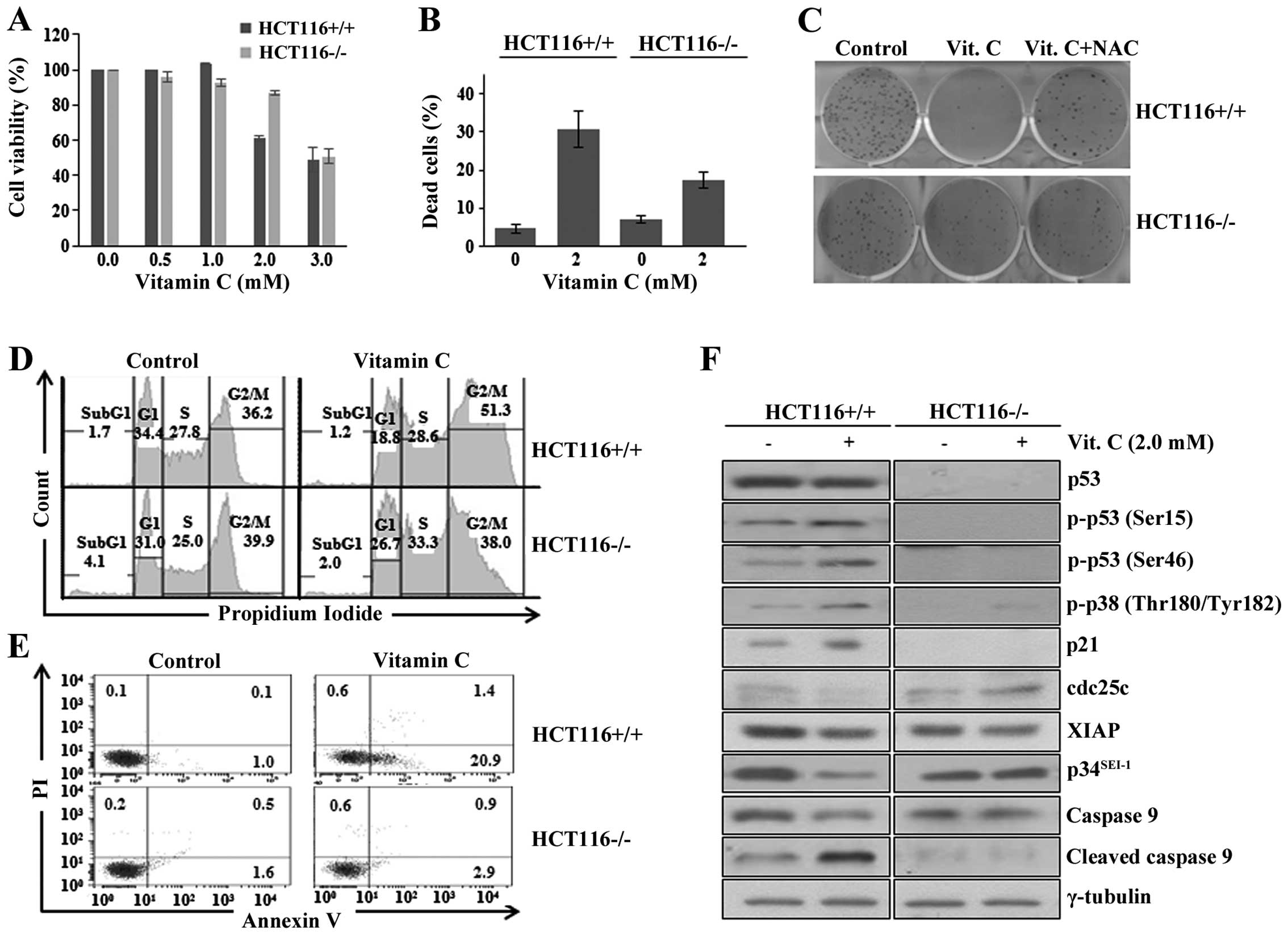
|
1
|
Du J, Cullen JJ and Buettner GR: Ascorbic
acid: chemistry, biology and the treatment of cancer. Biochim
Biophys Acta. 1826:443–457. 2012.PubMed/NCBI
|
|
2
|
Head KA: Ascorbic acid in the prevention
and treatment of cancer. Altern Med Rev. 3:174–186. 1998.PubMed/NCBI
|
|
3
|
Cameron E and Pauling L: Supplemental
ascorbate in the supportive treatment of cancer: prolongation of
survival times in terminal human cancer. Proc Natl Acad Sci USA.
73:3685–3689. 1976. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Chen Q, Espey MG, Sun AY, et al:
Pharmacologic doses of ascorbate act as a prooxidant and decrease
growth of aggressive tumor xenografts in mice. Proc Natl Acad Sci
USA. 105:11105–11109. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
De Laurenzi V, Melino G, Savini I,
Annicchiarico-Petruzzelli M, Finazzi-Agro A and Avigliano L: Cell
death by oxidative stress and ascorbic acid regeneration in human
neuroectodermal cell lines. Eur J Cancer. 31A:463–466. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Verrax J and Calderon PB: Pharmacologic
concentrations of ascorbate are achieved by parenteral
administration and exhibit antitumoral effects. Free Radic Biol
Med. 47:32–40. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Chen Q, Espey MG, Krishna MC, et al:
Pharmacologic ascorbic acid concentrations selectively kill cancer
cells: action as a pro-drug to deliver hydrogen peroxide to
tissues. Proc Natl Acad Sci USA. 102:13604–13609. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hoffer LJ, Levine M, Assouline S, et al:
Phase I clinical trial of i.v ascorbic acid in advanced malignancy.
Ann Oncol. 19:1969–1974. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Park S, Ahn ES, Lee S, et al: Proteomic
analysis reveals upregulation of RKIP in S-180 implanted BALB/C
mouse after treatment with ascorbic acid. J Cell Biochem.
106:1136–1145. 2009. View Article : Google Scholar
|
|
10
|
Belin S, Kaya F, Duisit G, Giacometti S,
Ciccolini J and Fontes M: Antiproliferative effect of ascorbic acid
is associated with the inhibition of genes necessary to cell cycle
progression. PLoS One. 4:e44092009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Chen Q, Espey MG, Sun AY, et al: Ascorbate
in pharmacologic concentrations selectively generates ascorbate
radical and hydrogen peroxide in extracellular fluid in vivo. Proc
Natl Acad Sci USA. 104:8749–8754. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hong SW, Jin DH, Hahm ES, et al: Ascorbate
(vitamin C) induces cell death through the apoptosis-inducing
factor in human breast cancer cells. Oncol Rep. 18:811–815.
2007.PubMed/NCBI
|
|
13
|
Kim J, Lee SD, Chang B, et al: Enhanced
antitumor activity of vitamin C via p53 in cancer cells. Free Radic
Biol Med. 53:1607–1615. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Hsu SI, Yang CM, Sim KG, Hentschel DM,
O’Leary E and Bonventre JV: TRIP-Br: a novel family of PHD zinc
finger- and bromodomain-interacting proteins that regulate the
transcriptional activity of E2F-1/DP-1. EMBO J. 20:2273–2285. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Li J, Muscarella P, Joo SH, et al:
Dissection of CDK4-binding and transactivation activities of
p34(SEI-1) and comparison between functions of p34(SEI-1) and
p16(INK4A). Biochemistry. 44:13246–13256. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ried T, Petersen I, Holtgreve-Grez H, et
al: Mapping of multiple DNA gains and losses in primary small cell
lung carcinomas by comparative genomic hybridization. Cancer Res.
54:1801–1806. 1994.PubMed/NCBI
|
|
17
|
Tang TC, Sham JS, Xie D, et al:
Identification of a candidate oncogene SEI-1 within a minimal
amplified region at 19q13.1 in ovarian cancer cell lines. Cancer
Res. 62:7157–7161. 2002.PubMed/NCBI
|
|
18
|
Hong SW, Shin JS, Lee YM, et al: p34
(SEI-1) inhibits ROS-induced cell death through suppression of
ASK1. Cancer Biol Ther. 12:421–426. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Hong SW, Kim CJ, Park WS, et al:
p34SEI-1 inhibits apoptosis through the stabilization of
the X-linked inhibitor of apoptosis protein: p34SEI-1 as
a novel target for anti-breast cancer strategies. Cancer Res.
69:741–746. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Roos-Mattjus P and Sistonen L: The
ubiquitin-proteasome pathway. Ann Med. 36:285–295. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ciechanover A and Schwartz AL: The
ubiquitin system: pathogenesis of human diseases and drug
targeting. Biochim Biophys Acta. 1695:3–17. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Komander D and Rape M: The ubiquitin code.
Annu Rev Biochem. 81:203–229. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Berkers CR and Ovaa H: Drug discovery and
assay development in the ubiquitin-proteasome system. Biochem Soc
Trans. 38:14–20. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Matsuzawa S, Takayama S, Froesch BA,
Zapata JM and Reed JC: p53-inducible human homologue of Drosophila
seven in absentia (Siah) inhibits cell growth: suppression by
BAG-1. EMBO J. 17:2736–2747. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Roperch JP, Lethrone F, Prieur S, et al:
SIAH-1 promotes apoptosis and tumor suppression through a network
involving the regulation of protein folding, unfolding, and
trafficking: identification of common effectors with p53 and
p21(Waf1). Proc Natl Acad Sci USA. 96:8070–8073. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Qi J, Kim H, Scortegagna M and Ronai ZA:
Regulators and effectors of Siah ubiquitin ligases. Cell Biochem
Biophys. 67:15–24. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Nemani M, Linares-Cruz G,
Bruzzoni-Giovanelli H, et al: Activation of the human homologue of
the Drosophila sina gene in apoptosis and tumor suppression. Proc
Natl Acad Sci USA. 93:9039–9042. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Telerman A and Amson R: The molecular
programme of tumour reversion: the steps beyond malignant
transformation. Nat Rev Cancer. 9:206–216. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Wu H, Lin Y, Shi Y, et al: SIAH-1
interacts with mammalian polyhomeotic homologues HPH2 and affects
its stability via the ubiquitin-proteasome pathway. Biochem Biophys
Res Commun. 397:391–396. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Yoshibayashi H, Okabe H, Satoh S, et al:
SIAH1 causes growth arrest and apoptosis in hepatoma cells through
beta-catenin degradation-dependent and -independent mechanisms.
Oncol Rep. 17:549–556. 2007.PubMed/NCBI
|
|
31
|
Kim SY, Choi DW, Kim EA and Choi CY:
Stabilization of HIPK2 by escape from proteasomal degradation
mediated by the E3 ubiquitin ligase Siah1. Cancer Lett.
279:177–184. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Matsuzawa SI and Reed JC: Siah-1, SIP, and
Ebi collaborate in a novel pathway for beta-catenin degradation
linked to p53 responses. Mol Cell. 7:915–926. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Matsuo K, Satoh S, Okabe H, et al: SIAH1
inactivation correlates with tumor progression in hepatocellular
carcinomas. Genes Chromosomes Cancer. 36:283–291. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Winter M, Sombroek D, Dauth I, et al:
Control of HIPK2 stability by ubiquitin ligase Siah-1 and
checkpoint kinases ATM and ATR. Nat Cell Biol. 10:812–824. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Herst PM, Broadley KW, Harper JL and
McConnell MJ: Pharmacological concentrations of ascorbate
radiosensitize glioblastoma multiforme primary cells by increasing
oxidative DNA damage and inhibiting G2/M arrest. Free Radic Biol
Med. 52:1486–1493. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Kang JS, Cho D, Kim YI, et al: Sodium
ascorbate (vitamin C) induces apoptosis in melanoma cells via the
down-regulation of transferrin receptor dependent iron uptake. J
Cell Physiol. 204:192–197. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Thomas CG, Vezyraki PE, Kalfakakou VP and
Evangelou AM: Vitamin C transiently arrests cancer cell cycle
progression in S phase and G2/M boundary by modulating the kinetics
of activation and the subcellular localization of Cdc25C
phosphatase. J Cell Physiol. 205:310–318. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Khanna KK, Keating KE, Kozlov S, et al:
ATM associates with and phosphorylates p53: mapping the region of
interaction. Nat Genet. 20:398–400. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Oda K, Arakawa H, Tanaka T, et al:
p53AIP1, a potential mediator of p53-dependent apoptosis, and its
regulation by Ser-46-phosphorylated p53. Cell. 102:849–862. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Perfettini JL, Castedo M, Nardacci R, et
al: Essential role of p53 phosphorylation by p38 MAPK in apoptosis
induction by the HIV-1 envelope. J Exp Med. 201:279–289. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Kim JE, Jin DH, Lee SD, et al: Vitamin C
inhibits p53-induced replicative senescence through suppression of
ROS production and p38 MAPK activity. Int J Mol Med. 22:651–655.
2008.PubMed/NCBI
|
|
42
|
Cheung HH, LaCasse EC and Korneluk RG:
X-linked inhibitor of apoptosis antagonism: strategies in cancer
treatment. Clin Cancer Res. 12:3238–3242. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Schimmer AD, Dalili S, Batey RA and Riedl
SJ: Targeting XIAP for the treatment of malignancy. Cell Death
Differ. 13:179–188. 2006. View Article : Google Scholar
|
|
44
|
Fraser M, Leung BM, Yan X, Dan HC, Cheng
JQ and Tsang BK: p53 is a determinant of X-linked inhibitor of
apoptosis protein/Akt-mediated chemoresistance in human ovarian
cancer cells. Cancer Res. 63:7081–7088. 2003.PubMed/NCBI
|
|
45
|
Srinivasula SM, Hegde R, Saleh A, et al: A
conserved XIAP-interaction motif in caspase-9 and Smac/DIABLO
regulates caspase activity and apoptosis. Nature. 410:112–116.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Datta R, Oki E, Endo K, Biedermann V, Ren
J and Kufe D: XIAP regulates DNA damage-induced apoptosis
downstream of caspase-9 cleavage. J Biol Chem. 275:31733–31738.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Frew IJ, Dickins RA, Cuddihy AR, et al:
Normal p53 function in primary cells deficient for Siah genes. Mol
Cell Biol. 22:8155–8164. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Cardaci S, Filomeni G, Rotilio G and
Ciriolo MR: Reactive oxygen species mediate p53 activation and
apoptosis induced by sodium nitroprusside in SH-SY5Y cells. Mol
Pharmacol. 74:1234–1245. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Guo J, Wu G, Bao J, Hao W, Lu J and Chen
X: Cucurbitacin B induced ATM-mediated DNA damage causes G2/M cell
cycle arrest in a ROS-dependent manner. PLoS One. 9:e881402014.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Yang J, Wu LJ, Tashino S, Onodera S and
Ikejima T: Protein tyrosine kinase pathway-derived ROS/NO
productions contribute to G2/M cell cycle arrest in
evodiamine-treated human cervix carcinoma HeLa cells. Free Radic
Res. 44:792–802. 2010. View Article : Google Scholar : PubMed/NCBI
|