZW, Agerberth B, Gell K, Andersson M,
Mutt V, Ostenson CG, Efendić S, Barros-Söderling J, Persson B and
Jörnvall H: Isolation and characterization of porcine
diazepam-binding inhibitor, a polypeptide not only of cerebral
occurrence but also common in intestinal tissues and with effects
on regulation of insulin release. Eur J Biochem. 174:239–245. 1998.
View Article : Google Scholar
|
|
34
|
Besman MJ, Yanagibashi K, Lee TD, Kawamura
M, Hall PF and Shively JE: Identification of
des-(Gly-Ile)-endozepine as an effector of corticotropin-dependent
adrenal steroidogenesis: stimulation of cholesterol delivery is
mediated by the peripheral benzodiazepine receptor. Proc Natl Acad
Sci USA. 86:4897–4901. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Boujrad N, Hudson JR Jr and Papadopoulos
V: Inhibition of hormone-stimulated steroidogenesis in cultured
Leydig tumor cells by a cholesterol-linked phosphorothioate
oligodeoxynucleotide antisense to diazepam-binding inhibitor. Proc
Natl Acad Sci USA. 90:5728–5731. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Papadopoulos V and Brown AS: Role of the
peripheral-type benzodiazepine receptor and the polypeptide
diazepam binding inhibitor in steroidogenesis. J Steroid Biochem
Mol Biol. 53:103–110. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Garnier M, Boujrad N, Oke BO, Brown AS,
Riond J, Ferrara P, Shoyab M, Suarez-Quian CA and Papadopoulos V:
Diazepam binding inhibitor is a paracrine/autocrine regulator of
Leydig cell proliferation and steroidogenesis: action via
peripheral-type benzodiazepine receptor and independent mechanisms.
Endocrinology. 132:444–458. 1993.PubMed/NCBI
|
|
38
|
Mukhin AG, Papadopoulos V, Costa E and
Krueger KE: Mitochondrial benzodiazepine receptors regulate steroid
biosynthesis. Proc Natl Acad Sci USA. 86:9813–9816. 1989.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Papadopoulos V, Nowzari FB and Krueger KE:
Hormone-stimulated steroidogenesis is coupled to mitochondrial
benzodiazepine receptors. Tropic hormone action on steroid
biosynthesis is inhibited by flunitrazepam. J Biol Chem.
266:3682–3687. 1991.PubMed/NCBI
|
|
40
|
Alho H, Varga V and Krueger KE: Expression
of mitochondrial benzodiazepine receptor and its putative
endogenous ligand diazepam binding inhibitor in cultured primary
astrocytes and C-6 cells: relation to cell growth. Cell Growth
Differ. 5:1005–1014. 1994.PubMed/NCBI
|
|
41
|
Venturini I, Zeneroli ML, Corsi L, Baraldi
C, Ferrarese C, Pecora N, Frigo M, Alho H, Farina F and Baraldi M:
Diazepam binding inhibitor and total cholesterol plasma levels in
cirrhosis and hepatocellular carcinoma. Regul Pept. 74:31–34. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Hansen JS, Faergeman NJ, Kragelund BB and
Knudsen J: Acyl-CoA-binding protein (ACBP) localizes to the
endoplasmic reticulum and Golgi in a ligand-dependent manner in
mammalian cells. Biochem J. 410:463–472. 2008. View Article : Google Scholar
|
|
43
|
Petrescu AD, Payne HR, Boedecker A, Chao
H, Hertz R, Bar-Tana J, Schroeder F and Kier AB: Physical and
functional interaction of Acyl-CoA-binding protein with hepatocyte
nuclear factor-4α. J Biol Chem. 278:1813–1824. 2003. View Article : Google Scholar
|
|
44
|
Wang L, Wei D, Huang S, Peng Z, Le X, Wu
TT, Yao J, Ajani J and Xie K: Transcription factor Sp1 expression
is a significant predictor of survival in human gastric cancer.
Clin Cancer Res. 9:6371–6380. 2003.PubMed/NCBI
|
|
45
|
Safe S and Abdelrahim M: Sp transcription
factor family and its role in cancer. Eur J Cancer. 41:2438–2448.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Yao JC, Wang L, Wei D, Gong W, Hassan M,
Wu TT, Mansfield P, Ajani J and Xie K: Association between
expression of transcription factor Sp1 and increased vascular
endothelial growth factor expression, advanced stage, and poor
survival in patients with resected gastric cancer. Clin Cancer Res.
10:4109–4117. 2003. View Article : Google Scholar
|
|
47
|
Yuan P, Wang L, Wei D, Zhang J, Jia Z, Li
Q, Le X, Wang H, Yao J and Xie K: Therapeutic inhibition of Sp1
expression in growing tumors by mithramycin A correlates directly
with potent antiangiogenic effects on human pancreatic cancer.
Cancer. 110:2682–2690. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Jiang Y, Wang L, Gong W, Wei D, Le X, Yao
J, Ajani J, Abbruzzese JL, Huang S and Xie K: A high expression
level of insulin-like growth factor 1 receptor is associated with
increased expression of transcription factor Sp1 and regional lymph
node metastasis of human gastric cancer. Clin Exp Metastasis.
21:755–764. 2004. View Article : Google Scholar
|
|
49
|
Lou Z, O’Reilly S, Liang H, Maher VM,
Sleight SD and McCormick JJ: Down-regulation of overexpressed sp1
protein in human fibrosarcoma cell lines inhibits tumor formation.
Cancer Res. 65:1007–1017. 2005.PubMed/NCBI
|
|
50
|
Nenoi M, Ichimura S, Mita K, Yukawa O and
Cartwright IL: Regulation of the catalase gene promoter by Sp1,
CCAAT-recognizing factors, and a WT1/Egr-related factor in hydrogen
peroxide-resistant HP 100 cells. Cancer Res. 61:5885–5894.
2001.PubMed/NCBI
|
|
51
|
Dhar SK, Xu Y, Chen Y and St Clair DK:
Specificity protein 1-dependent p53-mediated suppression of human
manganese superoxide dismutase gene expression. J Biol Chem.
281:21698–21709. 2006. View Article : Google Scholar : PubMed/NCBI
|